Abstract
Purpose
Whilst the use of dual-energy x-ray absorptiometry (DXA) has been widely adopted worldwide for the assessment of bone mineral density, the quality of DXA facilities is unknown. To address this, a global survey of Fracture Liaison Services (FLS) was conducted by the International Society for Clinical Densitometry (ISCD) and the International Osteoporosis Foundation (IOF) to assess the quality of their DXA facilities.
Methods
A questionnaire for the accessibility and quality of DXA services was co-created by representatives of the ISCD and the IOF and made available to institutions who participated in the Capture the Fracture Best Practice Framework. From a list of 331 contacted invitees, 124 FLS centres responded; analyses were based on 121 centres with suitable data.
Results
Over 70% of institutions reported that, for over 90% of the time, DXA access met service needs and the scanning/reporting quality was perceived as excellent. However, 25% of DXA facilities reported not being accredited by a professional/governmental organisation and adherence to some basic DXA quality assurance and reporting procedures was confirmed by <50% of services. Importantly, in excess of 50% of institutions stated that they desired ongoing education in osteoporosis and DXA for operators and interpreters.
Conclusion
There is significant variability in the access to and quality of DXA services for established FLS worldwide. Despite two decades of training initiatives in osteoporosis densitometry, many centres are falling short of the standards of the IOF-ISCD Osteoporosis Essentials criteria.
Keywords: osteoporosis, epidemiology, bone densitometry, dual energy x-ray absorptiometry, fracture liaison services, quality standards
Introduction
Osteoporosis is a major public health problem worldwide with approximately 8.9 million osteoporotic fractures occurring annually [1]. Osteoporotic fractures are associated with morbidity, mortality and significant economic costs; the economic burden associated with osteoporotic fractures was estimated at €37.4 billion in the European Union in 2010 [2].
Dual-energy x-ray absorptiometry (DXA) has been a widely adopted technology since 1987 for the assessment of bone mineral density (BMD). The World Health Organisation (WHO) operational definition of osteoporosis is based on a DXA measurement of BMD at least 2.5 standard deviations below the mean of a young adult reference population (T-score ≤ -2.5). Fracture risk is estimated to approximately double for every standard deviation decrease in BMD [3]. However, a higher total number of fragility fractures occur in individuals with BMD values above the threshold for osteoporosis diagnosis, resulting in under-treatment if BMD alone was used to determine treatment thresholds. Therefore, a clinical diagnosis of osteoporosis based on the occurrence of low trauma fractures and the use of clinical risk factors to enhance fracture risk prediction [4, 5] has developed in recent years.
Osteoporosis has been referred to as the silent epidemic [6] - silent because osteoporosis and bone fragility is asymptomatic prior to a fracture. As a consequence, osteoporosis often receives less attention from governments and healthcare providers when compared to other chronic non-communicable diseases worldwide [7]. The effectiveness of an individual nation at assessing and treating osteoporosis is greatly influenced by their policy on access to, and quality of, their DXA services. For example, an audit conducted by the International Osteoporosis Foundation (IOF), looking at access and reimbursement for osteoporosis assessment and treatment in Asia Pacific, found that whilst Australia, Hong Kong, Japan, New Zealand, Republic of Korea and Singapore were well resourced with 12-24 DXA machines per million of population, China, India, Indonesia, Pakistan, Philippines, Sri Lanka and Vietnam had less than 1 DXA machine per million [8]. An audit conducted by the IOF in conjunction with the European Federation of Pharmaceutical Industry Associations (EFPIA), looking at DXA provision in the European Union, found that Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Italy, Portugal and Slovenia had at least 11 DXA machines per million (previously estimated to be the minimum number required for adequate osteoporosis care [9]); however, 9 countries were found to have very inadequate provision (Bulgaria, Czech Republic, Hungary, Latvia, Lithuania, Luxembourg, Poland, Romania and the UK) [2]. The Latin America Regional Audit, published by the IOF in 2012, found the Latin American countries with the best access to DXA were Brazil and Chile with 10 DXA machines per million, while other countries ranged from 0.9 to 6.7 per million [10]. In North America, it has been estimated that a reduction in the number of DXA scans available in the office setting has resulted in a fall in the number of DXA providers and more than 1 million fewer DXA scans performed per annum [11].
