Abstract
Kisspeptin within the arcuate nucleus of the hypothalamus is a critical neuropeptide in the regulation of reproduction. Together with neurokinin B and dynorphin A, arcuate kisspeptin provides the oscillatory activity that drives the pulsatile secretion of GnRH, and therefore LH pulses, and is believed to be a central component of the GnRH pulse generator. It is well established that the amygdala also exerts an influence over gonadotrophic hormone secretion and reproductive physiology. The discovery of kisspeptin and its receptor within the posterodorsal medial amygdala (MePD), and our recent finding showing that intra-MePD administration of kisspeptin or a kisspeptin receptor antagonist results in increased LH secretion and decreased LH pulse frequency, respectively, suggests an important role for amygdala kisspeptin signalling in the regulation of the GnRH pulse generator. To further investigate the function of amygdala kisspeptin, the present study used an optogenetic approach to selectively stimulate MePD kisspeptin neurones and examine the effect on pulsatile LH secretion. MePD kisspeptin neurones in conscious Kiss1-Cre mice were virally infected to express the channelrhodopsin 2 protein and selectively stimulated by light via a chronically implanted fibre optic cannula. Continuous stimulation using 5 Hz resulted in an increased LH pulse frequency, which was not observed at the lower stimulation frequencies of 0.5 and 2 Hz. In wild-type animals, continuous stimulation at 5 Hz did not affect LH pulse frequency. These results demonstrate that selective activation of MePD Kiss1 neurons can modulate hypothalamic GnRH pulse generator frequency.
Introduction
It is well established that hypothalamic kisspeptin (Kp) is a critical neuropeptide for reproduction. Inactivating mutations of the genes encoding KISS1 or its receptor, KISS1R (a.k.a. GPR54), result in hypogonadotrophic hypogonadism and a failure to progress through puberty in humans and rodent models (1, 2). Of the major hypothalamic populations of Kiss1 neurones, in the anterior preoptic area (POA) and the arcuate nucleus (ARC), attention has focused on the ARC as a key component of the GnRH pulse generator (3, 4) These neurones, known as KNDy, because they co-express neurokinin B (NKB) and dynorphin A (DYN), innervate the distal processes of the GnRH neurones at the level of the median eminence (5) where kisspeptin can act directly to stimulate GnRH release (6). NKB acting through its receptor (TACR3) is thought to function as an excitatory signal to depolarise KNDy cells postsynaptically in the neural network, resulting in kisspeptin output to the GnRH neurones to initiate each GnRH pulse. The co-released DYN functions as an inhibitory signal within the KNDy neural network, acting presynaptically on kappa opioid receptors (KOR) to inhibit the release of NKB, thus terminating kisspeptin release and terminating the signal for GnRH secretion (7).
Despite the autonomous nature of the GnRH pulse generator, it is modulated by various signalling systems, including metabolic, circadian and stress, to regulate reproductive function. The amygdala, a key limbic brain structure commonly known for its role in higher-order emotional processing, is implicated in reproduction, including psychological stress-induced suppression of pulsatile LH secretion (8) and therefore it is reasonable to suggest that the amygdala is a component of upstream regulation of the hypothalamic GnRH pulse generator. The finding of extra-hypothalamic Kiss1 expression and its receptor in the medial amygdala, and more specifically its posterodorsal subnucleus (MePD) (9), has opened up new possibilities concerning its role in reproductive function. Indeed, we have shown that the MePD Kiss1 neurones are a key upstream regulator of pubertal timing (10), sexual motivation and social behaviour (11). Moreover, we have discovered through neuropharmacological studies that Kp signalling in the MePD per se robustly regulates hypothalamic GnRH pulse generator frequency (12), however, the underlying neural mechanisms are unknown.
In this study, we have used an optogenetic approach to selectively stimulate the MePD Kiss1 neurones in fully-conscious mice to characterise the parameters that alter LH pulse frequency. Additionally, since Kiss1 neurones in the ARC can be directly modulated by GABA (13, 14) and there are a large number of GABAergic neurones in the MePD (15, 16), this neurotransmitter might potentially mediate changes in LH pulse frequency observe during optogenetic stimulation of Kiss1 neurones in the MePD. Therefore, we will also determine whether tdTomato labelled Kiss1 neurones in the MePD co-expressed GABA.
Materials and methods
Animals
Adult female mice (strain 129S6Sv/Ev) weighing between 19-23 g and aged between 6-8 weeks were used. They were bred in house at King’s College London and genotyped with a multiplex PCR protocol to detect heterozygosity for the Kiss-Cre or wild-type allele (17). The primers to detect the wild-type allele were mKiss hetF3 (CCG TCA TCC AGC CTA AGT TTC TCA C) and mKiss hetR3 (ATA GGT GGC GAC ACA GAG GAG AAG C), and the primers to detect the mutant allele were mKiss a526 (GCT TTT ATT GCA CAA GTC TAG AAG CTC) and Asc403 (CAG CCG AAC TGT TCG CCA GGC TCA AGG). The Kiss-CRE;tdTomato mice carry a CRE-activated tdTomato transgene that is specifically expressed in Kiss1 neurons (17). Mice were kept singularly housed under controlled conditions (12 :12 h hour dark/light cycle, on at 07:00 h, 25 °C) and provided with food and water ad libitum. All animal procedures performed were approved by the Animal Welfare and Ethical Review Body (AWERB) Committee at King’s College London, and in accordance with the UK Home Office Regulations.
