Abstract
In this mini-review, we analyze the influence of cysteines in the structure and activity of mitochondrial outer membrane mammalian VDAC isoforms. The three VDAC isoforms show conserved sequences, similar structures and the same gene organization. The meaning of three proteins encoded in different chromosomes must thus be searched for subtle differences at the amino acid level. Among others, cysteine content is noticeable. In humans, VDAC1 has 2, VDAC2 has 9 and VDAC3 has 6 cysteines. Recent works have shown that, at variance from VDAC1, VDAC2 and VDAC3 exhibit cysteines predicted to protrude towards the intermembrane space, making them a preferred target for oxidation by ROS. Mass spectrometry in VDAC3 revealed that a disulfide bridge can be formed and other cysteine oxidations are also detectable. Both VDAC2 and VDAC3 cysteines were mutagenized to highlight their role in vitro and in complementation assays in Δporin 1 yeast. Chemico-physical techniques revealed an important function of cysteines in the structural stabilization of the pore. In conclusion, the works available on VDAC cysteines support the notion that the three proteins are paralogs with a similar pore-function and slightly different, but important, ancillary biological functions. This article is part of a Special Issue entitled 'EBEC 2016: 19th European Bioenergetics Conference, Riva del Garda, Italy, July 2–6,2016', edited by Prof. Paolo Bernardi.
1. Introduction
Cysteines are unique amino acids in proteins for their property to undergo reversible redox reactions as part of their normal function. One of the pathways originating from cysteines oxidation is the formation of cross-linked bonds with the aim to provide structural stability to the proteins. This reaction is in principle spontaneous (but not always, since specific enzymes evolved with the function of forming disulfide bonds) and it is associated with protein folding: in this sense, it is genetically pre-determined since spatial proximity to another cysteine is a prerequisite for disulfide formation. Disulfide formation indeed requires the proximity of two cysteines but also chemical conditions favoring this process like modifications of the thiol group reactivity. In normal conditions, at the physiological pH, cysteine sulfur shows a pK a value > 8.0, which means that the sulfur is present in the protonated state (or reduced, – SH). The reducing environment, predominant in the cytosol, together with enzymes and reagents, contributes in keeping the cysteine in this form (protonated). In the presence of factors lowering the cysteine pK a, like proximity of basic residues or a general oxidative environment (acid pH, imbalance of the redox agents like the glutathione redox couple, interaction with H-bonding partners (e.g. water molecules)) the cysteine sulfur can be deprotonated, becoming an highly reactive –S–, and, in water, yielding the reactive form –SOH. In the absence of another, close, sensitive sulfur, –SOH can undergo oxidation to –SO2H and –SO3H. These last oxidation states are seldom reversible in the cell. Among factors responsible for cysteine oxidation there are ROS species, in particular H2O2 (for a comprehensive review see [1]).
The voltage-dependent anion selective channels (VDACs) are a small family of proteins whose primary role is to form an aqueous pore through the outer mitochondrial membrane (OMM) that allows the exchange of metabolites and molecules [2–4]. OMM separates the cytosol from the mitochondrial intermembrane space (IMS). IMS has physico-chemical properties distinct from the mitochondrial matrix and cytosol: the pH is more acidic than cytosol by 0.2-0.7 units [5,6], and the glutathione redox buffer is more oxidizing [6]. In addition, ROS poured mainly by Complex III in IMS [7,8]. Thus, VDACs have the peculiar situation to have moieties exposed towards two different aqueous compartments, each one with a different composition and pH (IMS and cytosol), and to connect them through a water-filled barrel made by a narrow protein layer. VDACs are thus candidates to be subjected (and witness?) to any change in the chemical gradient existing between IMS and cytosol. Interestingly VDACs have set of cysteines presumed to be water-exposed [9, 10]. The number of cysteines interestingly is variable across isoforms, and specific for each isoform. In the last years, new information arose about modifications of VDACs in response to experimental or pathological or physiological unbalance of the ratio between IMS and cytosol. In this mini-review, we will try to rationalize the available information, to uncover the role of VDAC cysteines in the mitochondrial function.
2. VDAC structures: state of the art
In chordates, and in particular in mammals, three distinct VDAC iso-forms are encoded by distinct genes, located on different chromosomes [11,12]: the three genes share the same exon-intron organization indicating that they are the result of not evolutionary far duplications [11, 12,13]. The mammalian VDAC sequences are of about 280 amino acids, with the exception of VDAC2 that has the N-terminal fragment 11 residues longer than the other isoforms. The sequences are not particularly hydrophobic despite the proteins are embedded in the membrane. A striking difference is the number of cysteines in the VDAC sequences. In humans, VDAC1 shows 2 Cys, VDAC2 9 Cys and VDAC3 6 Cys. Interestingly in VDAC 2 and VDAC3 two cysteines are localized in the N-terminus moiety, but only one of them is conserved at the same position (Cys2, referring to VDAC1 and VDAC3 numbering).
Three experimental 3D structures of mouse and human VDAC1 iso-form have been determined using X-ray crystallography and NMR [14, 15,16]. These analyses revealed a β-barrel motif with 19 amphipathic β-strands. Each strand features a regular alternation of hydrophilic and hydrophobic residues, the former pointing to the water-accessible lumen of the channel and the latter interacting with the non-polar membrane environment. The barrel is organized as a regular antiparallel array of β-strands, with the exception of strands 1 and 19, running in parallel. This organization is peculiar of the eukaryotic VDAC family and contrasts with the rule of the even number of strands pertaining to bacterial porins [17]. The VDAC structure is completed by an N-terminal tail located inside the pore, with a strong tendency to fold as an amphipathic α-helix. However, the exact position and local structure of this segment are still elusive since these features are different in the available X-ray and NMR structures. The biophysical structural model of the VDAC protein has been questioned by Colombini [18], who objected that the artificial folding procedure used to prepare material for the crystallization experiments might produce more populations of stable VDAC structures, whose proposed biophysical structure might be just one of them. In accordance, he supports an alternative biochemical structure, based on mutagenesis and functional experiments [18]. However, recently, the structure of zebrafish VDAC2 was solved at high resolution confirming the same β-barrel arrangement as VDAC1 [19]. It has to be taken into account that zebrafish VDAC2 has no cysteine in the sequence and lacks the 11 amino acid longer N-terminal sequence that exists in mammalian VDACs. The VDAC3 structure has not yet been obtained. Several bioinformatic predictions have been put forward that, based on the large sequence similarity, proposed a barrel core basically identical to the other VDAC isoforms [20].
The topological sidedness of VDAC1 has been recently determined [21]. By topology, we refer to the assignment of a series of segments connecting alternate membrane-spanning β-strand as exposed to the cytosol or towards the IMS. Previous works were based on the analysis, in purified mitochondria, of VDAC1 protruding surfaces assayed by antibodies, proteases or by the inclusion of reporters [22,23,24]. These techniques had the glitch that the mitochondria intactness can never be 100% controlled, giving raise to possible misinterpretations of the results. In a next work, thus, the sidedness of VDAC was probed in intact cells by transformation with VDAC1 carrying a cleavable topological fluorescent probe. The fluorescence detection in living cells was thus the basis of the topology presented here: it reversed the previously proposed one revealing that the C-terminus points to the IMS [21].