Although variation in the access to DXA services worldwide has been reported, aspects of DXA quality at facilities are largely unknown. There is also reason to believe that suboptimal DXA testing and reporting is common and may adversely affect patient care [12–15]. To address this, in collaboration with the International Society for Clinical Densitometry (ISCD), the International Osteoporosis Foundation (IOF) invited Fracture Liaison Services (FLS) to participate in a survey assessing the access to, and quality of, their DXA facilities and DXA reporting. This report outlines the findings of this survey.
Methods
Representatives of the ISCD and IOF developed and refined the DXA access and quality survey using: educational content from the IOF / ISCD joint international course Osteoporosis Essentials (https://www.osteoporosis-essentials.org); ISCD-established Official Positions and Best Practices; and experience with ISCD’s Facility Accreditation Program [12]. Formatting for the survey followed a rubric familiar to FLS sites. The IOF contacted FLS sites participating in Capture the Fracture® (a global campaign to facilitate the implementation of coordinated, multi-disciplinary models of care for secondary fracture prevention) regarding completion of the online survey formatted and distributed using SurveyMonkey). FLS centres were contacted in November 2018 with a reminder message circulated in December 2018. From a list of 331 contacted invitees, 124 FLS centres responded. Three centres answered only one question and provided no other details, and their data were excluded. Therefore, the responses from 121 centres were used for analysis in this report.
The questions asked and additional variables derived are included in Appendix 1 (Online Resource), separated into two parts (Part I: FLS Service, Part II: DXA Service Provider). Responses to multiple choice questions from FLS were described using frequencies and percentages and presented in Appendix 2 (Online Resource). Missing responses were not used in the calculation of percentages. The number of missing responses for each question is shown in Appendix 3 (Online Resource). Key results are presented graphically in figures with the corresponding question number indicated in brackets in the title of the figure.
Results
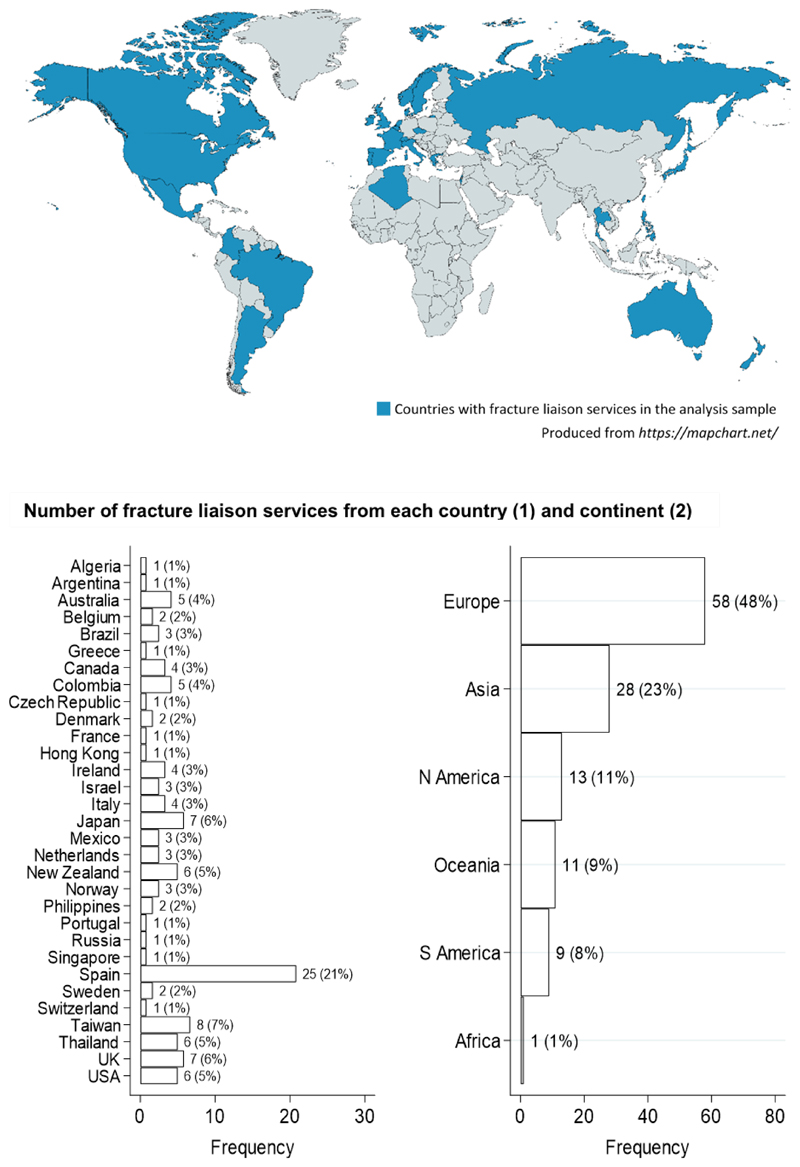
FLS from 31 countries and all inhabited continents were included in the analysis sample (Fig. 1). The country and continent with the highest number of FLS represented were Spain (n=25) and Europe (n=58), respectively.
