Abstract
Background & Aims
Immunoglobulin (Ig)A contributes to the homeostatic balance between host and the intestinal microbiota. Mechanisms that initiate the IgA response are unclear and likely to differ between humans and animal models. We used multiple experimental approaches to investigate the origin of the human intestinal plasma cells that produce IgA in the gastrointestinal (GI) tract.
Methods
The complexity of IgA-producing plasma cell populations in human GI mucosa and bone marrow and the specific response to oral cholera vaccine were compared by analysis of Ig genes. Flow cytometry, gene expression analysis, and immunohistochemistry were used to analyze signaling pathways induced by B-cell receptor (BCR) engagement in human gut-associated lymphoid tissue (GALT) and the involvement of innate immunity in B-cell activation in GALT, compared with non-intestinal sites.
Results
Human intestinal IgA-producing plasma cells appeared to be of germinal center origin; there was no evidence for the population complexity that accompanies the multiple pathways of derivation observed in bone marrow. In germinal center B cells of human GALT, Btk and Erk are phosphorylated, CD22 is downregulated, Lyn is translocated to the cell membrane, and Fos and Jun are upregulated; these features indicate BCR ligation during germinal center evolution. No differences in innate activation of B cells were observed in GALT, compared with peripheral immune compartments.
Conclusion
IgA-producing plasma cells appear to be derived from GALT germinal centers in humans. BCR engagement promotes formation of germinal centers of GALT, with no more evidence for innate immune receptor activation in the mucosa than non-intestinal immune compartments. Germinal centers in GALT should be the targets of mucosal vaccinations because they are the source of the human intestinal IgA response.
Keywords: Immunity, BCR, immunization, lymphocytes, antibodies
Background and Aims
Maintenance of the homeostatic equilibrium between the intestinal microbiota and the human host requires the secretion of copious amounts of the mucosal immunoglobulin IgA into the gut lumen daily. Failure to generate and transport a diverse IgA response into the lumen results in potentially pathogenic imbalance in the bacterial species in the gut and penetration of luminal antigens into the systemic compartment1–3.
Intestinal IgA is thought to provide two-tiered protection. IgA includes cross-reactive antibody that can bind sufficiently well but with low affinity to the commensal bacteria, mediating agglutination of luminal antigens and immune exclusion4 and an antigen specific component. IgA with specificity for lumenal bacterial strains has recently been demonstrated5. This diversity in function is consistent with two potential pathways to IgA production in animal models. In mice, the production of cross-reactive IgA has been associated with the self-renewing peritoneal B1 B cell lineage that has no direct homology in humans6,7. Microanatomically, the murine B1 B cell component of the IgA response has been localised to the diffuse connective tissue microenvironment of the lamina propria that has otherwise classically been considered to be the site of expression of the immune response where antibodies and cytokines are secreted8. This has resulted in considerable uncertainty over the relative importance of the gut-associated lymphoid tissue (GALT), consisting of Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) and the lamina propria as inductive sites for mucosal immune responses9.
In contrast, it is widely accepted that the antigen specific IgA response originates from the germinal centres (GCs) of PPs and ILFs10,11. However, unlike GC formation in the periphery, it has been recently suggested that GC formation in GALT, can be driven in absence of B cell receptor (BCR) involvement12, but through recognition of microbe-associated molecular patterns (MAMPs) of the intestinal microbiota, by innate receptors on B cells such as the toll-like receptors (TLRs)13.
Here we show that the human intestinal IgA+ plasma cell population as a whole has the hallmarks of GC origin, with no evidence of two pathways. We observe that activation of the B cell receptor is involved in the GC response in human PPs with no evidence of preferential TLR activation at this site. This study places PPs and specific antigen-dependent GCs at the heart of the human intestinal plasma cell response and shows unambiguously that the PPs and, by inference, the follicle-associated epithelium at the boundary between the lumen and the GALT, are the sites to target for protective human intestinal IgA responses.
Material and Methods
Bone marrow specimens
Bone marrow donors were 3 females aged 53, 60 and 66 years undergoing clinical investigation; two for lymphoma and one for thrombocytosis. All were found to have normal bone marrow specimens. 5ml samples were taken with informed consent and approval of the local Research Ethics Committee.
Cholera toxin vaccination and anti-CTB antibody detection
3 healthy volunteers were administered whole cell heat killed oral cholera vaccine containing recombinant cholera toxin B subunit (WC-rCTB (Dukoral) on 2 occasions, with ethics committee approval. Blood was taken at day 0, 7, 8, 14 and day 8 post booster. ELISA was used to assess the titres of cholera toxin B subunit (CTB)-specific IgA. Total PBMCs were isolated prior to immunisation and on days 8 and 14 after booster.
Isolation of blood and bone marrow mononuclear cells
Bone marrow and peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Paque centrifugation.
Peyer’s Patches sample isolation and single cell suspension
PP biopsies were taken from patients undergoing colonoscopy who had no signs of cancer or intestinal inflammation with informed consent and approval of the local Research Ethics Committee. Biopsies were processed as described previously14.
Study of lamina propria mononuclear cells
Surgical specimens were studied with informed consent and approval of the local Research Ethics Committee. Colonic mucosa was sampled from colectomy specimens removed at surgery for colon cancer, distant from the site of the tumour. Lamina propria mononuclear cells (LPMCs) were released from mucosa by collagenase digestion as described previously14.
Fluorescence-activated cell sorting (FACS)
To isolate bone marrow and lamina propria IgA plasma cells, cells were immunostained by direct immunofluorescence with RPE-conjugated anti-CD38 (1/50). Cells were permeabilised with an IntraStain kit (DAKO, Ely, UK) and incubated with FITC-conjugated anti-IgA. Surface CD38+, cytoplasmic IgA+ plasma cells were sorted with a MoFlo flow cytometer (Beckman Coulter) (Fig. S1). Single lamina propria plasma cells were sorted as described previously15. Blood mucosal IgA+ plasma cell precursors (cytoplasmic IgA+, CD79b+, α4β7hi) were isolated from the blood after Ficoll centrifugation. If required, biotinylated CTB was added to the intracellular staining and detected by avidin-APC. Specific CTB binding cells were sorted by flow cytometry. Cellular binding of CTB through GM1 was excluded by gating with the flow cytometer with pre-immunisation blood. To isolate mature mucosal homing and naïve B cells from single donor buffy coats, cells were immunostained for CD79 APC, IgD FITC, beta7 PECy5, and CD27 (antibodies used at 20ul per million cells in 100ul).