Despite the high sequence homology and the high structural similarity, the three VDAC isoforms appear to be functionally different and, in particular, the behavior of VDAC3 appears to be significantly different from the other two isoforms. Functional tests as reconstitution experiments have been carried out in planar lipid bilayers or in liposomes. The former kind of tests demonstrated that both native (purified from mitochondria) and recombinant VDAC1 and VDAC2 are able to form pores in lipid bilayers, with similar features [25,26]. VDAC3 was never purified from mitochondria, and only the recombinant form could be studied. This turned out to be poorly active as pore-forming protein either after reconstitution in artificial bilayers or incorporation in liposomes [26]. In a recent work, a careful analysis of the electrophysiological features of VDAC3 showed that the pore is dramatically smaller than for VDAC1 and VDAC2 [27].
The functional difference between the VDAC isoforms was also tested through yeast complementation assay, a general method to assess the ability of an externally supplemented protein to recover the physiological growth phenotype in a mutated strain [25,28]. In particular, Δporin1 yeast strains were transformed with plasmids expressing the mammalian VDAC isoforms, to assess their ability to restore the normal growth phenotype. The experiments showed that VDAC1 and VDAC2 were able to restore the growth phenotype while VDAC3 was not, or it was at a very low level [27].
3. VDAC1 cysteines
Mammalian VDAC1 contains only two cysteine residues at positions 127 and 232. They are conserved in the human, rat, rabbit, mouse, pig and bovine protein, but not in the model yeast Saccharomyces cerevisiae porin1 where two cysteines are present at positions 130 and 210. Cys127 is also conserved in VDAC2 but not in VDAC3. Cys127 is in the β-strand 8 and faces the phospholipidic moiety, while Cys 232 is in the strand 16, facing the water-filled inside of the pore [16].
Most of the information on VDAC1 cysteines have been proposed well before the elucidation of the structure (29) and were substantially confirmed later in [30]. By means of pure biochemical techniques, a correct description of the localization of the two cysteines was indeed proposed in [29]. Labeling of cysteines by both eosin-5-maleimide and N-(1-pyrenyl)-maleimide suggested their location at a boundary between water- and lipid-phase. Chromatographic materials able to bind cysteines in their reduced state and containing spacers with different lengths (Affi-Gel 501 with a 1.75 nm long spacer and Thiopropyl-Sepharose 6B with a 0.51 nm spacer) were utilized. Based on their influence on the chromatographic runs of VDAC1 micelles, it was suggested that at least one of the cysteines is localized between 0.51 and 1.75 nm deep in the protein micelle, as it was found later in the structure [16]. Cross-linking with a cysteine specific reagent (BMOE) showed the presence of dimers and trimers of VDAC1 but in a much less extent than upon utilization of other cross-linkers like EGS (not specific for sulfhydryl groups): this result suggests that VDAC1 cysteines are not involved in intermolecular disulfide formation and thus do not participate in oligomerization [30]. Assays performed to investigate the involvement of cysteines in apoptosis showed negative results [30].
The VDAC1 cysteines are subject to oxidation by ROS or other agents, as it was demonstrated in VDAC1 purified from bovine heart mitochondria ([29] and see below). Deletion of both cysteines does not affect the activity of the pore [30], which retains its voltage-dependence features, too [29,30]. The influence of the redox state of the cysteines was also examined, but no special difference in the functional activity was observed in precisely defined reduced or oxidized populations of the pore [29].
It is possible to conclude that VDAC1 cysteines have no specific relevance for the pore-conductance function neither for oligomerization nor apoptosis induction.
4. VDAC2 cysteines
Voltage-dependent anion channel isoform 2 (VDAC2) is also a 19-stranded transmembrane β-barrel protein residing in the outer mitochondrial membrane. Our current understanding of VDAC2 suggests that it has a wide interactome similar to VDAC1 [31] and VDAC3 [32] and is involved in several metabolic processes in the cell [33]. Similar to the more abundant VDAC1 isoform, VDAC2 conducts metabolites across the OMM and contributes to the control of metabolite flux [33,10]. Further, it plays a relevant role in calcium homeostasis in cardiac myocytes [34, 35]. There is a single work reporting the partial purification of VDAC2 (from bovine spermatozoa, [36]) and its functional characterization that reveals feature mostly shared with VDAC1.
Other than its metabolic importance, VDAC2 is known to be anti-apoptotic in nature [37], unlike its more abundant homologous protein VDAC1. Its upregulation has been observed in several debilitating diseases including Alzheimer's and cancer [38,39,40,41]. This property is possibly due to the unique ability of VDAC2 to sequester the pro-apoptotic protein Bak in the OMM and maintain it in the inactive state [37]. A recent report by the Hajnóczky group identified key segments of the VDAC2 barrel that may be important for constitutive Bak binding, and tBid import when apoptosis is triggered [42].
It is increasingly evident that the differences in the function of VDAC1 and VDAC2 lie in the primary sequence of both proteins. The mammalian VDAC2 possess an additional 11 amino acid long extension at the N-terminal helix, and an unusually high number of cysteine residues in its polypeptide sequence [9,10,11,33].
These two glaring differences in the primary protein sequence are thought to be responsible for the differential biophysical and functional characteristics of VDAC2. Our recent findings suggest that the 11-residue N-terminal extension is indeed critical to allow hVDAC2 folding and voltage-gated channel function [43]. Furthermore, the N-terminal extension is responsible for conferring equilibrium thermodynamic stability to the barrel structure [43]. Recent reports from our laboratory (de Pinto) have also revealed that the presence of the N-terminal additional residues confers higher helicity to this segment [44]. Similarly, our findings (Mahalakshmi) have revealed that cysteines also play a very important role in hVDAC2 folding, stability and function, and overall architecture [45,43].
The contribution of specific amino acids to the process of β-barrel folding and function is not widely studied for membrane proteins of human origin. Our current understanding, based on extensive studies with bacterial and human proteins, highlights the requirement of lipids and certain amino acids for the proper functioning of the protein [46,47, 48,49,50]. The thiol moiety of cysteine participates in several enzymatic reactions, metal binding and stabilizes the tertiary fold of the protein by modification upon oxidation into cystine [10,51].
A general approach to understanding the sequence contribution to protein behavior is through site-directed mutagenesis, wherein the impact of amino acid substitution on the overall architecture of protein is assessed [47,48]. In VDAC2, most of the cysteines are located towards the intermembrane space. To identify the importance of this unusual abundance of cysteine residues in hVDAC2, our laboratory (Mahalakshmi) has targeted the nine cysteines of this barrel by substituting them with the corresponding residue of the other two isoforms [45,43,52,53]. We replaced the unique cysteine in the N-terminal extension of hVDAC2 (Cys8) with serine. Using this Cys-null mutant (C0), and comparing its behavior to native hVDAC2 (WT), we were able to quantify the contribution of cysteines to the folding, function and stability of hVDAC2 [43,45,53].
