Abstract
Ribosomopathies are congenital disorders caused by mutations in ribosomal proteins (RP) or assembly factors and are characterized by cellular hypo-proliferation at an early stage. Paradoxically, many of these disorders have an elevated risk to progress to hyper-proliferative cancer at a later stage. Additionally, somatic RP mutations have recently been identified in various cancer types e.g. the recurrent RPL10-R98S mutation in T-cell acute lymphoblastic leukemia (T-ALL) and RPS15 mutations in chronic lymphocytic leukemia (CLL). We previously showed that RPL10-R98S promotes expression of oncogenes but also induces a proliferative defect due to elevated oxidative stress. In thisstudy, we demonstrate that this proliferation defect is eventually rescued by RPL10-R98S mouse lymphoid cells that acquire 5-fold more secondary mutations than RPL10-WT cells. The presence of RPL10-R98S and other RP mutations also correlated with a higher mutational load in T-ALLpatients, with an enrichment in NOTCH1-activating lesions. RPL10-R98S-associated cellular oxidative stress promoted DNA damage and impaired cell growth. Expression of NOTCH1 eliminated these phenotypes in RPL10-R98S cells, in part via downregulation of PKC-θ, with no effect on RPL10-WT cells. RP-mutant CLL patients also demonstrated a higher mutational burden, enriched for mutations that may diminish oxidative stress. We propose that oxidative stress due to ribosome dysfunction causes hypo-proliferation and cellular insufficiency in ribosomopathies and RP-mutant cancer. This drives surviving cells, potentiated by genomic instability, to acquire rescuing mutations which ultimately promote transition to hyper-proliferation.
Introduction
Impaired ribosome assembly and/or function stemming from mutations in ribosomal protein (RP) or ribosome assembly genes causes congenital disorders called ribosomopathies[e.g. Diamond-Blackfan Anemia (DBA) and Shwachman-Diamond Syndrome (SDS)].Despite the presence of identical ribosomal mutations in every cell, ribosomopathies display tissue-specific hypo-proliferative phenotypes which particularly affect the hematopoietic system. Intriguingly, these disorders also carry an increased risk of developing cancer later in life, particularly acute myeloid leukemia (AML) and myelodysplastic syndrome (1).Thisparadoxical transition from cellular hypo-to hyper-proliferation is reminiscent of a known phenomenonfirst observedin 1967.Here, William Dameshek described that patients who initially develop a hypo-proliferative disease, such as aplastic anemia, tend to be at higher risk of hyper-proliferative diseases, such as acute leukemia (2). Ribosomopathies are thus examples of the longstanding and unsolved “Dameshek’s Riddle.”Based on data obtained in yeast, we have previously proposed that compensatory mutations might be rescuing the proliferation defects imposed by ribosomal mutations over time (3).
Recurrent somatic mutations in ribosomal protein (RP) genes (RPS15, RPL5, RPL10, RPL11, and RPL22) have recently also been discovered in various solid tumortypes including breast cancer, glioblastoma and melanoma as well as in hematopoietic cancers such as T-cell acute lymphoblastic leukemia (T-ALL), chronic lymphocytic leukemia (CLL), and multiple myeloma (1).Of these, the R98S mutation in ribosomal protein L10 (RPL10-R98S) is the most recurrent known missense mutation,present in 8% of pediatric T-ALL (4).RPL10 (also called uL16)is an essential protein near the ribosomal catalytic core, and we have previously shown that the R98S mutation interferes with ribosome function and cell proliferation (4–6).Moreover, RPL10 interacts with the ribosome maturation protein SBDS during ribosome assembly, and SBDS mutations cause similar hypo-proliferative defects in SDS (7).In the present study, we explored the paradoxical transition from hypo- to hyper-proliferation and investigated how an initial ribosomal defect which restricts cell proliferation, exemplified by the RPL10-R98S mutation, can ultimately have an oncogenic impact.
Materials and Methods
Cell culture
Breeding and usage of transgenic Rpl10cKI R98S and control animals for cell isolations were approved by the local ethics committee (P145/2014). Ba/F3 cells were obtained from Leibniz-Institute DSMZ. Cells were not authenticated but all cell cultures were confirmed to be free of mycoplasma contamination.The NOTCH1-ICN construct has been previously described (8). Viable cell counts were obtained using a Guava EasyCyte (Merck Millipore) flow cytometer. Flow cytometry analysis of CellROX (ThermoFisher #C10422) and Phospho-H2A.X (Cell Signaling #9718S) to detect reactive oxygen species (ROS) and DNA damage, respectively, was performed using a MACSQuant VYB (Miltenyi) or BD FACS Canto and FlowJo software.
