Summary
The spindle checkpoint delays anaphase onset in cells with mitotic spindle defects. Here, we show that Chk1, a component of the DNA damage and replication checkpoints, protects vertebrate cells against spontaneous chromosome missegregation and is required to sustain anaphase delay when spindle function is disrupted by taxol, but not when microtubules are completely depolymerized by nocodazole. Spindle checkpoint failure in Chk1-deficient cells correlates with decreased Aurora-B kinase activity and impaired phosphorylation and kinetochore localization of BubR1. Furthermore, Chk1 phosphorylates Aurora-B and enhances its catalytic activity in vitro. We propose that Chk1 augments spindle checkpoint signaling and is required for optimal regulation of Aurora-B and BubR1 when kinetochores produce a weakened signal. In addition, Chk1-deficient cells exhibit increased resistance to taxol. These results suggest a mechanism through which Chk1 could protect against tumorigenesis through its role in spindle checkpoint signaling.
Introduction
The mitotic spindle checkpoint protects against chromosome missegregation by delaying sister chromatid separation until all sister kinetochores have achieved bipolar attachment to spindle microtubules (Kops et al., 2005). The checkpoint is triggered by kinetochores that lack bound microtubules, but also by lack of tension across attached sister kinetochores. Spindle checkpoint defects are associated with chromosomal instability, aneuploidy, and cancer predisposition (Kops et al., 2005).
Conserved components of the spindle checkpoint path-way include the Mad proteins Mad1, Mad2, and BubR1 (Mad3) and the Bub proteins Bub1 and Bub3 (Kops et al., 2005). Recruitment of Mad and Bub proteins to kinetochores of unaligned chromosomes is essential to prevent activation of the anaphase-promoting complex/cyclosome (APC/C) and to delay mitotic exit. Both BubR1 and Mad2 are recruited to unattached kinetochores, whereas only BubR1 is detectably recruited to kinetochores that are not under tension; however, Mad2 function is still required to delay mitotic exit in the absence of tension (Skoufias et al., 2001 Waters et al., 1998). Activated APC/C is an E3 ubiquitin ligase that targets key proteins, including cyclin B and securin, for degradation, leading to initiation of anaphase and segregation of sister chromatids (Kops et al., 2005).
The mechanisms that activate the spindle checkpoint are incompletely understood. In budding yeast, the Ipl1/Aurora protein kinase is required for the spindle checkpoint in the absence of tension, but not when kinetochore-microtubule attachment is disrupted (Biggins and Murray, 2001). It has been proposed that Ipl1/Aurora activates the checkpoint in response to tension defects and facilitates biorientation by promoting turnover of kinetochore-microtubule interactions until bipolar attachment is achieved (Cheeseman et al., 2002).
In higher eukaryotic cells, identification of the precise role of Aurora-B is complicated by the fact that lack of tension can generate unattached, or partially attached, kinetochores that activate the spindle checkpoint (King and Nicklas, 2000; Waters et al., 1998). Aurora-B catalytic activity is required for phosphorylation and recruitment of BubR1 to kinetochores, and inhibition of Aurora activity or depletion of proteins that regulate Aurora-B results in failure to sustain mitotic arrest in the absence of tension (Carvalho et al., 2003; Ditchfield et al., 2003; Lampson and Kapoor, 2005). The activity of Aurora-B is modulated by the chromosomal passenger proteins INCENP and Survivin. INCENP binds to Aurora-B via its conserved carboxy-terminal IN box and stimulates Aurora-B activity both in vitro and in vivo (Bishop and Schumacher, 2002; Honda et al., 2003). Survivin binds to INCENP and contributes to full Aurora-B activity in vivo, and both INCENP and Survivin are required for localization of Aurora-B to kinetochoresduring prometaphase (Honda et al., 2003). During anaphase and cytokinesis, the chromosomal passenger complex of Aurora-B, INCENP, and Survivin transfers to the spindle midzone and the midbody, respectively, and participates in chromosome segregation and completion of cytokinesis (Vagnarelli and Earnshaw, 2004).
Chk1 is a conserved protein kinase that is required to delay the entry of cells with damaged or unreplicated DNA into mitosis (Takai et al., 2000 Zachos et al., 2005). Chk1 was also proposed to delay the onset of anaphase in mitotic cells with damaged DNA in a Mad2-dependent manner (Collura et al., 2005 Royou et al., 2005) and to control the timing of mitotic entry during an unperturbed cell cycle (Kramer et al., 2004); however, a role for Chk1 in the spindle checkpoint has not been previously reported.
Using DT40 avian B-lymphoma cells in which Chk1 was genetically ablated by gene targeting (Zachos et al., 2003) and human cells in which Chk1 expression was depleted by using small interfering RNA (siRNA), we show that Chk1 protects against spontaneous chromosome missegregation and chromosomal instability. We also show that Chk1-deficient cells fail to sustain mitotic arrest in the presence of taxol, a drug that stabilizes microtubules, and that checkpoint failure is associated with decreased Aurora-B activity and defects in phosphorylation and localization of BubR1 to kinetochores. In contrast, Chk1 is not required for stable mitotic arrest when microtubules are completely depolymerized by nocodazole. Chk1 associates with kinetochores in prometaphase and is phosphorylated in cells treated with taxol and nocodazole, and Chk1 kinase activity is required to maintain mitotic arrest during treatment with taxol. Furthermore, Chk1 phosphorylates Aurora-B and enhances its catalytic activity in vitro. On the basis of the these findings, we propose that Chk1 augments spindle checkpoint signaling to ensure optimal regulation of Aurora-B and BubR1 and sustained mitotic arrest when kinetochores produce a weakened checkpoint signal. Remarkably, Chk1 is also required for optimal tumor cell killing by taxol.
Results
Chk1-Deficient Cells Exhibit Increased Levels of Chromosome Missegregation and Chromosomal Instability
To investigate a possible role for Chk1 in mitosis, DT40 and Chk1-deficient DT40 cells (Chk1−/−) stably expressing a histone 2B-GFP (H2B:GFP) fusion protein were monitored through cell division by time-lapse microscopy. This revealed that 4/12 (33%) of Chk1 −/− cells divided despite visible chromosome misalignment and/or missegregation of one or a few chromosomes, whereas 0/15 wild-type DT40 cells did so (Figure 1A).
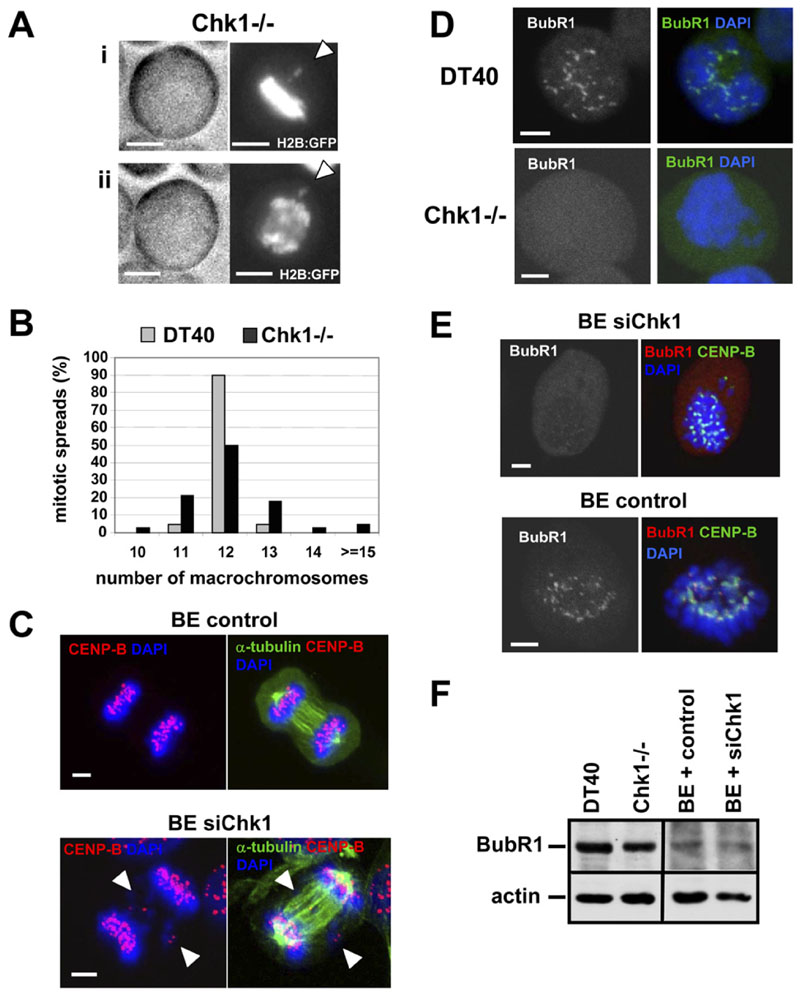
Figure 1. Chk1-Deficient Cells Exhibit Chromosome Missegregation, Chromosomal Instability, and Defects in Localization of BubR1 to Kinetochores during Unperturbed Mitosis.
