Abstract
Background
There is a substantial and unmet clinical need for pharmacological treatment of cannabis use disorders. Cannabidiol (CBD) could offer a novel treatment but it is unclear which doses might be effective or safe.
Methods
Participants meeting DSM-5 cannabis use disorder criteria were allocated to four-week treatment with oral CBD at 200mg, 400mg, 800mg or placebo during a cessation attempt using a double-blinded block randomisation sequence. All received a brief psychological intervention of motivational interviewing. An adaptive Bayesian dose-finding design was used to identify effective/ineffective doses at a priori interim and final analysis stages. The primary objective was to identify the Most Effective Dose (MED) of CBD for reducing cannabis use. The primary endpoint was lower urinary THC-COOH:creatinine concentrations and/or increased days per week abstinent from cannabis during treatment, evidenced by posterior probabilities exceeding Pr=0.9 for CBD versus placebo. All analyses were intention-to-treat.
Outcomes
Participants were initially randomised to placebo, 200mg, 400mg and 800mg CBD (n=48; 1:1:1:1). At interim analysis 200mg CBD was eliminated from the trial as an ineffective dose. Randomisation continued to 400mg CBD, 800mg CBD, and placebo (n=34; 1:1:1). At final analysis, both 400mg CBD and 800mg CBD exceeded primary endpoint criteria (Pr=0.9) for both primary outcomes: urinary THC-COOH:creatinine (Pr(400mg=MED | Data)=0.9995; Pr(800mg=MED | Data)=0.9965), days per week abstinent from cannabis (Pr(400mg=MED | Data)=0.9966; Pr(800mg=MED | Data)=0.9247). Compared to placebo, 400mg CBD decreased THC-COOH:creatinine concentrations by -94.21 ng/ml (95% Interval Estimate= -161.83, -35.56) and increased abstinence from cannabis by 0.48 days per week (95% Interval Estimate=0.15, 0.82). Compared to placebo, 800mg CBD decreased THC-COOH:creatinine concentrations by -72.02 ng/ml (95% Interval Estimate= -135.47, -19.52) and increased abstinence from cannabis by 0.27 days per week (95% Interval Estimate= -0.09, 0.64). CBD was well tolerated with no severe adverse events and 94% completed treatment.
Interpretation
In the first randomised clinical trial of CBD for cannabis use disorder, 400mg and 800mg CBD were safe and more effective than placebo at reducing cannabis use.
Trial registration
https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-000361-36/GB
Introduction
Cannabis is increasingly being legalised for medicinal and recreational use. The long-term effects of these policy reforms are unclear at present but may include substantial changes to the types of cannabis product sold and their availability to millions of people worldwide.1 When considering the potential health effects of cannabis use, its largest contribution to the global burden of disease is the impact of cannabis use disorders, which affect an estimated 22 million people worldwide – similar to the prevalence of opioid use disorders.2
Delta-9-tetrahydrocannabinol (THC), a partial cannabinoid receptor agonist, is the primary cannabinoid in cannabis products and causes dose-dependent intoxicating and reinforcing effects.1 Studies in Europe3 and the USA4 reported a two-fold increase in THC concentrations in cannabis during the last decade. Use of higher THC products has been associated with a greater severity of cannabis use disorder5,6 and increases in the treated incidence of cannabis use disorders.7 In the past two decades, the proportion of people seeking treatment for cannabis use disorders has risen in all world regions apart from Africa.8 Cannabis is now the primary drug cited by first-time clients presenting at addiction services across Europe, increasing by 76% in the last decade.9 Daily use of high-THC cannabis is associated with a five-fold increased risk of psychosis.10 Despite the substantial and increasing demand for treatment there are no pharmacotherapies recommended for the treatment of cannabis use disorders.11
Cannabidiol (CBD) is another cannabinoid found in many cannabis products4. CBD has minimal direct action at cannabinoid receptors but it has a broad pharmacology including inhibiting the hydrolysis and reuptake of endocannabinoids12 and negative allosteric modulation of cannabinoid receptors13. CBD has generated significant interest due to its potential medicinal uses14 and ability to interact with the effects of THC.15 CBD has shown therapeutic effects in human and preclinical models of addiction by reducing the impact of drug-related cues in attentional bias16,17 cue-induced craving18 and cue-induced reinstatement19 paradigms. Collectively, these studies suggest that CBD may have potential for treating a range of substance use disorders including cannabis16, opioids18,19 and tobacco.17,20 A meta-analysis of randomised clinical trials found that CBD was safe and well tolerated with few adverse effects, but interactions with other medications should be monitored carefully as CBD can inhibit cytochrome P450 enzymes.21
To date, no randomised trials have investigated CBD as a potential treatment for cannabis use disorder. Open-label case studies have reported that use of CBD products were associated with reduced cannabis withdrawal symptoms during cannabis abstinence.22,23 A 10-week open-label trial found that CBD administration was associated with improvements in psychological wellbeing and cognition in regular cannabis users who were not engaged in a cessation attempt.24 Nabiximols (a combination of THC and CBD at 1:1 ratio) has been found to reduce cannabis withdrawal symptoms and/or cannabis use in some randomised double-blind placebo-controlled trials.25–27 However, the causal role of CBD in these studies is unclear because they either used an open-label design22–24 or co-administered THC with CBD.25–27
Studies in human28 and rat29 models of anxiety have reported inverted-U shaped dose-response effects of CBD. This highlights the importance of conducting an initial dose-finding trial when investigating a novel indication for CBD. Trials testing a single dose against placebo may fail to select the most effective dose for that indication. Therefore, in this study we conducted a Phase IIa trial to identify potentially effective doses and eliminate ineffective doses using an adaptive Bayesian design. Bayesian methods are advantageous for adaptive clinical trials due to their efficiency, flexibility and ability to make use of all available evidence in a formal and principled way. As a result, they can reduce the amount of resources and participant burden required when conducting clinical trials. Furthermore, Bayesian analyses provide direct probabilistic measures of the likelihood of a hypothesis (i.e. that a treatment is more effective than placebo), given the evidence provided by the data. As such they provide results that can be more clinically meaningful than frequentist analyses, which test of the likelihood of the data given the null hypothesis (i.e. that a treatment does not differ from placebo). We selected a dose range informed by previous clinical trials of oral CBD30,31 of 200mg, 400mg and 800mg daily for four weeks. Our primary objective was to identify which (if any) dose of CBD was most effective at reducing cannabis use compared to placebo.