To isolate GC, marginal zone and mantle zone B cells, isolated PP cells were immunostained for CD79b-APC, CD10-FITC, IgD-PE, CD19-Pacific Blue (AbD Serotec) (the antibodies were used at the recommended concentration of 20ul per million cells in a total of 100ul). After immunostaining, cells were washed and resuspended in sterile PBS and immediately separated into different sub-populations with the BD FACSAria I cell sorter.
PCR amplification of IGL, IGK, IGH, cloning and DNA sequencing
Prior to IG gene analysis, sorted PBMCs and plasma cell populations were pelleted. Microdissected fragments of ileum from tissue sections confirmed to be free of lymphoid tissue were pelleted. DNA was released as described previously15.
Rearrangements of IGL and IGK were PCR amplified from single cells, IGLV1-J and IGLV2-J rearrangements from microdissected lamina propria and bone marrow plasma cells were PCR amplified, cloned, sequenced and analysed as described previously15. IGHV gene repertoire analysis of unsorted PBMCs at day 14 after the primary immunisation with the Dukoral vaccine was carried out by deep sequencing as described16.
A sequence was considered to be in-frame if the IGLJ segment was in the same reading frame as V across the VJ junction. Rearrangements with the same CDR3 were regarded as a single rearrangement. Sequences analyzed are accessible from EMBL/Genbank under accession numbers (FN550158–FN550364, AJ582973–AJ582987, AJ399965–AJ99969, AJ972151–209, AJ972210–AJ972375, AJ578180–AJ578199, AJ578201–AJ578211).
Gene expression profile analysis
Sorted PPMC B cells populations from 4 different patients were collected in nucleotide free tubes containing sterile PBS, pelleted and lysed with SuperAmp buffer (Miltenyi Biotec, Germany) before storage at -20. Tubes were sent on dry-ice to Miltenyi Biotech (Germany) for RNA isolation, quantitation and whole genome gene expression analysis. Results were further analyzed and compared by statistical analysis as mentioned below. Differences in gene expression were presented as fold difference as compared to average gene expression level in GCs (GC=1).
Intracellular phospho-staining for flow cytometry
Isolated PP cells were fixed immediately after isolation by adding paraformaldehyde directly into the culture medium to a final concentration of 1.5% and incubated at RT for 10 min. Cells were permeabilised by resuspending them in ice-cold 100% methanol and incubated on ice for 10 min. Cells were washed and resuspended in PBS/1% BSA. Fluorochrome-conjugated mAbs (p-Btk-Alexa Fluor 647(pY551) and p-Erk1/2-Pacific Blue (pT202/pY204) both from BD Bioscience, were added (the antibodies were used at the recommended concentration of 20ul per million cells in a total of 100ul). Cell surface immunostaining and intracellular phospho-staining was performed simultaneously for 25 min at RT. Cells were washed twice and immediately analysed. Flow cytometry was performed with the LSR II (Becton Dickinson) and data were analysed with FACS Diva software.
Frozen samples
Specimens of surgically resected ileum (n=8), tonsil (n=4) and spleen (n=1) were obtained from a collection of frozen, anonymous, histologically normal human tissues. They were studied with research ethics committee approval.
Immunohistochemistry
All antibodies and reagents were purchased from Dako (Cambridge, UK), unless otherwise stated. Frozen tissue sections (5 μm) were either fixed in 4% paraformaldehyde for 20 min and subjected to antigen retrieval by heating in Dako Target Retrieval solution at 95°C for 10 min, or fixed in ice-cold acetone for 20 min. After endogenous peroxidase blocking, single immunostaining was performed with anti phospho-Btk (Cell Signaling 1/100), TLR9 (1/100 Cell Signaling), CD21 (1/20 Dako), IRF-7 (Cell Signaling 1/100), followed by appropriate secondary antibodies and EnVision single immunostaining systems as previously described17. After immunostaining, sections were counterstained with hematoxylin. Isotype- and concentration-matched control antibodies were used as negative controls. Sections of human tonsil were immunostained to provide a positive control.
Microdissection
Laser capture microdissection was performed on Cresyl-violet (0.1% in ethanol)-stained cryosections of PPs (n=7), tonsil (n=5), and splenic GC (n=1) as previously described18.
mRNA isolation, quantitative RT-PCR
Messenger RNA (mRNA) was isolated, reverse transcribed and analysed by quantitative RT-PCR as previously described18. Primers and probes for TLR9, TLR4, IRF-7 were purchased from Applied Biosystems and were quantified with GAPDH as an endogenous control as previously described with an ABI PRISM 7900HT instrument. Results were analyzed with the ABI PRISM 7900HT Sequence Detection System Version 2.1 (SDS 2.3). Ct values were used for statistical analysis. For each gene, preliminary experiments to set a common threshold were performed.
Statistical analysis
Data were analyzed with either unpaired two-sided t-tests with Welch correction or Mann-Whitney U test with GraphPad Prism version 3.03 for Windows (GraphPad Software, San Diego, CA). A p value of < 0.05 was considered statistically significant. Comparisons of expected and observed numbers of in-frame and out-of-frame rearrangements were made with numbers rather than percentages with χ2 analysis in an excel spreadsheet.
Results
A single population of plasma cells is present in the intestinal mucosa
Population complexity in the human IgA response was studied by IG gene analysis. We initially considered two features of lambda light chain gene rearrangements (IGL); somatic hypermutation and biases in IGL reading frame.