(A)Example of chromosome missegregation. A Chk1−/− cell expressing H2B:GFP was analyzed by time-lapse microscopy. Phase-contrast (left panels) and fluorescence images (right panels) of (i) metaphase and (ii) anaphase are shown. (i) Misaligned and (ii) missegregated chromosomes are indicated by arrowheads. The scale bar is 5 μm.
(B)Distribution of macrochromosome numbers from mitotic spreads. A total of 60 metaphase spreads were counted for each cell line.
(C)BE cells transfected with negative siRNA (control) or Chk1 siRNA (siChk1) were analyzed by confocal microscopy. Examples of missegregated chromosomes during anaphase are indicated by arrowheads. Red, CENP-B; green, α-tubulin; blue, DNA. A single image plane is shown. The scale bar is 5 μm.
(D)Projected deconvolved image stacks of DT40 and Chk1−/− cells in prometaphase. Green, BubR1; blue, DNA. The scale bar is 5 μm.
(E)Proiected deconvolved image stacks of BE cells transfected as in (C). Red, BubR1; green, CENP-B; blue, DNA. The scale bar is 5 mm.
(F)Western blot analysis of BubR1 and actin.
Chromosome missegregation is predicted to result in chromosomal instability. To test for this directly, we prepared metaphase spreads of DT40 and Chk1 −/− cells and counted the number of macrochromosomes. Strikingly, whereas 90% of DT40 cells showed the expected number of 12 macrochromosomes (Sonoda et al., 1998), only 50% of Chk1 −/− cells were karyotypically normal; the remainder exhibited gain or loss of a small number of macrochromosomes (Figure 1B).
To verify these observations in a different cell type, we depleted Chk1 expression in human BE colon carcinoma cells by transient transfection of Chk1 siRNA (siChk1; Figure 3C). Examination of Chk1-depleted BE cells revealed that 10/30 (33%) of metaphases showed misaligned chromosomes and that 12/48 (25%) of cells in anaphase/cytokinesis showed one or few missegregated chromosomes (Figure 1C and Figure S1A, see the Supplemental Data available with this article online), compared to only 2/25 (8%) and 3/51 (6%), respectively, in cells transfected with negative siRNA (control). Similarly, increased chromosome missegregation during anaphase was observed in Chk1-depleted human colon carcinoma HCT116 and human embryonic kidney HEK293 cells (Figure S1C). Finally, time-lapse microscopy confirmed that 3/15 (20%) of Chk1-depleted BE cells divided with one or few missegregated chromosomes in anaphase, compared to 0/11 control BE cells (Figure S1B). These results show that Chk1 is required for accurate chromosome segregation in both avian and human cells.
Chk1-Deficient Cells Exhibit Defects in Recruitment of BubR1 to Kinetochores during Unperturbed Mitosis
Recruitment of BubR1 to kinetochores is essential for activation of the spindle checkpoint in response to misaligned chromosomes. In all (20/20) prometaphase DT40 cells examined by confocal microscopy, BubR1 was observed to localize to one or more kinetochores, indicating that the spindle checkpoint was activated (Figure 1D). In striking contrast, 8/25 (32%) of prometa-phase Chk1 −/− cells examined showed no detectable recruitment of BubR1 to any kinetochores (Figure 1D).
Similar results were obtained in Chk1-depleted BE cells, in which 11/20 (55%) prometaphases examined showed no detectable recruitment of BubR1 to any kinetochores (Figure 1E; mean BubR1/CENP-B fluorescence intensity of 0.08 ± 0.07; Table S1). In comparison, BubR1 was observed to localize to kinetochores in 20/20 of control prometaphase cells in which it was juxtaposed to CENPB, an inner centromere marker (Figure 1E), and the mean BubR1/CENP-B fluorescence intensity was 0.61 ± 0.37 (Table S1). However, BubR1 protein levels per se were not affected by loss of Chk1 (Figure 1F). Taken together, these results demonstrate that Chk1 is required for optimal localization of BubR1 to kinetochores during unperturbed mitosis, thus suggesting a novel role for Chk1 in spindle checkpoint function.
Chk1 Localizes to Kinetochores in Prometaphase
Proteins involved in the spindle checkpoint are often recruited to kinetochores during prometaphase. We therefore examined localization of Chk1 during unperturbed mitosis by using Chk1−/− cells reconstituted with a Chk1:GFP fusion protein. Chk1:GFP localized at kinetochores in prometaphase cells (Figure 2A), but not during metaphase or anaphase (data not shown). During cytokinesis, Chk1:GFP was observed to concentrate at the midbody, where it colocalized with INCENP (Figure S1D).
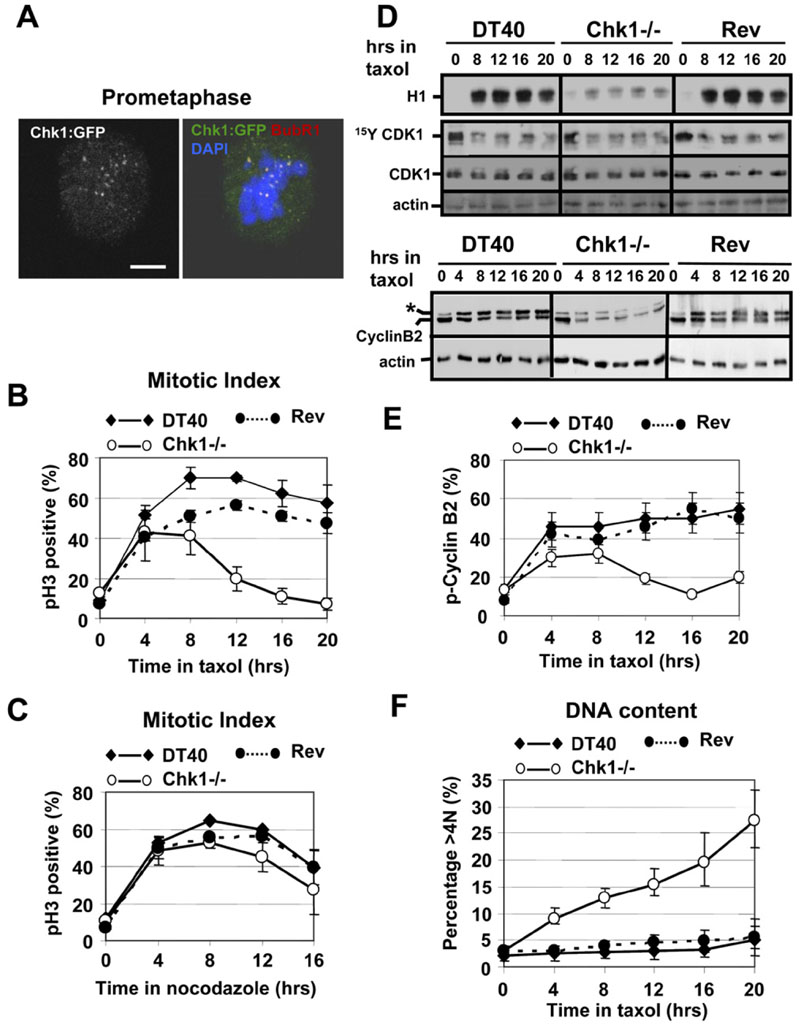
Figure 2. Chk1-Deficient Cells Fail to Sustain Taxol-Induced Mitotic Arrest.
