Summary
Condensins are essential protein complexes critical for mitotic chromosome organization. Little is known about the function of condensins during interphase, particularly in mammalian cells. Here we report the interphase-specific interaction between condensin I and the DNA nick-sensor poly(ADP-ribose) polymerase 1 (PARP-1). We show that the association between condensin I, PARP-1, and the base excision repair (BER) factor XRCC1 increases dramatically upon singlestrand break damage (SSB) induction. Damage-specific association of condensin I with the BER factors flap endonuclease 1 (FEN-1) and DNA polymerase δ/ε was also observed, suggesting that condensin I is recruited to interact with BER factors at damage sites. Consistent with this, DNA damage rapidly stimulates the chromatin association of PARP-1, condensin I, and XRCC1. Furthermore, depletion of condensin in vivo compromises SSB but not double-strand break (DSB) repair. Our results identify a SSB-specific response of condensin I through PARP-1 and demonstrate a role for condensin in SSB repair.
Introduction
Proper compaction of chromatin fibers into a higher order mitotic chromosome structure is critical for equal segregation of chromosomes during cell division. Failure of this process leads to aneuploidy and loss of genomic integrity. One essential factor in mitotic chromosome organization and segregation is the heteropentameric complex condensin. Condensin, originally identified in Xenopus, is conserved in eukaryotes (Hirano, 2002). In human cells, the canonical condensin complex is composed of the two structural maintenance of chromosomes (SMC) family proteins hCAP-C and hCAP-E as well as three non-SMC subunits termed CNAP1 (hCAP-D2/Eg7), hCAP-G, and hCAP-H (Kimura and Hirano, 2000; Schmiesing et al., 2000). The SMC family proteins hCAP-C and hCAP-E are unique coiled-coil ATPases that form a stable heterodimer and serve as the core of this complex. Recently, a second condensin complex that shares the same SMC components but differs in its non-SMC subunits was identified and termed condensin II (Ono et al., 2003). Both the original condensin (now termed condensin I) and condensin II affect the or-ganization and resolution of mitotic chromosomes in distinct ways, although the underlying mechanisms are not well understood (Ono et al., 2003). While condensin I is conserved from yeast to humans with the exception of C. elegans, condensin II is absent in yeast (Hirano, 2005). The focus of this study is on condensin I.
Human condensin I is expressed relatively abundantly throughout the cell cycle and exhibits dynamic cell-cycle-specific subcellular localization (Schmiesing et al., 2000). Although condensin I associates with chromosomes during mitosis, the majority of condensin I resides in the cytoplasm during interphase, which may be important for its cell-cycle-specific regulation. This is in contrast to the constitutive nuclear localization of condensin II (Ono et al., 2003; Yeong et al., 2003). However, a sub-population of condensin I forms chromatin-associated nuclear foci during interphase, suggesting a potential interphase-specific function for condensin I in the nucleus (Schmiesing et al., 2000). Supporting this notion, studies in S. pombe and Drosophila provided evidence for the involvement of condensin I in DNA repair and epigenetic regulation of transcription, respectively (Aono et al., 2002; Chen et al., 2004; Dej et al., 2004; Lupo et al., 2001). However, the underlying mechanisms were unclear and, thus far, there was no direct evidence indicating an interphase function for condensin I in vertebrates.
The non-SMC components of condensin I play critical regulatory roles in condensin’s function, including activation of ATPase activity, chromatin association, and cell-cycle-specific subcellular targeting (Ball et al., 2002; Kimura and Hirano, 2000). We have identified an approximately 113 amino acid (aa) domain at the C-terminal end of CNAP1/hCAP-D2, which functions autonomously in both nuclear and chromosome targeting and interacts with histones H1 and H3 (Ball et al., 2002). This domain was termed the nuclear- and chromosome-targeting domain (NCTD). To further address its function, we screened for interacting proteins by Far Western analysis. Here we report the identification of a 120 kDa nuclear protein that specifically binds to the CNAP1-NCTD. Mass spectrometric sequencing revealed that this protein is poly(ADP-ribose) polymerase 1 (PARP-1), a DNA nick sensor that plays a role in DNA repair and maintenance of genome integrity (Bouchard et al., 2003; Herceg and Wang, 2001). We found that this interaction occurs in vivo in the context of the native condensin I in an S/G2 phase nuclear-specific fashion. Intriguingly, this interaction is significantly enhanced following SSB damage induction and is required for stable complex formation between condensin I and the BER factor XRCC1. In a damage-specific manner, condensin I also binds to FEN-1 and DNA polymerase δ/ε, factors that are involved in long-patch BER (Klungland and Lindahl, 1997; Stucki et al., 1998). Finally, comet assays revealed that condensin depletion decreased the rate of SSB repair. Together, these results demonstrate that condensin I exerts an immediate SSB damage response through its interaction with the PARP-1-XRCC1 complex and plays an important role in SSB repair in vertebrate cells.
Results
CNAP1/hCAP-D2 NCTD Interacts Directly with PARP-1 In Vitro
To understand the regulation of the NCTD, we screened for interacting protein(s) by Far Western analysis using the NCTD as a probe. A GST-GFP-NCTD (GG-NCTD) fusion protein was found to bind specifically to a 120 kDa protein (p120) in HeLa nuclear extracts (Figure 1A). p120 was purified to near homogeneity by chromatography, byfollowing Far Western-positive fractions (Figures 1B and 1C). The p120 in the peak Q column fraction was excised from a silver-stained SDS-PAGE gel for mass spectrometric sequencing. The results showed 93% (40 out of 43) masses matched with PARP-1, indicating that p120 is the nuclear enzyme PARP-1. This was further confirmed by Western analysis of the peak Q column fractions positive for p120 using an antibody specific for PARP-1 (Figure 1C). The Far Western results were further confirmed using purified recombinant PARP-1 (see Figure S1 in the Supplemental Data available with this article online). These results demonstrate that the NCTD of CNAP1 directly interacts with PARP-1 in vitro.
Figure 1. Identification of a 120 kDa Protein that Interacts with the NCTD of CNAP1.
(A) The CNAP1 nuclear- and chromosome-targeting domain (NCTD) binds to a 120 kDa species (p120) in crude nuclear extracts. Far Western analysis of HeLa nuclear extracts (N.E.) and cytoplasmic extracts (C.E.) using a GST-GFP-NCTD (GG-NCTD) fusion protein as a radiolabeled probe. GST-GFP (GG) was used as a negative control. p120 is indicated by arrowhead. Coomassie stain of N.E. is also shown. A schematic diagram of the CNAP1-NCTD is shown underneath.
(B) Purification scheme for the isolation of p120 from HeLa N.E.
(c) Analysis of the purified p120. (Lanes 1-9) Far Western analysis of input, flowthrough (FT), and the final Q-column fractions. (Lanes 10-20) Silver staining of the same fractions showing the highly purified p120. (Lanes 21-30) Western blot analysis of the same fractions with anti-PARP-1 antibody. An arrowhead indicates p120 (PARP-1), while an asterisk indicates a PARP-1 cleavage product. Fraction numbers are shown at top.