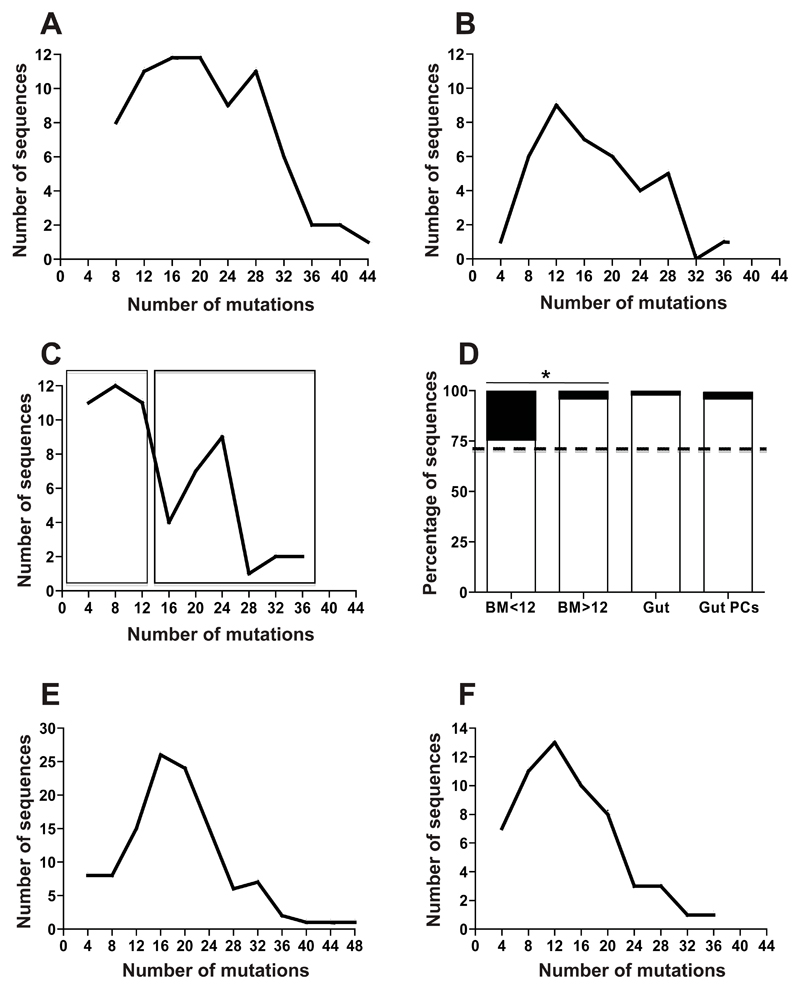
DNA was extracted from human small intestinal lamina propria isolated by microdissection from tissue sections of ileum (n=3) confirmed microscopically to be free of lymphoid tissue, and from single plasma cells derived from isolates of colonic LPMC (n=2). IGL were PCR amplified from these samples, cloned and sequenced. Human LPMCs and isolated plasma cells had a high mean frequency of somatic mutations in their immunoglobulin lambda variable (IGLV) genes, and mutational frequencies consistent with a normal distribution (Fig. 1A and B, and19, 20). DNA was also extracted from populations of IgA+ plasma cells isolated by FACS from bone marrow aspirates (n=3) (representative FACS plot in Fig S1). In contrast to intestinal plasma cells, the distribution of mutations in IGL gene rearrangements from bone marrow IgA+ plasma cells had two peaks in frequency of mutation (Fig. 1C), potentially identifying bone marrow plasma cells of peripheral and mucosal origin, as described in a recent report21. PCR error was 0.02% and did not contribute significantly to these data.
Figure 1. Mucosal plasma cells comprise a single dominant population.
Charts illustrating the numbers of IGLV sequences (vertical axis) with different numbers of point mutations acquired by somatic hypermutation. Sequences are grouped on the horizontal axis as those with 0-4, 5-8, 9-12 etc mutations. Points represent the total number of sequences in the sampled population with the specified number of mutations. A. Mutations in rearrangements of IGLV1 and IGLV2 from plasma cells sampled from sections of ileal lamina propria from 3 individuals checked microscopically to be free of lymphoid tissue. B. Mutations in IGLV from single isolated colonic IgA+ plasma cells from 2 individuals. The distribution of mutations illustrated in A and B approximates to a single curve. C. Mutations in IGLV1 and IGLV2 used by IgA plasma cells from bone marrow from 3 individuals show 2 peaks in frequency of somatic hypermutation. D. Ratios of productive (white bar) to non-productive (black bar) rearrangements of IGLV1 and IGLV2. Sequences analysed were: BM<12 (IGLV1 and IGLV2 from bone marrow plasma cells with 12 mutations or fewer, as boxed in C), BM>12 (IGLV1 and IGLV2 from bone marrow plasma cells with more than 12 mutations as boxed in C), Gut (rearrangements of IGLV1 and IGLV2 from microdissected lamina propria cells shown in A) and Gut PCs (rearrangements of IGLV1 and IGLV2 from single isolated lamina propria plasma cells in B). Expected 71% in-frame is indicated with a dotted line. The ratio of in-frame: out of frame rearrangements of IGLV1 and IGLV2 in bone marrow plasma cells is not biased away from expected. However, there is a significant bias away from expected 71% in-frame rearrangements in the more mutated sequences from bone marrow, Gut and Gut PC groups (p=0.02, p<0.001 and p=0.01 respectively) and a significant difference between the more and less mutated subsets of IGLV1 and IGLV2 bone marrow plasma cells (p=0.03). E. Frequency of mutations in IGHV from blood-borne IgA+, α4β7hi cells from 3 individuals F. Mutations in IGHV from GC derived, blood-borne IgA+, α4β7hi B cells that bind CTB after an oral vaccination of 3 individuals with vaccine containing CTB display similar features to those from pre-immune blood not selected for any specificity.
