Abstract
The origin of the oxytocin (OT)/vasopressin (VP) signaling system is thought to date back more than 600million years. OT/VP-like peptides have been identified in numerous invertebrate phyla including molluscs, annelids, nematodes and insects. However, to date we only have a limited understanding of the biological role(s) of this GPCR-mediated signaling system in insects. This chapter presents the current knowledge of OT/VP-like neuropeptide signaling in insects by providing a brief overview of insect OT/VP-like neuropeptides, their genetic and structural commonalities, and their experimentally tested and proposed functions. Despite their widespread occurrence across insect orders these peptides (and their endogenous receptors) appear to be absent in common insect model species, such as flies and bees. We therefore explain the known functionalities of this signaling system in three different insect model systems: beetles, locusts, and ants. Additionally, we review the phylogenetic distribution of the OT/VP signaling system in arthropods as obtained from extensive genome/transcriptome mining. Finally, we discuss the unique challenges in the development of selective OT/VP ligands for human receptors and share our perspective on the possible application of insect- and other non-mammalian-derived OT/VP-like peptide ligands in pharmacology.
1. Introduction
Neuropeptides acting as neurotransmitters or neurohormones are key players in the regulation of physiological, behavioral, and developmental processes in animals (Gäde, 2004). In mammals, oxytocin (OT) and vasopressin (VP) exert their effects via four G protein-coupled receptors (GPCRs): the oxytocin receptor, OTR, and three vasopressin receptors, V1aR, V1bR and V2R (Barberis, Mouillac, & Durroux, 1998; Gimpl & Fahrenholz, 2001). GPCRs are proteins with seven membrane-spanning α-helical segments. They transduce extracellular stimuli into intracellular signals and hence regulate various cellular processes (Gruber, Muttenthaler, & Freissmuth, 2010; Kroeze, Sheffler, & Roth, 2003; Vanden Broeck, 1996). Agonist binding results in a stabilized active receptor conformation that in turn recruits and regulates intracellular transducers, such as heterotrimeric G-proteins and arrestins (Wacker, Stevens, & Roth, 2017). GPCR agonists are diverse and can include neurotransmitters, hormones, growth factors, odorants, photons, small molecules, peptides and proteins (Gruber et al., 2010). The human genome contains more than 800 GPCR genes, making them the largest family of membrane proteins (Fredriksson, Lagerstrom, Lundin, & Schioth, 2003). OT and VP are synthesized by neurons in the paraventricular and supraoptic nuclei of the hypothalamus that project to and have release sites in the pituitary gland, from which both hormones are released into peripheral circulation and the brain. In addition, parvocellular neurons originating in the paraventricular nucleus also express OT and VP and project to discrete brain regions (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Central and peripheral functions of these two peptides include reproduction, osmoregulation, memory and learning (Donaldson & Young, 2008; McCall & Singer, 2012). In mammals and other vertebrates the two neuropeptides OT and VP exhibit distinct physiological roles. By contrast, invertebrates generally have only a single homolog of the OT/VP precursor and a single OT/VP-like receptor (Gruber, 2014; Liutkevičiūte & Gruber, 2016).
The OT/VP-like signaling system has an ancient origin and is found in groups whose last common ancestor lived over 600 million years ago, including insects, molluscs, annelids and nematodes (Gruber, 2014; Liutkevičiūte, Koehbach, Eder, Gil-Mansilla, & Gruber, 2016). It has been suggested that the vertebrate OT/VP-like peptides originated from a gene duplication of the ancestral peptide vasotocin (Liutkevičiūte & Gruber, 2016); refer to Chapter 4 of this volume).
All OT/VP-like molecules are nonapeptides possessing high sequence homology. They comprise a six residue N-terminal ring formed by a disulfide bond between the conserved cysteines in positions 1 and 6, and an amidated three residue C-terminal tail (Gruber, Koehbach, & Muttenthaler, 2012). They derive from larger precursor proteins, which contain a short N-terminal endoplasmic reticulum signal peptide followed by the mature nonapeptide domain and a canonical amidation processing sequence. The C-terminal domain is characterized by a protein called neurophysin. Neurophysins are primarily known to act as carrier proteins for OT and VP, but there is evidence that they have other OT/VP-independent biological functions as well (Elphick, 2010).
Despite a high degree of sequence similarity, OT/VP-like peptides are known by different names in different taxa such as annetocin (annelids, OT-like), conopressin (molluscs, VP-like), nematocin (nematodes, OT-like), inotocin (insects, OT/VP-like) or vasotocin (vertebrate ancestral form, OT/ VP-like) (Gruber, 2014). These names mask both fundamental differences and similarities between these molecules across species. The current names do not uniquely describe the peptide sequence (there are several different peptides in insects, for example, which are all referred to as “inotocin“) and the use of the suffixes “-pressin” or “-tocin” are arbitrary and not related to underlying functional similarities. In this chapter we use the terms “OT/ VP-like” or “inotocin signaling” as equivalent or synonymous when referring to insects.
OT/VP-like signaling appears to be involved in neurotransmission, metabolism, and osmoregulation in molluscs and annelids (Gruber, 2014); in the nematode Caenorhabditis elegans, it regulates gustatory associative learning and coordinates reproductive behavior (Beets et al., 2012; Garrison et al., 2012). Until very recently there has been relatively little information available on the role of this signaling in insects.
In this chapter we will provide an overview of the current knowledge of OT/VP-like signaling in beetles, locusts and ants as widely-studied insect model systems (summarized in Fig. 1). Interestingly, OT/VP-like signaling is absent in the European honey bee, Apis mellifera, and the fruit fly, Drosophila melanogaster. We provide an overview of the phylogenetic distribution of OT/VP signaling and highlight recent advances in the discovery of ligand and receptor genes in insects. Lastly, we will summarize available information about the possible application of insect OT/VP-like ligands in human pharmacology.
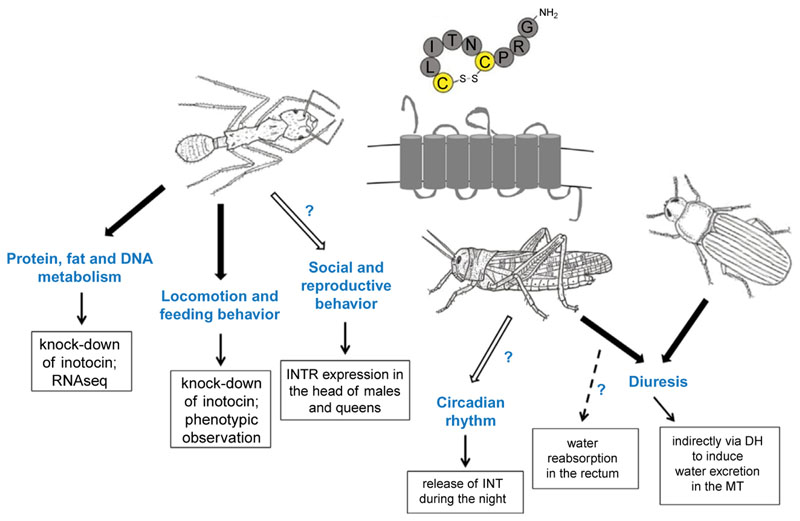
Fig. 1.
Summary of functional role of the inotocin signaling in ants, locusts and beetles. INT (inotocin), INTR (inotocin receptor), DH (diuretic hormone), MT (Malpighian tubules). Black arrows indicate experimentally tested functions and white arrows indicate suggested functions (based on weaker experimental evidence). Insect drawings were prepared by E. Gil-Mansilla.