(A)Chk1 colocalizes with BubR1 at kinetochores during prometaphase. Example of a Chk1−/−cell expressing Chk1:GFP and analyzed by confocal microscopy. Red, BubR1; green, Chk1:GFP; blue, DNA. A single image plane is shown. The scale bar is 5 μm.
(B and C) Mitotic index analysis of G2/M-elutriated DT40, Chk1−/−, and revertant (Rev) cells during treatment with (B) taxol or (C) nocodazole. pH3, phosphorylated Ser10 of histone H3. Error bars show the standard deviation from the mean from three experiments.
(D)Upper panels: CDK1-associated histone H1 kinase activity and western blot analysis of CDK1 (Tyr15-phosphorylated and total) and actin during treatment of G2/M-elutriated cells with taxol. Lower panels: western blot analysis of cyclin B2 and actin. Phosphorylated cyclin B2 is marked by an asterisk.
(E)Phosphorylated cyclin B2 as a percentage of the total cyclin B2 protein during treatment of G2/M-elutriated cells with taxol. Error bars show the standard deviation from the mean from three experiments.
(F)Endoreduplication in Chk1−/− cells during treatment with taxol. Cells were treated as in (B), and the percentage of cells with greater than 4N DNA content was determined. Error bars show the standard deviation from the mean from three experiments.
Chk1 Is Required for Sustained Mitotic Delay in Response to Microtubule Stabilization, but Not Depolymerization
To explore the role of Chk1 in the spindle checkpoint, we examined whether Chk1 was required for mitotic arrest when spindle function was perturbed by using the spindle poisons taxol and nocodazole. Taxol stabilizes microtubules by preventing tubulin depolymerization and primarily interferes with tension at kinetochores, although loss of microtubule attachment in a subset of kinetochores can occur (Schiff and Horwitz, 1980). Nocodazole, on the other hand, completely prevents microtubule polymerization and spindle formation; thus, all kinetochores remain completely unoccupied. Therefore, treatment with either taxol or nocodazole delays mitotic exit in checkpointproficient cells.
DT40, Chk1−/−, and revertant (Rev) cells were synchronized in G2/M by centrifugal elutriation to maximize accumulation of mitotic cells. The elutriated G2/M cells were then cultured with taxol or nocodazole, and the mitotic index over time was determined by staining for phosphorylated Serine 10 of histone H3 (pH3) and flow cytometry (Zachos et al., 2005). Rev cells are Chk1−/− cells that re-express Chk1 encoded by a transfected transgene (Zachos et al., 2003). As shown in Figure 2B, taxol treatment induced a pronounced accumulation of DT40 and Rev cells in mitosis that was maximal (60%-70%) between 8 and 12 hr and was sustained for up to 20 hr. In contrast, although the proportion of mitotic cells in Chk1−/− cultures treated with taxol initially increased, this increase was not sustained, and the mitotic index declined after 12 hr of treatment to baseline levels. In comparison, all three cell lines accumulated in mitosis similarly when treated with nocodazole (Figure 2C).
Time-lapse analysisfor up to 20 hr revealed that a similar percentage (˜70%) of DT40 and Chk1−/− cells expressing H2B:GFP entered mitosis during taxol or nocodazole treatment, as iudged by chromosome condensation. In the presence of taxol, all (40/40) mitotic DT40 cells monitored remained arrested with condensed chromosomes for the duration of the experiment, whereas the maiority (24/34, 71%) of mitotic Chk1−/− cells decondensed their chromosomes, which was preceded by the formation of aberrant cytokinetic furrows that did not progress to completion. In contrast, all mitotic DT40 (50/50) and Chk1−/−(52/52) cells monitored retained their condensed chromosomes during treatment with nocodazole. We conclude that Chk1-deficient cells are unable to sustain a mitotic delay in response to taxol, but that they can be arrested effectively in mitosis by nocodazole.
Consistent with mitotic exit during treatment with taxol, Chk1−/− cells failed to sustain high levels of CDK1-associated kinase activity compared to DT40 and Rev cells (Figure 2D). This was not due to reimposition of inhibitory phosphorylation of CDK1 on Tyrosine 15 (Tyr15), since the overall levels of CDK1 Tyr15 phosphorylation in Chk1−/−, DT40, and Rev cells treated with taxol were similar (Figure 2D).
Cyclin B2 is the predominant avian B-type cyclin, and a phosphorylated isoform accumulates during mitosis (Zachos et al., 2005). Decreased CDK1 kinase activity in Chk1−/− cells correlated with lower levels of both total and phospho-cyclin B2 compared to DT40 and Rev (Figure 2D). Only 10%-20% of total cyclin B2 was phosphorylated in Chk1−/− cells after 12-20 hr in taxol, compared to ˜50% in DT40 and Rev cells (Figure 2E). Collectively, these data demonstrate that Chk1−/− cells exit mitosis inthe presence of taxol with concomitant deactivation of CDK1 kinase activity and degradation of both total and phospho-cyclin B2. After mitotic exit, ˜30% of Chk1−/− cells underwent endoreduplication and accumulated DNA content greater than 4N, as determined by flow cytometry (Figure 2F).
Chk1 Is Required for Localization of BubR1 to Kinetochores in Response to Microtubule Stabilization
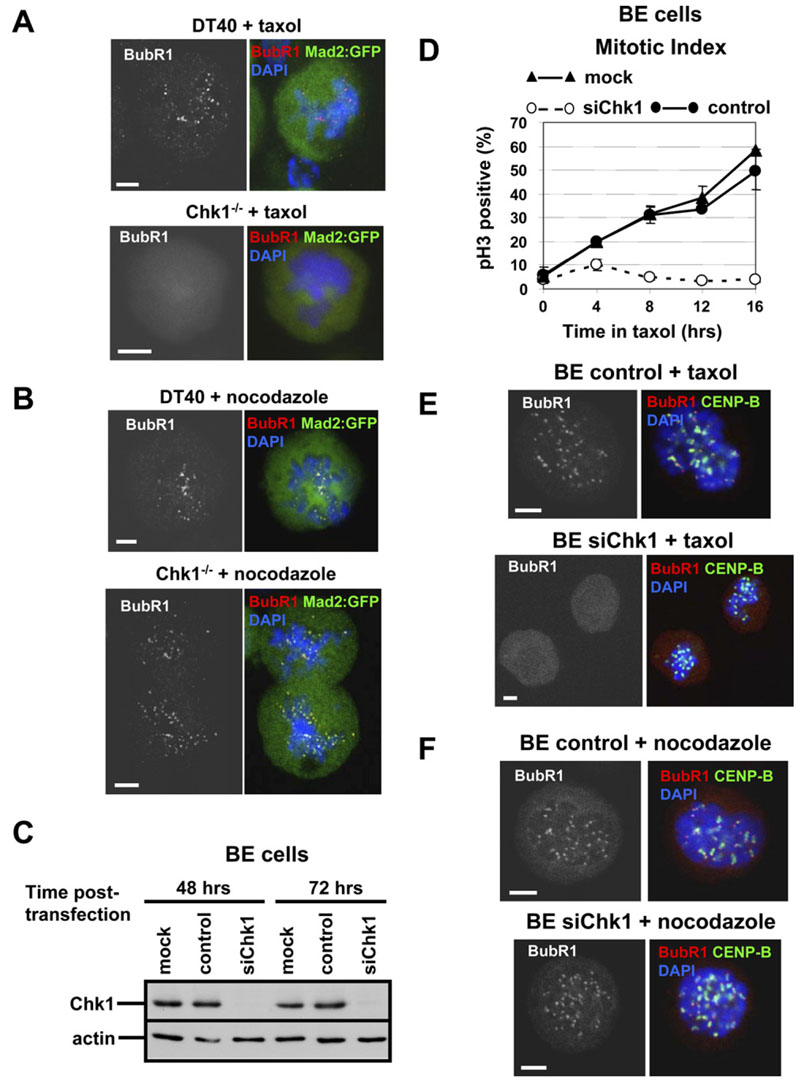
To determine whether Chk1 influenced localization of BubR1 or Mad2 to kinetochores during checkpoint activation by spindle poisons, we generated DT40 and Chk1−/− cells stably expressing a Mad2:GFP fusion protein and examined localization of BubR1 and Mad2 by confocal microscopy. DT40 cells that were arrested in prometaphase by taxol showed kinetochore localization of BubR1, but not Mad2:GFP (Figure 3A). In contrast, the majority of Chk1−/− cells in prometaphase failed to localize BubR1 or Mad2:GFPto kinetochores during treatment with taxol (Figure 3A). Importantly, BubR1 colocalized with Mad2:GFP to kinetochores both in DT40 and Chk1−/− cells treated with nocodazole, consistent with the sustained mitotic arrest elicited by this drug in both cell types (Figure 3B).
