Abstract
The diversity of the cellular proteome substantially exceeds the number of genes coded by the DNA of an organism because one or more residues in a majority of eukaryotic proteins are posttranslationally modified (PTM) by the covalent conjugation of specific chemical groups. We now know that PTMs alter protein conformation and function in ways that are not entirely understood at the molecular level. NMR spectroscopy has been particularly successful as an analytical tool in elucidating the themes underlying the structural role of PTMs. In this Perspective, we focus on NMR-based characterization of three abundant PTMs: phosphorylation, acetylation and glycosylation. We detail NMR methods that have found success in detecting these modifications at a site-specific level. We also highlight NMR studies that have mapped the conformational changes ensuing from these PTMs as well as evaluated their relation to function. The NMR toolbox is expanding rapidly with experiments available to probe not only the average structure of biomolecules but also how this structure changes with time on timescales ranging from picoseconds to seconds. The atomic resolution insights provided by NMR spectroscopy on the structure, dynamics, mechanism of biomolecules and biomolecular interactions ensure that NMR will continue to be a tool at the forefront of research in the structural biology of PTMs.
Introduction
Post-translational modifications (PTMs) are covalent alterations of polypeptide chains that provide a mechanism for expanding the cellular proteome after protein translation is complete 1. While the eukaryotic genome codes for approximately 6000 (yeast) - 30000 (human) genes, PTMs amplify the proteome to a staggering 100000 or more molecular protein variants. There are over 200 chemical moieties that are enzymatically conjugated to 15 of the 20 naturally occurring amino acids in a protein. PTMs vary considerably in complexity and size of the conjugated group, ranging from the simple oxidation of Cys residues to form a disulphide linkages or Pro to hydroxy Pro, to the attachment of heavy polyubiquitin or oligosaccharide units. Amino acids sidechains carrying a nucleophilic functional group, such as the hydroxyl group of Ser and Thr, the thiol in Cys and the amine variants of Lys, Arg and His are most susceptible to modification. PTMs modify the stability and expand the functional repertoire of a polypeptide sequence by altering physicochemical properties such as charge and conformation, as well as providing new interaction motifs and interfaces not present in the original protein molecule.
Unlike protein or nucleic acid synthesis, PTMs are not templated but are contingent upon stochastic recognition and modification by the concerned enzyme, resulting in considerable variability in the final protein product. The modification efficiency at consensus sequences such as the Asn-Xaa-Ser/Thr site required for N-linked glycosylation vary depending on the identity of the residue Xaa 2. In addition, PTMs are generally reversible: for example, amino acid-specific kinases add phosphate groups to Ser, Thr, His or Tyr residues, which are subsequently removed by the action of phosphatases; similarly, acetylase/deacetylase pairs control the acetylation of N-terminal or Lys sidechain amino groups. The relative activities of the enzymes responsible for addition and removal of the modification also play a role in controlling the extent of PTM at a particular protein site. Moreover, regulation of protein activity generally occurs via PTMs at multiple sites, and often via many different modifications 3. Such "cross-talk" between PTMs can be synergistic or competitive and result in either a graded or a cooperative response in the structure and function of the target protein. The large number and chemical diversity of PTMs and the heterogeneity that arises from conjugation at multiple different protein residues, as well as the possibility that each protein variant has a distinct conformation that informs function, makes the structural biology of PTMs a very challenging area of research.
The evolution of NMR as a tool to study PTMs has gone hand-in-hand with the development of pulse sequences and isotope-labeling methodologies in biomolecular NMR spectroscopy. NMR affords a number of advantages for this area of research including site-specific resolution, the ability to follow enzymatic PTM reactions in a time-dependent and quantitative manner, as well as the means for determining and contrasting the structure and dynamics in the unmodified and conjugated versions of the protein. In this Perspective, we focus on three frequently observed PTMs, phosphorylation, acetylation and glycosylation (Figure 1). We specifically highlight studies that have used NMR spectroscopy to detect the presence of PTMs, as well as to elucidate the effect of PTMs on the conformation and dynamics of the protein molecule.
Figure 1.
Four common post-translational modification of proteins, (A) phosphorylation, B) acetylation, C) O-glycosylation and D) N-glycosylation. The covalently conjugated chemical moieties are coloured red. The enzymes catalyzing the addition and removal of the modification are denoted in blue and green respectively, while the chemical source of the modification is indicated in magenta.
Phosphorylation: A small modification with giant implications
Detection
Phosphorylation is the enzymatic attachment of a phosphoryl group to Ser, Thr, Tyr or His residues (Figure 1A). Phosphorylation is one of the most prevalent PTMs in eukaryotes4, 5 and is catalyzed by kinases which use ATP as the source of PO3 2-. From an NMR perspective, the covalently bonded phosphate group carries the spin-1/2 31P nucleus, which is a very attractive analytical tool because of its 100% natural abundance and a gyromagnetic ratio that is only ~ 2.5-fold smaller than 1H 6. In addition, the two and three bond 2JCP and 3JHP scalar coupling values in phosphorylated residues are significant (4-10 Hz)7, 8 and have been leveraged for coherence transfer and spectral editing (see below). 31P NMR has been used to study phosphate units bonded to peptides for over three decades, ever since the pioneering work of Sykes, Whitaker and others 9–11, though multidimensional methods using 31P are only recently gaining traction.
2D 1H-15N and 1H-13C heteronuclear correlation methods have enjoyed a great deal of success in detecting protein phosphorylation. The phosphorylation of Ser and Thr residues in unstructured regions of proteins invariably results in characteristic downfield shifts of the backbone amide proton and nitrogen resonances of the modified amino acids by as much as 0.5 and 2.0 ppm respectively (Figure 2A) 12. These shifts, which are readily observable in 1H-15N HSQC spectra (Figure 2A, middle panel), have been traced to the intra-residue hydrogen bond formed by the amide -NH with the phosphate oxygen atom (Figure 2A, right panel) 13, 14. Such chemical shift changes become less pronounced at pH values (of ~ 5) below the pKa of the phosphorylated Ser or Thr, as the phosphate group can no longer act as an effective hydrogen bond acceptor 12, 15. The downfield shifts observable in 1H-15N HSQC spectra have been used to study phosphorylation in live oocyte cells to show that Ser112 of the SV40 virus large T antigen regulatory region is phosphorylated by casein kinase 2 (CK2) before Ser11116. Intriguingly, monophosphorylated substrate molecules dissociate from the CK2 in-between the two phosphorylation events. Phosphorylation-induced changes in Ser/Thr chemical shifts in HSQC spectra have also been observed as a function of time to decipher kinase mechanism16, 17 as well as cross-talk between acetylation and Ser/Thr phosphorylation 18.
Figure 2.
Detecting (A) Ser/Thr, (B) Tyr and (C,D) His phosphorylation. In panels A-C, the chemical structure of the modified region of the protein is highlighted as a yellow box. (A) Middle panel: Overlay of the 1H-15N HSQC spectra of unmodified (black) and multisitephosphorylated p53 TAD showing the downfield shifts in resonances of phosphorylated amino acids (reproduced with permission from Theillet et al.12). These large downfield shifts are a direct result of the intra-residue hydrogen bonding between the phosphate oxygen and the Ser/Thr NH (Right panel). (B) Right panel: Detection of Tyr phosphorylation through a 1H-13C HSQC where significant 13C shifts are seen for the aromatic -CHε (spectrum of DNA methyltransferase (134-144) reproduced with permission from Theillet et al.12). (C) The site at which His is phosphorylated (left panel) can be identified from distinct patterns in a 3D HNP-based experiment. 2D 1H-15N and 1H-31P strips from such a dataset are shown in the right panel (spectrum reproduced from Himmel et al.21 with permission). (D) Lower panel: 1H-15N HSQC spectral overlay of unmodified and His15-phosphorylated HPr22 (reproduced with permission from Theillet et al.12). While only moderate changes are observed for the modified His15, large downfield shifts are seen for Ala16 and Arg17 because of hydrogen bonding interactions between the phosphate oxygen and the amide group of Ala16 and Arg17 (top right panel; PDB ID: 1JEM)22.
In addition to changes in the 1H-15N spectrum, phosphorylation leads to 3-4 ppm chemical shift changes of the 13Cβ nucleus which can also be employed for diagnostic purposes8, 12, 19. Although downfield shifts in 1H-15N HSQC resonances upon phosphorylation are pronounced in intrinsically disordered proteins, these changes may be masked when Ser/Thr residues in folded proteins are modified; in such cases, 13Cβ shifts are more reliable indicators of the residue that is phosphorylated. Smet-Nocca et al. showed that when Ser16 of the Pin1 WW domain is phosphorylated by protein kinase A, backbone NH resonances of the neighbouring Ser18 and Ser19 are downfield shifted more than Ser16 15. However, the 2.0 ppm downfield shift of the Ser16 13Cβ resonance is diagnostic of phosphorylation (compared to < 0.1 ppm for Ser18).
Ser/Thr phosphorylation often occurs at multiple sites in a protein and can also give rise to global conformational changes, so that the NMR spectra of the phosphorylated variants are significantly different from that of the unmodified protein and have to be assigned independently. Backbone and sidechain assignments are generally obtained using standard triple resonance methods. Once these are available, methods utilizing 31P are available to identify the modified Ser/Thr residue. The 3D 1H-13C-31P pulse sequence originally developed for nucleic acids has been adapted to pSer/pThr because the H-C-O-P- moiety is common to both biomolecules. Brutscher and coworkers proposed a HCP-based pulse sequence for extracting the 1H-13Cα and 1H-13Cβ resonances from a 1H-13C constant time-(CT-)HSQC spectrum that relies on 13C-31P scalar couplings 8. 2JPCβ and 3JPCα are allowed to evolve during the constant time period of the CTHSQC and the resultant spectrum (transfer spectrum) is subtracted from a reference where 2JPCβ and 3JPCα are suppressed by moving the position of the 31P 180P refocusing pulse. The only peaks in the difference spectrum are the 1H-13Cα and 1H-13Cβ correlations of the phosphorylated Ser and Thr residues. The authors also proposed a simple extension of the CT-HSQC based pulse sequence to assign the 31P resonances of the pSer/pThr. Low power CW decoupling in the transfer spectrum at 31P resonance frequencies previously determined from a 1D 31P experiment helps in turning off 13C-31P scalar coupling evolution during the constant time duration, one 31P nucleus at a time, so that the 1H-13C resonances for this residue disappear from the difference spectrum and enable the mapping of 1H and 13C chemical shifts to 31P frequencies.
In contrast to phosphorylation of Ser and Thr, Tyr phosphorylation does not lead to downfield shifts in a 1H-15N HSQC because the phosphoryl moiety at the para position of the planar phenyl ring is too far from the backbone amide to engage in an intra-residue hydrogen bond. Instead, pTyr residues are best detected using a 1H-13C HSQC, which shows signature upfield shifts of the -CHε resonance of the phenyl ring by ~ 0.3 ppm in 1H and 3.5 ppm in 13C (Figure 2B).