Human Condensin I Interacts with PARP-1 In Vivo
To address whether CNAP1 in the context of condensin I interacts with PARP-1 in vivo, we carried out a coimmunoprecipitation (ColP) of the endogenous condensin I in HeLa nuclear extracts using antibody against CNAP1 followed by Western analysis with anti-PARP-1 antibody. Our anti-CNAP1 antibody, previously shown to precipitate the holo-condensin I complex (Schmiesing et al., 2000), also coprecipitated PARP-1 (Figure 2A). Similar results were obtained using antibody against hCAP-G but not with antibody against hSMC1 or control IgG. hSMC1 is a subunit of cohesin, a distinct SMC-containing complex required for sister chromatid cohesion (Schmiesing et al., 1998). Furthermore, poly- and monoclonal anti-PARP-1 antibodies copurified condensin I in reciprocal CoIPs (Figure 2B). PARP-1 is primarily a nuclear protein and, despite the presence of the major population of condensin I in the cytoplasm (80%-90% in the cytoplasm versus 10%-20% in the nucleus), the interaction between PARP-1 and condensin I is limited to the nucleus (Figure 2C). In addition, treatment with ethidium bromide (EtBr) had no effect on the association between condensin I and PARP-1, suggesting that the interaction is not mediated by DNA (Figures 1 and 2D). Taken together, the results demonstrate the nuclear-specific interaction of condensin I and PARP-1 in human cells.
Figure 2. PARP-1 Interacts with Condensin I In Vivo in a Nuclear and S/G2 Phase-Specific Manner.
(A) In vivo CoIP analysis of condensin interaction with PARP-1 using HeLa N.E. CoIPs with antibodies specific for condensin components CNAP1 and hCAP-G, cohesin component hSMC1, and preimmune IgG are indicated. Precipitates were eluted in two sequential steps using 1 M KCl and 2 M guanidine-HCl (2 M Guan). Eluted proteins from each step were TCA precipitated and analyzed by Western blotting using anti-PARP-1 antibody. Immunoprecipitated antigens are shown underneath.
(B) Reciprocal CoIP Western analysis using several anti-PARP-1 antibodies, followed by an anti-CNAP1 Western analysis. Goat polyclonal, mouse monoclonal, and rabbit polyclonal antibodies against PARP-1 were used for CoIP. Preimmune IgG served as a control.
(C) Nuclear-specific interaction of condensin and PARP-1. CoIP byanti-CNAP1 was performed using C.E. and N.E. Precipitates were analyzed by Western analysis for the presence of PARP-1. CoIP efficiency was assessed by anti-hCAP-G Western blot as shown underneath.
(D) The EtBr-resistant interaction between condensin I and PARP-1. CoIP of control (—) or EtBr treated (+) N.E. was carried out using an anti-hCAP-G antibody. Western blot detection of PARP-1 (arrowhead) and hCAP-G (bottom) are indicated.
(E) The condensin I-PARP-1 interaction peaks at S phase. HeLa cells were synchronized to different cell cycle stages (Figure S2A). N.E. were immunoprecipitated with anti-CNAP1 (lanes 2-4), preimmune IgG (lanes 5-7), oranti-hCAP-G antibodies (lanes 8-10) and analyzed byWestern blotting with anti-PARP-1 antibody. An anti-hCAP-G Western blot indicates comparable precipitations of condensin I at different cell cycle stages.
PARP-1 is not, however, a subunit of condensin. PARP-1 association is sensitive to a 1 M KCl wash, while condensin subunit interactions are mostly resistant to 1M KCl in condensin CoIPs (Figure 2) (Schmiesing et al., 2000). Although condensin was found in both the 1 M KCl and 2 M guanidine fractions in the reciprocal anti-PARP1 CoIPs (Figure 2B), this apparent discrepancy most likely reflects differences in the antibody targets. It is possible that a stable PARP-1-condensin complex with a certain conformation may be preferentially recognized by anti-PARP-1 antibodies. Nonetheless, sucrose gradient fractionation of HeLa nuclear extracts revealed that the major peak of condensin I corresponding to 13S (Kimura et al., 2001) and that of PARP-1 do not coincide (Figure 4F). Thus, only a subpopulation of each factor is engaged in this interaction.
Figure 4. Analysis of Condensin I Interactions with BER Factors.
(A) CoIP of N.E. from H2O2-treated cells with anti-hCAP-G antibody probed with antibodies against PARP-1, XRCC1, FEN-1, or DNA ligase III as indicated. Cells were exposed to 20 mM H2O2for20 min and were either allowed no recovery (0 hr) or 1 hr of recovery (1 hr) as indicated. Control untreated HeLa cells (Cont) were harvested at the same time. Beads were probed with guinea pig anti-hCAP-G antibody to show IP efficiency.
(B) Similar CoIP in the absence (Cont) or presence of damage (H2O2) as in (A) followed by Western analysis using anti-DNA polymerase δ, ε, or β antibodies.
(C) Anti-hCAP-G CoIP of N.E. from H2O2-exposed HeLa treated with EtBr or micrococcal nuclease (MNase). Western blots were performed using antibodies specific for PARP-1 and XRCC1. Beads were probed for hCAP-G.
(D) The association between PARP-1 and XRCC1 increases upon damage induction. Anti-PARP-1 (polyclonal) CoIP of H2O2-exposed HeLa N.E. followed by anti-XRCC1 Western blot. Lanes 7 and 8 (beads) were reprobed with monoclonal PARP-1 antibody to show IP efficiency.
(E) Condensin association with XRCC1 is mediated through PARP-1. Nuclear extracts from wild-type (PARP+/+) and PARP-1 knockout (PARP-/-) MEFs treated with 20 mM H2O2 for 20 min were immunoprecipitated with anti-hCAP-G antibody and probed for XRCC1 by Western blotting. A CNAP1 Western analysis shows IP efficiency.
(F) Western analysis of sucrose gradient ultracentrifugation of N.E. from control or H2O2-treated HeLa cells. Fraction numbers from the bottom to the top of the gradient (1-12) and the fraction corresponding to 13S (arrowhead at the top) are indicated. Western blot analysis was done using antibodies against hCAP-G, PARP-1, XRCC1, FEN1, or ligase III as indicated.
Condensin I Interaction with PARP-1 Is Interphase Specific
To gain insight into a functional role for the PARP-1-condensin I interaction, we examined the cell cycle specificity of this association. Anti-CNAP1 antibody coprecipitated PARP-1 specifically in S phase and less prominently in G2 phase, while no significant interaction was detected during mitosis or G1 phase (Figure 2E and Figure S2A). A similar observation was made with an anti-hCAP-G CoIP despite an abundance of condensin I and PARP-1 throughout the cell cycle (Figure 2E) (Schmiesing et al., 2000). This suggests that PARP-1 is not involved in the mitotic function of condensin I. Consistent with this premise, mitotic chromosome morphology and condensin association with mitotic chromosomes were not affected in PARP-1-/- mouse embryonic fibroblast (MEF) cells (Figure S2B). These results suggest that the interaction with PARP-1 is involved in an interphase nuclear function of condensin I.