Fig. 1. Countries where fracture liaison services in the analysis sample were located (1).
In total, 80% of FLS had access to DXA within the institution with the remainder having access to DXA outside the institution. Overall, 50% of FLS stated that they requested DXA for new patients regularly (over 90% of the time); the proportion of FLS regularly requesting DXA to monitor therapy in treated patients <75 years and ≥75 years was 52% and 44% respectively. Although, 83% said they performed DXA at specific intervals for patients initiating treatment, the length of the DXA testing intervals varied substantially between FLS.
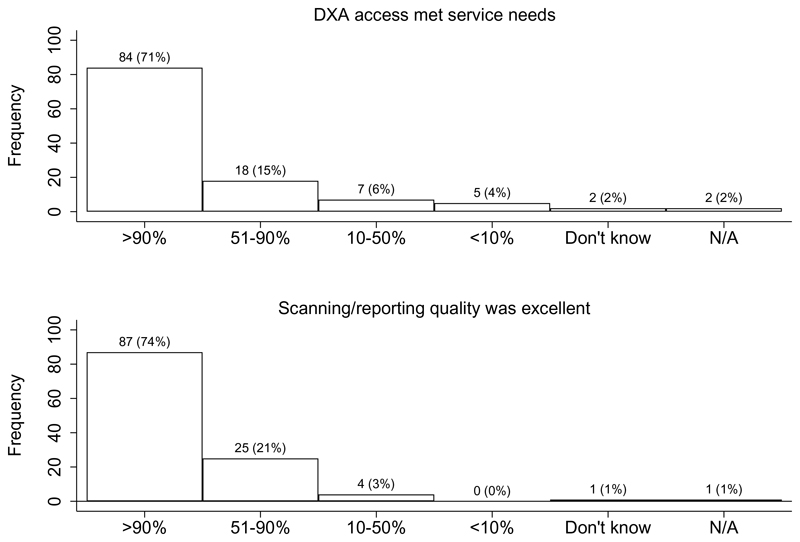
DXA access met service needs over 90% of the time for 71% of FLS, and DXA services (scanning and reporting) were reported as excellent over 90% of the time for 74% of FLS (Fig. 2). The quality of a DXA report was only questioned weekly or daily among 12% of FLS. Only 45% said their facilities had been accredited by a professional or governmental organisation, with 30% not knowing.
Fig. 2. Proportion of time DXA access met service needs (9) and scanning/reporting quality was described as excellent (10).
