Abstract
Many phosphorylation signal transduction pathways in the eukaryotic cell are modulated by posttranslational modification of specific serines/threonines with N-acetylglucosamine (O-GlcNAc). Levels of O-GlcNAc on key proteins regulate biological processes as diverse as the cell cycle, insulin signalling and protein degradation. The two enzymes involved in this dynamic and abundant modification are the O-GlcNAc transferase and O-GlcNAcase. Structural data has recently revealed that the O-GlcNAcase possesses an active site with significant structural similarity to the human lysosomal hexosaminidases HexA/HexB. PUGNAc, an O-GlcNAcase inhibitor widely used to raise levels of O-GlcNAc in human cell lines, also inhibits these hexosaminidases. Here, we have exploited recent structural information of an O-GlcNAcase-PUGNAc complex to design and synthesize a glucoimidazole-based inhibitor, GlcNAcstatin, which is a 5 picomolar competitive inhibitor of enzymes of the O-GlcNAcase family, shows 100000-fold selectivity over HexA/B and binds to the O-GlcNAcase active site by mimicking the transition state as revealed by X-ray crystallography. This compound is able to raise O-GlcNAc levels in human HEK 293 and SH-SY5Y neuroblastoma cell lines, and thus provides a novel, potent tool for the study of the role of O-GlcNAc in intracellular signal transduction pathways.
Many proteins in the eukaryotic cell are modified by O-linked N-acetylglucosamine (O-GlcNAc) on serines and threonines1. O-GlcNAcylation has been shown to be important for regulation of the cell cycle, DNA transcription and translation, insulin sensitivity and protein degradation2,3. Misregulation of O-GlcNAcylation is associated with diabetes and Alzheimer’s disease2,4,5. Two enzymes are involved in the dynamic cycling of this posttranslational modification, the O-GlcNAc transferase (OGT, classified as CAZY6 family GT41) and O-GlcNAcase (OGA, GH84). PUGNAc (O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate), a nanomolar inhibitor of OGA, has been extensively used to induce and study the effects of raised O-GlcNAc levels in the cell7,8. However, PUGNAc is also a potent inhibitor of the human lysosomal hexosaminidases HexA and HexB, inactivation of which has been associated with the Tay-Sachs and Sandhoff lysosomal storage disorders. While more selective derivatives of PUGNAc and other OGA inhibitors have recently been reported9,10,11, these are also associated with weaker (micromolar) inhibition of OGA. Here we report on a rationally designed glucoimidazole, GlcNAcstatin ((1), Fig. 1), which inhibits a bacterial OGA (bOGA) with a K i of 4.6 pM and 100000-fold selectivity over HexA/B, is active against human OGA (hOGA) in human cell lines and is tethered in the active site as revealed by X-ray crystallography.
Figure 1.
Structure of GlcNAcstatin and Lineweaver-Burk analysis of bOGA steady-state kinetics in the presence of inhibitor concentrations.
Recent structural data of bOGA in complex with PUGNAc has shown that this compound mimics the sp 2 configuration of the C1 atom in the transition state and binds with the acetamido group in a deep pocket that is significantly smaller in HexA/HexB than in OGA12, providing an inroad to engineering selectivity. The PUGNAc phenylcarbamate moiety extends out of the active site towards a solvent exposed tryptophan. In an effort to increase affinity and selectivity in parallel, we decided to investigate the somewhat isosteric gluco-configured derivatives13 of the naturally occurring hexosaminidase inhibitor nagstatin14. In 1995 Tatsuta reported the total synthesis of this compound15 and a series of related glycoimidazole derivatives with variable configuration of the sugar ring, showing up to nanomolar inhibition against a panel of glycosidases16,17. Subsequent work by the Vasella group defined the structure-activity relationships of nagstatin analogues (tetrahydroimidazo[1,2-a]pyridines) as glycosidase inhibitors, making use of an original synthetic approach to the bicyclic core structure18, showing that the molecular architecture of these compounds in the ground state accurately mimics the assumed flattened half-chair/envelope conformation of the sugar ring in the transition state, while protonation of the imidazole ring effectively emulates the charge distribution in the oxocarbenium ion. Furthermore, critical evaluation of the nature of the C2 substituent showed that a glycon-mimicking group in this position results in stronger inhibition19,20. Combining the current body of knowledge on the glycoimidazoles together with the structural data of the bOGA-PUGNAc complex, we designed a series of GlcNAc configured nagstatin derivatives (GlcNAcstatins) that address specificity towards the OGA enzymes through elaborated N8 (tetrahydroimidazopyridine numbering) acyl derivatives while providing a source of increased affinity through the incorporation of suitable C2 substituents. Here we report the synthesis of GlcNAcstatin (1, Fig. 1), a glucoimidazole with noticeable similarity to the molecular architecture of PUGNAc, but bearing a larger iso-butanamido group on N8 and a phenethyl group on C2.
