Abstract
There is great global disparity in the outcome of infants born with gastroschisis. Mortality approaches 100% in many low income countries. Barriers to better outcomes include lack of antenatal diagnosis, deficient pre-hospital care, ineffective neonatal resuscitation and venous access, limited intensive care facilities, poor access to the operating theatre and safe neonatal anesthesia, and lack of neonatal parenteral nutrition. However, lessons can be learned from the evolution in management of gastroschisis in high-income countries, generic efforts to improve neonatal survival in low- and middle-income countries as well as specific gastroschisis management initiatives in low-resource settings. Micro and meso-level interventions include educational outreach programs, and pre and in hospital management protocols that focus on resuscitation and include the delay or avoidance of early neonatal anesthesia by using a preformed silo or equivalent. Furthermore, multidisciplinary team training, nurse empowerment, and the intentional involvement of mothers in monitoring and care provision may contribute to improving survival. Macro level interventions include the incorporation of ultrasound into World Health Organisation antenatal care guidelines to improve antenatal detection and the establishment of the infrastructure to enable parenteral nutrition provision for neonates in low- and middle-income countries. On a global level, gastroschisis has been suggested as a bellwether condition for evaluating access to and outcomes of neonatal surgical care provision.
Keywords: gastroschisis, low-resource settings, low and middle-income countries, bellwether
Introduction
The global disparity in the outcome of gastroschisis (GS) is glaring. Survival in high-income countries (HICs) has improved significantly over the last half century; from approximately 10% in the 1960’s to current survival rates of over 95%1,2. Such improvements have not been duplicated in most low and lower middle-income countries with recently reported survival rates of 0-2% in Uganda, 0% in Cote d’Ivoire, and 16% in Zimbabwe3–5. In an international survey, two-thirds of paediatric surgery centres in sub-Saharan Africa reported a mortality rate from GS of between 75-100% and the remaining third between 50-75%6. Outcomes vary widely in middle-income countries globally with reported survival rates of 20% in Iran, 21% in Jamaica, 25% in Nigeria, 66% in Turkey, 35-71% in South Africa, 43-77% in China, 90% in Malaysia and 92% in Thailand7–13.
It has been suggested by some that GS is a disease of HICs. However, the literature suggests a truly global congenital anomaly with a rising incidence2,3,12,14–27. In low- and middle-income countries (LMICs), the number of patients with GS presenting to a healthcare facility is increasing15,28. In Pretoria, there was a 35-fold increase in presenting cases between 1981 and 200115. Indeed, GS is a condition regularly encountered by paediatric surgical teams in LMICs with, in one survey, an estimated 22 cases/ institution/ year in low-income countries and 12 cases/ institution/ year in middle-income countries6. The aetiology remains unknown2. The associated risk factors such as low maternal age, low body mass index, smoking, use of anti-depressants, exposure to contraceptive hormones during the first trimester, pre-gestational or gestational diabetes, alcohol, cocaine and other drugs have mostly been derived from HIC data2,14,25,27,29–41. Very little epidemiological data from LMICs is available. In a prospective cohort study in Uganda the majority of mothers were between 20-29 years of age despite a high proportion of teenage pregnancies in the country compared to HICs. Furthermore, mothers denied smoking or taking drugs4. Investigating risk factors for GS in different settings across the globe may provide fresh aetiological clues4,42.
The paucity of data on GS from LMICs is reflected in studies investigating clinical management, interventional strategies and outcomes. This paper describes the particular challenges of managing infants with GS in the low-resource setting, potential solutions, and the use of GS as a bellwether procedure for global health evaluation and planning.
Challenges of Managing Gastroschisis in Low-Resource Settings
The current successful management of GS in HICs results from a multi-faceted approach; antenatal diagnosis, planned delivery at a tertiary paediatric surgery centre or adequate pre-hospital management and safe transfer, pre-intervention resuscitation, bowel reduction and defect closure, and post-interventional neonatal care including the provision of parenteral nutrition until enteral feeding is established. Each component of this care package presents different challenges in the low-resource setting.
Antenatal diagnosis and pre-hospital management
In LMICs, the majority of women now receive some antenatal care as per the World Health Organisation (WHO) guidelines43. However, the WHO does not currently recommend an ultrasound scan as part of that antenatal care package43. Antenatal ultrasound scans that do happen, are often performed in the private sector with varying levels of reliability4. In a prospective study of 42 neonates with GS in Kampala, 24% (n=10) of mothers had undergone an antenatal ultrasound scan, but only one had been given the correct diagnosis4. Hence, the majority of neonates with GS in low-resource settings are born outside of a tertiary paediatric surgery centre with no prior warning or advice regarding how to manage a neonate born with this condition4,5,42. Awareness and education in the community and district level hospitals regarding the pre-hospital management is commonly deficient. In Kampala, 81% of neonates with GS were born in a first or second level healthcare facility, but for most neonates, appropriate care was not initiated; 81% were without appropriate bowel coverage, 54% without intravenous (IV) access or IV fluids, 83% were without a nasogastric (NG) tube, 52% were breastfeeding and only 58% arrived within 12-hours of delivery4. Only 35% travelled by ambulance4.
Delays in accessing neonatal surgical care and deficient pre-hospital management result in many neonates with gastroschisis presenting with hypothermia, hypovolaemia, coagulopathy and sepsis4,5,9,42. In addition, 25% present with complex GS. In some infants this may reflect postnatal factors such as bowel exposure, contamination, damage and/ or torsion of the vascular pedicle resulting in intestinal ischaemia and necrosis44. Even those with simple GS commonly present with very edematous and matted bowel, making reduction and closure even more challenging.