Methods
Study design and participants
We conducted a Phase IIa, randomised, double-blind, placebo-controlled, single-site, parallel group clinical trial to investigate CBD as a pharmacological treatment for cannabis use disorder. The trial was prospectively registered before data collection began: https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-000361-36/GB and https://clinicaltrials.gov/ct2/show/results/NCT02044809 The trial was approved by the UK Health Research Authority (13/EE/0303) and the UK Medicines and Healthcare Regulatory Agency (20363/0325/001-0001). It was conducted according to Good Clinical Practice (GCP) and reported according to the CONSORT checklist (appendix pp 1-3).
Following telephone screening and a screening visit to determine eligibility, participants engaged in a cannabis cessation attempt scheduled to begin at their baseline visit (week 0). At the end of their baseline visit they were randomised to parallel treatment arms receiving either CBD (200mg, 400mg, 800mg) or placebo. Participants attended site visits weekly during treatment (weeks 1-4). Follow up occurred at weeks 6 (site visit), 8 (telephone), 12 (site visit) and 16 (site visit), 20 (telephone) and 24 (telephone).
Participants were recruited through advertisements on websites, forums and flyers in the local community. We initially intended to restrict eligibility to those aged 16-26 with vital signs in normal limits, but removed these criteria to increase the generalizability of our findings to a wider population who might stand to benefit from this treatment. Inclusion criteria were age 16-60 years, meeting DSM-5 criteria for a cannabis use disorder (at least moderate severity), to express a desire to stop using cannabis and intending to do so in the next month based on an adapted Motivation To Stop Scale.32 Participants were additionally required to report ≥1 failed quit attempt for their cannabis use, to report co-administering their cannabis together with tobacco, which is the most common method of using cannabis in Europe33, to provide a urine sample positive for THC-COOH (Urine Cup 10A: methamphetamine, cocaine, THC, benzodiazepines, tricyclic antidepressants, barbituates, phencyclidine, amphetamines, morphine, methadone; Alere Toxicology, Abingdon, UK), and capacity to give informed consent as defined by GCP guidelines.
Females of childbearing potential were required to have a negative pregnancy test within seven days of starting treatment. Additional inclusion criteria for females of childbearing potential and all males were to use an effective method of contraception including oral, injected or implanted hormonal methods of contraception; placement of an intrauterine system or device, a barrier method of contraception, or true abstinence from the time consent was signed until six weeks after treatment discontinuation.
Exclusion criteria were current breastfeeding or pregnancy, allergies to CBD, microcrystalline cellulose or gelatin, prescribed psychotropic drug use at screening assessments or during treatment weeks, use of other illicit drugs more than twice a month at screening, evidence of inaccurate self-reported drug use due to a positive urine test for a drug that was not reported during screening assessment, current or prior self-reported diagnosis of a psychotic disorder, any physical health problem deemed clinically significant by the investigator team, and not speaking English (due to verbal assessments). All data were collected at the Clinical Psychopharmacology Unit, UCL, UK.
Procedures
Synthetic CBD (99.9% purity) was obtained from STI Pharmaceuticals (Brentwood, UK) and manufactured by Nova Laboratories (Leicester, UK). Identical size 2 gelatin capsules contained microcrystalline cellulose filler and CBD (0mg, 50mg, 100mg, or 200mg). Participants were instructed to take two capsules twice daily to achieve daily doses of placebo, 200mg, 400mg and 800mg respectively. The bioavailability of oral CBD is increased by food, and twice-daily administration is recommended on the basis of pharmacokinetic and safety data.34 Participants received scheduled text messages twice daily reminding them to take their medication, spaced 12 hours apart. Dosette boxes were provided for each week of treatment to aid compliance. Compliance was assessed by self-report at weekly assessments as well as return of capsules. If participants failed to show adequate compliance on any treatment week (either ≥ 30% capsules returned, or ≥ 30% self-reported doses missed), or if they failed to attend a site visit within 2 days of the scheduled appointment during treatment, or if any concomitant psychotropic medication was taken during treatment, they were not provided with additional medication for the duration of the trial but continued all other aspects of the protocol.
All participants received a brief psychological intervention of motivational interviewing.35 Motivational interviewing is widely used in healthcare settings and has been found to reduce cannabis use in randomised trials of cannabis use disorder.36 Six 30 minute sessions of motivational interviewing were delivered by trained psychologists at the screening visit, baseline visit, and treatment weeks 1-4. During the first motivational interviewing session, a target quit date was planned to coincide with the baseline visit. All sessions were audio recorded for clinical supervision. Training and supervision were provided by a lead clinical psychologist based in specialist National Health Service drug services throughout the duration of the trial.
Outcomes
Primary outcomes were cannabis use, as measured in urine (THC-COOH:creatinine concentrations) and by self-report (days per week abstinent from cannabis). These were the two variables that were most strongly associated with cannabis use disorder severity in a study testing 15 different biological and self-report measures of cannabis use.37 Urine samples were collected at site visits using temperature monitored cups to ensure compliance (Galle pot, Synergy Health, Abergavenny, UK). They were stored in 10ml polypropylene tubes at -80°C prior to analysis using Liquid Chromotography-Tandem Mass Spectrometry by ABS Laboratories (Hertford, UK) with a lower limit of THC-COOH quantification of 1 ng/ml. Self-reported days abstinent from cannabis were assessed weekly using the Timeline Follow-back method.38 The primary endpoint was a reduction in urinary THC-COOH:creatinine concentrations and/or increased days per week abstinent from cannabis for CBD versus placebo during treatment, evidenced by posterior probabilities exceeding Pr = 0.9. All primary endpoint data were double-entered independently by two researchers and were 100% verified against source data by an independent clinical trial monitor. Reductions in cannabis use up to the final follow up were analysed as a secondary endpoint.
