Abstract
Background
Because of a high risk to develop fatal anaphylaxis, early detection of immunoglobulin E (IgE)-dependent allergy is of particular importance in patients with mastocytosis.
Objective
We examined whether microarray-based screening for allergen-reactive IgE (allergen-chip) is a sensitive and robust approach to detect specific IgE in patients with mastocytosis.
Methods
Sera for 42 patients were analyzed, including 4 with cutaneous mastocytosis, 2 with mastocytosis in the skin, and 36 with systemic mastocytosis. In addition, sera from an age-and sex-matched control cohort (n = 42) were analyzed.
Results
In 15 of 42 patients with mastocytosis (35.7%), specific IgE was detected by allergen-chip profiling. Ves v 5 and Bet v 1 were the most frequently detected allergens (Ves v 5: 16.7% of patients; Bet v 1: 11.9% of patients). Allergen reactivity was confirmed by demonstrating upregulation of CD203c on blood basophils upon exposure to the respective allergen(s) in these patients. Specific IgE was identified by chip studies in 11 of 26 patients with mastocytosis with mediator-related symptoms (42.3%) and in 4 of 14 patients with mastocytosis without symptoms (28.6%). In the cohort with known allergy, 9 of 9 patients (100%) had a positive allergen-chip result. In patients with mastocytosis without a known allergy (n = 31), the chip identified 6 positive cases (19.5%). The prevalence of chippositive patients was slightly lower in the mastocytosis group (35.7%) compared with age- and sex-matched controls (40.5%).
Conclusions
Although specific IgE may not be detectable in all sensitized patients with mastocytosis, allergy chip-profiling is a reliable screening approach for the identification of patients with mastocytosis suffering from IgE-dependent allergies.
Keywords: Allergy, IgE, Mastocytosis, Allergen-chip
Introduction
The term mastocytosis denotes a heterogeneous group of mast cell (MC) disorders defined by abnormal growth and pathological accumulation of clonal MCs in the skin and/or internal organs1–4. According to the classification of the World Health Organization, patients can be divided into cutaneous mastocytosis (CM) and systemic mastocytosis (SM). The group of SM includes indolent SM (ISM), smoldering SM (SSM), aggressive SM (ASM), MC leukemia, and SM with an associated hematologic neoplasm (SM-AHN).1,4–7 These disease variants differ from each other regarding symptoms, clinical course, and prognosis.8–10
Mediator-related symptoms are found in all variants of mastocytosis.1–8 Although the MC burden is higher in advanced SM, the severity of mediator-related symptoms does not correlate with the SM variant.11,12 Examples for mediator-related symptoms are skin rash, flushing, diarrhea, and hypotension.11–17 In patients with mastocytosis, allergic reactions can be severe or even life-threatening, especially when an immunoglobulin E (IgE)-dependent allergy is also present14–18. These patients often suffer from an MC activation syndrome.19–21 A particularly high risk for anaphylaxis is found in patients allergic to bee or wasp venoms.16–18
Based on the high risk to develop fatal anaphylaxis, early detection of IgE sensitization is of particular importance in patients with mastocytosis. However, in these patients, results of conventional serologic tests measuring specific IgE are often negative, which may have several explanations. For example, parts or even most of the IgE may be fixed to MC surfaces via IgE receptors. Even the skin test may show false-negative results because of treatment with “antiallergic” drugs. Moreover, the skin test has a certain risk for anaphylaxis and new sensitizations. Nevertheless, it is important to detect patients with mastocytosis at risk for IgE-dependent reactions and there is a need for specific diagnostic assays that cover most or all relevant allergens.
Chips containing microarrayed allergens are powerful tools for detecting IgE-dependent sensitization.22,23 Using this approach, IgE reactivity of patients’ sera with more than 100 allergens can be assessed in a single test.23 Allergen-chips have been validated successfully in several trials and found to be a valuable approach to screen for allergen-specific IgE in patients with allergy.22–26 However, so far, the allergen-chip technology has not been tested in patients with mastocytosis.
We analyzed sera of 42 patients suffering from mastocytosis as well as sera of 42 age- and sex-matched controls without a known mastocytosis or another malignant disease using the Immuno Solid-phase Allergen-Chip technology. Our results show that the allergen-chip is a robust approach to detect IgE sensitivities in patients with mastocytosis.
Methods
Patients’ characteristics
A total of 42 patients with mastocytosis (median age, 45.5 years; range, 21-73 years; female:male ratio, 1:0.7) and 42 age- and sex-matched controls without mastocytosis were analyzed for specific IgE in the allergen-chip. Patients with mastocytosis were classified according to World Health Organization criteria.1,2 Baseline characteristics of patients are summarized in Table I and in Table E1 in this article’s Online Repository at www.jaci-inpractice.org. Patients were seen at the Medical University of Vienna between 1998 and 2018. Various disease-related symptoms, including mediator-related symptoms and bone pain (due to osteopenia/osteoporosis), were recorded during follow-up. ISM was the most common disease variant (23 patients, 54.8%). Five patients had SSM (11.9%), 5 SM-AHN (11.9%), 3 ASM (7.1%), and 4 CM (9.5%). In 2 patients, no bone marrow data were available; these patients were diagnosed as mastocytosis in the skin (MIS) (4.8%). The median serum tryptase level was 53.6 ng/mL (range, 6.1-1617 ng/mL). Hypersensitivity reactions were reported in 9 patients (21%). Eight of these patients had confirmed IgE-mediated allergies and suffered from mediator-induced symptoms. In 1 patient, the case history revealed a hypersensitivity reaction against tetracyclines. In 26 patients (61.9%), a history of mediator-related symptoms was documented. In all patients, the chip analysis was performed during routine blood examinations. In 2 patients (no. 5, ISM, and no. 26, SSM; see Table E1), the chip analysis was performed at 2 (no. 5) or 3 (no. 26) different time points.
