Abstract
Synthetic immune-stimulatory drugs such as agonists of the Toll-like receptors (TLR) 7/8 are potent activators of antigen-presenting cells (APCs), however, they also induce severe side effects due to leakage from the site of injection into systemic circulation. Here, we report on the design and synthesis of an amphiphilic polymer-prodrug conjugate of an imidazoquinoline TLR7/8 agonist that in aqueous medium forms vesicular structures of 200 nm. The conjugate contains an endosomal enzyme-responsive linker enabling degradation of the vesicles and release of the TLR7/8 agonist in native form after endocytosis, which results in high in vitro TLR agonist activity. In a mouse model, locally administered vesicles provoke significantly more potent and long-lasting immune stimulation in terms of interferon expression at the injection site and in draining lymphoid tissue compared to a nonamphiphilic control and the native TLR agonist. Moreover, the vesicles induce robust activation of dendritic cells in the draining lymph node in vivo.
Introduction
Immunotherapy has gained increasing importance in clinical management of diseases with high mortality, including cancer and infectious diseases. Especially for cancer treatment, recent advances in immune therapy using adoptively transferred T cells and monoclonal antibodies targeting the programmed death/ligand 1 (PD-1/PD-L1) axis have achieved ground-breaking clinical outcomes, including complete cure and long-term disease-free survival of late-stage cancer patients.1 Unfortunately, the remarkable therapeutic benefit of PD-1/L1 antibodies are only achieved in a fraction of patients.2 Hence, a huge unmet clinical need remains to render immune therapy more efficiently in a broad population of patients.
Evidence emerges that the response to immunotherapy is highly dependent on pre-existing immunity in patients, especially the status of cellular immunity.3 Therefore, strategies that mount tumor-specific immunity in patients are highly promising to increase the response rate of the treatment.4 Among several classes of immune-modulatory molecules, agonists of Toll like receptors (TLR) are of major interest to induce activation of antigen presenting cells (APCs) and promote antigen presentation to T cells in secondary lymphoid organs.5
TLR7/8 agonists bind to receptors located on endosomal membranes.6 Two small molecule TLR7/8 agonists (imiquimod/Aldara and resiquimod/R-848) are on the market as topical formulations, but are not suitable for parenteral administrations due to severe systemic inflammation caused by their rapid diffusion from the site of injection when administered locally (i.e. subcutaneous or intramuscular).7 Furthermore, the latter shortens the duration of such agonists, which is a downside for effective immune-modulation.8 So far, various attempts have been performed to enable site-specific immune-activation and to avoid systematic inflammation by TLR agonists, including modification with lipid motifs9–11, chemical conjugation to bio/nanomaterials12–17 and physical entrapment into nanoparticles via hydrophobic and electrostatic interactions18–22. However, these strategies do not allow for efficient release of the TLR agonist in native form in cellular organelles where the corresponding TLR receptors are located and hence might reduce the biological activity.
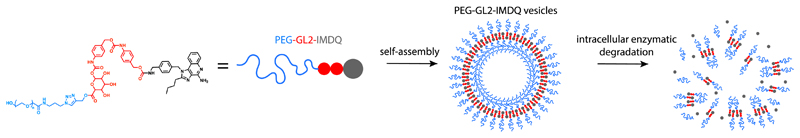
Here, we report on conjugation of an imidazoquinoline (IMDQ) TLR7/8 agonist to poly(ethylene glycol) (PEG) through a hydrophobic enzyme-responsive and self-immolative linker (i.e. prodrug formation), which induces self-assembly of the resulting amphiphiles into vesicular nanoparticles (Figure 1). These structures traffic to lymph nodes, disassemble under endosomal conditions and provoke robust immune activation in vivo.
Figure 1.
Self-assembly of PEG-modified imidazoquinoline (IMDQ) amphiphile prodrug into vesicular nanoparticles. An optimized amphiphile prodrug bearing two benzyl repeating units in the linker and PEG5k self-assembles into vesicles. Upon endocytosis by antigen presenting cells, the vesicles degrade and native IMDQ is released, thereby binding to TLR7/8 receptors and triggering immune-activation.