BaF3 cells
generation and culturing of isogenic cell lines expressing RPL10-R98S and RPL10-WT has been described (6).As long as the cells were cultured, stable expression of RPL10-R98S was confirmed on a weekly basis by cDNA sequencing of the RPL10-R98S locus as previously reported (6).Cells were transduced with NOTCH1-ICN-IRES-GFP, NOTCH1-L1601P-ΔPEST-IRES-GFP or empty IRES-GFP vector, sorted for GFP, and treated with 10 µM of the NOTCH1 transcriptional complex inhibitor SAHM1 or DMSO. Cells were plated at a density of 100,000/mL and counted after 48h.
Lineage-negative cells
generation and culturing of lineage-negative cells on semi-solid media was recently reported (9).Briefly, isolation of lineage-negative cells from the bone marrow of 6-8 week old transgenic MX1-Cre Rpl10cKI R98S and from Mx1-Cre control mice was performed using an EasySep Mouse Hematopoietic Progenitor Cell enrichment kit (Stemcell Technologies). Isolated cells were retrovirally transduced with NOTCH1-ICN-IRES-GFP, NOTCH1-L1601P-ΔPEST-IRES-GFP or empty IRES-GFP vector, sorted for GFP, and plated at 2000 cells /mL in Methocult with recombinant cytokines (GFM3534, Stemcell Technologies), supplemented with 10 µM SAHM1 or DMSO. The cells were replated after 12 days, and cell count measurements and flow cytometry stainings carried out at the second replating are shown in the figures. For experiments involving the anti-oxidant N-acetyl-cysteine (NAC), lin- cells were suspended in liquid cytokine-rich media, and cultured for 3 hours in the presence or absence of 20 µM NAC (Sigma) followed by analysis by flow cytometry or western blotting.
Pro-T cells
cell cultures were established as described (10) from CD2-Cre Rpl10cKI R98S and Rpl10cKI R98S control mice. Briefly, isolated lineage negative cells were seeded onto 12-well cell culture plates that were coated with DL4/Fc fusion protein in the presence of murine stem cell factor and IL-7 (Final concentration 20ng/ul, Stemcell Technologies). After 2-3 weeks, the differentiation of lineage negative cells into T-cell progenitors with a retained DN2-DN3 phenotype was confirmed by flow cytometry analysis of CD25, CD44, and CD117 markers.
Western blotting
Standard western blotting was carried out as previously reported (9) using primary antibodies targeting Phospho-H2A.X (Cell Signaling #9718), catalase (Cell Signaling #14097) and PKC-θ (Cell signaling #13643) and a secondary Goat Anti-Rabbit IgG-HRP antibody (Thermo Fischer). Proteins were visualized using chemiluminescence on an Azure C600 (Azure Biosystems). Quantification was performed using LI-COR Image Studio Lite software version 5.2. Vinculin (Sigma Aldrich) was used to normalize for protein input.
Exome sequencing and patient data analysis
Exome sequencing of Ba/F3 cell clones was performed by BGI-Tech using Agilent SureSelect exome capture and Illumina sequencing. Three independent clones were analyzed. The SNPs and INDELs unique to each clone were first identified. These gene sets were subsequently subjected to several filtering steps to exclude silent and germline mutations, and those mutations falling in intergenic regions.
The variant allelic frequencies (VAFs) of RPL10-R98S and NOTCH1 activating lesions from the Cools (11) and Mullighan (12) T-ALL cohorts were calculated by dividing the number of reads of the specific allele in the tumor sample by the total number of reads in that tumor sample. For male patients, VAF values of RPL10-R98S were divided by 2 to correct for the X-chromosome location of this gene. VAFs higher than 0.6 were divided by 2 to allow comparison of heterozygous and homozygous mutations.
Mutational signature analysis
Mutational signature analysis as described by Alexandrov et al. (13) was performed using the R package ‘mutSignatures’ and was applied on the Mullighan T-ALL whole exome sequencing dataset (12), after dividing the samples according to ribosomal protein mutation status (RP-WT; RPL10-R98S, other RP-mutant). The first two signatures were used because analysis of the lowest Root mean squared error (RMSE) indicated that the lowest RMSE was obtained by taking 1 or 2 signatures for each group, and because the first two signatures could explain all the mutations for each group. The first and second mutational signatures were thus used to compare the three groups using a Wilcoxon rank sum test.
Statistical analysis
The statistical tests that were used are indicated in the figure legends.