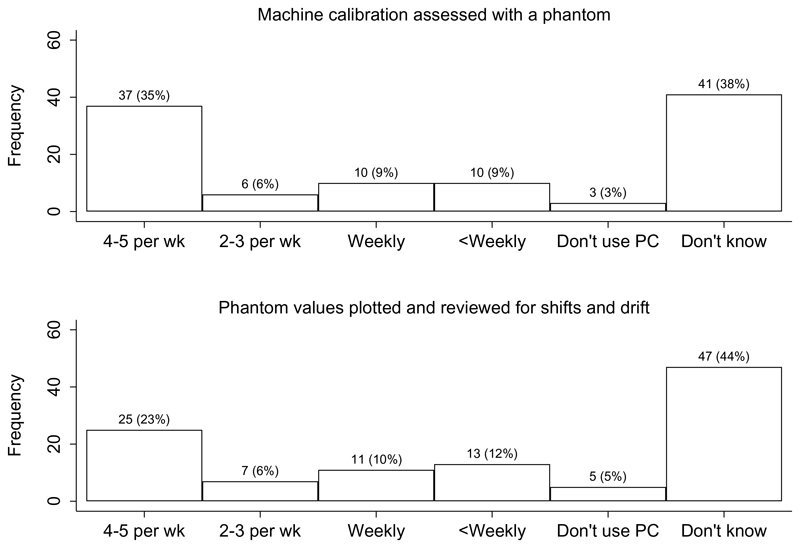
In total, 72% stated that they designated operators as supervisors of DXA activities; 59% confirmed that they required ongoing education in osteoporosis or DXA for operators; and 61% confirmed this requirement for interpreters. A facility-specific standard operating procedures manual was used in 69% of FLS (22% not knowing) with 72% of services stating that the manufacturer’s operations manual was immediately available in the DXA suite. A high proportion of FLS were unaware how often machine calibration was assessed with a phantom or how often phantom values were plotted and reviewed for shifts (sudden decline in machine accuracy/precision) and drift (gradual decline in machine accuracy/precision). However, most that were aware reported a regular occurrence of these activities (Fig. 3). Overall, 58% of FLS had numerical quality assurance rules for acceptable DXA machine performance with 35% not knowing.
Fig. 3. Frequency machine calibration was assessed with a phantom (17) and frequency phantom values were plotted and reviewed for shifts and drift (18).
PC: Phantom calibration; wk: week
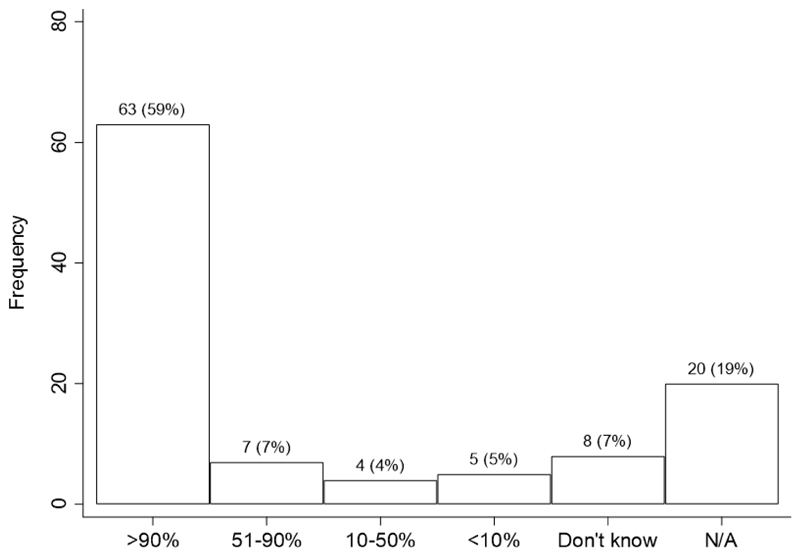
The use of questionnaires to gather patient information was common. For example, 82% of FLS used questionnaires; 59% used a standardised questionnaire to gather clinical patient information over 90% of the time (Fig. 4). Of those using questionnaires, the majority of FLS included questions about the following: indications for DXA scans (76%); clinical risk factors for fracture risk calculations (89%); use of bone-detrimental medications (83%); fracture history (94%); and treatment with osteoporotic medications (90%). The most common primary source for collecting the DXA questionnaire responses was the technologist who collected the information at the time of scan or validated patient-supplied data (31%), followed by the patient (26%) and then the referring physician (20%); only 5% did not know.
Fig. 4. Proportion of time a standardised questionnaire was used to gather clinical patient information (21).