These observations were also verified in human cells. Unlike control cells, BE cells depleted for Chk1 by transfection of Chk1 siRNAs that target different regions of Chk1, either as a pool or individually, failed to arrest in mitosis when exposed to taxol (Figure 3D and Figure S2A). Similarly, HCT116 and HEK293 cells depleted for Chk1 exhibited decreased accumulation of mitotic cells during treatment with taxol compared to controls (Figure S2B). Importantly, mitotic arrest of Chk1-depleted human cells was essentially restored in the presence of nocodazole (Figure S2C).
Failure of Chk1-depleted human cells to arrest in mitosis during treatment with taxol was also associated with defects in BubR1 recruitment. As shown in Figure 3E, all (20/20) control BE cells examined showed localization of BubR1 to kinetochores after taxol treatment, and BubR1 was juxtaposed to CENP-B (mean BubR1/CENP-B fluorescence intensity of 0.52 ± 0.35; Table S1). In contrast, 14/21 (67%) of Chk1-depleted cells showed no detectable localization of BubR1 to kinetochores (Figure 3E) and a mean BubR1/CENP-B fluorescence intensity of 0.05 ± 0.04 (Table S1). During treatment with nocodazole, BubR1 localized to kinetochores in all control (25/25) and Chk1-depleted (17/17) mitotic BE cells examined (Figure 3F), and the mean BubR1/CENP-B fluorescence intensity was similar in both cell types (0.44 ± 0.14 and 0.47 ±0.19, respectively; Table S1). Taken together, these results demonstrate that Chk1 is required for sustained mitotic arrest and recruitment of BubR1 to kinetochores in cells treated with taxol, but not nocodazole.
Figure 3. Chk1 Is Required for Localization of BubR1 to Kinetochores during Treatment with Taxol.
(A and B) Projected deconvolved image stacks of DT40 and Chk1−/− cells expressing Mad2:GFP and treated with (A) taxol or (B) nocodazole for 45 min. Red, BubR1; green, Mad2:GFP; blue, DNA. The scale bar is 5 μm.
(C)Western blot analysis of total Chk1 and actin in BE cells mock transfected (mock), transfected with negative siRNA (control), or transfected with Chk1 siRNA (siChk1).
(D)Mitotic index analysis of BE cells transfected as in (C) and treated with taxol. pH3, phosphorylated Ser10 of histone H3. Error bars show the standard deviation from the mean from three experiments.
(E and F) Projected deconvolved image stacks of BE cells transfected as in (C) and treated with (E) taxol or (F) nocodazole for 2 hr. Red, BubR1; green, CENP-B; blue, DNA. The scale bar is 5 μm.
Chk1 Catalytic Activity Is Required for Sustained Mitotic Arrest in Response to Taxol
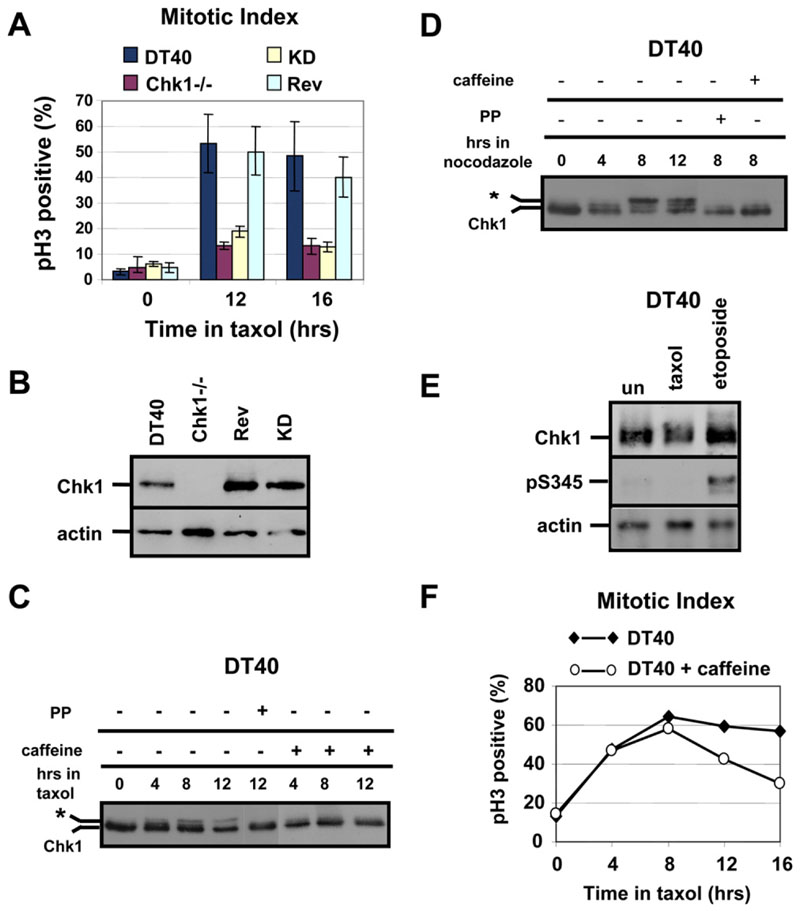
To test whether Chk1 catalytic activity was required for taxol-induced mitotic arrest, the mitotic index of Chk1−/− cells stably expressing a kinase-dead (KD) Chk1 protein devoid of basal catalytic activity was determined. Unlike DT40 and Rev cells, both Chk1−/− and KD cells failed to accumulate in mitosis during taxol treatment (Figure 4A). In addition, ˜25% of KD and Chk1−/− cells treated with taxol for 16 hr exhibited DNA content >4N, indicative of polyploidy, compared to only 5% for DT40 and Rev cells (data not shown). The relative levels of Chk1 expression in DT40, Chk1−/−, Rev, and KD cells areshown in Figure 4B. These results indicate that Chk1 kinase activity is required to sustain mitotic arrest during treatment with taxol.
Figure 4. Phosphorylation of Chk1 during Treatment of Cells with Spindle Poisons.
(A)Chk1 kinase activity is required for taxol-induced mitotic arrest. Mitotic index analysis of G2/M-elutriated DT40, Chk1−/−, kinase-dead (KD), and revertant (Rev) cells during treatment with taxol. pH3, phosphorylated Ser10 of histone H3. Error bars show the standard deviation from the mean from three experiments.
(B)Western blot analysis of total Chk1 and actin in asynchronous cells.
(C and D) Western blot analysis of total Chk1 during treatment of G2/M-elutriated DT40 cells with (C) taxol or (D) nocodazole, in the absence or presence of caffeine. The asterisk marks phosphorylated Chk1. Treatment of sample with λ protein phosphatase (PP) is shown.
(E)Western blot analysis of total Chk1, phosphorylated Chk1 at Serine 345 (pS345), and actin. G2/M-elutriated DT40 cells were untreated (un) or were treated with taxol or etoposide for 8 hr.
(F)Taxol-induced mitotic arrest is weakened by caffeine. Mitotic index analysis of G2/M-elutriated DT40 cells during treatment with taxol, in the presence or absence of caffeine. pH3, phosphorylated Ser10 of histone H3.
Chk1 Is Phosphorylated at Noncanonical Sites during Spindle Checkpoint Activation
Chk1 became phosphorylated during taxol nocodazole treatments, as judged by the appearance of slower-migrating forms of the protein, which were sensitive to lambda phosphatase (Figures 4C and 4D). This phosphorylation was inhibited by caffeine (Figures 4C and 4D), an inhibitor of upstream kinases ATM and ATR (Kumagai et al., 1998). Surprisingly, however, exposure to taxol or nocodazole did not result in detectable phosphorylation of Chk1 at Serines 317 and 345 (Gatei et al 2003), although modification of both sites was readily observed in cells treated with the DNA-damaging agent etoposide(Figure 4E and data not shown). These results indicate that Chk1 is phosphorylated at noncanonical residues via a caffeine-sensitive pathway in cells exposed to spindle poisons.