5. VDAC3 cysteines
VDAC3 is undoubtedly the least characterized of the VDAC isoforms. Differently from VDAC1 and VDAC2 that are mainly co-localized within the same area of the outer mitochondrial membrane, VDAC3 is distributed all over the surface of the mitochondrion [54]. Moreover, together with VDAC2, it is highly expressed in testis and, in particular, in cyto-skeletal structures of the sperm tail called outer dense fibers (ODF) [55]. Despite VDAC3 shares about 70% of sequence homology with VDAC1 and 2, the phylogenetic analysis seems to indicate that it is evolutionary distinct and, therefore, could play a different role within the cell. It has been shown the involvement of VDAC3 in ciliary disassembly during the cell cycle [56] and in microtubule doublet formation [57]. VDAC3 knock out (K.O.) mice have been reported to show male infertility due to reduced or lack of sperm mobility. The authors associated the defective sperm mobility with the VDAC3 gene K.O. [57]. Moreover, overexpression of VDAC isoforms in yeast cells lacking the endogenous porin (Δpor1) showed that only VDAC3 is not able to restore the wild-type phenotype on not-fermentable carbon sources at 37 °C. This behavior may be linked to the peculiar electrophysiological properties of VDAC3. Until a short time ago, all attempts to characterize VDAC3 channel activity were unsuccessful, since its insertion in artificial membranes was observed very rarely, only after the addition of large amounts of reducing agents [26,58]. Recently, our group discovered that the recombinant human VDAC3 forms small-conductance channels of about 100 ps that probably had been until then unnoticed. These observations, together with the finding that VDAC3 overexpression in Δpor1 yeast exerts very weak protection against ROS compared to VDAC1 [9], let us suppose a key role for cysteine residues in the protein activity.
A substantial difference between the three VDAC isoforms lies precisely in the number and distribution of cysteines. In particular, VDAC3 has more cysteines (six in human and seven in rat) than VDAC1, which has only two. Based on the transmembrane model of VDAC1 [21] it has been predicted that most of the cysteine residues of VDAC3 (Cys2, 36, 65, 122 and 229) are exposed towards the inter membrane space (IMS). The oxidizing properties of this cellular compartment render cysteines highly reactive and susceptible to modifications. Mass spectrometry confirmed that cysteines in VDAC3 can exist in different oxidation states and that a disulfide bridge is formed between cysteines 2 and 8 [51]. The removal of selected cysteines (Cys2, 8 and 122 in different combinations) reduced the range of oxidation state, modified the electrophoretic migration and strongly affected the protein activity both in vivo and in vitro. In particular, the deletion of Cys2 together with Cys8 and/or Cys122 completely changed the electrophysiological features of VDAC3 that became able to insert easily into a phospholipid bilayer and to adopt a higher conductance state than the wild type protein [51]. These data were further confirmed by the ability of those mutants to complement the absence of porin1 in yeast cells [51]. It is interesting to note that almost at the same time, an independent group came to the same conclusions about the importance of cysteines in VDAC3 channel activity [59]. Based on the electrophysiological characterization of some cysteine mutants (mainly Cys2–8Ala and Cys122Ala), Okazaki et al. stated that the decreased channel gating observed for VDAC3 was due to fixation of the N-terminal domain to the bottom of the pore through a disulfide bridge between residues Cys 2 and 122. Although the presence of the disulfide bridge at this location was not detected by mass spectrometry [51], it is not possible to exclude that beside C2–8 other disulfide bridges can form between other cysteines pairs.
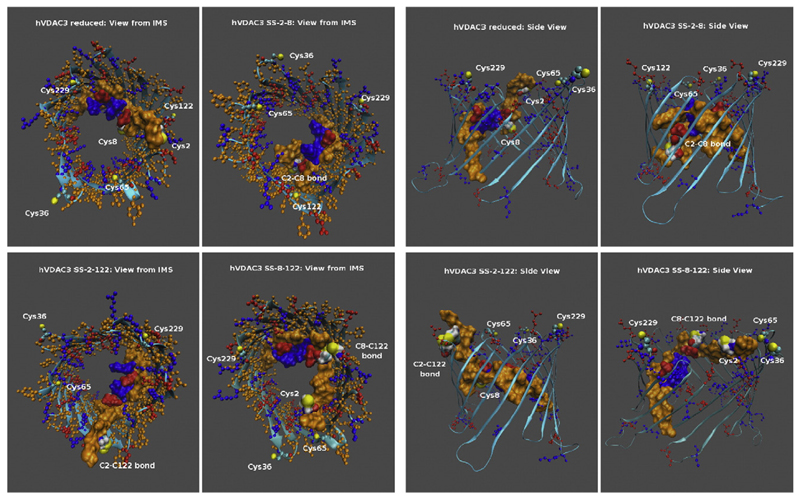
Various VDAC3 intra-chain disulfide bridges were simulated by molecular dynamics (Fig. 1) [51,60]. The bioinformatic analysis not only provided supposed structures of the rigid barrel containing the disulfides: but also was useful to calculate the measured reduction of the current flow in the presence of the same disulfide bridges [51,60]. Considering the bond between Cys 2 and 8 (found in VDAC3 by mass spectrometry [51]): it occludes only slightly the channel diameter, but hinders that the N-terminal tip protrudes towards the exterior of the pore (Fig. 1). The disulfide (SS) 2–8 (and also the SS 2–122, hypothesized in [59], does not substantially reduce the channel diameter, while SS 8–122, the only SS where the amino acid sequence 1–7 of the N-tail crosses the top part of the channel, leads to a predicted 20% conductance drop [60]. From the predicted structures containing the disulfide bridges, it looks intriguing that an important consequence of the disulfide bridge might be not only the reduction of the available diameter of the pore but also the possibility to have some residue exposed outside the pore (Fig. 1). At the end, we must highlight that the simulations were based on the available, but rigid, structures derived by [16]: this means that any unpredicted conformational change, leading to closer cysteine pairs, cannot be visualized or supposed, in the absence of experimental support.
Fig. 1.
Predicted structures of the hVDAC3 channel in the reduced state and of the same protein containing respectively disulfide SS-2–8, SS-2–122 and SS-8–122. Left Panel. The figures show a view of the pores from the intermembrane mitochondrial space (IMS). The N-terminal tail (residues 1–25) containing cysteines 2 and 8, both involved in the predicted disulfide bridges, is shown in a space-filling representation to highlight its position with respect to the pore. Basic and acidic residues as well as cysteines are shown in a ball-and-stick representation. Basic residues are colored in blue while the acidic ones are in red and the neutral ones in orange. Sulfur atoms are colored in yellow. The structures were generated through a 400 ns MD simulation in the NVT ensemble as detailed in [60]. The Figure was produced using the VMD program [76]. Right Panel. The figures show a side-view of the pores. The same conventions and the same program were used in this panel. The authors are indebted to Carlo Guardiani (Cagliari, Warwick) for the figure drawing.
6. Cysteines and VDAC oligomerization
Proteins with a high cysteine content usually form different oligomeric states through disulfide bonds. Oligomerization of VDAC has been investigated previously [53,61,62,63]. Under physiological conditions, VDAC1 is suspected form contacts between single poreforming units, at strands 19,1 and 2 [64]. When apoptosis is induced, it is believed that VDAC1 undergoes conformational changes, which initiates the formation of higher order oligomers involving other weakly stabilized strands, such as strand 16 [64]. These oligomerization events, however, do not occur through disulfide bond formation.