Neonatal resuscitation and ward care
On arrival at the tertiary paediatric surgery centre, additional barriers to optimal care may exist; neonatal resuscitation may be delayed or ineffective. In many LMIC settings, newborns with GS are nursed on the general paediatric surgical ward rather than the neonatal unit, or neonatal intensive care unit (NICU) if one is available. This is often because they are considered ‘dirty’ and an infection risk to other patients. Severe shortages of the paediatric surgical workforce exist in most LMICs and hence a neonatologist, paediatric surgeon and trained neonatal nurse may be unavailable or significantly delayed following presentation45. In such settings, each nurse may have to care for many sick newborns and may intentionally focus time and energy caring for other infants considered more likely to survive4. Wesonga noted that nurses are used to sending these infants home to die suggesting that the mind-set of key members of the medical team may be a barrier to improved survival4,6. Similarly, newborns with GS are not prioritised for the limited operating theatre space in such settings, resulting in significantly delayed surgical care or no care even after arrival to a tertiary paediatric surgical care facility4. Finally, mothers are often separated from their infants negating the opportunity for them to contribute to their monitoring and basic care.
Gastroschisis reduction and closure
The optimal method of gastroschisis reduction and closure in HICs remains controversial. The two most commonly utilised methods are primary closure under general anesthesia in the operating room or serial reductions using a preformed silo over a number of days followed by either bedside or operating room closure, with or without a general anesthetic46,47. Allotey compared 53 consecutive neonates that underwent either primary closure or preformed silo application and reported lower mean airway pressures and inspired oxygen requirement, higher urine output and no inotropic support in the latter group; 43% of those undergoing primary closure required inotropes48. A randomised controlled trial comparing primary closure with preformed silo reported a lower requirement for ventilation in the silo group with no difference in other outcomes49. A meta-analysis comparing primary closure with all methods of staged closure also reported fewer ventilator days (p<0.0001), reduced time to first feed (p=0.04) and lower infection rates (p=0.03) in the latter group amongst studies with least selection bias50. Subsequently, a systematic review and metaanalysis comparing preformed silo with all alternative strategies reported lower ventilatory requirements with the former. Indeed, many neonates in the silo group required no ventilation47. These findings are consistent with a lower risk of abdominal compartment syndrome with use of the preformed silo.
In HICs the increased cardiorespiratory support required after primary closure can, typically, be provided in the context of a neonatal intensive care unit (NICU). This is often unavailable in LMICs; 36% availability in an international survey6. Furthermore, the very edematous and matted bowel that results from late presentations in LMICs may predispose to more severe abdominal compartment syndrome if primary closure is undertaken. This should mean that the preformed silo could result in improved outcomes through reduced NICU requirements. Reasons for the limited use of silos in LMICs include lack of availability, expertise and expense6.
It is estimated that 63-79% of infants with GS in LMICs undergo general anesthesia for bowel reduction and abdominal wall closure6. Neonatal anesthesia can be life-threatening in this setting due to a lack of specialist training, resources and the higher American Association of Anaesthesiologists (ASA) score of the newborn at the time of surgery due to the limited pre-hospital management and in-hospital resuscitation44,51. In addition, neonates with GS are often born early; in Durban, South Africa 64% were preterm and 72% <2.5kg and in Harare, Zimbabwe 43% were preterm and 72% <2.5kg5,42. This increases the risk of neonatal anesthesia further51.
Intravenous access and parenteral nutrition
Maintaining consistent intravenous (IV) access in the newborn infant is challenging. In HICs, the challenge is usually overcome as a result of appropriately trained personnel that can be dedicated to the task, a wide range of central lines that can be inserted via peripheral and central veins. In addition, the deployment of specialist equipment such as ultrasound aids effective venous access44. Dedicated personnel such as nurse specialists, equipment such as mobile ultrasound machines and suitable consumables are often unavailable in low-resource settings. Where central venous lines are available, infection control practices may not be well established, resulting in a higher rates of sepsis. Writing from Durban, Sekabira noted that despite having access to NICU and PN, the mortality from gastroschisis was still 43% with central line sepsis being a leading cause of death42.
For neonates with simple gastroschisis, the median duration of PN requirement in HICs is 23 days1. Despite PN being available for adults and older children in many tertiary level hospitals in LMICs, it is commonly unavailable for neonates. In an international survey, only 19% of tertiary paediatric surgery centres in LICs had access to PN6. The challenges for provision of PN to neonates in low-resources settings are two-fold. There is a lack of infrastructure and availability of neonatal specific PN bags, as well as difficulties in achieving central IV access. The shorter bench-life of neonatal PN compared to adult PN as purchased by manufacturing companies in LMICs adds to the complexity in terms of transportation and risk of waste. In sub-Saharan Africa, South Africa manufactures neonatal PN and can transport it efficiently within the country and to adjacent countries. However, transportation further afield is not currently deemed feasible. Many other countries in sub-Saharan Africa do not have an in-country PN manufacturing company and do not have the inhospital facilities, equipment and training to prepare it locally.
Strategies for Optimising Gastroschisis Outcomes in Low-Resource Settings
Antenatal diagnosis and pre-hospital management
To achieve the consistent and accurate diagnosis of gastroschisis and other congenital anomalies in LMICs, WHO guidelines on antenatal care will need to be amended to include ultrasound scanning. This would lead to the incorporation of prenatal scans in national guidelines and protocols as part of routine antenatal care. Antenatal ultrasound scanning currently undertaken in the private sector varies considerably in reliability. This is related to the varying level of training undertaken by the providers and possibly the quality of the equipment available4. National standards for training and service provision for antenatal ultrasound scanning may help to improve diagnostic accuracy. Incorporating education regarding parental advice and referral when a congenital anomaly is detected is also vital. Consideration would have to be given to the ethical implications of antenatally diagnosed congenital anomalies and suitable guidelines and safeguards would be required.