For secondary outcomes, we recorded the total score of the Cannabis Withdrawal Scale39, tobacco use assessed in urine (cotinine:creatinine concentration, lower limit of cotinine quantification 1 ng/ml; ABS Laboratories) and by self-report (number of cigarettes smoked using the Timeline Follow-back method).38 Alcohol consumption was recorded using a Timeline Follow-back38 of each beverage consumed and its alcohol by volume, which was converted to the number of standard UK alcohol units (8g alcohol). Sleep quality was recorded using the total score on the Pittsburgh Sleep Quality Index.40 Depression and anxiety were recorded using total scores on the Beck Depression Inventory41 and Beck Anxiety Inventory.42 Secondary endpoints were reduced cannabis withdrawal symptoms, cigarette and alcohol consumption, urinary cotinine concentrations, depression and anxiety symptoms, and improved sleep quality, assessed (i) during treatment weeks, (ii) up to the final follow up. Additional secondary outcomes included cognitive, biological and physiological measures which will be reported elsewhere.
Blood samples were collected at site visits, which were arranged at a time that was most convenient for each participant. Blood samples were drawn into a 6ml lithium heparin vacutainer and centrifuged immediately at 2800 RPM for 5 minutes. Plasma samples were stored in 2ml cryotubes at -80°C prior to analysis using Gas Chromotography-Mass Spectrometry (ABS Laboratories) with a lower limit of quantification of 0.1 ng/ml.
Participants were asked about possible adverse events at each assessment from week 1 to week 16. Adverse events were categorized as mild (does not interfere with the participant’s daily routine and does not require intervention; it causes slight discomfort), moderate (interferes with some aspects of daily routine, or requires intervention, but is not damaging to health; it causes moderate discomfort) or severe (results in alteration, discomfort or disability which is clearly damaging to health). All adverse events were verified with a medical supervisor and an independent trial monitor throughout the trial on an ongoing basis.
As no previous trials investigating CBD as a treatment for cannabis use disorder were available, our effect size estimates were informed by a pilot study testing the effects of one-week’s CBD treatment on cigarette consumption in tobacco smokers who intended to quit.20 On the basis of these data, we estimated that a sample size of n=12 per group would provide 80% power to detect a similar effect of CBD in this study (d=1.21). Due to uncertainty in these estimates, we planned analyses at n=12 (interim analysis) and n=24 per group (final analysis) in a two-stage adaptive design.
Randomisation and masking
The randomisation sequence was generated by the trial statistician using block randomisation (R command: ‘blockrand’) with a block size equivalent to the number of treatment arms in the randomisation code. The randomisation code was held by the emergency un-blinding service (www.sealedenvelope.com) and the drug manufacturer for labelling prior to shipping to the trial site. Medication packages were labelled by the manufacturer and sent to the trial site with anonymous participant numbers. All investigators and participants remained blinded throughout the duration of the trial. Only blinded investigators enrolled participants, assigned participants to interventions, conducted assessments and entered data. Unblinding did not occur until after the database had been locked by the trial statistician.
Statistical analysis
All analyses were intention-to-treat. Missing data were handled using (Bayesian) multiple imputation under the assumption of Missing at Random. The missing outcomes are automatically simulated from the Bayesian procedure, in accordance with the modelling and distributional assumptions. In order to test our primary endpoint, THC-COOH/creatinine concentrations and days per week abstinent from cannabis were analysed during treatment weeks 1-4. We ran a Bayesian model for each dose of CBD compared to placebo to compute the predictive distribution of the outcome given the evidence that had become available up to that point. Based on these joint posterior distributions (obtained using Markov Chain Monte Carlo algorithms, with model convergence assessed using the Gelman-Rubin Statistic) we computed the probability that each dose of CBD was the Most Effective Dose (MED) compared to placebo. If this probability was below a pre-specified lower threshold of Pr = 0.1, the dose was dropped. Similarly, if a dose exceeding a pre-specified upper threshold Pr = 0.9, then it was considered the MED.43 All analyses included time as a fixed effect (treatment weeks 1-4) and were adjusted for baseline scores at week 0. Participant was fitted as a random intercept. For continuous, positive and skewed outcomes (e.g. THC-COOH:creatinine concentrations), generalised linear regression models were used assuming a Gamma distribution. Logistic regression models were used for binomial count outcomes (e.g. the number of days abstinent from cannabis in a week). Post hoc sensitivity analyses were conducted adding age and sex to primary endpoint models. Cannabis use (urinary THC-COOH:creatinine, days per week abstinent) were analysed up to the final follow up as a secondary endpoint. Secondary outcomes were analysed separately for treatment week data only (weeks 1-4) and for all data (all treatment week and follow up data combined) as additional secondary endpoints. Models were selected assuming Gamma, Binomial or Poisson distributions as appropriate. Absolute differences between CBD and placebo were used to estimate treatment effects for all primary and secondary endpoints. These were obtained from statistical models including fixed effects of time, adjusted for baseline scores at week 0, and with a random intercept of participant. Summary statistics stratified by treatment group and time point were obtained from raw data.
Role of the funding source
The UK Medical Research Council is a public-funded research council. As part of their Developmental Pathway Funding Scheme, the Medical Research Council panel provide expert guidance on clinical trial design. The funder played no role in the collection, analysis or interpretation of data, the writing of the report, or the decision to submit for publication.