Table I. Characteristics of the patients with mastocytosis analyzed on the chip (mechanisms for development of allergies).
| Mastocytosis: Variants and subvariants | n (%) | F/M | Age (y), median (min-max) | Tryptase (ng/mL) median, (min-max) | WBC (cells/μL) median, (min-max) | Eosinophils (cells/μL) median, (min-max) | Platelets (cells/μL), Median (min-max) | History of allergies, n (%) | Mediator symptoms, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Nonadvanced mastocytosis | |||||||||
| CM | 4 (9.5) | 3/1 | 37 (33-45) | 13.05 (6-17) | 7,210 (5,720-10,000) | 164.4 (57.6-259.8) | 266,500 (158,000-378,000) | 2 (50.0) | 2 (50.0) |
| MIS | 2 (4.8) | 2/0 | 33 (31-35) | 13.5 (14-14) | 7,800 (7,800-7,800) | 0 (0-0) | 305,000 (305,000-305,000) | 0 (0.0) | 1 (50.0) |
| ISM | 23 (54.8) | 14/9 | 45 (26-73) | 46.5 (15-938) | 6,620 (3,750-10,880) | 102.2 (0-488) | 265,000 (204,000-341,000) | 6 (26.1) | 14 (60.9) |
| SSM | 5 (11.9) | 4/1 | 48 (35-55) | 352.5 (175-1,090) | 4,860 (3,820-6,800) | 0 (0-0) | 212,000 (193,000-280,000) | 1 (20.0) | 4 (80.0) |
| Advanced mastocytosis | |||||||||
| ASM | 3 (7.1) | 0/3 | 59 (21-72) | 285 (54-1,617) | 5,030 (4,370-6,760) | 349.6 (100.6-1,487.2) | 115,000 (25,000-269,000) | 0 (0.0) | 2 (66.7) |
| SM-AHN | 5 (11.9) | 2/3 | 53 (47-72) | 379 (78-615) | 17,600 (2,950-35,490) | 797.5 (59-2,515.5) | 93,500 (54,000-287,000) | 0 (0.0) | 3 (60.0) |
| All patients | 42 | 25/17 | 45.5 (21-73) | 53.6 (6-1,617) | 6,535 (2,950-35,490) | 100 (0-2,515.5) | 253,500 (274-378,000) | 9 (21.0) | 26 (65.0) |
F, Female; M, male; WBC, white blood cell.
Mediator symptoms include flush, vascular instability, headache, bone pain, and diarrhea.
Collection of serum samples
Serum samples were collected from patients with mastocytosis and from age- and sex-matched controls without mastocytosis (Austrian individuals). The study was approved by the Ethics Committee of the Medical University of Vienna (nos. 1549/2012 and 1787/2017) and conducted in accordance with the Declaration of Helsinki.
Monoclonal antibodies and other reagents
The anti-IgE monoclonal antibody (mAb) E124.2.8 and the phycoerythrin (PE)-conjugated mAb 97A6 (CD203c) were purchased from Immunotech (Marseille, France). The recombinant (r) allergens rBet v 1, rPhl p 1, and rPhl p 5 were obtained from Biomay (Vienna, Austria). Recombinant Der p 2 and rVes v 5 were expressed and purified as described.27,28
Analysis of serum samples using microarrayed allergens
Sera were analyzed for the presence of IgE directed against microarrayed allergens using ImmunoCAP Solid-phase Allergen-Chip technology capable of analyzing specific IgE for more than 100 allergens (Thermo Fisher/Phadia AB, Uppsala, Sweden) essentially as described.23 A list of allergens spotted on the chip is presented in Table E2 in this article’s Online Repository at www.jaci-inpractice.org. After microarrays had been washed, aliquots of undiluted, centrifuged sera (35 μL per sample) were applied and incubated in a humid chamber for 2 hours.23 Thereafter, arrays were again washed and dried. Then, 35 μL of detection antibody was applied for 30 minutes in a humid chamber. After rinsing, washing, and drying, arrays were scanned in a confocal laser-scanner (LuxScan-10 K microarray scanner, CapitalBio, Beijing, People’s Republic of China) and evaluated by Microarray Image Analyzer v3.1.2 software (Thermo Fisher/Phadia AB). Each of the allergen molecules was spotted in triplicates on the chip. Fluorescent signal intensities of the dots were coded, with red dots representing high levels of allergen-specific IgE and blue dots corresponding to low IgE levels, measured by a confocal laser-scanner (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). Levels of specific IgE were semi-quantified by Microarray Image Analyzer v3.1.2 software and expressed by ImmunoCAP Solid-phase Allergen-Chip standardized units (ISU). A positive result was defined by a minimal cutoff level of 0.3 ISU. Grading by ISU was as follows: <0.3 ISU, below cutoff; ≥0.3 to <1 ISU, low; >1 to <10 ISU, moderate to high; >10 ISU, very high.