In addition to a high mean mutation frequency, human intestinal plasma cells have a distinctive bias in the ratio of productive to non-productive IGL rearrangements15. Rearrangements that involve the IGLV1 and IGLV2 families of IGLV gene segments in the mucosal plasma cell population are almost all in the correct genetic reading frame. In contrast, rearrangements of IGLV1 and IGLV2 in naïve B cells approximate to the expected 71% in-frame rearrangements15 and Fig. S2). We divided 59 gene rearrangements involving IGLV1 and IGLV2 derived from bone marrow plasma cells into two groups according to the number of mutations in the variable regions; those with 12 mutations or fewer and those with more than 12, as illustrated by boxes in Fig. 1C. We observed that the IGLV1-J and IGLV2-J gene rearrangements from bone marrow plasma cells with the lower range of mutational frequency comprised 76% productive rearrangements (not significantly different to the expected 71%). However, 96% of the sequences with the higher mutational frequencies were in the correct genetic reading frame (significantly more than both expected [p=0.02] and than the group with lower mutational frequency [p=0.03]), consistent with the properties of mucosal plasma cells (Fig. 1D).
Therefore combined analysis of mutational frequency and the genetic reading frame of light chain gene rearrangements enabled identification of a mixed plasma cell population in bone marrow and, consistently, a single population of intestinal lamina propria plasma cells.
IgA response to oral cholera vaccination shows hallmarks of a GC response, and is indistinguishable from the IgA response in general
The high mean frequency of mutations in IGV genes characteristic of intestinal plasma cells and blood-borne mucosal IgA+ plasma cell precursors suggests that they are derived from GCs. To determine whether a GC derived antigen specific IgA response would appear different to the IgA response in general, we analysed immunoglobulin heavy chain variable region gene (IGHV) sequences contributing to a specific IgA response to CTB following oral immunisation with the whole cell killed oral cholera vaccine containing recombinant CTB (WC-rCTB). CTB is a well-characterised T-cell dependent B cell antigen22 known to stimulate an IgA response through the GALT, and was used as a positive control to identify the hallmarks of a human mucosal GC response. Mucosal IgA+ plasma cell precursors were isolated from the blood of three healthy volunteers before oral immunisation. The increase in titre of specific antibody following oral immunisation was tracked (Fig. S3) and on day 8 following the booster immunisation, CTB binding and non-binding mucosal IgA+ plasma cell precursors were sorted by FACS (Fig. S3). IGHV genes from sorted cells were amplified by PCR, and the PCR products cloned and sequenced. An example of clonal evolution of the anti-CTB response by acquisition of mutations characteristic of a GC response was observed (Fig. S4).
We observed no difference in the V, D or J usage between CTB binding and non-binding mucosal IgA+ plasma cell precursors from the 3 individuals studied. The curves that illustrate the mutational frequency in the CTB binding and non-binding sorted populations had similar profiles to each other (Fig. 1E and F) and to the profiles of mutation in IGL derived from intestinal lamina propria plasma cells and their immediate precursors (Fig 1A and B). Thus, the GC derived specific CTB response was indistinguishable from the mucosal IgA+ response whether analysed as microdissected ileal lamina propria cells, isolated plasma cells from colon, or circulating cells expressing high αβ and cytoplasmic IgA, demonstrating a common GC origin.
BCR activation in Peyer’s Patch GCs
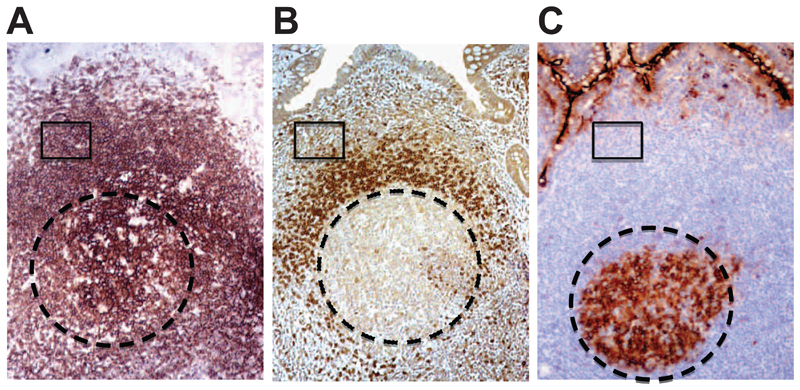
Three major microanatomically defined B cell compartments can be identified histologically in human PPs; GC, mantle zone and marginal zone (Fig. 2 A-C, and Fig. S5). These B cell populations were identified by FACS for the first time in this study with the following combination of antibodies: PP GC B cells (CD79b+,IgD−, CD10+), PP mantle zone B cells (CD79b+,IgD+,CD10−) and PP marginal zone B cells (CD79b+,IgD−,CD10−) (Fig.3A).
Figure 2. B cell microenvironments in the Peyer’s Patches.
Sequential sections of PPs illustrating the organization of B cell populations. A. CD20 (brown) is expressed by the majority of B cells in the PPs, B. naïve B cells in the mantle express IgD, while marginal zone B cells, representing the largest population of B cell in the PPs are IgD-. C. GC B cell express CD10, while both marginal zone and mantle zone are CD10-. Circle indicates the GC areas, while boxes illustrate an area in the CD20+IgD-CD10- marginal zone. Original magnification 100x.
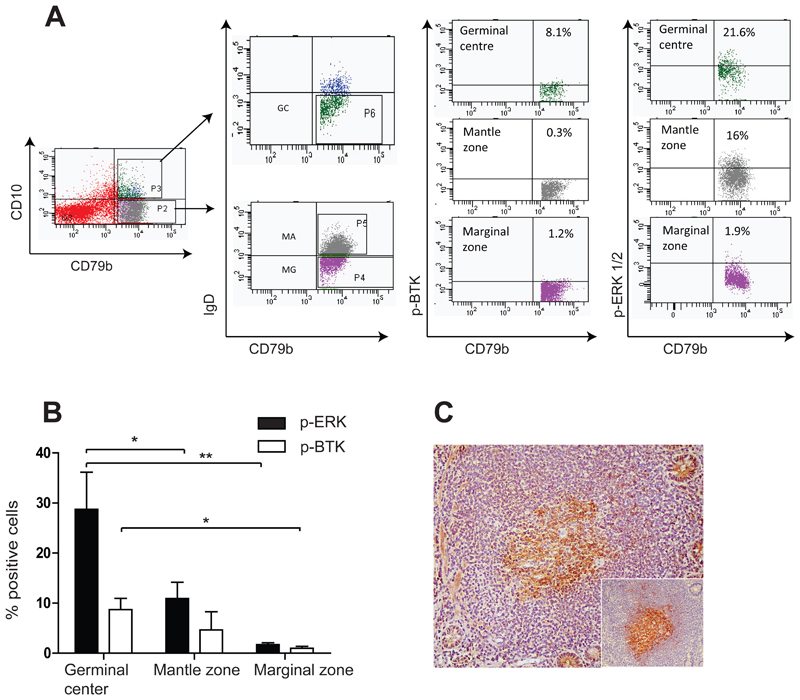
Figure 3. Btk and Erk are phosphorylated in PP GC B cells.