GlcNAcstatin was synthesized using a combination of the Tatsuta and Vasella approaches, as set out in the Supporting Information. Kinetic analysis using a previously published assay12 (adapted for use with picomolar enzyme concentrations, see Supporting Information) shows that GlcNAcstatin is a very potent, competitive inhibitor of bOGA with a K i of 4.6 ± 0.1 pM (Fig. 1). Direct measurement of a binding K d either by isothermal titration calorimetry or intrinsic tryptophan fluorescence was not possible due to complete depletion of free inhibitor concentration in the picomolar range by even the lowest experimentally possible concentration of enzyme (data not shown).
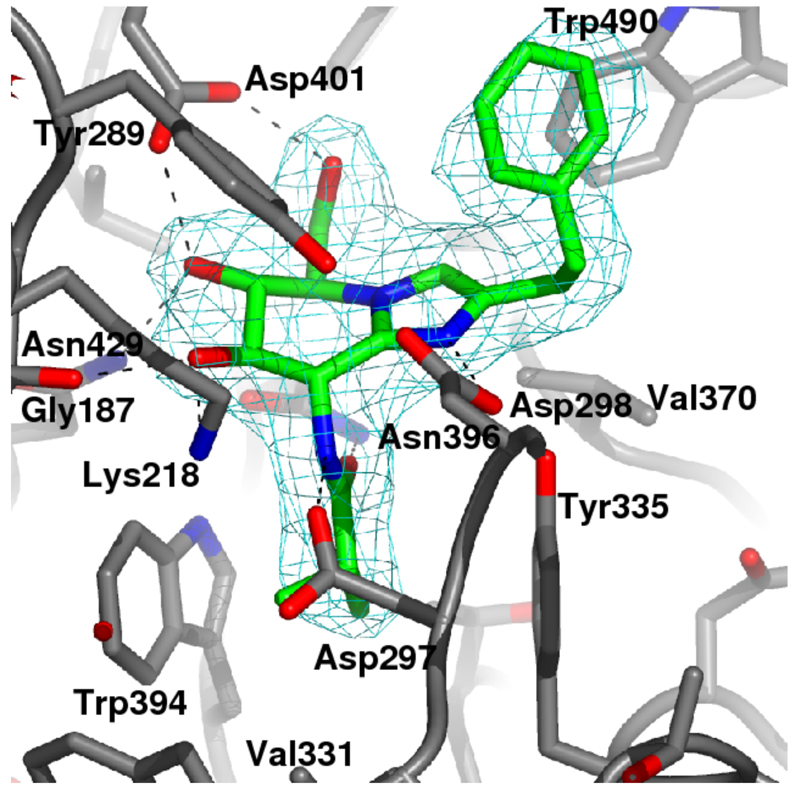
Inhibition of HexA/B was also evaluated, giving a K i of 0.52 μM (see Supporting Information). Thus, GlcNAcstatin is 100000-fold selective for the O-GlcNAcase active site compared to the structurally most closely related enzymes in the human cell. To demonstrate the molecular basis of this selectivity, we characterized the binding mode of the inhibitor by determining the crystal structure of the bOGA-GlcNAcstatin complex to a resolution of 2.25 Å (Fig. 2). Similar to the previously determined PUGNAc12 and thiazoline21 complexes, the sugar moiety of the inhibitor occupies a pocket in the enzyme and interacts with four conserved hydrogen bond donors/acceptors. The pyranose ring adopts a 4E conformation, similar to that observed in previously determined enzyme-glucoimidazole complexes22,23 and the PUGNAc complex12. Interaction with Asp297 and Asn396 forces the iso-butanamido group to adopt a conformation compatible with the proposed substrate-assisted catalysis mechanism9,12,21, with the carbonyl oxygen approaching the sp 2 configured “anomeric” carbon to within 3.4 Å (Fig. 2). The terminal methyls of the iso-butanamido group penetrate a pocket formed by the conserved Tyr335 and Trp394 (Fig. 2). In HexA/B this pocket is significantly smaller, explaining why the larger acyl groups on the previously reported inhibitors9,10,11 significantly increase the Ki against these enzymes. Asp298, the catalytic acid, is precisely positioned for the lateral protonation mechanism as proposed by Vasella18. The phenethyl group extends away from the active site, interacting with the solvent-exposed Trp490.
Figure 2.
Crystal structure of GlcNAcstatin (sticks with green carbon atoms) complexed to bOGA. Unbiased 2.25 Å |Fo|–|Fc|, ϕcalc (2.5σ) electron density for the inhibitor is shown in cyan.
To evaluate the usefulness of GlcNAcstatin as a chemical tool to study the effects of inhibition of OGA in human cells, activity of the compound in human SH-SY5Y human neuroblastoma cell lysates and the HEK 293 cell line was evaluated qualitatively by Western blot analysis with an anti-O-GlcNAc antibody. Fig. 3A shows that GlcNAcstatin appears more active than PUGNAc in inhibiting bOGA from removing O-GlcNAc from proteins in SH-SY5Y cell lysates. More importantly, when used to treat HEK 293 cells, GlcNAcstatin qualitatively appears more efficient at raising O-GlcNAc levels than PUGNAc (Fig. 3B).