Unilateral stereotaxic injection of channelrhodopsin viral construct and implantation of fibre optic cannula
All surgical procedures were performed under aseptic conditions. General anaesthesia was achieved using ketamine (Vetalar, 100 mg/kg, i.p.; Pfizer, Sandwich, UK) and xylazine (Rompun, 10 mg/kg, i.p.; Bayer, Leverkusen, Germany). Animals were firstly bilaterally ovariectomised immediately before being secured in a motorised Kopf stereotaxic frame. All cranial and brain surgical procedures were performed using a robot stereotaxic system (Neurostar, Tubingen, Germany). A small hole was drilled in the skull at a location above the MePD. The stereotaxic injection coordinates used to target the MePD were obtained from the mouse brain atlas of Paxinos and Franklin (18) (2.1 mm lateral, 1.70 mm posterior to bregma and at a depth of 5.1 mm). Using a 2-μL Hamilton micro-syringe (Esslab, Essex, UK) attached to the robot stereotaxic frame, 1 μl of the AAV-construct (AAV9.EF1.dflox.hChR2(H134R)-mCherry.SPRE.hGH (4.35 x 1013 GC/ml; Penn Vector Core, University of Pennsylvania, USA) was injected unilaterally into the MePD at a rate of 100 nl/min. The needle was left in position for a further 5 min and then removed slowly over 1 min. A fibre optic cannula (200 μm, 0.39NA, 1.25 mm ceramic ferrule; Thorlabs LTD, Ely, UK) was then inserted at the same co-ordinates as the injection site, but to a depth of 4.85 mm, so that the fibre optic cannula was situated immediately above the latter. A glue composite (RS Pro 20 g Super Glue, RS Components, Corby, UK) was then used to fix the cannula in place, and the skin incision closed with suture. All mice, both test (Cre+) and wild-type (Cre-), received the AAV injection and implantation of a fibre optic cannula. Following a 1 week recovery period from surgery, the mice were handled daily to acclimatise them to the tail-tip blood sampling procedure (19).
In vivo optogenetic stimulation of MePD kisspeptin neurones and blood samplings for LH measurement
All experiments were conducted in the absence of gonadal hormone replacement, in order to mitigate the modulatory effects of circulating oestrogen on the neuroendocrine control of the GnRH pulse generator. Experiments were carried out at least 4 weeks following surgery, to ensure sufficient opsin expression as well as to allow for sufficient habituation to the bleeding protocol. Prior to optogenetic stimulation, the very tip of the mouse’s tail was excised using a sterile scalpel for subsequent blood sample collection (20). The chronically implanted fibre optic cannula was then attached via a ceramic mating sleeve to a multimode fibre optic rotary joint patch cables (Thorlabs), allowing freedom of movement of the animal, for delivery of blue light (473 nm wavelength) using a Grass SD9B stimulator controlled DPSS laser (Laserglow Technologies, Toronto, Canada). Laser intensity at the tip of the fibre optic patch cable was 5 mW. After 1 h acclimatisation, blood samples (4 μl) were collected every 5 min for 2.5 h. After 1 h controlled blood sampling, continuous optic stimulation (5-ms pulse width) was initiated at 0.5, 2 or 5 Hz for 90 min. To control for the effects of viral infection and surgical procedure, AAV-infected and fibre optic-implanted Kiss-Cre+ animals were bled for LH pulse detection in the absence of optic stimulation. Furthermore, to control for the effects of optogenetic stimulation, Cre- animals, which would therefore not express the ChR2, were bled in the presence of 5 Hz optic stimulation. The Kiss-Cre+ mice received all the stimulation protocols in random order, with at least two days, but typically one week, between experiments. Cre- (n = 5) animals received 5 Hz optic stimulation only.
The blood samples were processed by ELISA as reported previously (19). Mouse LH standard and antibody were purchased from Harbour-UCLA (California, USA) and secondary antibody (NA934) was from VWR International (Leicestershire, UK). The intra-assay and inter-assay variations were 4.6% and 10.2%, respectively.
Validation of AAV injection site and fibre optic cannula placement
After completion of experiments, mice were anaesthetised with a lethal dose of ketamine and transcardially perfused with heparinised saline for 5 min, followed by 10 min of ice-cold 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4) for 15 min using a pump (Minipuls, Gilson, Villiers Le Bel, France). Brains were rapidly collected and postfixed sequentially at 4 °C in 15% sucrose in 4% PFA and in 30% sucrose in phosphate-buffered saline until they sank. Afterwards, brains were snap-frozen on dry ice and stored at -80 °C until processing. Brains were coronally sectioned (30-μm) using a cryostat (Bright Instrument Co., Luton, UK) and every third section was collected between -1.34 mm to -2.70 mm from the bregma. Sections were mounted on microscope slides, air-dried and cover slipped with ProLong Antifade mounting medium (Molecular Probes, Inc. OR, USA). Precise injection sites were verified and evaluated and only animals expressing mCherry fluorescent protein in the MePD were included in the analysis. Positive neurones expressing mCherry fluorescent protein throughout the MePD were quantified using an Axioskop 2 Plus microscope (Zeiss, Gottingen, Germany). The neuroanatomical landmarks bordering the MePD were determined using a reference guide from the mouse brain atlas (17). The number of mCherry positive neurones was counted in the MePD of each animal using 5-6 section and the total number was used to calculate the group mean (mean ± SEM). Images were taken using Axioskop 2 Plus microscope (Zeiss) equipped with Axiovision version 4.7 (Zeiss). Additionally, correct fibre optic cannula placement in the MePD was confirmed by microscopic inspection of the same 30-μm brain sections. Only data from animals with correct AAV-injection and cannula placement were analysed.