Results and Discussion
For the design of the enzyme responsive linker, we made use of benzyl carbamate residues capped by a β-glucuronidase (β-GUS) sensitive glucuronide23,24, which is conjugated to a PEG chain through an ester bond (Figure 1). Esterases as well as β-GUS are highly expressed in endosomes, and upon cleavage of the ester bond and β-GUS-mediated cleavage of the glucuronide, a self-immolative elimination reaction takes place within the benzyl carbamate residues, resulting in the release of IMDQ in native form. Note that small molecule enzyme-responsive prodrugs of imidazoquinolines have been reported before25, targeting metabolization by cancer cells that overexpress β-galactosidase. This is an elegant approach to induce innate immune activation in the tumor microenvironment in response to cancer cell metabolism-mediated secretion of native imidazoquinoline. However, these prodrugs have been designed to be hydrophilic which did not alter the pharmacokinetic profile towards improved lymphatic delivery.
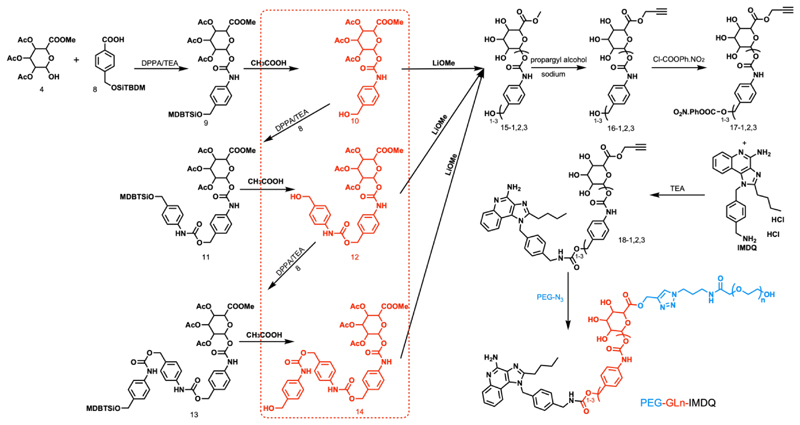
The convergent synthesis route for the prodrug amphiphiles which we abbreviate as PEG-GLn-IMDQ (n: number of benzyl carbamate residues in the β-GUS-sensitive linker (GL)) is presented in Scheme 1. Firstly, building blocks 4 and 8 were coupled via a carbamate bond to yield compound 9 which contain a single benzyl moiety. After deprotection, the obtained compound 10 was reacted with 8 yielding compound 11 which contains two benzyl moieties. The same procedure was repeated to obtain compound 13 which contains three benzyl moieties. Deacetylation of 10, 12 and 14 yielded compounds 15-1,2,3 (“1,2,3” represents the number of benzyl repeating units in the linker). The methyl groups of 15-1,2,3 were then exchanged with propargyl alcohol to install an alkyne moiety (compound 16-1,2,3). Next, the hydroxyl group of 16-1,2,3 was activated and conjugated to the aliphatic amine of IMDQ. Finally, the IMDQ derivatives with 1-3 benzyl repeating units (18-1,2,3) were conjugated with PEG (MW=5, 2 and 0.75 kDa) bearing an azide group via copper(I)-catalyzed azide-alkyne cycloaddition to yield the final compounds PEG-GLn-IMDQ (n=1-3, number of benzyl moieties). As a control, 5kDa PEG directly conjugated IMDQ (PEG5k-IMDQ) without the β-GUS-sensitive linker was synthesized (synthesis and characterization details in Supporting information).
Scheme 1.
Synthesis route for PEG-GLn-IMDQ (n=1-3).
