Introduction
Congenital myasthenic syndromes (CMS) are a group of rare inherited disorders of neuromuscular transmission.1 Clinical presentations range from predominant ptosis, ophthalmoparesis, facial and bulbar weakness, and generalized muscle weakness to predominant limb-girdle weakness with sparing of the eye and face muscles. Symptoms may appear during the neonatal period, late childhood, adolescence or even adulthood. Clinical presentation and response to treatment may be influenced by the underlying molecular mechanism.
Mutations in more than 30 genes have been identified as causing CMS.1 The primary pathogenic mechanism is defective neuromuscular junction (NMJ) transmission but may include central nervous system and skeletal muscle involvement. Biallelic loss-of-function (LOF) genetic mutations in SCN4A encoding skeletal muscle sodium channel NaV1.4 are a rare cause of CMS.2–6 Heterozygous carriers are asymptomatic, demonstrating recessive inheritance. NaV1.4 conducts the depolarizing current of the skeletal muscle action potential that when reduced results in attenuated action potentials and muscle force.
Biallelic SCN4A LOF mutations can also be found in patients diagnosed with congenital myopathy6–8 and occasionally hypokalemic periodic paralysis (hypoPP).9,10 A common pathogenic mechanism can account for the notion that patients diagnosed with SCN4A-associated CMS may present with additional features of myopathy5 or hypoPP4. The mutant NaV1.4 channels within SCN4A LOF clinical spectra show distinct functional defects. Mutations associated with congenital myopathy show a range of alterations on NaV1.4 channel function but one allele is often null.6–8 Hitherto reported CMS-associated mutations enhance channel inactivation2–5 and typically affect fourth voltage sensing domain (VSD) of NaV1.4, the key VSD implicated in control of channel inactivation. Depolarization of the muscle may lead to excess accumulation of the mutant channels in an inactivated state leading to episodes of muscle weakness. Mutant NaV1.4 channels associated with hypoPP typically show mixed loss- and gain-of-function (GOF) features, but dominant inheritance and depolarized muscle fibers in hypoPP patients indicate GOF features as the main pathogenic mechanism.6 However, on occasion, hypoPP shows recessive inheritance suggesting a contribution of LOF features to the clinical presentation.9,10
Results
We report a 25-year-old consanguineous Turkish woman who presented from infancy with fluctuating stridor, dysphonia, dyspnea and limb weakness that persisted for days or weeks and aggravated during menstruation. She gave informed consent for this study. Clinical examination showed mild hyperlordosis, dysphonia, and speech-induced stridor. Amyotrophy, fixed muscle weakness, palpebral ptosis, diplopia, dyskalemia or myotonia were not observed. Tendon reflexes, serum CK levels and serum potassium levels were normal during episodes. Nerve conduction studies, 3-Hertz repetitive nerve stimulations (RNS), post-exercise RNS, electromyography (EMG) were normal but long exercise test (LET) (supplementary methods) exemplified a 35% ulnar nerve CMAP decrease from baseline (53% from peak). Pulmonary function tests, electrocardiogram, echocardiogram, thymus CT-scan, and muscle biopsy were unremarkable. Anti-AchR & anti-MUSK serum antibodies were absent. Single-fiber EMG was not performed. As she presented with childhood-onset fluctuating muscle weakness predominantly involving bulbar muscles, she was eventually diagnosed with CMS. Pyridostigmine, amifanpridine, and acetazolamide treatments were unsuccessful.