LH Pulses and Statistical Analysis
Detection of LH pulses was established by use of the Dynpeak algorithm (21). The effect of optogenetic stimulation on parameters of LH secretion was calculated by comparing the mean LH inter-pulse interval within the 90 min stimulation period with the 60 min pre-stimulation control period. For the non-stimulated control animals, the same timepoints were compared. The mean interval between LH pulses, within the 90 min stimulation period, or equivalent, was also compared between experimental groups. Statistical significance was tested using a two-tailed paired t-test. The LH pulse amplitude was calculated as the difference between the peak of an LH pulse and the baseline LH level, and the means were compared between the 60 min pre-stimulation period and 90 min stimulation period. The LH pulse amplitude was also compared between experimental groups within the 90 min stimulation period. P < 0.05 was considered statistically significant. Data are presented as the mean ± SEM.
Immunohistochemistry
Ovary intact 6-8 weeks old Kiss/CRE - tdTomato mice (strain 129S6Sv/Ev;C57B6) in dioestrus (determined via vaginal cytology) were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) pH 7.6. (Sigma-Aldrich, UK). The brains were removed and post-fixed in the same fixative at room temperature for 1 hour and then transferred into 30% sucrose solution in PBS until they sunk. The brains were cut on a microtome with a freezing stage into 30μm coronal sections and stored in cryoprotectant media at -20°C. Free-floating sections were washed in 1X Tris-buffered saline (TBS, pH 7.5) three times to remove the cryoprotectant media, then subjected to antigen retrieval in citrate buffer (pH 6.0) at 80°C for 30 min. The sections were washed in Tris-buffered saline containing 0.1% Triton X-100 (TBST) three times then blocked with 5% BSA (Sigma-Aldrich, UK) and 0.5% Triton X (Sigma-Aldrich, UK) for 30 min at room temperature on an orbital shaker. Sections were incubated with rabbit anti-GABA (A2052,1:600 dilution, Sigma-Aldrich, UK) in incubation media (0.25% Triton-X-100, 0.3% bovine serum albumin, 2% normal serum (NGS; Sigma-Aldrich, UK) in 1X TBS, pH 7.5) for 48 h at 4 °C on an orbital shaker. After the primary antibody incubation, sections were washed three times in TBST and then incubated with fluorophore 488-conjugated goat anti-rabbit (1:400 dilution; Thermo Fisher Scientific, UK) for 2 h at room temperature in the dark on an orbital shaker. Sections were then washed three times in 1X TBS and mounted on poly-lysine-coated slides (VWR, UK) in mounting medium (Fluoromount-G, ThermoFisher), cover slipped and stored at 4°C in the dark until being imaged using epi-fluorescence and confocal microscopy (Leica SP2 Laser Scanning Confocal Microscope, Cambridge Advanced Imaging Centre, UK). The brain sections were analysed for co-localization between the tdTomato fluorescence (red) and the GABA immunostaining signal (green) by photomicroscopy of each channel and combining the images for overlap of the signals indicated by a yellow colour. A signal was judged to be positive of the shape of the cell was co-incident between the two channels. To eliminate signals were not erroneously generated by overlapping cells, thin 30um sections were used and the focal plane of the signal was restricted by con-focal microscopy. For each section, the analysis was performed bilaterally.