Results
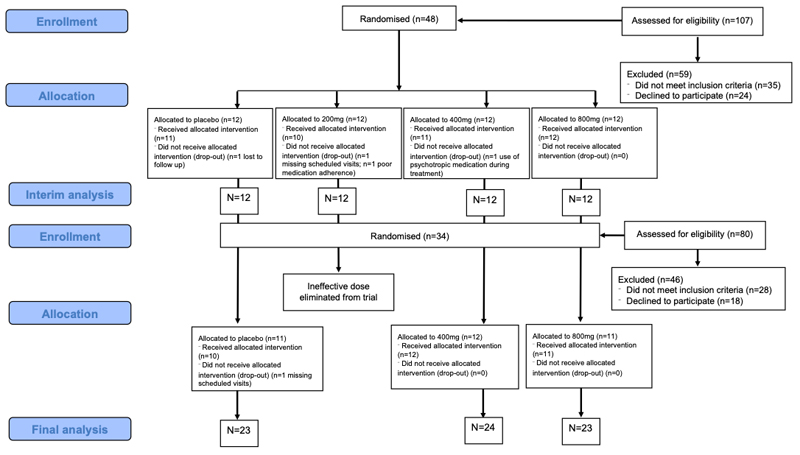
A total of 82 participants were randomised across both stages of the trial (Figure 1, Table 1). Across the trial, 94% of participants completed treatment as evidenced by medication compliance of at least 70% at each treatment week (for both self-report and returned medication) and attending all treatment week visits within two days of the scheduled appointment. Comparisons at both the interim analysis (appendix p4) and the final analysis (appendix p5) stages of the adaptive trial showed that CBD and placebo groups were similar for demographics and drug use at baseline.
Figure 1.
CONSORT diagram showing enrolment, allocation and analysis at interim and final stages of the adaptive trial
Table 1.
Characteristics of all participants included in both interim and final analysis stages of the trial. Data show frequencies, percentages, means and 95% Interval Estimates for the placebo (n=23), 200mg CBD (n=12), 400mg CBD (n=24) and 800mg CBD (n=23) groups
| Placebo | 200mg CBD | 400mg CBD | 800mg CBD | |
|---|---|---|---|---|
| Age | 24.87 (18.55, 43.35) |
27.33 (19.28, 39.08) |
26.58 (19.15, 41.25) |
27.43 (19.00, 36.90) |
| Sex (%male, male/female) | 73.91%, 17/6 | 75.00%, 9/3 | 70.83%, 17/7 | 69.57%, 16/7 |
| DSM-5 CUD symptoms at screening assessment | 8.61 (7.63, 9.58) |
8.67 (7.63, 9.70) |
9.00 (8.29, 9.71) |
8.48 (7.39, 9.57) |
Between 28th May 2014 and 12th August 2015, 48 participants were randomised to placebo, 200mg CBD, 400mg CBD and 800mg CBD (1:1:1:1). At interim analysis, CBD was more effective than placebo at reducing cannabis use at doses of 400mg and 800mg but not at 200mg. For urinary THC-COOH/creatinine, both 400mg and 800mg CBD exceeded the primary endpoint criteria of Pr=0.9 (Pr(200mg=MED | Data)=0.4191; Pr(400mg=MED | Data)=0.9827; Pr(800mg=MED | Data)=0.9488). For days per week abstinent from cannabis, the 400mg CBD arm exceeded primary endpoint criteria of Pr=0.9, while the 200mg CBD group exceeded the lower threshold criteria (Pr=0.1) indicating that it should be dropped (Pr(200mg=MED | Data)=0.0082; Pr(400mg=MED | Data)=0.9354; Pr(800mg=MED | Data)=0.8660), appendix p6. Therefore, the 200mg CBD arm was eliminated from the trial and no further participants were randomised to this arm. Post hoc sensitivity analyses including age and sex did not change the pattern of results.
Between 24th May 2016 and 12th January 2017 an additional 34 participants were randomised to the remaining arms of placebo, 400mg CBD and 800mg CBD (1:1:1). At final analysis, both 400mg CBD and 800mg CBD exceeded the primary endpoint criteria of Pr=0.9 for both primary outcomes: urinary THC-COOH:creatinine (Pr(400mg=MED | Data=0.9995; Pr(800mg=MED | Data=0.9965), days per week abstinent from cannabis (Pr(400mg=MED | Data=0.9966; Pr(800mg=MED | Data=0.9247), Figure 2, Table 2. Compared to placebo, 400mg CBD decreased THC-COOH:creatinine concentrations by -94.21 ng/ml (95% Interval Estimate= -161.83, -35.56) and increased abstinence from cannabis by 0.48 days per week (95% Interval Estimate=0.15, 0.82). Compared to placebo, 800mg CBD decreased THC-COOH:creatinine concentrations by -72.02 ng/ml (95% Interval Estimate= -135.47, -19.52) and increased abstinence from cannabis by 0.27 days per week (95% Interval Estimate= -0.09, 0.64). Post hoc sensitivity analyses including age and sex did not change the pattern of results.