In many LMICs stigma towards infants born with a congenital anomaly and indeed their families remains a problem. Consequently, at present it is likely that many neonates with gastroschisis or other congenital anomalies never reach a healthcare facility. Hence, community engagement and education regarding congenital anomalies and the availability of treatment is required. Improving pre-hospital care at first and second level healthcare facilitates and safe transfer for neonates with gastroschisis has the potential to make a significant impact on the outcomes4,5. Potential methods for achieving this include production of a pre-hospital management protocol to be distributed throughout such facilities, outreach training led by the tertiary paediatric surgical team, and/ or inviting district hospital care providers to a neonatal surgery study day held centrally at the tertiary paediatric surgery centre. This would have the added benefit of enhancing networking and communications between different members of the multi-disciplinary team at the different levels of healthcare. The protocol could be tailored to the local environment and may include the use of a clear plastic covering for the bowel, training on how to avoid torsion of the intestinal vessels and hence ischaemia, administration of IV fluids and NG tube insertion if available, kangaroo care, and safe, efficient transfer to the tertiary paediatric surgery centre.
Neonatal resuscitation and ward care
There is evidence that implementation of protocols can improve care and outcomes of critically ill paediatric patients52. This would help to both standardise care, particularly for neonates with simple gastroschisis, and also help to gain a team consensus regarding the roles of the various multidisciplinary team members in the patient’s care. Establishing secure venous access should be an early priority to ensure effective resuscitation. Failure of venous access often precedes demise of the surgical newborn in LMICs3. To prevent this eventuality, medium term venous access options should be achieved early on, before peripheral veins are used up. These include percutaneous central venous access and possibly use of the umbilical vein for initial resuscitation.
Ekenze et al undertook a quality improvement (QI) project focussed on improving neonatal surgical outcomes in Nigeria through co-ordinated interdisciplinary collaboration53. This involved both individual specialist training and multidisciplinary team training between paediatric surgeons, anaesthetists and nurses. It resulted in a significant reduction in overall mortality from 48.9 to 22.7% (p<0.05)53. Khan et al undertook a QI project focused on reducing surgical site infection rates after congenital heart surgery in Pakistan54. Pivotal to their implementation strategy was nurse empowerment through appointment of a senior nurse to oversee the project, liaise with key stakeholders and train/ supervise local nurses on clinical skills, actively participate on ward rounds, and demonstrate assertive communication skills54. They reported a reduction in surgical site infection and bacterial sepsis from 30 to 1% (p=0.0001)54.
Numerous studies focused on reducing neonatal mortality in LMICs have highlighted the benefit of involving mothers in the monitoring and basic care for their baby55–57. This is particularly important in low-resource settings where nursing staff are commonly overburdened58–60. Bhutta et al undertook a QI project in Pakistan utilising maternal training and empowerment; this reduced the LOS for their very low birth weight neonates from 34 to 16-days without increasing complications57. Although these strategies have been shown to improve outcomes in isolation, most successful initiatives for improving neonatal survival in LMICs involve a multi-faceted approach55,57,61. Agarwal et al implemented a simple bundle of interventions aimed at improving neonatal survival on a busy neonatal unit in Pakistan55. This included protocol-based management with abandonment of unnecessary interventions, nurse training and empowerment, training and utilisation of mothers as caregivers, aggressive enteral feeding, infection control measures, and rational use of antibiotics55. This resulted in a significant reduction in overall mortality from 29.3/1000 to 20.3/100055. Another step towards improving outcomes for neonates with GS may be to move their primary care from the paediatric surgical ward to the neonatal care unit or NICU, if available. All these QI measures require whole team co-ordination, motivation and buy-in.
Gastroschisis reduction and closure
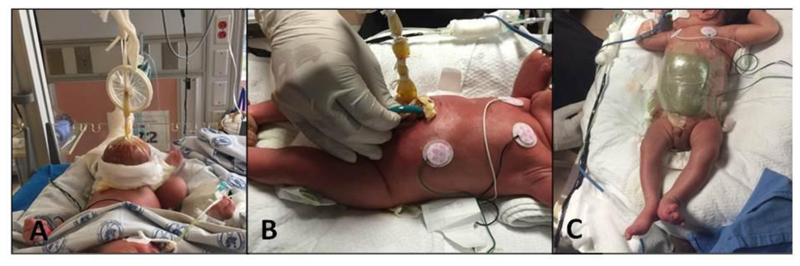
As noted above, preformed silos have the potential for improving survival in neonates with gastroschisis in low-resource settings by minimising the risk of compartment syndrome and need for neonatal intensive care47–50. They also have the added benefit that they can be applied by a suitably trained medical officer / registrar or specialist nurse at the bedside, negating the need for an emergency theatre slot and consultant paediatric surgeon which may not be available47. In the United Kingdom, a pre-formed silo has been used routinely in many centres for sutureless closure of GS. These silos cost approximately $300 each, a price deemed by many as too expensive for the low-resource setting6,62. While it could be argued that this option is still cheaper than surgery, cost-effectiveness studies are currently unavailable62. In some middle- and high-income countries including Mexico, Malaysia, France and Japan, the Alexis Wound Protector and Retractor (Applied Medical ®) has been used as an alternative (Figure 1). While this device has the potential disadvantage of an intra-abdominal ring which is stiffer than pre-formed silos manufactured specifically for abdominal wall defects, good outcomes have been reported in the limited studies available63–66. The Alexis wound protectors costs just $25-$30 each and hence are much more affordable67. A multi-centre interventional study using the Alexis device in LMICs would help to evaluate its effectiveness in this setting and promote its widespread use if found to be effective.
Figure 1.
A) Alexis WP&R in situ 3-days after application, B) Alexis WP&R being removed 24 hours after complete bowel reduction, C) Dressing applied following sutureless closure and left in situ for 14-days.