Results
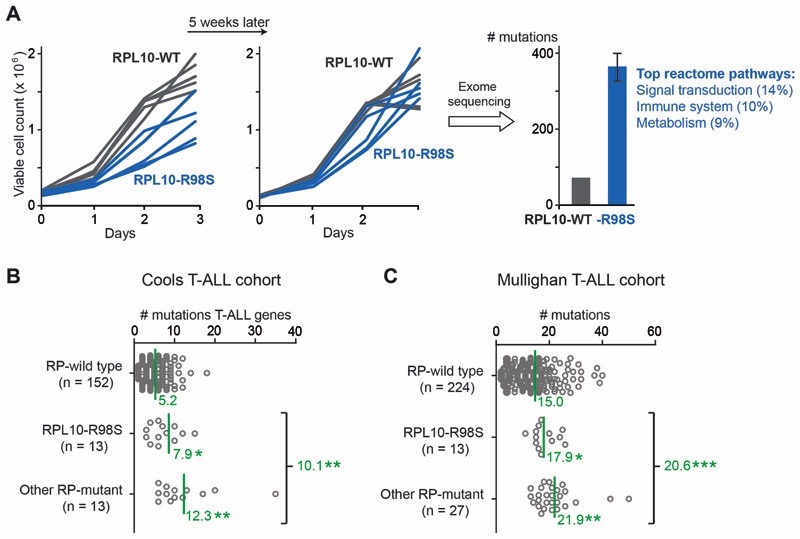
The T-ALL associated RPL10-R98S mutation is one of the best described somatic ribosomal mutations in cancer. Confirming previous observations (4), introduction of the RPL10-R98S mutation into lymphoid Ba/F3 mouse cells, a well-established hematopoietic model for oncogenic studies, caused a proliferation defect compared to WT cells (Figure 1A, left). However, consistent with previous research in an RPL10-R98S yeast model (5),this proliferation defect disappearedafter culturing the cells for 5 additional weeks (Figure 1A, middle). We confirmed that this proliferative recovery of RPL10-R98S Ba/F3 cells was not due to loss of expression of the RPL10-R98S mutation, and performed exome sequencing of one RPL10-WT and two RPL10-R98S Ba/F3 cell clones collected after this additional culturing period to gain insights in the proliferation rescue. Exome sequencing revealeda ~5-fold higher mutational load in the mutant cells (average of 368 vs. 72 mutations, Figure 1A, right). These mutations were evenly distributed among a variety of cellular pathways in WT cells, butclustered into pathwaysofsignal transduction, immune system, and metabolism in RPL10-R98S cells (Figure 1A, right). These results prompted us to explore a potential mutagenic phenotype in ribosome-mutant T-ALL. To this end, we surveyed available T-ALL targeted resequencing data (11)(Cools cohort)of genes known to be recurrently mutated in T-ALL (T-ALL genes). Notably, T-ALL patients carrying RPL10-R98S, as well asmutations in other RPs, presented approximately twice as many mutations in T-ALL genes, with an average of 10.1 mutations for all RP-mutant cases vs. 5.2 mutations for RP-WT cases (Figure 1B). Lesions in other T-ALL genes with similar incidences as the RPL10-R98S defect in pediatric T-ALL, such as IL7R and EZH2, did not correlate with an increased number of mutations (5.8 and 4.9 average number of mutations, respectively). These findings were further confirmed by analysis of a recent whole exome sequencing dataset from 264 pediatric T-ALL patients (12) (Mullighan cohort). In this comprehensive study, RP-mutant T-ALL patients displayed a ~37% higher total mutational load compared to RP-WT patients (Figure 1C).
Figure 1. RP lesions increase the mutagenic burdenin T-ALL.
(A) Left and middle: growth curves of Ba/F3 cell clones expressing RPL10-R98S or RPL10-WT at different time points.Right: number of mutations as determined by exome sequencing on three cell clones (1 RPL10-WT and 2 RPL10-R98S) from the left panel of Figure 1A. The percentages in the reactome pathways correspond to the fraction of mutated genes in the RPL10-R98S clones belonging to the mentioned reactome pathway. (B)The number of mutations in genes known to be recurrently mutated in T-ALL (T-ALL genes) in RP-mutant vs. RP-WT T-ALL patient samples, mined from targeted resequencing data (11). The RPL10-R98S category includes patients with RPL10-R98S as the only RP mutation. The “other RP-mutant” category includespatients with mutations in RPL5, RPL22, or in multiple RPs including RPL10-R98S. Mean values are indicated by a green line and the corresponding green number. (C) The number of mutations in RP-mutant vs. RP-WT T-ALL, mined from patient whole exome data (12). The RPL10-R98S category includes patients with RPL10-R98S as the only RP mutation. The “other RP-mutant” category includes a patient with the RPL10-R98C mutation, a patient with the RPL10-Q123P mutation, and patients with mutations in RPL4, RPL5, RPL9, RPL11, RPL22, RPS3, RPS13, or in multiple RPs. Mean values are indicated by a green line. P-values were calculated using a T-test after accounting for equality of variances using an F-test (panels B and C). ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05.
