Standfirst
Nano-formulating dexamethasone, and administering it via intravenous injection or inhalation, may help to improve anti-COVID-19 treatment efficacy by targeting the potent corticosteroid drug to hyper-activated immune cells, by potentiating its anti-edema activity and by exploiting its anti-fibrotic effects.
Dexamethasone is the first drug to show life-saving efficacy in patients infected with COVID-19. In the world's largest randomized controlled trial (RCT) of COVID-19 treatments, the so-called RECOVERY trial1, six interventions are being evaluated. Besides the potent anti-inflammatory corticosteroid drug dexamethasone, these include the antimalaria drug hydroxychloroquine, the antibiotic azithromycin, the anti-HIV drug cocktail lopinavir-ritonavir, the anti-inflammatory antibody tocilizumab, and convalescent plasma from cured patients. The first and the third statement published by the chief investigators of the RECOVERY trial reported no clinical benefit for hydroxychloroquine and lopinavir-ritonavir in hospitalized patients1. The second statement announced that dexamethasone (6 mg/day; administered orally or intravenously for 10 days) reduces the numbers of COVID-19-related deaths by 35% in patients on the intensive care unit (ICU) who require mechanical ventilation1,2. In non-ventilated patients on oxygen therapy, the mortality rate was reduced by 20%. Moreover, dexamethasone treatment resulted in a shorter hospitalization time (12 days for the dexamethasone group vs. 13 days for standard-of-care), and in a higher probability of hospital discharge within the 28 days of the clinical trial (65% vs. 61%)2. These findings are in line with recently published findings on dexamethasone efficacy in acute respiratory distress syndrome3, and they are expected to have massive global impact. Not only because dexamethasone is the first and thus far only drug to significantly improve survival in a RCT in COVID-19 patients, but also because it is a very well-known drug, which is extensively used, widely available and very cheap4.
While the main focus of initial RCT against COVID-19 has been on antiviral drugs, such as remdesivir, it has become clear that a significant number of severe cases and fatalities are due to an overreaction of the immune system, leading to hyperinflammation and to the macrophage activation syndrome. It is important to note in this regard that the recent results from the RECOVERY trial indicate that at 1 week post COVID-19 infection, the health status of patients is primarily debilitated by immunopathological phenomena, rather than by viral replication2. Hyperinflammation and the macrophage activation syndrome result in an overproduction of proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α (i.e. cytokine storm), as well as in coagulation abnormalities, which contribute to organ failure and fatalities5,6. Recognizing the importance of proinflammatory cytokines in COVID-19 severity and mortality, highly specific anti-cytokine biologics have been proposed as potential treatments for critically ill patients, including e.g. the anti-IL-6 antibody tocilizumab, which is included in the RECOVERY trial. While we are all eagerly awaiting further announcements from this and other ongoing RCT, it is striking that a well-known and widely available broad-spectrum cytokine suppressor drug, which is at least a hundred times cheaper than specific anti-cytokine antibodies, is the first to show live-saving efficacy against COVID-19. Sure, dexamethasone's mechanisms of action go way beyond suppressing cytokines. But in the current indication and situation, in which we cannot yet causally link disease progression and patient mortality to specific molecular features, this broad mechanism of action may actually be beneficial. Especially if we would be able to improve the delivery of dexamethasone to target cells and tissues that play a key role in the acute and progressive phase of COVID-19.
We here propose to reformulate dexamethasone as a nanodrug to improve the management of COVID-19 complications. At the preclinical level, several different diseases have already been successfully treated with dexamethasone nanomedicine formulations, including e.g. rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, liver fibrosis, wound healing and cancer7–12. In the case of cancer, liposomal dexamethasone has shown promising efficacy in syngeneic and xenograft mouse models, particularly also in multiple myeloma13, a disease in which dexamethasone has long been a cornerstone drug as part of both induction and maintenance therapy. At the University Medical Center at RWTH Aachen, a first-in-man clinical trial of PEGylated liposomal dexamethasone has been initiated in 2017 in patients with progressive multiple myeloma14. The results obtained thus far show good tolerability at doses up until 40 mg/kg (dexamethasone-equivalent), as well as initial signs of efficacy.