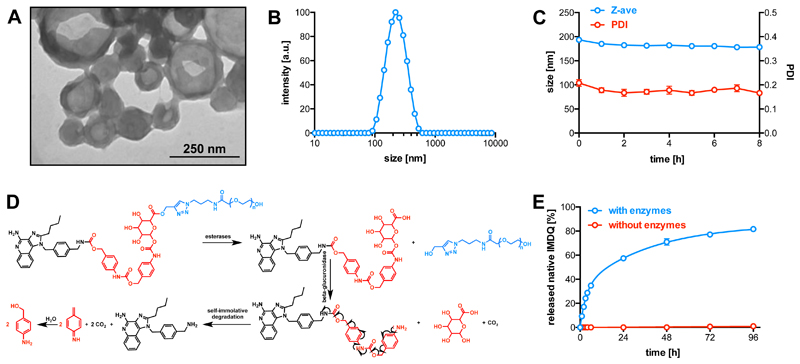
The PEG-GLn-IMDQ with different number (1-3) of the benzyl repeating units and MW of PEG (5, 2 and 0.75 kDa) were used to investigate the self-assembly behavior in aqueous medium. Hereto, the conjugates were dissolved in tetrahydrofuran (THF) and added dropwise to deionized water (v/v=1/1) in a low-energy sonication bath. THF was evaporated under room temperature. As shown in Figure S1, PEG5k-IMDQ was fully water-soluble and did not form any ordered structures (e.g. PDI=1). PEG5k-GL1-IMDQ formed random aggregates in water as shown by transmission electron microscopy (TEM) and dynamic light scattering (DLS) (Figure S1). Interestingly, PEG5k-GL2-IMDQ self-assembled into vesicular nanoparticles (Figure 1) with a clearly depicted hollow structure by TEM (Figure 2A) and a mean hydrodynamic diameter of 195 nm (PDI=0.2) measured by DLS (Figure 2B). PEG5k-GL3-IMDQ formed larger particles (264 nm, PDI 0.2, Figure S1). In addition, also PEG2/0.75k-GL2-IMDQ formed nanoparticles in aqueous medium, however, these appeared to be unstable over time (cfr, Figure S2 in Supporting Information) and TEM did not indicate the formation of vesicular structures (Figure S1). By contrast, PEG5k-GL2-IMDQ formed stable vesicles (Figure 2C), which was selected for further investigation. Vesicle-like polymeric nanostructures (e.g. polymersomes) are considered particularly attractive drug delivery systems.26–35 Notably, vesicles based on synthetic polymer-drug conjugates were very rarely reported (e.g. conjugates of oligo(ethylene glycol)-camptothecin and its analogue36,37).
Figure 2.
(A) TEM image of self-assembled vesicles based on PEG5k-GL2-IMDQ (B) DLS plot of the PEG5k-GL2-IMDQ vesicles with a mean diameter of 195 nm and low dispersity (0.2). (C) Stability of the PEG5k-GL2-IMDQ vesicles after dilution in PBS 7.4 and incubated at 37 °C. (n=3) (D) Enzymatic degradation scheme of PEG5k-GL2-IMDQ. (E) In vitro release of IMDQ from PEG5k-GL2-IMDQ vesicles in the presence of an esterase and β-GUS at pH 5.0 and 37 °C (n=3).
Next, we investigated the enzyme-responsive prosperities of the PEG5k-GL2-IMDQ vesicles, under conditions that mimic the endosomal milieu. The vesicles were incubated at pH 5.0 (i.e. endosomal pH) and 37 °C with an esterase to cleave the ester bond between the PEG chain and GL2-IMDQ and β-GUS which subsequently hydrolyses the glucuronide motif. The residual linker was expected to be prone to a self-immolative reaction, thereby releasing IMDQ in native form (Figure 2D). Of note, β-GUS is predominantly present in endosomal compartments, which is also the intracellular location of TLR7/8 receptors. Hence, the PEG5k-GL2-IMDQ is optimal to selectively release IMDQ upon cellular uptake to trigger the TLR7/8 signaling pathway. To quantify the release kinetics of native IMDQ from of the PEG5k-GL2-IMDQ vesicles, we measured native IMDQ by high-performance liquid chromatography (HPLC). As sown in Figure 2E, when the vesicles were incubated in the absence of the enzymes, minimal release of IMDQ could be detected. By contrast, in the presence of the enzymes, quantitative IMDQ release reached a plateau after 4 days. This data suggests efficient release of IMDQ from the vesicles under endosome-mimicking.