Effects of DNA Damage on the Condensin I-PARP-1 Interaction
PARP-1 acts as a DNA nick and DSB sensor and is recruited to damage sites where it participates in BER and DSB repair processes (Masutani et al., 2003). Therefore, we investigated whether the condensin I-PARP-1 interaction is involved in DNA repair. Cells were treated with the DNA damaging agent hydroxyurea (HU), which stalls DNA replication and subsequently generates DNA strand breaks. We detected a 30- to 40-fold increase in the amount of PARP-1 coprecipitated with anti-hCAP-G antibody after 24 hr HU treatment, as compared to untreated cells (Figure 3A). A similar result was obtained with camptothecin (CPT) treatment, which also induces replication stall-mediated DNA breaks (data not shown). Furthermore, the interaction is not only increased but appears to be stabilized as some fraction of the PARP-1 population became 1 M KCl resistant and eluted in the 2 M guanidine-HCl fraction in HU-treated cells (Figure 3A). Similarly, a robust enhancement of the interaction was observed in cells treated with the alkylating agent methyl methanesulfonate (MMS) and the oxidizing agent H2O2, which primarily induce SSBs and base damage that results in SSBs (Figures 3B–3D). Antibodies specific for hCAP-G, hCAP-E, orCNAP1 used inCoIPsexhibited consistent results, confirming that this enhancement of the interaction involves the holo-condensin I complex (Figures 3C and 3D; data not shown). Under the same conditions, we observed no significant association of condensin II with condensin I (Figure S3), suggesting that this SSB response of condensin I is independent of condensin II. Furthermore, although the normal interaction between condensin I and PARP-1 is prominent only in S phase (Figure 2E), the SSB-stimulated interaction occurs in both G1 and S/G2 phases, indicating that this response is active throughout interphase (Figure 3D).
Figure 3. Treatment of Cells with Genotoxic Agents Stimulates the Interaction between PARP-1 and Condensin I.
After treatment of cells with HU (A) or MMS (B), nuclear extracts were immunoprecipitated with anti-hCAP-G antibody and analyzed by Western blot using anti-PARP-1 antibody. To ensure equal IP efficiency, the 2 M Guan fractions were also probed with anti-hCAP-H or CNAP1 antibody as indicated.
(A) Cells were treated for 24 hr with different concentrations of HU.
(B) Cells were harvested immediately following exposure to either 10 mM or 30 mM of MMS for 30 min or 1 hr as indicated.
(C) Extracts from cells treated with 20 mM H2O2 for 20 min were used in an anti-hCAP-E CoIP followed by an anti-PARP-1 Western blot.
(D) The PARP-1-condensin I interaction is induced throughout interphase in response to damage. HeLa cells synchronized to G1 or S/G2 phases were treated with H2O2 and harvested immediately. CoIP with anti-hCAP-G antibody was followed by anti-PARP-1 Western analysis. The 2 M Guan fractions were reprobed for hCAP-E to show IP efficiency.
Condensin I Forms a Stable Complex with PARP-1 and XRCC1 in Response to SSB Damage
H2O2 and other SSB-inducing agents are known to activate BER, which is a major repair pathway for SSBs (Slupphaug et al., 2003). As PARP-1 participates in the BER pathway (Dantzer et al., 2000; Trucco et al., 1998), the results described above raise the possibility that condensin I may also play a role in BER. Therefore, we next tested the potential interaction of condensin I with other BER factors. In response to H2O2 treatment, antibody against hCAP-G coprecipitated PARP-1 as well as XRCC1, a binding partner of PARP-1 and important scaffold protein in the BER pathway (Masson et al., 1998) (Figure 4A). In addition, anti-hCAP-G antibody weakly but specifically coprecipitated FEN-1, an enzyme involved in AP (apurine/apyrimindine) site removal in long-patch BER (Klungland and Lindahl, 1997) (Figure 4A). Consistent with this, interactions of DNA polymerases δ and ε with condensin I were also observed (Figure 4B). We failed, however, to observe any significant interaction of condensin I with ligase III, RPA, PCNA, or DNA polymerase β (Figures 4A and 4B; data not shown). Similar results were obtained following MMS treatment (data not shown). Therefore, human condensin I appears to interact with a specific subset of BER factors following SSB damage induction.
The enhanced interactions of condensin I with PARP-1 and XRCC1 peaked immediately upon damage induction (0 hr), coinciding with rapid SSB repair, and were also observed at a lower level at 1 hr postdamage (Figure 4A). These enhanced interactions are not due to an increase of protein expression (Figure 5C, inputs). In addition, Western analysis of condensin I subunits in cytoplasmic and nuclear fractions and immunolocalization analysis indicate that these enhanced interactions are not due to relocalization of cytoplasmic condensin to the nucleus (data not shown). Furthermore, they are not mediated by the increased DNA association of PARP-1, XRCC1, or condensin I, as neither EtBr nor micrococcal nuclease treatment had any effect on the interaction (Figure 4C).