A. Example of flow cytometry scatter plot on human PP B cells showing immunostaining with anti-phosphorylated Btk (p-Btk) and phosphorylated Erk (p-Erk). Subsets of isolated B cells (CD79+) were identified on the basis of CD10 and IgD expression. GC cells (IgDCD10+) showed increased expression of phosphorylated Btk and Erk compared with mantle zone (IgD+CD10-) and marginal zone (IgD-CD10-) B cells. B. Diagram illustrating summary of replicate experiments with cells from 4 different individuals, immunostained with the antiphospho antibodies, demonstrating significantly increased p-Erk expression (p=0.05 and p=0.03 for GC v.s mantle zone and GC v.s. marginal zone, mean +SD) and increased p-Btk expression in GC B cells. C. Photomicrograph illustrating p-Btk expression within PP GCs (Original magnification 100x).
We evaluated the activation state of the proteins involved in the BCR signalling cascade in GC, mantle zone and marginal zone B cells from human PPs by flow cytometry (Fig. 3A). PP GC B cells consistently showed higher percentage of cells with phosphorylated Btk and Erk compared to mantle and marginal zone B cells (Fig. 3A and B), demonstrating preferential BCR engagement in this population. This was further validated by immunohistochemistry, which identified phosphorylated Btk in GCs of PPs (Fig. 3C).
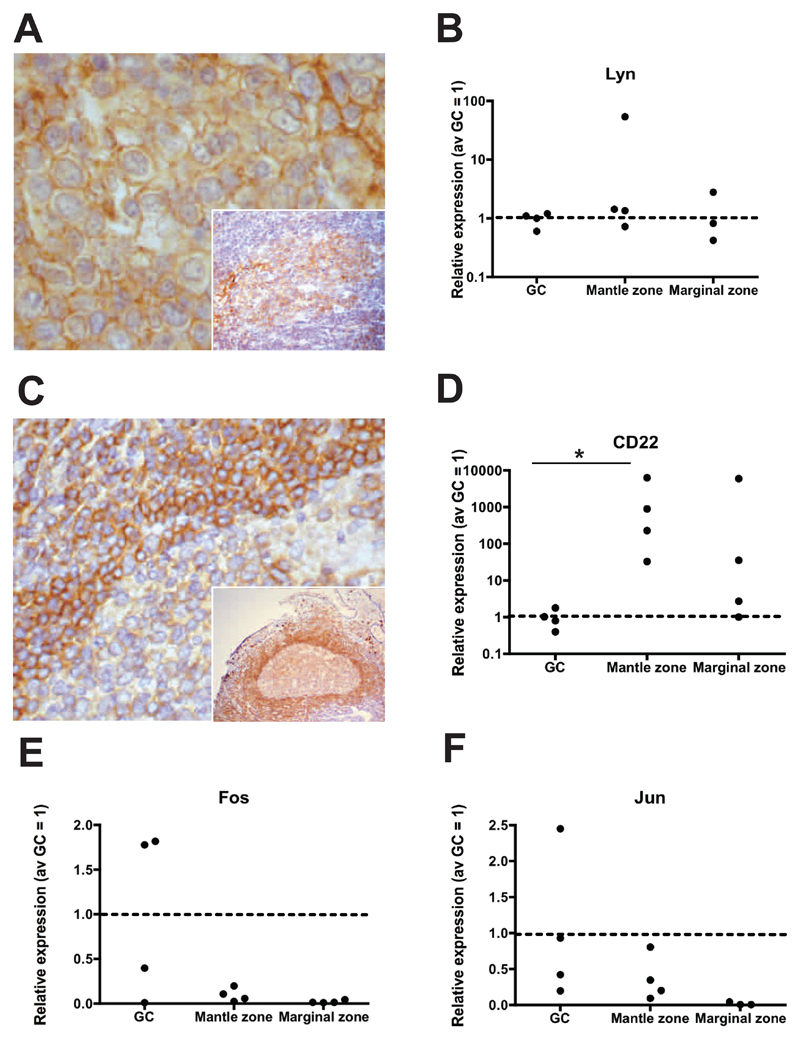
BCR activation is initiated by several src-family kinases such as Lyn, Fyn and Blk. However, only Lyn can both activate and uniquely attenuate BCR signalling, providing a negative feedback loop. Upon BCR ligation, Lyn translocates to the cell membrane and phosphorylates the immunoreceptor tyrosine-based activation motif regions of alpha and beta chains of the BCR and the immunoreceptor tyrosine-based inhibition motif regions of inhibitory receptors, such as CD22. CD22 is subsequently down-regulated as B cells enter the GC response. Translocation of Lyn to the cell membrane was visualised in the GCs of PPs in sections immunostained with anti-Lyn antibody (Fig. 4 A and inset). By gene expression analysis we confirmed that Lyn was expressed at similar levels in the three B cell populations (Fig. 4B). Consistent with BCR ligation down-regulation of CD22 in PP GC was observed by immunohistochemistry (Fig.4C). Accordingly gene expression analysis showed significant down-regulation of CD22 in isolated GC B cells (p=0.03) (Fig. 4D). Jun and Fos comprise the AP1 transcription factor complex that is transcriptionally upregulated upon BCR crosslinking and internalization (Fig. 4E and F). By analysis of gene expression, we observed a tendency for raised Jun and Fos expression in PP GC B cells, further supporting the findings above.