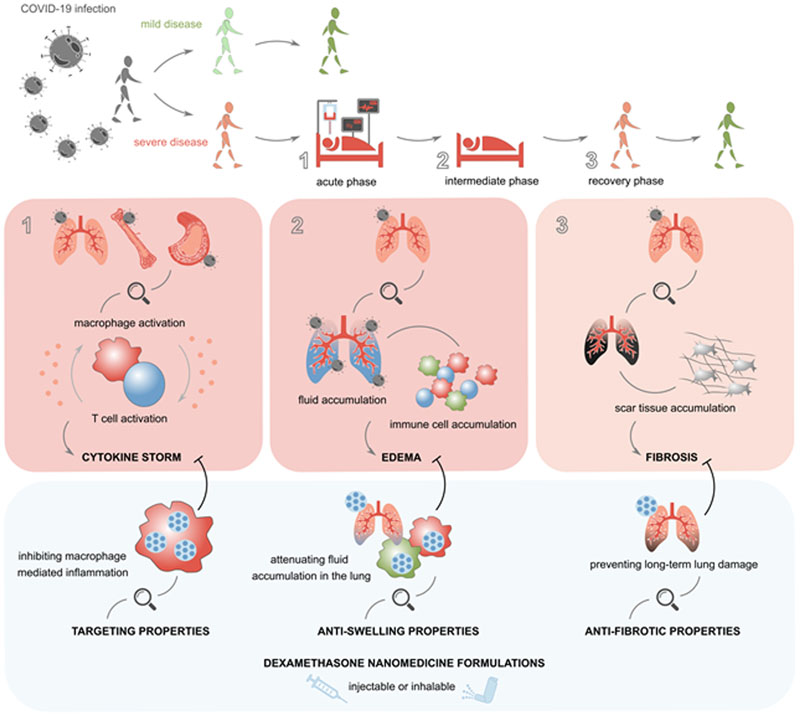
The proposition that dexamethasone nanomedicine formulations are useful for the treatment of COVID-19 is based on the widely recognized notion that nanoparticles potently accumulate in macrophages, upon intravenous administration as well as upon inhalation (Fig. 1). In this context, it is worth mentioning the liposomal amikacin product Arikayce®, which was approved by the FDA in 2019 for treating Mycobacterium avium complex lung disease. As a nanomedicine formulation, Arikayce® efficiently targets the pulmonary macrophages in which the bacterial pathogens reside and it has been shown to thereby improve disease treatment as compared to free amikacin15. Along the same line of thinking, pulmonary delivery of dexamethasone liposomes may outperform free dexamethasone when targeting alveolar macrophages as a strategy to intervene in the (sub)acute phase of COVID-19. Intravenous administration, on the other hand, provides the possibility of using liposomes and other nanomedicine formulations to target dexamethasone to myeloid and lymphoid tissues which are enriched with phagocytes, such as spleen and bone marrow. It furthermore enables efficient and relatively selective delivery of the potent corticosteroid drug to sites of inflammation where the vasculature is leaky and where large numbers of phagocytes have infiltrated, attenuating the production of proinflammatory cytokines, of matrix degrading enzymes and of other signalling molecules which contribute to edema formation and progressive tissue damage in COVID-19. It is crucial in this regard to impart long-circulating behaviour (e.g. via PEGylation) upon the intravenously injected nanomedicine formulations, as this promotes accumulation in inflammatory macrophages infiltrated at the pathological site, while avoiding rapid capture by the liver- and spleen-resident macrophage populations that are responsible for clearing nanomedicine formulations from the blood stream16.
Fig. 1. Dexamethasone nanomedicines for COVID-19.
COVID-19 infection causes several acute and longer-lasting life-threatening symptoms, including cytokine storm, edema formation and fibrosis development. Dexamethasone nanomedicines may help to better manage the severity of these disease manifestations, promoting better control of life-threatening symptoms at the acute and intermediate phase, and more rapid and more complete recovery in the post-ICU phase.
Dexamethasone nanomedicines are nowhere near a vaccine in terms of global impact and control of COVID-19 disease burden. In a number of cases, however, dexamethasone nanomedicines may really help in the day-to-day management of the disease:
-
(1)
As alluded to above, nanomedicine formulations can help to target the potent corticosteroid drug to inflammation-initiating and -propagating phagocytic cells in the lung, in the blood, and in myeloid and lymphoid tissues. This assists in better controlling MAS and cytokine storm, which have been implicated in COVID-19-related fatalities5. As a consequence, critically ill patients on ventilation or on oxygen therapy are expected to recover faster and more efficiently than upon treatment with the free drug.
-
(2)
Dexamethasone is a highly active anti-edema agent. Its potent anti-swelling properties contribute to its mechanism of action in multiple different diseases, including in high-grade inflammatory disorders and in glioblastoma, and this assumingly also contributes to its activity in COVID-19. Nanoformulating dexamethasone could further potentiate this effect, by increasing drug availability and drug activity over time in hyperactivated immune cell populations in the inflamed parts of the lung. Dexamethasone nanomedicine formulations may furthermore help to sustain anti-inflammatory and anti-edema drug activity in the days and weeks after patients have been released from the hospital.