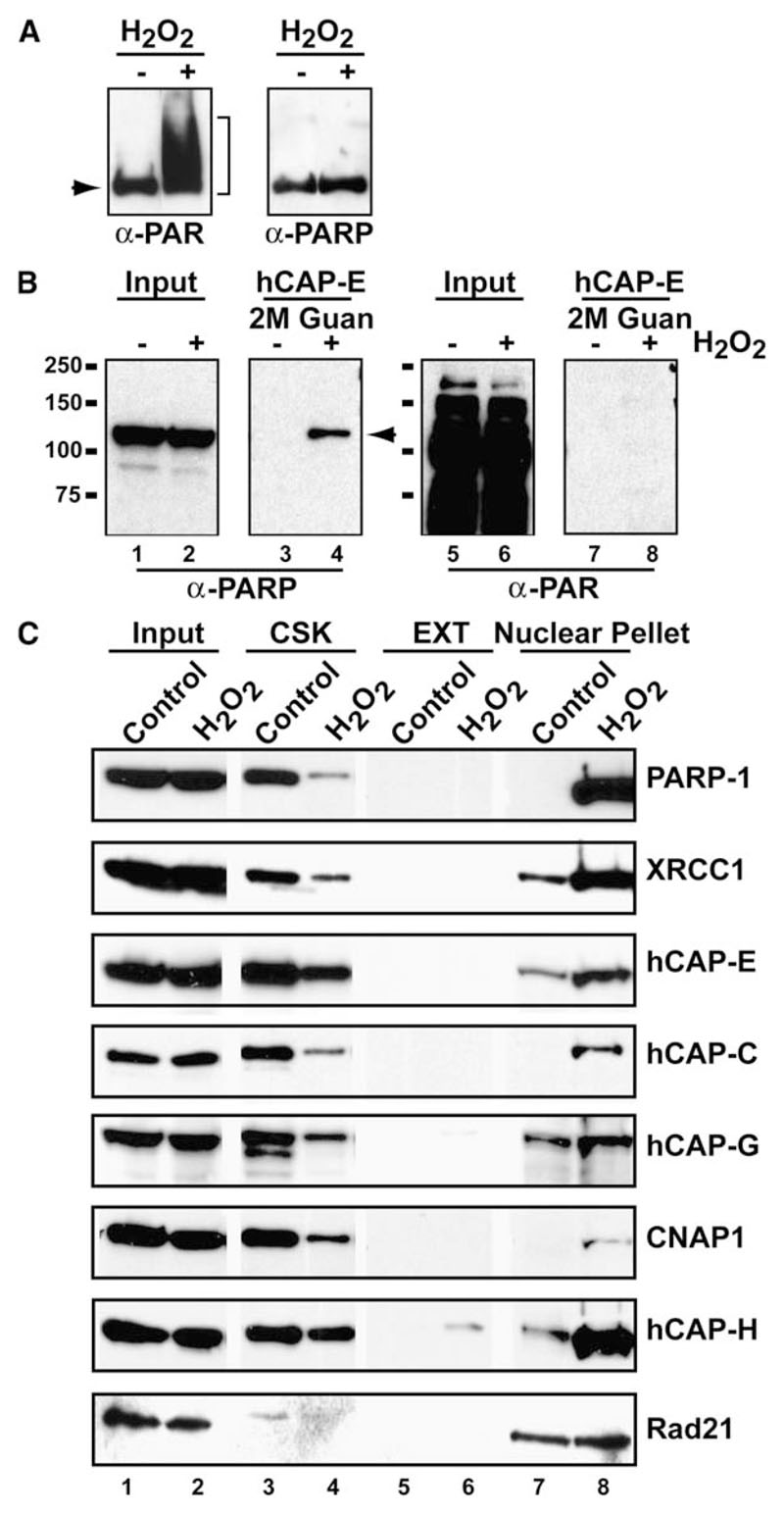
Figure 5. Condensin I Interacts with Hypo-(ADP-ribosyl)ated PARP-1 and Associates with Damaged Chromatin.
(A) Detection of auto-(ADP-ribosyl)ation of PARP-1 following H2O2 treatment. PARP-1 immunopurified using anti-PARP-1 polyclonal antibody from cells untreated (—) or treated (+) with H2O2 was analyzed by Western blot using monoclonal anti-PAR and anti-PARP-1 antibodies. Arrowhead indicates hypo-(ADP-ribosyl)ated form of PARP-1.
(B) Condensin I interacts with a non- or hypo-(ADP-ribosyl)ated form of PARP-1. Nuclear extracts from cells untreated (—) or treated (+) with H2O2 were used in anti-hCAP-E CoIPs. Precipitates were eluted in 2 M Guan after a 1 M KCl wash and probed with monoclonal anti-PARP-1 (lanes 1-4) and anti-PAR (lanes 5-8) antibodies to determine the ADP-(ribosyl)ation status of PARP-1.
(C) Sequential extraction of HeLa cells without and with H2O2 treatment. CSK and EXT fractions contain cytoplasmic, cytoskeletal, and soluble nuclear proteins. Insoluble nuclear pellet fractions contain proteins that are tightly bound to chromatin and/or the nuclear matrix. The blot was probed for PARP-1, XRCC1, the five condensin I subunits, and the cohesin subunit RAD21 (loading control). The apparent decrease of these proteins (except for RAD21) in the CSK fraction reflects the decrease of soluble nuclear proteins, which now associate tightly with the chromatin-enriched nuclear pellet.
CoIP analysis using anti-PARP-1 antibody revealed that the association between PARP-1 and XRCC1 was also enhanced in response to DNA damage (Figure 4D). The interaction between condensin I and XRCC1 appears to be abrogated in PARP-1-/- MEF cells, while a clear interaction was observed between condensin I and XRCC1 in the presence of DNA damage in wild-type+/+ MEFs (Figure 4E). This observation indicates that PARP-1 is required for the interaction between condensin I and XRCC1. Together, these results suggest that condensin I and XRCC1 are induced to associate with PARP-1 in response to SSBs.
The interaction observed between condensin I and FEN-1, which was 1M KCl sensitive in the condensin CoIP, was abolished upon EtBr or micrococcal nuclease treatment (data not shown). This suggests that, while condensin I, PARP-1, and XRCC1 form a stable complex in a DNA-independent fashion (Figure 4C), the condensin I-FEN-1 association is likely promoted by the proximal binding of the two factors at damage lesions on DNA. Consistent with this, sucrose gradient ultracentrifugation revealed a striking shift of subpopulations of PARP-1 and XRCC1, but not FEN-1, to fractions that closely overlap with condensin I upon H2O2 treatment (Figure 4F). A damage-induced shift of condensin I is not obvious, possibly due to insufficient resolution of the fractions close to the bottom of the gradient. A slight shift of ligase III fractions was also observed, but not to the extent of the PARP-1 and XRCC1 fractions. These results further indicate that ligase III is not present in this complex (see also Figure 4A) even though ligase III was shown to be a binding partner for XRCC1 (Caldecott et al., 1994; Nash et al., 1997). Taken together, these results demonstrate that condensin I, PARP-1, and (ligase III-free) XRCC1 are engaged in a stable complex in response to SSBs.
Condensin I Interacts with the Hypo-(ADP-ribosyl)ated Form of PARP-1
After initial recruitment to DNA lesions, PARP-1 becomes hyper-(ADP-ribosyl)ated, which induces its dissociation from DNA (Lindahl et al., 1995). To examine the (ADP-ribosyl)ation status of PARP-1 in this interaction, we immunopurified PARP-1 using polyclonal anti-PARP-1 antibody in the presence of H2O2. This revealed heterogeneous populations of PARP-1 that are (ADP-ribosyl)ated to varying degrees as detected by anti-poly(ADP-ribose) (PAR) antibody (Figure 5A). The PAR signal of PARP-1 in undamaged cells most likely reflects the presence of endogenous damage that activates PARP activity (e.g., during DNA replication). We found that a monoclonal anti-PARP-1 antibody (Biomol, SA-250) used to detect PARP-1 in condensin CoIPs only detects the non- and/or hypo-(ADP-ribosyl)ated form (Figure 5A). Therefore, in order to determine whether the enhanced interaction between PARP-1 and condensin is mediated by (ADP-ribosyl)ation, anti-PAR antibody was used to analyze the condensin CoIP. Interestingly, antibody against condensin I preferentially coprecipitated a non- or hypo-(ADP-ribosyl)ated form of PARP-1 even in the presence of damage (Figure 5B). These results indicate that the DNA damage-induced enhancement of the condensin I-PARP-1 complex formation is not mediated by the hyper-(ADP-ribosyl)ation of PARP-1 and that the population of PARP-1 that is hypo-(ADP-ribosyl)ated is preferentially involved in the complex formation between condensin I, PARP-1, and XRCC1. Since hyper-(ADP-ribosyl)ation induces dissociation of PARP-1 from DNA (Zahradka and Ebisuzaki, 1982), these results further suggest that the PARP-1 in this complex is capable of interacting with DNA. Consistent with this, we observed the increased nuclear retention of condensin I, PARP-1, and XRCC1 in response to DNA damage following sequential cell extraction (Figure 5C). A similar “nuclear retention” assay was used previously to demonstrate the increased association of ATM with damaged DNA (Andegeko et al., 2001). The stable complex formation and concomitant nuclear retention of condensin I, PARP-1, and XRCC1 suggest that they together associate with damaged DNA in vivo.