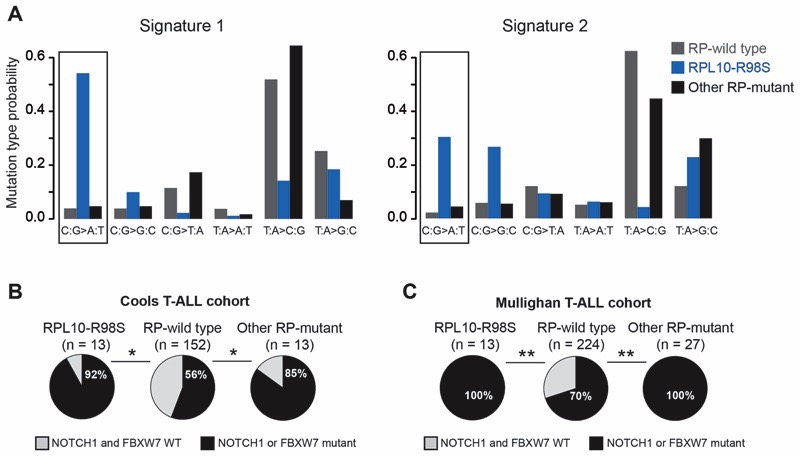
To gain insight into the mutagenic processes in RP-mutant T-ALL, we applied a mutational signature analysis on the Mullighan dataset as previously described (13). Two signatures were selected for analysis (see methods), and RPL10-R98S samples displayed a significant difference for signature 1 compared to RP-WT samples (Figure 2A). We previously described that RPL10-R98S elevates oxidative stress in mouse cell models, and that reduction of reactive oxygen species (ROS) levels by means of an anti-oxidant can rescue the proliferation defect of RPL10-R98S cells (9). Furthermore, oxidative stress is known to induce DNA damage which can in turn promote mutagenesis. In agreement with these findings, the mutational signature of RPL10-R98S patient samples mainly consists of C:G>A:T transversion mutations (Figure 2A, left bars in signature 1 indicated by a black rectangle), which correspondto mutations that have been linked to oxidative DNA damage (14). Signature 2 also shows a 14-fold higher probability for this mutational type in RPL10-R98S samples as compared to RP-WT samples. These findings thus underscore that mutational processes in RPL10-R98S samples differ from those in RP-WT T-ALL, and that oxidative DNA damage is a major mutational cause in RPL10-R98S leukemia.
Figure 2. RP-mutant T-ALL displays unique mutational signatures and enrichment in NOTCH1 pathway activating mutations.
(A)Mutational signature analysis (13) performed on a T-ALL whole exome sequencing cohort (12). RPL10-R98S samples showed a significant differencefor signature 1 compared to RP-WT samples (P = 0.0044). The C:G>A:T transversion mutations, which mainly correspond to the type of mutations linked to oxidative DNA damage, are indicated by black rectangles. (B) Fraction of patients with mutations in NOTCH1 pathway genes in RP-mutant vs. RP-WT T-ALL, mined from targeted resequencing data (11).The RPL10-R98S category includes patients with RPL10-R98S as the only RP mutation. The “other RP-mutant” category includes patients with mutations in RPL5, RPL22, or in multiple RPs including RPL10-R98S. (C) Fraction of patients with mutations in NOTCH1 pathway genes in RP-mutant vs. RP-WT T-ALL, mined from whole exome data (12). The RPL10-R98S category includes patients with RPL10-R98S as the only RP mutation. The “other RP-mutant” category includes a patient with the RPL10-R98C mutation,a patient with the RPL10-Q123P mutation, and patients with mutations in RPL4, RPL5, RPL9, RPL11, RPL22, RPS3, RPS13, or in multiple RPs. A Wilcoxon rank sum test was performed in Panel A, and a two-tailed Fisher’s test in Panels B-C. **P ≤ 0.01, *P ≤ 0.05.
We next investigated whether RP-mutant cases are enriched for mutations in particular genes. After accounting for multiple testing (Benjamini-Hochberg correction) in the Cools cohort, the top three genes mutated at higher incidences in RP-mutant T-ALL patients, and particularly in RPL10-R98S cases,consisted ofthetranscription factors BCL11B, NOTCH1, and FBXW7 (P = 0.08 each). The last two belong to the NOTCH1 signaling pathway, andactivating mutations in NOTCH1 or inactivating mutations in its inhibitor FBXW7 were significantly enriched in RP-mutant cases (Figure 2B). This was confirmed in the Mullighan cohort, revealing a 100% co-occurrence of RP-mutant cases with NOTCH1 pathway mutations (Figure 2C). Given the known roles of NOTCH1 in malignant T-cell transformation and the availability of clinical NOTCH1 inhibitors, we explored the effects of hyper-activating this pathway on ribosome-defective cells.