Results
Validation of AAV injection site and fibre optic cannula placement
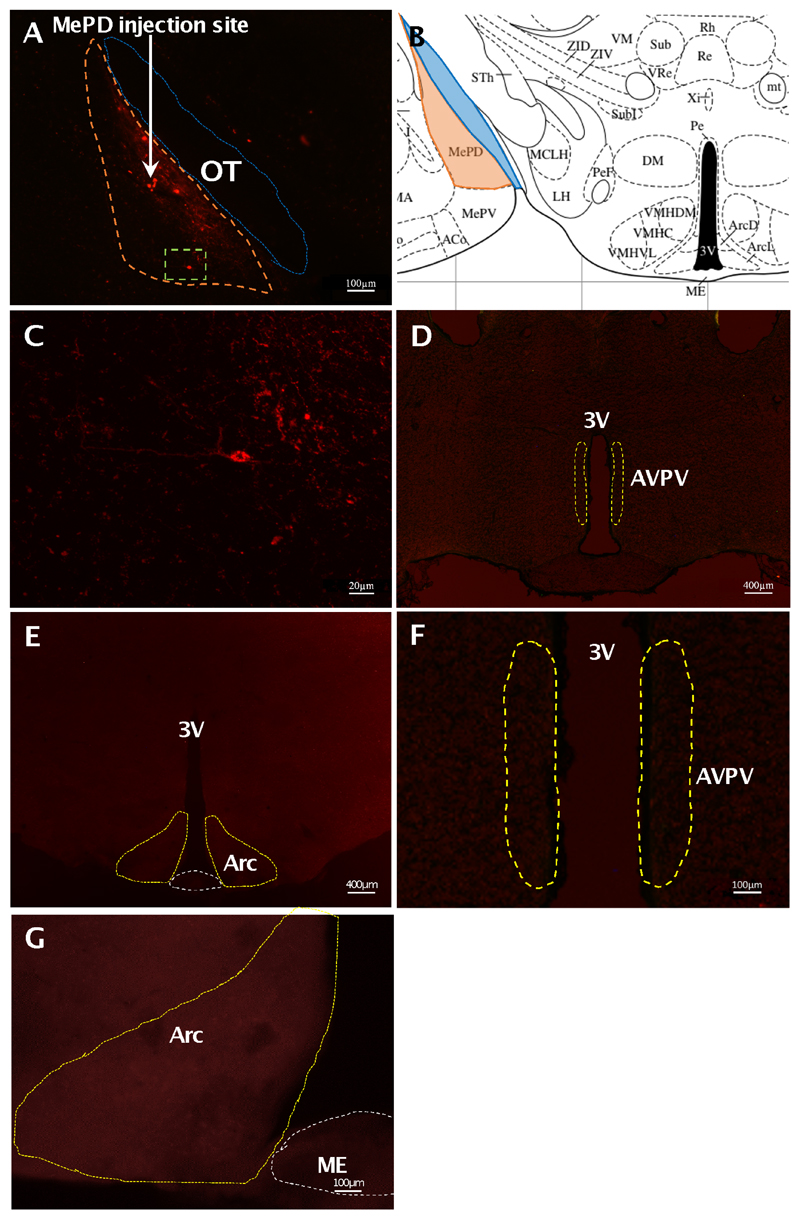
The AAV-ChR2 virus used to infect the cells in Kiss-Cre+ mice was tagged with fluorescent mCherry, allowing it to be visualised under a microscope. Analysis of images acquired from coronal sectioning of the mouse brains showed that 6 out of the 8 animals had successful stereotaxic injection of AAV-ChR2 virus into the MePD. The mean number of mCherry-positive cells in unilaterally-injected brain sections was 22.00 ± 4.81 per animal. A representative example of a coronal brain section is presented in figure 1. Animals with misplaced injections did not have altered LH pulse frequencies following optogenetic stimulation (data not shown). mCherry cell bodies were not seen within the AVPV or ARC, indicating targeted ChR2 expression to Kiss1 neurones in the MePD (Fig. 1D-G). Additionally, there was an absence of mCherry expression within the MePD of the AAV-ChR2 injected WT animals (data not shown). All fibre optic cannulae were correctly placed in the MePD.
Figure 1. Expression of ChR2-mCherry in MePD kisspeptin neurones in Kiss-CRE mice.
Coronal section showing red mCherry fluorescence positive neurones (orange line) in the MePD (A) and the white arrow indicates the injection site of AAV9.EF1.dflox.hChR2(H134R)-mCherry.WPRE.hGH into the MePD of Kiss-Cre mice in the same section. Higher-power view shows the MePD kisspeptin neurones tagged with mCherry (red fluorescence), which indicates ChR2 receptor expressing kisspeptin neurones (C); this image is a magnification of the area of Fig. 1A encased within the green dotted line. The absence of mCherry fluorescence in the AVPV (D) and ARC or median eminence (ME) (E) shows the specific infection of MePD kisspeptin cells with the channelrhodopsin. Higher-power view of the AVPV (F) and ARC (G) further confirms the absence of mCherry-infected cells in these regions. Schematic representation of MePD and its spatial relationship with the optic tract (B). ME, median eminence; OT, optic tract; 3V, third ventricle.