His phosphorylation differs from phosphorylation of Ser, Thr and Tyr in that there are two sites, Nδ and Nε, that can get phosphorylated (Figure 2C,D). Moreover, the N-P bond is weaker than the O-P bond, leading to Nδ-Nε phospho transfer under basic conditions and spontaneous dephosphorylation under acidic ones 12, 20, making the characterization of His phosphorylation a quintessential NMR challenge. Griesinger and coworkers have developed an out-and-back 3D HNP-based pulse sequence that distinguishes the different phosphorylation states of His 21. Sidechain 1H magnetization is first transferred to 15N via a 2-bond 1H-15N scalar coupling (5-10 Hz), and from 15N to 31P via 1JNP (5-17 Hz). Nδ&-pHis is expected to give a single major peak at the chemical shifts of [Hε1,Nδ1,Pδ1] with a minor peak at [Hδ2,Nδ1,Pδ1] due to residual 3JNH (~2 Hz) (Figure 2C, right panel). Nε2-pHis generates two peaks at [Hε1,Nε2,Pε2] and [Hδ2,Nε2,Pε2] that are of comparable intensity. Nδ1,Nε2-diphosphoHis results in three peaks at three corners of a rectangle with chemical shifts of [Hε1,Nδ1,Pδ1], [Hε1,Nε2,Pε2] and [Hδ2,Nε2,Pε2]; the fourth corner is occupied by the weak peak which originates from the three-bond 15N-1H coupling (Figure 2C, right panel). The authors applied this sequence to show that two bacterial proteins, the phosphocarrier protein HPr and the phosphotransfrease regulatory protein PRDI have different His phosphorylation states, with HPr phosphorylated at Nδ1 and PRDI phosphorylated at Nε2 21. Earlier studies had shown that the folded α/β HPr protein phosphorylated at His15 Nδ1 exhibited significant chemical shift changes for the backbone amide resonances of Ala16 and Arg17 rather than His15 itself (Figure 2D, bottom panel) that are a direct consequence of hydrogen bonding of their amides with the oxygen atoms of the phosphate acceptor (Figure 2D, top right panel) 22, 23.
All the above methods probing phosphorylation require 15N and 13C isotope-labeled samples. However, NMR techniques that can detect phosphorylation in unlabeled proteins would be powerful complements, for example in optimizing conditions for in vitro enzymatic phosphorylation or for measuring the kinetics of conjugation, provided they do not compromise the resolution available from 1H/13C/15N-based NMR datasets. Live and Edmondson developed one such method applicable to natural abundance samples that uses 31P-1H 2D heteronuclear correlation NMR to study phosphorylation in unlabeled flavodoxin (20 kDa) and ovalbumin (43 kDa) 7. The dataset in these cases was acquired using the simple 2D HMQC pulse sequence, which suffers from passive coupling to geminal (Ser), methyl (Thr) and Hα protons in compared to a HSQC-based pulse sequence. However, the demonstration of proof-of-principle on two large proteins over three decades ago suggests that this method maybe underutilized in studying protein phosphorylation.
The phosphate group in phosphorylated residues itself can be mono- or di-anionic; the pKa for loss of the first proton in phosphate monoesters is ~ 2, while the pKa for the second proton is between 5.5 and 7. These pKa values imply that phosphate groups conjugated to Ser/Thr have average charges between -1 and -220. The protonation state of the phosphate can be monitored at high sensitivity using 31P chemical shifts, which move downfield by as much as ~4 ppm upon deprotonation of the mono-anion19, 24.
Conformational changes arising from phosphorylation
The conformational changes caused by PTMs have been explored most extensively in the context of phosphorylation. Covalent conjugation of phosphate groups to Ser, Thr, His or Tyr transform neutral or partially positively charged residues (in the case of His) to negatively charged mono- or di-anionic species. Phosphorylation thus modifies the net charge per residue of the protein, a parameter that is known to influence the globule-coil transition in intrinsically disordered proteins (IDPs) 25, 26. Given that phosphorylation/dephosphorylation events mediated by pairs of kinases and phosphatases are central events in signaling cascades, protein degradation, transactivation and localization27, it is of paramount importance to understand the extent to which phosphorylation can remodel protein conformation. Intriguingly, we now have examples of phosphorylation inducing the complete spectrum of conformational changes ranging from local structural perturbations all the way to global order-to-disorder transitions.
One of the best examples of large-scale conformational changes resulting from phosphorylation, as well as its relation to function, is seen in the 4E-BP2/eIF4E system (Figure 3A-C)28, 29. 4EBP2 is an IDP that binds tightly to eIF4E via the YXXXXLΦ motif to suppress cap-dependent translation initiation30, 31 and the YXXXXLΦ motif folds into a helical conformation in the eIF4E-bound state32. Upon phosphorylation, the affinity of 4E-BP2 for eIF4E reduces sufficiently enough for eIF4G to compete with and displace 4E-BP2 from the eIF4E complex, triggering translation initiation33–35. In order to decipher the molecular mechanism underlying this phospho-regulation, Forman-Kay and coworkers phosphorylated 4E-BP2 in vitro at T37, T46, S65, T70 and S83 (pWT) 29. The 1H-15N HSQC spectrum dramatically changes upon phosphorylation from the IDP-like appearance of unmodified 4E-BP2, characterized by poor chemical shift dispersion in the 1H dimension, to that of a folded protein with several peaks resonating upfield of 8 ppm and downfield of 9 ppm (Figure 3A). The phosphorylation of two residues T37 and T46 was found to be necessary and sufficient to induce the folding of 4E-BP2 (p2-4E-BP2). The structure of p2-4E-BP2 was determined to be a 4-stranded β-sheet that buried the YXXXXLΦ motif in the central β-strand, rendering it inaccessible for binding to eIF4E and thereby furnishing a mechanism for the lower affinity of interaction between p2-4E-BP2 and eIF4E (Figure 3B). The folding transition itself is triggered by the hydrogen bonding of the backbone amide protons of G39 and G48 with the phosphate groups conjugated to T37 and T46 respectively, resulting in two tight turns of the β-sheet (Figure 3C) 29. The imprint of this hydrogen bonding pattern is seen in the HSQC spectrum, where the 1HN nuclei of G39 and G46 show dramatic downfield shifts and resonate at ~ 11 ppm. Intriguingly, phosphorylation of pT37 or pT46 alone results in partially folded forms where either the first or the second β-turn is present. This observation suggests that the restructuring of intrinsically disordered 4E-BP2 to the four-stranded β-sheet is not a cooperative event, but rather occurs via intermediate incompletely folded states where discrete structural elements are stabilized by the covalently attached phosphate groups.
Figure 3.
Conformational changes upon phosphorylation. (A) Overlay of 1H-15N HSQC spectra of unmodified (blue) and pT37pT46 4EBP2 showing the dramatic changes occurring in the spectrum upon phosphorylation of T37 and T46 (spectra reproduced with permission from Bah et al.29). (B) Four-stranded β-sheet structure of pT37pT46 4EBP2 (PDB ID: 4EBP). The canonical eIF4E binding motif YXXXXLc is coloured yellow, while the two tight turns formed as a result of phosphorylation are in red. (C) Stick representations of the amino acids in the two turn regions showing the hydrogen bonds formed by G39 and G48, as well as by the two phosphate groups, that facilitates the formation of these two turns. (D) Overlay of 1H-15N HSQC spectra of unmodified (black) and Ser10-phosphorylated cysteine string protein (CSP). (E) Residue-specific chemical shift perturbations upon phosphorylation. The inset shows the 7-helix structure of unmodified CSP (PDB ID: 2N05) on which amino acids showing chemical shift changes greater than 0.3 ppm are plotted in yellow. (F) A close-up of the regions surrounding Ser10 and Lys58 that displays the change in compaction of the protein occurring upon phosphorylation and the subsequent establishment of an electrostatic interaction between the negatively charged phosphate group and the positive Lys sidechain (PDB IDs: 2N04 and 2N05; spectra and figures reproduced with permission from Patel et al.36).
The disorder-to-order transition that is observed in 4E-BP2 upon phosphorylation of T37 and T46 raises two questions: first, how many phosphates are necessary for significant remodeling of the polypeptide chain, and second, what sort of structural changes can we expect when folded proteins are phosphorylated. The study by Morgan and coworkers on a phosphorylated cysteine string protein (CSP) addresses both these questions (Figure 3D-F) 36. CSP belongs to the Hsp40 family of heat shock chaperones37 and localizes to neuronal synaptic vesicles, where it promotes the folding of SNAP-2538 and syntaxin39 involved in endo/exocytosis. The N-terminal segment of CSP is phosphorylated in organisms as diverse as worms and humans40, and phosphorylation of Ser10 in mammalian CSP modulates the binding to client proteins such as synaptotagmin41. The authors first determined the structure of CSP1-100 to be composed of seven α-helices (α1-α7)36. The spectrum of unmodified CSP1-100 has significant chemical shift dispersion, as expected from a stably folded protein (Figure 3D, red). Phosphorylation of Ser10 results in large chemical shift changes of peaks arising from residues in the N-terminus of the protein surrounding α1, but also at α5 and the linker connecting α5 and α6 (Figure 3E). Nevertheless, the spectrum retains its chemical shift dispersion, confirming that the protein still preserves a stable fold (Figure 3D, black). The structure in the phosphorylated state reveals a helix-to-coil transition of α1 caused by phosphorylation. The electrostatic interaction between the positively charged Lys58 sidechain and the anionic phosphate at Ser10 draws the disordered N-terminus towards α4, resulting in an overall more compact tertiary structure (Figure 3F). The ability of CSP to switch between two distinct native folds is reminiscent of the recently discovered class of metamorphic proteins42 that exist in equilibrium between two functional native state structures43–45. This report on CSP phosphorylation also demonstrates that a single phosphorylation event is sufficient to tilt the free energy balance between conformations and push the protein to an alternate native state structure.
Studies exploring the consequences of phosphorylation on folded proteins are fewer in number than reports focusing on IDPs, presumably because IDPs are hotspots for PTMs such as phosphorylation46, but also because NMR spectroscopy remains the only tool for characterizing the intrinsically disordered and dynamic IDPs at atomic resolution47. Nevertheless, the existing studies confirm that single-site phosphorylation can result in global transitions such as those seen above for CSP. Kainosho and coworkers elucidated the structural impact of Thr38 phosphorylation on myosin phosphatase inhibitor protein CPI-17 using NMR chemical shift perturbations48. CPI-17 (residues 35-120) is a four-helix bundle with the phosphorylation site (T38) residing in an N-terminal loop, and phosphorylated CPI-17 is a much more potent inhibitor than the unmodified protein49. Chemical shift changes resulting from T38 phosphorylation are localized to the N-terminal helix, suggesting a picture of CPI-17 that consists of a stable three-helix base onto which the N-terminal helix is packed as a movable arm. Phosphorylation triggers the motion of the arm, without or with associated unfolding, exposing the buried inhibitor site and providing a molecular basis for the 1000-fold higher potency of pCPI-17 as a phosphatase inhibitor48.
Phosphorylation of IDPs elicits diverse responses in structure ranging from global disorder-order transitions29, either resulting from the specific hydrogen bonds established by the phosphate group or because of a non-specific electrostatic effect, to virtually no change in conformation50. The phosphate group can act as a N-cap to modulate the helicity of downstream residues. Phosphorylation of S235 in the Pro-rich region of tau protein (S208-Q244) increases the helicity in the region between P236 and L24351, while phosphorylation at T231 results in the formation of a salt bridge between the phosphate group and the sidechain of R23052. Similarly, hydrogen bonds formed between the phosphate group on Ser133 of KID and the Tyr658 hydroxyl group, as well as with the positively charged K662 sidechain of KIX53, 54 are critical for the binding of the KIX domain of the transcriptional co-activator CBP and the kinase inducible domain (KID) of the cAMP-regulated transcriptional factor. The hydrogen bond acceptor capability of the phosphate unit can also enhance the cis content of pSer- or pThr-Pro bonds, with accompanying functional consequences. Phosphorylation increases the population of the cis isomer of the pThr20-Pro21 peptide bond in amyloid precursor protein (APP)13 which alters the affinity of APP for cytosolic binding partners. Showalter and colleagues24 demonstrated that Ser hyperphosphorylation of heptad repeats (consensus sequence YSPTSPS) in the intrinsically disordered C-terminal domain (CTD) of RNA polymerase II favours the cis isomer in pSer-Pro linkages. The extent of increase is upto six-fold for the sequence YSPTpSPN, in which the Asn7 sidechain can hydrogen bond to the Thr4 carbonyl, thereby stabilizing the cis isomer and demonstrating a sequence-specific regulatory effect of phosphorylation. Intriguingly, the CTD recruits cis Pro-specific regulatory factors, and it is this heptad with the maximum cis isomer content that is best recognized and dephosphorylated by the Ssu72 phosphatase.