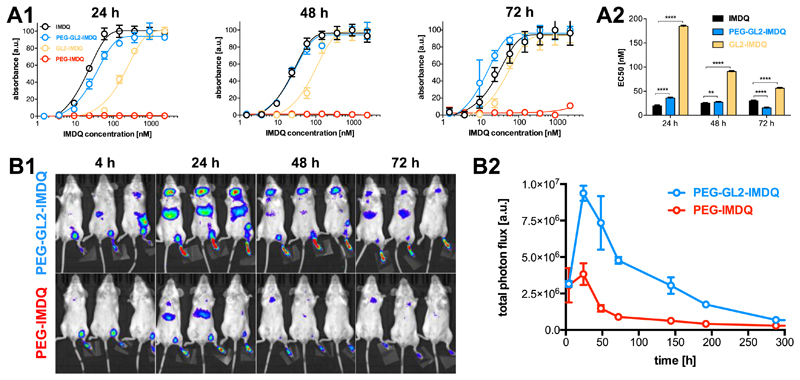
Afterwards, we assessed the TLR agonistic activity of the PEG5k-GL2-IMDQ vesicles in vitro using the RAW Blue reporter cell assay, which is based on an engineered mouse macrophage cell line that secretes embryonic alkaline phosphatase (SEAP) in response to TLR triggering and downstream nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling11. In these experiments, PEG5k-GL2-IMDQ was compared to native IMDQ, PEG5k-IMDQ (i.e. IMDQ conjugated to a PEG chain through an amide bond) and GL2-IMDQ (IMDQ modified with the β-GUS-sensitive linker but lacking the PEG chain) as controls. The dose-response curves shown in Figure 3A indicate that PEG5k-GL2-IMDQ vesicles and GL2-IMDQ showed increased activity in time, likely due to the release of IMDQ through endosomal enzymatic activity. PEG5k-GL2-IMDQ vesicles were more potent than the small molecule compound GL2-IMDQ, which we speculate is due to the enhanced dispersibility in aqueous medium of PEG5k-GL2-IMDQ and/or improved cellular uptake of nanomaterials over small molecules by antigen presenting cells.11 To underscore the amphiphilic nature of PEG5k-GL2-IMDQ and their high cellular uptake, we loaded the vesicles with a hydrophobic Cyanine3 derivative and assessed its cellular uptake by confocal laser scanning microscopy. Whereas the vesicles promoted substantial cellular internalization of the dye (Figure S3), PEG5k-IMDQ did not have this ability. Additionally, the fully water-soluble PEG5k-IMDQ did not show any TLR agonistic activity (Figure 3A) in the Raw Blue reporter cell assay, thereby hinting a poor cellular uptake of this construct and/or hindrance in TLR7/8 triggering. Interestingly, the vesicles were even more potent than native IMDQ after 72 hours of incubation (Figure 3A). Such behavior, showing increased potency of a chemically modified IMDQ conjugate over native IMDQ has not been observed in any of our previous research on IMDQ-conjugated nanomaterials7,13,38,11, which highlights the role of the enzyme degradable linker in conferring the PEG5k-GL2-IMDQ vesicles with high biological activity.
Figure 3.
In vitro RAW Blue assay andin vivo immune activation. (A1) Time dependent activation curves. (A2) EC50 values of the compounds. (n=6: Student t-test; ****:p<0.0001, **:p<0.01) (B1) IVIS imaging of IFNβ+/Δβ-luc reporter mice upon subcutaneous injection of PEG5k-GL2-IMDQ vesicles and soluble PEG-IMDQ into the footpad. (B2) Fluorescence signal detected at the local injection site and draining LN of the IFNβ+/Δβ-luc reporter mice. (n=3)
To assess the in vivo biological behavior of the PEG5k-GL2-IMDQ vesicles, we employed a transgenic IFNβ+/Δβ-luc mouse model39 engineered with a luciferase reporter gene linked to the expression of the type I interferon, IFNβ. The vesicles were injected subcutaneously into the footpad of the mice, followed by non-invasive luciferase bioluminescence imaging. At 24 hours post-injection, potent innate immune activation occurred in the draining popliteal lymph node (LN) and at the site of injection, while PEG5k-IMDQ showed much less potency (Figure 3B). Mice receiving soluble IMDQ developed a strong innate response throughout their whole body immediately after injection (Figure S4), due to the rapid leakage of IMDQ from the injection site and subsequent entry into systemic circulation. It is important to note that the signal in mice receiving PEG5k-GL2-IMDQ vesicles was even detectable more than one week post injection (Figure 3B), indicating prolonged immune stimulation due to the sustained degradation and release of IMDQ. Such long-acting immune stimulation has been shown to enable less frequent administration and induce more potent immune-modulation/vaccination effects.8,40,41 By contrast, the signal from PEG-IMDQ and IMDQ rapidly decayed to baseline levels 72 hours post-injection (Figure 3B and Figure S4).