The Rapid Repair of SSBs Is Impaired in Condensin-Depleted Cells
We tested whether condensin contributes to the efficient repair of SSBs in vivo. Using ScII (hCAP-E homolog) conditional knockout chicken DT40 cells (Hudson et al., 2003), the relative amounts of DNA damage were quantified by an alkaline comet assay at different time points following H2O2 treatment. ScII knockout cells were complemented with recombinant ScII, which can be shut off by treatment with doxycycline (Dox). In the absence of any exogenous damage, 90% of the wild-type cells treated with Dox exhibited a tail moment of less than 5, while ScII knockout cells either without or with Dox treatment (“ScII ON” and “ScII OFF”, respectively) contained some residual damage at low levels. This damage, however, is not sufficient to affect cell viability (Table S1). Upon H2O2 treatment, similar amounts of damage were induced in the wild-type cells with Dox and ScII ON and ScII OFF cells (Figure 6A, immediate). However, subsequent repair of damaged DNA was significantly delayed in ScII OFF cells (Figure 6A, 45 min and 2 hr). Only 20% of the ScII OFF cells had recovered to a tail moment of less than 20, compared to 60% and 90% of the wild-type and ScII ON cells, respectively, at 45 min after damage induction. Furthermore, while more than 90% of both the wild-type and ScII ON cells exhibited a tail moment of less than 20 after 2 hr, only 50% of the ScII OFF cells showed a similar recovery from SSB damage.
Figure 6. Condensin-Depleted Cells Are Compromised in SSB Repair.
(A) An alkaline comet assay performed on ScII (CAP-E)-depleted chicken DT40 cells. Parental wild-type cells treated with Dox (ScII ON), and ScII conditional knockout cells without Dox (ScII ON) or with Dox (ScII OFF), were exposed to 150 μM H2O2 for 10 min and allowed no recovery (immediate) or 45 min to 2 hr of recovery. Tail moment was used as a relative indicator of severity of damage within a cell. A Western blot shows the efficient depletion of ScII following a 36 hr exposure to Dox. A β-tubulin Western blot was used as a loading control.
(B) An alkaline comet assay on HeLa cells treated with siRNA against CNAP1. Following depletion of CNAP1, cells were treated as described in (A) and analyzed immediately, 1 hr and 2 hr after damage induction by comet assay. A Western blot demonstrates depletion of CNAP1 in siRNA-treated cells compared to mock and control siRNA (nonsilencing) treated cells. A Western analysis of cohesin component Rad21 was used as a loading control.
(C) A neutral comet assay to detect DSBs following IR. The siRNA-treated HeLa cells were exposed to 10 Gy g irradiation and analyzed as in (B).
Similar experiments were carried out in human cells using siRNA directed against condensin I-specific CNAP1/hCAP-D2. Following two siRNA transfections, CNAP1 was efficiently depleted (Figure 6B). In these cells, the rate of SSB repair was significantly decreased compared to mock or nonsilencing control siRNA-treated cells. A similar effect was observed in cells treated with siRNA against XRCC1 (Figure S4A). Although depletion of non-SMC subunits of condensin I causes mitotic chromosome abnormality and mitotic delay, it does not lead to any significant mitotic arrest (Hirota et al., 2004; Ono et al., 2003). Consistent with this, no perturbation of the cell cycle was observed after CNAP1 depletion under our conditions (Figure S4B). Thus, the inhibitory effect of CNAP1 depletion on SSB repair is not a secondary effect due to the accumulation of mitotic cells that may have a different repair capability. Taken together, these results demonstrate that condensin I is important for efficient SSB repair in vertebrate cells.
Condensin I Does Not Play a Significant Role in DSB Repair
As PARP-1 also interacts with DSB repair factors (Galande and Kohwi-Shigematsu, 2000; Ruscetti et al., 1998), we examined whether condensin I is involved in DSB repair. There was no significant effect of CNAP1/hCAP-D2 depletion on ionizing irradiation-induced DSB repair as measured by a neutral comet assay, which detects DSBs but not SSBs (Figure 6C). Also, no significant in vivo interactions of condensin I with factors involved in different aspects of DSB repair (Mre11, Nbs1, Ku, XRCC4, Rad51, and RPA) were detected, either before or after exposure to ionizing irradiation (Figure S5A). Furthermore, we observed no change in the frequency of sister chromatid exchange (SCE) in ScII-depleted DT40 cells after mitomycin C treatment, in contrast to the depletion of cohesin, which led to a decrease of SCE (Figure S5B). Since SCE was shown to be caused by homologous recombination (HR) (Sonoda et al., 1999), the results indicate that condensin does not play a major role in HR repair.
Discussion
Although the critical function of condensin I in mitotic chromosome structure organization is well established, its role during interphase remains unclear. Our results demonstrate that condensin I exhibits a rapid response to DNA SSB damage through its highly inducible interactions with PARP-1, the BER machinery, and chromatin. Consistent with this, SSB repair but not DSB repair is significantly compromised in condensin-depleted cells, indicating the involvement of condensin I in SSB repair.
Condensin I Interactions with a Subset of BER Factors
BER is the major pathway to repair base damage and SSBs caused by both endogenous and exogenous agents, such as reactive oxygen species, alkylating agents, and ionizing irradiation (Slupphaug et al., 2003). BER utilizes two distinct pathways that include single nucleotide gap repair (short patch BER) and two to eight nucleotide gap repair (long-patch BER). Short-patch BER involves AP endonuclease, DNA polymerase b, and ligase III, while the long-patch pathway utilizes FEN-1, DNA polymerase δ/ε (or β), and ligase I. While XRCC1 appears to participate in both processes, our observation that condensin I interacts with FEN-1 and DNApolymer-ase δ/ε suggests the involvement of condensin I in the long-patch BER pathway. Although DNA polymerase β is also involved in long-patch BER, we failed to detect any significant interaction between condensin I and DNA polymerase β. It was previously shown that DNA polymerase β does not play an active role in the repair of oxidation-induced SSBs (Fortini et al., 2000). Since condensin I responds strongly to this type of damage, it is possible that DNA polymerase β and condensin-PARP-1-XRCC1 have different preferences for damage type.