The electrostatic field from phosphate groups can also influence protein conformation, particularly for proteins undergoing multisite phosphorylation. In the case of the intrinsically disordered Ser/Arg-rich C-terminal domain of the splicing factor SRSF1, multiple phosphate groups partially rigidify the polypeptide into an arch-like structure, though the lack of resolvable NOE cross-peaks in a 15N-edited NOESY experiment, as well the low (< 0.5) heteronuclear 15N-1H NOE values confirm that the phosphorylated chain is not stably folded55. pH titrations indicated that the charge state of the pSer groups was responsible for this conformational change. The electrostatic field from multiple phosphate units on the cyclin-dependent kinase inhibitor Sic1 have been proposed to contribute significantly to its binding to the positively charged interface on Cdc4 56. An increase in negative charge upon phosphorylation of Thr51 in the acidic patch of the intrinsically disordered cancer/testis antigen PAGE4 allows the acidic region to interact more favorably with a basic N-terminal motif, as seen from chemical shifts, dihedral angle data, NOEs and PRE measurements. The compaction of PAGE4 that occurs as a consequence disrupts the interaction of a transient helix near the acidic patch with a binding partner c-Jun57.
Finally, apart from structural effects, phosphorylation can also cause changes in the dynamics of the protein chain. Landrieu and coworkers demonstrated using 15N R1, 1H-15N heteronuclear NOE and amide hydrogen exchange rates that the phosphate binding loop of the Pin1 WW domain becomes more rigid upon phosphorylation, while the reduced 15N R2 values suggested that the phosphate group had quenched microsecond-millisecond conformational exchange in the surrounding region15. Phosphorylation also switches the population of Ets-1 transcriptional activator molecules from a flexible DNA binding-competent state to a folded and inhibited state, resulting in a graded DNA binding response that depends on the number of phosphate groups attached to the Ser-rich region of Ets-158.
Acetylation: A simple but powerful addition to the PTM toolbox
Detection
Acetylation can occur co-translationally at the N-terminal -NH3 + or at Lys sidechain Nε groups of a protein. Acetylation is catalyzed by acetyltransferases that use acetyl-CoA as the precursor molecule, while deacetylation is carried out by the deacetylase family of enzymes (Figure 1B). Unlike phosphorylation where multisite modification is common, acetylation is more sparse and results in smaller upfield shifts of the amide 1H (~ 0.05 ppm) and 15N (0.5 ppm) resonances of the modified residues (Figure 4)12, 59. However, acetylation converts the Lys sidechain amino group that is generally not visible in 1H-15N HSQC datasets because of exchange with solvent, to an amide which resonates at 8.0/128 ppm in HSQC spectra and constitutes the best diagnostic imprint of Lys acetylation (Figure 4, right panel, blue box).
Figure 4.
Monitoring Lys acetylation (left panel) by comparing 1H-15N HSQC spectra of unmodified (black) and CBP-acetylated (red) c-myc (reproduced with permission from Liokatis et al.59). The overlapping acetamide resonances from the modified Lys323 and Lys326 sidechains are boxed in blue. The chemical structure of the modification is shown in the left panel within a yellow box.
For proteins carrying a single acetylation site, the site of acetylation can be determined simply by noting the Lys backbone amide resonance that has been perturbed. Acetylation generally does not result in large scale perturbations in the HSQC spectrum, facilitating assignment60, 61. However, assignment of multiple acetylation sites poses more of a problem, because of degeneracies in Lys sidechain 1H and 13C chemical shifts especially in intrinsically disordered protein regions that are prone to acetylation. One novel approach to address this problem is to monitor the disappearance of Lys backbone amide peaks in conjunction with the appearance of Lys-acetyl NH resonances. Once the assignments of the acetylated backbone amides are obtained, sidechain acetamide assignments can be directly inferred from the rates at which they appear in the HSQC. Selenko and coworkers have used this approach to assign acetamide peaks at Lys373 and Lys382 of the p53 transactivation domain (TAD), a disordered region that is acetylated stepwise by CBP/p30012.
Another approach that has been used to study acetylation sites in proteins is to employ an acetyllysine mimic, trifluoroacetyl-L-lysine (TFAcK). Li and coworkers introduced this mimic into recombinant proteins by expanding the genetic code and evolving an orthogonal tRNA/aminoacyl-tRNA synthetase pair capable of incorporating trifluoroacetyl-L-lysine at sites specified by the amber stop codon62. 19F NMR benefits from the high gyromagnetic ratio of 19F6, and also has the advantage that there is no spectroscopic background signal arising from the rest of the protein. However, acetylation sites have to be previously known in order to use this method.
Conformational changes upon acetylation
Acetylation changes the physicochemical properties of the polypeptide chain by converting the polar positively charged Lys sidechain primary amine into a neutral and more hydrophobic acetamide group. The acetylated protein also has an additional hydrogen bond acceptor in the form of the carbonyl moiety. Chemical shift perturbations observed in 1H-15N HSQC spectra upon acetylation are usually restricted to peaks from residues surrounding the acetylation site and are generally small (0.05 ppm in 1H and 0.5 ppm in 15N) 12, 61, suggesting that acetylation does not induce major conformational changes in the protein.
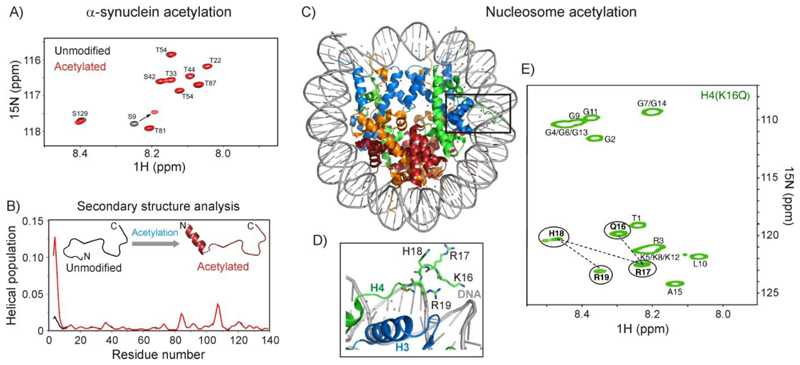
Perhaps the best characterized consequence of acetylation on protein conformation is the ability of an N-terminal acetyl group to serve as a helix cap and prevent the fraying of a helix at its N-terminus 63, 64. Bax and coworkers studied the role of N-terminal acetylation on the structural and dynamic properties of α-synuclein, a protein implicated in the pathophysiology of Parkinson's disease (Figure 5A,B)65. A substantial fraction of α-synuclein isolated from erythrocytes is acetylated, though the molecular implications of acetylation are not understood. The authors showed that acetylation of the N-terminus induces helicity in the first 10 residues of α-synuclein (Figure 5B), while leaving the properties of the rest of the chain unchanged. The perturbation of synuclein peaks as far as residue 12 indicates that the transient N-terminal helix is formed cooperatively, aided by the N-terminal acetamide cap. The basic N-terminus and acidic C-terminus of α-synuclein are known from paramagnetic relaxation enhancement (PRE)66 data to form charge-guided transient contacts with each other67. Intriguingly, Bax and coworkers detected a small decrease in the translational diffusion coefficient of α-synuclein upon acetylation that points to a slight expansion in the chain dimension. This appears to be a direct manifestation of the removal of the N-terminal positive charge whereupon the compaction from electrostatic interactions is lost. A similar effect is seen when Ser129 near the C-terminus of α&synuclein is phosphorylated to reduce the overall positive charge in this region67. In order to follow the membrane association of unmodified and acetylated α-synuclein, the authors used the attenuation in peak intensity upon adding lipids as a probe65. These measurements show that acetylated α-synuclein has a two-fold higher affinity for lipid, as well as that the N-terminus of the acetylated form might be responsible for initiating membrane association.
Figure 5.
Conformational changes observed upon acetylation. (A) 1H-15N spectra of unmodified (black) and N-terminal-acetylated (red) α-synuclein showing the chemical shift perturbation of S9 near the N-terminus. (B) Acetylation results in an increase in helical propensity near the N-terminus of α-synuclein. Figure panels in (A) and (B) reproduced with permission from Maltsev et al.65 (C) Structure of the Drosophila nucleosome (PDB ID: 2PYO71). The histone proteins H2A, H2B, H3 and H4 are coloured orange, red, blue and green respectively. DNA is coloured grey. The region surrounding the H4 tail is indicated by a black box and a close-up is shown in panel D. (E) 1H-15N HSQC spectrum of the nucleosome reconstituted with 13C/15N H4 carrying the acetylation mimic mutation K16Q. The peaks that appear in this spectrum that are not present in the spectrum of the wild type nucleosome are circled. Spectrum is reproduced with permission from Zhou et al.70
The conclusion that N-terminal acetylation of α-synuclein increases the helicity of the first 9 residues has been corroborated independently by Kang et al. who also demonstrated that acetylation reduces the aggregation propensity of the protein68. Interestingly, Lee and coworkers showed that Ca2+-calmodulin (Ca-CaM) but not apo-calmodulin (apo-CaM) binds to a peptide encompassing the N-terminal region of α-synuclein, and that acetylation increases the strength of the interaction 10-fold69. Given that both CaM and α-synuclein colocalize to neurons and play important roles in neurotransmission, it is possible that CaM modulates the membrane association of α-synuclein in a Ca2+-dependent manner by competing for the same binding site.
The difficulty of producing high concentration homogeneous site-specifically acetylated samples of complex systems has also prompted researchers to use acetylation mimics in querying the role of acetylation on protein structure and function. Bai and coworkers examined the structural perturbations of the nucleosome in response to histone acetylation (Figure 5C-E)70. The nucleosome is the structural unit of eukaryotic chromatin, in which an approximately 146 bp DNA segment is wound around an octameric histone core containing two copies each of histone proteins H2A, H2B, H3 and H4 (Figure 5C)71. Histones are among the most important classes of proteins whose function is regulated by the activity of histone acetyltransferases (acetylation) and histone deacetylases (deacetylation)72. The N-terminal regions of histone proteins generally lack secondary structure and are known as "histone tails". The tail of H4 carries a basic patch 16KRHR19 that is essential for formation of higher-order chromatin structure73 as well as ATP-dependent nucleosome sliding catalyzed by the ISWI enzymes74, with acetylation of K16 hindering both processes75, 76. The crystal structure of the nucleosome shows that K16 forms electrostatic interactions with the sugar-phosphate DNA backbone (Figure 5D). Bai and coworkers reconstituted the nucleosome with Drosophila histone proteins and the 167 bp Widom 601 DNA sequence70. In each sample, one histone protein at a time was isotope-labeled with 15N and 13C. 1H-15N HSQC spectra showed only the resonances from the flexible N-terminal histone tails (residues 1-15 for H4) and few resonances from the C-terminus, with the rest of the peaks broadened out because of the high molecular weight (> 200 kDa) and fast transverse relaxation rates of the nucleosome. The position of the histone H4 tail with respect to the rest of the nucleosome was verified using methyl-TROSY NMR77 in conjunction with paramagnetic relaxation enhancement (PRE)66 data. In order to carry out PRE measurements, A15 in the H4 tail was mutated to Cys and a nitroxide spin label (MTSL) was covalently linked to the Cys sidechain. Deuterated and ILV-13CH3 protonated H3 was used in nucleosome reconstitution for PRE experiments in order to take advantage of the methyl-TROSY effect in the 1H-13C HMQC, which has pushed the size limits of protein NMR spectroscopy to > 1 MDa78. 1H PRE data show the enhanced relaxation of L65, L70, V71, L74, L82 and V89 in H3 which are proximal to H4 A15, confirming that the location of the H4 tail is the same as that observed in the crystal structure of the nucleosome. Intriguingly, when the nucleosome is reconstituted with the K16Q H4 variant, which partially mimics acetylation of K16, peaks arising from residues Q16, R17, H18 and R19 belonging to the N-terminal tail also become visible (Figure 5E). Since only disordered and flexible residues retain intensity in the 1H-15N HSQC spectrum, the appearance of Q16-R19 peaks demonstrates that the K16Q acetylation-mimic mutation induces structural disorder in the H4 histone tail. The local disorder generated in the H4 tail upon acetylation thus directly points to a molecular mechanism by which acetylation can influence physiological processes such as nucleosome remodeling that involve the H4 basic patch (K16-R19)70.