2. OT/VP-like signaling in beetles
The first evidence for the existence of an OT/VP-like signaling system in beetles was obtained in the early 1980s after immunostaining of the central nervous system (CNS) of the Colorado potato beetle, Leptinotarsa decemlineata with anti-vasopressin and anti-oxytocin antibodies (Veenstra, 1984; Veenstra, Romherg-Privee, & Schooneveld, 1984). More recently, the presence of an OT/VP-like peptide, designated as inotocin, was predicted from the genome sequence data of the red flour beetle, Tribolium castaneum, and cloned (Aikins et al., 2008; Stafflinger et al., 2008). Inotocin-encoding genes are likely present in many other beetle species based on genome or transcriptome predictions, while L. decemlineata even appears to have two different copies of this gene (Liutkevičiūte et al., 2016). On available evidence, CLITNCPRG*a is the most common peptide sequence in beetles, while L. decemlineata has both CLITNCPKG* and CLITNCPIG* as putative inotocin-like peptides (Liutkevičiūte et al., 2016).
An inotocin receptor has been predicted in several beetle species based on available genome or transcriptome sequences (Liutkevičiūte et al., 2016), but has only been cloned and characterized in T. castaneum (Aikins et al., 2008; Stafflinger et al., 2008). As expected, this receptor is a GPCR and is activated by the monomeric form of inotocin (CLITNCPRG*). The T. castaneum receptor could also be activated by two other analogs, which were tested because they had been previously identified by high performance liquid chromatography (HPLC) in the migratory locust, Locusta migratoria (Proux et al., 1987). These are an antiparallel and a parallel dimer of the nonapeptide, which activated the receptor as partial agonists and had ~10- and ~80-fold lower potency than the monomer, respectively (Aikins et al., 2008; Keov, Liutkevičiūte, Hellinger, Clark, & Gruber, 2018). Furthermore, oxytocin, vasotocin, isotocin (OT-like peptide in fishes; Godwin & Thompson, 2012) and conopressin could all induce a response (but only at higher concentrations than any of the inotocin forms), whereas vasopressin and other insect neuropeptides [such as crustacean cardioactive peptide (CCAP), adipokinetic hormones I and II (AKH I and AKH II), and corazonin (CRZ)] could not. From these results, it seems that the inotocin receptor from T. castaneum is more OT-like than VP-like (cf. the preference for isoleucine at position 3 of the peptide). This is supported by the amino acid sequence homology of the beetle receptor to its human counterparts (~40% similarity with the human OT receptor compared to 37–38% with the human VP receptors; Aikins et al., 2008; Keov et al., 2018; Stafflinger et al., 2008).
In situ hybridization and immunohistochemistry studies on CNS tissues from T. castaneum, L. decemlineata and the mealworm Tenebrio molitor showed that inotocin is synthesized by a single pair of neurons with cell bodies situated in the subesophageal ganglion (Aikins et al., 2008; Veenstra, 1984; Veenstra et al., 1984). These two neurons project symmetrically throughout the entire CNS, from the brain to the terminal abdominal ganglion. Interestingly, there was no immunostaining observed in peripheral nerves in T. castaneum, suggesting a major central role and possibly a minor one peripherally (Aikins et al., 2008). Moreover, expression analysis of the inotocin receptor in this species revealed that it is highly expressed in the CNS (mainly in the head), while there is little expression in the Malpighian tubules and the hindgut, further supporting this idea (Aikins et al., 2008; Stafflinger et al., 2008). Although little is known about the in vivo roles of inotocin in beetles, there is an indication that it could play a role in diuresis in both T. castaneum and T. molitor. Injecting monomeric inotocin in adult T. castaneum induced an immediate diuretic response, as measured in an evaporation chamber, whereas neither dimer was active. In T. molitor, an ex vivo experiment was performed by incubating Malpighian tubules with different tissues and the inotocin peptides. The only condition under which inotocin (monomer) induced an increase in excretion was when incubated with the CNS together with the corpora cardiaca/corpora allata (CC/CA) complex. Without the CC/CA complex (only the CNS) or when incubated only with inotocin, Malpighian tubules did not excrete any water. Again, the antiparallel dimer of inotocin was not able to induce any response. Diuretic hormone, which has been shown to induce diuresis in the Malpighian tubules (Aikins et al., 2008; Furuya, Schegg, Wang, Kingt, & Schooley, 1995), is stored and released by the CC/CA complex of T. molitor (Wiehart, Torfs, Van Lommel, Nicolson, & Schoofs, 2002). Hence, this experimental result suggested an indirect role of inotocin on diuresis (for instance via central signaling instead of peripheral action). Indeed, it was hypothesized that inotocin could be acting on the CC/CA of T. molitor to induce the release of diuretic hormone that in turn triggers water excretion from the Malpighian tubules (Aikins et al., 2008) and the same could occur in T. castaneum (Fig. 1).
Knockdown studies of OT/VP-like signaling using RNA interference (RNAi) have also been performed in T. castaneum, but downregulating the precursor and receptor transcripts in this animal did not influence any of the processes monitored: general mortality, developmental time in the pupal stage, egg-laying behavior, egg hatching, and mortality in high salt loads (Aikins et al., 2008). Although these knockdown experiments had no observable effect, there is still the possibility that inotocin has additional roles in beetles other than diuresis. Other approaches that could be used to study OT/VP-like signaling include modulation of the receptor by inotocin analogs, which can be injected into the animal. Potentially useful analogs that could be used for this purpose are atosiban, a well-known synthetic oxytocin analog, and kalata B7, a plant-derived cyclotide (Keov et al., 2018). In addition, their structures may also serve as leads for development of additional receptor ligands.
3. OT/VP-like signaling in locusts
Locusts are swarm-forming insects, which can be distinguished from other grasshoppers (Orthoptera:Acrididae) by their ability to switch between solitarious and gregarious phases upon changes in population density, a process known as phase polyphenism (Simpson & Sword, 2008). The first evidence for an OT/VP-like peptide in insects came from the migratory locust, L. migratoria, nearly 40 years ago (Rémy, Girardie, & Dubois, 1979), when immunostaining of the subesophageal ganglion with an antimammalian VP antibody revealed a single pair of immunoreactive neurons. Inotocin was subsequently isolated using HPLC and identified as being a nonapeptide with the sequence CLITNCPRG (Proux et al., 1987), which is the most common inotocin sequence across insect taxa (Liutkevičiūte et al., 2016). Proux et al. (1987) isolated two forms of inotocin: a monomer and its antiparallel dimer; later a parallel dimer was also found (Baines & Bacon, 1994). The anti-mammalian VP antibody was effective in insects because VP and inotocin share a common C-terminus (residues in positions 6–9). The N-terminals, however, differ (residues at positions 2, 3 and 4; Donaldson & Young, 2008).
The widespread presence of inotocin in other grasshoppers is supported by immunostaining studies of the whole CNS in 16 grasshopper species across several subfamilies with the same AVP antibody used previously by Remy and co-workers (Evans & Cournil, 1990; Rémy et al., 1979; Tyrer, Davis, Arbas, Thompson, & Bacon, 1993). Unfortunately, the peptide has not been isolated and sequenced in any species other than L. migratoria, hence these immunostaining results are the only evidence for widespread OT/VP-like signaling in grasshoppers, and it is unknown whether there are multiple variants of the inotocin sequence across the Orthoptera.
In common with other animal species, inotocin in grasshoppers is formed by the post-translational modification of a large precursor hormone containing a signal peptide, followed by the nonapeptide itself, and finally a neurophysin domain (Aikins et al., 2008; Caldwell & Young, 2006; Di Giglio et al., 2017; Liutkevičiūte et al., 2016). The presence of a neurophysin co-expressed with inotocin was supported by Rémy et al. (1979), who discovered that a mammalian anti-neurophysin II antibody stained the same pair of neurons in the subesophageal ganglion that expressed inotocin. It was only with the recent publication of the genome of L. migratoria, that this finding was confirmed and the full precursor sequence determined (Wang et al., 2014). A single OT/VP-like peptide receptor has also been predicted in the genome of L. migratoria, based on sequence similarities with other known OT/VP-like peptide receptors. As with all other OT/VP-like peptide receptors so far identified, the predicted L. migratoria OT/VP-like peptide receptor belongs to the GPCR family (Liutkevičiūte et al., 2016). To our knowledge, this inotocin receptor has yet to be cloned or characterized.