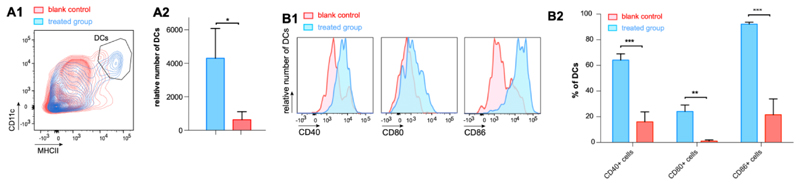
Finally, we sought prove that PEG5k-GL2-IMDQ vesicles are capable of activating dendritic cells (DCs) which are the most potent class of professional APCs and a major target cell population in immune therapy and vaccination. Flow cytometry analysis of the draining lymph node 24 hours post subcutaneous injection of the vesicles showed a strong increase (~5-fold) in the number of DCs (Figure 4A), indicating the capacity of the vesicles to induce robust recruitment of DCs to secondary lymphoid tissues. Furthermore, analysis of the expression of maturation markers CD40, CD80 and CD86 on the surface of DCs, indicated potent (over ~4-fold) induction of DC maturation in mice by PEG5k-GL2-IMDQ vesicles compared to untreated mice (Figure 4B). Taken together, our in vitro reporter cell assay data and our in vivo data underscore the ability of the PEG5k-GL2-IMDQ vesicles to induce robust activation of DCs through TLR7/8 triggering.
Figure 4.
In vivo recruitment and activation of dendritic cells (DCs) in draining lymphoid tissue. (A) Flow cytometry analysis of the DC population in the draining lymph node in response to subcutaneous injection of PEG5k-GL2-IMDQ vesicles. (A1) contour plots and (A2) relative numbers of DCs. (n=3: Student t-test; *:p<0.05) (B) Flow cytometry analysis of the expression of maturation markers on DCs in the draining lymph node in response to subcutaneous injection of PEG5k-GL2-IMDQ vesicles. (B1) histograms and (B2) percentages of DCs expressing a specific maturation marker. (n=3 Student t-test; ***:p<0.001, **:p<0.05)
In addition, in both in vivo studies, neither signs of adverse reactogenicity such as severe swelling, bruising and redness at the site of injection, not any abnormal behavior of mice treated with PEG5k-GL2-IMDQ vesicles was observed, which gives a first indication regarding an acceptable safety profile of the vesicles.
Conclusions
In summary, we designed and synthesized PEG5k-GL2-IMDQ, a novel amphiphilic polymeric prodrug of an imidazoquinoline TLR7/8 agonist that forms vesicles in aqueous medium. These vesicles degraded specifically in response to endosomal enzymes, thereby showing high in vitro TLR agonistic potency. In vivo, in mouse models, PEG5k-GL2-IMDQ vesicles provoked robust and long-acting innate immune-stimulation in draining lymphoid tissue and avoided systemic inflammation. Furthermore, PEG5k-GL2-IMDQ vesicles efficiently promoted recruitment of DCs to lymph nodes and induced robust DC maturation.
In previous studies, IMDQ-conjugated nanoparticles were shown to induce, upon intratumoral injection, potent tumor-specific immune responses. When admixed with protein antigens in a vaccination setting, robust antigen-specific T cell and humoral response in mice were mounted.7,42,38,43 Based on the highly efficient activation of DCs in vivo by PEG5k-GL2-IMDQ vesicles, we expect also these vesicles to be a highly potent immunotherapeutic and vaccine adjuvant, which will be assessed in future studies, including strategies to co-formulate antigen by either entrapment into the vesicle core or conjugation to the vesicle surface.
Supplementary Material
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Experimental details on chemical synthesis and characterization data, as well as methods for vesicle formulation and in vitro/in vivo characterizations are presented.
Acknowledgments
S.V.H. acknowledges Ghent University for a postdoctoral scholarship. This work is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ImmunoBioSynth (817938), Picelles (813086), Meta-Targeting (864121), the China Scholarship Council, the European Union (European Fund for Regional Development: I3-STM (0800387) and TAKTIRA (EFRE-0801767), the German Research Foundation (D.F.G.: GRK/RTG 2375 Tumor-targeted Drug Delivery (project no. 331065168) and SFB 1066), the Aachen Interdisciplinary Center for Clinical Research (IZKF; Project O3-2).