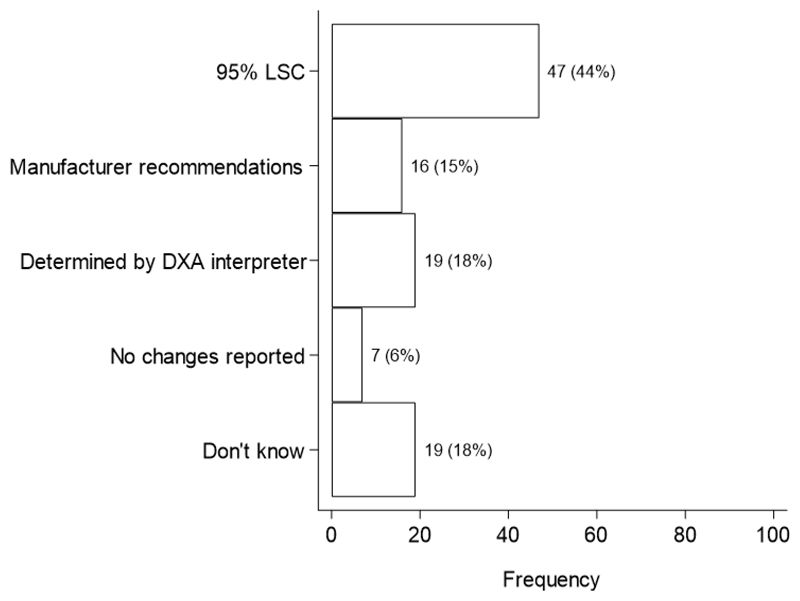
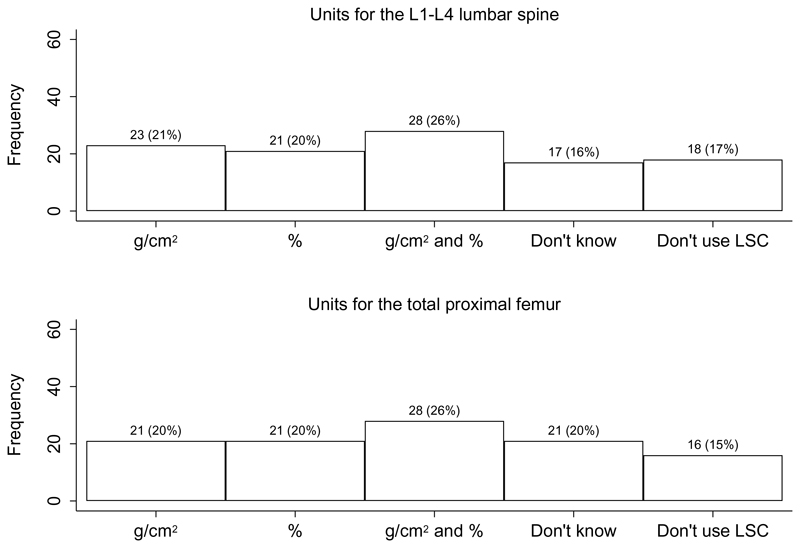
For the assessment of significant BMD changes over time, the most common method was least significant change at the 95% significance level (95% LSC), reported among 44% of FLS (Fig. 5). However, some followed manufacturer recommendations (15%) and others relied on the DXA interpreters to determine significant BMD changes (18%); only 6% of FLS did not report significant changes in BMD. The units for reporting the 95% LSC for the L1-L4 lumbar spine and total proximal femur included g/cm2, percentages and also a combination of these units (Fig. 6). Although most FLS reported one skeletal diagnostic category per measurement site (56%), some reported this per patient (35%) and a small minority did not know (9%).
Fig. 5. Method for assessing significant BMD change over time (29).
95% LSC: Least significant change at the 95% significance level
Fig. 6. Units for reporting 95% least significant change for the L1-L4 lumbar spine (30) and total proximal femur (31).
LSC: Least significant change
The majority of FLS (79%) used validated quantitative fracture risk instruments to report DXA results with 91% of those using such instruments reporting the use of FRAX®. Overall, 42% stated that they reported quantitative fracture risk over 90% of the time. Regarding the content of DXA reports, 84% of FLS confirmed that they identified body site, region of interest and body side for each valid BMD reported over 90% of the time; 78% of FLS said they had DXA reports containing information about the scanner manufacturer and model. There was more variability between FLS regarding the frequency of the following occurrences: DXA report states technical adequacy or limitations of the DXA study; DXA interpreters comment on differences in standard acquisitioning and analysis parameters; and DXA facility report recommends repeat scan intervals.
Discussion
This report provides the first worldwide sample of DXA access and quality in the FLS setting. Over 70% of institutions reported that, for over 90% of the time, DXA access met service needs and the scanning/reporting quality was perceived as excellent. A high proportion of centres used questionnaires to gather clinical patient information and FRAX® was the most commonly used tool for reporting quantitative fracture risk. Routine use of DXA for new patients and monitoring was very common as well. Conversely, adherence to some basic DXA quality assurance and reporting procedures, as taught in the IOF / ISCD joint international course Osteoporosis Essentials and reflected in an ISCD Best Practices white paper [12], was confirmed by <50% of services.