Although both XRCC1 and PARP-1 were shown to interact with ligase III (Caldecott et al., 1996), we did not observe any significant interaction between condensin I and ligase III. This can be explained by the finding that ligase III preferentially interacts with the (ADP-ribosyl)ated form of PARP-1 (Leppard et al., 2003), whereas condensin I associates only with the non- or hypo-(ADP-ribosyl)ated form of PARP-1 (this study). Our results suggest that the condensin I-hypo-(ADP-ribosyl)ated PARP-1-XRCC1 complex formation represents an immediate early step of the damage response and protein assembly at the damage sites (Figure 7). It is possible that, once hyper-(ADP-ribosyl)ated, PARP-1 dissociates from condensin I and recruits ligase III. Alternatively, the condensin I-containing complex may be formed separately from the populations of PARP-1 and XRCC1 that interact with ligase III, and/or the condensin I-containing complex may be involved in a ligase III-independent subpathway of BER.
Figure 7. A Model for the Recruitment of Condensin I to SSB Sites.
SSB damage induces complex formation between condensin I, PARP-1, and XRCC1. This complex is recruited to the DNA lesion and interacts with additional BER factors such as FEN-1 and DNA polymerases β and ε. Condensin I may be involved in local chromatin/DNA structural organization that allows efficient BER.
What mediates the damage-induced increased affinity of the condensin I-PARP-1 interaction is currently unclear. Since there is no significant change in levels of condensin I and PARP-1 proteins in the cell, the enhancement is most likely mediated by posttranslational modification(s). Although our results demonstrated that hyper-(ADP-ribosyl)ation of PARP-1 is not responsible for this change, it is still possible that limited (ADP-ribosyl)ation of either PARP-1 or condensin I may facilitate the interaction. Characterization of the interaction domains of these factors should address the mechanism further.
A Distinct Role of Condensin I in DNA Repair
We have previously shown that human cohesin interacts with the Mre11 complex and is recruited to DSB damage sites in a S/G2 phase-specific and Mre11/Rad50-dependent manner (Kim et al., 2002). Cohesin is important for postreplicative repair, and its depletion led to a decrease of SCE (Sjögren and Nasmyth, 2001; Sonoda et al., 2001). Unlike condensin, no significant change in nuclear retention of cohesin was observed in response to SSBs (Figure 5C). Thus, the two essential SMC complexes appear to participate in distinct DNA damage repair processes.
Although an interphase-specific function of condensin in DNA repair was suggested by studies in yeast, its underlying mechanism and the affected DNA repair pathway remain undefined (Aono et al., 2002; Chen et al., 2004). As PARP-1 is not found in yeast, the observed SSB-specific damage response of condensin involving PARP-1 is unique to higher eukaryotes, and condensin’s role in DNA repair may differ in yeast.
Previously, hCAP-E was reported to interact with the NHEJ factor ligase IV in human cells (Przewloka et al., 2003). We found no effect of condensin I depletion on DSB repair, and thus far failed to identify any significant interaction of condensin I with DSB repair factors, including XRCC4, the partner of ligase IV. Thus, the relevance of the ligase IV interaction with hCAP-E in DNA repair and whether this interaction involves holo-condensins remain to be examined. A role for condensin II in DNA repair is currently unclear. However, as condensin II is constitutively in the nucleus, it may also have interphase functions in such processes as DNA replication or repair. It was shown that condensin I and II do not interact with each other in mitotic chromosome condensation (Hirota et al., 2004; Ono et al., 2003). Similarly, we observed no significant association of condensin II with the condensin I-PARP-1-XRRCC1 complex in the presence of SSBs.
Condensin I on Damaged Chromatin DNA
What is the function of condensin I at the damage site? There are several possibilities that are not necessarily mutually exclusive. First, condensin I may organize local DNA structure for efficient BER. The condensin SMC heterodimer in S. pombe was shown to exhibit DNA annealing activity in vitro (Sakai et al., 2003), and condensin I in higher eukaryotes can affect the topology of a naked DNA plasmid in vitro (Kimura and Hirano, 1997; Kimura et al., 1999). These activities may contribute to the BER process. Alternatively, condensin I may serve as a scaffold factor by interacting with BER factors and stabilizing the assembly of the BER machinery at damage sites. Thus far, however, we observed no significant effect of condensin depletion on the damage-induced nuclear retention of PARP-1 and other BER factors that interact with condensin, suggesting that the function of condensin I is not simply factor recruitment (data not shown). A third possibility is that condensin’s effect on BER may be mediated through the reorganization or stabilization of surrounding chromatin either directly through its interaction with nucleosomes or by further recruiting a necessary factor(s). Thus, it is possible that condensin I, together with PARP-1-XRCC1, prepares chromatin structures to facilitate SSB repair in higher eukaryotes. Furthermore, the S phase-specific interaction of condensin I and PARP-1 as well as the selective interactions of condensin with FEN-1 and DNA polymerase δ/ε raise the possibility that condensin I functions in normal DNA replication by either directly participating in lagging strand DNA processing or by facilitating the repair of SSBs inadvertently generated during DNA replication. Taken together, our results demonstrate an interphase-specific function of condensin I in SSB damage repair.
Experimental Procedures
Cell Lines, Synchronization, and Extracts
HeLa and 293T cells and PARP-1+/+ and PARP-/- MEFs were grown as published (Ball et al., 2002; Wang et al., 1995). Parental wild-type and ScII-conditional knockout chicken DT40 cells (Hudson et al., 2003) were cultured and treated with 1 μg/ml Dox for 36 hr prior to H2O2 treatment. HeLa cells were synchronized by double thymidine block in combination with nocodazole, and extracts were made as described (Schmiesing et al., 1998). The efficiency of cell synchronization was confirmed by FACS analysis (Figure S1A).
DNA-Damaging Agents
Cells were treated with HU, H2O2, MMS (Sigma-Aldrich), or CPT (A.G. Scientific). Treatments were carried out at 37°C for times and concentrations indicated in the figures.
Antibodies
Anti-hCAP-E and hCAP-C antibodies were previously described (Schmiesing et al., 1998). Anti-hCAP-H polyclonal rabbit antibody was generated against a 211 aa amino-terminal fragment of hCAP-H expressed in E. coli and affinity purified with the same peptide. Antibodies against hCAP-G (aa 238-572) and PARP-1 (aa 323-603) were prepared similarly. Goat polyclonal anti-PARP-1 (R&D Systems) and mouse monoclonal antibodies specific for PARP-1 (Biomol), XRCC1, FEN-1, ligase III, Mre11, Rad51, DNA pol δ and ε (GeneTex), PAR polymers (Biomol), XRCC1 (Trevigen), DNA pol β (NeoMarkers), Ku-70 (Novus), NBS1 (BD Biosciences), and a polyclonal antibody specific for XRCC1 (Santa Cruz) were also used.