References
- (1).Ribas A, Wolchok JD. Cancer Immunotherapy Using Checkpoint Blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Galon J, Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- (4).Zappasodi R, Merghoub T, Wolchok JD. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell. 2018;33(4):581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).O’Neill LAJ, Golenbock D, Bowie AG. The History of Toll-like Receptors — Redefining Innate Immunity. Nat Rev Immunol. 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- (6).Kawai T, Akira S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- (7).Nuhn L, Vanparijs N, Beuckelaer AD, Lybaert L, Verstraete G, Deswarte K, Lienenklaus S, Shukla NM, Salyer ACD, Lambrecht BN, Grooten J, et al. pH-Degradable Imidazoquinoline-Ligated Nanogels for Lymph Node-Focused Immune Activation. PNAS. 2016;113(29):8098–8103. doi: 10.1073/pnas.1600816113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Boopathy AV, Mandal A, Kulp DW, Menis S, Bennett NR, Watkins HC, Wang W, Martin JT, Thai NT, He Y, et al. Enhancing Humoral Immunity via Sustained-Release Implantable Microneedle Patch Vaccination. PNAS. 2019;116(33):16473–16478. doi: 10.1073/pnas.1902179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine Adjuvant Activity of 3M-052: An Imidazoquinoline Designed for Local Activity without Systemic Cytokine Induction. Vaccine. 2011;29(33):5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- (10).Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Vrieze JD, Louage B, Deswarte K, Zhong Z, Coen RD, Herck SV, Nuhn L, Frich CK, Zelikin AN, Lienenklaus S, Sanders NN, et al. Potent Lymphatic Translocation and Spatial Control Over Innate Immune Activation by Polymer–Lipid Amphiphile Conjugates of Small-Molecule TLR7/8 Agonists. Angew Chem Int Ed. 2019;58(43):15390–15395. doi: 10.1002/anie.201905687. [DOI] [PubMed] [Google Scholar]
- (12).Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, et al. In Vivo Characterization of the Physicochemical Properties of Polymer-Linked TLR Agonists That Enhance Vaccine Immunogenicity. Nat Biotechnol. 2015;33(11):1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Herck SV, Deswarte K, Nuhn L, Zhong Z, Catani JPP, Li Y, Sanders NN, Lienenklaus S, Koker SD, Lambrecht BN, David SA, et al. Lymph-Node-Targeted Immune Activation by Engineered Block Copolymer Amphiphiles–TLR7/8 Agonist Conjugates. J Am Chem Soc. 2018;140(43):14300–14307. doi: 10.1021/jacs.8b08595. [DOI] [PubMed] [Google Scholar]
- (14).Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N, Gammon JM, et al. Peptide–TLR-7/8a Conjugate Vaccines Chemically Programmed for Nanoparticle Self-Assembly Enhance CD8 T-Cell Immunity to Tumor Antigens. Nat Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wilson DS, Hirosue S, Raczy MM, Bonilla-Ramirez L, Jeanbart L, Wang R, Kwissa M, Franetich J-F, Broggi MAS, Diaceri G, Quaglia-Thermes X, et al. Antigens Reversibly Conjugated to a Polymeric Glyco-Adjuvant Induce Protective Humoral and Cellular Immunity. Nat Mater. 2019;18(2):175–185. doi: 10.1038/s41563-018-0256-5. [DOI] [PubMed] [Google Scholar]