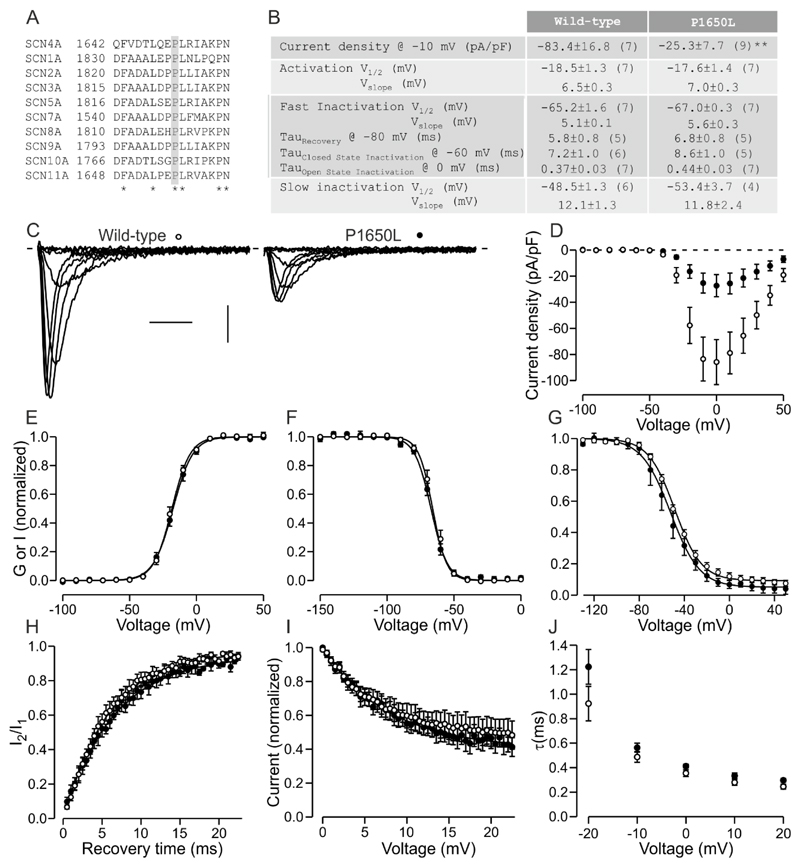
Targeted exon sequencing (supplementary methods) found c.4949C>T variation in exon 24 of the SCN4A gene (NM_000334.4) leading to a p.(Pro1650Leu) (P1650L) in C-terminus of NaV1.4 while CHRNE was normal. The mutated proline is conserved among NaV isoforms (Fig 1A) and the mutation is extremely rare in the gnomAD database (4/277124 alleles). The variation was homozygous in the patient but heterozygous in her asymptomatic parents and two brothers suggesting recessive inheritance.
Figure 1.
Functional characterization of the P1650L variant. A. Sequence alignment of Nav family. Fully conserved proline is highlighted in grey. Fully conserved residues are indicated by *. B. Table of parameters for wild-type and P1650L channels. Number of cells is shown in parenthesis for each parameter. **: p<0.01. C. Representative traces of wild-type and P1650L activation in response to voltage steps ranging from -60 mV to + 10 mV. X-axis: 2 ms, y-axis: 20 pA/pF. D-J. Wild-type data is shown in open symbols, P1650L data in closed symbols. Solid lines show fit of Boltzmann (E-G) or exponential (H-I) equation to mean data. D. Current density is plotted against test voltage. E. Voltage dependence of activation. Peak conductance is plotted against test voltage. F-G. Voltage dependence of fast (F) and slow (G) inactivation. Current in response to test pulse is plotted against the pre-pulse voltage. H. Recovery from inactivation. Current in response to a second test pulse relative to current in response to first pulse is plotted against the duration at recovery voltage -80 mV. I. Onset of close state inactivation. Current in response to test pulse is plotted against duration of pre-pulse to -60 mV. J. Open state inactivation. Time constant of a fit of an exponential curve to inactivation following channel opening is plotted against the test voltage.
Patch clamp analysis (supplementary methods) of wild-type and P1650L mutant channels did not reveal changes in the voltage dependence of activation, but the current density of the cells expressing the mutant channels was less than 30% of the cells expressing the wild-type channel (p<0.01, Fig 1). Voltage dependence of fast- or slow inactivation, or time constants of onset of open- or closed state inactivation or of recovery from inactivation did not differ between mutant and wild-type channels (Fig 1). These data indicate that P1650L mutation reduces functional expression levels of NaV1.4 channel.
Discussion
Our patient was clinically diagnosed with CMS based on the presentation of fluctuating weakness predominantly affecting bulbar muscles. The genetic diagnosis of biallelic LOF SCN4A variants places the patient on a clinical spectra of congenital myopathy-CMS-hypoPP. Fluctuating weakness suggests a myasthenic rather than myopathic disorder. The very early onset of symptoms and absence of dyskalemia although not excluding a diagnosis of hypoPP are atypical. Although CMS best describes the clinical picture EMG investigations did not reveal indications for NMJ dysfunction. EMG signs can be negative in myasthenic disorders and are variable or absent in other SCN4A CMS cases.2–5 The prolonged episodes of weakness can also be a feature of PP however, and the LET was positive with a CMAP profile reminiscent of that described for a hypoPP patient with homozygous NaV1.4 mutation.11 Similar features of CMS with overlapping EMG evidence of hypoPP has been reported previously in a patient with biallelic LOF NaV1.4 variant.4
Unlike NaV1.4 variants associated with episodic weakness in other cases of CMS and hypoPP, P1650L reduced functional expression of the channel without gain- or loss-of function changes in biophysical properties of the channel. This suggests that biallelic NaV1.4 variants with reduced baseline availability without changes in channel biophysical profile can underlie episodic weakness. Reduced baseline availability of functional NaV1.4 channels likely increases the susceptibility of the muscle force to depolarization-induced channel inactivation.9 Accordingly, heterozygous NaV1.4 knock-out mice present with latent myasthenia.12
Mutation P1650L affects the intracellular C-terminus rather than transmembrane VSDs as described for other CMS-associated SCN4A mutations. This suggests that mutations outside the transmembrane regions should also be considered when searching for genetic cause of CMS. P1650 is located in an EF hand-like motif that was recently shown to be important in the control of trafficking of other NaV channel isoforms.13 This suggests attenuated trafficking as a potential mechanism for reduced functional expression of P1650L channel although this needs to be experimentally confirmed.