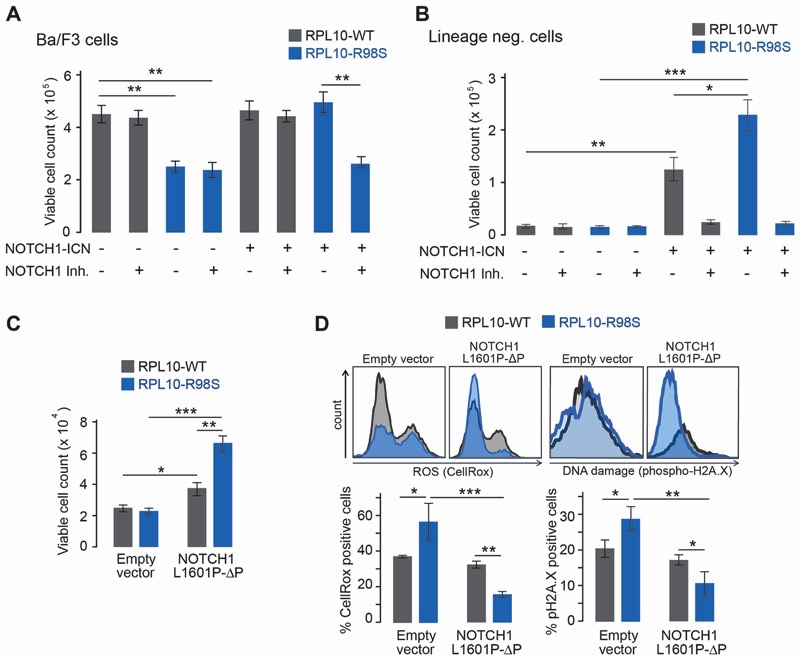
To gain insights into the biological mechanisms driving frequent co-occurrence of NOTCH1 activation and the RPL10-R98S mutation, we introduced the transcriptionally active form of NOTCH1(Intracellular NOTCH1 - ICN) into three RPL10-R98S and RPL10-WT cell models. First, expression of ICN rescued the proliferation defect ofRPL10-R98S Ba/F3 cells (Figure 3A), which was reversed by addition of the NOTCH1 transcriptional complex inhibitor SAHM1.Second, we isolated lineage negative (lin-) hematopoietic stem cells from RPL10-R98S and RPL10-WTknock-in mice (7) and cultured them on cytokine-rich semi-solid medium.Here, while the proliferation of both mutant and WT cells benefited from ICN expression, RPL10-R98S cells displayed a markedly increased proliferative benefitcompared to cells isolated from WT mice (Figure 3B). Finally,we generated NOTCH1 ligand (Delta-like 4, DL4) dependent pro-T cultures from RPL10-R98S and WT lin- cells. Such pro-T cell lines approximate thestage of T-cell differentiation at which T-ALL cells are blocked. Pro-T cells generated from RPL10-R98S lin- cells displayed a proliferative advantage compared to cultures generated from WT lin- cells in this NOTCH1 stimulated system. Amore pronounced proliferative decline of the mutant cells was moreover observed upon DL4 removal (Figure S1). These data from three independent, genetically defined cellular models suggest that NOTCH1 cooperates with RPL10-R98S to accelerate cellular proliferation.
Figure 3. NOTCH1 rescues theproliferation and functional defectsof a RP-mutant T-ALL model.
(A) Cell count of Ba/F3 clones expressing RPL10-R98S or RPL10-WT and NOTCH1-ICN or empty vector, with or without 10 µM of the NOTCH1 inhibitor (NOTCH1 Inh.) SAHM1. (B) Cell count of lin- cells in a serial replating assay expressing RPL10-R98S or RPL10-WT and NOTCH1-ICN or empty vector, with or without the NOTCH1 inhibitor (NOTCH1 Inh.) SAHM1. (C)Cell count of lin- cells cultured on cytokine-rich semi-solid media expressing RPL10-R98S or RPL10-WT, transduced with NOTCH1-L1601P-?PEST (NOTCH1-L1601P-ΔP) or empty vector. (D)Levels of ROS (CellRox) and DNA damage (phospho-H2A.X) in viable lin- cells expressing RPL10-R98S or RPL10-WT, transduced with NOTCH1-L1601P-ΔPEST (NOTCH1-L1601P-ΔP) or empty vector. A representative staining for each condition is depicted, along with bar graphs indicating the average values from at least 3 biological replicates. Error bars denote standard deviation. P-values were calculated using a T-test after accounting for equality of variances using an F-test. ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05. N = 3-6.
We next investigated the molecular mechanism of the RPL10-R98S - NOTCH1 cooperation by employing a clinically relevant mutant form of NOTCH1 that is recurrently found in T-ALL patients (NOTCH1-L1601P-ΔPEST). Expression of this active form of NOTCH1also strongly promoted growthin RPL10-R98S lin- cells (Figure 3C). In agreement with the mutational signature analysis as well as previous observations (9), RPL10-R98S induced elevated ROS levels in these cells. Furthermore, analysis of the phosphorylated form of histone H2A.X, a marker of double strand DNA breaks, demonstrated that the higher RPL10-R98SlinkedROS levelswere associated with enhanced DNA damage. Importantly, expression of NOTCH1-L1601P-ΔPEST or ICN reduced the oxidative stress and DNA damagein RPL10-R98S but not in WT lin- cells (Figure 3D + Figure S2, left panel). To confirm the causative role of oxidative stress on DNA damage in RPL10-R98S cells, we treated lin- cells with the anti-oxidant N-acetyl-cysteine (NAC) followed by ROS and DNA damage staining as above. Similarly to NOTCH1, this NAC-treatment decreased ROS and DNA damage levels in RPL10-R98S cells (Figure S3).