Figure 4. Signature of BCR activation in PP GCs.
A. Photomicrograph illustrating Lyn translocation to the cell membrane, in keeping with Lyn activation upon BCR engagement by antigen (and inset higher magnification). B. Relative quantitation of Lyn gene expression (4 individuals studied) by isolated GC (IgD-CD10+), mantle zone (IgD+CD10-) and marginal zone (IgD-CD10-) cells. Data is represented as relative quantitation normalized to average GC=1 (red dotted line). B cells isolated from PPs show no significant difference in Lyn mRNA expression in the three populations. C. IHC on PP GC showing low protein expression in the PP GCs immunostained with anti-CD22 monoclonal antibody, as compared with the mantle or marginal zones (and inset lower magnification). D. Accordingly, significant down-regulation of CD22 transcription in PP GCs was observed (p=0.03 GC vs. mantle zone). (Original magnification 200x in A and C and 100x in inset). E and F. Isolated PP GC cells show increased transcription of the BCR regulated genes, Jun and Fos.
No evidence for involvement of TLRs in the activation of B cells in human PPs
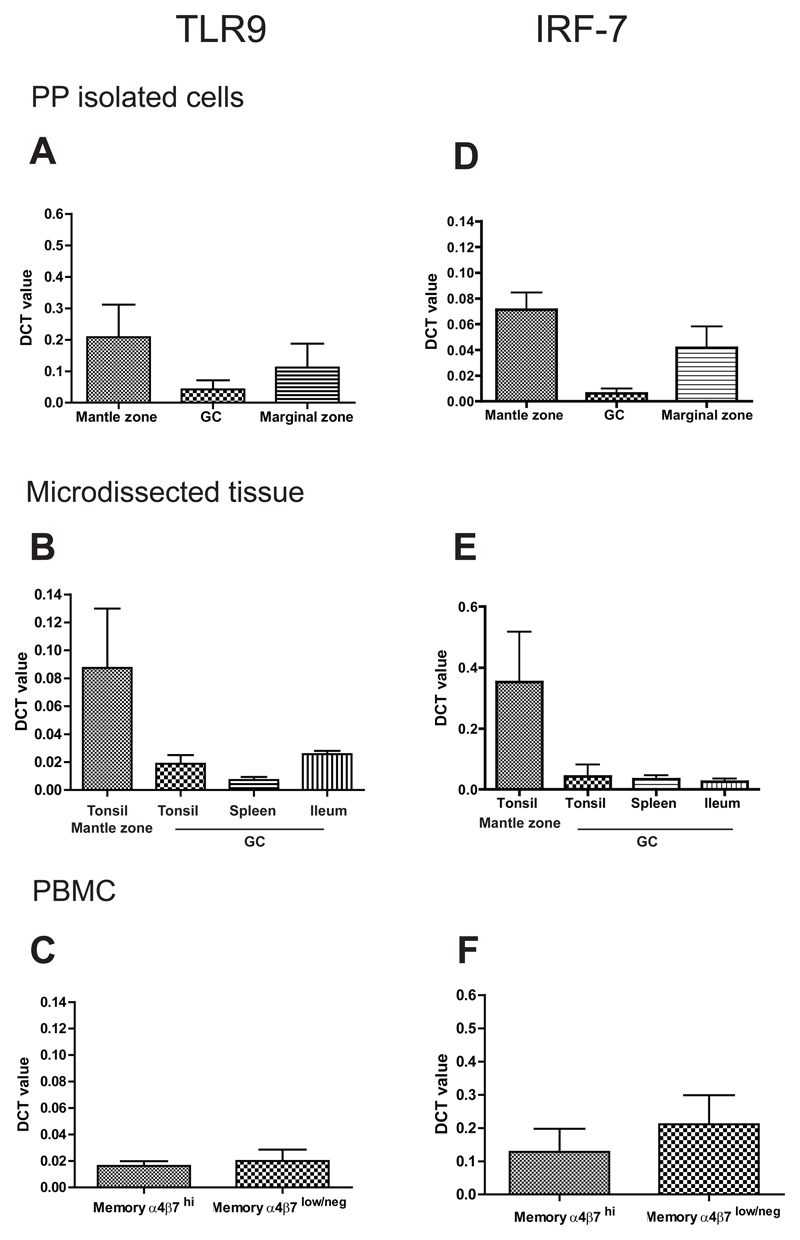
It has been suggested that germline-encoded receptors such as TLRs may be involved in the activation of B cells and formation of GC in the gut, as a distinctive feature of intestinal B cell responses. Gene expression analysis performed on B cell subsets isolated from PPs did not identify any differential expression of TLR genes (TLR9, TLR4, TLR5 and TLR7) or molecules transcriptionally regulated upon TLR involvement in any PP microanatomical compartments. TLR9 expression was investigated in more detail because there is convincing evidence that TLR9 is involved in human B cell activation23. TLR9 mRNA expression was quantified in isolated PP GC, mantle and marginal zone B cells Fig. 5A; for sorting strategy see Fig. 3A), laser capture microdissected tonsil mantle zone and GC, spleen GC and PP GC (Fig. 5B) and blood-borne CD27+ memory cells associated with mucosal (α4β7hi) and peripheral (α4β7lo/-) immunity (Fig. 5C). There was no evidence of increased TLR9 expression in the isolated cells from PP GC, microdissected tonsil GC, PP GC and spleen GC as compared to mantle or marginal zone isolated cells (Fig. 5A and B). TLR9 mRNA expression level did not differ significantly in circulating memory B cells with mucosal or non-mucosal phenotype (α4β7hi or α4β7lo/- respectively) (Fig. 5C).
Figure 5. No difference in TLR9 or IRF-7 expression in the GCs of Peyer’s Patches compared to GC from other lymphoid tissues.