Adhering to these reported DXA quality assurance and reporting procedures are important for ensuring high quality care. For example, ongoing education for operators/interpreters ensures their skills are maintained as the scientific field evolves. Identifying the skeletal site, region of interest and body side for BMD measurements enables the same region to be scanned for follow-up measurements. Established facility-specific standard operating procedures ensures the processes for enabling high quality scanning such as machine calibration, testing and reporting are implemented systematically. Frequent phantom scanning verifies the accuracy of the BMD values reported. The use of LSC values for assessing BMD change allows clinicians to determine whether follow-up measurements reflect genuine changes in BMD. Further details on the rationale for specific DXA measurement and reporting guidelines are outlined in the ISCD Best Practices paper [12].
Overall, 55% of facilities were either not accredited by a professional/governmental organisation or the accreditation status was unknown. However, information was not available on whether DXA facility accreditation was possible or required at the FLS location. Over half of FLS programs stated that they required ongoing education in osteoporosis and DXA for operators and interpreters. Many were not aware how often machine calibration was assessed, although this was generally reported as regular among those that were aware. This suggests a widespread need for educational initiatives targeting DXA measurement technology and reporting. Perhaps adaptation of the joint IOF ISCD Osteoporosis Essentials course may contribute to such an educational initiative.
There was considerable variation in the method for assessing significant BMD change over time. Less than 50% of DXA facilities confirmed the use of Least Significant Change (LSC) values for assessing significant change in BMD over time – a cornerstone of critically assessing these changes. Furthermore, of the participating centres reporting 95% LSC for the L1-L4 lumbar spine and total proximal femur, a substantial proportion did not use g/cm2, contravening current ISCD guidelines. Finally, the recommended practice of a solitary skeletal classification based on T-score results occurred only 35% of the time with 9% not knowing. These findings point to unmet needs in osteoporosis assessment and monitoring worldwide.
A few other themes also emerged; monitoring was less routine in over 75s; there was inconsistency of the DXA testing interval in patients initiating treatment; perception of excellent scanning and reporting quality could be contrasted with poor performance for some technical quality factors such as phantom scanning; the high frequency of DXA errors reported elsewhere contrasted with 73% of facilities in this study questioning reports less than monthly or never.
Responses also pointed at a need for instrument calibration; around 40% of facilities failed to state frequency of phantom calibration and only 35% reported this being undertaken 4-5 times per week. Finally, there appears to be a need for standardisation on reporting of clinical patient information; 35% of centres did not state regular use (>50% of the time) of a pro forma questionnaire for this information but 72% stated use of FRAX® to estimate fracture risk. Clearly, the clinical risk factors included within FRAX® are being recorded; standardisation of these within a more extensive risk factor questionnaire may be given consideration.
It is well established that FLS models are beneficial for reducing the osteoporosis care gap as demonstrated in systematic reviews [16, 17]. National and international guidelines [18–20] also recommend the FLS model to improve osteoporosis assessment and management. Our findings provide evidence, however, that even where FLS models are in place, there is still significant variability in the quality of DXA services. This is consistent with previous studies which have shown that there is currently huge variation, both within and between countries, in the availability, quality and scope of secondary prevention facilities. For example, in a prospective observational study comprising over 60,000 older women recruited in 10 countries from primary care practices, it was shown that in excess of 80% did not receive osteoporosis treatment following a fragility fracture [21]. Therefore, there is a clear need for strategies that set and measure standards of care within individual FLS services to reduce variability and improve osteoporosis care; clinical or quality standards for FLS have already been developed in Canada [22], New Zealand [23] and the UK [24]. However, apart from the Royal Osteoporosis Society (ROS) in the UK, suggesting sufficient quality assured DXA scanning and reporting in a timely manner, these do not include specific standards for DXA.