Similarly, the crystal structure of VDAC2 from zebrafish reveals a broader dimerization interface in this protein, which can be mapped to strands 17, 18, 19, 1 and 3 [19]. Further, lipidic bicelles promote zfVDAC2 dimerization by an additional 20% when compared to lauryldimethylamine oxide (LDAO) micelles. As seen for VDAC1, oligomerization ofVDAC2 does not involve cysteine residues. Using the hVDAC2 C0 construct, it was possible to demonstrate that ~40% protein oligomerization can be obtained in LDAO micelles, suggesting that oligomer formation is independent of cysteines [53]. This oligomer population is also unaffected by the LDAO-to-protein ratio, whereas the protein structure depends on the overall detergent concentration.
Interestingly, hVDAC2 does not show the formation of the distinct dimeric bands seen for VDAC1, and instead shows a higher tendency to form multimeric species upon cross-linking [53]. Mass spectrometric mapping of potential disulfides in refolded hVDAC2 has established that a large population of the protein possessed cysteines in the reduced form [45]. Put together, these studies support that cysteines are not involved in hVDAC2 barrel dimerization.
Until now VDAC3 oligomerization, and the role of cysteines in it, has not been investigated in detail.
7. Cysteines and pore stability
Physico-chemical experiments on VDACs have been largely carried out on the isoform 2, and to a minor extent on VDAC3, from humans. The role of cysteines has been investigated by comparing the behavior of WT isoforms to Cys-deleted C0 VDAC. hVDAC2 requires an optimal detergent- or lipid-to-protein ratio (DPR or LPR) to fold. Refolding is completed within timescales of seconds-minutes in LDAO [53], and is characterized by an initial rapid burst phase, which is marginally faster in the Cys-less hVDAC2 C0 construct that lacks the N-terminal extension of 11 residues [43]. The slower folding phase is in the range of 0.385 ± 0.037 min–1 for hVDAC2 WT and 0.185 ± 0.015 min–1 for the hVDAC2 C0 mutant, and is marginally faster for the WT protein. The overall initial slow folding kinetics of the native protein has implications in preventing the formation of off-pathway protein aggregates [43].
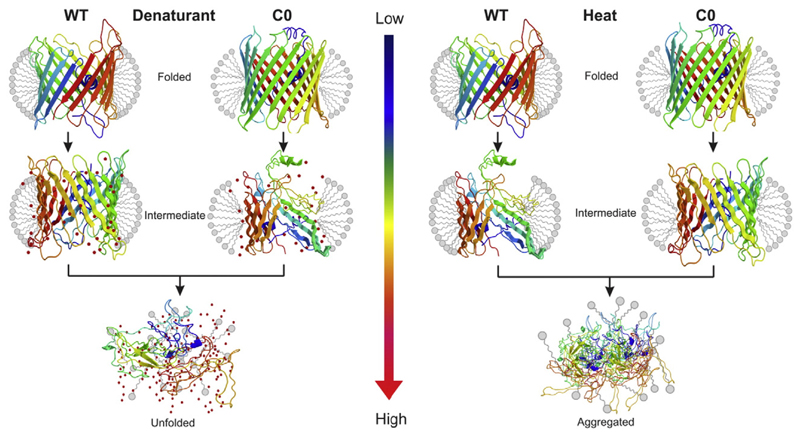
Studies have revealed that high curvature stress and hydrophobic mismatch does not allow the formation of stably refolded hVDAC2 in lipid vesicles made of 1,2-dimyristoyl-sn-glycero-3-phosphocholine [43]. Further, the folding efficiency is reduced in the presence of cysteines. However, once folded, hVDAC2 WT exhibits kinetic stability in both detergent micelles and lipid membranes. This is evident from the irreversible thermal denaturation and fast unfolding event displayed by hVDAC2, which is coupled with protein aggregation (Fig. 2). An increase in the DPR stabilizes the barrel by proportionately increasing the protein unfolding temperature (T m) in thermal denaturation [52].
Fig. 2.
Schematic representation of the effect of denaturant on hVDAC2 WT and C0 mutants. Cartoon representation of the barrel (rainbow color) is shown in the folded conformation, and is surrounded by a detergent micelle (gray color). The colored arrow represents an increasing denaturant concentration (gradient increases from blue to red color). The left panel highlights the interaction of WT and C0 with a chemical denaturant, such as guanidine hydrochloride. Proteins remain folded when the denaturant is absent, but increasing the chemical denaturant results in unfolding of protein by disrupting protein–lipid interaction. As WT exhibits stronger protein–lipid interactions, the barrel retains considerable structure at intermediate denaturant concentrations, compared to the C0 protein. The right panel represents the response of hVDAC2 mutants to thermal denaturation. Here, increasing the temperature disrupts protein–protein and protein–lipid interaction, and finally gives rise to the formation of irreversible protein aggregates. C0 is well structured and forms stronger intra-protein contacts. Hence, these non-covalent interactions allow the barrel to remain structured at temperatures that cause WT unfolding.
The Cys-less hVDAC2 C0 mutant exhibits greater thermal stability, as it forms a barrel that is well structured. However, this structural advantage in C0 comes at the expense of protein–lipid interactions. The Cys-less barrel is readily solvated by chemical denaturants such as guanidine hydrochloride, suggesting the lack of strong protein-micelle interactions. The slow unfolding of the native protein and the resistance to unfolding with increasing denaturant levels re-affirm the role of cysteine residues in anchoring the folded hVDAC2 barrel to the surrounding lipid environment (Fig. 2).
Estimation of the activation energy barrier of aggregation in hVDAC2, which exhibits kinetic stability, suggests that the unfolding rates are strongly cysteine dependent. Cysteine removal increases the activation energy in the measurable DPR conditions of 2600:1–6000:1. Here, an E act value of ∼17 kcal mol–1 was obtained for hVDAC2 WT and ∼23 kcal mol–1 for C0 [52]. Hence, optimal lipid–protein interaction, malleability to the lateral pressure from the lipid, and the coupled kinetic and thermodynamic components that together govern hVDAC2 stability, are modulated by the presence of cysteines (Fig. 2). The affinity of cysteines to interact with detergent/lipids confers barrel anchoring to the WT protein, and may be extrapolated to its differential behavior from VDAC1.
It has been shown that the prolonged incubation of both hVDAC2 WT and C0 in the presence of high urea content does not cause barrel denaturation when LDAO is used as the refolding agent. Surprisingly, however, dodecyl β-D-maltoside (DDM)-refolded protein is susceptible to denaturation by urea, which indicates the importance of headgroup- and micelle shape-mediated stabilization of the protein [53]. Cysteine removal destabilizes the hVDAC2 barrel, and, as a result, unfolding of C0 is seen at much lower denaturant concentrations than WT. This observation highlights the lowered affinity of C0 to LDAO and DDM [53].
Removal of cysteine residue increases the susceptibility of hVDAC2 to changes in DPR, indicating that minimal changes to the residues in the loop regions can cause drastic destabilization of the barrel structure. Apparent Gibbs free energy values derived for both proteins reveal an added stability of ∼0.7–1.0 kcal mol–1 in the presence of cysteines [53]. In the case of DDM, where the system shows equilibrium re/unfolding and the absence of hysteresis, a marginally greater stability of ∼0.3 kcal mol–1 is observed [43].