The acetylation of a Lys sidechain generates a hydrophobic methyl group that acts as an epitope for recognition by bromodomains which are specific domains that bind acetylated proteins79. Mujtaba et al. solved the structure of HIV1 Tat peptide acetylated at Lys50 in complex with the bromodomain of p300/CBP-associated factor (PCAF)80. The peptide binds in an extended conformation with the acetyllysine moiety making contacts with hydrophobic amino acids lining the binding cavity. Barring recognition by bromodomains, however, very little is understood about how the hydrophobic acetyl groups on Lys sidechains alter the conformational and protein-protein interaction tendencies of a polypeptide.
Glycosylation: the most complex PTM of all
Detection
Glycosylation is a substantially more complex PTM from a biochemical standpoint than phosphorylation and acetylation81, and a detailed discussion of the biochemistry of glycoconjugation is outside the scope of this review. Glycoproteins are glycoconjugates which have oligosaccharide units or glycans covalently bonded to the protein, generally via N- or O-linkages (Figure 1C,D), though Cys-S-linked and Trp-C-linked glycoproteins are also known. N-linked glycoproteins have glycans bonded to the Asn amide sidechain, while O-glycosylation occurs at Ser or Thr hydroxyl groups. The glycan itself can range in complexity from a single sugar unit (glucose, fucose, etc.) to large branched oligosaccharides carrying over a dozen sugar residues81.
O-glycans are usually connected to Ser or Thr hydroxyls through N-acetyl galactosamine (GalNAc) (Figure 6A, top panel)81 and the core glycan can be linear or branched. The biosynthesis of O-GalNAc glycans occurs in Golgi bodies and glycosyltransferases catalyze the conjugation of UDP-activated sugar units to the target protein. The cellular activity and substrate specificity of glycosyltransferases determines the composition of the glycan that eventually modifies the protein. In addition to these O-GalNAc glycans, many proteins also have a single N-acetyl glucosamine (GlcNAc) covalently linked to Ser or Thr amino acids81. Single O-GlcNAc modifications are similar to PTMs such as phosphorylation and acetylation in being reversibly added and removed, often in response to metabolic cues and nutrient levels82. Moreover, there is extensive cross-talk between O-GlcNAcylation and phosphorylation83, wherein OGlcNAcylation affects the efficiency of site-specific phosphorylation and also competes with the same Ser residues for modification84.
Figure 6.
(A) Detecting O-glycosylation using 1H-15N HSQC spectra of unmodified (black) and S400 O-GlcNAcylated tau protein (red). Spectra reproduced with permission from Smet-Nocca et al.90 and (B) Bottom left panel: The anomeric region of the 1H-13C HSQC spectrum of pineapple stem bromelain carrying an N-glycosylation unit (shown as an inset in the spectrum). Bottom right panel: 1H-1H TOCSY spectrum of the same protein. Spectra are reproduced with permission from Schubert et al.88 The chemical structures of the modifications are shown in the top panels in each case within yellow boxes.
N-glycans generally possess a common pentasaccharide core with GlcNAc serving as the point of attachment to the Asn residue (Figure 6B, top panel)81. In case of N-glycosylation, the assembly of the glycan begins at the endoplasmic reticulum (ER) membrane on a polyisoprenoid lipid diphosphate called dolichol phosphate. The oligosaccharide is then transferred to the Asn residue at specific Asn-X-Ser/Thr sequons (where X can be any non-Pro amino acid)2 of proteins translocated into the ER. The glycan is then processed by a number of competing glycosidases and glycosyltransferases in the ER as well as in the Golgi to give rise to the mature glycoprotein. Glycan synthesis is not templated and the final glycoprotein is the result of enzymes whose activity depends on the organism, cell type and environmental cues. As a consequence, the set of glycoproteins present in an organism is highly variable and dynamic, generating considerable biological complexity and diversity81.
For over three decades, NMR spectroscopy has served as a pivotal analytical tool for the atomic resolution characterization of glycoproteins85–87, where the problem is not only to identify the presence of a glycan, but also to pinpoint its chemical structure. Multinuclear multidimensional NMR techniques have been shown to be applicable for studying glycoproteins without need for isotope-labeling88, 89, making them powerful alternatives to mass spectrometry for obtaining complementary chemical information on glycan modifications in proteins. Arguably the most reliable fingerprint for the presence of a glycan appears in the 1H-13C HSQC spectrum from the anomeric CH group which resonates at ~ 4.4-5.2 ppm and ~ 100-110 ppm in the 1H and 13C dimensions respectively (Figure 6B, bottom left panel)12, 88, 90, 91. The resonance frequencies of the anomeric group localize to a region of the spectrum where virtually no other -CH peak in a glycoprotein would appear, making these resonances important diagnostic indicators. Additionally, N-linked glycans have one sugar residue that is linked to Asn via the N-glycosidic bond. The "anomeric" carbon for this residue is bonded to an oxygen and a nitrogen (instead of two oxygens for the canonical anomeric carbon) and this -CH resonates at yet another unique region around 5.0/80-85 ppm, separated not only from other protein and glycan peaks, but also from other anomeric carbons (Figure 6B)88. Aside from the anomeric carbons, O-glycosylation also causes an upfield shifts of 13Cα by ~ 2 ppm and downfield shift of 13Cβ by ~ 6 ppm for the modified Ser/Thr residue, with small accompanying changes of ~ 0.1-0.2 ppm for Hα and Hβ90,92. Glycans containing acetylated sugars such as N-acetylglucosamine and N-acetylgalactosamine can also be identified from a 1H-15N HSQC which shows extra peaks at ~ 8.2/122 ppm originating from the acetamide group of the acetylated sugar unit90, 92 (Figure 6A, bottom panel).
The constituent sugar residues of N- and O-glycans are typically identified and their resonances assigned using a combination of homonuclear (1H-1H) and heteronuclear (1H-13C) correlation-, TOCSY- and NOESY-based NMR methods86, 88, 93, 94. The sugar units themselves, as well as the linkage between these units, result in fingerprint patterns in 2D NMR datasets that have been extensively used for characterizing glycans. A novel approach that improves on these methods that is, to first order, independent of the size of the target glycoprotein has been developed by Schubert and coworkers (Figure 6B, bottom panels)88. The authors used 7M urea to denature the glycoprotein and acquired NMR data under denaturing conditions in which the glycan chain does not adopt higher order structures. The resonances of the glycans can thus be easily assigned by comparing to database chemical shift values, and the spectral sensitivity and resolution are dominated by the local mobility of the protein and the glycan rather than the global tumbling of the glycoprotein as a whole, making the measurements relatively insensitive to the size of the molecule. This methodology was used to assign glycan resonances of four glycoproteins from diverse organisms, the bacterial AtaC, invertase from the yeast Saccharomyces cerevisiae, pineapple stem bromelain (Figure 6B) and human serum albumin88. A similar approach of denaturing the glycoprotein and determining glycan composition by comparing with a free glycan chemical shift database has been adopted by Peng et al. to profile glycans conjugated to antibodies89.
Conformational tendencies of glycoproteins
The effects of glycosylation on protein conformation can be quite heterogeneous depending on the attached glycan moiety. While glycosylation does not change the net charge per residue of the protein, the glycan furnishes hydrogen bond donors and acceptors that can interact with the solvent-exposed polar groups on the protein surface. Nevertheless, global folding/unfolding or disorder-order transitions have not yet been observed upon glycan conjugation.
In a seminal piece of work, Wagner and colleagues solved the three-dimensional structure of human CD2 covalently bound to a high-mannose N-glycan (hsCD2105)95, 96 using 1H, 15N and 13C NMR methodology (Figure 7A). CD2 is a cell surface glycoprotein that mediates cellular adhesion via its N-terminal adhesion domain that is modified at Asn65 with a GlcNAc2Man7 in 40% of the glycosylated molecules. N65Q97 or T67A98 mutants of CD2 are expressed on the cell surface but do not bind ligands or antibodies suggesting that the attached glycan is responsible for stabilizing the native structure of the protein. The protein part of hsCD2105 adopts a V-set immunoglobulin fold composed of one anti-parallel 3-stranded and one anti-parallel 5-stranded β-sheet, with an additional β-strand A interacting in parallel orientation with the 5-stranded sheet (Figure 7A)96. The high-mannose glycan is curved towards the 3-stranded sheet and establishes polar contacts with charged Lys residues (K61, K69 and K71), stabilizing the overall tertiary fold of the protein (Figure 7A). 13C linewidth measurements for the anomeric carbons showed that the mobility of the glycan increased outward, with the terminal mannose units enjoying maximum flexibility. In contrast, the two GlcNAc residues closest to the point of attachment to the protein chain are rigid and display NOE cross-peaks to N18 and D20, as well as to Lys residues K61 and K69. While glycans had been previously known to stabilize proteins, this study provided a molecular basis for how glycans fortify the native polypeptide fold.
Figure 7.
Conformational changes in glycosylated proteins. (A) The structure of CD2 (PDB ID: 1GYA96) showing the proximity of the attached N-glycan to the positively charged Lys cluster. Lys residues are shown as blue sticks, while the N-glycan (including N65 to which the oligosaccharide is attached) is shown as spheres. K71, which forms a hydrogen bond with mannose unit-7, is also explicitly identified. (B) The 26 lowest-energy NMR structures of glycan-bound HCG (PDB ID: 1HD4100). N78 to which the glycan is attached is shown as green sticks. The glycan unit is depicted as lines. Residues that are influenced by the presence of the glycan are shown as blue spheres. (C) NMR structures of unmodified (PDB ID: 2K32102) and glycosylated (PDB ID: 2K33102) AcrA61-210ΔΔ. (D) Chemical shift perturbations and secondary structure propensity changes (as seen from differences in Cα residual chemical shifts) upon glycosylation of AcrA61-210ΔΔ>at N42. A small decrease in helical propensity is observed in the vicinity of N42 when the glycan is conjugated. Figure is reproduced with permission from Slynko et al.102 (E) 13C CPMG relaxation dispersion data depicting the modulation in the effective transverse relaxation rate (R2,eff) as a function of the CPMG frequency (νCPMG) acquired at 800 MHz (blue) or 900 MHz (red) on the 13C2-galactose-labeled glycan with the galactose label on the α1-3 arm (triangles, diamonds) or the α1-6 arm (squares, circles). (F) A structural model for the two states involved in conformational exchange, with the α1-6 arm of the glycan interacting with the protein in the major state (PDB ID: 1L6X107) and mobile in the minor state. Figures in panels (E) and (F) are reproduced with permission from Barb et al.104 The glycan units attached to the proteins in panels A, B, C and F are indicated in boxes alongside, with the glycan units numbered accordingly numbered on the structure in panels A-C. In panel B, one arm of the branched glycan is coloured in green while the other is coloured as pale pink. A legend for all the glycan representations is given at the bottom of the figure.
Vliegenthart and coworkers solved the structure of the α-subunit of human chorionic gonadotropin glycosylated at Asn78 with either a single GlcNAc residue or with a nine-residue biantennary glycan (hCGg) (Figure 7B)99, 100. The core structure of the protein component consists of two twisted β-hairpins (10-28 and 59-85) connected by three disulfide bonds between Cys28-Cys82, Cys10-Cys60 and Cys32-Cys84 (Figure 7B). The glycan points outwards from N78 in direction of β-hairpin 1, interacting with the β-turn formed by residues 14-23 and influencing the chemical shifts and NOE patterns of a number of residues in its proximity (Figure 7B, blue spheres). Increased NOE cross-peak intensities are observed for Phe18/Ile25, Gln20/Ala23, and Ala23/Pro24 pairs; taken in conjunction with the larger observed backbone order parameters for residues 20-23, these indicate that the glycan stabilizes the β-turn of the first hairpin and providing a mechanism by which the glycan alters the conformational free energy surface of the polypeptide. The mobility and dynamics of the glycan beyond the mannose branching point101 is reflected in the inability to constrain its conformation during NMR structure calculations, with the result that every member of the NMR ensemble has a different orientation for the two arms of the glycan (Figure 7B).