Relative quantitation (DCT) of mRNA expression levels for TLR9 (A, B, C) in A. B cell subsets isolated from PP (GC, mantle and marginal zone; n=9 individual donors),B. microdissected areas of tonsils (GC and mantle zone n= 5 different donors), PP GCs (n= 7 individual donors) and spleen GCs (one donor). C. isolated mature mucosal (IgD-CD27+α4β7hi) and non mucosal (IgD-CD27+aα4β7lo/-) cells (n= 6 individual donors), showing no significant up-regulation of TLR9 transcript in GC B cells isolated from PP or microdissected tissue or mucosal B cells. D. Relative quantitation (DCT) of mRNA for IRF-7 in the same subsets analyzed for TLR9 (n=6 individual donors for each subset analyzed) showing lack of induction of IRF-7 gene in GC cells isolated from PP, E. microdissected GCs and F. sorted blood mucosal memory cells.
Since the level of TLR9 expression may not reflect activation status, we analysed the expression of IRF7, an intracellular mediator of TLR9 signalling. Upon TLR9 activation, IRF7 is regulated both at the transcriptional and post-transcriptional level, translocating to the nucleus upon activation. Quantitative RT-PCR analysis of IRF7 transcripts did not show signs of IRF-7 upregulation in PP GC, nor significant differential IRF-7 expression in PP and tonsil or spleen GC (Fig. 5D and E) or between α4β7hi and α4β7lo/- B cells (Fig. 5F). IRF7 expression was similar to the expression of TLR9 throughout.
Immunohistochemical analysis of PP and tonsil GCs consistently demonstrated the presence of TLR9 expression with similar staining intensity and distribution in both sites (Supplementary figure 6). Similarly, PP tissue sections immunostained with anti IRF-7 antibody did not show significant difference in the distribution and percentage of IRF-7 positive cells in the PP GC as compared to tonsil GC (data not shown).
Therefore although there appears to be differential expression of TLR9 between lymphoid microanatomical compartments, there is no difference in TLR9 expression between comparable compartments in the intestine, tonsil and spleen and no evidence for differential engagement of TLR9 in PPs.
Discussion
We have shown that in healthy humans, despite the apparent functional divergence of the IgA response into cross-reactive ‘natural’ IgA and specific IgA, intestinal IgA plasma cells are in fact components of a single dominant population generated in the GCs of GALT via a mechanism that involves activation of the BCR. This should reinforce the drive to target vaccines to GALT in order to induce T cell-dependent B cell responses and GC formation for effective mucosal immunization.
Several features of IG gene rearrangements carried by intestinal plasma cells unite them as a single population and also discern them from those associated with peripheral immunity. Notably, human intestinal plasma cells have a high average frequency of somatic mutations and IGV genes of human intestinal plasma cells are all mutated15. If mutations are acquired outside the GC, this occurs at low frequency24. In this respect, the human intestinal plasma cell population is markedly different to that of mice that includes cells with low or unmutated rearrangements of VDJ13. This feature has been linked to the murine B1 B cell lineage that has no direct equivalent in humans. Also, human intestinal plasma cells and their precursors have a distinctive profile of IGL. Rearrangements involving the IGLV1 and IGLV2 families are almost exclusively in the correct genetic reading frame in intestinal plasma cells but not the subsets of bone marrow IgA+ plasma cells with lower mutational frequency, peripheral B cells or circulating IgG expressing cells (15 and Fig. S2). These features unify the human intestinal plasma cell population as a separate and distinct entity, unlike the intestinal plasma cells in animal models.
The frequency and distribution of mutations in rearrangements of VDJ in the IgA in response to vaccination with WC-rCTB resembled that observed in the intestinal IgA+ plasma cell population as a whole. A single example of acquisition of mutations in a WC-rCTB specific cell over time was consistent with the dynamics of a normal GC response. Indeed, the cellular proliferation required to accumulate the commonly observed high frequency of mutations in VDJ in intestinal plasma cells and their precursors is only observed in a GC microenvironment, and not in other sites that have been linked to induction of IgA including the lamina propria25. The close resemblance between the response to CTB and the IgA response as a whole is again consistent with GC origin of the human intestinal IgA response.
PP GC B cells show definitive features of BCR activation; margination of Lyn, down-regulation of the BCR inhibitor CD22, phosphorylation of Btk and Erk and transcriptional up-regulation of Fos and Jun, demonstrating that the BCR is involved in human PP GC formation. Btk phosphorylation mediates expression of AID and T-bet in BCR-stimulated cells, both events linked to B cell activation26. We have previously described AID expression in GALT18, consistent with the observations reported in the current study that could support the generation of the high number of somatic mutations in IGV genes. However, intestinal AID expression is not restricted to GCs of GALT and the significance of large interfollicular B cells that express AID is not known. GC independent, AID mediated class switch to IgA in HIV infection and CD40 ligand deficiency have been observed though whether these pathways make a significant contribution in healthy GALT remains unclear.
One previous study has proposed that PP GC in mice form independently of BCR signalling stating that “gut-associated lymphoid tissue (GALT) can be driven into GCs through the interaction of innate immune receptors with intestinal microbial antigens, in the absence of BCR engagement”12. However, this study that is widely cited in this context, substitutes the requirement for BCR for survival with engineered expression of a latent membrane protein of Epstein Barr Virus and does not exclude the involvement or requirement for BCR ligation12.
The proposition that innate receptors contribute directly to human intestinal B cell activation directly has scant evidence to support it, other than the local abundance of MAMPs that could support such activity. In terms of hierarchy, TLR9 signalling is dependent on BCR activation, since B cells cannot internalize antigens by pinocytosis28. Engagement of TLR9 by CpG has the potential to reduce the threshold for BCR mediated B cell activation downstream of antigen uptake and this is therefore an important possibility to consider29. Nonetheless, we saw no evidence for differential TLR expression in mucosal B cell subsets. We saw no difference in TLR9 expression between equivalent populations in the tonsil or spleen and the gut or any evidence of differential transcriptional up-regulation of the IFN-regulatory gene IRF-7 that is regulated at transcriptional level and phosphorylated in the endosomal compartment upon TLR9 engagement by unmethylated DNA30. Therefore, although involvement of innate receptors is an interesting proposition, there is no evidence to support their preferential or differential use in the human intestinal plasma cell response. A model in which the threshold for BCR activation is reduced either through the properties of mucosal B cells or T cells or as a consequence of antigen excess, could alone explain the ability of GALT GCs to support both the natural IgA and specific IgA components of the PP derived human intestinal IgA+ plasma cell response.