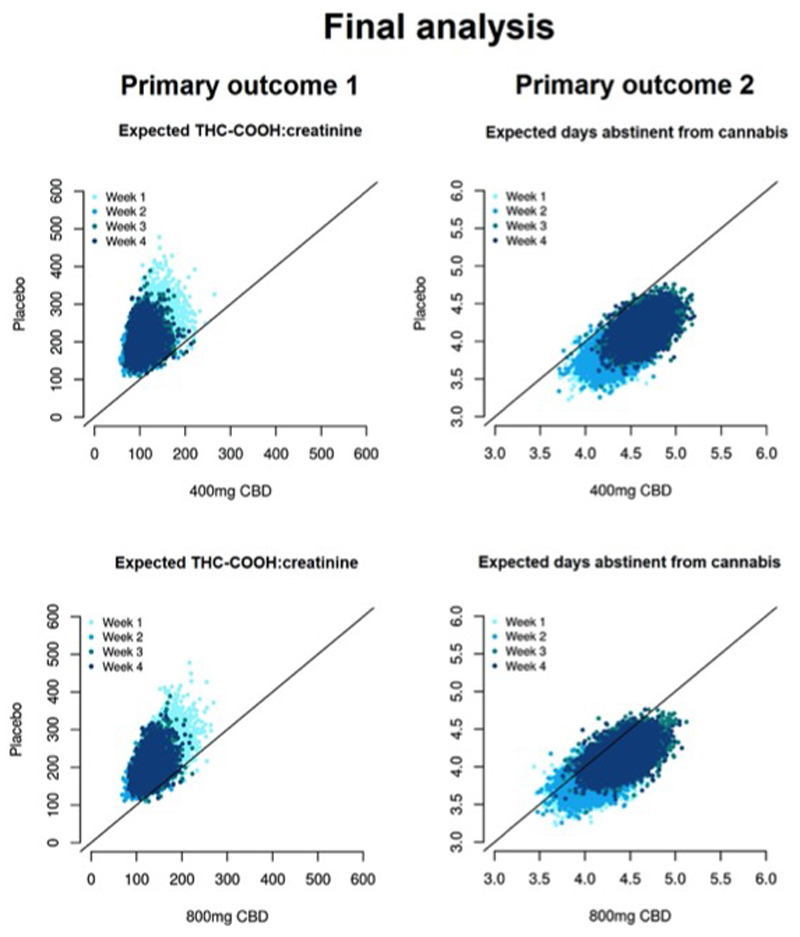
Figure 2.
Primary endpoint: final analysis. Each cloud of points represents the expected value of the primary outcome for participants randomised to CBD or placebo, simulated from the joint posterior distribution of treatment arms. Both 400mg and 800mg CBD exceed the upper threshold (Pr=0.9) for primary endpoint criteria, showing reduced THC-COOH:creatinine concentrations (left panel) and increased days per week abstinent from cannabis (right panel) compared to placebo.
Table 2.
Primary endpoint data. Means (95% Interval Estimates) stratified by group and time point at final analysis of placebo (n=23), 400mg CBD (n=24) and 800mg CBD (n=23) treatment arms
| Placebo |
400mg |
800mg |
||||
|---|---|---|---|---|---|---|
| Urinary THC-COOH: creatinine (ng/ml) | Days abstinent from cannabis | Urinary THC-COOH: creatinine (ng/ml) | Days abstinent from cannabis | Urinary THC-COOH: creatinine (ng/ml) | Days abstinent from cannabis | |
|
|
|
|
||||
| Baseline | 343.09 (188.41, 497.78) |
1.17 (0.48, 1.87) |
521.00 (316.55, 725.44) |
0.79 (0.34, 1.24) |
315.31 (150.00, 480.61) |
1.65 (0.68, 2.62) |
| Week 1 | 202.99 (68.59, 337.38) |
4.17 (3.29, 5.05) |
267.60 (71.61, 463.60) |
4.25 (3.40. 5.10) |
142.27 (70.65.213.90) |
4.04 (3.07. 5.01) |
| Week 2 | 187.53 (89.46.285.60) |
3.83 (2.77.4.88) |
227.17 (77.62.376.72) |
4.17 (3.26. 5.07) |
98.04 (53.89. 142.20) |
4.43 (3.55.5.32) |
| Week 3 | 185.53 (88.22. 282.84) |
4.36 (3.26. 5.46) |
272.31 (62.70. 481.92) |
4.67 (3.75. 5.58) |
125.73 (60 51.190.94) |
4.52 (3.63. 5.41) |
| Week 4 | 195.00 (92.08.297.92) |
4.14 (3.20.5.08) |
251.24 (95.38.407.11) |
4.38 (3.39. 5.36) |
144.07 (48 53.239.62) |
4.91 (4.05. 5.78) |
At final analysis, secondary endpoints were analysed for 400mg CBD versus placebo and 800mg CBD versus placebo (Table 3, appendix pp 7-15). Analysis of cannabis use up to the final follow up showed that 400g CBD reduced urinary THC-COOH:creatinine by -29.18 ng/ml (95% Interval Estimate= -52.08, -7.25) and increased abstinence from cannabis by 0.03 (95% Interval Estimate= 0.00, 0.07) days per week compared to placebo. However, the 800mg CBD group was similar to placebo up to the final follow up, with an estimate of -13.20 ng/ml (95% Interval Estimate= -37.58, 12.10) for urinary THC-COOH:creatinine and -0.02 (95% Interval Estimate= -0.06, 0.03) days per week abstinent from cannabis. Secondary outcomes were mixed, but some findings were consistent across analyses of treatment and follow up. Compared to placebo, 400mg CBD decreased the number of cigarettes smoked per week by -5.04 (95% Interval Estimate= -6.57, -3.47) during treatment and by -1.32 (95% Interval Estimate= -1.89, -0.60) up to the final follow up. However, compared to placebo, 400mg CBD increased Pittsburgh Sleep Quality Index scores by 0.84 (95% Interval Estimate= 0.15, 1.57) during treatment and by 0.55 (95% Interval Estimate= 0.21, 0.92) up to the final follow up, indicating poorer sleep quality following CBD. Compared to placebo, 800mg CBD reduced Cannabis Withdrawal Scale scores by -1.26 (95% Interval Estimate= -2.13, -0.39) during treatment and by -2.50 (95% Interval Estimate= -3.08, -1.93) up to the final follow up, indicating a reduction in cannabis withdrawal symptoms following CBD. Compared to placebo, 800mg CBD reduced Beck Anxiety Inventory scores by -1.29 (95% Interval Estimate= -1.97, -0.62) during treatment and by -0.52 (95% Interval Estimate= -0.82, -0.27) up to the final follow up, indicating a reduction in anxiety symptoms following CBD.
Table 3.