One major obstacle that has stymied atomic resolution studies of glycoproteins using NMR spectroscopy is the difficulty in producing homogeneous isotope-labeled samples from E.coli, a benefit that protein NMR spectroscopy has enjoyed for decades93. Allain and colleagues circumvented this problem by overexpressing and purifying the components required for glycosylation, 15N/13C-labeled AcrA, the oligosaccharyltransferase PglB and the lipid-linked oligosaccharide (LLO), from E.coli and carrying out the glycosylation of AcrA in vitro102. The LLO was expressed in E.coli cells carrying a plasmid with the pgl locus which encodes 12 proteins making up the Campylobacter jejuni glycosylation machinery that can be transferred to E.coli without loss of functionality103. The glycosylation site on AcrA61-210ΔΔ (N42) is in a 30 residue-long loop that has partial helicity in the region surrounding N42 (Q36-44) in the unmodified form (Figure 7C, top)102. This helicity decreases upon glycosylation, probably because of steric constraints imposed by the added glycan chain (Figure 7D). However, the overall fold of the protein remains unchanged upon covalent conjugation to the N-glycan (Figure 7C, bottom). The methodology introduced in this report for producing isotope-labeled glycoproteins is appealing, though it does not appear to be easily generalizable to other systems, considering the fact that the nature of the glycan conjugated to the protein is dependent on the organism and cell-type, and that 30L of E.coli LB culture was required to make adequate LLO for one NMR sample.
The three examples described above demonstrate that significant changes in conformation do not occur as a result of N-glycosylation, though polar interactions between the glycan and the protein do stabilize the native protein fold. These examples also show that the picoseconds-nanoseconds mobility of the glycan chain increases gradually as we progress from the derivatized Asn residue towards the end of the chain. However, the glycan can also show dynamics on the μs-ms timescale and adopt functionally relevant alternate conformations, and the best evidence for this comes from the work of Prestegard and colleagues who studied the effect of N-glycosylation on the immunoglobulin IgG1 Fc fragment (Figure 7F)104. IgG molecules are important constituents of the adaptive immune system that target foreign bodies in the blood for destruction. The Fc fragment (crystallizable fragment) of the heavy chain of IgG is glycosylated at N297 and the biantennary glycan performs a variety of functions including stabilizing the Fc fragment105 and presenting a binding site for lectins, cell-surface receptors and glycan-modifying enzymes. However, crystal structures show that the termini of the oligosaccharide are buried between two Cγ2 domains and are not accessible for interaction with receptor proteins (Figure 7F, state A)106, 107. Prior NMR studies had shown that while the α1-3 Man branch had limited mobility, the α1-6 Man branch is completely immobilized on the protein surface (Figure 7F, state A)108, 109. The authors used samples of IgG1-Fc selectively labeled with 13C at specific galactose units purified from myeloma cells for their experiments. Initially, 13C/1H resonance assignments for the galactose units of the α1-6 Man and α1-3 Man branch were obtained a sample with 13C-labeled galactose present only at the α1-6 branch. A comparison of galactose chemical shifts with those from the free glycan showed significant differences especially for the α1-6 Man branch, confirming the notion that this galactose residue interacts with the protein surface in the ground state conformation of the glycoprotein. Using 13C2-galactose labeled IgG1-Fc, the authors then ran 13C CPMG relaxation dispersion NMR experiments110–113 which can detect sparsely (> 0.5 %) and transiently (0.2 - 5 ms) populated 'excited' biomolecular conformations (Figure 7E). These experiments unequivocally demonstrate the existence of an alternate thermally accessible conformation for the glycan where the α1-6 Man galactose 13C2 shows a modulation of the effective transverse relaxation rate with CPMG frequency, indicating that this nucleus has different chemical shifts in the ground and excited conformations (Figure 7E). In contrast, the α1-3 Man galactose 13C2 shows flat dispersions which confirm that the ground and excited state chemical shifts are identical. The chemical shifts of α1-6 Man galactose in the excited conformation were obtained via a temperature titration which assumes that the chemical shifts of the ground and excited state are invariant to temperature and that temperature merely alters the relative populations of the two states. In contrast to the ground state, the excited state chemical shift resembles that of the free glycan, painting a picture of the higher energy conformation where the glycan is free from the steric constraints imposed by the protein and accessible for interaction with binding partners (Figure 7F, state B). More generally, this study reveals the presence of dynamics and functional excited conformations in glycans conjugated to proteins, that adds to our knowledge of how transiently populated conformations influence function and malfunction in proteins114–119 and nucleic acids120–123.
One of the earliest studies probing the role of N-glycosylation on protein conformation came from the group of Raymond Dwek, who studied the effect of glycosylating Asn34 of RNase B with five glycans, Man5-9GlcNAc2 124, 125. Compared to the unmodified protein RNase A, RNase B retained the same protein fold. However, the stability of the protein increased upon glycosylation, and amide protons of residues near the glycosylation site (29-35), as well as others distal to Asn34 (10-13, 57-61, 75-76) enjoyed a higher degree of protection from the solvent, showing that glycosylation reduced the protein fluctuations required for H/D exchange. Interestingly, a fraction of these residues is more protected in the Man5 glycoform than in RNase B125, suggesting that specific contacts that are possible with a smaller glycan maybe disrupted with the larger Man6-9GlcNAc2.
Other studies have confirmed that the interaction between the glycan and the protein, as well as the changes in protein conformation upon glycan conjugation, appear to be dependent on the specific glycoform. A recent report on the soluble domain of the high-affinity IgE receptor (FcεRI) that is glycosylated at seven distinct Asn residues has probed a heterogeneous mixture of glycoforms expressed and purified from HEK cells94. Samples were subjected to NMR spectroscopy under native or denaturing conditions to determine that oligomannose N-glycans were more likely to interact with the protein than hybrid or complex type N-glycans. Kato and coworkers prepared a series of truncated glycoforms of Fc-IgG1 glycosylated at N297 with a biantennary glycan using glycosidases in conjunction with enzymatic galactosylation126. 13C/15N isotope-labeled samples revealed increasing degrees of chemical shift perturbation in the protein with the level of truncation; even regions of the protein that were not in contact with the glycan were affected, suggesting the presence of allosteric effects of the glycan on protein conformation. Similar to phosphorylation, N-glycosylation can alter cis/trans isomer ratios at Xaa-Pro peptide bonds. N-glycosylation with a truncated glycan GlcNAc2 at a conserved Asn residue in the nicotinic acetylcholine receptor favours the trans conformation of a Phe-Pro bond that is five residues upstream of the modified Asn, despite the fact that 2D NMR did not reveal any stable interactions between the peptide and the carbohydrate127. However, the molecular mechanism underlying much of the impact of glycans on protein structure remain poorly understood.
O-glycosylation has been known to induce diverse local conformational changes in the protein backbone. O-GalNAcylation of the peptide hormone calcitonin at either Thr6 or Thr21 results in local reduction in α-helical content as seen from Hα secondary chemical shifts and supported by NOE data and H/D exchange128. Conjugation with O-GalNAc also causes the formation of extended structures in glycopeptides derived from mucin129–131. Interestingly, the conformational properties of O-GalNAcylated proteins depend on whether the anchoring amino acid is Ser or Thr. Avenoza, Peregrina and coworkers have shown that soybean and Vicia villosa agglutinins prefer to bind O-GalNAc-Thr, while Helix pomatia agglutinin favours O-GalNAc-Ser132. The differences in epitope recognition have been traced to the local conformational preferences of OGalNAc-Ser and O-GalNAc-Thr, which also adopt different dihedral angles at the glycosidic linkage with O-GalNAc-Thr observed in the eclipsed conformation, while other rotameric states are preferred by O-GalNAc-Ser132, 133. In contrast to O-GalNAcylation, conjugation with glucose or N-acetylglucosamine elicits the formation of α-helical134 or turn-like conformations135, 136.
Conclusions and future perspectives
Biomolecules are now understood to be dynamic objects that leverage not only their native state structure but also other thermally accessible higher energy conformations to perform their function113. Organisms use PTMs to alter the physicochemical nature of the protein chains and modulate their conformational free energy landscape, thereby expanding the repertoire of structures available to fill functional niches. In this review, we have highlighted the ways in which NMR spectroscopy has contributed as an analytical tool for detecting PTMs and for elucidating the conformational changes arising from the modifications. There is now evidence not only for PTMs causing global conformational transitions but also for functional dynamics in the post-translationally modified proteins.
A number of important questions pertaining to the biological structure-function paradigm in the context of PTMs still remain open. First, how do PTMs alter the conformational free energy surface of their target proteins? While most studies have focused on the ground state, very little is understood about low lying thermally accessible conformations in the unconjugated and modified forms of the protein. Second, when proteins such as 4E-BP229 or CSP36 are phosphorylated, does phosphorylation remodel the polypeptide chain or does it switch the population between two pre-existing conformations on the free energy landscape of the unmodified protein? This question seems particularly relevant when a small number of PTMs (one or two) induce global transitions between states that are presumably several kcal/mol apart in free energy in the unmodified protein. Indeed, biological processes such as protein maturation137, signal transduction138 and enzyme catalysis114 have been shown to occur via population switch mechanisms. Third, can multiple PTMs competing for the same protein sites (for eg. phosphorylation and O-glycosylation)84, 139 especially in malleable IDPs coax the protein into distinct conformations or alter the relative stabilities of conformations on the free energy landscape in different ways to modulate function? These and many other questions make the structural biology of PTMs a fascinating and rewarding area of research.
The NMR toolkit is constantly expanding140–144 and methodological breakthroughs have now enabled us to visualize not only the ground state structure of biomolecules, but also the structures of thermally accessible yet invisible states within a free energy of ~ 2.5 kcal/mol from the ground state. Furthermore, NMR techniques are available to probe the dynamics of biomolecules from picoseconds to seconds and beyond. Underlying all of this methodology is the quintessential "forbidden fruit" of NMR of being able to obtain atomic resolution insights into biomolecules in solution state, a feature that will remain of prime importance as we continue to explore the mysteries hidden in the structural biology of PTMs.
Acknowledgements
This work was supported by the Wellcome Trust/DBT India Alliance Fellowship (grant number: IA/I/18/1/503614) awarded to A.S. The authors would like to thank Prof. Siddhartha P. Sarma and Dr. Jayashree Nagesh for a critical reading of the manuscript. A.K. thanks the DBT-RA program for fellowship support.