We have previously identified clonally related cells in human PP GCs and ileal lamina propria demonstrating the existence of this pathway of plasma cell derivation in humans31. Here we show that B cells in GCs of PP, driven through the BCR, are the major source of human intestinal plasma cells with no evidence of dual origin, or preferential role of innate receptors expressed by B cells.
Supplementary Material
Acknowledgements
This work was funded by projects grants from the BBSRC (Grant BB/E000371/1 funding FB), MRC (funding AV), Guy’s and St Thomas’ Charity (funding LB) and the Wellcome Trust (Grant 074576 funding WS). We thank Sandra Martins and Gary Warnes for their help with the cell sorting and Tom MacDonald for collaboration.
Abbreviations
- BCR
B cell receptor
- CTB
Cholera toxin B submunit;
- GALT
gut-associated lymphoid tissue
- GC
germinal center
- IGK
immunoglobulin kappa light chain
- IGL
immunoglobulin lambda light chain
- ILF
isolated lymphoid follicle
- LPMC
lamina propria mononuclear cell
- MAMPs
microbe-associated molecular patterns
- PBMC
Peripheral blood mononuclear cell
- PP
Peyer’s patch
- PPMC
Peyer’s patch mononuclear cell
- TLR
toll-like receptor
- WR-rCTB
whole cell killed oral cholera vaccine containing recombinant CTB.
Footnotes
The authors declare that there is no conflict of interest.
Involvement of authors in study:
Francesca Barone and Anna Vossenkamper: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Laurent Boursier, Wen Su, Alan Watson, Deborah K. Dunn-Walters, Sonali Wijetilleka: acquisition of data; analysis and interpretation of data.
Susan John, Paul Fields: analysis and interpretation of data; obtained funding; technical and material support
Jonathan D. Edgeworth and Jo Spencer: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
References
- 1.Johansen FE, Pekna M, Norderhaug IN, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagarasan S, Muramatsu M, Suzuki K, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–7. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 3.Braathen R, Sandvik A, Berntzen G, et al. Identification of a polymeric Ig receptor binding phage-displayed peptide that exploits epithelial transcytosis without dimeric IgA competition. J Biol Chem. 2006;281:7075–81. doi: 10.1074/jbc.M508509200. [DOI] [PubMed] [Google Scholar]
- 4.Walker WA, Isselbacher KJ, Bloch KJ. Immunologic control of soluble protein absorption from the small intestine: a gut-surface phenomenon. Am J Clin Nutr. 1974;27:1434–40. doi: 10.1093/ajcn/27.12.1434. [DOI] [PubMed] [Google Scholar]
- 5.Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroese FG, Ammerlaan WA, Kantor AB. Evidence that intestinal IgA plasma cells in mu, kappa transgenic mice are derived from B-1 (Ly-1 B) cells. Int Immunol. 1993;5:1317–27. doi: 10.1093/intimm/5.10.1317. [DOI] [PubMed] [Google Scholar]
- 7.Spencer J, Dogan A. A common migratory highway between human spleen and mucosa-associated lymphoid tissues; data from nature's own experiments. Mucosal Immunol. 2009;2:380–2. doi: 10.1038/mi.2009.91. [DOI] [PubMed] [Google Scholar]
- 8.Fagarasan S, Kinoshita K, Muramatsu M, et al. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–15. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 10.Cebra JJ, Gearhart PJ, Kamat R, et al. Origin and differentiation of lymphocytes involved in the secretory IgA responses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):201–15. doi: 10.1101/sqb.1977.041.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji M, Suzuki K, Kitamura H, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Casola S, Otipoby KL, Alimzhanov M, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–27. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–71. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- 14.Vossenkamper A, Marches O, Fairclough PD, et al. Inhibition of NF-kappaB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE. J Immunol. 185:4118–27. doi: 10.4049/jimmunol.1000500. [DOI] [PubMed] [Google Scholar]
- 15.Su W, Gordon JN, Barone F, et al. Lambda light chain revision in the human intestinal IgA response. J Immunol. 2008;181:1264–71. doi: 10.4049/jimmunol.181.2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YC, Kipling D, Leong HS, et al. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 116:1070–8. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barone F, Bombardieri M, Rosado MM, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–40. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 18.Barone F, Patel P, Sanderson JD, et al. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009;2:495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, Kuppers R. Human IgA- and IgM-secreting intestinal plasma cells carry heavily mutated VH region genes. Eur J Immunol. 1998;28:2971–7. doi: 10.1002/(SICI)1521-4141(199809)28:09<2971::AID-IMMU2971>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Boursier L, Dunn-Walters DK, Spencer J. Characteristics of IgVH genes used by human intestinal plasma cells from childhood. Immunology. 1999;97:558–64. doi: 10.1046/j.1365-2567.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei HE, Yoshida T, Sime W, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113:2461–9. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 22.Cong Y, Bowdon HR, Elson CO. Identification of an immunodominant T cell epitope on cholera toxin. Eur J Immunol. 1996;26:2587–94. doi: 10.1002/eji.1830261108. [DOI] [PubMed] [Google Scholar]
- 23.Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 24.Weller S, Faili A, Garcia C, et al. CD40–CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–70. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boursier L, Gordon JN, Thiagamoorthy S, et al. Human intestinal IgA response is generated in the organized gut-associated lymphoid tissue but not in the lamina propria. Gastroenterology. 2005;128:1879–89. doi: 10.1053/j.gastro.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Halcomb KE, Musuka S, Gutierrez T, et al. Btk regulates localization, in vivo activation, and class switching of anti-DNA B cells. Mol Immunol. 2008;46:233–41. doi: 10.1016/j.molimm.2008.08.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu W, Santini PA, Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–17. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–77. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 30.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 31.Dunn-Walters DK, Boursier L, Spencer J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur J Immunol. 1997;27:2959–64. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.