Final analysis of primary and secondary endpoints. Data show the absolute difference (95% Interval Estimate) between 400mg CBD (n=24) and placebo (n=23) and between 800mg (n=23) and placebo (n=23). Separate analyses are presented for treatment weeks only and up to the final follow up
| Treatment weeks | Up to final follow up | |||||||
|---|---|---|---|---|---|---|---|---|
| 400mg vs Placebo | 800mg vs Placebo | 400mg vs Placebo | 800mg vs Placebo | |||||
|
|
|
|
|
|||||
| THC-COOH creatinine (ng/ml) | -94.21a | (-161.83, -35 56) | -72.02a | (-135.47, -19.52) | -29.18 | (-52.08, -7.25) | -13.20 | (-37.58, 12.10) |
| Days abstinent | 0.48a | (015, 0.82) | 0.27a | (-0.09, 0.64) | 0.03 | (0.00, 0.07) | -0.02 | (-0.06, 0.03) |
| Cannabis Withdrawal Scale | -0.34 | (-1.14, 0.50) | -1.26 | (-2.13, -0.39) | -1.32 | (-1.89, -0.06) | -2.50 | (-3.08, -1.93) |
| Urinary cotinine:creatinine (ng/nl) | -72.31 | (-194.35, 36.57) | -36.08 | (-163.46, 104.28) | -66.52 | (-157.06, 13.10) | -56.60 | (-145.36, 35.19) |
| Cigarettes smoked | -5.04 | (-6.57, -3.47) | 8.66 | (6-89, 10.26) | -1.32 | (-1.89, -0.60) | -2.50 | (-3.08, -1.93) |
| Alcohol units consumed | I5.32 | (-3.60, 49.27) | -8.49 | (-17.27, 0.44) | 0.86 | (-2.82, 3.84) | -I.50 | (-4.83, 1.04) |
| Pittsburgh Sleep Quality Index | 0.84 | (0.15, 1.57) | 0.16 | (-0.57, 0.81) | 0.55 | (0.21, 0.92) | 0.46 | (0.08, 0.82) |
| Beck Depression Inventory | 0.34 | (-0.47, 1.17) | 0.14 | (-0.70, 1.00) | -0.48 | (-0.76, -0.21) | -0.21 | (-0.49, 0.07) |
| Beck Anxiety Inventory | 1.41 | (0.65, 2.17) | -1.29 | (-1.97, -0.62) | 0.01 | (-0.25, 0.34) | -0.52 | (-0.82, -0.27) |
Primary endpoint
There were dose-response increases in plasma CBD concentrations during treatment (Table 4). Compared to placebo, 200mg CBD increased plasma CBD concentrations by 9.37 ng/ml (95% Interval Estimate=5.80, 14.66), 400mg CBD increased plasma CBD concentrations by 29.90 ng/ml (95% Interval Estimate=19.62, 49.44) and 800mg CBD increased plasma CBD concentrations by 46.30 ng/ml (95% Interval Estimate=33.90, 63.13).
Table 4.
Mean (95% Interval Estimates) plasma concentrations of CBD (ng/ml) stratified by group and time point in the Placebo (n=23), 200mg (n=12), 400mg (n=24) and 800mg (n=23) treatment arms
| Placebo | 200mg CBD | 400mg CBD | 800mg CBD | |
|---|---|---|---|---|
| Week 0 | 0.12 (-0.01, 0.24) |
0.02 (-0.01, 0.06) |
0.15 (-0.06, 0.36) |
0.02 (-0.02, 0.07) |
| Week 2 | 0.06 (-0.03, 0.15) |
10.28 (4.20, 16.36) |
40.42 (19.12, 61.72) |
59.31 (17.95, 100.67) |
| Week 4 | 0.07 (0.00, 0.13) |
10.24 (2.65, 17.83) |
21.63 (11.06, 32.20) |
63.33 (30.09, 96.57) |
| Week 6 | 0.09 (-0.04, 0.21) |
0.49 (0.32, 0.66) |
4.14 (0.12, 8.16) |
3.91 (2.42, 5.41) |
| Week 12 | 0.02 (-0.03, 0.08) |
0.00 (0.00, 0.00) |
0.19 (0.06, 0.32) |
0.34 (0.14, 0.55) |
In the placebo arm (n=23) there were 65 mild adverse events and 9 moderate adverse events. In the 200mg CBD arm (n=12) there were 42 mild adverse events and 4 moderate adverse events. In the 400mg CBD arm (n=24) there were 96 mild adverse events and 8 moderate adverse events. In the 800mg CBD arm (n=23) there were 78 mild adverse events and 8 moderate adverse events. The number of mild adverse events did differ between placebo and 200mg CBD (Relative Risk=1.24, 95% Interval Estimate=0.73, 2.09), 400mg CBD (Relative Risk=1.39, 95% Interval Estimate=0.91, 2.14), or 800mg CBD (Relative Risk=1.19, 95% Interval Estimate=0.77, 1.86), appendix p16. The number of moderate adverse events did not differ between placebo and 200mg CBD (Relative Risk=0.85, 95% Interval Estimate=0.26, 2.58), 400mg CBD (Relative Risk=0.84, 95% Interval Estimate=0.35, 2.24), or 800mg CBD (Relative Risk=0.89, 95% Interval Estimate=0.40, 2.45), appendix p17. There were no severe adverse events.
The final follow-up assessment was conducted on 5th June 2017. After successfully achieving primary endpoint criteria, the phase IIa dose finding trial was stopped. Due to lack of funding a subsequent phase IIb stage (efficacy of most effective dose) was not initiated and the trial ended on 30th May 2018.
Discussion
In the first randomised clinical trial of CBD for the treatment of cannabis use disorder, we used an adaptive Bayesian design to establish which (if any) dose of CBD was more effective than placebo at reducing cannabis use. We eliminated 200mg CBD at an early stage and continued randomising to 400mg CBD, 800mg CBD and placebo treatment arms. At final analysis of the primary endpoint, both 400mg CBD and 800mg CBD were more effective than placebo at reducing cannabis use. These treatment effects were found over and above a brief psychological intervention typically delivered in drug treatment settings.