DT40 or BE cells treated with caffeine exit mitotic arrest more rapidly than control cells in the presence of taxol, which suggests that mitotic phosphorylation of Chk1 is important for taxol-induced mitotic delay (Figure 4F and Figure S3A). However, Chk1 kinase activity was not detectably increased during treatment of DT40 cells with taxol or nocodazole (Figure S3B), and localization of Chk1:GFP protein to kinetochores in prometaphase cells during taxol treatment was not inhibited by caffeine (Figure S3C). Therefore, further studies are required to elucidate the functional significance of the mitotic phosphorylation of Chk1.
Chk1 Is Required for Aurora-B Activity during Treatment with Taxol
The kinase activity of Aurora-B is required to sustain mitotic arrest during exposure to taxol, but not nocodazole (Ditchfield et al., 2003). Since this phenotype is reminiscent of the one observed in Chk1-deficient cells, we investigated whether Chk1 and Aurora interact functionally during activation of the spindle checkpoint.
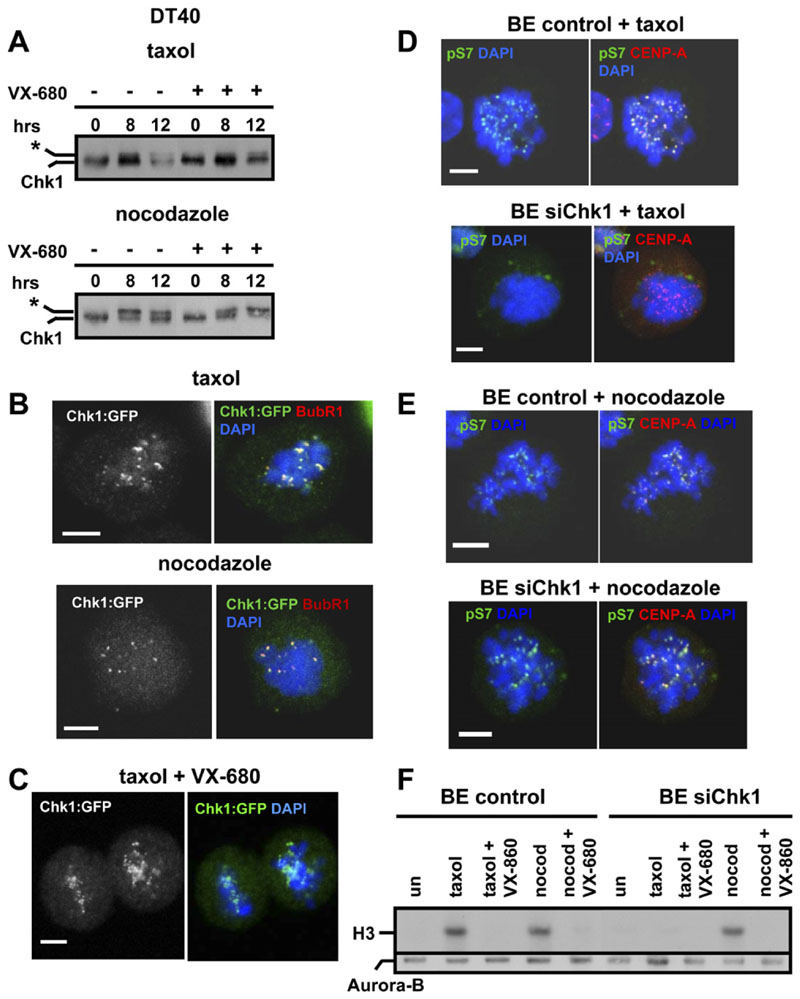
First, we examined whether mitotic phosphorylation of Chk1 was dependent on Aurora. Inhibition of Aurora activity by VX-680 abolished phosphorylation of Serine 10 of histone H3 (S10 H3), a physiological substrate of Aurora, in mitotic DT40 cells (data not shown; Harrington et al., 2004). However, phosphorylation of Chk1 in response to taxol and nocodazole was not inhibited by VX-680, as judged by the appearance of slower-migrating forms of the protein (Figure 5A). Furthermore, localization of Chk1:GFP to kinetochores during treatment of cells with taxol or nocodazole (Figure 5B) was not sensitive to treatment with VX-680 (Figure 5C and data not shown). These results show that mitotic phosphorylation and localization of Chk1 to kinetochores do not require Aurora activity.
Figure 5. Chk1-Deficient Cells Exhibit Decreased Aurora Activity during Treatment with Taxol.
(A) Mitotic phosphorylation of Chk1 does not require Aurora activity. Western blot analysis of total Chk1 during treatment of G2/M-elutriated DT40 cells with taxol or nocodazole, in the absence or presence of VX-680. The asterisk marks phosphorylated Chk1.
(B and C) Localization of Chk1 to kinetochores is not dependent on Aurora activity. Chk1−/− cells expressing Chk1:GFP were treated with taxol or nocodazole for 2 hr in the (B) absence or (C) presence of VX-680 and were analyzed by confocal microscopy. Red: BubR1; green, Chk1:GFP; blue, DNA. A single image plane is shown. The scale bar is 5μm
(D and E) Projected deconvolved image stacks of BE cells transfected with negative siRNA (control) or with Chk1 siRNA(siChk1) and treated with (D) taxol or (E) nocodazole for 2 hr. Red, CENP-A; green, phosphorylated Serine 7 of CENP-A (pS7); blue, DNA. The scale bar is 5 mm.
(F) BE cells transfected as in (D) were treated with taxol, nocodazole (nocod), or VX-680 for 6 hr. Upper panel: Aurora-B-associated histone H3 kinase activity. Lower panel: western blot analysis of immunoprecipitated Aurora-B.
To test whether Aurora activity is dependent on Chk1, we first examined phosphorylation of Serine 7 of CENPA (pS7) as a surrogate marker of Aurora kinase activity (Zeitlin et al., 2001). During treatment of BE cells with taxol, all (15/15) control cells examined showed strong phosphorylation of pS7, indicative of activated Aurora (Figure 5D). In contrast, 10/18 (56%) of Chk1-depleted cells showed weak or undetectable phosphorylation of pS7, suggesting that Aurora kinase activity is diminished in the absence of Chk1 (Figure 5D). During treatment with nocodazole, both control (20/20) and Chk1-depleted (22/22) cells showed strong phosphorylation of pS7 (Figure 5E).
To measure Aurora-B kinase activity directly, Aurora-B was immunoprecipitated from control or Chk1-depleted BE cells, and kinase assays were performed with histone H3 as a substrate. During treatment with taxol, Aurora-B activity was induced in control cells, but not in Chk1depleted cells (Figure 5F). In contrast, during treatment with nocodazole, Aurora-B activity was similarly induced in both control and Chk1-depleted cells and was inhibited by VX-680 (Figure 5F). Taken together, our results show that Chk1 is required for optimal activity of Aurora-B during treatment with taxol, but not nocodazole. Kinetochore localization of Aurora-B during taxol treatment, however, was not dependent on Chk1 (Figure S4).
Finally, mitotic accumulation of DT40 and Chk1−/− cells during treatment with taxol was examined in the presence or absence of VX-680 by using morphological criteria (condensed chromosomes) since VX-680 eliminates phospho-S10 H3 staining (data not shown). Inhibition of Aurora activity by VX-680 diminished mitotic accumulation of DT40 cells and exacerbated the checkpoint defect in Chk1−/− cells (Figure 6A). This suggests that both Chk1-dependent and Chk1-independent mechanisms contribute to Aurora activation in response to spindle disruption by taxol.