References
- [1].Walsh C. Posttranslational modification of proteins: expanding nature's inventory. Roberts and Company Publishers; 2006. [Google Scholar]
- [2].Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- [3].Csizmok V, Forman-Kay JD. Complex regulatory mechanisms mediated by the interplay of multiple post-translational modifications. Curr Opin Struct Biol. 2018;48:58–67. doi: 10.1016/j.sbi.2017.10.013. [DOI] [PubMed] [Google Scholar]
- [4].Humphrey SJ, James DE, Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol Metab. 2015;26:676–687. doi: 10.1016/j.tem.2015.09.013. [DOI] [PubMed] [Google Scholar]
- [5].Barber KW, Rinehart J. The ABCs of PTMs. Nat Chem Biol. 2018;14:188–192. doi: 10.1038/nchembio.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levitt MH. Spin dynamics: basics of nuclear magnetic resonance. John Wiley & Sons; 2001. [Google Scholar]
- [7].Live DH, Edmondson DE. Studies of phosphorylated sites in proteins using proton-phosphorus-31 two-dimensional NMR further evidence for a phosphodiester link between a seryl and a threonyl residue in Azotobacter flavodoxin. J Am Chem Soc. 1988;110:4468–4470. [Google Scholar]
- [8].McIntosh LP, Kang H-S, Okon M, Nelson ML, Graves BJ, Brutscher B. Detection and assignment of phosphoserine and phosphothreonine residues by 13 C–31 P spin-echo difference NMR spectroscopy. J Biomol NMR. 2009;43:31–37. doi: 10.1007/s10858-008-9287-6. [DOI] [PubMed] [Google Scholar]
- [9].Vogel HJ, Bridger WA, Sykes BD. Frequency-dependent phosphorus-31 nuclear magnetic resonance studies of the phosphohistidine residue of succinyl-CoA synthetase and the phosphoserine residue of glycogen phosphorylase a. Biochemistry (Mosc) 1982;21:1126–1132. doi: 10.1021/bi00535a003. [DOI] [PubMed] [Google Scholar]
- [10].Brauer M, Sykes BD. Methods Enzymol. Elsevier; 1984. Phosphorus-31 nuclear magnetic resonance studies of phosphorylated proteins; pp. 36–81. [DOI] [PubMed] [Google Scholar]
- [11].Matheis G, Whitaker JR. 31P NMR chemical shifts of phosphate covalently bound to proteins. Int J Biochem. 1984;16:867–873. doi: 10.1016/0020-711x(84)90145-9. [DOI] [PubMed] [Google Scholar]
- [12].Theillet F-X, Smet-Nocca C, Liokatis S, Thongwichian R, Kosten J, Yoon M-K, Kriwacki RW, Landrieu I, Lippens G, Selenko P. Cell signaling, post-translational protein modifications and NMR spectroscopy. J Biomol NMR. 2012;54:217–236. doi: 10.1007/s10858-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ramelot TA, Nicholson LK. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J Mol Biol. 2001;307:871–884. doi: 10.1006/jmbi.2001.4535. [DOI] [PubMed] [Google Scholar]
- [14].Du J-T, Li Y-M, Wei W, Wu G-S, Zhao Y-F, Kanazawa K, Nemoto T, Nakanishi H. Low-barrier hydrogen bond between phosphate and the amide group in phosphopeptide. J Am Chem Soc. 2005;127:16350–16351. doi: 10.1021/ja054568p. [DOI] [PubMed] [Google Scholar]
- [15].Smet-Nocca C, Launay H, Wieruszeski J-M, Lippens G, Landrieu I. Unraveling a phosphorylation event in a folded protein by NMR spectroscopy: phosphorylation of the Pin1 WW domain by PKA. J Biomol NMR. 2013;55:323–337. doi: 10.1007/s10858-013-9716-z. [DOI] [PubMed] [Google Scholar]
- [16].Selenko P, Frueh DP, Elsaesser SJ, Haas W, Gygi SP, Wagner G. In situ observation of protein phosphorylation by high-resolution NMR spectroscopy. Nat Struct Mol Biol. 2008;15:321–329. doi: 10.1038/nsmb.1395. [DOI] [PubMed] [Google Scholar]
- [17].Landrieu I, Lacosse L, Leroy A, Wieruszeski J-M, Trivelli X, Sillen A, Sibille N, Schwalbe H, Saxena K, Langer T. NMR analysis of a Tau phosphorylation pattern. J Am Chem Soc. 2006;128:3575–3583. doi: 10.1021/ja054656+. [DOI] [PubMed] [Google Scholar]
- [18].Liokatis S, Stützer A, Elsässer SJ, Theillet F-X, Klingberg R, Van Rossum B, Schwarzer D, Allis CD, Fischle W, Selenko P. Phosphorylation of histone H3 Ser10 establishes a hierarchy for subsequent intramolecular modification events. Nat Struct Mol Biol. 2012;19:819. doi: 10.1038/nsmb.2310. [DOI] [PubMed] [Google Scholar]
- [19].Bienkiewicz EA, Lumb KJ. Random-coil chemical shifts of phosphorylated amino acids. J Biomol NMR. 1999;15:203–206. doi: 10.1023/a:1008375029746. [DOI] [PubMed] [Google Scholar]
- [20].Hunter T. Why nature chose phosphate to modify proteins. Philos Trans R Soc Lond, Ser B Biol Sci. 2012;367:2513–2516. doi: 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Himmel S, Wolff S, Becker S, Lee D, Griesinger C. Detection and Identification of Protein-Phosphorylation Sites in Histidines through HNP Correlation Patterns. Angew Chem Int Ed. 2010;49:8971–8974. doi: 10.1002/anie.201003965. [DOI] [PubMed] [Google Scholar]
- [22].Rajagopal P, Waygood EB, Klevit RE. Structural consequences of histidine phosphorylation: NMR characterization of the phosphohistidine form of histidinecontaining protein from Bacillus subtilis and Escherichia coli. Biochemistry (Mosc) 1994;33:15271–15282. doi: 10.1021/bi00255a008. [DOI] [PubMed] [Google Scholar]
- [23].Jones BE, Rajagopal P, Klevit RE. Phosphorylation on histidine is accompanied by localized structural changes in the phosphocarrier protein, HPr from Bacillus subtilis. Protein Sci. 1997;6:2107–2119. doi: 10.1002/pro.5560061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gibbs EB, Lu F, Portz B, Fisher MJ, Medellin BP, Laremore TN, Zhang YJ, Gilmour DS, Showalter SA. Phosphorylation induces sequence-specific conformational switches in the RNA polymerase II C-terminal domain. Nat Commun. 2017;8 doi: 10.1038/ncomms15233. 15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mao AH, Crick SL, Vitalis A, Chicoine CL, Pappu RV. Net charge per residue modulates conformational ensembles of intrinsically disordered proteins. Proc Natl Acad Sci U S A. 2010;107:8183–8188. doi: 10.1073/pnas.0911107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Das RK, Pappu RV. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proc Natl Acad Sci U S A. 2013;110:13392–13397. doi: 10.1073/pnas.1304749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohen P. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- [28].Lin T-A, Kong X, Haystead T, Pause A, Belsham G, Sonenberg N, Lawrence JC. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994;266:653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- [29].Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, Zhao C, Sonenberg N, Kay LE, Forman-Kay JD. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature. 2015;519:106–110. doi: 10.1038/nature13999. [DOI] [PubMed] [Google Scholar]
- [30].Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marcotrigiano J, Gingras A-C, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell. 1999;3:707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- [32].Tait S, Dutta K, Cowburn D, Warwicker J, Doig AJ, McCarthy JE. Local control of a disorder–order transition in 4E-BP1 underpins regulation of translation via eIF4E. Proc Natl Acad Sci U S A. 2010;107:17627–17632. doi: 10.1073/pnas.1008242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomoo K, Abiko F, Miyagawa H, Kitamura K, Ishida T. Effect of N-terminal region of eIF4E and Ser65-phosphorylation of 4E-BP1 on interaction between eIF4E and 4E-BP1 fragment peptide. J Biochem. 2006;140:237–246. doi: 10.1093/jb/mvj143. [DOI] [PubMed] [Google Scholar]
- [35].Lukhele S, Bah A, Lin H, Sonenberg N, Forman-Kay JD. Interaction of the eukaryotic initiation factor 4E with 4E-BP2 at a dynamic bipartite interface. Structure. 2013;21:2186–2196. doi: 10.1016/j.str.2013.08.030. [DOI] [PubMed] [Google Scholar]
- [36].Patel P, Prescott GR, Burgoyne RD, Lian L-Y, Morgan A. Phosphorylation of cysteine string protein triggers a major conformational switch. Structure. 2016;24:1380–1386. doi: 10.1016/j.str.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sharma M, Burré J, Südhof TC. CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol. 2011;13:30. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- [39].Chamberlain LH, Graham ME, Kane S, Jackson JL, Maier VH, Burgoyne RD, Gould GW. The synaptic vesicle protein, cysteine-string protein, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4. J Cell Sci. 2001;114:445–455. doi: 10.1242/jcs.114.2.445. [DOI] [PubMed] [Google Scholar]
- [40].Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- [41].Evans GJ, Morgan A. Phosphorylation-dependent interaction of the synaptic vesicle proteins cysteine string protein and synaptotagmin I. Biochem J. 2002;364:343–347. doi: 10.1042/BJ20020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Murzin AG. Metamorphic proteins. Science. 2008;320:1725–1726. doi: 10.1126/science.1158868. [DOI] [PubMed] [Google Scholar]
- [43].Tuinstra RL, Peterson FC, Kutlesa S, Elgin ES, Kron MA, Volkman BF. Interconversion between two unrelated protein folds in the lymphotactin native state. Proc Natl Acad Sci U S A. 2008;105:5057–5062. doi: 10.1073/pnas.0709518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Luo X, Yu H. Protein metamorphosis: the two-state behavior of Mad2. Structure. 2008;16:1616–1625. doi: 10.1016/j.str.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Markley JL, Kim JH, Dai Z, Bothe JR, Cai K, Frederick RO, Tonelli M. Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron–sulfur cluster biosynthesis and delivery. FEBS Lett. 2013;587:1172–1179. doi: 10.1016/j.febslet.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bah A, Forman-Kay JD. Modulation of intrinsically disordered protein function by post-translational modifications. J Biol Chem. 2016;291:6696–6705. doi: 10.1074/jbc.R115.695056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jensen MR, Ruigrok RW, Blackledge M. Describing intrinsically disordered proteins at atomic resolution by NMR. Curr Opin Struct Biol. 2013;23:426–435. doi: 10.1016/j.sbi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- [48].Ohki S-y, Eto M, Kariya E, Hayano T, Hayashi Y, Yazawa M, Brautigan D, Kainosho M. Solution NMR structure of the myosin phosphatase inhibitor protein CPI-17 shows phosphorylation-induced conformational changes responsible for activation. J Mol Biol. 2001;314:839–849. doi: 10.1006/jmbi.2001.5200. [DOI] [PubMed] [Google Scholar]
- [49].Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- [50].Martin EW, Holehouse AS, Grace CR, Hughes A, Pappu RV, Mittag T. Sequence determinants of the conformational properties of an intrinsically disordered protein prior to and upon multisite phosphorylation. J Am Chem Soc. 2016;138:15323–15335. doi: 10.1021/jacs.6b10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sibille N, Huvent I, Fauquant C, Verdegem D, Amniai L, Leroy A, Wieruszeski JM, Lippens G, Landrieu I. Structural characterization by nuclear magnetic resonance of the impact of phosphorylation in the proline-rich region of the disordered Tau protein. Proteins: Struct Funct Bioinform. 2012;80:454–462. doi: 10.1002/prot.23210. [DOI] [PubMed] [Google Scholar]
- [52].Schwalbe M, Kadavath H, Biernat J, Ozenne V, Blackledge M, Mandelkow E, Zweckstetter M. Structural impact of tau phosphorylation at threonine 231. Structure. 2015;23:1448–1458. doi: 10.1016/j.str.2015.06.002. [DOI] [PubMed] [Google Scholar]