Effects of continuous optogenetic stimulation of MePD kisspeptin neurones on LH pulse parameters
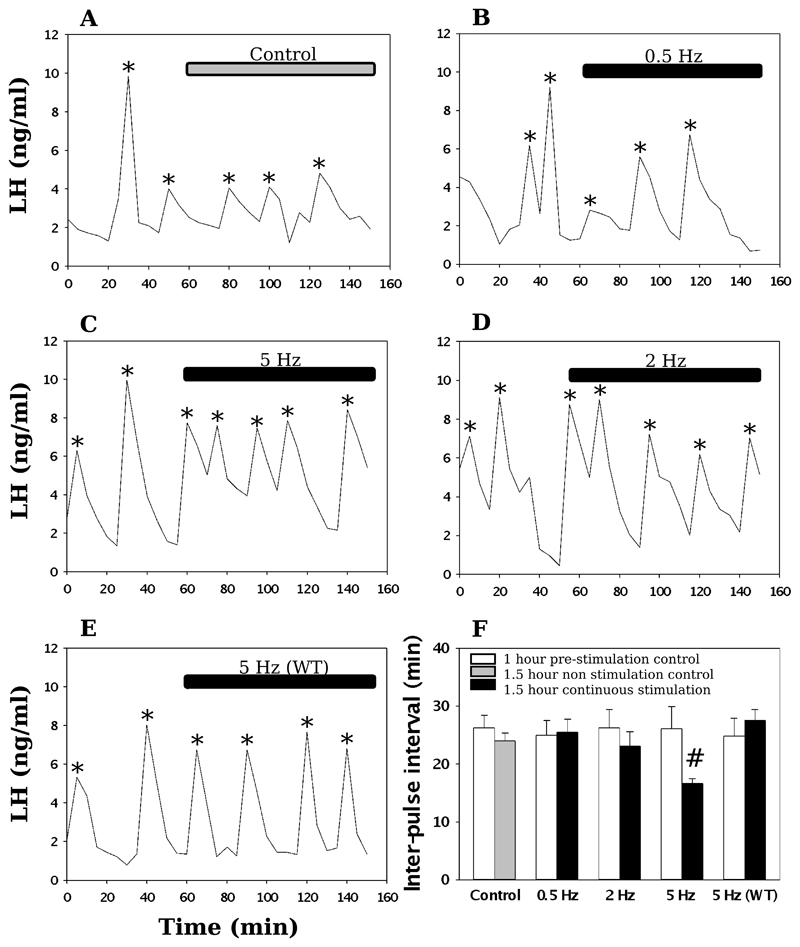
Following 1 h blood sampling with no light stimulation, MePD kisspeptin neurones were stimulated with blue light at 0.5, 2, or 5 Hz for 90 min. In Kiss1-Cre+ mice, only continuous stimulation at 5 Hz resulted in a significant increase in LH pulse frequency from a mean inter-pulse interval (IPI) of 26.07 ± 3.81 min to 16.64 ± 0.83 min (Fig. 2). No significant change in IPI was observed at 0.5 or 2 Hz optic stimulation, or in the non-stimulated controls (Fig. 2). Additionally, in Kiss1-Cre- mice, there was no change in LH pulse frequency at 5 Hz optic stimulation (Fig. 2). In terms of LH pulse amplitude, there was no statistically significant difference between the 60 min control period and the 90 min stimulation, or equivalent non-stimulation period for each group (Table 1).
Figure 2. Effect of continuous optogenetic stimulation of MePD Kiss1 neurones on LH pulse frequency.
Representative examples showing the effects of no stimulation (A), 0.5 Hz (B), 2 Hz (C), 5 Hz stimulation (D) on pulsatile LH secretion in ovariectomised mice. A representative example is also shown for the effect of 5 Hz stimulation on ovariectomised wild-type mice (E). (F), The mean LH inter-pulse interval for increasing frequencies before and after the onset of stimulation. Continuous stimulation at 5 Hz resulted in a significant reduction in the LH inter-pulse interval. LH pulses detected by the DynePeak algorithm are indicated with an asterisk. #P < 0.05 vs control, (n = 6 per treatment group). Results represent mean ± SEM.
Table 1. LH pulse amplitude before and during optogenetic stimulation of the MePD Kiss1 neurones.
The mean (± SEM) values for LH pulse amplitude before and during optic stimulation showed no statistically significant difference in each experimental group between the mean LH pulse amplitude before and during 5 Hz optic stimulation n = 6 per group.
| Frequency of optic stimulation | Mean LH pulse amplitude (ng/ml) | |
|---|---|---|
| Control period | Stimulation period | |
| Control (no optic stimulation) | 3.50 ± 2.05 | 4.26 ± 1.91 |
| 0.5 Hz | 3.31 ± 1.71 | 3.46 ± 0.76 |
| 1 Hz | 3.19 ± 1.59 | 3.22 ± 1.20 |
| 2 Hz | 3.91 ± 2.70 | 4.45 ± 2.88 |
| 5 Hz | 4.38 ± 3.45 | 3.67 ± 2.34 |
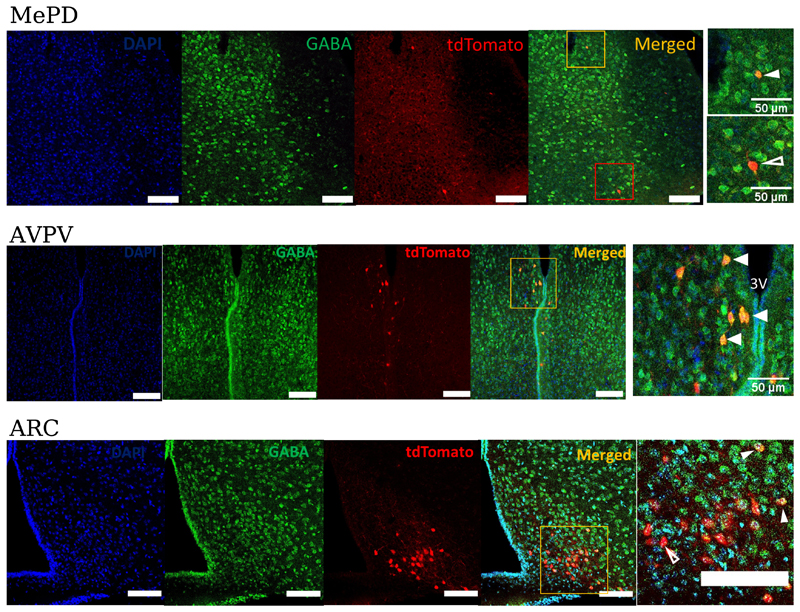
Kiss1 neurones in the medial amygdala do not co-express GABA
Kiss1 neurones in the ARC can be directly modulated by GABA (13, 14) and as there are a large number of GABAergic neurones in the MePD, we hypothesised that this neurotransmitter might mediate the changes in LH pulse frequency we observe after optogenetic activation of Kiss1 neurones in the MePD. GABA expression was analysed by immunohistochemistry (IHC) in tdTomato positive Kiss1 neurons in the MePD AVPV, and ARC (Fig. 3). The anti-GABA antibody has been used widely by other groups and its specificity is supported by IHC studies using tissues from GAD65 knock-out mice, which have reduced GABA production (22, 23) and in mice lacking both GAD65 and GAD67 where no GABA immunoreactivity is found (24). In the AVPV region, we found that 71% of tdTomato positive Kiss1 neurones co-expressed GABA (n = 5 mice, 105 out of 147 neurones) but in the MePD, only 10% co-expression was found (n = 6 mice, 9 out of 93 neurones). In the ARC, 14% of tdTomato positive neurones expressed GABA (n = 3 mice, 139 out of 984 neurones).