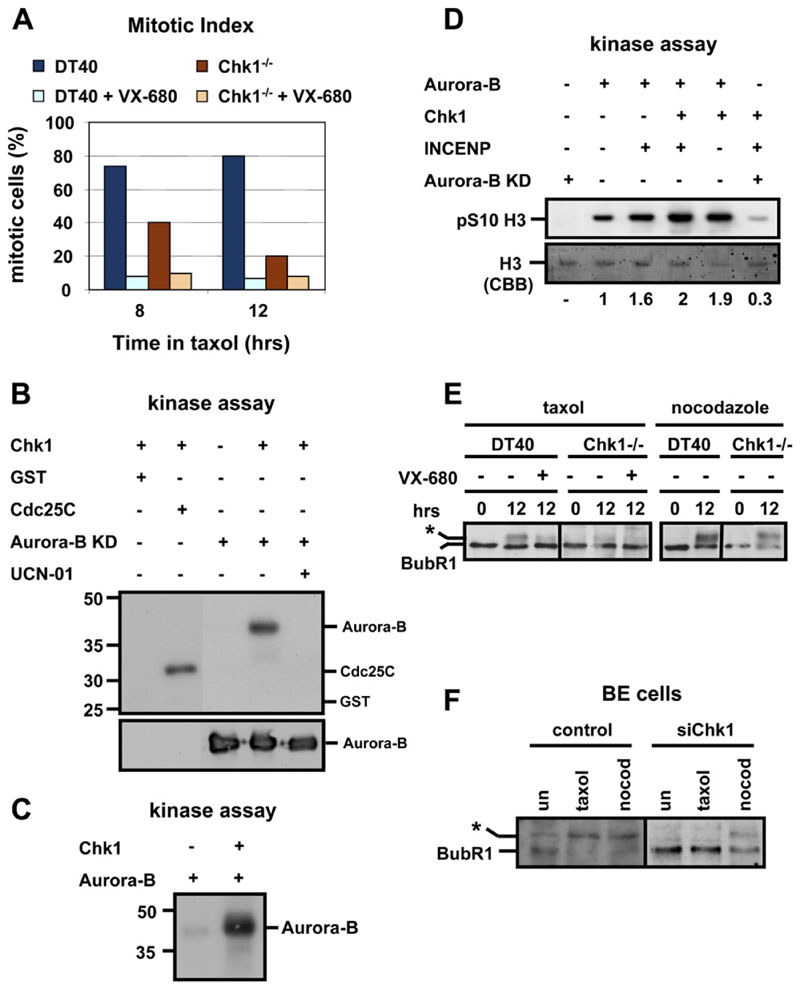
Figure 6. Chk1 Phosphorylates and Enhances Aurora-B Catalytic Activity In Vitro.
(A)Treatment with VX-680 exacerbates the checkpoint defect in Chk1−/− cells. Mitotic index analysis of G2/M-elutriated DT40 and Chk1−/− cells during treatment with taxol, in the presence or absence of VX-680.
(B)Upper panel: in vitro Chk1 kinase assay with GST, GST-Cdc25C (200-256), and kinase-dead (KD) Aurora-B (D200A) as substrates. Lower panel: western blot analysis of Aurora-B.
(C)In vitro Chk1 kinase assay with kinase-active Aurora-B as substrate.
(D)Phosphorylation of histone H3 in vitro, with complexes of Aurora-B, Chk1, GST-INCENP (826-919), and kinase-dead (KD) Aurora-B (D200A). pS10, phosphorylated Ser10. Values indicate the relative levels of pS10 of histone H3 with phosphorylation in the presence of Aurora-B arbitrarily set to 1. CBB, Colloidal Brilliant Blue.
(E and F) Chk1 is required for taxol-induced phosphorylation of BubR1. (E) Western blot analysis of BubR1 during treatment of G2/M-elutriated cells with taxol/nocodazole, in the presence or absence of VX-680. (F) Western blot analysis of BubR1 in BE cells transfected with negative siRNA (control) orChk1 siRNA (siChk1). Cells were untreated (un) or were treated with taxol/nocodazole (nocod) for 12 hr. The asterisk marks phosphorylated BubR1.
Chk1 Phosphorylates and Enhances Aurora-B Catalytic Activity In Vitro
To investigate whether regulation of Aurora-B by Chk1 occurs through a direct mechanism, we examined phosphorylation of Aurora-B by Chk1 in vitro. Recombinant Chk1 protein was incubated with purified GST, GST-Cdc25C (200-256), or KD recombinant Aurora-B (D200A) to rule out autophosphorylation (Figure 6B). Chk1 phosphorylated GST-Cdc25C (200-256), but not GST alone, as previously reported (Peng et al., 1997). Remarkably, Chk1 also phosphorylated KD Aurora-B, and this phosphorylation was abolished in the presence of the Chk1 inhibitor UCN-01 (Figure 6B) (Graves et al., 2000). Chk1 also phosphorylated kinase-active Aurora-B (Figure 6C and data not shown). We conclude that Chk1 phosphorylates Aurora-B in vitro.
We also examined the ability of Chk1 to enhance Aurora-B-mediated phosphorylation of S10 H3 in vitro. Recombinant Aurora-B phosphorylated S10 H3, and this phosphorylation was enhanced by incubating Aurora-B with GST-INCENP (826-919) (Figure 6D). Remarkably, recombinant Chk1 also enhanced phosphorylation of S10 H3 compared to Aurora-B alone, both in the presence and absence of GST-INCENP (826-919) (Figure 6D). This phosphorylation was dependent on the catalytic activity of Aurora-B, as incubating recombinant KD Aurora-B (D200A) with Chk1 and GST-INCENP (826-919) resulted in very low levels of phosphorylated S10 H3 (Figure 6D). In contrast, recombinant Chk1 was not detectably phosphorylated by Aurora-B in vitro (data not shown). These results show that Chk1 can modulate Aurora-B catalytic activity in vitro.
Chk1 Is Required for Phosphorylation of BubR1 during Treatment with Taxol
Recruitment to kinetochores during mitosis correlates with Aurora-dependent phosphorylation of BubR1 (Ditchfield et al., 2003;Lampson and Kapoor, 2005). Consistently, phosphorylation of BubR1 during treatment with taxol was impaired in Chk1−/− cells compared to DT40 cells, as judged by the appearance of slowermigrating electrophoretic forms of BubR1 (Figure 6E). In contrast, phosphorylation of BubR1 in response to nocodazole was similar in both cell types, and inhibition of Aurora kinase activity with VX-680 decreased phosphorylation of BubR1 in DT40 cells (Figure 6E).
Similarly, depletion of Chk1 expression by siRNA in BE cells resulted in a substantial decrease in the amount of phosphorylated BubR1 compared to control in response to taxol, but not when cells were treated with nocodazole (Figure 6F). We conclude that Chk1 is required for optimal phosphorylation of BubR1, specifically during treatment of cells with taxol.
Chk1 Is Required for Optimal Tumor Cell Killing by Taxol
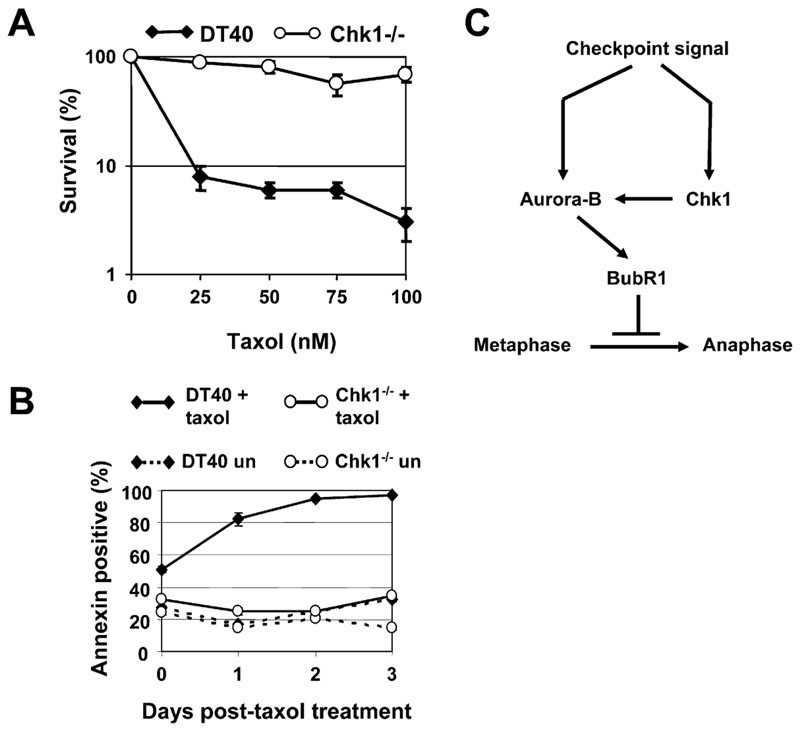
Antimitotic chemotherapeutic drugs are widely used in cancer treatment (Kops et al., 2005). To examine whether loss of Chk1 affects killing of tumor cells by taxol DT40 and Chk1−/− cells were treated with increasing concentrations of taxol for 12 hr and washed free of the drug, and clonogenic survival determined (Figure 7A). Strikingly, at all doses tested, Chk1−/− cells were more resistant to killing than DT40 cells under these conditions (Figure 7A).