Chromatography and Peptide Sequencing
HeLa nuclear extracts were passed through a P-11 phosphocellulose column (Whatman, 4071050) and eluted using a KCl gradient in Buffer D (20 mM HEPES, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA [pH 7.9], 25% glycerol, 0.5 mM DTT, 1 mM AEBSF). The Far Western-positive fractions were pooled and purified over HiTrap heparin. Peak heparin fractions were subjected to a Mono Q HR 5/5 (Amersham Biosciences) column. The 120 kDa polypeptide species in the peak Q fraction was excised from a silver-stained SDS-PAGE gel and subjected to MALDI-TOF mass spectrometric analysis.
Far Western Analysis
Crude nuclear and cytoplamic extracts or column fractions were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were denatured and renatured by sequential dilution of the 6 M guanidine-HCl. GST-GFP (GG) and GG-NCTD (the C-terminal 113 aa of CNAP1) were expressed in E. coli as probes, purified on glutathione-Sepharose beads, and labeled in vitro with protein kinase A and [γ-32P] ATP. After labeling, GG and GG-NCTD were eluted from beads with glutathione and were hybridized to the membrane (Ball et al., 2002).
Immunofluorescent Staining and Image Analysis
Mitotic chromosome spreads were prepared and stained as described (Schmiesing et al., 1998). 2,6-diamidinophenylindole (DAPI) was used for DNA detection.
Coimmunoprecipitation
CoIP was carried out as previously described (Schmiesing et al., 1998). Briefly, the antibody-protein complex was washed four times with HEMG buffer (25 mM HEPES [pH 7.6], 0.1 mM EDTA, 12.5 mM MgCl2, 10% glycerol) containing 0.1M KCl in the presence of 0.1% Nonidet P-40. Bound proteins were eluted from the beads with HEMG-1.0M KCl (1.0 M KCl) followed by 2 M guanidine-HCl. Eluted proteins were precipitated with trichloroacetic acid (TCA) and analyzed by SDS-PAGE and Western blotting.
Western Blot Analysis
Colorimetric or enhanced chemiluminescence Western blot was performed as previously described (Schmiesing et al., 1998). For quantification of the fold increase of the PARP-1:condensin interaction upon DNA damage induction, BioRad’s Quantity One software (version 4.4.1) was used.
Sucrose Gradient Ultracentrifugation
Nuclear extracts from HeLa cells either untreated or treated with H2O2 for 20 min at 37°C were subjected to a 5%-30% sucrose gradient centrifugation at 155,000 × g for 15 hr at 4°C with a SW50.1 rotor (Beckman Instruments). Each fraction was TCA precipitated and analyzed by SDS-PAGE and Western blotting. Sedimentation coefficient values were calculated based on a standard curve using ferritin, catalase, and BSA.
Sequential Extraction
Sequential extraction was performed as previously described (Gregson et al., 2001). Briefly, cells were extracted with CSK (10 mM PIPIES [pH 6.8], 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2) with 0.5% Triton X-100 for 5 min at 4°C (the “CSK” fraction) and extraction buffer (42.5 mM Tris-HCL [pH 8.3], 8.5 mM NaCl, 2.6 mM MgCl2, 0.5% deoxycholic acid) with 1% Tween-20 for 5 min at 4°C (the “EXT” fraction). The pellet consists of nuclear proteins that associate with DNA and/or nuclear matrix.
Comet Assay
The alkaline and neutral comet assays were performed using aTrevigen CometAssay kit (4250-050-K) according to the manufacturer’s recommendations. DNA was visualized using Trevigen SYBR Green I fluorescent staining as provided in the kit. Typically, more than 100 cells per sample were analyzed, and experiments were repeated at least three times. Comet data was analyzed using CometScore (TriTek Corp).
siRNA Transfection
HeLa cells were transfected twice 18 hr apart with siRNA directed against CNAP1 (5′-TAGGGTGACCTGGAATTCGAA-3′) ora negative control (5′-AATTCTCCGAACGTGTCACGT-3′) at a final concentration of 10 nM using HiPerFect Transfection Reagent per the manufacturer’s instructions (Qiagen). Cells were used for the comet assay and Western blot analyses at 48 hr after the second transfection.
Supplementary Material
Supplemental Data include five figures, one table, and Supplemental References and can be found with this article online at http://www.molecule.org/cgi/content/full/21/6/837/DC1/.
Acknowledgments
We would like to thank Drs. Zhao-Qi Wang and Mark Smulson for providing the PARP-1 knockout MEFs, Drs. Masanao Miwa and Mitsuko Masutani for the PARP-1 cDNA clone, Dr. Shunichi Takeda for Scc1-knockout DT40 cells, and Dr. Tim Yen for hSgo siRNA. This work was supported in part by NIH predoctoral fellowship GM070411 (to J.T.H.) and NIH GM59150 and ES013511 (to K.Y.). K.Y. was a Leukemia & Lymphoma Society Scholar.