Recently, the IOF has developed internationally endorsed standards for FLS in the form of the Capture the Fracture (CTF) Best Practice Framework (BPF) (http://www.capturethefracture.org/). The initiative comprises a comprehensive suite of 13 standards for: Patient Identification; Patient Evaluation; Post-fracture Assessment Timing; Vertebral Fracture; Assessment Guidelines; Secondary Causes of Osteoporosis; Falls Prevention Services; Multifaceted Health and Lifestyle Risk-factor Assessment; Medication Initiation; Medication Review; Communication Strategy; Long-term Management and Database Standard. CTF also includes a global map with a quality grading scheme on which, subject to application, secondary fracture prevention services can be documented [25]. Using this tool, the CTF initiative, aimed at raising the quality and coverage of fracture liaison services providing secondary prevention for osteoporosis, has already been shown to be effective. In 2015, a report was published which analysed the first 60 FLS to apply for Best Practice Recognition and showed significant heterogeneity in service provision. This suggests that a single framework with set criteria was able to benchmark services across healthcare systems worldwide [26]. The findings of the current study suggest heterogeneity in the quality of DXA services provided within the FLS global network, an area not specifically addressed within the CTF BPF. This may benefit from establishing DXA quality indicators to further support the successes achieved through the CTF BPF.
This study has some limitations. First, fewer countries in parts of Africa and Asia were represented in this survey and 21% of FLS were from a single country (Spain). Although the opportunity to explore variation within countries was limited, those for which more than 6 centres were available (Spain, Taiwan, UK and Japan) did reveal heterogeneity in responses, particularly in DXA request frequency and testing interval. Second, we were unable to externally verify the details of an institution’s submission and, therefore, relied on the individual institutions to properly complete the questionnaire. However, a key strength of this global study is that FLS from 31 countries and all inhabited continents were included. Third, for the question on how often the quality of a DXA report is questioned, it was not possible to know who questioned the report and any impact of this on clinical care. Fourth, trabecular bone score was unfortunately not able to be included in the initial questionnaire and its use in the FRAX® risk calculator therefore remains unknown. Fifth, for questions about the DXA service provider, it was not possible to know whether staff at the FLS facility or DXA facility provided responses. Finally, bias may have been introduced due to the level of non-response to questionnaires from FLS centres, potentially resulting in an overrepresentation of higher performance facilities which may limit the generalisability of findings. However, this would suggest that our key findings of significant variability in access to, and quality of, DXA services and the high number of centres falling short of quality standards are even greater than reported. Although some questions had a high number of non-responses among the FLS centres that were included in the analysis, the majority of questions had a low non-response rate.
In conclusion, our report confirms that there is significant variability in the access to, and quality of, DXA services worldwide. To improve osteoporosis care, strategies are required to reduce this variability. This could be achieved through defining CTF BPF standards for DXA quality with ongoing training and assessment, building on the educational collaboration between the IOF and ISCD. Such improvements are anticipated to create an even more robust and effective CTF network worldwide, and strengthen the mission of improving skeletal health around the globe.
Supplementary Material
Mini-abstract.
In a global survey of fracture liaison services, most reported that DXA access met needs. However, adherence to basic DXA quality and reporting procedures was confirmed by only around 50% of institutions and many required education for operators/interpreters. Overall, there is significant variability in the access to, and quality of, DXA services worldwide.
Acknowledgments
This study was funded by the following organisations: International Society for Clinical Densitometry; International Osteoporosis Foundation; UK Medical Research Council; University of Southampton.
Funding information
This study was funded by the following organisations: International Society for Clinical Densitometry; International Osteoporosis Foundation; UK Medical Research Council; University of Southampton.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest
MAC reports personal fees (outside the submitted work) from UCB, Pfizer, and Eli Lilly for conference attendance; CC reports personal fees (outside the submitted work) from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB. EMD reports personal fees (outside the submitted work) from Pfizer Healthcare and from the UCB Discussion panel. NCH reports consultancy, lecture fees and honoraria (outside the submitted work) from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Kyowa Kirin, Consilient Healthcare, Radius Health and Internis Pharma. JAK reports grants (outside the submitted work) from Radius Health, Amgen and UCB. CRS reports Consultant for Amgen and President of the International Society for Clinical Densitometry (2019-2020). All other authors declare that they have no conflicts of interest.