Upregulation of CD203c on basophils
Peripheral blood was obtained in 7 patients with mastocytosis, namely 4 with specific IgE (3 with documented allergy, 1 sensitized patient) and 3 in whom no specific IgE was detected by chip analysis. In the basophil CD203c activation test, 45 μL of heparinized peripheral blood was incubated with 5 μL of recombinant allergens (rBet v 1, rDer p 2, rPhl p 1, rPhl p 5, rVes v 5; each 1 μg/mL), anti-IgE antibody E124.2.8 (1 μg/mL) as positive control or control buffer, and 2.5 μL of PE-conjugated mAb 97A6 (CD203c) at 37°C for 15 minutes.29,30 Then, samples were subjected to erythrocytelysis using 1 mL FACS Lysing Solution (BD Biosciences, San Jose, Calif). Thereafter, cells were washed and analyzed by multicolor flow cytometry on a FACS Calibur (BD Biosciences). Basophils were detected by their forward side-scatter characteristics and expression of CD203c (see Figure E2 in this article’s Online Repository at www.jaci-inpractice.org). Antibody reactivity was measured using FlowJo-Software (BD Biosciences).29,30 Allergen-induced upregulation of CD203c was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells and expressed as stimulation index (MFIstim/MFIcontrol) as described.29,30 Antibody reactivity was controlled by isotype-matched PE-labeled mouse IgG1κ mAb MOPC-21 (BD Biosciences).
Results
Chip-based detection of specific IgE in patients with mastocytosis
Of the 42 patients with mastocytosis tested, 15 (35.7%) showed IgE reactivity in the allergen-chip. Most of these patients (8 of 15 [53.3%]) were found to be polysensitized against multiple allergens. The most frequently detected allergen sensitivities in our mastocytosis patients were Ves v 5 (16.7%) and Bet v 1 (11.9%), followed by Aln g 1 (9.5%), Phl p 1 (9.5%), Cora 1.0401 (7.1%), Phl p 5 (7.1%), and Ole e 1 (7.1%) (Table II; see Figure E3 in this article’s Online Repository at www.jaci-inpractice.org). Detailed results for all patients are presented in Table E3 in this article’s Online Repository at www.jaci-inpractice.org.
Table II. Allergens that were most frequently detectable by chip technology in the patient group with mastocytosis and in individuals of the control cohort.
| Allergen | Mastocytosis (n = 42) | Nonadvanced mastocytosis (n = 34) | Advanced mastocytosis (n = 8) | Controls (n = 42) |
|---|---|---|---|---|
| Ves v 5 | 16.7 | 17.6 | 12.5 | 16.7 |
| Bet v 1 | 11.9 | 14.7 | 0 | 9.5 |
| Aln g 1 | 9.5 | 11.8 | 0 | 7.1 |
| Cor a 1.0401 | 7.1 | 8.8 | 0 | 7.1 |
| Phl p 1 | 9.5 | 11.8 | 0 | 14.3 |
| Phl p 5 | 7.1 | 8.8 | 0 | 14.3 |
| Ole e 1 | 7.1 | 8.8 | 0 | 7.1 |
Values represent % of patients. Sera obtained from 42 patients with mastocytosis and 42 controls without mastocytosis were analyzed for specific IgE by the chip. Results for the chip-positive cases are shown as a percentage of all patients in each group. Allergen designation: Aln g 1, pathogenesis-related protein (PR-10) from alder; Bet v 1, PR-10, pollen from birch; Cor a 1.0401, PR-10 from hazelnut; Ole e 1, common olive group 1 (olive); Phl p 1, beta-expansin, pollen from timothy grass; Phl p 5, pollen from timothy grass; Ves v 5, antigen 5, venom from yellow jacket.
Comparison with an age- and sex-matched control population
In our control cohort, specific IgE was detected in 17 of 42 individuals (40.5%) by chip-profiling. In most of these cases (64.7% [11 of 17]), reactivity against several different allergens was detected. The prevalence of chip-positive cases was slightly higher in healthy controls compared with patients with mastocytosis, where 35.7% of the cases displayed specific IgE by chip-profiling (P > .05). Indeed, for most allergens tested, the numbers of IgE reactivities in the chip (number of allergens detected) was higher in controls compared with our patients with mastocytosis (Table II). In both groups, most chip-positive cases were found to be polysensitized against various allergens (mastocytosis, 53.3%; controls, 64.7%). As shown in Figure E3 (and Table E4) in this article’s Online Repository at www.jaci-inpractice.org, some of the allergens, such as Phl p 6, Der p 2, or Bet v 2, were recognized only in controls but not in patients with mastocytosis.