Recent reports that extend the physico-chemical studies on hVDAC2 to hVDAC3 have also observed similar results. As seen in hVDAC2, hVDAC3 is also under kinetic control, and undergoes irreversible unfolding upon denaturation by heat [51]. In LDAO micelles, cysteine 8 is particularly important to ensure the cooperative unfolding of hVDAC-3. Studies have shown that a single Cys → Ala mutation at this residue can lead to nearly 20% unfolding of the hVDAC3 barrel, before barrel aggregation begins in thermal denaturation experiments [51]. Cysteine residues of hVDAC3 are, therefore, important to maintain the folded state of the barrel. This is further established in hVDAC3 with a dual Cys2 and Cys8 mutation, wherein the protein shows less stability, compared to the hVDAC3 C8A [51].
Overall, the presence of cysteine residues strongly influences protein behavior. Differences in biophysical characteristics mediated by cysteines can also give rise to structural as well as functional variations, as seen in hVDAC2 and hVDAC3.
8. Cysteines and pore gating
As mentioned above, cysteines in mammalian VDAC1 have no specific influence on the pore-forming activity and in conductance features like the voltage-dependence [29,30]. It has been proposed that the conductance of hVDAC1 can be controlled by dynamic movement of the N-terminal helix and is independent of the cysteines [65].
Cysteines are instead important for channel functionality of hVDAC2 and hVDAC3. Similar to VDAC1, variants of hVDAC2 (native as well as the Cys-less C0 protein) exhibit voltage-gated channel activity. Surprisingly, however, the hVDAC2 WT barrel shows noisier channel activity in planar bilayer measurements, while the protein lacking cysteines shows clear transitions between closed and open states [53]. Noisier channel forming feature of WT is thus correlated with the presence of cysteines, and may be the consequence of local variation in the overall protein scaffold. The noisier behavior can be correlated to the redox state of available cysteines that can be not homogeneous among single protein molecules (see below).
hVDAC2 exhibits similar loss of voltage gating behavior when the N-terminal helix is deleted [43,66], as seen in VDAC1 [67]. The N-terminal helix of hVDAC2, as for hVDAC3, contains two cysteines. Thus, it is surprising to find that the N-terminal helix deleted hVDAC2 shows a greater frequency of channel insertion in the open state, compared to the Cys-less counterpart [43].
hVDAC3 is the least effective isoform in pore conductance, and its gating looks heavily influenced by the state of cysteines. This information mostly comes from experiments carried out on recombinant hVDAC3 reconstituted in artificial planar bilayers [26,27,51,59]. The insertion of recombinant hVDAC3 in the membrane was found to be more difficult than for the other isoforms. The presence of reducing agents and the pH of the medium influenced both the channel insertion in the membrane and its activity [51,59], indicating that cysteines in VDAC3 play a very important functional role. The conductance of the pore formed by WT VDAC3 showed a large range of values [59] and the most prevailing form was a very low conductance [27], in comparison to VDAC1. Reduced cysteines are relevant to determine a wider conductance level [51,59]. VDAC3 is not clearly voltage dependent as VDAC1. Site-directed mutagenesis experiments where specific Cys residues were mutated in Ala, showed that a VDAC1-like conductance state could be restored through the C122A and C2A/C8A mutations. These results were interpreted as it was that the gating of VDAC3 can be blocked by a disulfide bond established between Cys122 and Cys2 [59].
In our opinion, based on the experiments reported in [51] for hVDAC3, the gating properties of VDAC2 and VDAC3, can be not univocal but presenting a variegated range of responses, depending on the redox state of the set of exposed cysteines.
9. Cysteines and redox sensitivity
It has been established that the pore-forming structure in the OMM is a main way of escape from the mitochondria of an aliquot of the produced, in particular, by the complex III [68]. superoxide radical is very active but has a short life, while hydrogen peroxide (H2O2), its catabolite produced by the SOD1 (superoxide dismutase 1) in the IMS, is permeable and able to oxidize cysteines [69]. It is an easy guess to predict that VDAC pores showing a large, available surface (the connecting loops between β-strands protruding towards the IMS and the inside of the channel) are good, available targets to water soluble ROS.
With the availability of very powerful proteomic instrumentations, several studies have reported large surveys of protein oxidation in different conditions: very often VDACs are listed among the modified proteins. Carbonylation is a frequent oxidative modification also found for VDACs [70,71]. Thiols oxidation of VDAC was demonstrated in yeast, in a systematic study of the oxidized proteome of unstressed cells [72]. Upon downregulation of glutaredoxin1 (Grx1) in cells, the oxidation of thiol groups of VDAC was observed in [73]. Another oxidative modification is S-nitrosylation specifically found in VDAC3 in spermatozoa [74]. More recently VDAC3 was found to be the target of mitochondrial ROS specifically generated by the complex III. In a redox DIGE (two-dimensional fluorescence difference gel electrophoresis) proteomic study performed on intact and highly coupled rat heart mitochondria, VDAC3 featured a strikingly high increase in thiol oxidation by ROS [68].
Also VDAC2, in a study of reversible protein cysteine thiol oxidation in heart lysates from adult wild type and Cat Tg mice obtained through iodoacetyl 'Tandem Mass Tags' (iodoTMT) spectrometry, was found to be one of the targets of oxidation by ROS [75]. In this work, the ratio of reversibly oxidized cysteine thiols to total available cysteines — referred to as thiol occupancy — was determined on a site-specific basis for both wild type and Cat Tg mice. Significant differences in thiol occupancy were found for VDAC1 and VDAC2. In particular, in VDAC1, Cys232, showed a 2.1-fold decrease in thiol occupancy; VDAC2 showed a 2.0-fold decrease in thiol occupancy for Cys36, 2.8-fold for Cys65, and 23.6-fold for in Cys199 and Cys216, considering Cat Tg mice versus WT mice [75].
The oxidative modification of VDACs' cysteines is directly visible for VDAC1 [29] and VDAC3 [51], where the electrophoretic mobility of these proteins is changed by the oxidation state of cysteines. By incubating VDAC1 purified from bovine heart mitochondria, but also by subjecting whole mitochondria, to reducing or oxidizing reagents, three bands in the molecular weight range of 30–35 kDa were visualized. They were identified as a reduced form (35 kDa) and two oxidized forms at 33.5 and 30 kDa [29]. This latter form was supposed to contain an internal disulfide bridge established upon denaturation or partial de-folding of the protein because it was the most compact in SDS-PAGE and fully reversible by reduction. The oxidized form with 33.5 kDa corresponds to the mobility of strongly oxidized cysteines as seen after treatment with performic acid [29].