- (16).Wei L, Zhao Y, Hu X, Tang L. Redox-Responsive Polycondensate Neoepitope for Enhanced Personalized Cancer Vaccine. ACS Cent Sci. 2020;6(3):404–412. doi: 10.1021/acscentsci.9b01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ignacio BJ, Albin TJ, Esser-Kahn AP, Verdoes M. Toll-like Receptor Agonist Conjugation: A Chemical Perspective. Bioconjugate Chem. 2018;29(3):587–603. doi: 10.1021/acs.bioconjchem.7b00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-Agonist-Loaded Nanoparticles Promote the Polarization of Tumour-Associated Macrophages to Enhance Cancer Immunotherapy. Nat Biomed Eng. 2018;2(8):578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat Commun. 2016;7(1):1–13. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, Ferguson DM, Griffith TS, Panyam J. Polymeric Nanoparticles Encapsulating Novel TLR7/8 Agonists as Immunostimulatory Adjuvants for Enhanced Cancer Immunotherapy. Biomaterials. 2018;164:38–53. doi: 10.1016/j.biomaterials.2018.02.034. [DOI] [PubMed] [Google Scholar]
- (21).Rodell CB, Ahmed MS, Garris CS, Pittet MJ, Weissleder R. Development of Adamantane-Conjugated TLR7/8 Agonists for Supramolecular Delivery and Cancer Immunotherapy. Theranostics. 2019;9(26):8426–8436. doi: 10.7150/thno.35434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gale EC, Roth GA, Smith AAA, Alcántara-Hernández M, Idoyaga J, Appel EA. Immunoengineering: A Nanoparticle Platform for Improved Potency, Stability, and Adjuvanticity of Poly(I:C) Adv Ther. 2020;3(1) 2070001. [Google Scholar]
- (23).Houba PHJ, Leenders RGG, Boven E, Scheeren JW, Pinedo HM, Haisma HJ. Characterization of Novel Anthracycline Prodrugs Activated by Human β-Glucuronidase for Use in Antibody-Directed Enzyme Prodrug Therapy. Biochem Pharmacol. 1996;52(3):455–463. doi: 10.1016/0006-2952(96)00248-1. [DOI] [PubMed] [Google Scholar]
- (24).de Groot FMH, Albrecht C, Koekkoek R, Beusker PH, Scheeren HW. “Cascade-Release Dendrimers” Liberate All End Groups upon a Single Triggering Event in the Dendritic Core. Angew Chem Int Ed. 2003;42(37):4490–4494. doi: 10.1002/anie.200351942. [DOI] [PubMed] [Google Scholar]
- (25).Hantho JD, Strayer TA, Nielsen AE, Mancini RJ. An Enzyme-Directed Imidazoquinoline for Cancer Immunotherapy. ChemMedChem. 2016;11:2496–2500. doi: 10.1002/cmdc.201600443. [DOI] [PubMed] [Google Scholar]
- (26).Abdelmohsen LKEA, Williams DS, Pille J, Ozel SG, Rikken RSM, Wilson DA, Hest JCMV. Formation of Well-Defined, Functional Nanotubes via Osmotically Induced Shape Transformation of Biodegradable Polymersomes. J Am Chem Soc. 2016;138(30):9353–9356. doi: 10.1021/jacs.6b03984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zou Y, Zheng M, Yang W, Meng F, Miyata K, Kim HJ, Kataoka K, Zhong Z. Virus-Mimicking Chimaeric Polymersomes Boost Targeted Cancer SiRNA Therapy In Vivo. Adv Mater. 2017;29(42) doi: 10.1002/adma.201703285. 1703285. [DOI] [PubMed] [Google Scholar]
- (28).Tian X, Angioletti-Uberti S, Battaglia G. On the Design of Precision Nanomedicines. Sci Adv. 2020;6(4) doi: 10.1126/sciadv.aat0919. eaat0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Oliveira H, Pérez-Andrés E, Thevenot J, Sandre O, Berra E, Lecommandoux S. Magnetic Field Triggered Drug Release from Polymersomes for Cancer Therapeutics. J Control Release. 2013;169(3):165–170. doi: 10.1016/j.jconrel.2013.01.013. [DOI] [PubMed] [Google Scholar]
- (30).Liu G, Wang X, Hu J, Zhang G, Liu S. Self-Immolative Polymersomes for High-Efficiency Triggered Release and Programmed Enzymatic Reactions. J Am Chem Soc. 2014;136(20):7492–7497. doi: 10.1021/ja5030832. [DOI] [PubMed] [Google Scholar]
- (31).Xi Y, Wang Y, Gao J, Xiao Y, Du J. Dual Corona Vesicles with Intrinsic Antibacterial and Enhanced Antibiotic Delivery Capabilities for Effective Treatment of Biofilm-Induced Periodontitis. ACS Nano. 2019;13(12):13645–13657. doi: 10.1021/acsnano.9b03237. [DOI] [PubMed] [Google Scholar]