References
- Andegeko Y, Moyal L, Mittelman L, Tsarfaty I, Shiloh Y, Rotman G. Nuclear retention of ATM at sites of DNA double strand breaks. J Biol Chem. 2001;276:38224–38230. doi: 10.1074/jbc.M102986200. [DOI] [PubMed] [Google Scholar]
- Aono N, Sutani T, Tomonaga T, Mochida S, Yanagida M. Cnd2 has dual roles in mitotic condensation and interphase. Nature. 2002;417:197–202. doi: 10.1038/417197a. [DOI] [PubMed] [Google Scholar]
- Ball AR, Jr, Schmiesing JA, Zhou C, Gregson HC, Okada Y, Doi T, Yokomori K. Identification of a chromosome targeting domain in the human condensin subunit CNAP1/hCAP-D2/Eg7. Mol Cell Biol. 2002;22:5769–5781. doi: 10.1128/MCB.22.16.5769-5781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–454. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott KW, Aoufouchi S, Johnson J, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;15:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ES, Sutani T, Yanagida M. Cti1/C1D interacts with condensin SMC hinge and supports the DNA repair function of condensin. Proc Natl Acad Sci USA. 2004;101:8078–8083. doi: 10.1073/pnas.0307976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is im-paired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- Dej KJ, Ahn C, Orr-Weaver TL. Mutations in the Drosophila condensin subunit dCAP-G: defining the role of condensin for chromosome condensation in mitosis and gene expression in in-terphase. Genetics. 2004;168:895–906. doi: 10.1534/genetics.104.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini P, Pascucci B, Belisario F, Dogliotti E. DNA polymerase beta is required for efficient DNA strand break repair induced by methyl methanesulfonate but not by hydrogen peroxide. Nucleic Acids Res. 2000;28:3040–3046. doi: 10.1093/nar/28.16.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galande S, Kohwi-Shigematsu T. Caught in the act: binding of Ku and PARP to MARs reveals novel aspects of their functional interaction. Crit Rev Eukaryot Gene Expr. 2000;10:63–72. [PubMed] [Google Scholar]
- Gregson HC, Schmiesing JA, Kim J-S, Kobayashi T, Zhou S, Yokomori K. A potential role for human cohesin in mitotic spindle aster assembly. J Biol Chem. 2001;276:47575–47582. doi: 10.1074/jbc.M103364200. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Wang Z-Q. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477:97–110. doi: 10.1016/s0027-5107(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. [DOI] [PubMed] [Google Scholar]
- Hirano T. Condensins: organizing and segregating the genome. Curr Biol. 2005;15:R265–R275. doi: 10.1016/j.cub.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Hirota T, Gerlich D, Koch B, Ellenberg J, Peters JM. Distinct functions of condensin I and II in mitotic chromosome assembly. J Cell Sci. 2004;117:6435–6445. doi: 10.1242/jcs.01604. [DOI] [PubMed] [Google Scholar]
- Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell. 2003;5:323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Krasieva TB, LaMorte VJ, Taylor AMR, Yoko-mori K. Specific recruitment of human cohesin to laser-induced DNA damage. J Biol Chem. 2002;277:45149–45153. doi: 10.1074/jbc.M209123200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hirano T. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell. 1997;90:625–634. doi: 10.1016/s0092-8674(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hirano T. Dual roles of the 11S regulatory subcomplex in condensin functions. Proc Natl Acad Sci USA. 2000;97:11972–11977. doi: 10.1073/pnas.220326097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR. 13S condensin actively reconfigures DNA by introducing global positive writhe: implication for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Kimura K, Cuvier O, Hirano T. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J Biol Chem. 2001;276:5417–5420. doi: 10.1074/jbc.C000873200. [DOI] [PubMed] [Google Scholar]
- 3348.Klungland A, Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase (FEN1) EMBO J. 1997;16:3341. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard JB, Dong Z, Mackey ZB, Tomkinson AE. Physical functional interaction between DNA ligase III alpha poly(ADP-Ribose) polymerase 1 in DNA single-strand break repair. Mol. Cell Biol. 2003;23:5919–5927. doi: 10.1128/MCB.23.16.5919-5927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Satoh MS, Poirier GG, Klungland A. Posttranslational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995;20:405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Lupo R, Breiling A, Bianchi ME, Orlando V. Drosophila chromosome condensation proteins topoisomerase II Barren colocalize with Polycomb maintain Fab-7 PRE silencing. Mol Cell. 2001;7:127–136. doi: 10.1016/s1097-2765(01)00161-7. [DOI] [PubMed] [Google Scholar]
- Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-Ribose) polymerase negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani M, Nakagama H, Sugimura T. Poly(ADP-ribose) carcinogenesis. Genes Chromosomes Cancer. 2003;38:339–348. doi: 10.1002/gcc.10250. [DOI] [PubMed] [Google Scholar]
- Nash RA, Caldecott KW, Barnes DE, Lindahl T. XRCC1 protein interacts with one of two distinct forms of DNA ligase III. Biochemistry. 1997;36:5207–5211. doi: 10.1021/bi962281m. [DOI] [PubMed] [Google Scholar]
- Ono T, Losada A, Hirano M, Myers MP, Neuwald AF, Hirano T. Differential contributions of condensin I condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109–121. doi: 10.1016/s0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Przewloka MR, Pardington PE, Yannone SM, Chen DJ, Cary RB. In vitro in vivo interactions of DNA ligase IV with a subunit of the condensin complex. Mol Cell Biol. 2003;14:685–697. doi: 10.1091/mbc.E01-11-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti T, Lehnert BE, Halbrook J, Trong HL, Hoekstra MF, Chen DJ, Peterson SR. Stimulation of the DNA-dependent protein kinase by Poly(ADP-Ribose) polymerase. J Biol Chem. 1998;273:14461–14467. doi: 10.1074/jbc.273.23.14461. [DOI] [PubMed] [Google Scholar]
- Sakai A, Hizume H, Sutani T, Takeyasu K, Yanagida M. Condensin but not cohesin SMC heterodimer induces DNA reannealing through protein-protein assembly. EMBO J. 2003;22:2764–2775. doi: 10.1093/emboj/cdg247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiesing JA, Ball AR, Gregson HC, Alderton J, Zhou S, Yokomori K. Identification of two distinct human SMC protein complexes involved in mitotic chromosome dynamics. Proc Natl Acad Sci USA. 1998;95:12906–12911. doi: 10.1073/pnas.95.22.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiesing JA, Gregson HC, Zhou S, Yokomori K. A human condensin complex containing hCAP-C/hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol Cell Biol. 2000;20:6996–7006. doi: 10.1128/mcb.20.18.6996-7006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren C, Nasmyth K. Sister chromatid cohesion is re-quired for postreplicative double-strand break repair in Saccharomyces cerevisiae . Curr Biol. 2001;11:991–995. doi: 10.1016/s0960-9822(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Slupphaug G, Kavli B, Krokan HE. The interacting pathways for preventing repair of oxidative DNA damage. Mutat Res. 2003;531:231–251. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Sasaki MS, Morrison C, Yamaguchi-Iwai Y, Takata M, Takeda S. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol Cell Biol. 1999;19:5166–5169. doi: 10.1128/mcb.19.7.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Matsusaka T, Morrison C, Vagnarelli P, Hoshi O, Ushiki T, Nojima K, Fukagawa T, Waizenegger IC, Peters JM, et al. Scc1/Rad21/Mcd1 is required for sister chromatid cohesion kinetochore function in vertebrate cells. Dev. Cell. 2001;1:759–770. doi: 10.1016/s1534-5807(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Stucki M, Pascucci B, Parlanti E, Fortini P, Wilson SH, Hübscher U, Dogliotti E. Mammalian base excision re-pair by DNA polymerases δ and ε. Oncogene. 1998;17:835–843. doi: 10.1038/sj.onc.1202001. [DOI] [PubMed] [Google Scholar]
- Trucco C, Oliver FJ, de Murcia G, Menissier-de Murcia J. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res. 1998;26:2644–2649. doi: 10.1093/nar/26.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner EF. Mice lacking ADPRT poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Yeong FM, Hombauer H, Wendt KS, Hirota T, Mudrak I, Mechtler K, Loregger T, Marchler-Bauer A, Tanaka K, Peters J-M, Ogris E. Identification of a subunit of a novel kleisin-β/SMC complex as a potential substrate of protein phosphatase 2A. Curr Biol. 2003;13:2058–2064. doi: 10.1016/j.cub.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Zahradka P, Ebisuzaki K. A shuttle mechanism for DNA-protein interactions. The regulation of poly(ADP-ribose) polymerase. Eur J Biochem. 1982;127:579–585. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.