Correlation between chip results, type of mastocytosis, and disease burden
The prevalence of “chip-positive” cases was 50.0% in CM (2 of 4), 50.0% in MIS (1 of 2), 47.8% in ISM (10 of 23), 20% in SSM (1 of 5), 0% in ASM (0 of 3), and 20% in SM-AHN (1 of 5). Two patients, 1 with SSM and 1 with ISM, were also analyzed during follow-up and became chip-positive after several years of observation. Overall, the percentage of chip-positive cases was substantially higher in nonadvanced mastocytosis (CM/MIS/ISM/SSM: 41.2%) compared with advanced SM (ASM/SM-AHN: 12.5%). We also examined correlations between chip-positivity and serum tryptase levels, a marker of the total burden of MC. Median serum tryptase levels were higher in the cohort without specific IgE (n = 27; median, 69.7 ng/mL; range, 6.1-1617 ng/mL) compared with those with specific IgE against at least 1 allergen (n = 15; median, 40 ng/mL, range, 13.7-1090 ng/mL). Overall, a moderate inverse correlation between total IgE and tryptase levels was found (Pearson correlation coefficient: r = 0.41; P < .05; see Figure E4 in this article’s Online Repository at www.jaci-inpractice.org). However, no correlation between specific IgE detected by microarray and total IgE levels was found in our patients (r = 0.24).
Allergen-induced upregulation of CD203c on basophils
To confirm the functional relevance of IgE reactivities detected by chip-profiling, we performed basophil activation experiments. In these experiments, recombinant allergens (rVes v 5, rBet v 1, rPhl p 1, rPhl p 5, rDer p 2) were applied to examine their ability to upregulate CD203c on basophils derived from patients with mastocytosis. In all chip-positive patients tested, the recombinant allergen(s) increased the expression of CD203c on basophils (see Figure 1 and Figure E5, A, in this article’s Online Repository at www.jaci-inpractice.org). For example, in patient number 8, specific IgE against Bet v 1, Phl p 1, Phl p 5, and Ves v 5 was detected in the chip, and all these allergens were all found to induce upregulation of CD203c on basophils (see Figure E5, A). Patient number 38 showed low IgE reactivity against Phl p 5 (0.56 ISU) in the chip, but rPhl p 5 was still able to promote CD203c expression. Interestingly, in this patient, rPhl p 1 and rBet v 1 also augmented CD203c expression on basophils although the chip did not detect Phl p 1– or Bet v 1–specific IgE (see Figure E5, A). We also tested 3 other patients with mastocytosis in whom no IgE reactivity was detected by chip-profiling. In these patients, no allergen-induced upregulation of CD203c on basophils was found (see Figure E5, B).
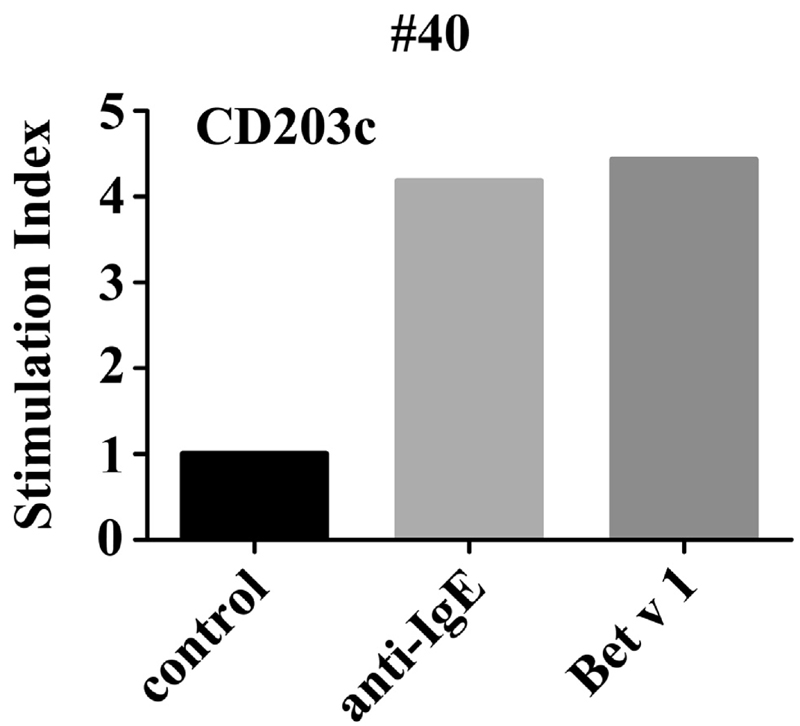
Figure 1.
Confirmation of specific IgE reactivity detected in the chip by basophil CD203c activation test. Heparinized blood from patient number 40 was incubated with recombinant Bet v 1 (1 μg/mL), anti-IgE (1 μg/mL) or control buffer and PE-conjugated antibody 97A6 (CD203c) for 15 minutes (37°C). Basophils were detected by side-scatter characteristics and expression of CD203c. Allergen-induced upregulation of CD203c was calculated from MFIs obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells and expressed as stimulation index (MFIstim/MFIcontrol).