The single pair of neurons that biosynthesize inotocin in grasshoppers closely resemble those found in beetles, having cell bodies of about 50–60 μm diameter lying near the ventral midline of the subesophageal ganglion (Evans & Cournil, 1990; Rémy & Girardie, 1980; Rémy et al., 1979; Thompson, Tyrer, May, & Bacon, 1991; Tyrer et al., 1993). These two neurons extend parallel axons that project both anteriorly into the brain and posteriorly to the terminal abdominal ganglion. The exact pattern of arborization appears to vary between different subfamilies of grasshopper. In the Cyrtacanthacridinae, which includes the desert locust, Schistocerca gregaria, there is extensive central branching within the brain and in each ganglion of the ventral nerve cord but no fibers enter the optic lobes or the peripheral nerves (except in males, see below). In the Gomphocerinae, such as the field grasshopper, Chorthippus brunneus, there is likewise extensive arborization within the CNS but there is also some branching within the lobulae of the optic lobes. Conversely, grasshoppers in the Oedipodinae, which includes L. migratoria, have relatively sparse branching in the central neuropils of the brain and ventral nerve cord, predominately in dorsal regions, but have extensive arborizations throughout the entire optic lobe. A distinctive feature of the neuron in this subfamily is a proliferation of fine fibers that exit the CNS to form plexuses in most peripheral nerves (Evans & Cournil, 1990; Thompson et al., 1991; Tyrer et al., 1993).
Sexually dimorphic branching of the inotocin expressing neurons has so far only been found in S. gregaria, which occurs in both the seventh and terminal (fused segments 8–11) abdominal ganglia. In females no neuronal processes exit any of these ganglia, while in males some fibers enter nerves innervating the genitals and accessory reproductive structures.
The contrasting arborization patterns in different subfamilies of grasshoppers raise important questions as to the extent to which inotocin acts as a neurotransmitter or neuromodulator within the CNS, is released onto peripheral effectors, or enters into general circulation in the hemolymph in different acridid lineages (Evans & Cournil, 1990; Tyrer et al., 1993). Hemolymph concentrations of inotocin are far below what would be expected for a circulating hormone (10−12M vs 10−9M), supporting a central role or targeted peripheral delivery (Baines, Thompson, Rayne, & Bacon, 1995).
Several studies in L. migratoria have suggested that inotocin expression varies with the light-dark cycle over the day (Picquot & Proux, 1987; Thompson & Bacon, 1991) (Fig. 1). In mammals, VP expression varies according to an intrinsic circadian rhythm, which is entrained to the external light intensity (Li et al., 2008; Li, Burton, Zhang, Hu, & Zhou, 2009; Reppert, Schwartz, & Uhl, 1987). In locusts the inotocin-containing neurons are constantly active under dark conditions firing at 2–8 spikes s−1, but silent in daylight, an effect seen even when the optic lobes and ocellar nerves were detached from the rest of the brain (Thompson & Bacon, 1991). This led Thompson and Bacon (1991) to propose that locusts, like some other insect species, may possess extraocular photoreceptors on the surface of the brain that detect light independently from the ocelli and compound eyes. The authors suggest that these extraocular photoreceptors synapse with a descending interneuron in the pars intercerebralis of the brain that descends via the circumesophageal connectives to synapse with the inotocin-expressing neurons in the subesophageal ganglion.
Neither the extraocular receptors nor descending neuron have been positively identified, but their properties can be inferred from their effects on the inotocin-expressing neurons. Physiological saline with high Ca2+ concentrations promotes continuous synaptic release and under these conditions the inotocin-expressing neurons are active even under strong light conditions, suggesting that their activity is driven by a continuous excitatory drive from the descending neuron (Baines & Bacon, 1994). In dark conditions, the descending interneuron is spontaneously active and continually excites the inotocin-expressing neurons (Baines & Bacon, 1994; Lundquist, Baines, & Bacon, 1996; Thompson & Bacon, 1991). Pharmacological experiments suggest that the descending neuron is cholinergic, and that the inotocin-expressing neurons have both fast nicotinic and slow muscarinic receptors leading to transient and long-lasting (several seconds) depolarizations, respectively (Baines & Bacon, 1994). Pressure injections of histamine, an inhibitory neurotransmitter of insect primary photoreceptors (Schlemermeyer, Schütte, & Ammermüller, 1989), into the brain near the putative site of the extraocular receptors led to the suppression of spontaneous firing in the inotocin expressing neurons, mimicking the effect of light conditions, with the inference that the extraocular photoreceptors inhibit the firing of the descending neuron which in turn removes the excitatory drive to the inotocin-expressing neurons (Lundquist et al., 1996).
The inotocin-expressing neurons in non-oedipodinine grasshoppers do not show the light-dark response characteristic of L. migratoria (Lundquist, Baines, Thompson, & Bacon, 1998). In these grasshoppers the inotocin expressing neurons are tonically active under both light and dark conditions. Mechanosensory stimulation from anywhere on the body is a potent stimulus of the inotocin-expressing neurons in all grasshopper species, and appears to act via similar cholinergic descending interneurons as mediate the light response in L. migratoria (Lundquist et al., 1998).
As in beetles, the in vivo function of inotocin in locusts is unclear. There is some evidence supporting a role in diuresis in L. migratoria, even though this is controversial (Fig. 1). Diuresis occurs essentially at two sites: at the Malpighian tubules where water is excreted and forms the primary urine, and in the rectum where there is some water, ion and metabolite reabsorption (Spring, 1990). It is likely that inotocin acts in an indirect way to promote water excretion at the level of the Malpighian tubules. Incubation of the Malpighian tubules attached to the posterior part of the midgut with inotocin or an extract of the subesophageal ganglion led to an increase in excretion, in a concentration-dependent manner (Proux et al., 1987; Proux & Herault, 1988; Proux, Picquot, Herault, & Fournier, 1988; Proux, Rougon, & Cupo, 1982), whereas if only the Malpighian tubules were put in the incubation medium, nothing was observed (Coast et al., 1993). It is now known that the posterior midgut and the ampullae possess endocrine cells containing diuretic hormone and that this neuropeptide induces strong diuresis by elevating cAMP levels in the Malpighian tubules (Montuenga et al., 1996). Hence, it is possible that inotocin could affect diuresis indirectly by acting on the endocrine cells of the midgut and the ampullae to induce the release of diuretic hormone, which in turn, induces a rise in cAMP that triggers water excretion. There is some additional evidence supporting a possible role of inotocin in diuresis. Relative atmospheric humidity has some influence on inotocin concentration in the subesophageal ganglion and ventral nerve cord. Decreasing relative humidity to 30–40% induced a significant decrease in inotocin expression, but no significant differences were observed following an increase to 80–100% relative humidity (Picquot & Proux, 1989; Proux & Rougon-Rapuzzi, 1980), supporting a role in the reduction of water loss in dry environments. Changing the concentration of physiological saline, mimicking the effect of altered water balance in the hemolymph had no effect on the firing rate of the inotocin-expressing neurons, however (Thompson & Bacon, 1991). Furthermore, there was only a loose correlation between variation in the concentration of inotocin in the hemolymph and diuresis in locusts over a 24h period, with the peak of both occurring 4h after feeding in the morning, but being otherwise unrelated (Picquot & Proux, 1987).