Discussion
We employed the RPL10-R98S mutation found in T-ALL as a tool to investigatethe oncogenic mechanisms of a ribosome-mutant cancer. This mutation also mimics the cellular transition of hypo-to hyper-proliferation of ribosomopathies in cell culture, and is functionally linked to SBDS which is in turn mutated in the ribosomopathy SDS, characterized by elevated cancer incidences. In addition to being a model of recurrent somatic ribosomal lesions in cancer, RPL10-R98Sthus offers an appropriate and useful system to also model the oncogenic transition of ribosomopathies.
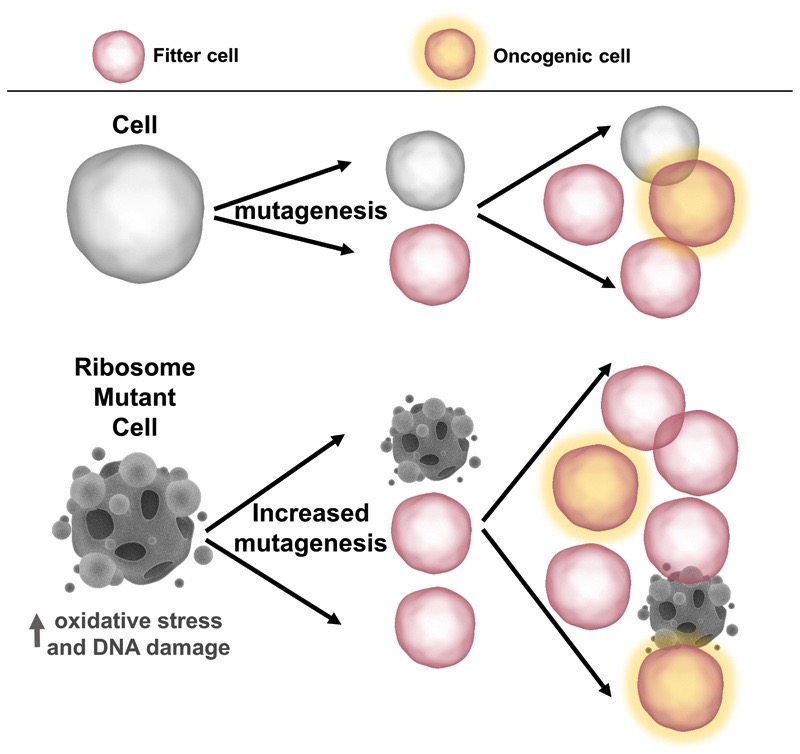
Our results allow the generation of a model linking Dameshek’s Riddle in congenital ribosomopathies and the growing list of somatic ribosome defects in cancer (Figure 4). RP mutations first cause insufficient functional ribosomes to be produced, which affects hematopoietic cells in particular,for reasons that are only beginning to emerge(e.g. specialized ribosome composition in hematopoietic tissue). This causesearly hematopoietic insufficiency, withthe surviving and ribosome-defective cells facing high oxidative stress, DNA damage,and pressure to select for compensatory mutations that can rescuethe oxidative stress and associated proliferation defect. Of note, only ribosomopathies associated with hematopoietic deficiencies progress to cancer. For example,DBA and SDSare characterized by early anemia and bone marrow failure, and a high risk to develop predominantly hematologic cancers, such as AML, later in life. In contrast, Treacher Collins Syndrome does not display any hematopoietic abnormalities and is not correlated with increased cancer risk (1).Fanconi anemia is another congenital disorder, without RP mutations, thatinitially presentswith anemia followed by a higher risk of progression to AML. The genes affected in Fanconi anemia have important roles in DNA repair, and the elevated cancer risk in this disease waspartly attributed to faulty DNA repair (15).
Figure 4. Model: the role of RP lesions in ribosome-defective disorders.
By promoting oxidative stress and DNA damage, RP lesions provide a fertile ground for elevated mutagenesis. This increases the likelihood of acquiring disease-specific rescuing and/or cooperating lesions, such as mutations in the NOTCH1 and TP53 pathways in T-ALL and CLL, respectively.