Correlation of clinical data with results from the allergen-chip
In patients with mastocytosis presenting with MC mediatoreinduced symptoms, 11 of 26 cases (42.3%) showed IgE reactivity in the chip. In those without mediator-related symptoms, the percentage of patients with specific IgE was lower (4 of 14 [28.6%]) although the difference was not significant (P > .05). In 1 of the 4 patients with specific IgE without mediator-related symptoms, a hypersensitivity reaction to tetracycline was reported in the case history, and in 2 patients no information on mediator-related symptoms was available (Figure E6, A, in this article’s Online Repository at www.jaci-inpractice.org).
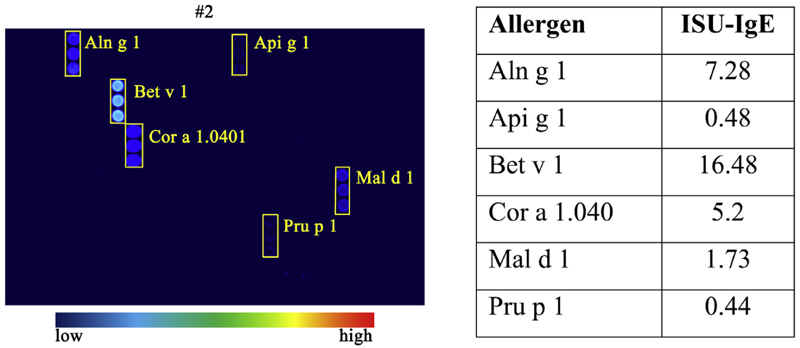
All 9 patients with mastocytosis with a history of allergies had a positive chip result (see Figure E6, B). An example (patient no. 2, ISM) with specific IgE against Aln g 1, Api g 1, Bet v 1, Cor a 1.040, Mal d 1, and Pru p 1 is shown in Figure 2. In another patient with a reported reaction to tetracyclins (presumably IgE-independent), specific IgE against Api m 1 was detected. In the mastocytosis cohort without allergies in their case history (n ¼ 31), the percentage of positive cases in the chip was 19.3% (6 of 31). In this cohort, 3 patients (62.5%) reported suffering from MC mediatoreinduced symptoms. We also compared the predictive value of the allergen-chip (frequency of positive cases with allergies) in mastocytosis with published data published in individuals with allergy or healthy controls. As presented in Table E5 in this article’s Online Repository at www.jaci-inpractice.org, the frequency of positive cases was similar in the control cohorts compared to patients with mastocytosis.
Figure 2.
Results from the chip approach in a patient with confirmed history of allergies. Sera from patient number 2 (ISM) with known allergy against birch pollen, hazelnut, and wasp was analyzed by chip-profiling (triplicates). IgE reactivity was quantified by Microarray Image Analyzer v3.1.2 software and expressed by ISU. ISU values are listed in the right panel. ISU score: <0.3 ISU, below cutoff; >0.3 to <1 ISU, low; ≥1 to <10 ISU, moderate to high; >10 ISU, very high. For more details, see Table E3.
Use of the allergen-chip in the follow-up studies of patients with mastocytosis
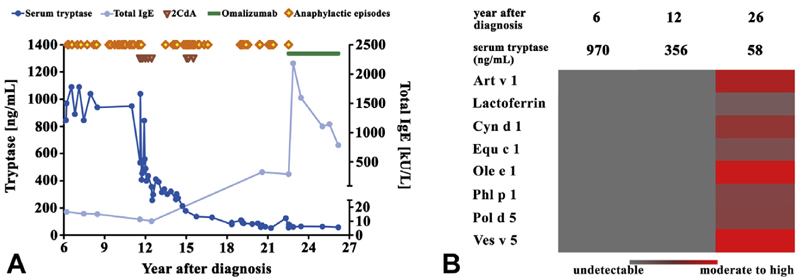
Next, we asked whether the microarray results are reproducible in individual patients and are able to detect specific IgE after sensitization. To address this point, sera from 2 patients (no. 5 and no. 26) were examined at different time points. Patient number 5 was stung by a wasp 3 years after diagnosis, and samples were taken at diagnosis and 3.5 years later. In this patient, Ves v 5 was detected by chip-profiling in 2005 but not in 2001. No other IgE reactivities were detected in this patient by chip-profiling. Thirteen years after diagnosis the patient was stung again by a wasp and developed anaphylaxis. Patient number 26 was suffering from SSM with a high MC burden and recurrent anaphylaxis, requiring resuscitation and hospitalization (at least 3 times a year) (Figure 3, A).31 The first chip-reactivity patterns were obtained 6 and 12 years after diagnosis and showed a negative result (Figure 3, B). Because of the severity of the symptoms, the high MC burden, and suspected progression to ASM, cytoreductive therapy with 2-chlorodeoxyadenosine (¼cladribine) was initiated (Figure 3, A). Treatment resulted in a substantial decrease in the MC burden and in the basal serum tryptase level (Figure 3, A). During and shortly after therapy, no severe symptoms were observed. However, 12 months after the last cycle of cladribine, severe reactions (MC activation syndrome) were again recorded. Later, a second series of cladribine cycles was given, followed again by a symptom-free period. In addition, serum tryptase levels further decreased. Three years later, when symptoms were again severe and life-threatening, treatment with omalizumab was initiated and resulted in a substantial improvement (see Figure 3, A, and Table E6 in this article’s Online Repository at www.jaci-inpractice.org). Twenty-six years after diagnosis, chip analysis was repeated and revealed specific IgE against Ole e 1, Ves v 5, and Art v 1 (Figure 3, B). At that time, total IgE levels were also markedly higher compared with those found in samples taken 6 and 12 years after diagnosis. A detailed description of the case report is provided in this article’s Online Repository at www.jaci-inpractice.org.32,33
Figure 3.