All participants in this trial met a DSM-5 diagnosis of cannabis use disorder, with 96% in the severe range. They expressed a desire to quit using cannabis in the next month, smoked tobacco with their cannabis, and had failed to quit cannabis use on at least one previous cessation attempt. This is an important population for which there is substantial and rising need for treatment and for whom no pharmacotherapies are recommended at present.11 The four-week treatment was completed by 94% of participants, as evidenced by self-report and behavioural compliance data of at least 70%, and attending all weekly treatment visits within 2 days of scheduled appointments. CBD did not differ from placebo in the number of mild or moderate adverse events at 200mg, 400mg or 800mg. There were no severe adverse events, and no participants dropped out due to treatment. The excellent safety and tolerability data and exceptionally high retention rates in our trial suggest that these doses of CBD offer a safe and acceptable treatment for this population.
To our knowledge, this is the first adaptive Bayesian dose-finding trial of CBD for a new medical indication. The primary objective of this Phase IIa trial was to establish which (if any) dose of CBD was more effective than placebo at reducing cannabis use. Both 400mg CBD and 800mg CBD exceeded primary endpoint criteria (Pr = 0.9) for reducing cannabis use during treatment, with converging evidence from biological and self-report primary outcomes. Our estimates showed that 400mg CBD decreased THC-COOH:creatinine concentrations by -94.21 ng/ml (95% Interval Estimate= -161.83, -35.56) and increased abstinence from cannabis by 0.48 days per week (95% Interval Estimate=0.15, 0.82), while 800mg CBD decreased THC-COOH:creatinine concentrations by -72.02 ng/ml (95% Interval Estimate= -135.47, -19.52) and increased abstinence from cannabis by 0.27 days per week (95% Interval Estimate= -0.09, 0.64).
The effects of the doses tested are suggestive of an inverted-U dose response curve. The 200mg arm was eliminated as an ineffective dose, and there was some indication that 400mg CBD was marginally more effective than 800mg CBD. Secondary endpoints showed that the reductions in cannabis were maintained up to the final follow up in the 400mg CBD arm but not the 800mg CBD arm. From a treatment perspective, our findings indicate that doses ranging from 400mg to 800mg CBD have the potential to reduce cannabis use in clinical settings, and it is unlikely that additional benefit would be gained from doses exceeding 800mg CBD. It is important to be aware that this dose range (400mg to 800mg CBD) is considerably higher than those in CBD products widely available without a prescription (e.g. 25mg per day).14 These products lack quality assurance and should not be used for medicinal purposes.
There were mixed results for other secondary endpoints, but some findings were consistent across treatment weeks and follow up. Compared to placebo, 400mg CBD decreased the number of cigarettes smoked in line with previous studies of CBD in tobacco smokers.17,20 However, sleep quality was lower in the 400mg CBD group compared to placebo, which may be interpreted in the context of greater reductions in cannabis use in this group. Compared to placebo, 800mg CBD reduced cannabis withdrawal symptoms, consistent with previous case reports22,23 and reduced anxiety symptoms in line with experimental studies in humans28 and rats.29
Key strengths of this study include its novel indication for which there is substantial clinical need, and its adaptive Bayesian dose-finding design. This design enabled us to test a range of doses in an efficient manner, which would have required considerably greater resources and participant burden when using a typical trial design. In terms of limitations, this Phase IIa dose-finding trial was not designed to estimate efficacy. Further evidence is needed to improve the precision of the estimates obtained in this study. Although there was strong evidence for dose-response effects of CBD on plasma CBD concentrations, factors such as food consumption could have contributed to variation in the bioavailability of CBD. This trial used a four-week treatment period, consistent with a previous clinical trial for psychosis.30 Further studies are needed to investigate the extent to which these findings translate to different durations of treatment. Additional research is needed to investigate if CBD reduces cannabis use independently, or through mechanisms shared with other mental health symptoms such as anxiety.
In conclusion, our trial provides the first causal evidence supporting CBD as a treatment for cannabis use disorders. These findings are important in light of major policy changes surrounding the production and sale of cannabis products, increases in the number of people entering treatment for cannabis use disorders worldwide8,9 and the absence of recommended pharmacotherapies at present.11
Supplementary Material
Research in context.
Evidence before the study
We searched the Cochrane database and peer-reviewed journal articles in Google Scholar using the terms ‘CBD’, ‘Cannabis’ and ‘Marijuana’ up to 12th March 2020. No language restrictions were imposed. A Cochrane review on pharmacotherapies for cannabis use disorders published in 2019 did not recommend any pharmacotherapies for reducing cannabis use in clinical settings. There were no randomised trials testing CBD as a treatment for cannabis use disorder.
Added value of this study
This is the first randomised clinical trial of CBD for the treatment of cannabis use disorder. It used an adaptive Bayesian dose-finding design with doses ranging from 200mg to 800mg CBD to maximise trial efficiency and likelihood of success in identifying a potentially effective dose.
Implications of all available evidence
At daily oral doses of 400mg or 800mg, CBD shows potential as a safe and effective treatment for reducing cannabis use in people with a cannabis use disorder, assessed by both biological and self-report measures.
Acknowledgements
This trial was supported by a UK Medical Research Council Developmental Pathway Funding Scheme award (MR/K015524/1) to VC, CM, GB, TF and JK. TF was supported by a Senior Academic Fellowship from the Society for the Study of Addiction.
Funding
Medical Research Council.
Footnotes
Competing interests
Dr. Bloomfield reports personal fees from Spectrum Therapeutics, outside the submitted work. No other authors report any competing interests.
Data sharing
Participants did not provide consent for data sharing.