A role of inotocin in diuresis of locusts is consistent with a conserved function of the VP-like system across the animal kingdom and is similar to the role attributed to inotocin in T. castaneum and T. molitor, (see above), although in mammals VP is an anti-diuretic rather than diuretic hormone (Caldwell & Young, 2006). In mammals, annelids and chordates OT/ VP-like peptides play a more extensive role in regulating osmoregulatory function (Caldwell & Young, 2006; Gruber, 2014). However, it is still possible that inotocin also plays a role in water reabsorption in the rectum of insects similar to the role of VP in the kidneys of mammals. This was suggested by Proux and co-workers (Proux et al., 1988; Proux & Herault, 1988) in early studies, where they observed that low inotocin concentrations were capable of inducing fluid reabsorption in the rectum.
From the three identified forms of inotocin, only one was shown to be effective in inducing diuretic effects (or water reabsorption) in locusts, the antiparallel dimer (Proux et al., 1987, 1988; Proux & Herault, 1988). The biological function of the monomer form is not known, but it was hypothesized that it is just a precursor of the antiparallel dimer. Serine proteases were able to transform the monomer into the antiparallel dimer and induce diuresis, and the dimer could be degraded by enzymes extracted from the rectum. When those degradation products were analyzed by HPLC, a product similar to the monomer could be detected (Picquot & Proux, 1990). It is possible that the monomer has a role as a neurotransmitter or neuromodulator in the CNS: in a coarse biochemical assay, the monomer induced a net decrease in cAMP levels throughout the thoracic CNS, but neither dimer had any measurable effect (Baines et al., 1995). It is clear that much remains to be done to understand the roles of inotocin in locusts, both as a potential hormone and neurochemical, and how the tactile and visual stimuli that drive the activity of the inotocin expressing neurons affect physiology or behavior.
4. OT/VP-like signaling in ants
Gruber and Muttenthaler (2012) reported the discovery of the precursor protein encoding genes and mature OT/VP-like peptide sequences using a simple genome-mining approach in three ant species from the subfamilies Myrmicinae (Atta cephalotes), Formicinae (Camponotus floridanus) and Ponerinae (Harpegnathos saltator). The identified OT/VP-related peptides with the amino acid sequences CLITNCPRG* (in A. cephalotes and H. saltator) and CLIVNPRG* (in C. floridanus), exhibited high similarity to vasotocin and contained elements of both mammalian OT (CYIQNCPLG*) as well as VP (CYFQNCPRG*) peptides. Further analysis showed that the precursor proteins shared considerable sequence homology to the precursors of the other insect OT/VP-like proteins as well as mammalian OT and VP precursors. Apart from the mature nonapeptide sequences, each precursor protein contained a canonical amidation sequence and a neurophysin domain (Gruber & Muttenthaler, 2012). In addition to the discovered inotocin nonapeptides, Gruber and Muttenthaler (2012) also identified putative receptor sequences in ant genomes that are similar to inotocin receptors of other insects such as the red flour beetle T. castaneum. Another important finding was the absence of inotocin signaling system in another hymenopteran, the honey bee (A. mellifera) (Stafflinger et al., 2008). A subsequent transcriptomic study confirmed these initial findings and identified inotocin peptides and receptor sequences in 17 ant species (Liutkevičiūte et al., 2016).
These observations not only provided an exciting opportunity to advance our understanding of the evolution of inotocin signaling but led to the elucidation of the molecular mechanisms of pharmacological and physiological actions of inotocin peptides and their receptors in ants, as an important group of social insects. Di Giglio et al. (2017) cloned the inotocin peptide precursor and its putative receptor from the black garden ant Lasius niger, before determining the pharmacological properties of a synthetic inotocin ligand on the receptor. The results provided evidence that the endogenous inotocin peptide was indeed the cognate ligand of the identified receptor. Shortly afterwards, Chérasse and Aron (2017) studied the gene expression levels of the inotocin receptor in L. niger ant queens during reproductive, social and aggressive behavior. Using qPCR, they observed that the inotocin receptor is expressed in the heads, with the highest expression levels occurring early in the queens’ lives under crowded and mated conditions (Chérasse & Aron, 2017). The authors suggested that the inotocin receptor could play an important role in the control of social and reproductive behaviors in ants, which has been further studied by Gruber and colleagues. Liutkevičiūte et al. (2018) studied the functionality of inotocin signaling in two Lasius species, L. neglectus and L. niger using qPCR, gene-knockdown (RNAi), transcriptomics, as well as phenotypic screening of physiology and behavior. They initially characterized inotocin peptide-receptor signaling in L. neglectus and observed that inotocin is a potent agonist of its cognate receptor for the Gq- and Gs-signaling pathways (Liutkevičiūte et al., 2018). Radioligand binding studies showed that a tritiated inotocin analog was able to bind to membranes transiently expressing the inotocin receptor. The authors measured the expression levels in both ant species in different developmental stages, castes, parts of the body and organs (Liutkevičiūte et al., 2018). The results demonstrated that the expression of the inotocin peptide and receptor are low in larvae and were higher in pupae, with the highest expression levels detected in adult ants. Compared to workers, the expression of the inotocin precursor in mated queens was about 10-fold lower. When comparing all castes of ants used in this study, the expression of the inotocin precursor was most pronounced in the heads, while the receptor was expressed throughout the body with the highest expression found in the heads of males (Liutkevičiūte et al., 2018). These gene expression data were confirmed using immunostaining of the mature peptide. The authors analyzed cellular localization of the inotocin peptide in both ant species and revealed that inotocin localizes in the brain, and notably in the subesophageal ganglion. Further experiments aimed to determine changes in expression driven by external stimuli. The expression profile of the inotocin peptide and receptor were affected by seasonal changes. The data suggest that inotocin peptide and receptor expression in L. neglectus is lower in winter compared to summer conditions, and lower in nursing ants compared to foragers (Liutkevičiūte et al., 2018). Furthermore, starvation led to the downregulation of the inotocin peptide mRNA levels. RNAi was used to downregulate the inotocin peptide mRNA in an attempt to alter phenotypes and gene expression. An mRNA-sequencing analysis uncovered approximately 100 differentially expressed genes related to lipid, protein and DNA metabolism. Given that OT and VP are involved in the regulation of social behavior in humans, Liutkevičiūte et al. (2018) then conducted experiments with the RNAi-knockdown ants to examine whether inotocin signaling affected behavior. The knockdown of inotocin peptide in ants resulted in higher walking activity, enhanced self-grooming in the brood chamber, and a higher motivation for feeding (Fig. 1) (Liutkevičiūte et al., 2018).
In summary, these data support a role for inotocin signaling in ants, particularly with respect to metabolism, activity and feeding behavior.b Additionally, inotocin in ants could play an important role in the control of energy status linking appetite and locomotion. Ants have provided valuable insights into the roles of inotocin signaling, but there are limitations: for example, due to their small size, ant tissues cannot be readily dissected and employed in physiological and biochemical experiments. Moreover, ants do not possess a very robust RNAi response compared to several other insects. Nevertheless, in our view these studies constitute an excellent step toward better understanding of inotocin signaling in insects.