Follow-up in a case of SSM with a history of recurrent episodes of life-threatening anaphylactic reactions. (A) Serum tryptase levels ( ) and IgE (
) and IgE ( ) in patient number 26 (SSM) suffering from recurrent life-threatening anaphylaxis (
) in patient number 26 (SSM) suffering from recurrent life-threatening anaphylaxis ( ). Following 2 series of 2CdA-cycles (
). Following 2 series of 2CdA-cycles ( ), a temporary control of symptoms was achieved. After recurrence of severe anaphylactic episodes, omalizumab-therapy (
), a temporary control of symptoms was achieved. After recurrence of severe anaphylactic episodes, omalizumab-therapy ( ) was initiated, followed by marked improvement. (B) Allergen-chip was performed 6 years after diagnosis, before 2CdA, 12 years after diagnosis, and 26 years after diagnosis. 2CdA, 2-Chlorodeoxyadenosine (=cladribine).
) was initiated, followed by marked improvement. (B) Allergen-chip was performed 6 years after diagnosis, before 2CdA, 12 years after diagnosis, and 26 years after diagnosis. 2CdA, 2-Chlorodeoxyadenosine (=cladribine).
Discussion
Patients with mastocytosis suffering from a concomitant hymenoptera venom allergy, other allergies, or drug hypersensitivity reactions have an increased risk for severe anaphylaxis.14–18 Early detection of such concomitant allergies is therefore of particular importance. In the current study, the presence of specific IgE was analyzed in the sera of patients with mastocytosis by chip testing. The prevalence of chip-positive cases was higher in patients with mastocytosis with mediator-related symptoms compared with those without symptoms. Moreover, all patients with mastocytosis with a known IgE-dependent allergy were positive in the allergen-chip.
The prevalence of IgE-dependent allergies in the Western world is increasing, which may be due to several factors, including changes in our lifestyle, industrialization, pollution, and climate changes. Today, the prevalence of IgE-dependent allergies in Europe is considered to range between 20% and 30%. A similar prevalence has been reported for the Austrian population (25%-27%).34 It has also been described that IgE reactivity alone may not necessarily lead to an overt allergic disease.35 In our study, 40.5% of the age- and sex-matched Austrian controls had a positive chip result, and in most of these cases, the chip detected 2 or more IgE-reactive allergens. The criterion to select cases for the control cohort was the absence of mastocytosis and the absence of other neoplastic disorders. Otherwise, these controls were randomly collected without asking for allergies in the case history. These results suggest that the chip technology is a sensitive test detecting low quantities of IgE, including clinically “silent” IgE sensitivities, which is supported by results from birth cohorts where clinically silent sensitization often preceded “aeroallergen” symptomatic allergy.25,36,37
So far, little is known about the overall IgE-reactivity status of patients with mastocytosis. In our study, 42 patients with confirmed mastocytosis were examined by the chip technology. Compared with other methods to detect IgE, a large panel of allergens (>100) can be tested with a small amount of patient-derived serum in this allergen-chip. Probably the most important result obtained in our study was that the prevalence of chip-positive cases was lower in our patients with mastocytosis compared with our age- and sex-matched controls (35.7% compared with 40.5%). The lower percentage of affected patients with mastocytosis (compared with controls) was found in most subgroups defined by specific allergens. One explanation for this phenomenon would be that in patients with mastocytosis, most of the IgE is fixed to the high-affinity IgE receptor expressed on neoplastic MCs. IgE measurements in our patient receiving cytoreductive therapy (cladribine) confirmed this hypothesis. An alternative explanation would be that in symptomatic patients with a high MC burden, IgE is often (or even permanently) internalized together with IgE receptors and is then degraded. Finally, it cannot be excluded that in patients with mastocytosis, treatment with glucocorticosteroids or cytoreductive drugs suppresses growth and function of plasma cells producing IgE. However, even in patients receiving cytoreductive drugs such as cladribine, the allergen-chip still disclosed positive results.