Figure 3.
Immunoblot detection of O-GlcNAc modifications on cellular proteins, compared to molecular weight standards in the far left lanes. A: bOGA (5 μM) activity on SH-SY5Y neuroblastoma cell lysates is inhibited by increasing amounts of PUGNAc/GlcNAcstatin, B: HEK293 cells incubated for 12 h with increasing amounts of inhibitors.
In conclusion, compound (1), GlcNAcstatin reported here represents a novel, potent and highly selective tool to study the role of the O-GlcNAc modification in the human cell. Several other inhibitors such as the thiazolines9 and PUGNAc derivatives10,11 carrying larger N2 acyl groups have very recently been reported. Whilst these compounds did improve the selectivity, showing weaker inhibition of HexA/B, they did so at the cost of also significantly reducing inhibition of OGA, with K is increasing to the micromolar range. The work described here shows that it is possible to achieve both picomolar inhibition and exquisite selectivity with rationally designed glucoimidazoles. The structural data of the GlcNAcstatin complex will allow for further fine tuning of the inhibitory properties of GlcNAcstatin derivatives by elaboration of the N8 acyl group and the aromatic substituents off the imidazole ring.
Supplementary Material
Supporting Information Available. Experimental details of synthesis of (1), enzyme inhibition and X-ray crystallography.
Acknowledgements
We thank the ESRF for the time at ID14-4. DvA is supported by a Wellcome Trust SRF and the Lister Prize, HCD by the College of Life Sciences Alumni Studentship. We thank Alan Fairlamb for fruitful discussions.
References
- (1).Torres CR, Hart GW. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- (2).Zachara NE, Hart GW. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- (3).Love DC, Hanover JA. Science STKE. 2005;312:1–14. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- (4).Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. J Biol Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- (5).Lehman DM, et al. Diabetes. 2005;54:1214–1221. doi: 10.2337/diabetes.54.4.1214. [DOI] [PubMed] [Google Scholar]
- (6). http://afmb.cnrs-mrs.fr/CAZY
- (7).Horsch M, Hoesch L, Vasella A, Rast DM. Eur J Biochem. 1991;197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- (8).Haltiwanger RS, Grove K, Philipsberg GA. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- (9).Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. J Biol Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- (10).Stubbs KA, Zhang N, Vocadlo DJ. Org Biomol Chem. 2006;4:839–845. doi: 10.1039/b516273d. [DOI] [PubMed] [Google Scholar]
- (11).Kim EJ, Perreira M, Thomas CJ, Hanover JA. J Am Chem Soc. 2006;128:4234–4235. doi: 10.1021/ja0582915. [DOI] [PubMed] [Google Scholar]
- (12).Rao FV, Dorfmueller HC, Villa F, Allwood M, Eggleston IM, van Aalten DMF. EMBO J. 2006;25:1569–1578. doi: 10.1038/sj.emboj.7601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Terinek M, Vasella A. Helv Chim Acta. 2005;88:10–22. [Google Scholar]
- (14).Aoyagi T, Suda H, Uotani K, Kojima F, Aoyama T, Horiguchi K, Hamada M, Takeuchi T. J Antibiot. 1992;45:1404–1408. doi: 10.7164/antibiotics.45.1404. [DOI] [PubMed] [Google Scholar]
- (15).Tatsuta K, Miura S. Tetrahedron Lett. 1995;36:6721–6724. [Google Scholar]
- (16).Tatsuta K, Miura S, Ohta S, Gunji H. J Antibiot. 1995;48:286–288. doi: 10.7164/antibiotics.48.286. [DOI] [PubMed] [Google Scholar]
- (17).Tatsuta K, Miura S, Ohta S, Gunji H. Tetrahedron Lett. 1995;36:1085–1088. [Google Scholar]
- (18).Heightman TD, Vasella AT. Angew Chem-Int Edit. 1999;38:750–770. doi: 10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- (19).Panday N, Canac Y, Vasella A. Helv Chim Acta. 2000;83:58–79. [Google Scholar]
- (20).Shanmugasundaram B, Vasella A. Helv Chim Acta. 2005;88:2593–2602. [Google Scholar]
- (21).Dennis RJ, Taylor EJ, Macauley MS, Stubbs KA, Turkenburg JP, Hart SJ, Black GN, Vocadlo DJ, Davies GJ. Nat Struct Mol Biol. 2006;13:365–371. doi: 10.1038/nsmb1079. [DOI] [PubMed] [Google Scholar]
- (22).Hrmova M, Streltsov VA, Smith BJ, Vasella A, Varghese JN, Fincher GB. Biochemistry. 2005;44:16529–16539. doi: 10.1021/bi0514818. [DOI] [PubMed] [Google Scholar]
- (23).Gloster TM, Roberts S, Perugino G, Rossi M, Moracci M, et al. Biochemistry. 2006;45:11879–11884. doi: 10.1021/bi060973x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.