Figure 3. GABA expression in Kiss1-tdTomato labelled neurones in different regions of the hypothalamus.
Brain sections from gonadally intact Kiss-CRE;tdTomato female mice were examined for co-localization between tdTomato and GABA. tdTomato was detected by intrinsic fluorescence in the brain slices and GABA by immunohistochemistry. In the MePD, the white filled arrowhead indicates a tdTomato neurone (red) that co-expresses GABA (green). Unfilled arrowhead indicates a tdTomato neurone (red) that does not co-express GABA. The fifth panel is a magnified area of the region shown by the orange and red box in the merged panel respectively. n = 6 mice, 7-11 sections per mouse. In the AVPV region, arrowheads indicate tdTomato neurones (red) that co-express GABA (green). The fifth panel is a magnified area of the region shown by the orange box in the merged panel. 3V, third ventricle. n = 5 mice, 4-5 sections per mouse. In the ARC region, very few GABA positive neurones were found and only 14% of tdTomato positive neurones expressed GABA. White filled arrowhead indicates Kiss1-tdTomato neurones (red) that co-express GABA (green). Unfilled arrowheads indicate Kiss1-tdTomato neurones (red) that do not co-express GABA. The fifth panel is a magnified area of the area encased by the orange box in the merged panel. Scale bar =100μm. n = 3 mice, 4-5 sections per mouse. For all sections nuclei were counterstained with DAPI (blue).
Discussion
The present study demonstrates that optogenetic stimulation of Kiss1 neurones within the MePD increases LH pulse frequency and provides clear evidence that MePD Kiss1 neuronal activity can modulate the frequency of the hypothalamic GnRH pulse generator. These data extend our earlier neuropharmacological findings where intra-MePD administration of Kp dose-dependently increased LH levels (12) and more recent studies showing chemogenetic activation of MePD Kiss1 neurones increased mean circulating levels of LH (25, 26). The relationship between the amygdala and its regulation of gonadotrophic hormone secretion has received considerable attention, with evidence to support the notion that the medial amygdala in fact has a negative output on the reproductive system. Stimulating the medial amygdala results in delayed pubertal onset (27), whilst lesions advance the onset of puberty (28) and increase the secretion of LH (29). Indeed, the findings that up to 70% of the neuronal outputs arising from the MePD are GABAergic (16), including a significant percentage of those reaching reproductive centres (15), support the idea of an MePD inhibitory modulation of reproduction. We have recently shown MePD neurones projecting directly onto ARC Kiss1 neurones, although their neurochemical phenotype remains unknown (30). The identification of the latter connection to the KNDy network might help to establish the underlying mechanisms by which the MePD regulates the GnRH pulse generator. Interestingly, MePD Kiss1 neurones have also been shown to project to GnRH neurones in the medial preoptic area (POA) (31). However, our previous data showing Kiss1 receptor antagonism in the MePD reduced LH pulse frequency (12) would suggest that MePD Kiss1 signalling per se rather than Kiss1 output to the POA GnRH neurones underlies the upstream regulation of the hypothalamic GnRH pulse generator. Moreover, chemogenetic activation of MePD Kiss1 fails to activate GnRH neurones (25). These data and our present finding that continuous lower-frequency optic stimulation increased the frequency of LH pulses supports the concept that MePD control of LH secretion is via activation of the KNDy neurones in the ARC, which are thought to be a major component of the neural construct underlying GnRH pulse generation (3, 4).
How can we reconcile the findings that classical stimulation and lesion studies suggest an inhibitory influence of the amygdala, whilst selective optical stimulation of MePD Kiss1 enhances LH secretion? Clearly, these experimental manipulations are different, with lesion and stimulation studies affected a wider range of neurons than specific activation of Kiss1 neurones. In addition, although GABAergic neurones are present in the MePD, we did not find extensive co-localisation between GABA and tdTomato labelled Kiss1 neurones in this region. In contrast, we found that GABA was co-localized with around 70% of tdTomato positive Kiss1 neurons in the AVPV which is similar to that found by others (32). In the ARC, GABA staining was found in only 14% of Kiss1 neurones, which is lower than the 25-50% reported elsewhere (32, 33). This discrepancy may reflect different methodological approaches as we have measured GABA directly while the other groups used less direct approaches such as in situ hybridization for Gad67 expression (32) or reporter activity driven from the Vgat promoter (33). Alternatively, it is possible that the anti-GABA antibody may not detect all GABAergic neurones in the ARC although we consider this to be unlikely given the data for robust staining in the AVPV. Since very few Kiss1 neurones in the MePD are GABAergic, we would not expect these to be inhibitory on LH secretion.