- [53].Radhakrishnan I, Pérez-Alvarado GC, Parker D, Dyson HJ, Montminy MR, Wright PE. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator: coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- [54].Zor T, Mayr BM, Dyson HJ, Montminy MR, Wright PE. Roles of phosphorylation and helix propensity in the binding of the KIX domain of CREB-binding protein by constitutive (c-Myb) and inducible (CREB) activators. J Biol Chem. 2002;277:42241–42248. doi: 10.1074/jbc.M207361200. [DOI] [PubMed] [Google Scholar]
- [55].Xiang S, Gapsys V, Kim H-Y, Bessonov S, Hsiao H-H, Möhlmann S, Klaukien V, Ficner R, Becker S, Urlaub H. Phosphorylation drives a dynamic switch in serine/arginine-rich proteins. Structure. 2013;21:2162–2174. doi: 10.1016/j.str.2013.09.014. [DOI] [PubMed] [Google Scholar]
- [56].Mittag T, Orlicky S, Choy W-Y, Tang X, Lin H, Sicheri F, Kay LE, Tyers M, Forman-Kay JD. Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proc Natl Acad Sci U S A. 2008;105:17772–17777. doi: 10.1073/pnas.0809222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].He Y, Chen Y, Mooney SM, Rajagopalan K, Bhargava A, Sacho E, Weninger K, Bryan PN, Kulkarni P, Orban J. Phosphorylation-induced conformational ensemble switching in an intrinsically disordered cancer/testis antigen. J Biol Chem. 2015;290:25090–25102. doi: 10.1074/jbc.M115.658583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pufall MA, Lee GM, Nelson ML, Kang H-S, Velyvis A, Kay LE, McIntosh LP, Graves BJ. Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science. 2005;309:142–145. doi: 10.1126/science.1111915. [DOI] [PubMed] [Google Scholar]
- [59].Liokatis S, Dose A, Schwarzer D, Selenko P. Simultaneous detection of protein phosphorylation and acetylation by high-resolution NMR spectroscopy. J Am Chem Soc. 2010;132:14704–14705. doi: 10.1021/ja106764y. [DOI] [PubMed] [Google Scholar]
- [60].Kamah A, Huvent I, Cantrelle FoX, Qi H, Lippens G, Landrieu I, Smet-Nocca C. Nuclear magnetic resonance analysis of the acetylation pattern of the neuronal Tau protein. Biochemistry (Mosc) 2014;53:3020–3032. doi: 10.1021/bi500006v. [DOI] [PubMed] [Google Scholar]
- [61].Smet-Nocca C, Wieruszeski JM, Melnyk O, Benecke A. NMR-based detection of acetylation sites in peptides. J Pept Sci. 2010;16:414–423. doi: 10.1002/psc.1257. [DOI] [PubMed] [Google Scholar]
- [62].Zhang F, Zhou Q, Yang G, An L, Li F, Wang J. A genetically encoded 19 F NMR probe for lysine acetylation. Chem Commun. 2018;54:3879–3882. doi: 10.1039/c7cc09825a. [DOI] [PubMed] [Google Scholar]
- [63].Doig AJ, Chakrabartty A, Klingler TM, Baldwin RL. Determination of Free Energies of N-Capping in. alpha.-Helixes by Modification of the Lifson-Roig Helix-Coil Theory To Include N-and C-Capping. Biochemistry (Mosc) 1994;33:3396–3403. doi: 10.1021/bi00177a033. [DOI] [PubMed] [Google Scholar]
- [64].Decatur SM. IR spectroscopy of isotope-labeled helical peptides: Probing the effect of N-acetylaion on helix stability. Biopolymers. 2000;54:180–185. doi: 10.1002/1097-0282(200009)54:3<180::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [65].Maltsev AS, Ying J, Bax A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochemistry (Mosc) 2012;51:5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Clore GM. Exploring sparsely populated states of macromolecules by diamagnetic and paramagnetic NMR relaxation. Protein Sci. 2011;20:229–246. doi: 10.1002/pro.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Paleologou KE, Schmid AW, Rospigliosi CC, Kim H-Y, Lamberto GR, Fredenburg RA, Lansbury PT, Fernandez CO, Eliezer D, Zweckstetter M. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of αsynuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kang L, Moriarty GM, Woods LA, Ashcroft AE, Radford SE, Baum J. N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012;21:911–917. doi: 10.1002/pro.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gruschus JM, Yap TL, Pistolesi S, Maltsev AS, Lee JC. NMR structure of calmodulin complexed to an N-terminally acetylated α-synuclein peptide. Biochemistry (Mosc) 2013;52:3436–3445. doi: 10.1021/bi400199p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou B-R, Feng H, Ghirlando R, Kato H, Gruschus J, Bai Y. Histone H4 K16Q mutation, an acetylation mimic, causes structural disorder of its N-terminal basic patch in the nucleosome. J Mol Biol. 2012;421:30–37. doi: 10.1016/j.jmb.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Clapier CR, Chakravarthy S, Petosa C, Fernández-Tornero C, Luger K, Müller CW. Structure of the Drosophila nucleosome core particle highlights evolutionary constraints on the H2A-H2B histone dimer. Proteins: Struct Funct Bioinform. 2008;71:1–7. doi: 10.1002/prot.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- [73].Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell. 2004;116:259–272. doi: 10.1016/s0092-8674(04)00044-3. [DOI] [PubMed] [Google Scholar]
- [74].Clapier CR, Längst G, Corona DF, Becker PB, Nightingale KP. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol. 2001;21:875–883. doi: 10.1128/MCB.21.3.875-883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shogren-Knaak M, Ishii H, Sun J-M, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- [76].Allahverdi A, Yang R, Korolev N, Fan Y, Davey CA, Liu C-F, Nordenskiöld L. The effects of histone H4 tail acetylations on cation-induced chromatin folding and self-association. Nucleic Acids Res. 2010;39:1680–1691. doi: 10.1093/nar/gkq900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rosenzweig R, Kay LE. Bringing dynamic molecular machines into focus by methyl-TROSY NMR. Annu Rev Biochem. 2014;83:291–315. doi: 10.1146/annurev-biochem-060713-035829. [DOI] [PubMed] [Google Scholar]
- [78].Wiesner S, Sprangers R. Methyl groups as NMR probes for biomolecular interactions. Curr Opin Struct Biol. 2015;35:60–67. doi: 10.1016/j.sbi.2015.08.010. [DOI] [PubMed] [Google Scholar]
- [79].Filippakopoulos P, Knapp S. The bromodomain interaction module. FEBS Lett. 2012;586:2692–2704. doi: 10.1016/j.febslet.2012.04.045. [DOI] [PubMed] [Google Scholar]
- [80].Mujtaba S, He Y, Zeng L, Farooq A, Carlson JE, Ott M, Verdin E, Zhou M-M. Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Mol Cell. 2002;9:575–586. doi: 10.1016/s1097-2765(02)00483-5. [DOI] [PubMed] [Google Scholar]
- [81].Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, et al. Essentials of Glycobiology. Third ed. Cold Spring Harbor Laboratory Press; 2017. [PubMed] [Google Scholar]
- [82].Slawson C, Copeland R, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kaasik K, Kivimäe S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptáček LJ. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell metabolism. 2013;17:291–302. doi: 10.1016/j.cmet.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Neglia CI, Cohen HJ, Garber AR, Thorpe SR, Baynes JW. Characterization of glycated proteins by 13C NMR spectroscopy. Identification of specific sites of protein modification by glucose. J Biol Chem. 1985;260:5406–5410. [PubMed] [Google Scholar]
- [86].Weisshaar G, Hiyama J, Renwick AG, Nimtz M. NMR investigations of the N-linked oligosaccharides at individual glycosylation sites of human lutropin. Eur J Biochem. 1991;195:257–268. doi: 10.1111/j.1432-1033.1991.tb15702.x. [DOI] [PubMed] [Google Scholar]
- [87].Gerken TA, Butenhof KJ, Shogren R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry (Mosc) 1989;28:5536–5543. doi: 10.1021/bi00439a030. [DOI] [PubMed] [Google Scholar]
- [88].Schubert M, Walczak MJ, Aebi M, Wider G. Posttranslational modifications of intact proteins detected by NMR spectroscopy: application to glycosylation. Angew Chem. 2015;127:7202–7206. doi: 10.1002/anie.201502093. [DOI] [PubMed] [Google Scholar]
- [89].Peng J, Patil SM, Keire DA, Chen K. Chemical structure and composition of major glycans covalently linked to therapeutic monoclonal antibodies by middle-down nuclear magnetic resonance. Anal Chem. 2018;90:11016–11024. doi: 10.1021/acs.analchem.8b02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Smet-Nocca C, Broncel M, Wieruszeski J-M, Tokarski C, Hanoulle X, Leroy A, Landrieu I, Rolando C, Lippens G, Hackenberger CP. Identification of OGlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol Biosyst. 2011;7:1420–1429. doi: 10.1039/c0mb00337a. [DOI] [PubMed] [Google Scholar]
- [91].Yamaguchi Y, Kato K. Modern magnetic resonance. Springer; 2008. Structural glycobiology by stable-isotope-assisted NMR spectroscopy; pp. 223–229. [Google Scholar]
- [92].Dehennaut V, Hanoulle X, Bodart J-F, Vilain J-P, Michalski J-C, Landrieu I, Lippens G, Lefebvre T. Microinjection of recombinant O-GlcNAc transferase potentiates Xenopus oocytes M-phase entry. Biochem Biophys Res Commun. 2008;369:539–546. doi: 10.1016/j.bbrc.2008.02.063. [DOI] [PubMed] [Google Scholar]
- [93].Yanaka S, Yagi H, Yogo R, Yagi-Utsumi M, Kato K. Stable isotope labeling approaches for NMR characterization of glycoproteins using eukaryotic expression systems. J Biomol NMR. 2018;71:193–202. doi: 10.1007/s10858-018-0169-2. [DOI] [PubMed] [Google Scholar]
- [94].Unione L, Lenza MP, Ardá A, Urquiza P, Laín A, Falcón-Pérez JM, Jiménez-Barbero Js, Millet O. Glycoprofile Analysis of an Intact Glycoprotein As Inferred by NMR Spectroscopy. ACS Central Science. 2019 doi: 10.1021/acscentsci.9b00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wyss DF, Choi JS, Wagner G. Composition and Sequence Specific Resonance Assignments of the Heterogeneous N-Linked Glycan in the 13.6 kDa Adhesion Domain of Human Cluster of Differentiation 2 (CD2) as Determined by NMR on the Intact Glycoprotein. Biochemistry (Mosc) 1995;34:1622–1634. doi: 10.1021/bi00005a019. [DOI] [PubMed] [Google Scholar]
- [96].Wyss DF, Choi JS, Li J, Knoppers MH, Willis KJ, Arulanandam A, Smolyar A, Reinherz EL, Wagner G. Conformation and function of the N-linked glycan in the adhesion domain of human CD2. Science. 1995;269:1273–1278. doi: 10.1126/science.7544493. [DOI] [PubMed] [Google Scholar]
- [97].Recny MA, Luther MA, Knoppers MH, Neidhardt EA, Khandekar SS, Concino M, Schimke PA, Francis MA, Moebius U, Reinhold B. N-glycosylation is required for human CD2 immunoadhesion functions. J Biol Chem. 1992;267:22428–22434. [PubMed] [Google Scholar]
- [98].Arulanandam A, Withka JM, Wyss DF, Wagner G, Kister A, Pallai P, Recny MA, Reinherz EL. The CD58 (LFA-3) binding site is a localized and highly charged surface area on the AGFCC'C" face of the human CD2 adhesion domain. Proc Natl Acad Sci U S A. 1993;90:11613–11617. doi: 10.1073/pnas.90.24.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].De Beer T, Van Zuylen CW, Leeflang BR, Hård K, Boelens R, Kaptein R, Kamerling JP, Vliegenthart JF. NMR Studies of the Free α Subunit of Human Chorionic Gonadotropin: Structural Influences of N-Glycosylation and the β Subunit on the Conformation of the α Subunit. Eur J Biochem. 1996;241:229–242. doi: 10.1111/j.1432-1033.1996.0229t.x. [DOI] [PubMed] [Google Scholar]