In addition, the recombinant and refolded hVDAC3, like bovine heart VDAC1 [29], showed changes in the electrophoretic migration pattern [51]. The reason for this alteration was oxidation state of cysteines, since reduction by DTT restored hVDAC3 migration at the expected molecular weight [51]. This result indicates that VDAC3 is very prone to oxidation. Thus, the oxidation state of hVDAC3 cysteines was investigated by mass spectrometry. The analysis of VDAC3 from rat liver mitochondria led to the identification of a disulfide bridge between the cysteine 2 and 8 in the N-terminal domain. Interestingly C2 and C8, together with C36, C65, and C229 were also found in different oxidation states: sulfonic, sulfinic and –SH reduced form. This was a rather surprising finding since the sulfonic acid is an irreversible oxidation state. Evidence for "overoxidation" of thiols, meaning the irreversible oxidation of a cysteine thiol to sulfinic or sulfonic acid was reported in other works [67].
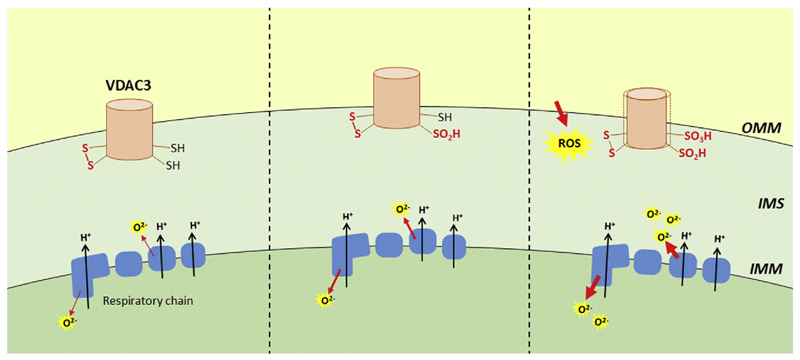
These very recent results deserves further investigations for getting conclusions but it is clear that VDACs in the outer membrane look to have a dynamic oxidation range of cysteine oxidation giving rise to a complex set of individual protein modification whose consequences are still far to be deciphered (Fig. 3).
Fig. 3.
Change in VDAC3 oxidation states due to elevated ROS level can lead to pore modifications. VDAC3 cysteines are in a stationary situation, most likely mainly reduced, and protruding towards the mitochondrial intermembrane space (left). Complex III and other proteins pour ROS in the IMS. The progressive accumulation of ROS increases the amount of oxidized cysteines in VDAC3. Also sulfinic and sulfonic states can be reached, randomly, in various exposed cysteines (central panel). Continuous increase of ROS in IMS can heavily modify VDAC3 cysteines and these irreversible modifications can be supposed to produce conformational changes with various possible outputs like definitive damaging of the protein or changes signaling the ROS level to surrounding actors (right panel). The oxidation state of cysteines to –SO2H and –SO3H is considered "irreversible" since neither of these oxoforms can be reduced directly by cellular thiols.
10. Evolutionary considerations
It is interesting to observe that the most relevant difference in amino acid composition of VDAC isoforms is the number of cysteines. As presented in a previous review [10], the composition of the three paralogs is very similar with the exception of cysteine content which, in human is 2 (VDAC1), 6 (VDAC3), 9 (VDAC2) but in rat is even more spread being 2 (VDAC1), 7 (VDAC3), 11 (VDAC2). VDAC3 is considered the oldest isoform, and VDAC1 and VDAC2 diverged more recently [13]. VDAC1 is thus considered the most recent mitochondrial porin. If this scheme is correct, the ramification of the VDAC family is associated with a branch leading to the reduction of cysteines in the sequence (VDAC1), associated with a robust pore-forming activity and a pro-apoptotic function, and a branch with increased number of cysteines (VDAC2), associated with an anti-apoptotic function.
11. Conclusions
The different number of cysteine residues in otherwise similar poreforming VDACs has inspired several investigators. A clearer picture is becoming available thanks to these works. Cysteines per se are of limited importance in the ion-conductance activity of a large pore-like VDAC1. Their increasing number can however influence the structure and the stability of the channel, since they can facilitate the protein-lipid interactions. In mammalian VDAC2 and VDAC3, where more cysteines have been evolutionarily selected at certain sites in the polypeptide, they contribute to maintaining a defined structure, conferring stability and function. However, the more surprising result of VDACs cysteine studies is the evidence that they are available to be oxidized at various extents. Not only the possible formation of internal disulfide bridges has been demonstrated [51] or envisaged [59], but also further oxidized states have been found with molecular techniques [51]. The picture emerging is that VDAC2 and VDAC3 exist in the OMM not as many identical, hollow pores but as a highly dynamic population of slightly different channels wallpapered inside by different sets of oxidized sulfurs. These different sets of oxidation should depend on the local features of the IMS bathing the pores, and will modify the pore conformation and conductance with still unknown consequences. It is thus predictable a direct involvement of VDAC3 and, possibly of VDAC2 due to its similar cysteine residues arrangement, in the modulation and communication of mitochondrial redox state and ROS production.
An emerging notion is whether the three VDACs should be viewed as paralogs and not isoforms [33]. As proposed some years ago [10], the results reviewed in this work clearly indicate that the most important discriminating feature among the VDAC isoforms is their behavior or susceptibility towards ROS: this topic will need a complete unveiling in the future.
Acknowledgments
We are grateful to dr. Carlo Guardiani for Fig. 1, Ms. Svetlana Maurya for generating Fig. 2, and to dr. Andrea Magrì for Fig. 3. This work was supported by the Italian Ministero dell'Istruzione, dell'Universita e della Ricerca, MIUR, (PRIN project 2010CSJX4F) to VDP, SR, and AM, by the ARISLA project ALSINTERACTORS 2013 to AM, by the FIR-UNICT project 350CD1 2014 to AM and VDP, by funding from the Wellcome Trust/ DBT (Department of Biotechnology) India Alliance award number IA/I/14/1/501305 to RM. AG thanks IISER Bhopal for research fellowship. RM is a Wellcome Trust/ DBT India Alliance Intermediate fellow.