Several other strategies for gastroschisis reduction and closure have been trialled in low-resource settings. Du et al advocate for immediate reduction of the bowel and defect closure in an OR adjacent to the delivery room in those that are antenatally diagnosed10. Similarly, the Bianchi technique can be utilised with bedside reduction and closure of the defect immediately after resuscitation68–70. This technique has the benefit of avoiding neonatal anesthesia, however it may expose the neonate to an increased risk of abdominal compartment syndrome and need for intensive care. In order to minimise this risk, an umbilical flap or ‘turban’ can be utilised without closing the fascia defect underneath thus reducing the tension and intra-abdominal pressure71–73.
Parenteral nutrition and intravenous access
Provision of short-term PN can be life-saving for neonates with gastroschisis and other gastrointestinal congenital and acquired conditions requiring surgical intervention. Although deemed an expensive resource, PN can in fact be cost-effective in terms of disability-adjusted life years (DALYs) averted. This is particularly true for neonates with conditions such as gastroschisis, which can potentially be cured with the use of a short period of PN resulting in a full, normal life74. Urgent work is required to evaluate and develop existing supply chains so that PN can become available for neonates in LMICs. Such a venture would require collaboration between numerous stakeholders including manufacturing companies, paediatricians, gastroenterologists, nutritionists, laboratory team members, paediatric surgeons, hospital management and procurement teams. Collaboration with international partners could help to facilitate this. Similarly, an interventional study aimed at improving outcomes from gastroschisis that incorporated use of PN in the low-resource setting may provide the evidence and incentive required to get such a programme off the ground. At present, the majority of neonates with gastroschisis die within the first week of life, hence one might consider providing PN only to those who survive to 1-week to optimise resource allocation75. This would also be consistent with studies suggesting outcomes are better for children in intensive care if PN is started after a week rather than immediately when they are so sick during the first few days of admission76.
In the immediate resuscitative period, studies have shown that umbilical vein catheterisation can be used successfully in neonates with gastroschisis77. In the longer term, provision of central lines for neonates requiring short-term PN has the potential to be life-saving. Again, studies proving that gastroschisis can be successfully managed in the low-resource setting utilising these basic resources may be required to help incentivise local procurement and management teams to provide such resources. Such research is required to help overcome the current beliefs that gastroschisis is a futile condition not worthy of precious resource utilisation4.
An early and aggressive enteral feeding program has the potential to minimise PN requirements. Earlier time to first enteral feed has been associated with a shorter duration of PN and length of hospital stay in both HIC and LMIC settings without increasing the risk of necrotizing enterocolitis29,78,79. When PN is not available, there may be the potential for some survivors without this resource13. Term neonates are estimated to have the ability to survive up to 1-month without nutrition80. Hence, those few who are delivered at term or close to, have simple gastroschisis, and do not succumb to sepsis have a chance of survival without PN. In Blantyre, Malawi, the mortality from gastroschisis is reported to be 60% without routine availability of PN3. Similarly, in Malaysia, Naidu reports some survivors without the use of PN13.
Gastroschisis as a Bellwether Condition
Bellwether surgical procedures were widely reported in The Lancet Commission on Global Surgery published in 201581. These procedures can be seen as surrogates for the overall quality of healthcare and availability of resources. The three proposed procedures were laparotomy, caesarean section, and treatment of an open fracture. Following outcome analyses from a wider range of operations, these three were considered the best proxies for estimating the capacity of an institution to provide a broader range of surgical care. Hence, if an institution can provide these three procedures effectively, then it should also be able to manage a wide range of general surgical, obstetric and orthopaedic emergencies. However, the provision of surgical care for neonates or young children was not considered during this process and indeed provision of these three bellwether procedures provides little information about whether a centre has the capacity to provide neonatal surgical care.
The ability to assess institutional capacity and access to surgical services is vital for global health planning. The Lancet Commission bellwether procedures have been used to map 2-hour access to emergency and essential surgical care globally82–85. This helps to identify areas to prioritise global health funding and efforts to help reach the target of 80% coverage of essential surgical and anaesthetic care per country by 203081. Such data is not available regarding access to neonatal or paediatric surgical care. Yet up to 50% of the population in LMICs are children45. Indeed congenital anomalies are a major global health problem, now listed as the 5th leading cause of death in children under 5-years of age globally86. The overwhelming majority (97%) of the deaths from congenital anomalies are in LMICs and it is estimated that up to two-thirds of the disability and deaths related to congenital anomalies could be averted through the provision of surgical care87,88.
GS is one of the commonest congenital anomalies and has been suggested as a bellwether condition for assessing the capacity of an institution to provide neonatal surgical care3,89. This is because in most cases it is an isolated condition and caring for neonates with GS requires all the components of a neonatal surgical care system. Hence, if an institution is able to effectively care for neonates with GS, it is likely to have the skills and resources available to effectively manage a wide range of other neonatal surgical conditions. In order to ensure neonates are appropriately represented in plans to scale up access to surgical care globally, it will be vital to first map current access and outcomes; GS could be used as a proxy for this. In addition to the tertiary level care setting, GS tests the ability of first and second level care facilities to resuscitate, stabilise and safely transfer a surgical neonate.
Conclusion
The current disparity in outcomes for GS between HICs and LMICs is glaring and reflects poorly on the global community. This paper outlines potential solutions including a practical bundle of intervention for use in LMICs. There is very limited published literature from LMICs using similar interventions and further research would be informative. In addition to GS service delivery, the results of such research could aid strategic planning for neonatal surgical services more widely as many of the recommended interventions may also help to improve outcomes for other neonatal surgical conditions. This is a neglected area on the global health agenda which should now be prioritised if neonatal and under-5 mortality targets set in the Sustainable Development Goals are to be met90.
Acknowledgements
We thank Dr. Alejandro A. Peñarrieta Daher, Dr. Eduardo Bracho Blanchet, Dr. Roberto Dávila Pérez and Dr. Cristian R. Zalles Vidal from Hospital Infantil de México Federico Gomez, México, for information provided. We also acknowledge Ms Kat Ford, Ms Sarah Bradley and Ms Kate Tavener for sharing information from fieldwork in Uganda.