5. Mining of ligand and receptor genes in other insects
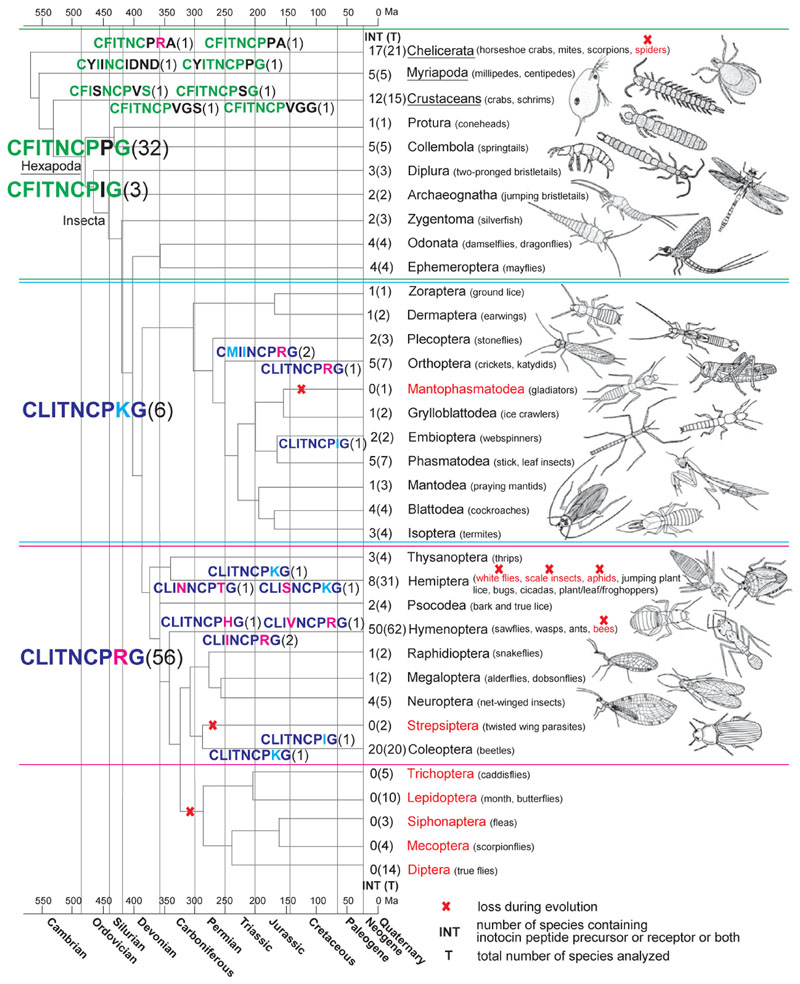
Driven by the intriguing distribution of the OT/VP encoding genes in insects–present in ants, beetles and grasshoppers, but absent in flies and bees–Liutkevičiūte et al. (2016) further investigated the phylogenetic distribution of the inotocin signaling system in arthropods using the extensive library of insect sequence data compiled by Misof et al. (2014). Liutkevičiūte et al. (2016) searched for genes encoding components of the inotocin GPCR signaling system in genomes and/or transcriptomes of more than 260 species of insects and other arthropods. They found that inotocin signaling-related genes are present in all subphyla of the Arthropoda, and in 25 out of the 32 orders of the subphylum Hexapoda (Fig. 2). Closer analysis, however, revealed a more complex evolutionary history of OT/ VP-like signaling in these groups. For instance, all 20 beetle (Coleoptera) species analyzed had the inotocin signaling system (receptor and/or precursor),c whereas in the Hymenoptera (and as outlined above), this pathway appeared to be present in sawflies (Symphyta), parasitoid wasps (Parasitica), social wasps (Vespidae) and ants (Formicoidea) but has seemingly been lost in bees (Apoidea). A similar pattern is observed in the Hemiptera: inotocin being apparently present in just some Heteroptera, widely present in plant, leaf and froghoppers (Auchenorrhyncha, but apparently absent in Cicadas), and totally absent in aphids (Sternorrhynca: Aphidiformes). Likewise, in the Chelicerata, OT/VP-like signaling appears to be present in mites (Acari) and scorpions (Scorpiones) but absent in spiders (Aranea; Liutkevičiūte et al., 2016). The seven insect orders that appeared to have completely lost inotocin signaling are the Mantophasmatodea, Strepsiptera, Trichoptera, Lepidoptera, Siphonaptera, Mecoptera and Diptera (although both propeptide and receptor sequences somewhat resembling those of OT/VP have been found in the New Zealand glow worm, Arachnocampa luminosa, which belongs to a basal family of Diptera; Sharpe, Dearden, Gimenez, & Krause, 2015). These results support the hypothesis that inotocin signaling was present in ancestral arthropod lineages but has since been lost multiple times (Liutkevičiūte et al., 2016). In addition to their phylogenetic study, Liutkevičiūte et al. (2016) further analyzed the sequence variability of inotocin peptides and receptors. Most analyzed species have one gene encoding the inotocin receptor and one gene encoding the cognate inotocin peptide. Liutkevičiūte and colleagues (Liutkevičiūte et al., 2016) observed that all identified inotocin precursor proteins exhibit a high degree of homology. There are several exceptions to this general observation: for instance, in the crustacean, Xibalbanus tulumensis (formerly known as Speleonectes tulumensis), belonging to the Remipedes, a sister group to the Hexapoda, no amidation processing signal could be identified. Additionally, while all other beetle data comprise only one precursor copy encoding the mature inotocin peptide CLITNCPRG*, the Colorado potato beetle (L. decemlineata) contains two copies encoding two distinct inotocin-like peptides (CLITNCPKG* and CLITNCPIG*). Liutkevičiūte et al. (2016) also identified inotocin-like decapeptides in the centipede (Myriapoda), Scolopendra subspinipes, and the Copepod sea-lice (Crustacea), Lepeophtheirus salmonis and Caligus rogercresseyi. Interestingly, six of the identified multi-copy receptor species were discovered in the Chelicerata and one in Myriapoda, but not a single species within the Hexapoda. Liutkevičiūte et al. (2016) also analyzed four different OT/VP-like receptor transcripts from the crustacean water flea (Cladocera) Daphnia magna and found that they were almost identical except for minor deletions and insertions present in the intracellular loop 3 and transmembrane domains 3 and 5.
Fig. 2.
Map of inotocin peptide GPCR signaling in arthropods. A phylogenetic map based on the recently established insect phylogeny (Misof et al., 2014) is shown. The absence of the inotocin signaling system is highlighted in red. Different putative peptide sequences are shown in different colors to indicate the diversity and distribution throughout the phylogeny. The numbers in brackets next to the peptide sequences indicate the frequencies of occurrence (from a total of 121 analyzed precursor sequences that contained a mature peptide domain). Number of species where the inotocin signaling system (receptor and/or precursor) is present (INT) as well as the total number of sampled species(T) has been indicated next to the tree branches. The inotocin signaling system is confined to specific groups of arthropods. For clarity of this phylogenetic illustration, the upper three groups of Arthropoda (Chelicerata, Myriapoda and Crustaceans; underlined) represent subphyla. All other groups denote orders of the subphylum Hexapoda; the class of Insecta comprises the orders Archaeognatha to Diptera. Reproduced from Liutkevičiūte, Z., Koehbach, J., Eder, T., Gil-Mansilla, E., & Gruber, C. W. (2016). Global map of oxytocin/vasopressin-like neuropeptide signalling in insects. Scientific Reports, 6,39177. 10.1038/srep39177 with permission under a Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http:/creativecommons.org/licenses/by/4.0/.
Overall, these data have considerably extended our knowledge regarding the phylogenetic distribution and molecular structure of inotocin GPCR signaling in insects and other arthropods. Nevertheless, sequence similarity between different insect species does not always correspond to functional similarity and vice versa, so it is of crucial importance that we continue to functionally characterize and compare inotocin GPCR signaling in different insect species. Such comparative analysis may enable the identification of common, as well as distinct, features of OT/VP physiology across the animal kingdom.