Intriguingly, other RP-mutant T-ALL samples outside of RPL10-R98S did not show a signature mainly driven by oxidative stress in our analysis. This suggests that other types of stress may cause the elevated mutation rate seen in these samples. However, emerging literature supports a more general connection between ribosomal lesions, oxidative stress and mutagenesis. For example, a mouse model of the ribosomopathy SDS was shown to be characterized by oxidative stress and activation of DNA damage responses in hematopoietic cells (16). Furthermore, oxidative stress induced DNA damage has recently been described in DBA samples (17). Finally, increased oxidative stress was shown to be involved in accelerating DNA damage and mutagenesis in myeloid cells (18). By inducing oxidative stress, RP lesions maythus act aspromoters ofDNA damage in RP-mutant diseases. In addition, ribosomal RNA has been shown to be a target for oxidative nucleobase damage which interferes with ribosome function (19).This oxidative “snowball” effect might even further enhance a mutagenic phenotype of RP lesions.
A larger mutagenic poolis expected to increase the cellular oncogenic potential.Fittingly, we observed a ~2-fold higher load of known T-ALL mutations inRP-mutant T-ALLcompared to patients with WT ribosomes (Figure 1B–C). One such mutation type affects the NOTCH1 pathway and our data support that cells expressing RPL10-R98S, and likely other RP mutations (Figure 2B–C), display elevated dependence on NOTCH1. This dependence might stem from the ability of NOTCH1 to protect ribosome-mutant cells from increased generation of ROS. We have previously shown that RPL10-R98S ribosomes are functionally rewired and lead to the over-expression of oncogenes such as JAK-STAT signaling mediators and the anti-apoptotic factor BCL-2 (6,9). However, this alone seemsinsufficient to drive cellular transformation, as increased ROS levels interfere with cellular proliferation. Selecting for mutations that diminish oxidative stress could remove a major impediment towards achieving transformation. To this end, NOTCH1 has known roles in modulating the cellular responses to high oxidative stress.In particular, NOTCH1 signaling has been shown to reduceROS production by elevating catalase levels (20)and by repression of protein kinase C θ (PKC-θ), which in turn represses ROS accumulation (21). While we did not observe NOTCH1-induced changes in catalase levels, lin- RPL10-R98S cells displayed a more significant reduction of PKC-θ levels upon expression of NOTCH1 (Figure S2), thereby corroborating this previously proposed mechanism of ROS repression via NOTCH1. Our model assumes that RPL10-R98S precedes the NOTCH1 rescuing mutations. While determining the exact mutational order at the pre-leukemic stage is not possible, analysis of the variant allelic frequencies in the Cools and Mullighan T-ALL cohorts indicates that NOTCH1-activating mutationsare acquired after RPL10-R98S in the majority of cases (Figure S4). In addition to NOTCH1, a recent genomic study also described frequent co-occurrence of translocations driving overexpression of the transcription factor NKX2-1 with RP-mutationsin T-ALL (12).Because we focused on point mutations and INDELS in our studies (4,11) and not on translocations, this association was not picked up in our analyses. Investigating the relationship between RP mutations and overexpression of NKX2-1 can be an important aim for future studies.
Recurrent ribosomal mutations in CLL have also been described, with mutations in ribosomal protein S15 (RPS15) reported as early clonal events with poor prognosis (22,23). We analyzed an available CLL whole exome dataset (22), and alsoobserved ~30% higher mutational burden in RP-mutant CLL (Figure S5). While RP mutations did not correlate with NOTCH1 lesions here, an enrichment of TP53 aberrations in RPS15-mutant CLL was previously reported (23).Moreover, a recent study described the acquisition of TP53 mutations in patients with the ribosomopathy SDS as early events in the transformation to AML (24).A mouse model of SDS furthermore displayed oxidative stress and activation of DNA damage responses in hematopoietic cells (16). The role of TP53 inactivation in RP-mutant CLL and SDS is unclear, butit may also alleviate oxidative stress as TP53 activation upon a cellular burden (i.e. ribosome assembly defects) is known to promote oxidative stress.
Hematopoietic cancers depend on multiple mutagenic events. We suggest that hematopoietic RP lesions actas intrinsic cellular stressors that make transformation more accessible by predisposing cells to acquire mutations that alleviate RP-lesion associated stress and stimulate cellular proliferation. These findings provide novel perspectives for prognosis and treatmentof RP-mutant patients.For example, our data indicate that RP-mutant T-ALL patients might particularly benefit from NOTCH1-targeted therapy. Moreover, tumor mutational burden was recently demonstrated as a biomarker of response to checkpoint inhibitor immunotherapy, with higher mutational burdens corresponding to higher response rates in a variety of cancers (25). Immunotherapy therefore represents a compelling targeted therapy opportunity for RP-mutant patients,a number that keeps on growing.
Supplementary Material
Significance.
The discovery of ribosomal mutations in cancer raised questions regarding their oncogenic mechanisms. We show that ribosomal lesions cause oxidative stress and increase mutagenesis, promoting acquisition of rescuing mutations that stimulate proliferation.