References
- 1.Bradnock T, Marven S, Owen A, et al. Gastroschisis: one year outomes from national cohort study. BMJ. 2011;343(d6749) doi: 10.1136/bmj.d6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldkamp ML, Botto LD. Developing a research and public health agenda for gastroschisis: how do we bridge the gap between what is known and what is not? Am J Med Genet C Semin Med Genet. 2008;148C(3):155–61. doi: 10.1002/ajmg.c.30183. [DOI] [PubMed] [Google Scholar]
- 3.Ford K, Poenaru D, Moulot O, et al. Gastroschisis: Bellwether for neonatal surgery capacity in low resource settings? J Pediatr Surg. 2016;51(8):1262–7. doi: 10.1016/j.jpedsurg.2016.02.090. [DOI] [PubMed] [Google Scholar]
- 4.Wesonga AS, Fitzgerald TN, Kabuye R, et al. Gastroschisis in Uganda: Opportunities for improved survival. J Pediatr Surg. 2016;51(11):1772–7. doi: 10.1016/j.jpedsurg.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Apfeld JC, Wren SM, Macheka N, et al. Infant, maternal, and geographic factors influencing gastroschisis related mortality in Zimbabwe. Surgery. 2015;158(6):1475–80. doi: 10.1016/j.surg.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Wright NJ, Zani A, Ade-Ajayi N. Epidemiology, management and outcome of gastroschisis in Sub-Saharan Africa: Results of an international survey. Afr J Paediatr Surg. 2015;12(1):1–6. doi: 10.4103/0189-6725.150924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askarpour S, Ostadian N, Javaherizadeh H, Chabi S. Omphalocele, gastroschisis: epidemiology, survival, and mortality in Imam Khomeini hospital, Ahvaz-Iran. Pol Przegl Chir. 2012;84(2):82–5. doi: 10.2478/v10035-012-0013-4. [DOI] [PubMed] [Google Scholar]
- 8.Marshall Niles SG, Mitchell-Fearon K, Gill MI, et al. Mortality-related factors in gastroschisis - a Jamaican perspective. J Pediatr Surg. 2017;52(4):530–3. doi: 10.1016/j.jpedsurg.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Stevens P, Muller E, Becker P. Gastroschisis in a developing country: poor resuscitation is a more significant predictor of mortality than postnasal transfer time. S Afr J Surg. 2016;54(1):4–9. [PubMed] [Google Scholar]
- 10.Du L, Pan WH, Cai W, Wang J, Wu YM, Shi CR. Delivery room surgery: an applicable therapeutic strategy for gastroschisis in developing countries. World J Pediatr. 2014;10(1):69–73. doi: 10.1007/s12519-014-0455-3. [DOI] [PubMed] [Google Scholar]
- 11.Erdogan D, Azili MN, Cavusoglu YH, et al. 11-year experience with gastroschisis: factors affecting mortality and morbidity. Iran J Pediatr. 2012;22(3):339–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Niramis R, Suttiwongsing A, Buranakitjaroen V, et al. Clinical outcome of patients with gastroschisis: what are the differences from the past? J Med Assoc Thai. 2011;94(Suppl 3):S49–56. [PubMed] [Google Scholar]
- 13.Naidu RR, Lee FH, Teh KH. Management of gastroschisis in a peripheral hospital setting. Med J Malaysia. 1996;51(4):444–6. [PubMed] [Google Scholar]
- 14.Salemi JL, Pierre M, Tanner JP, et al. Maternal nativity as a risk factor for gastroschisis: a population-based study. Birth Defects Res A Clin Mol Teratol. 2009;85(11):890–6. doi: 10.1002/bdra.20612. [DOI] [PubMed] [Google Scholar]
- 15.Arnold M. Is the incidence of gastroschisis rising in South Africa in accordance with international trends? A retrospective analysis at Pretoria Academic and Kalafong Hospitals, 1981-2001. S Afr J Surg. 2004;42(3):86–8. [PubMed] [Google Scholar]
- 16.Benjamin BG, Ethen MK, Van Hook CL, Myers CA, Canfield MA. Gastroschisis prevalence in Texas 1999-2003. Birth Defects Res A Clin Mol Teratol. 2010;88(3):178–85. doi: 10.1002/bdra.20642. [DOI] [PubMed] [Google Scholar]
- 17.Di Tanna GL, Rosano A, Mastroiacovo P. Prevalence of gastroschisis at birth: retrospective study. BMJ. 2002;325(7377):1389–90. doi: 10.1136/bmj.325.7377.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazaura MR, Lie RT, Irgens LM, et al. Increasing risk of gastroschisis in Norway: an age-period-cohort analysis. Am J Epidemiol. 2004;159(4):358–63. doi: 10.1093/aje/kwh051. [DOI] [PubMed] [Google Scholar]
- 19.Loane M, Dolk H, Bradbury I, Group EW. Increasing prevalence of gastroschisis in Europe 1980-2002: a phenomenon restricted to younger mothers? Paediatr Perinat Epidemiol. 2007;21(4):363–9. doi: 10.1111/j.1365-3016.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 20.Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332(7538):423–4. doi: 10.1136/bmj.332.7538.423-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Materna-Kiryluk A, Wieckowska B, Wisniewska K, et al. Geospatial clustering of gastroschisis in Poland: Data from the Polish Registry of Congenital Malformations (PRCM) Int J Occup Med Environ Health. 2016;29(3):461–70. doi: 10.13075/ijomeh.1896.00624. [DOI] [PubMed] [Google Scholar]