6. Possible applications of insect inotocin ligands in pharmacology
Following this review of the discovery, distribution and physiology of the insect OT/VP signaling system, we will now provide a few examples of how these discoveries could be exploited. Amongst the diversity of insect inotocin ligands, there could be novel chemical probes or pharmacological ligands for human OT/VP receptors. The OT/VP signaling system plays a role in a number of physiological functions and behaviors, such as antidiuretic action, vasoconstriction and lactation (summarized in Gruber et al., 2010; Jurek & Neumann, 2018), aggression (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005; Wersinger, Ginns, O’Carroll, Lolait, & Young, 2002), attachment (Insel & Young, 2001), anxiety-related behavior (Landgraf, 2006), fear conditioning (Eckstein et al., 2015), labor (Gruber & O’Brien, 2011), social recognition (Kirsch et al., 2005), and memory and learning (Alescio-Lautier & Soumireu-Mourat, 1998). Consequently, malfunctioning of this signaling system may contribute to various disorders and diseases, including autism, social anxiety disorder, schizophrenia, borderline personality disorder, depression, preterm-labor (Gruber & O’Brien, 2011), cardiovascular diseases and cancer (Goldman, Marlow-O’Connor, Torres, & Carter, 2008; Gruber & O’Brien, 2011; Keri, Kiss, & Kelemen, 2009; McQuaid, McInnis, Abizaid, & Anisman, 2014; Meyer-Lindenberg et al., 2011; Scantamburlo et al., 2007). Despite progress in the development of probes for the modulation of OT/VP receptors, assigning specific functions to particular receptors is still challenging. This problem could be addressed by the development of novel and more receptor-specific ligands, which could then lead to highly targeted therapeutics for diseases associated with different OT/VP receptors.
There has been sustained industrial and academic research over the past half-century to design and synthesize novel OT and VP analogs, resulting in several commercial OT/VP agonists and antagonists. The main challenge in the development of selective ligands is the high sequence similarity of the OT/VP receptors, particularly in the extracellular region, where the approximately 80% homology is one of the main obstacles in drug development (Koehbach, Stockner, Bergmayr, Muttenthaler, & Gruber, 2013). Receptor selectivity should ideally be greater than 2 orders of magnitude (defined as the difference in affinity of a compound to bind to a single receptor isoform over the remaining ones). In reality this has only been achieved by very few VP analogs (e.g., desmopressin, d[Cha4]AVP, d[Leu4]AVP; Manning et al., 2012) and selectivity is generally lost at higher doses. Additionally, receptor selectivity is species dependent: a compound selective for mouse receptors is not necessarily selective in humans, which poses an additional obstacle in the use of animal models for drug development. Furthermore, in vivo selectivity is enhanced through various other factors such as receptor up- and downregulation, local ligand production and degradation (Gruber et al., 2012). Finally, there have been reports of functional OT and VP receptor homo- and heterodimers, which make it difficult to predict the signaling profile and selectivity of any given ligand (Terrillon et al., 2003).
All of the above have hampered the development of specific small molecule agonists and antagonists, to the point where companies such as Merck, Pfizer and Sanofi have abandoned their development programs of non-peptide based compounds (Gruber et al., 2012; Manning et al., 2012). Peptide ligands, however, have a unique potential to overcome these constraints. Despite their handicaps of a short half-life, and difficulty crossing the blood-brain barrier, an advantage of peptides over small molecules is their potential to interact with a larger surface area of the receptor, as well as their greater stereochemical and structural complexity.
Rational design of peptide ligands has yielded several semi-selective therapeutics. For example, atosiban is a competitive OT/VP receptor antagonist first described in 1985. It is a nonapeptide, desamino-oxytocin analog most commonly used intravenously to halt preterm labor (Akerlund, Carlsson, Melin, & Trojnar, 1985). Another example, desmopressin (1-desamino-8-d-arginine vasopressin), is a synthetic human VP derivative. In contrast to VP, the first cysteine residue has been deaminated via replacement with β-mercaptopropionic acid and the arginine residue in position eight has been switched from levorotation to dextrorotation. It is relatively selective for the human V2 receptor, where it is used as an agonist in the treatment of diabetes insipidus (Vavra et al., 1968).
An alternative to the rational design of ligands is the screening of naturally occurring compounds, utilizing the evolutionary conservation of peptide-ligands as a starting point. This approach has recently been successfully used to develop a selective human V1a receptor antagonist. Inotocin was isolated from the black garden ant, L. niger, and investigated for its pharmacological properties on human OT/VP receptors. It was discovered that inotocin activates the human V1b receptor while inhibiting the human V1a receptor. This functional dichotomy was then exploited by replacing the arginine residue in position eight of inotocin with its stereoisomer d-arginine, thereby creating a stable, competitive V1a receptor-antagonist with a 3000-fold increased binding selectivity toward the V1a receptor compared to the other human receptor subtypes (Di Giglio et al., 2017). Another rich source of bioactive peptides are so-called conus-toxins, derived from cone snails (Conus spp.). Conopressin-T, a peptide isolated from C. tulipa venom, is a selective antagonist of the human V1a receptor. Substitution of the valine in position nine with glycine switches the peptide from antagonism to agonism (Dutertre et al., 2008).
The distribution of OT/VP-like peptides of pharmacological interest is therefore not limited to vertebrates but also extends to invertebrates, which provide a considerable pool of candidates and vantage points for the development of superior, selective molecular probes for both OT and VP receptors. The survey by Liutkevičiūte et al. (2016) revealed 11 different OT/VP-like peptide sequences just within insects, and more probably remain to be discovered. Despite limited knowledge about the function of this system in insects, where there is evidence for regulation of metabolism and locomotor activity, inotocin-like peptide precursors and cognate receptors have been identified in hundreds of arthropod species (Liutkevičiūte et al., 2016, 2018), such as arachnotocin in the mite Varroa destructor. Moreover, distinct OT-, VP- and inotocin-like peptides have been identified in a growing number of other invertebrates, including molluscs (octopressin and cephalotocin in Octopus vulgaris), annelids (annetocin in oligochaetes), nematodes (nematocin) and starfish (asterotocin) where they generally modulate social and reproductive behavior (Donaldson & Young, 2008; Gruber, 2014), thus indicating the presence of an even wider pool of neuropeptide candidates.
Plants are another potential source of therapeutic lead compounds. Ethnopharmacological investigations of uterotonic plant preparations have identified the cyclotide kalata B7, which has been successfully employed as a template for the design of synthetic OT-like peptides with nanomolar affinity and selectivity for the oxytocin receptor (Koehbach, O’Brien, et al., 2013).
In conclusion, we believe that invertebrate OT/VP-like signaling systems are worthwhile targets for future research. Due to the functional conservation of the system, any new knowledge gleaned from insects and other invertebrates might be applicable for translational therapeutic research in humans. In addition, studying insect OT/VP signaling systems could also provide insights and valuable lead compounds for the development of peptide-derived OT/VP ligands. In contrast to small molecule ligands, these compounds could overcome the problem of receptor selectivity, an important limitation in the pursuit of highly effective OT/VP therapeutics and probes. Breakthroughs in this area will result in a deeper understanding of this fascinating neuroendocrine system both in humans and other animals, and could also provide novel treatments for a diverse range of disorders and diseases.
Acknowledgments
The study of insect OT/VP-like signaling in the laboratories of Christian Gruber and Jozef Vanden Broeck is supported by the Austrian Science Fund FWF (I3243) and the Fund for Scientific Research–Flanders FWO (G0F2417N).
Footnotes
*C-terminal amidation.
During production of this chapter, the following manuscript was published (Koto et al., 2019), which adds further information to the function of inotocin signalling in ants.
A functional signaling system requires the expression of precursor and receptor; lack of either may be due to low quality of transcriptome data.