Footnotes
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- 1.Bachi A, Dalle-Donne I, Scaloni A. Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem Rev. 2013;113:596–698. doi: 10.1021/cr300073p. [DOI] [PubMed] [Google Scholar]
- 2.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 3.Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- 4.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Asp Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Cortese JD, Voglino AL, Hackenbrock CR. The ionic strength of the intermembrane space of intact mitochondria is not affected by the pH or volume of the intermembrane space. Biochim Biophys Acta. 1992;1100:189–197. doi: 10.1016/0005-2728(92)90081-c. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchi J-O, Ryu SY, Jhun BS, Hurst S, Sheu SS. Mitochondral ion channels/transporters as sensors and regulators of cellular redox signaling. Antioxid Redox Signal. 2014;21:987–1006. doi: 10.1089/ars.2013.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleier L, Wittig I, Heide H, Steger M, Brandt U, Dröse S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med. 2015;78:1–10. doi: 10.1016/j.freeradbiomed.2014.10.511. [DOI] [PubMed] [Google Scholar]
- 9.Reina S, Palermo V, Guarnera A, Guarino F, Messina A, Mazzoni C, De Pinto V. Swapping of the N-terminus of VDAC1 with VDAC3 restores full activity of the channel and confers anti-aging features to the cell. FEBS Lett. 2010;584:2837–2844. doi: 10.1016/j.febslet.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 10.Messina A, Reina S, Guarino F, De Pinto V. VDAC isoforms in mammals. Biochim Biophys Acta. 2012;1818:1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Sampson MJ, Lovell RS, Craigen WJ. The murine voltage-dependent anion channel gene family Conserved structure and function. J Biol Chem. 1997;272:18966–18973. doi: 10.1074/jbc.272.30.18966. [DOI] [PubMed] [Google Scholar]
- 12.De Pinto V, Guarino F, Guarnera A, Messina A, Reina S, Tomasello FM, Palermo V, Mazzoni C. Characterization of human VDAC isoforms: a peculiar function for VDAC3? Biochim Biophys Acta. 2010;1797:1268–1275. doi: 10.1016/j.bbabio.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Young MJ, Bay DC, Hausner G, Court DC. The evolutionary history of mitochondrial porins. BMC Evol Biol. 2007;7:31. doi: 10.1186/1471-2148-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, et al. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ujwal R, Cascioc D, Colletierc JP, Fahama S, Zhanga J, Torod L, Pinga P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci U S A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz GE. beta-Barrel membrane proteins. Curr Opin Struct Biol. 2000;10:443–447. doi: 10.1016/s0959-440x(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 18.Colombini M. The published 3D structure of the VDAC channel: native or not? Trends Biochem Sci. 2009;34:382–389. doi: 10.1016/j.tibs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, et al. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amodeo GF, Scorciapino MA, Messina A, De Pinto V, Ceccarelli M. Charged residues distribution modulates selectivity of the open state of human isoforms of the voltage dependent anion-selective channel. PLoS ONE. 2014;9:e103879. doi: 10.1371/journal.pone.0103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasello FM, Guarino F, Reina S, Messina A, De Pinto V. The voltage-dependent anion selective channel 1 (VDAC1) topography in the mitochondrial outer membrane as detected in intact cell. PLoS ONE. 2013;8:e81522. doi: 10.1371/journal.pone.0081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Pinto V, Prezioso G, Thinnes F, Link TA, Palmieri F. Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry. 1991;30:10191–10200. doi: 10.1021/bi00106a017. [DOI] [PubMed] [Google Scholar]
- 23.Stanley S, Dias JA, D'Arcangelis D, Mannella CA. Peptide-specific antibodies as probes of the topography of the voltage-gated channel in the mitochondrial outer membrane of Neurospora crassa . J Biol Chem. 1995;270:16694–16700. doi: 10.1074/jbc.270.28.16694. [DOI] [PubMed] [Google Scholar]
- 24.McDonald BM, Wydro MM, Lightowlers RN, Lakey JH. Probing the orientation of yeast VDAC1 in vivo. FEBS Lett. 2009;583:739–742. doi: 10.1016/j.febslet.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, et al. Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- 26.Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr Biol. 1999;170:89–102. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- 27.Checchetto V, Reina S, Magrì A, Szabo I, De Pinto V. Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell Physiol Biochem. 2014;34:842–853. doi: 10.1159/000363047. [DOI] [PubMed] [Google Scholar]
- 28.Antos N, Budzinska M, Kmita H. An interplay between the TOM complex and porin isoforms in the yeast Saccharomyces cerevisiae mitochondria. FEBS Lett. 2001;500:12–16. doi: 10.1016/s0014-5793(01)02575-3. [DOI] [PubMed] [Google Scholar]
- 29.De Pinto V, a al Jamal J, Benz R, Genchi G, Palmieri F. Characterization of SH groups in porin of bovine heart mitochondria. Porin cysteines are localized in the channel walls. Eur J Biochem. 1991;202:903–911. doi: 10.1111/j.1432-1033.1991.tb16450.x. [DOI] [PubMed] [Google Scholar]
- 30.Aram L, Geula S, Abel N, Shoshan-Barmatz V. VDAC1 cysteine residues: topology and function in channel activity and apoptosis. Biochem J. 2010;427:445–454. doi: 10.1042/BJ20091690. [DOI] [PubMed] [Google Scholar]
- 31.Roman I, Figys J, Steurs G, Zizi M. Hunting interactomes of a membrane protein: obtaining the largest set of voltage-dependent anion channel-interacting protein epitopes. Mol Cell Proteomics. 2006;5:80–1667. doi: 10.1074/mcp.T600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Messina A, Reina S, Guarino F, Magrì A, Tomasello F, Clark RE, Ramsay RR, De Pinto V. Live cell interactome of the human voltage dependent anion channel 3 (VDAC3) revealed in HeLa cells by affinity purification tag technique. Mol BioSyst. 2014;10:2134–2145. doi: 10.1039/c4mb00237g. [DOI] [PubMed] [Google Scholar]
- 33.Maurya SR, Mahalakshmi R. VDAC-2: Mitochondrial outer membrane regulator masquerading as a channel? FEBS J. 2016 Dec 28; doi: 10.1111/febs.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22:97–381. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberio T, Mammucari C, D'Agostino G, Rizzuto R, Fasano M. Altered dopamine homeostasis differentially affects mitochondrial voltage-dependent anion channels turnover. Biochim Biophys Acta. 2014;1842:22–1816. doi: 10.1016/j.bbadis.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 36.Menzel VA, Cassarà MC, Benz R, De Pinto V, Messina A, Cunsolo V, R Saletti, Hinsch KD, Hinsch E. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci Rep. 2009;29:351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- 37.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 38.Yoo BC, Fountoulakis M, Cairns N, Lubec G. Changes of voltage-dependent anion-selective channel proteins VDAC1 and VDAC2 brain levels in patients with Alzheimer's disease and Down syndrome. Electrophoresis. 2001;22:9–172. doi: 10.1002/1522-2683(200101)22:1<172::AID-ELPS172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Reddy PH. Is the mitochondrial outermembrane protein VDAC1 therapeutic target for Alzheimer's disease? Biochim Biophys Acta. 2013;1832:67–75. doi: 10.1016/j.bbadis.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asmarinah A, Paradowska-Dogan A, Kodariah R, Tanuhardja B, Waliszewski P, Mochtar CA, Weidner W, Hinsch E. Expression of the Bcl-2 family genes and complexes involved in the mitochondrial transport in prostate cancer cells. Int J Oncol. 2014;45:96–1489. doi: 10.3892/ijo.2014.2576. [DOI] [PubMed] [Google Scholar]