- 22.Nichols CR, Dickinson JE, Pemberton PJ. Rising incidence of gastroschisis in teenage pregnancies. J Matern Fetal Med. 1997;6(4):225–9. doi: 10.1002/(SICI)1520-6661(199707/08)6:4<225::AID-MFM8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Tan KB, Tan KH, Chew SK, Yeo GS. Gastroschisis and omphalocele in Singapore: a ten-year series from 1993 to 2002. Singapore Med J. 2008;49(1):31–6. [PubMed] [Google Scholar]
- 24.Vo LU, Langlois PH. Time trends in prevalence of gastroschisis in Texas, 1999 to 2011: Subgroup analyses by maternal and infant characteristics. Birth Defects Res A Clin Mol Teratol. 2015;103(11):928–40. doi: 10.1002/bdra.23438. [DOI] [PubMed] [Google Scholar]
- 25.Skarsgard ED, Meaney C, Bassil K, et al. Maternal risk factors for gastroschisis in Canada. Birth Defects Res A Clin Mol Teratol. 2015;103(2):111–8. doi: 10.1002/bdra.23349. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez SM, Burd RS. Increasing prevalence of gastroschisis repairs in the United States: 1996-2003. J Pediatr Surg. 2007;42(6):943–6. doi: 10.1016/j.jpedsurg.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Kirby RS, Marshall J, Tanner JP, et al. Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstet Gynecol. 2013;122(2 Pt 1):275–81. doi: 10.1097/AOG.0b013e31829cbbb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agugua NE, Nwako FA. Gastroschisis a fifteen-year experience. West Afr J Med. 1990;9(2):147–50. [PubMed] [Google Scholar]
- 29.Sharp M, Bulsara M, Gollow I, Pemberton P. Gastroschisis: early enteral feeds may improve outcome. J Paediatr Child Health. 2000;36(5):472–6. doi: 10.1046/j.1440-1754.2000.00552.x. [DOI] [PubMed] [Google Scholar]
- 30.Feldkamp ML, Carey JC, Sadler TW. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet A. 2007;143A(7):639–52. doi: 10.1002/ajmg.a.31578. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Li X, Dai L, et al. Assessing the trend of gastroschisis prevalence in China from 1996 to 2007 using two analytical methods. Birth Defects Res A Clin Mol Teratol. 2011;91(3):177–84. doi: 10.1002/bdra.20753. [DOI] [PubMed] [Google Scholar]
- 32.Bargy F, Beaudoin S. Comprehensive developmental mechanisms in gastroschisis. Fetal Diagn Ther. 2014;36(3):223–30. doi: 10.1159/000360080. [DOI] [PubMed] [Google Scholar]
- 33.Forrester MB, Merz RD. Comparison of trends in gastroschisis and prenatal illicit drug use rates. J Toxicol Environ Health A. 2006;69(13):1253–9. doi: 10.1080/15287390500361750. [DOI] [PubMed] [Google Scholar]
- 34.Given JE, Loane M, Garne E, et al. Gastroschisis in Europe - A Case-malformed-Control Study of Medication and Maternal Illness during Pregnancy as Risk Factors. Paediatr Perinat Epidemiol. 2017 doi: 10.1111/ppe.12401. [DOI] [PubMed] [Google Scholar]
- 35.Lam PK, Torfs CP. Interaction between maternal smoking and malnutrition in infant risk of gastroschisis. Birth Defects Res A Clin Mol Teratol. 2006;76(3):182–6. doi: 10.1002/bdra.20238. [DOI] [PubMed] [Google Scholar]
- 36.Schulz AC, Stressig R, Ritgen J, et al. A classic twin study of isolated gastroschisis. Fetal Pediatr Pathol. 2012;31(5):324–30. doi: 10.3109/15513815.2012.659393. [DOI] [PubMed] [Google Scholar]
- 37.Root ED, Meyer RE, Emch M. Socioeconomic context and gastroschisis: exploring associations at various geographic scales. Soc Sci Med. 2011;72(4):625–33. doi: 10.1016/j.socscimed.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Siega-Riz AM, Herring AH, Olshan AF, Smith J, Moore C, National Birth Defects Prevention S The joint effects of maternal prepregnancy body mass index and age on the risk of gastroschisis. Paediatr Perinat Epidemiol. 2009;23(1):51–7. doi: 10.1111/j.1365-3016.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 39.Torfs CP, Curry CJ. Familial cases of gastroschisis in a population-based registry. Am J Med Genet. 1993;45(4):465–7. doi: 10.1002/ajmg.1320450414. [DOI] [PubMed] [Google Scholar]
- 40.Richardson S, Browne ML, Rasmussen SA, et al. Associations between periconceptional alcohol consumption and craniosynostosis, omphalocele, and gastroschisis. Birth Defects Res A Clin Mol Teratol. 2011;91(7):623–30. doi: 10.1002/bdra.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robledo-Aceves M, Bobadilla-Morales L, Mellin-Sanchez EL, et al. Prevalence and risk factors for gastroschisis in a public hospital from west Mexico. Congenit Anom (Kyoto) 2015;55(2):73–80. doi: 10.1111/cga.12087. [DOI] [PubMed] [Google Scholar]
- 42.Sekabira J, Hadley GP. Gastroschisis: a third world perspective. Pediatr Surg Int. 2009;25(4):327–9. doi: 10.1007/s00383-009-2348-4. [DOI] [PubMed] [Google Scholar]
- 43.Lincetto O, Mothebesoane-Anoh S, Gomex P, Munjanja S. Opportunities for Africa’s newborns. Antenatal care. [accessed 9th February 2018];World Health Organisation. http://www.who.int/pmnch/media/publications/africanewborns/en/index1.html.