An alternative, but less simple explanation might involve a disinhibitory system whereby Kiss1 in the MePD activates inhibitory GABAergic interneurons, which in turn inhibit GABAergic efferents to the ARC. Whilst this remains hypothetical, it is important to realise that the MePD is of pallidal origin (34), therefore suggesting that a neural circuit within this structure is, atleast in part, of a classical GABAergic disinhibitory nature. Although the endogenous firing pattern of MePD Kiss1 neurones is unknown, we have shown that continuous optogenetic activation of this population at the relatively low frequency of 5 Hz increased LH pulse frequency, thus demonstrating the minimal activational requirements to modulate the hypothalamic GnRH pulse generator. Further work is required to establish the functional link between MePD Kiss1 neurone activation and GABAergic signalling to affect pulsatile LH secretion.
Evidence that Kiss1 neurones within the MePD have a stimulatory role modulating the GnRH pulse generator is an important development in reproductive neuroendocrinology, as it provides further mechanistic insight into why KISS1R receptor antagonism in the MePD was shown to delay puberty, disrupt oestrous cyclicity and reduce the occurrence of the preovulatory LH surge in the rat (10). The MeA is highly-active in response to a range of external stressors such as restraint (35), footshock (36), and predatory odour (37), and is involved in the activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to external anxiogenic stimuli; increased levels of ACTH and glucocorticoids are accompanied with stimulation of the MeA (38). Additionally, as part of the limbic brain, the MePD is an upstream regulator that likely acts as a neural hub integrating several external signals, including olfactory chemosignals and anxiogenic stimuli, with the function of the GnRH pulse generator. Kp and the amygdala have been shown to be critically involved in the integration of olfactory cues with the promotion of sexual behaviour. Lesioning of the MePD results in a diminution of sexual partner preference in rodents (39), and in the “ram effect”, whereby the introduction of asexually active ram can overcome reproductive inactivity in anoestrous ewes, the resurgence of functional LH pulsatility coincides with an increase in Kiss1 neurone activity (FOS) in the ARC, POA, and MeA (40, 41). Our previous study using pharmacological activation of MePD Kiss1 neurones with the DREADD (Designer Receptors Exclusively Activated by Designer Drugs) methodology built upon previous understanding of the facilitatory role of the MePD in sexual behaviour. We showed that stimulating MePD Kiss1 neurones in this way produces a dual result of augmenting sexual partner preference whilst attenuating anxiety behaviour in male mice (11). Furthermore, in studies of men viewing sexual images, intravenous administration of kisspeptin results in increased neuronal activity in the amygdala, detected with fMRI neuroimaging, corresponding to a reduction in negative mood and reduced aversion to sex (42). Taken together, these are important developments in reproductive neuroendocrinology. Investigating kisspeptin in the limbic brain has been able to further our understanding of the elusive neural mechanisms governing reproductive physiology, including pubertal development, as well as the mechanisms by which stress has its inhibitory effects on reproductive capability.
Table 2. Number of Kiss1-tdTomato labelled neurones co-localised with GABA in different regions of the hypothalamus and amygdala.
The mean (± SEM) values of co-localisation of GABA in Kiss1-tdTomato labelled neurones in each region. n = 3 - 6 animals per group.
| Region | Number of Kiss1-tdTomato labelled cells | Number of colocalization with GABA |
|---|---|---|
| MePD | 18.6 ± 3.5 | 1.8 ± 0.7 |
| AVPV | 36.75 ± 14.9 | 26.25 ± 10.9 |
| ARC | 328 ± 3.1 | 46.33 ± 5.7 |
Acknowledgements
We are extremely grateful for all the help and advice on optogenetics provided by Dr. Matt Grubb, Centre for Developmental Neurobiology, Faculty of Life Sciences and Medicine, King's College London, UK. The authors gratefully acknowledge the financial support from the MRC (MR/N022637/1). GL was funded by an MRC PhD studentship.
Footnotes
Data Availability Statement: All data contained within the manuscript have been deposited in the King’s Research Data Management System and are freely available to public access (www.kcl.ac.uk/library/researchsupport/research-data-management/preserve/deposit-your-data-with-kings3).