References
- Aikins MJ, Schooley DA, Begum K, Detheux M, Beeman RW, Park Y. Vasopressin-like peptide and its receptor function in an indirect diuretic signaling path-way in the red flour beetle. Insect Biochemistry and Molecular Biology. 2008;38:740–748. doi: 10.1016/j.ibmb.2008.04.006.. [DOI] [PubMed] [Google Scholar]
- Akerlund M, Carlsson AM, Melin P, Trojnar J. The effect on the human uterus of two newly developed competitive inhibitors of oxytocin and vasopressin. Acta Obstetricia et Gynecologica Scandinavica. 1985;64(6):499–504. doi: 10.3109/00016348509156728. [DOI] [PubMed] [Google Scholar]
- Alescio-Lautier B, Soumireu-Mourat B. Role of vasopressin in learning and memory in the hippocampus. Progress in Brain Research. 1998;119:501–521. doi: 10.1016/s0079-6123(08)61590-3. [DOI] [PubMed] [Google Scholar]
- Baines RA, Bacon JP. Pharmacological analysis of the cholinergic input to the locust VPLI neuron from an extraocular photoreceptor system. Journal of Neurophysiology. 1994;72:2864–2874. doi: 10.1152/jn.1994.72.6.2864. [DOI] [PubMed] [Google Scholar]
- Baines RA, Thompson KSJ, Rayne RC, Bacon JP. Analysis of the peptide content of the locust vasopressin-like immunoreactive (VPLI) neurons. Peptides. 1995;16:799–807. doi: 10.1016/0196-9781(95)00038-L. [DOI] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. The Journal of Endocrinology. 1998;156(2):223–229. doi: 10.1677/joe.0.1560223. [DOI] [PubMed] [Google Scholar]
- Beets I, Janssen T, Meelkop E, Temmerman L, Suetens N, Rademakers S, et al. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans. Science. 2012;338(6106):543–545. doi: 10.1126/science.1226860. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. The Journal of Neuroscience. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Young WS. Oxytocin and vasopressin: Genetics and behavioral implications. 3rd ed. Vol. 495 Springer-Verlag; New York Inc: 2006. [Google Scholar]
- Chérasse S, Aron S. Measuring inotocin receptor gene expression in chronological order in ant queens. Hormones and Behavior. 2017;96:116–121. doi: 10.1016/j.yhbeh.2017.09.009.. [DOI] [PubMed] [Google Scholar]
- Coast GM, Rayne RC, Hayes TK, Mallet AI, Thompson KSJ, Bacon JP. A comparison of the effects of two putative diuretic hormones from Locusta migratoria on isolated locust malpighian tubules. The Journal of Experimental Biology. 1993;175:1–14. doi: 10.1242/jeb.175.1.1. [DOI] [PubMed] [Google Scholar]
- Di Giglio MG, Muttenthaler M, Harpsøe K, Liutkevičiūte Z, Keov P, Eder T, et al. Development of a human vasopressin V1a-receptor antagonist from an evolutionary-related insect neuropeptide. Scientific Reports. 2017;7 doi: 10.1038/srep41002. 41002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Croker D, Daly NL, Andersson A, Muttenthaler M, Lumsden NG, et al. Conopressin-T from Conus tulipa reveals an antagonist switch in vasopressin-like peptides. Journal of Biological Chemistry. 2008;283(11):7100–7108. doi: 10.1074/jbc.M706477200. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, et al. Oxytocin facilitates the extinction of conditioned fear in humans. Biological Psychiatry. 2015;78(3):194–202. doi: 10.1016/j.biopsych.2014.10.015.. [DOI] [PubMed] [Google Scholar]
- Elphick MR. NG peptides: A novel family of neurophysin-associated neuropeptides. Gene. 2010;458(1-2):20–26. doi: 10.1016/j.gene.2010.03.004.. [DOI] [PubMed] [Google Scholar]
- Evans PD, Cournil I. Co-localization of FLRF- and vasopressin-like immuno- reactivity in a single pair of sexually dimorphic neurones in the nervous system of the locust. The Journal of Comparative Neurology. 1990;292:331–348. doi: 10.1002/cne.902920302.. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein- coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular Pharmacology. 2003;63(6):1256–1272. doi: 10.1124/mol.63.6.1256.. [DOI] [PubMed] [Google Scholar]
- Furuya K, Schegg KM, Wang H, Kingt DS, Schooley DA. Isolation and identification of a diuretic hormone from the mealworm Tenebrio molitor (water balance/corticotropin-releasing factor/cyclic AMP/Malpighian tubules) Proceedings of the National Academy of Sciences. 1995;92:12323–12327. doi: 10.1073/pnas.92.26.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäde G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annual Review of Entomology. 2004;49:93–113. doi: 10.1146/annurev.ento.49.061802.123354. [DOI] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338(6106):540–543. doi: 10.1126/science.1226201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Hormones and Behavior. 2012;61(3):230–238. doi: 10.1016/j.yhbeh.2011.12.016.. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Research. 2008;98(1-3):247–255. doi: 10.1016/j.schres.2007.09.019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Experimental Physiology. 2014;99(1):55–61. doi: 10.1113/expphysiol.2013.072561.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, Koehbach J, Muttenthaler M. Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future Medicinal Chemistry. 2012;4(14):1791–1798. doi: 10.4155/fmc.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, Muttenthaler M. Discovery of defense- and neuropeptides in social ants by genome-mining. PLoS One. 2012;7(3):e32559. doi: 10.1371/journal.pone.0032559.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, Muttenthaler M, Freissmuth M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Current Pharmaceutical Design. 2010;16(28):3071–3088. doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, O’Brien M. Uterotonic plants and their bioactive constituents. Planta Medica. 2011;77(3):207–220. doi: 10.1055/s-0030-1250317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jurek B, Neumann ID. The oxytocin receptor: From intracellular signaling to behavior. Physiological Reviews. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Keov P, Liutkevičiūte Z, Hellinger R, Clark RJ, Gruber CW. Discovery of peptide probes to modulate oxytocin-type receptors of insects. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-28380-3. 10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: Oxytocin and trust in schizophrenia. Social Neuroscience. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, O’Brien M, Muttenthaler M, Miazzo M, Akcan M, Elliott AG, et al. Oxytocic plant cyclotides as templates for peptide G protein-coupled receptor ligand design. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(52):21183–21188. doi: 10.1073/pnas.1311183110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochemical Society Transactions. 2013;41(1):197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koto, et al. Oxytocin/vasopressin-like peptide inotocin regulates cuticular hydrocarbon synthesis and water balancing in ants. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(12):5597–5606. doi: 10.1073/pnas.1817788116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. Journal of Cell Science. 2003;116(24):4867–4869. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- Landgraf R. The involvement of the vasopressin system in stress-related disorders. CNSNeurological Disorders Drug Targets. 2006;5(2):167–179. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;296(3):R824–R830. doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Predel R, Neupert S, Hauser F, Tanaka Y, Cazzamali G, et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Research. 2008;18:113–122. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liutkevičiūte Z, Gil-Mansilla E, Eder T, Casillas-Perez B, Di Giglio MG, Muratspahić E, et al. Oxytocin-like signaling in ants influences metabolic gene expression and locomotor activity. The FASEB Journal. 2018;32 doi: 10.1096/fj.201800443.. fj201800443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liutkevičiūte Z, Gruber CW. Evolutionary aspects of physiological function and molecular diversity of the oxytocin/vasopressin signaling system. In: Murphy D, Gainer H, editors. Molecular neuroendocrinology: From genome to physiology. Wiley-Blackwell; 2016. pp. 5–23. [Google Scholar]