- (32).Sun G, Fang H, Cheng C, Lu P, Zhang K, Walker AV, Taylor J-SA, Wooley KL. Benzaldehyde-Functionalized Polymer Vesicles. ACS Nano. 2009;3(3):673–681. doi: 10.1021/nn8007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Mable CJ, Derry MJ, Thompson KL, Fielding LA, Mykhaylyk OO, Armes SP. Time-Resolved SAXS Studies of the Kinetics of Thermally Triggered Release of Encapsulated Silica Nanoparticles from Block Copolymer Vesicles. Macromolecules. 2017;50(11):4465–4473. doi: 10.1021/acs.macromol.7b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Blackman LD, Varlas S, Arno MC, Houston ZH, Fletcher NL, Thurecht KJ, Hasan M, Gibson MI, O’Reilly RK. Confinement of Therapeutic Enzymes in Selectively Permeable Polymer Vesicles by Polymerization-Induced Self-Assembly (PISA) Reduces Antibody Binding and Proteolytic Susceptibility. ACS Cent Sci. 2018;4(6):718–723. doi: 10.1021/acscentsci.8b00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Qi W, Zhang Y, Wang J, Tao G, Wu L, Kochovski Z, Gao H, Chen G, Jiang M. Deprotection-Induced Morphology Transition and Immunoactivation of Glycovesicles: A Strategy of Smart Delivery Polymersomes. J Am Chem Soc. 2018;140(28):8851–8857. doi: 10.1021/jacs.8b04731. [DOI] [PubMed] [Google Scholar]
- (36).Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, Wang J, Tang J, Fan M, Kirk EV, Murdoch WJ. Prodrugs Forming High Drug Loading Multifunctional Nanocapsules for Intracellular Cancer Drug Delivery. J Am Chem Soc. 2010;132(12):4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- (37).Wang J, Sun X, Mao W, Sun W, Tang J, Sui M, Shen Y, Gu Z. Tumor Redox Heterogeneity-Responsive Prodrug Nanocapsules for Cancer Chemotherapy. Adv Mater. 2013;25(27):3670–3676. doi: 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- (38).Yoo E, Salyer ACD, Brush MJH, Li Y, Trautman KL, Shukla NM, Beuckelaer AD, Lienenklaus S, Deswarte K, Lambrecht BN, Geest BGD, et al. Hyaluronic Acid Conjugates of TLR7/8 Agonists for Targeted Delivery to Secondary Lymphoid Tissue. Bioconjugate Chem. 2018;29(8):2741–2754. doi: 10.1021/acs.bioconjchem.8b00386. [DOI] [PubMed] [Google Scholar]
- (39).Lienenklaus S, Cornitescu M, Ziętara N, Łyszkiewicz M, Gekara N, Jabłońska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, Staeheli P, Weiss S. Novel Reporter Mouse Reveals Constitutive and Inflammatory Expression of IFN-β In Vivo. J Immunol. 2009;183(5):3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- (40).Zhang Y, Cui Z, Kong H, Xia K, Pan L, Li J, Sun Y, Shi J, Wang L, Zhu Y, Fan C. One-Shot Immunomodulatory Nanodiamond Agents for Cancer Immunotherapy. Adv Mater. 2016;28(14):2699–2708. doi: 10.1002/adma.201506232. [DOI] [PubMed] [Google Scholar]
- (41).Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, et al. Sustained Antigen Availability during Germinal Center Initiation Enhances Antibody Responses to Vaccination. PNAS. 2016;113(43):E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Nuhn L, Koker SD, Lint SV, Zhong Z, Catani JP, Combes F, Deswarte K, Li Y, Lambrecht BN, Lienenklaus S, Sanders NN, et al. Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Adv Mater. 2018;30(45) doi: 10.1002/adma.201803397. 1803397. [DOI] [PubMed] [Google Scholar]
- (43).Nuhn L, Hoecke LV, Deswarte K, Schepens B, Li Y, Lambrecht BN, Koker SD, David SA, Saelens X, Geest BGD. Potent Anti-Viral Vaccine Adjuvant Based on pH-Degradable Nanogels with Covalently Linked Small Molecule Imidazoquinoline TLR7/8 Agonist. Biomaterials. 2018;178:643–651. doi: 10.1016/j.biomaterials.2018.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.