- 44.Wright N, PaedSurg Africa Research Collaboration . Paediatric Surgery across SubSaharan Africa: A Multi-Centre Prospective Cohort Study. King’s College London; MSc Dissertation; 2017. [Google Scholar]
- 45.Krishnaswami S, Nwomeh BC, Ameh AE. The pediatric surgery workforce in low- and middle-income countries: problems and priorities. Semin Pediatr Surg. 2016;25(1):32–42. doi: 10.1053/j.sempedsurg.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Zani A, Ruttenstock EM, Davenport M, Ade-Ajayi N. Is there unity in Europe? First survey of EUPSA delegates on the management of gastroschisis. Eur J Pediatr Surg. 2012;23(1):19–24. doi: 10.1055/s-0032-1326954. [DOI] [PubMed] [Google Scholar]
- 47.Ross AR, Eaton S, Zani A, Ade-Ajayi N, Pierro A, Hall NJ. The role of preformed silos in the management of infants with gastroschisis: a systematic review and meta-analysis. Pediatr Surg Int. 2015;31(5):473–83. doi: 10.1007/s00383-015-3691-2. [DOI] [PubMed] [Google Scholar]
- 48.Allotey J, Davenport M, Njere I, et al. Benefit of preformed silos in the management of gastroschisis. Pediatr Surg Int. 2007;23:1065–9. doi: 10.1007/s00383-007-2004-9. [DOI] [PubMed] [Google Scholar]
- 49.Pastor AC, Phillips JD, Fenton SJ, et al. Routine use of a SILASTIC spring-loaded silo for infants with gastroschisis: a multicentre randomized controlled trial. J Pediatr Surg. 2008;43:1807–12. doi: 10.1016/j.jpedsurg.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Kunz SN, Tieder JS, Whitlock KJ, Jackson C, Avansino JR. Primary fascial closure versus staged closure with silo in patients with gastroschisis: a meta-analysis. J Pediatr Surg. 2013;48(4):845–57. doi: 10.1016/j.jpedsurg.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosenberg AT. Neonatal anesthesia with limited resources. Paediatr Anaesth. 2014;24(1):98–105. doi: 10.1111/pan.12291. [DOI] [PubMed] [Google Scholar]
- 52.Meade M, Ely EW. Protocols to improve care of critically ill pediatric and adult patients. JAMA. 2002;288(20):2601–3. doi: 10.1001/jama.288.20.2601. [DOI] [PubMed] [Google Scholar]
- 53.Ekenze SO, Modekwe VI, Ajuzieogu OV, Asinobi IO, Sanusi J. Neonatal surgery in a developing country: Outcome of co-ordinated interdisciplinary collaboration. J Paediatr Child Health. 2017;53:976–80. doi: 10.1111/jpc.13610. [DOI] [PubMed] [Google Scholar]
- 54.Khan A, Abdullah A, Ahmad H, et al. Impact of International Quality Improvement Collaborative on Congenital Heart Surgery in Pakistan. Heart. 2017;103(21):1680–6. doi: 10.1136/heartjnl-2016-310533. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R, Agarwal K, Acharya U, Christina P, Sreenivas V, Seetaraman S. Impact of simple interventions on neonatal mortality in a low-resource teaching hospital in India. J Perinatol. 2007;27(1):44–9. doi: 10.1038/sj.jp.7211620. [DOI] [PubMed] [Google Scholar]
- 56.Bastani F, Abadi TA, Haghani H. Effect of Family-centered Care on Improving Parental Satisfaction and Reducing Readmission among Premature Infants: A Randomized Controlled Trial. J Clin Diagn Res. 2015;9(1):SC04–8. doi: 10.7860/JCDR/2015/10356.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhutta ZA, Khan I, Salat S, Raza F, Ara H. Reducing length of stay in hospital for very low birthweight infants by involving mothers in a stepdown unit: an experience from Karachi (Pakistan) BMJ. 2004;329(7475):1151–5. doi: 10.1136/bmj.329.7475.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crouse HL, Torres F, Vaides H, et al. Impact of an Emergency Triage Assessment and Treatment (ETAT)-based triage process in the paediatric emergency department of a Guatemalan public hospital. Paediatr Int Child Health. 2016;36(3):219–24. doi: 10.1179/2046905515Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 59.Namazzi G, Waiswa P, Nakakeeto M, et al. Strengthening health facilities for maternal and newborn care: experiences from rural eastern Uganda. Glob Health Action. 2015;8:24271. doi: 10.3402/gha.v8.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert C, Darlow B, Zin A, et al. Educating neonatal nurses in Brazil: a before-and-after study with interrupted time series analysis. Neonatology. 2014;106(3):201–8. doi: 10.1159/000362532. [DOI] [PubMed] [Google Scholar]
- 61.Leng H, Wang H, Lin B, Cheng G, Wang L. Reducing Transitional Hypothermia in Outborn Very Low Birth Weight Infants. Neonatology. 2016;109(1):31–6. doi: 10.1159/000438743. [DOI] [PubMed] [Google Scholar]
- 62.Medicina. Medicina Silo user guide. Innovative surgical solutions. [accessed 27th February 2018]; http://www.eurosteriel-medical.nl/sites/default/files/folders/medicina/07_silo%20IFU.pdf.