- 41.Shoshan-Barmatz V, Mizrachi D. VDAC1: from structure to cancer Front therapy. Oncologia. 2012;2:164. doi: 10.3389/fonc.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naghdi S, Vàrnai P, Hajnòczky G. Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis. Proc Natl Acad Sci U S A. 2015;112:9–E5590. doi: 10.1073/pnas.1510574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurya SR, Mahalakshmi R. N-helix and cysteines inter-regulate human mitochondrial VDAC-2 function and biochemistry. J Biol Chem. 2015;290:52–30240. doi: 10.1074/jbc.M115.693978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guardiani C, Scorciapino MA, Amodeo GF, Grdadolnik J, Pappalardo G, De Pinto V, Ceccarelli M, Casu M. The N-terminal peptides of the three human isoforms of the mitochondrial voltage-dependent anion channel have different helical propensities. Biochemistry. 2015;54:56–5646. doi: 10.1021/acs.biochem.5b00469. [DOI] [PubMed] [Google Scholar]
- 45.Maurya SR, Mahalakshmi R. Modulation of human mitochondrial voltage-dependent anion channel 2 (hVDAC-2) structural stability by cysteine-assisted barrel-lipid interactions. J Biol Chem. 2013;288:92–25584. doi: 10.1074/jbc.M113.493692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurya SR, Chaturvedi D, Mahalakshmi R. Modulating lipid dynamics and membrane fluidity to drive rapid folding of a transmembrane barrel. Sci Rep. 2013;3 doi: 10.1038/srep01989. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta A, Zadafiya P, Mahalakshmi R. Differential contribution of tryptophans to the folding and stability of the attachment invasion locus transmembrane β-barrel from Yersinia pestis. Sci Rep. 2014;4 doi: 10.1038/srep06508. 6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iyer BR, Mahalakshmi R. Residue-dependent thermodynamic cost and barrel plasticity balances activity in the PhoPQ-activated enzyme PagP of Salmonella typhimurium . Biochemistry. 2015;54:22–5712. doi: 10.1021/acs.biochem.5b00543. [DOI] [PubMed] [Google Scholar]
- 49.Chaturvedi D, Mahalakshmi R. Juxtamembrane tryptophans have distinct roles in defining the OmpX barrel–micelle boundary and facilitating protein-micelle association. FEBS Lett. 2014;588:71–4464. doi: 10.1016/j.febslet.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Dawaliby R, Trubbia C, Delporte C, Masureel M, Van Antwerpen P, Kobilka BK, Govaerts C. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol. 2016;12:35–39. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reina S, Checchetto V, Saletti R, Gupta A, Chaturvedi D, Guardiani C, Guarino F, Scorciapino MA, Magrì A, Foti S, Ceccarelli M, et al. VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: the putative role of cysteine residue modifications. Oncotarget. 2016;7:2249–2268. doi: 10.18632/oncotarget.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurya SR, Mahalakshmi R. Influence of protein–micelle ratios and cysteine residues on the kinetic stability and unfolding rates of human mitochondrial VDAC-2. PLoS ONE. 2014;9:e87701. doi: 10.1371/journal.pone.0087701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maurya SR, Mahalakshmi R. Cysteine residues impact the stability and micelle interaction dynamics of the human mitochondrial β-barrel anion channel hVDAC-2. PLoS ONE. 2014;9:e92183. doi: 10.1371/journal.pone.0092183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann D, Bückers J, Kastrup L, Hell SW, Jakobs S. Two-color STED microscopy reveals different degrees of colocalization between hexokinase-I and the three human VDAC isoforms. PMC Biophys. 2010;3:4. doi: 10.1186/1757-5036-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinsch KD, De Pinto V, Aires VA, Schneider X, Messina A, Hinsch E. Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J Biol Chem. 2004;279:15281–15288. doi: 10.1074/jbc.M313433200. [DOI] [PubMed] [Google Scholar]
- 56.Majumder S, Fisk HA. VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle. 2013;12:849–858. doi: 10.4161/cc.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, Craigen WJ. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 58.Reina S, Magrì A, Lolicato M, Guarino F, Impellizzeri A, Maier E, Benz R, Ceccarelli M, De Pinto V, Messina A. Deletion of β-strands 9 and 10 converts VDAC1 voltage-dependence in an asymmetrical process. Biochim Biophys Acta. 2013;1827:793–805. doi: 10.1016/j.bbabio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Okazaki M, Kurabayashi K, Asanuma M, Saito Y, Dodo K, Sodeoka M. VDAC3 gating is activated by suppression of disulfide-bond formation between the N-terminal region and the bottom of the pore. Biochim Biophys Acta. 2015;1848:3188–3196. doi: 10.1016/j.bbamem.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Guardiani C, Leggio L, Scorciapino MA, De Pinto V, Ceccarelli M. A computational study of ion current modulation in hVDAC3 induced by disulfide bonds. Biochim Biophys Acta Biomembr. 2016;1858:813–823. doi: 10.1016/j.bbamem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Betaneli V, Petrov EP, Schwille P. The role of lipids in VDAC oligomerization. Biophys J. 2012;102:523–531. doi: 10.1016/j.bpj.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 63.Zalk R, Israelson A, Garty ES, Azoulay-Zohar H, Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem J. 2005;386:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geula S, Naveed H, Liang J, Shoshan-Barmatz V. Structure-based analysis of VDAC1 protein: defining oligomer contact sites. J Biol Chem. 2012;287:2179–2190. doi: 10.1074/jbc.M111.268920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geula S, Ben-Hail D, Shoshan-Barmatz V. Structure-based analysis of VDAC1: N-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem J. 2012;444:85–475. doi: 10.1042/BJ20112079. [DOI] [PubMed] [Google Scholar]
- 66.Gattin Z, Schneider R, Laukat Y, Giller K, Maier E, Zweckstetter M, Griesinger C, Benz Y, Becker S, Lange A. Solid-state NMR electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR. 2015;61:311–320. doi: 10.1007/s10858-014-9876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zachariae U, Schneider R, Briones R, Gattin Z, Demers JP, Giller K, Maier E, Zweckstetter M, Griesinger C, Becker S, Benz R, et al. A Lange, β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure. 2012;20:1540–1549. doi: 10.1016/j.str.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bleier L, Wittig I, Heide H, Steger M, Brandt U, Dröse S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med. 2015;78:1–10. doi: 10.1016/j.freeradbiomed.2014.10.511. [DOI] [PubMed] [Google Scholar]
- 69.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death, Annu Rev Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 70.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 71.Simamura E, Hirai K, Shimada H, Koyama J, Niwa Y, Shimizu S. Furanonaphthoquinones cause apoptosis of cancer cells by inducing the production of reactive oxygen species by the mitochondrial voltage dependent anion channel. Cancer Biol Ther. 2006;5:1523–1529. doi: 10.4161/cbt.5.11.3302. [DOI] [PubMed] [Google Scholar]
- 72.O'Brien KM, Dirmeier M, Engle M, Poyton RO. Mitochondrial protein oxidation in yeast mutants lacking MnSOD or CuZnSOD. J Biol Chem. 2004;279:51817–51827. doi: 10.1074/jbc.M405958200. [DOI] [PubMed] [Google Scholar]
- 73.Le Moan N, Clement G, Le Maout S, Tacnet F, Toledano MB. The Saccharomyces cerevisiae proteome of oxidized protein thiols. J Biol Chem. 2006;281:10420–10430. doi: 10.1074/jbc.M513346200. [DOI] [PubMed] [Google Scholar]
- 74.Lefièvre L, Chen Y, Conner SJ, Scott JL, Publicover SJ, Ford WC, Barratt CL. Human spermatozoa contain multiple targets for protein S-nitrosylation: an alternative mechanism of the modulation of sperm function by nitric oxide? Proteomics. 2007;7:3066–3084. doi: 10.1002/pmic.200700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao C, Behring JB, Shao D, Sverdlov AL, Whelan SA, Elezaby A, et al. Overexpression of catalase diminishes oxidative cysteine modifications ofcardiac proteins. PLoS ONE. 2015;10:e0144025. doi: 10.1371/journal.pone.0144025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33-38–27-8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]