Author contributions
Study design: TF, CH, GB, JK, CM, VC; Trial coordination: TF, CH; Trial supervision: VC; Medical trial supervision: MB, AM; Project Management: JK; Training and supervision of motivational interviewing: DO; Conducting assessments and delivering motivational interviewing: TF, CH, NS, ET, DA, AF; Database management: NS, ET, DA, RL, SC; Statistical analysis: GB; Writing first draft of the manuscript: TF; critically revising the manuscript: all authors.
References
- 1.Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nature Reviews Neuroscience. 2016;17(5):293–306. doi: 10.1038/nrn.2016.28. [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Charlson F, Ferrari A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Psychiatry. 2018 doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman TP, Groshkova T, Cunningham A, Sedefov R, Griffiths P, Lynskey MT. Increasing potency and price of cannabis in Europe, 2006–16. Addiction. 2019;114(6):1015–23. doi: 10.1111/add.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008–2017) European archives of psychiatry and clinical neuroscience. 2019:1–11. doi: 10.1007/s00406-019-00983-5. [DOI] [PubMed] [Google Scholar]
- 5.Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychological medicine. 2015;45(15):3181–9. doi: 10.1017/S0033291715001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier MH. Associations between butane hash oil use and cannabis-related problems. Drug and alcohol dependence. 2017;179:25–31. doi: 10.1016/j.drugalcdep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Freeman TP, van der Pol P, Kuijpers W, et al. Changes in cannabis potency and first-time admissions to drug treatment: a 16-year study in the Netherlands. Psychological medicine. 2018:1–7. doi: 10.1017/S0033291717003877. [DOI] [PubMed] [Google Scholar]
- 8.World Drug Report 2019. United Nations publication; Sales No. E.19.XI.8. [Google Scholar]
- 9.EMCDDA. European Drug Report 2018: Trends and Developments. Publications Office of the European Union; Luxembourg: 2018. [Google Scholar]
- 10.Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. The Lancet Psychiatry. 2019 doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen S, Gowing L, Sabioni P, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2019;(1) doi: 10.1002/14651858.CD008940.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisogno T, Hanuš L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British journal of pharmacology. 2001;134(4):845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laprairie R, Bagher A, Kelly M, Denovan-Wright E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British journal of pharmacology. 2015;172(20):4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman TP, Hindocha C, Green SF, Bloomfield MA. Medicinal use of cannabis based products and cannabinoids. Bmj. 2019;365:l1141. doi: 10.1136/bmj.l1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman AM, Petrilli K, Lees R, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neuroscience & Biobehavioral Reviews. 2019 doi: 10.1016/j.neubiorev.2019.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Δ 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35(9):1879. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindocha C, Freeman TP, Grabski M, et al. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. 2018 doi: 10.1111/add.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. American Journal of Psychiatry. 2019 doi: 10.1176/appi.ajp.2019.18101191. appi. ajp. 2019.18101191. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. Journal of Neuroscience. 2009;29(47):14764–9. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addictive behaviors. 2013;38(9):2433–6. doi: 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020:1–10. doi: 10.1038/s41386-020-0667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crippa JAdS, Hallak JEC, Machado-de-Sousa J, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. Journal of clinical pharmacy and therapeutics. 2013;38(2):162–4. doi: 10.1111/jcpt.12018. [DOI] [PubMed] [Google Scholar]
- 23.Shannon S, Opila-Lehman J. Cannabidiol oil for decreasing addictive use of marijuana: a case report. Integrative Medicine: A Clinician's Journal. 2015;14(6):31. [PMC free article] [PubMed] [Google Scholar]
- 24.Solowij N, Broyd SJ, Beale C, et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis and Cannabinoid Research. 2018;3(1):21–34. doi: 10.1089/can.2017.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allsop D, Lintzeris N, Copeland J, Dunlop A, McGregor I. Cannabinoid replacement therapy (CRT): Nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clinical Pharmacology & Therapeutics. 2015;97(6):571–4. doi: 10.1002/cpt.109. [DOI] [PubMed] [Google Scholar]
- 26.Lintzeris N, Bhardwaj A, Mills L, et al. Nabiximols for the Treatment of Cannabis Dependence: A Randomized Clinical Trial. JAMA internal medicine. 2019;179(9):1242–53. doi: 10.1001/jamainternmed.2019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trigo JM, Soliman A, Quilty LC, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PloS one. 2018;13(1):e0190768. doi: 10.1371/journal.pone.0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Frontiers in pharmacology. 2017;8:259. doi: 10.3389/fphar.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199(2):223. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- 30.Leweke F, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational psychiatry. 2012;2(3):e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha JM, Carlini E, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 32.Kotz D, Brown J, West R. Predictive validity of the Motivation To Stop Scale (MTSS): a single-item measure of motivation to stop smoking. Drug and alcohol dependence. 2013;128(1-2):15–9. doi: 10.1016/j.drugalcdep.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Hindocha C, Freeman TP, Ferris JA, Lynskey MT, Winstock AR. No smoke without tobacco: a global overview of cannabis and tobacco routes of administration and their association with intention to quit. Frontiers in psychiatry. 2016;7:104. doi: 10.3389/fpsyt.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS drugs. 2018;32(11):1053–67. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller WR, Rollnick S. Motivational interviewing: Helping people change. Guilford press; 2012. [Google Scholar]
- 36.Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database of Systematic Reviews. 2016;(5) doi: 10.1002/14651858.CD005336.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran HV, Hindocha C, Morgan CJA, Shaban N, Das RK, Freeman TP. Which biological and self-report measures of cannabis use predict cannabis dependency and acute psychotic-like effects? Psychological medicine. 2018 doi: 10.1017/S003329171800226X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobell LC, Sobell MB. Measuring alcohol consumption. Springer; 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- 39.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug and alcohol dependence. 2011;119(1-2):123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Berry SM, Carlin BP, Lee JJ, Muller P. Bayesian adaptive methods for clinical trials. CRC press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.