When correlating chip results with clinical parameters, we found that only 21.4% of our patients reported having an allergy in their case history had a positive chip result, whereas 35.7% of all patients with mastocytosis tested positive in the chip. These data might argue for a high sensitivity of the chip regarding detection of allergen-specific IgE. Because early detection of patients at risk for allergic reactions is of importance, especially in patients with mastocytosis, the high sensitivity might represent a major advantage of the chip. Indeed, recently published data have shown that in patients suffering from hymenoptera venom allergy, severe reactions may be observed already when Ves v 5especific IgE levels are below 1 ISU.28 In our study, the most prevalent allergen in the mastocytosis group was Ves v 5 (16.7%; median, 1.7 ISU). In this regard, it is noteworthy that 1 of our patients was tested positive for Ves v 5 9 years before a life-threatening anaphylactic reaction to a wasp sting.
There are several limitations of currently used chip-based assays to detect IgE in patients with SM. One is that the assays may not cover all relevant hymenoptera venom species. For example, the chip assay applied did not detect Ves v 2, Ves v 3, Api m 3, Api m 5, or Api m 10. In addition, the chip did not detect drugs known to cause hypersensitivity reactions, such as penicillin.38 However, severe drug reactions are rare in these patients and may have different etiologies, including noneIgE-dependent mechanisms.39,40 Another disadvantage is that in patients with SM who have a very high MC burden, a negative result by chip testing does not exclude the presence of an IgE-mediated allergy, because most of the IgE is fixed to the MC surface and is therefore not detectable by a serologic test, especially when the levels of produced IgE are low and the burden of MCs is high. However, as exemplified in 1 patient with SSM in this study, specific IgE may become detectable when the burden of neoplastic MCs decreased substantially after cytoreductive therapy. Indeed, in patient number 26, an increase in total IgE was already observed after cladribine before the administration of omalizumab, which typically increases serum IgE levels. To confirm the clinical value of the chip, we performed functional experiments with basophils in 7 patients. In these analyses, the recombinant allergens were found to promote CD203c expression on basophils in all patients carrying specific IgE detected by chip-profiling. On the other hand, however, the chip was not able to detect all allergen reactivities that were documented in the CD203c assay. For example, in patient number 38 (ISM), moderate IgE reactivity for Phl p 5 was found in the chip and was confirmed by allergen-induced CD203c upregulation on basophils. However, in the same patient, recombinant Phl p 1 and Bet v 1 also induced CD203c upregulation on basophils although the chip did not detect IgE against Phl p 1 or Bet v 1. Another interesting observation was that the chip was positive in 6 of 31 patients with mastocytosis (3 symptomatic and 3 non-symptomatic) in whom no previous allergy had been documented. Unfortunately, we were not able to apply skin tests or the CD203c assay in these cases.
Together, the allergen-chip approach represents a novel, simple, and easily applicable tool for detection of specific IgE in patients with mastocytosis. This assay is able to early detect patients with mastocytosis with IgE-dependent sensitization to a large panel of different allergens, including venom allergens, which are the most relevant allergens in mastocytosis, in a single test. Whether this approach is able to predict allergies in all patients with mastocytosis remains to be determined in future studies.
Supplementary Material
What is already known about this topic?
Patients with mastocytosis are at high risk to develop fatal anaphylaxis. Therefore, early detection of immunoglobulin E (IgE) sensitization is of particular importance in these patients.
What does this article add to our knowledge?
A broad screen by allergen-chip profiling was found to identify multiple, potentially clinically relevant, IgE species in patients with mastocytosis in a single test.
How does this study impact current management guidelines?
Allergen-chip profiling represents a useful tool to screen for allergen-specific IgE species in patients with mastocytosis and may thus help in early detection of life-threatening IgE- sensitivities in high-risk patients.
Acknowledgments
This study was supported by the Austrian Science Fund (FWF) (grant nos. DK W 1248-B30, F4603-B20, F4605-B20, F4704-B20, and P32470-B).