Acknowledgments
This study was supported by an José Carreras EHA junior research grant to S.O.Sulima, by a postdoctoral fellowship from Kom op tegen Kanker (Stand up to Cancer)to K.R.Kampen, an FWO-SB fellowship to S.Vereecke (1S49817N), a grant from the NIH (R01 GM117177) to J.D.Dinman, an ERC starting grant (334946), FWO funding (G084013N and 1505917N) and a Stichting Tegen Kanker grant (2012-176) to K.De Keersmaecker. The NOTCH1-L1601P-ΔPEST-GFP construct was a kind gift from Roger Habets and the lab of Bart De Strooper (VIB & KU Leuven Center for Brain & Disease Research).
Footnotes
The authors declare no competing financial interests
References
- 1.Sulima SO, Hofman IJF, De Keersmaecker K, Dinman JD. How Ribosomes Translate Cancer. Cancer Discov. 2017;7:1069–1087. doi: 10.1158/2159-8290.CD-17-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dameshek W. Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and “hypoplastic” leukemia have in common? Blood. 1967;30:251–254. [PubMed] [Google Scholar]
- 3.De Keersmaecker K, Sulima SO, Dinman JD. Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood. 2015;125:1377–1382. doi: 10.1182/blood-2014-10-569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Keersmaecker K, Atak ZK, Li N, Vicente C, Patchett S, Girardi T, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet. 2012;45:186–190. doi: 10.1038/ng.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulima SO, Patchett S, Advani VM, De Keersmaecker K, Johnson AW, Dinman JD. Bypass of the pre-60S ribosomal quality control as a pathway to oncogenesis. Proc Natl Acad Sci USA. 2014;111:5640–5645. doi: 10.1073/pnas.1400247111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girardi T, Vereecke S, Sulima SO, Khan Y, Fancello L, Briggs JW, et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2017;32:809–819. doi: 10.1038/leu.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weis F, Giudice E, Churcher M, Jin L, Hilcenko C, Wong CC, et al. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat Struct Mol Biol. 2015;22:914–919. doi: 10.1038/nsmb.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. The Journal of Experimental Medicine. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampen KR, Sulima SO, Verbelen B, Girardi T, Vereecke S, Rinaldi G, et al. The ribosomal RPL10 R98S mutation drives IRES-dependent BCL-2 translation in T-ALL. Leukemia. 2018 doi: 10.1038/s41375-018-0176-z. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehre N, Nusser A, Muenchow von L, Tussiwand R, Engdahl C, Capoferri G, et al. A stromal cell free culture system generates mouse pro-T cells that can reconstitute T-cell compartments in vivo. Eur J Immunol. 2014;45:932–942. doi: 10.1002/eji.201444681. [DOI] [PubMed] [Google Scholar]
- 11.Vicente C, Schwab C, Broux M, Geerdens E, Degryse S, Demeyer S, et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100:1301–1310. doi: 10.3324/haematol.2015.130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211–1218. doi: 10.1038/ng.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 2013;3:246–259. doi: 10.1016/j.celrep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 15.Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. 2018;18:168–185. doi: 10.1038/nrc.2017.116. [DOI] [PubMed] [Google Scholar]
- 16.Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell. 2016;19:613–627. doi: 10.1016/j.stem.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Kapralova K, Saxova Z, Kralova B, Lanikova L, Pospisilova D, Divoky V, et al. Inflammatory Signature, Oxidative Stress, and DNA Damage Response in DBA Pathogenesis. Blood. 2017;130:2452. meeting abstract. [Google Scholar]
- 18.Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 2017;32:869–883.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Willi J, Küpfer P, Evéquoz D, Fernandez G, Katz A, Leumann C, et al. Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center. Nucleic Acids Res. 2018;46:1945–1957. doi: 10.1093/nar/gkx1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang H, Zhang Y, Shi X, Wei T, Lou J. Role of Notch-1 signaling pathway in PC12 cell apoptosis induced by amyloid beta-peptide (25-35) Neural Regen Res. 2014;9:1297–1302. doi: 10.4103/1673-5374.137577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giambra V, Jenkins CR, Wang H, Lam SH, Shevchuk OO, Nemirovsky O, et al. NOTCH1 promotes T cell leukemia-initiating activity by RUNX-mediated regulation ofPKC-θ and reactive oxygen species. Nat Med. 2012;18:1693–1698. doi: 10.1038/nm.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526:525–530. doi: 10.1038/nature15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ljungström V, Cortese D, Young E, Pandzic T, Mansouri L, Plevova K, et al. Whole-exome sequencing in relapsing chronic lymphocytic leukemia: clinical impact of recurrent RPS15 mutations. Blood. 2015;127:1007–1016. doi: 10.1182/blood-2015-10-674572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J, Miller CA, Baty J, Ramesh A, Jotte MRM, Fulton RS, et al. Somatic mutations and clonal hematopoiesis in congenital neutropenia. Blood. 2017;131:408–416. doi: 10.1182/blood-2017-08-801985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.