References
- 1.Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis J, Odén A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 5.Kanis J, Johnell O, Odén A, Johansson H, McCloskey E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanis J. Osteoporosis--the silent epidemic. Health Visit. 1989;62:14–15. [PubMed] [Google Scholar]
- 7.Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. doi: 10.1016/j.bone.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mithal A, Bansal B, Kyer CS, Ebeling P. The Asia-Pacific Regional Audit-Epidemiology, Costs, and Burden of Osteoporosis in India 2013: A report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014;18:449–454. doi: 10.4103/2230-8210.137485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanis J, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int. 2005;16:229–238. doi: 10.1007/s00198-004-1811-2. [DOI] [PubMed] [Google Scholar]
- 10.Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster J-Y, Rizzoli R, Cooper C, Kanis JA. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2007;28:1507–1529. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overman RA, Farley JF, Curtis JR, Zhang J, Gourlay ML, Deal CL. DXA utilization between 2006 and 2012 in commercially insured younger postmenopausal women. J Clin Densitom. 2015;18:145–149. doi: 10.1016/j.jocd.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Binkley N, Morgan SL, Shuhart CR, Camargos BM, Carey JJ, Gordon CM, Jankowski LG, Lee J-K, Leslie WD. Best practices for dual-energy X-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry Guidance. J Clin Densitom. 2016;19:127–140. doi: 10.1016/j.jocd.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Messina C, Bandirali M, Sconfienza LM, D’Alonzo NK, Di Leo G, Papini GDE, Ulivieri FM, Sardanelli F. Prevalence and type of errors in dual-energy x-ray absorptiometry. Eur Radiol. 2015;25:1504–1511. doi: 10.1007/s00330-014-3509-y. [DOI] [PubMed] [Google Scholar]
- 14.Promma S, Sritara C, Wipuchwongsakorn S, Chuamsaamarkkee K, Utamakul C, Chamroonrat W, Kositwattanarerk A, Anongpornjossakul Y, Thamnirat K, Ongphiphadhanakul B. Errors in patient positioning for bone mineral density assessment by dual X-ray absorptiometry: effect of technologist retraining. J Clin Densitom. 2018;21:252–259. doi: 10.1016/j.jocd.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Krueger D, Shives E, Siglinsky E, Libber J, Buehring B, Hansen K, Binkley N. DXA errors are common and reduced by use of a reporting template. J Clin Densitom. 2019;22:115–124. doi: 10.1016/j.jocd.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Ganda K, Puech M, Chen J, Speerin R, Bleasel J, Center J, Eisman J, March L, Seibel M. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24:393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 17.Sale J, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int. 2011;22:2067–2082. doi: 10.1007/s00198-011-1544-y. [DOI] [PubMed] [Google Scholar]
- 18.Marsh D, Åkesson K, Beaton D, Bogoch E, Boonen S, Brandi M-L, McLellan A, Mitchell P, Sale J, Wahl D. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22:2051–2065. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 19.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 20.Kanis J, Cooper C, Rizzoli R, Reginster J. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenspan SL, Wyman A, Hooven FH, Adami S, Gehlbach S, Anderson FA, Jr, Boone S, Lacroix AZ, Lindsay R, Netelenbos J, Pfeilschifter J, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60:455–461. doi: 10.1111/j.1532-5415.2011.03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osteoporosis Canada. Quality Standards for Fracture Liaison Services in Canada. [Accessed 15 April 2020];2014 https://www.osteoporosis.ca/wp-content/uploads/OC-Quality-Standards-ENG-Nov-2014.pdf.
- 23.Osteoporosis New Zealand. Clinical Standards for Fracture Liaison Services in New Zealand. [Accessed 15 April 2020];2016 https://osteoporosis.org.nz/wp-content/uploads/ONZ-FLS-Clinical-Standards-WEB.pdf.
- 24.Royal Osteoporosis Society. Effective Secondary Prevention of Fragility Fractures: Clinical Standards for Fracture Liaison Services. [Accessed 15 April 2020];2019 https://theros.org.uk/media/1eubz33w/ros-clinical-standards-for-fracture-liaison-services-august-2019.pdf.
- 25.Åkesson K, Marsh D, Mitchell PJ, McLellan A, Stenmark J, Pierroz D, Kyer C, Cooper C. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javaid M, Kyer C, Mitchell P, Chana J, Moss C, Edwards M, McLellan A, Stenmark J, Pierroz D, Schneider M, Kanis J, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int. 2015;26:2573–2578. doi: 10.1007/s00198-015-3192-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.