Abbreviations used
- ASM
Aggressive systemic mastocytosis
- CM
Cutaneous mastocytosis
- IgE
Immunoglobulin E
- ISM
Indolent systemic mastocytosis
- ISU
ImmunoCAP Solid-phase Allergen-Chip standardized units
- mAb
Monoclonal antibody
- MC
Mast cell
- MFI
Mean fluorescence intensity
- MIS
Mastocytosis in the skin
- PE
Phycoerythrin
- SM
Systemic mastocytosis
- SM-AHN
Systemic mastocytosis with an associated hematologic neoplasm
- SSM
Smoldering systemic mastocytosis
Footnotes
Conflicts of interest: C. Lupinek received honoraria from Thermo Fisher for lectures given at symposia for continuing medical education. R. Valenta received research grants from Biomay AG, Vienna, Austria, and Viravaxx, Vienna, Austria, and serves as a consultant for these companies. W. R. Sperr received a research grant from Thermo Fisher and honoraria from Novartis, Pfizer, Deciphera, Jazz, Teva, and Celgene. P. Valent received research grants from Celgene, Incyte, Pfizer, Deciphera, and Blueprint and honoraria from Novartis, Pfizer, Deciphera, Accord, and Celgene. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3–11. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 5.Horny HP, Akin C, Arber DA, Peterson LA, Tefferi A, Metcalfe DD, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol. 3. Lyon, France: IARC Press; 2017. pp. 62–9. [Google Scholar]
- 6.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77:1261–70. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valent P, Akin C, Metcalfe DD. Mastocytosis. 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–32. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, et al. Somatic c-kit activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–4. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 10.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–64. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 11.Valent P, Akin C, Arock M. Diagnosis and treatment of anaphylaxis in patients with mastocytosis. Curr Treat Options Allergy. 2014;1:247–61. [Google Scholar]
- 12.Akin C. Anaphylaxis and mast cell disease: what is the risk? Curr Allergy Asthma Rep. 2010;10:34–8. doi: 10.1007/s11882-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 13.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–72. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 14.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–32. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 15.Valent P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin Exp Allergy. 2014;44:914–20. doi: 10.1111/cea.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera anaphylaxis and C-kit mutations: an unexpected association. Curr Allergy Asthma Rep. 2015;15:49. doi: 10.1007/s11882-015-0550-0. [DOI] [PubMed] [Google Scholar]
- 17.Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera allergy and mast cell activation syndromes. Curr Allergy Asthma Rep. 2016;16:5. doi: 10.1007/s11882-015-0582-5. [DOI] [PubMed] [Google Scholar]
- 18.Niedoszytko M, de Monchy J, van Doormaal JJ, Jassem E, Oude Elberink JN. Mastocytosis and insect venom allergy: diagnosis, safety and efficacy of venom immunotherapy. Allergy. 2009;64:1237–45. doi: 10.1111/j.1398-9995.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 19.Akin C. Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140:349–55. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013;68:417–24. doi: 10.1111/all.12126. [DOI] [PubMed] [Google Scholar]
- 22.Anto JM, Bousquet J, Akdis M, Auffray C, Keil T, Momas I, et al. Mechanisms of the Development of Allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139:388–99. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- 23.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asarnoj A, Hamsten C, Lupinek C, Melén E, Andersson N, Anto JM, et al. Prediction of peanut allergy in adolescence by early childhood storage protein-specific IgE signatures: the BAMSE population-based birth cohort. J Allergy Clin Immunol. 2017;140:587–90. doi: 10.1016/j.jaci.2016.12.973. [DOI] [PubMed] [Google Scholar]
- 25.Asarnoj A, Hamsten C, Wadén K, Lupinek C, Andersson N, Kull I, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. 2016;137:813–21. doi: 10.1016/j.jaci.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørensen M, Klingenberg C, Wickman M, Sollid JUE, Furberg AS, Bachert C, et al. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy. 2017;72:1548–55. doi: 10.1111/all.13175. [DOI] [PubMed] [Google Scholar]
- 27.Chen KW, Focke-Tejkl M, Blatt K, Kneidinger M, Gieras A, Dall’Antonia F, et al. Carrier-bound non allergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients. Allergy. 2012;67:609–21. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinger P, Lupinek C, Kalogiros L, Silar M, Zidarn M, Korosec P, et al. The culprit insect but not severity of allergic reactions to bee and wasp venom can be determined by molecular diagnosis. PLoS One. 2018;13:e0199250. doi: 10.1371/journal.pone.0199250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 30.Kneidinger M, Schmidt U, Rix U, Gleixner KV, Vales A, Baumgartner C, et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111:3097–107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- 31.Boehm T, Reiter B, Ristl R, Petroczi K, Sperr W, Stimpfl T, et al. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy. 2019;74:583–93. doi: 10.1111/all.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckl-Dorna J. Omalizumab’s impact on total and allergen-specific IgE levels: a polyclonal story. Int Arch Allergy Immunol. 2016;169:69–70. doi: 10.1159/000444998. [DOI] [PubMed] [Google Scholar]
- 33.Mizuma H, Tanaka A, Uchida Y, Fujiwara A, Manabe R, Furukawa H, et al. Influence of omalizumab on allergen-specific IgE in patients with adult asthma. Int Arch Allergy Immunol. 2015;168:165–72. doi: 10.1159/000442668. [DOI] [PubMed] [Google Scholar]
- 34.Österreichische Gesundheitsbefragung 2014 Hauptergebnisse des Austrian Health Interview Survey (ATHIS) und methodische Dokumentation. Vienna, Austria: Statistik Austria; 2015. [Accessed July 27, 2017]. p. 245. Available from: https://broschuerenservice.sozialministerium.at/Home/Download?publicationId=542. [Google Scholar]
- 35.Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens: a GA2LEN project. Allergy. 2006;61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 36.Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. Mechanisms for the Development of Allergies Consortium. J Allergy Clin Immunol. 2015;135:1199–206. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickman M, Lupinek C, Andersson N, Belgrave D, Asarnoj A, Benet M, et al. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91–9. doi: 10.1016/j.ebiom.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338–51. doi: 10.1056/NEJMra1807761. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton RG, Oppenheimer J. Serological IgE analyses in the diagnostic algorithm for allergic disease. J Allergy Clin Immunol Pract. 2015;3:833–40. doi: 10.1016/j.jaip.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Warrington R, Silviu-Dan F, Wong T. Drug allergy. Allergy Asthma Clin Immunol. 2018;14:60. doi: 10.1186/s13223-018-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.