- 63.Kanamori Y. Operative general surgery in neonates and infants. Gastroschisis and Omphalocele: Springer; Japan: 2016. [Google Scholar]
- 64.Kusafuka J, Yamataka A, Okazaki T, et al. Gastroschisis reduction using ‘Applied Alexis”, a wound protector and retractor. Pediatr Surg Int. 2005;21:925–7. doi: 10.1007/s00383-005-1518-2. [DOI] [PubMed] [Google Scholar]
- 65.Ogasawara Y, Okazaki T, Kato Y, Lane GJ, Yamataka A. Spontaneous sutureless closire of the abdominal wall defect in gastroschisis using a commercial wound retractor system. Pediatr Surg Int. 2009;25:973–6. doi: 10.1007/s00383-009-2450-7. [DOI] [PubMed] [Google Scholar]
- 66.Ferreira CG, Lacreuse I, Geslin D, et al. Staged gastroschisis closure using Alexis wound retractor: first experiences. Pediatr Surg Int. 2014;30:305–11. doi: 10.1007/s00383-013-3440-3. [DOI] [PubMed] [Google Scholar]
- 67.eSutures. Applied Medical Alexis Wound Protector/Retractor. [accessed 27th February 2018]; https://www.esutures.com/product/0-in-date/48-applied-medical/485-alexis/46208432-applied-medical-alexis-wound-protector--retractor-x-large-C8304/
- 68.Bianchi A, Dickson AP. Elective delayed reduction and no anesthesia: ‘minimal intervention management’ for gastroschisis. J Pediatr Surg. 1998;33:1338–40. doi: 10.1016/s0022-3468(98)90002-1. [DOI] [PubMed] [Google Scholar]
- 69.Kimble RM, Singh SJ, Bourke C, Cass DT. Gastroschisis reduction under analgesia in the neonatal unit. J Pediatr Surg. 2001;36(11):1672–4. doi: 10.1053/jpsu.2001.27957. [DOI] [PubMed] [Google Scholar]
- 70.Duncan ND, Brown B, Dundas SE, et al. “Minimal intervention management” for gastroschisis: a preliminary report. West Indian Med J. 2005;54(2):152–4. doi: 10.1590/s0043-31442005000200014. [DOI] [PubMed] [Google Scholar]
- 71.Sandler A, Lawrence J, Meehan J. A “plastic” sutureless abdominal wall closure in gastroschisis. J Pediatr Surg. 2004;39(5):738–41. doi: 10.1016/j.jpedsurg.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 72.Bisaliev BN, Tsap NA. [Experience of Treatment of Newborn Children with Gastroschisis] Vestn Khir Im I I Grek. 2015;174(6):46–51. [PubMed] [Google Scholar]
- 73.Emami CN, Youssef F, Baird RJ, et al. A risk-stratified comparison of fascial versus flap closure techniques on the early outcomes of infants with gastroschisis. J Pediatr Surg. 2015;50(1):102–6. doi: 10.1016/j.jpedsurg.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Lunzer H, Menardi G, Brezinka C. Long-term follow-up of children with prenatally diagnosed omphalocele and gastroschisis. J Matern Fetal Med. 2001;10(6):385–92. doi: 10.1080/714052779. [DOI] [PubMed] [Google Scholar]
- 75.van Puffelen E, Polinder S, Vanhorebeek I, et al. Cost-effectiveness study of early versus late parenteral nutrition in critically ill children (PEPaNIC): preplanned secondary analysis of a multicentre randomised controlled trial. Crit Care. 2018;22(1):4. doi: 10.1186/s13054-017-1936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fivez T, Kerklann D, Mesotten D, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374:1111–22. doi: 10.1056/NEJMoa1514762. [DOI] [PubMed] [Google Scholar]
- 77.Gazula S. Umbilical venous catheter insertion in gastroschisis. Indian J Pediatr. 2014;81(11):1261–2. doi: 10.1007/s12098-014-1377-8. [DOI] [PubMed] [Google Scholar]
- 78.Dama M, Rao U, Gollow I, Bulsara M, Rao S. Early Commencement of Enteral Feeds in Gastroschisis: A Systematic Review of Literature. Eur J Pediatr Surg. 2017 doi: 10.1055/s-0037-1598086. [DOI] [PubMed] [Google Scholar]
- 79.Ameh EA, Chirdan LB. Ruptured exomphalos and gastroschisis: a retrospective analysis of morbidity and mortality in Nigerian children. Pediatr Surg Int. 2000;16(1–2):23–5. doi: 10.1007/s003830050006. [DOI] [PubMed] [Google Scholar]
- 80.Coran AG, Krummel TM, Laberge J-M. Pediatric Surgery. Philadelphia: Elsevier Saunders; 2012. [Google Scholar]
- 81.Meara JG, Leather A, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare and economic development. Lancet. 2015;386(9993):569–624. doi: 10.1016/S0140-6736(15)60160-X. [DOI] [PubMed] [Google Scholar]
- 82.Masenburg B, Saluja S, Jenny H, Raykar N, Ng-Kamstra J, Guilloux A. Assessing the Brazilian surgical system with six surgical indicators: a descriptive and modelling study. BMJ Glob Health. 2017;2(2):e000226. doi: 10.1136/bmjgh-2016-000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson G, Ilcisin L, Abesiga L, Mayanja R, Portal Benetiz N, Ngonzi J. Surgical volume and postoperative mortality rate at a referral hospital in Western Uganda: Measuring the Lancet Commission on Global Surgery indicators in low-resource settings. Surgery. 2017;161(6):1710–9. doi: 10.1016/j.surg.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Guest G, McLeod E, Perry W, Tangi V, Pedro J, Ponifasio P. Collecting data for global surgical indicators: a collaborative approach in the Pacific Region. BMJ Glob Health. 2017;2(4):e000376. doi: 10.1136/bmjgh-2017-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Health Organisation. Global Reference List of 100 Core health Indicators. [accessed 27th February 2018];2015 http://apps.who.int/iris/bitstream/10665/173589/1/WHO_HIS_HSI_2015.3_eng.pdf.
- 86.GBD Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1725–74. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farmer D, Sitkin N, Lofberg K, Donkor P, Ozgediz D. Surgical Interventions for Congenital Anomalies. In: Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN, editors. Essential Surgery: Disease Control Priorities. Third edition. Vol. 1 Washington (DC): 2015. [Google Scholar]
- 88.Institute of Health Metrics and Evaluation. Global Health Data Exchange. GBD Results Tool. [accessed 27th February 2018];2018 http://ghdx.healthdata.org/gbd-results-tool.
- 89.Public Health England. National congenital anomaly and rare disease registration service. Congenital anomaly statistics 2015. [accessed 27th February 2018];2015 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/630736/Congenital_anomaly_statistics_2015.pdf.
- 90.United Nations. Sustainable Development Goals. [accessed 27th February 2018];2015 http://www.un.org/sustainabledevelopment/health/