Figure 7. Chk1-Deficient Cells Are Resistant to Killing by Taxol.
(A and B) (A) Clonogenic survival and (B) Annexin V staining of DT40 and Chk1−/− cells treated with taxol. Error bars show the standard deviation of the mean from two experiments. un, untreated.
(C)Model for the role of Chk1 in the spindle checkpoint.
To investigate the mechanism of taxol-induced cell death, DT40 and Chk1−/− cells were treated with 100 nM taxol for 12 hr and washed free of the drug, and apoptosis was measured by using Annexin V staining. DT40 cells exhibited high levels of taxol-induced apoptosis, whereas Chk1−/− cells were relatively resistant (Figure 7B). Taken together, these results suggest that Chk1-dependent functions are required for optimal tumor cell killing by taxol.
Discussion
Chk1 is a key effector of the DNA damage and replication checkpoints; however, a role for Chk1 in spindle checkpoint signaling has not been previously described. Here, we show that Chk1-deficient cells exhibit increased levels of spontaneous chromosome missegregation and defects in recruitment of the spindle checkpoint protein BubR1 to kinetochores during unperturbed mitosis. We believe that premature mitotic entry of Chk1-deficient cells is unlikely to fully account for this missegregation phenotype for two reasons. First, Chk1-deficient cells that enter mitosis prematurely exhibit a different phenotype in which the majority of chromosomes localize outside the spindle poles (Loffler et al., 2006 Zachos et al., 2005), and this premature entry is typically followed by failed cytokinesis and chromosome decondensation or nuclear fragmentation (unpublished data). Second, inhibition or overexpression of checkpoint proteins that control mitotic entry reportedly does not result in chromosome missegregation (Loffler et al., 2006 Stumpff et al., 2004). Instead, our data suggest a role for Chk1 in the spindle checkpoint.
To further explore this novel, to our knowledge, role of Chk1, we investigated whether Chk1 was required for mitotic arrest induced by taxol, which stabilizes microtubules and primarily interferes with tension at kinetochores, or nocodazole, which completely depolymerizes microtubules and abolishes both microtubule attachment and tension. These experiments demonstrated that Chk1 was required to sustain mitotic arrest during treatment with taxol, but not nocodazole. The failure to sustain taxol-induced mitotic arrest was associated with impaired Aurora-B kinase activity and diminished phosphorylation and localization of BubR1 to kinetochores. Furthermore, Chk1 itself underwent phosphorylation in response to spindle poisons and colocalized with BubR1 at kinetochores.
The requirement for Chk1 to sustain mitotic arrest could be interpreted in two main ways in the light of current models. One possibility is that lack of tension per se is sensed via a mechanism distinct from that which detects unattached kinetochores (Li and Nicklas, 1995; Stern and Murray, 2001) and would predict a specific role for Chk1 in the tension-sensing mechanism.
Alternatively, because loss of tension can produce unoccupied or partially occupied kinetochores (King and Nicklas, 2000; Waters et al., 1998), the checkpoint signal generated when all kinetochores are completely unoccupied during microtubule depolymerization by nocodazole may simply be stronger than that which emanates from a subset of partially occupied kinetochores during microtubule stabilization by taxol. According to this model, sustained activation of the spindle checkpoint under the latter conditions might require Chk1 to amplify the relatively weaker checkpoint signal.
Although we cannot distinguish unambiguously between these possibilities at present, it is possible to propose a model for the role of Chk1 in the spindle checkpoint that accommodates our observations (Figure 7C). During activation of the spindle checkpoint, Chk1 becomes phosphorylated via a caffeine-sensitive pathway and is available to associate with kinetochores. In response to checkpoint signals emanating from a minority of kinetochores or to microtubule stabilization by taxol, Chk1 is required for optimal Aurora-B kinase activity, phosphorylation and localization of BubR1 to kinetochores, and sustained mitotic delay. By contrast, checkpoint signals generated in response to loss of microtubule attachment at many or all kinetochores during treatment with nocodazole achieve a sufficient level of Aurora-B kinase activity to sustain mitotic arrest independently of Chk1.
It was recently reported that depletion of Chk1 resulted in mitotic arrest with misaligned chromosomes, rather than in a spindle checkpoint defect (Tang et al., 2006). Although this appears to be at odds with findings from our study, a similar paradox has been reported for several kinetochore proteins (Nuf2R, Ndc80Hec1, Nnf1R); partial depletion of the proteins (˜80%) engages the spindle checkpoint, whereas almost complete depletion (˜99%) inactivates it (McAinsh et al., 2006 Meraldi et al., 2004). Therefore, the difference between the results of Tang et al. (2006) and our observations may be due to different degrees of Chk1 depletion.
Our model raises several important questions regarding regulation of Chk1 in response to spindle perturbations, modulation of Aurora-B activity by Chk1, and the potential role of Chk1 in tumor cell killing by antimitotic agents.
The first concerns the identity of the kinase responsible for mitotic Chk1 phosphorylation and the functional signif-icance of this modification. ATM and ATR are widely accepted as caffeine-sensitive upstream regulators of Chk1 (Kumagai et al., 1998); however, phosphorylation of Chk1 during spindle checkpoint signaling does not affect the Ser317 or Ser345 sites conventionally targeted by these kinases (Gatei et al., 2003). This suggests either that ATM and ATR have the potential to phosphorylate Chk1 at alternative, noncanonical sites during mitosis, or that mitotic phosphorylation of Chk1 is mediated by a different caffeine-sensitive kinase. Recently, the spindle checkpoint protein TTK/hMps1 was shown to phosphorylate Chk2 (Wei et al., 2005), and Chk1 was proposed to be a substrate for CDK1 (Shiromizu et al., 2006). Mitotic phosphorylation of Chk1 was not required for its subcellular localization, and Chk1 catalytic activity was not detectably induced during treatment with spindle poisons, although it was necessary for sustained mitotic arrest. It is possible that mitotic phosphorylation stimulates the kinase activity of a subset of Chk1 molecules; however, this is difficult to detect in our kinase assay. Alternatively, the basal catalytic activity of Chk1 might be sufficient for its role in the spindle checkpoint, and phosphorylation of Chk1 might facilitate recognition of spindle checkpointrelevant substrates. Further work, including mapping of the unidentified modification sites, is required to distinguish between these alternatives.
INCENP directly modulates Aurora-B catalytic activity through allosteric interaction, whereas Survivin regulates binding of INCENP to Aurora-B (Carvalho et al., 2003 Honda et al., 2003). We showed that Chk1 phosphorylates Aurora-B and enhances its catalytic activity in vitro. It is possible that Chk1 phosphorylates Aurora-B to directly upregulate its kinase activity. Alternatively, Chk1 might enhance Aurora-B activity in vitro through some form of allosteric interaction. In the latter case, phosphorylation of Aurora-B by Chk1 might serve in vivo to stabilize the interaction between INCENP and Aurora-B under conditions of checkpoint activation. To our knowledge, Chk1 is the first kinase shown to phosphorylate Aurora-B, although regulatory autophosphorylation has been reported (Yasui et al., 2004). Further studies are under way to map the Chk1-phosphorylation sites of Aurora-B and to investigate the importance of this phosphorylation for Aurora-B activity and spindle checkpoint signaling.