We conclude that reduced functional expression of NaV1.4 channel without changes in its biophysical properties may underlie episodic weakness and present clinically with an overlap of CMS and hypoPP.
Supplementary Material
Acknowledgements
We thank Dr Damien Sternberg for helpful discussion and Nicolas Dondaine for technical assistance.
Funding Statement
The molecular study (MYOdiagHTS panel) was supported by Association Française contre les Myopathies (AFM-16992) and CREGEMES. The work was supported by the UK Medical Research Council (grant MR/M006948/1).
Footnotes
Disclosures
Dr. A. ECHANIZ-LAGUNA reports no disclosures.
Dr. V. BIANCALANA reports no disclosures.
Dr A. NADAJ-PAKLEZA reports no disclosures.
Dr E. FOURNIER reports no disclosures.
Dr. E. MATTHEWS reports no disclosures.
Dr. M.G. HANNA reports no disclosures.
Dr. R. MANNIKKO reports no disclosures.
Competing Interests Statement.
The authors (AEL, VB, ANP, EF, EM, MGH & RM) disclose all potential competing interests.
Contributorship Statement.
AEL, VB, ANP, EF, EM, MGH & RM designed and performed research, and collected the data. AEL, EM & RM wrote the manuscript. VB, ANP, EF & MGH critically revised the manuscript for important intellectual content.
References
- 1.Vanhaesebrouck AE, Beeson D. The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies. Curr Opin Neurol. 2019;32:696–703. doi: 10.1097/WCO.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsujino A, Maertens C, Ohno K, et al. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A. 2003;100:7377–82. doi: 10.1073/pnas.1230273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold WD, Feldman DH, Ramirez S, et al. Defective fast inactivation recovery of NaV1.4 in congenital myasthenic syndrome. Ann Neurol. 2015;77:840–50. doi: 10.1002/ana.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habbout K, Poulin H, Rivier F, et al. A recessive NaV1.4 mutation underlies congenital myasthenic syndrome with periodic paralysis. Neurology. 2016;86:161–9. doi: 10.1212/WNL.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elia N, Palmio J, Castaneda MS, et al. Myasthenic congenital myopathy from recessive mutations at a single residue in NaV1.4. Neurology. 2019;92:e1405–e15. doi: 10.1212/WNL.0000000000007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2018;246:309–330. doi: 10.1007/164_2017_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaharieva IT, Thor MG, Oates EC, et al. Loss-of-function mutations in SCN4A cause severe foetal hypokinesia or 'classical' congenital myopathy. Brain. 2016;139:674–91. doi: 10.1093/brain/awv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonorazky HD, Marshall CR, Al-Murshed M, et al. Congenital myopathy with “corona” fibres, selective muscle atrophy, and craniosynostosis associated with novel recessive mutationsin SCN4A . Neuromuscul disord. 2017;27:574–80. doi: 10.1016/j.nmd.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Luo S, Sampedro Castaneda M, Matthews E, et al. Hypokalaemic periodic paralysis and myotonia in a patient with homozygous mutation p.R1451L in NaV1.4. Sci Rep. 2018;8:9714. doi: 10.1038/s41598-018-27822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groome JR, Lehmann-Horn F, Fan C, et al. NaV1.4 mutations cause hypokalaemic periodic paralysis by disrupting IIIS4 movement during recovery. Brain. 2014;137:998–1008. doi: 10.1093/brain/awu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arzel-Hezode M, Sternberg D, Tabti N, et al. Homozygosity for dominant mutations increases severity of muscle channelopathies. Muscle nerve. 2010;41:470–7. doi: 10.1002/mus.21520. [DOI] [PubMed] [Google Scholar]
- 12.Wu F, Mi W, Fu Y, et al. Mice with an NaV1.4 sodium channel null allele have latent myasthenia, without susceptibility to periodic paralysis. Brain. 2016;139:1688–99. doi: 10.1093/brain/aww070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sizova DV, Huang J, Akin EJ, et al. A 49-residue sequence motif in the C terminus of Nav1.9 regulates trafficking of the channel to the plasma membrane. J Biol Chem. 2020;24(295):1077–1090. doi: 10.1074/jbc.RA119.011424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.