- [100].Erbel PJ, Karimi-Nejad Y, van Kuik JA, Boelens R, Kamerling JP, Vliegenthart JF. Effects of the N-linked glycans on the 3D structure of the free αsubunit of human chorionic gonadotropin. Biochemistry (Mosc) 2000;39:6012–6021. doi: 10.1021/bi992786n. [DOI] [PubMed] [Google Scholar]
- [101].Leeflang B, Boelens R, Kaptein R, Kamerling J, Vliegenthart J. Mobilities of the inner three core residues and the Man (alpha 1--6) branch of the glycan at Asn78 of the alpha-subunit of human chorionic gonadotropin are restricted by the protein. Biochemistry (Mosc) 1998;37:1933–1940. doi: 10.1021/bi9718548. [DOI] [PubMed] [Google Scholar]
- [102].Slynko V, Schubert M, Numao S, Kowarik M, Aebi M, Allain FHT. NMR structure determination of a segmentally labeled glycoprotein using in vitro glycosylation. J Am Chem Soc. 2009;131:1274–1281. doi: 10.1021/ja808682v. [DOI] [PubMed] [Google Scholar]
- [103].Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- [104].Barb AW, Prestegard JH. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol. 2011;7:147. doi: 10.1038/nchembio.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- [106].Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9-and 2.8-. ANG. resolution. Biochemistry (Mosc) 1981;20:2361–2370. [PubMed] [Google Scholar]
- [107].Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- [108].Wormald MR, Rudd PM, Harvey DJ, Chang S-C, Scragg IG, Dwek RA. Variations in oligosaccharidea protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry (Mosc) 1997;36:1370–1380. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- [109].Yamaguchi Y, Kato K, Shindo M, Aoki S, Furusho K, Koga K, Takahashi N, Arata Y, Shimada I. Dynamics of the carbohydrate chains attached to the Fc portion of immunoglobulin G as studied by NMR spectroscopy assisted by selective 13 C labeling of the glycans. J Biomol NMR. 1998;12:385–394. doi: 10.1023/a:1008392229694. [DOI] [PubMed] [Google Scholar]
- [110].Palmer AG, Kroenke CD, Loria JP. Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Methods Enzymol. 2000;339:204–238. doi: 10.1016/s0076-6879(01)39315-1. [DOI] [PubMed] [Google Scholar]
- [111].Mulder FA, Skrynnikov NR, Hon B, Dahlquist FW, Kay LE. Measurement of slow (μs-ms) time scale dynamics in protein side chains by 15N relaxation dispersion NMR spectroscopy: Application to Asn and Gln residues in a cavity mutant of T4 lysozyme. J Am Chem Soc. 2001;123:967–975. doi: 10.1021/ja003447g. [DOI] [PubMed] [Google Scholar]
- [112].Hansen DF, Vallurupalli P, Lundström P, Neudecker P, Kay LE. Probing chemical shifts of invisible states of proteins with relaxation dispersion NMR spectroscopy: how well can we do? J Am Chem Soc. 2008;130:2667–2675. doi: 10.1021/ja078337p. [DOI] [PubMed] [Google Scholar]
- [113].Sekhar A, Kay LE. An NMR view of protein dynamics in health and disease. Annu Rev Biophys. 2019;48:297–319. doi: 10.1146/annurev-biophys-052118-115647. [DOI] [PubMed] [Google Scholar]
- [114].Boehr DD, McElheny D, Dyson HJ, Wright PE. The dynamic energy landscape of dihydrofolate reductase catalysis. Science. 2006;313:1638–1642. doi: 10.1126/science.1130258. [DOI] [PubMed] [Google Scholar]
- [115].Korzhnev DM, Religa TL, Banachewicz W, Fersht AR, Kay LE. A transient and low-populated protein-folding intermediate at atomic resolution. Science. 2010;329:1312–1316. doi: 10.1126/science.1191723. [DOI] [PubMed] [Google Scholar]
- [116].Neudecker P, Robustelli P, Cavalli A, Walsh P, Lundström P, Zarrine-Afsar A, Sharpe S, Vendruscolo M, Kay LE. Structure of an intermediate state in protein folding and aggregation. Science. 2012;336:362–366. doi: 10.1126/science.1214203. [DOI] [PubMed] [Google Scholar]
- [117].Bouvignies G, Vallurupalli P, Hansen DF, Correia BE, Lange O, Bah A, Vernon RM, Dahlquist FW, Baker D, Kay LE. Solution structure of a minor and transiently formed state of a T4 lysozyme mutant. Nature. 2011;477:111. doi: 10.1038/nature10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Whittier SK, Hengge AC, Loria JP. Conformational motions regulate phosphoryl transfer in related protein tyrosine phosphatases. Science. 2013;341:899–903. doi: 10.1126/science.1241735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sekhar A, Rumfeldt JA, Broom HR, Doyle CM, Bouvignies G, Meiering EM, Kay LE. Thermal fluctuations of immature SOD1 lead to separate folding and misfolding pathways. eLife. 2015;4:e07296. doi: 10.7554/eLife.07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Nikolova EN, Kim E, Wise AA, O’Brien PJ, Andricioaei I, Al-Hashimi HM. Transient Hoogsteen base pairs in canonical duplex DNA. Nature. 2011;470:498–502. doi: 10.1038/nature09775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Dethoff EA, Petzold K, Chugh J, Casiano-Negroni A, Al-Hashimi HM. Visualizing transient low-populated structures of RNA. Nature. 2012;491:724. doi: 10.1038/nature11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhao B, Guffy SL, Williams B, Zhang Q. An excited state underlies gene regulation of a transcriptional riboswitch. Nat Chem Biol. 2017;13:968. doi: 10.1038/nchembio.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kimsey IJ, Szymanski ES, Zahurancik WJ, Shakya A, Xue Y, Chu C-C, Sathyamoorthy B, Suo Z, Al-Hashimi HM. Dynamic basis for dG• dT misincorporation via tautomerization and ionization. Nature. 2018;554:195. doi: 10.1038/nature25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Joao HC, Scragg IG, Dwek RA. Effects of glycosylation on protein conformation and amide proton exchange rates in RNase B. FEBS Lett. 1992;307:343–346. doi: 10.1016/0014-5793(92)80709-p. [DOI] [PubMed] [Google Scholar]
- [125].Joao HC, Dwek RA. Effects of glycosylation on protein structure and dynamics in ribonuclease B and some of its individual glycoforms. Eur J Biochem. 1993;218:239–244. doi: 10.1111/j.1432-1033.1993.tb18370.x. [DOI] [PubMed] [Google Scholar]
- [126].Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 2006;1760:693–700. doi: 10.1016/j.bbagen.2005.10.002. [DOI] [PubMed] [Google Scholar]
- [127].Rickert KW, Imperiali B. Analysis of the conserved glycosylation site in the nicotinic acetylcholine receptor: potential roles in complex assembly. Chem Biol. 1995;2:751–759. doi: 10.1016/1074-5521(95)90103-5. [DOI] [PubMed] [Google Scholar]
- [128].[128] Tagashira M, Iijima H, Toma K. An NMR study of O-glycosylation induced structural changes in the α-helix of calcitonin. Glycoconjugate J. 2002;19:43–52. doi: 10.1023/a:1022532930708. [DOI] [PubMed] [Google Scholar]
- [129].Kirnarsky L, Prakash O, Vogen SM, Nomoto M, Hollingsworth MA, Sherman S. Structural effects of O-glycosylation on a 15-residue peptide from the mucin (MUC1) core protein. Biochemistry (Mosc) 2000;39:12076–12082. doi: 10.1021/bi0010120. [DOI] [PubMed] [Google Scholar]
- [130].Coltart DM, Royyuru AK, Williams LJ, Glunz PW, Sames D, Kuduk SD, Schwarz JB, Chen X-T, Danishefsky SJ, Live DH. Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J Am Chem Soc. 2002;124:9833–9844. doi: 10.1021/ja020208f. [DOI] [PubMed] [Google Scholar]
- [131].Hashimoto R, Fujitani N, Takegawa Y, Kurogochi M, Matsushita T, Naruchi K, Ohyabu N, Hinou H, Gao XD, Manri N. An Efficient Approach for the Characterization of Mucin-Type Glycopeptides: The Effect of O-Glycosylation on the Conformation of Synthetic Mucin Peptides. Chem Eur J. 2011;17:2393–2404. doi: 10.1002/chem.201002754. [DOI] [PubMed] [Google Scholar]
- [132].Madariaga D, Martínez-Sáez N, Somovilla VJ, García-García L, Berbis M Á, Valero-Gónzalez J, Martín-Santamaíra S, Hurtado-Guerrero R, Asensio JL, Jiménez-Barbero J. Serine versus Threonine Glycosylation with α-O-GalNAc: Unexpected Selectivity in Their Molecular Recognition with Lectins. Chem Eur J. 2014;20:12616–12627. doi: 10.1002/chem.201403700. [DOI] [PubMed] [Google Scholar]
- [133].Corzana F, Busto JH, Jiménez-Osés G, García de Luis M, Asensio JL, Jiménez-Barbero J, Peregrina JM, Avenoza A. Serine versus threonine glycosylation: the methyl group causes a drastic alteration on the carbohydrate orientation and on the surrounding water shell. J Am Chem Soc. 2007;129:9458–9467. doi: 10.1021/ja072181b. [DOI] [PubMed] [Google Scholar]
- [134].Corzana F, Busto JH, Engelsen SB, Jiménez-Barbero J, Asensio JL, Peregrina JM, Avenoza A. Effect of β-O-Glucosylation on l-Ser and l-Thr Diamides: A Bias toward α-Helical Conformations. Chem Eur J. 2006;12:7864–7871. doi: 10.1002/chem.200600128. [DOI] [PubMed] [Google Scholar]
- [135].Simanek EE, Huang D-H, Pasternack L, Machajewski TD, Seitz O, Millar DS, Dyson HJ, Wong C-H. Glycosylation of threonine of the repeating unit of RNA polymerase II with β-linked N-acetylglucosame leads to a turnlike structure. J Am Chem Soc. 1998;120:11567–11575. [Google Scholar]
- [136].Wu W-g, Pasternack L, Huang D-H, Koeller KM, Lin C-C, Seitz O, Wong C-H. Structural study on O-glycopeptides: glycosylation-induced conformational changes of O-GlcNAc, O-LacNAc, O-sialyl-LacNAc, and O-sialyl-lewis-X peptides of the mucin domain of MAdCAM-1. J Am Chem Soc. 1999;121:2409–2417. [Google Scholar]
- [137].Culik RM, Sekhar A, Nagesh J, Deol H, Rumfeldt JA, Meiering EM, Kay LE. Effects of maturation on the conformational free-energy landscape of SOD1. Proc Natl Acad Sci U S A. 2018;115:E2546–E2555. doi: 10.1073/pnas.1721022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yao X, Rosen MK, Gardner KH. Estimation of the available free energy in a LOV2-Jα photoswitch. Nat Chem Biol. 2008;4:491. doi: 10.1038/nchembio.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Olivier-Van Stichelen S, Dehennaut V, Buzy A, Zachayus J-L, Guinez C, Mir A-M, El Yazidi-Belkoura I, Copin M-C, Boureme D, Loyaux D. OGlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41. FASEB J. 2014;28:3325–3338. doi: 10.1096/fj.13-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yuwen T, Kay LE, Bouvignies G. Dramatic Decrease in CEST Measurement Times sing Multi-Site Excitation. ChemPhysChem. 2018;19:1707–1710. doi: 10.1002/cphc.201800249. [DOI] [PubMed] [Google Scholar]
- [141].Yuwen T, Huang R, Vallurupalli P, Kay LE. A Methyl-TROSY-Based 1H Relaxation Dispersion Experiment for Studies of Conformational Exchange in High Molecular Weight Proteins. Angew Chem Int Ed. 2019;58:6250–6254. doi: 10.1002/anie.201900241. [DOI] [PubMed] [Google Scholar]
- [142].Vallurupalli P, Tiwari VP, Ghosh S. A Double Resonance CEST Experiment to Study Multistate Protein Conformational Exchange: An Application to Protein Folding. J Phys Chem Lett. 2019;10:3051–3056. doi: 10.1021/acs.jpclett.9b00985. [DOI] [PubMed] [Google Scholar]
- [143].Gibbs E, Kriwacki R. Direct detection of carbon and nitrogen nuclei for high-resolution analysis of intrinsically disordered proteins using NMR spectroscopy. Methods. 2018;138:39–46. doi: 10.1016/j.ymeth.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kazimierczuk K, Orekhov V. Non-uniform sampling: post-Fourier era of NMR data collection and processing. Magn Reson Chem. 2015;53:921–926. doi: 10.1002/mrc.4284. [DOI] [PubMed] [Google Scholar]