- Liutkevičiūte Z, Koehbach J, Eder T, Gil-Mansilla E, Gruber CW. Global map of oxytocin/vasopressin-like neuropeptide signalling in insects. Scientific Reports. 2016;6:39177. doi: 10.1038/srep39177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist C, Baines RA, Bacon JP. Evidence that histamine is a neurotransmitter in an insect extraocular photoreceptor pathway. Journal of Experimental Biology. 1996;199:1973–1982. doi: 10.1242/jeb.199.9.1973. [DOI] [PubMed] [Google Scholar]
- Lundquist CT, Baines RA, Thompson KSJ, Bacon JP. Differential regulation of a homologous peptidergic neuron in grasshoppers: Evolution of a neural circuit. Journal of Comparative Physiology A. 1998;182:755–765. [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nature Neuroscience. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neuroscience and Biobehavioral Reviews. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. http://www.nature.com/nrn/journal/v12/n9/suppinfo/nrn3044_S1.html. [DOI] [PubMed] [Google Scholar]
- Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346(6210):763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- Montuenga LM, Zudaire E, Prado MA, Audsley N, Burrell MA, Coast GM. Presence of Locusta diuretic hormone in endocrine cells of the ampullae of locust Malpighian tubules. Cell and Tissue Research. 1996;285:331–339. doi: 10.1007/s004410050650.. [DOI] [Google Scholar]
- Picquot M, Proux JP. Relationship between excretion of primary urine and haemolymph level of diuretic hormone in the migratory locust. Physiological Entomology. 1987;12:455–460. doi: 10.1111/j.1365-3032.1987.tb00772.x. [DOI] [Google Scholar]
- Picquot M, Proux JP. Influence of hydration on the concentration of two arginine-vasopressin-like neuropeptides coexisting in the ventral nerve cord of the migratory locust, Locusta migratoria. Journal of Insect Physiology. 1989;35:571–577. [Google Scholar]
- Picquot M, Proux JP. Biosynthesis and degradation of the arginine-vasopressin-like insect diuretic hormone, a neurohormone in the migratory locust. Regulatory Peptides. 1990;31:139–156. doi: 10.1016/0167-0115(90)90001-D. [DOI] [PubMed] [Google Scholar]
- Proux JP, Herault J-P. Cyclic AMP: A second messenger of the newly characterized AVP-like insect diuretic hormone, the migratory locust diuretic hormone. Neuropeptides. 1988;12:7–12. doi: 10.1016/S0143-4179(98)90003-8. [DOI] [PubMed] [Google Scholar]
- Proux JP, Miller CA, Li JP, Carney RL, Girardie A, Delaage M, et al. Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochemical and Biophysical Research Communications. 1987;149:180–186. doi: 10.1016/0006-291X(87)91621-4.. [DOI] [PubMed] [Google Scholar]
- Proux JP, Picquot M, Herault J-P, Fournier B. Diuretic activity of a newly identified neuropeptide–The arginine-vasopressin-like insect diuretic hormone: Use of an improved bioassay. Journal of Insect Physiology. 1988;34:919–927. doi: 10.1016/0022-1910(88)90127-8. [DOI] [Google Scholar]
- Proux JP, Rougon G, Cupo A. Enhancement of excretion across locust Malpighian tubules by a diuretic vasopressin-like hormone. General and Comparative Endocrinology. 1982;47:449–457. doi: 10.1016/0016-6480(82)90123-X. [DOI] [PubMed] [Google Scholar]
- Proux JP, Rougon-Rapuzzi G. Evidence for vasopressin-like molecule in migratory locust. Radioimmunological measurements in different tissues: Correlation with various states of hydration. General and Comparative Endocrinology. 1980;42:378–383. doi: 10.1016/0016-6480(80)90168-9. [DOI] [PubMed] [Google Scholar]
- Rémy C, Girardie J. Anatomical organization of two vasopressin-neurophysin- like neurosecretory cells throughout the central nervous system of the migratory locust. General and Comparative Endocrinology. 1980;40:27–35. doi: 10.1016/0016-6480(80)90092-1. [DOI] [PubMed] [Google Scholar]
- Rémy C, Girardie J, Dubois MP. Vertebrate neuropeptide-like substances in the subesophageal ganglion of two insects: Locusta migratoria R. and F. (Orthoptera) and Bombyx mori L. (Lepidoptera) immunocytological investigation. General and Comparative Endocrinology. 1979;37:93–100. doi: 10.1016/0016-6480(79)90050-9. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ, Uhl GR. Arginine vasopressin: A novel peptide rhythm in cerebrospinal fluid. Trends in Neurosciences. 1987;10:76–80. doi: 10.1016/0166-2236(87)90029-4. [DOI] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schlemermeyer E, Schutte M, Ammermuller J. Immunohistochemical and electrophysiological evidence that locust ocellar photoreceptors contain and release histamine. Neuroscience Letters. 1989;99:73–78. doi: 10.1016/0304-3940(89)90267-X. [DOI] [PubMed] [Google Scholar]
- Sharpe ML, Dearden PK, Gimenez G, Krause KL. Comparative RNA seq analysis of the New Zealand glowworm Arachnocampa luminosa reveals bioluminescence-related genes. BMC Genomics. 2015;16:825. doi: 10.1186/s12864-015-2006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Sword GA. Locusts. Current Biology. 2008;18:R364–R366. doi: 10.1016/j.cub.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Spring JH. Endocrine regulation of diuresis in insects. Journal of Insect Physiology. 1990;36:13–22. doi: 10.1016/0022-1910(90)90146-7. [DOI] [Google Scholar]
- Stafflinger E, Hansen KK, Hauser F, Schneider M, Cazzamali G, Williamson M, et al. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3262–3267. doi: 10.1073/pnas.0710897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, et al. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Molecular Endocrinology. 2003;17(4):677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- Thompson KSJ, Bacon JP. The vasopressin-like immunoreactive (VPLI) neurons of the locust, Locusta migratoria. II. Physiology. Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology. 1991;168:619–630. doi: 10.1007/BF00215084. [DOI] [PubMed] [Google Scholar]
- Thompson KS, Tyrer NM, May ST, Bacon JP. The vasopressin-like immunoreactive (VPLI) neurons of the locust, Locusta migratoria. I. Anatomy. Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology. 1991;168:605–617. doi: 10.1007/BF00215083. [DOI] [PubMed] [Google Scholar]
- Tyrer NM, Davis NT, Arbas EA, Thompson KSJ, Bacon JP. Morphology of the vasopressin-like immunoreactive (VPLI) neurons in many species of grasshopper. The Journal of Comparative Neurology. 1993;329:385–401. doi: 10.1002/cne.903290309. [DOI] [PubMed] [Google Scholar]
- Vanden Broeck JJ. G-protein-coupled receptors in insect cells. International Review of Cytology. 1996;164:189–268. doi: 10.1016/s0074-7696(08)62387-6. [DOI] [PubMed] [Google Scholar]
- Vavra I, Machova A, Holecek V, Cort JH, Zaoral M, Sorm F. Effect of a synthetic analogue of vasopressin in animals and in patients with diabetes insipidus. Lancet. 1968;1(7549):948–952. doi: 10.1016/s0140-6736(68)90904-5. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Immunocytochemical demonstration of a homology in peptidergic neurosecretory cells in the suboesophageal ganglion of a beetle and a locust with antisera to bovine pancreatic polypeptide, FMRFamide, vasopressin and α-MSH. Neuroscience Letters. 1984;48:185–190. doi: 10.1016/0304-3940(84)90017-X. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Romherg-Privee HM, Schooneveld H. Immunocytochemical localization of peptidergic cells in the neuro-endocrine system of the Colorado potato beetle Leptinotarsa decemlineata, with antisera against vasopressin, vasotocin and oxytocin. Histochemistry. 1984;81:29–34. doi: 10.1007/BF00495397. [DOI] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, Roth BL. Howligands illuminate GPCR molecular pharmacology. Cell. 2017;170(3):414–427. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang X, Yang P, Jiang X, Jiang F, Zhao D, et al. The locust genome provides insight into swarm formation and long-distance flight. Nature Communications. 2014;5 doi: 10.1038/ncomms3957. 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Molecular Psychiatry. 2002;7(9):975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wiehart UIM, Torfs P, VanLommel A, Nicolson SW, Schoofs L. Immu-nocytochemical localization of a diuretic hormone of the beetle Tenebrio molitor, Tenmo-DH(37), in nervous system and midgut. Cell and Tissue Research. 2002;308:421–429. doi: 10.1007/s00441-002-0552-9.. [DOI] [PubMed] [Google Scholar]