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Jr, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 2.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E23. doi: 10.1073/pnas.1713897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voliotis M, Feng Li X, De Burgh R, Lass G, Lightman SL, O’Byrne KT, Tsaneva-Atanasova K. The origin of GnRH pulse generation: An integrative mathematical-experimental approach. J Neurosci. 2019 doi: 10.1523/JNEUROSCI.0828-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation GnRH release. J Neuroendocrinol. 2011;23(10):863–70. doi: 10.1111/j.1365-2826.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 6.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–32. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. Elife. 2016;5 doi: 10.7554/eLife.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Li X, Lupi M, Kinsey-Jones JS, Shao B, Lightman SL, O’Byrne KT. The role of the medial and central amygdala in stress-induced suppression of pulsatile LH secretion in female rats. Endocrinology. 2011;152(2):545–55. doi: 10.1210/en.2010-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020–30. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adekunbi DA, Li XF, Li S, Adegoke OA, Iranloye BO, Morakinyo AO, Lightman SL, Taylor PD, Poston L, O’Byrne KT. Role of amygdala kisspeptin in pubertal timing in female rats. PLoS One. 2017;12(8):e0183596. doi: 10.1371/journal.pone.0183596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adekunbi DA, Li XF, Lass G, Shetty K, Adegoke OA, Yeo SH, Colledge WH, Lightman SL, O’Byrne KT. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol. 2018 doi: 10.1111/jne.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, Jayasena CN, Ghatei MA, Bloom SR, Matthews PM, O’Byrne KT, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016;221(4):2035–47. doi: 10.1007/s00429-015-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 2014;34(49):16296–308. doi: 10.1523/JNEUROSCI.3057-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Ronnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114(9):2413–8. doi: 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–60. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Keshavarzi S, Sullivan RK, Ianno DJ, Sah P. Functional properties and projections of neurons in the medial amygdala. J Neurosci. 2014;34(26):8699–715. doi: 10.1523/JNEUROSCI.1176-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, Chen C, Colledge WH. Visualisation of Kiss1 Neurone Distribution Using a Kiss1-CRE Transgenic Mouse. J Neuroendocrinol. 2016;28(11) doi: 10.1111/jne.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 19.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adultmale mice. Endocrinology. 2013;154(12):4939–45. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE. Pulse and Surge Profiles of Luteinizing Hormone Secretion in the Mouse. Endocrinology. 2016;157(12):4794–802. doi: 10.1210/en.2016-1351. [DOI] [PubMed] [Google Scholar]
- 21.Vidal A, Zhang Q, Medigue C, Fabre S, Clement F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 2012;7(7):e39001. doi: 10.1371/journal.pone.0039001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi J, Kim M, Sanchez R, Ziaee SM, Kohtz JD, Koh S. Enhanced susceptibility to stress and seizures in GAD65 deficient mice. PLoS One. 2018;13(1):e0191794. doi: 10.1371/journal.pone.0191794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94(12):6496–9. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33(3):187–94. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S, Tang C, Sing K, Kim HW, Millar RP, Tello JA. Medial Amygdala Kiss1 Neurons Mediate Female Pheromone Stimulation of Luteinizing Hormone in Male Mice. Neuroendocrinology. 2019;108(3):172–89. doi: 10.1159/000496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fergani C, Leon S, Padilla SL, Verstegen AM, Palmiter RD, Navarro VM. NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice. Elife. 2018;7 doi: 10.7554/eLife.40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Sela M, Critchlow V. Delayed puberty following electrical stimulation of amygdala in female rats. Am J Physiol. 1966;211(5):1103–7. doi: 10.1152/ajplegacy.1966.211.5.1103. [DOI] [PubMed] [Google Scholar]
- 28.Stephens SB, Raper J, Bachevalier J, Wallen K. Neonatal amygdala lesions advance pubertal timing in female rhesus macaques. Psychoneuroendocrinology. 2015;51:307–17. doi: 10.1016/j.psyneuen.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawton IE, Sawyer CH. Role of amygdala in regulating LH secretion in the adult female rat. Am J Physiol. 1970;218(3):622–6. doi: 10.1152/ajplegacy.1970.218.3.622. [DOI] [PubMed] [Google Scholar]
- 30.Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PLoS One. 2019;14(3):e0213927. doi: 10.1371/journal.pone.0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology. 2017;104(3):223–38. doi: 10.1159/000445895. [DOI] [PubMed] [Google Scholar]
- 32.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Atkin S, Jr, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. Neuroendocrinology. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong RY, Czieselsky K, Porteous R, Herbison AE. Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice. J Neurosci. 2015;35(43):14533–43. doi: 10.1523/JNEUROSCI.1776-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. 2012;6:33. doi: 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J Neuroendocrinol. 2005;17(1):22–8. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796(1–2):132–42. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- 37.Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos 427 immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104(4):1085–97. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 38.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 2012;105(2):554–9. doi: 10.1016/j.physbeh.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabre-Nys C, Cognie J, Dufourny L, Ghenim M, Martinet S, Lasserre O, Lomet D, Millar RP, Ohkura S, Suetomi Y. The Two Populations of Kisspeptin Neurons Are Involved in the Ram-Induced LH Pulsatile Secretion and LH Surge in Anestrous Ewes. Endocrinology. 2017;158(11):3914–28. doi: 10.1210/en.2017-00429. [DOI] [PubMed] [Google Scholar]
- 41.Gelez H, Fabre-Nys C. Neural pathways involved in the endocrine response of anestrous ewes to the male or its odor. Neuroscience. 2006;140(3):791–800. doi: 10.1016/j.neuroscience.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 42.Comninos AN, Wall MB, Demetriou L, Shah AJ, Clarke SA, Narayanaswamy S, Nesbitt A, Izzi-Engbeaya C, Prague JK, Abbara A, Ratnasabapathy R, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest. 2017;127(2):709–19. doi: 10.1172/JCI89519. [DOI] [PMC free article] [PubMed] [Google Scholar]