-
(3)
Dexamethasone is a highly potent anti-fibrotic agent. Multiple preclinical studies, in various different disease models, have shown that the anti-fibrotic effects of dexamethasone can be potentiated by reformulating it as a nanomedicine formulation7–12. In this context, dexamethasone nanomedicines have been shown to be particularly useful for preventing fibrosis. Since pulmonary fibrosis has recently emerged as a key complication in the long-term follow-up management of COVID-19 (especially in patients that have been ventilated for prolonged periods of time)17, inhaled or intravenously injected dexamethasone nanomedicines could meet an urgent medical need also at this level of COVID-19 management.
When realistically and holistically reflecting upon the potential of dexamethasone nanomedicines for the treatment of COVID-19, we conclude that money and time are important issues. The mere fact that dexamethasone is an already widely available and very cheap drug with - now proven - life-saving capability in COVID-19 substantially raises the bar for any new nanomedicine product based on dexamethasone. A dexamethasone nanomedicine product obviously entails a higher level of complexity in terms of composition and manufacturing, and it would first have to be clinically tested and registered before it becomes available on the market, where it would have to fetch at least $100 per treatment to make it economically viable. We believe the key undertaking here is to carefully design the clinical studies with the nanomedicine product so as to unambiguously prove what the actual added value is. If targeted delivery of dexamethasone in COVID-19 patients using a nanomedicine formulation leads to better outcomes, for instance in terms of a reduction of the number of days that patients need mechanical ventilation and/or require costly ICU hospitalization 18,19, then this is already a huge gain that can easily offset against the higher level of complexity and cost of the nanodrug. And if in these clinical studies dexamethasone nanomedicines would also turn out to be able to outperform the free drug in terms of improving the survival of critically ill patients, then that would be another major leap forward in the worldwide battle against COVID-19.
Acknowledgements
T.L. and J.M.M. gratefully acknowledge support by the European Research Council (ERC) and the German Research Foundation (DFG).
Footnotes
Reprints and permission information is available online at http://www.nature.com/reprints.
References
- 1.Results — RECOVERY Trial. [Accessed: 2nd July 2020]; Available at: https://www.recoverytrial.net/results.
- 2.Horby P, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. 2020.06.22.20137273. [DOI] [Google Scholar]
- 3.Villar J, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 4.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020 doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 5.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan L, et al. Nanomedicines for Inflammatory Arthritis: Head-to-Head Comparison of Glucocorticoid-Containing Polymers, Micelles, and Liposomes. ACS Nano. 2014;8:458–466. doi: 10.1021/nn4048205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banciu M, Metselaar JM, Schiffelers RM, Storm G. Liposomal glucocorticoids as tumor-targeted anti-angiogenic nanomedicine in B16 melanoma-bearing mice. J Steroid Biochem Mol Biol. 2008;111:101–110. doi: 10.1016/j.jsbmb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Crielaard BJ, et al. Macrophages and liposomes in inflammatory disease: Friends or foes? Int J Pharm. 2011;416:499–506. doi: 10.1016/j.ijpharm.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Bartneck M, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials. 2015;37:367–382. doi: 10.1016/j.biomaterials.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Kroon J, et al. Liposomal delivery of dexamethasone attenuates prostate cancer bone metastatic tumor growth In Vivo. Prostate. 2015;75:815–824. doi: 10.1002/pros.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier A, et al. Glucocorticoid-loaded liposomes induce a pro-resolution phenotype in human primary macrophages to support chronic wound healing. Biomaterials. 2018;178:481–495. doi: 10.1016/j.biomaterials.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Deshantri AK, et al. Liposomal dexamethasone inhibits tumor growth in an advanced human-mouse hybrid model of multiple myeloma. J Control Release. 2019;296:232–240. doi: 10.1016/j.jconrel.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 14.A Evaluation of the Safety of Oncocort IV Pegylated Liposomal Dexamethasone Phosphate in Patients With Progressive Multiple Myeloma - Full Text View - ClinicalTrials.gov. [Accessed: 2nd July 2020]; Available at: https://clinicaltrials.gov/ct2/show/NCT03033316. [Google Scholar]
- 15.Zhang J, et al. Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages. Front Microbiol. 2018;9:915. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metselaar JM, Wauben MHM, Wagenaar-Hilbers JPA, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 17.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;0 doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaier K, Heister T, Wolff J, Wolkewitz M. Mechanical ventilation and the daily cost of ICU care. BMC Health Serv Res. 2020;20:267. doi: 10.1186/s12913-020-05133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasta J, McLaughlin T, Mody S, Piech C. Daily Cost of an Intensive Care Unit Day: The Contribution of Mechanical Ventilation. Crit Care Med. 2005;33 doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]