Inhibition of spindle checkpoint function is a potential anticancer strategy because it can induce massive chro-mosome missegregation, which leads to tumor cell death (Harrington et al., 2004). Strikingly, we observed that Chk1−/− tumor cells are more resistant to taxol-induced apoptotic cell death. It is possible that Chk1 is required to prevent cells with chromosomal instability from adapting, or that Chk1-mediated apoptosis prevents the survival of aneuploid cells that breach the spindle checkpoint. Similarly, BubR1 is required for apoptosis of abnormal mitotic cells (Shin et al., 2003). Understanding the mechanisms of Chk1-dependent cell killing during chronic checkpoint-mediated mitotic arrest may provide new prospects for improving cancer treatment.
Experimental Procedures
For antibodies, cell lines, plasmids, and proteins, see Supplemental Data.
Cell Culture and Treatments
Avian B-lymphoma DT40 cells were grown and elutriated as described (Zachos et al., 2005). Human colon carcinoma BE (a gift from S. Wilkinson and C. Marshall), HCT116 cells, and human embryonic kidney HEK293 cells were grown in DMEM (GIBCO) containing 10% fetal bovine serum at 37°C in 5% CO2. Cells were treated with 1 mg/ml nocodazole, 10 μM taxol, 5 mM caffeine, 60 mM aphidicolin, 1 mM etoposide (all from Sigma), and 3 mM VX-680 (Kava Technology), as appropriate.
RNA Interference
Negative siRNA or siRNA duplexes designed to repress Chk1 (Dharmacon) were transfected into BE, HCT116, or HEK293 cells 48 hr prior to analysis or treatment with drugs by using OligofectAMINE (Invitrogen). A pool of Chk1 siRNAs was used unless otherwise stated. The siRNA sequences are available upon request.
Indirect Immunofluorescence Microscopy
Cells were fixed in 4% paraformaldehyde in cytoskeleton buffer (1.1 M Na2HPO4, 0.4 M KH2PO4, 137 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM EGTA, 5 mM PIPES, 5 mM glucose [pH 6.1]) for 5 min at 37°C; permeabilized in 0.15% Triton X-100 in cytoskeleton buffer, and immunostained as appropriate. Fluorescence intensities were quantified by using ImageJ (NIH, USA). Background readings were subtracted, and the BubR1 values were normalized against the CENP-B signal.
Western Blotting
Cells were lysed in ice-cold whole-cell extract buffer (20 mM HEPES, 5 mM EDTA, 10 mM EGTA, 0.4 M KCl, 0.4% Triton X-100, 10% glycerol, 5 mM NaF, 50 ng/ml Okadaic acid, 1 mM DTT, 5 mg/μl Leupeptin, 50 mg/ml PMSF, 1 mM Benzamidine, 5 mg/ml Aprotinin, 1 mM Na3VO4) for 30 min on ice. Lysates were cleared by centrifugation at 15,000 x g for 10 min. Densitometric analysis was performed with Quantity One (Biorad).
Kinase Assays
Immunoprecipitation-kinase assays for CDK1 with histone H1 as a substrate were performed as described (Zachos et al., 2005).
For Chk1 immunoprecipitation-kinase assays, see Supplemental Data.
For in vitro Chk1 kinase assays, 0.5 mg human recombinant Chk1 was incubated with 1 mg protein substrate in 20 ml kinase buffer (20 mM MOPS [pH 7.2], 5 mM EGTA, 10 mM MgCl2, 25 mM sodium β-glycerophosphate, 1 mM NagVO, 1 mM DTT, 100 μM ATP, 1 μCi γ-ATP) for 20 min at 30°C, prior to analysis by SDS-PAGE. Where appropriate, 145 ng UCN-01 was included in the reaction.
Aurora-B immunoprecipitation-kinase and in vitro kinase assays were performed essentially as described (Honda et al., 2003). For in vitro Aurora-B kinase assays, reactions were analyzed by western blotting with polyclonal antibody against phospho-Ser10 of histone H3 (Upstate Biotechnology). After transferring to nitrocellulose, histone H3 that remained on the gel was visualized by staining with Colloidal Brilliant Blue (Sigma).
Metaphase Spreads
Cells were treated with 100 ng/ml colchicine (Sigma) for 3 hr, swollen in 0.075 M KCl at 37°C for 20 min, fixed twice in fresh fixative (methanol:glacial acetic acid, 3:1) for 10 min at room temperature, and dropped onto slides. Spreads were stained with DAPI and were visualized by fluorescence microscopy.
Clonogenic Cell Survival and Annexin Assays
Clonogenic survival assays were performed by using methylcellulose (Sigma) semisolid medium as described (Zachos et al., 2003). An Annexin V kit (Pharmigen) was used to detect apoptotic cells by flow cytometry.
Supplementary Material
Supplemental Data include a description of the antibodies, cell lines, plasmids, and recombinant proteins used and are available at http://www.developmentalcell.com/cgi/content/full/12/2/247/DC1/.
Acknowledgments
We thank K. Vousden for comments on the manuscript and M. O’Prey for help with the time-lapse microscopy. We also thank E. Nigg, S. Takeda, T. Fukagawa, N. Mailand, S. Wilkinson, and C. Marshall for useful materials. This work was supported by the Association for International Cancer Research (G.Z.) and Cancer Research UK (E.J.B., M.W., M.S., and D.A.F.G.).
References
- Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Collura A, Blaisonneau J, Baldacci G, Francesconi S. The fission yeast Crb2/Chk1 pathway coordinates the DNA damage and spindle checkpoint in response to replication stress induced by topoisomerase I inhibitor. Mol Cell Biol. 2005;25:7889–7899. doi: 10.1128/MCB.25.17.7889-7899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei M, Sloper K, Sorensen C, Syljuasen R, Falck J, Hobson K, Savage K, Lukas J, Zhou BB, Bartek J, Khanna KK. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J Cell Sci. 2000;113:3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Clevel DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA, Bartek J, Lukas J. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–891. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Loffler H, Rebacz B, Ho AD, Lukas J, Bartek J, Kramer A. Chk1-dependent regulation of Cdc25B functions to coordinate mitotic events. Cell Cycle. 2006;5:2543–2547. doi: 10.4161/cc.5.21.3435. [DOI] [PubMed] [Google Scholar]
- McAinsh AD, Meraldi P, Draviam VM, Toso A, Sorger PK. The human kinetochore proteins Nnf1R and Mcm21R are required for accurate chromosome segregation. EMBO J. 2006;25:4033–4049. doi: 10.1038/sj.emboj.7601293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Royou A, Macias H, Sullivan W. The Drosophila Grp/Chk1 DNA damage checkpoint controls entry into anaphase. Curr Biol. 2005;15:334–339. doi: 10.1016/j.cub.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4:483–497. doi: 10.1016/s1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- Shiromizu T, Goto H, Tomono Y, Bartek J, Totsukawa G, Inoko A, Nakanishi M, Matsumura F, Inagaki M. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1) Genes Cells. 2006;11:477–485. doi: 10.1111/j.1365-2443.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc Natl Acad Sci USA. 2001;98:4492–4497. doi: 10.1073/pnas.081076898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shino-hara A, Ogawa H, Takata M, Yamaguchi-Iwai Y, Takeda S. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern BM, Murray AW. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr Biol. 2001;11:1462–1467. doi: 10.1016/s0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Stumpff J, Duncan T, Homola E, Campbell SD, Su TT. Drosophila Wee1 kinase regulates Cdk1 and mitotic entry during embryogenesis. Curr Biol. 2004;14:2143–2148. doi: 10.1016/j.cub.2004.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Naga-hama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1) Proc Natl Acad Sci USA. 2006;103:11964–11969. doi: 10.1073/pnas.0604987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- Waters JC, Chen RH, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JH, Chou YF, Ou YH, Yeh YH, Tyan SW, Sun TP, Shen CY, Shieh SY. TTK/hMps1 participates in the regulation of DNA damage checkpoint response by phosphorylating CHK2 on threonine 68. J Biol Chem. 2005;280:7748–7757. doi: 10.1074/jbc.M410152200. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Auto-phosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- Zachos G, Rainey MD, Gillespie DA. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 2003;22:713–723. doi: 10.1093/emboj/cdg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos G, Rainey MD, Gillespie DA. Chk1-dependent S-M checkpoint delay in vertebrate cells is linked to maintenance of viable replication structures. Mol Cell Biol. 2005;25:563–574. doi: 10.1128/MCB.25.2.563-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–1157. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.