Summary
Osteoporosis is the most prevalent metabolic bone disease, characterized by low bone mass and microarchitectural deterioration. Here, we show that warmth exposure (34°C) protects against ovariectomy–induced bone loss by increasing trabecular bone volume, connectivity density and thickness, leading to improved biomechanical bone strength in adult female, and in young male mice. Transplantation of the warm–adapted microbiota phenocopies the warmth–induced bone effects. Both warmth and warm–microbiota transplantation revert the ovariectomy–induced transcriptomics changes of the tibia, and increase periosteal bone formation. Combinatorial metagenomics/metabolomics analysis shows that warmth enhances bacterial polyamine biosynthesis, resulting in higher total polyamine levels in vivo. Spermine and spermidine supplementation increases bone strength, while inhibiting polyamine biosynthesis in vivo limits the beneficial warmth effects on the bone. Our data suggest warmth exposure as a potential treatment option for osteoporosis while providing a mechanistic framework for its benefits in bone disease.
Introduction
External temperature is an environmental parameter that affects various aspects of physiology and requires constant adaptation by living organisms to its fluctuations. To dissipate heat, rodents increase skin vasodilation at specific locations where the surface–to–body ratio is high. They also adapt to increased temperatures partly by enlarging the tail and ear length/surface (Meyer, 2017) (Alhilli, 1983; Ashoub, 1958; Harland, 1960), allowing further heat dissipation. Warmth exposure also has effects on development; for example, promoting femur growth (Romsos, 1985; Serrat, 2008) and favoring denser trabecular and cortical microarchitecture (Iwaniec, 2016). Unilateral heating of the limb from weaning is associated with elongation of the extremities on the heat–exposed side only (Serrat, 2015), and chondrocyte proliferation in vitro is higher at warmer incubation temperatures (Serrat, 2008; Serrat, 2014).
Osteoporosis is the most prevalent metabolic bone disease, characterized by low bone mass and microarchitectural deterioration (Sozen, 2017), leading to weaker bones and increased fracture risk. Bone remodeling is enabled by the coordinated action of the two major type of cells present in the bone: osteoblasts, which are responsible for bone formation, and osteoclasts, which are involved in bone resorption. The most common type of primary osteoporosis occurs as a result of post-menopausal estrogen deficiency (Reginster, 2006), and as such, it is exceedingly common in aging females, but can also occur in men. Whether heat administration post-development and during late adulthood in healthy, or during osteoporotic states, can affect bone health, remodeling and physiology is unknown.
The intestinal flora has emerged as an important regulator of host physiology, including the bone (Li, 2019; Hsu, 2018; Jones, 2017; Li, 2019; Ohlsson, 2015; Ohlsson, 2018; Parvaneh, 2014; Sjogren, 2012). We (Chevalier, 2015), and others (Zietak, 2016), have previously shown that the host adaptation to cold is in part mediated by alterations of the gut microbiota composition. However, it is not clear whether elevated environmental temperature can affect the microbiota composition. It is also not known whether such alterations would have any effect on bone morphology and strength.
In the present study, we show that warmth exposure applied at later stages of development improves bone microarchitecture and strength during healthy conditions. We further demonstrate that this phenomenon can be used in pathological conditions, where it prevents the deleterious effect of estrogen depletion in a mouse model of osteoporosis. These osteological improvements are mediated by warm temperature–induced alterations of the gut microbiota composition and are sufficient to prevent bone loss, indicating an existence of a signaling axis between warmth exposure and the bone that is mediated by the microbiota. In terms of possible translation, we performed human metadata analysis and found an inverse correlation between the incidence of osteoporotic hip fractures and external temperature that is independent of Vitamin D and calcium levels. Mechanistically, through a combinatorial metagenomic, targeted metabolomic and functional approach we show that the warm-adapted microbiota has a higher potency to produce polyamines; in particular, acetylated spermidine and putrescine. Polyamine biosynthesis inhibition limits the benefits of warmth exposure, while polyamine supplementation mimics the effects of warmth in vivo in the mouse osteoporosis model. Our data suggest warmth exposure as a potential treatment option for the prevention of osteoporosis, while providing a mechanistic understanding of the role of a microbiota-host interaction during warmth exposure and bone disease.
Results
Warmth Exposure Improves Bone Strength in Adulthood
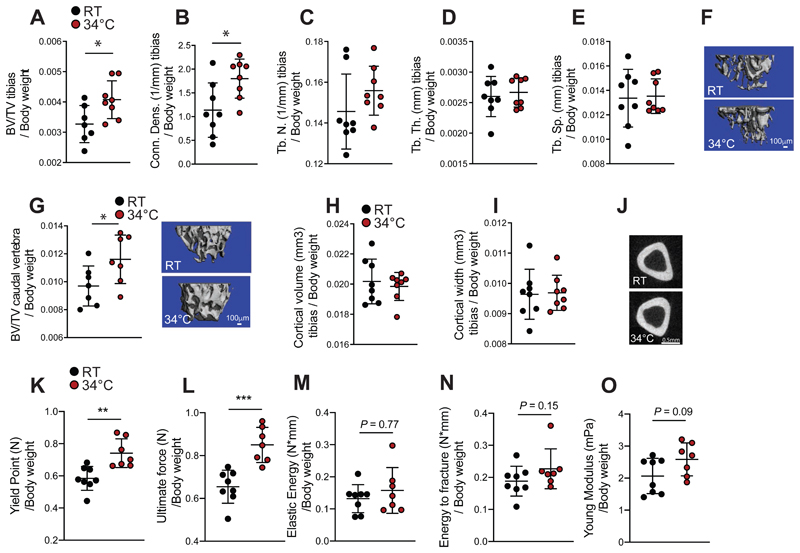
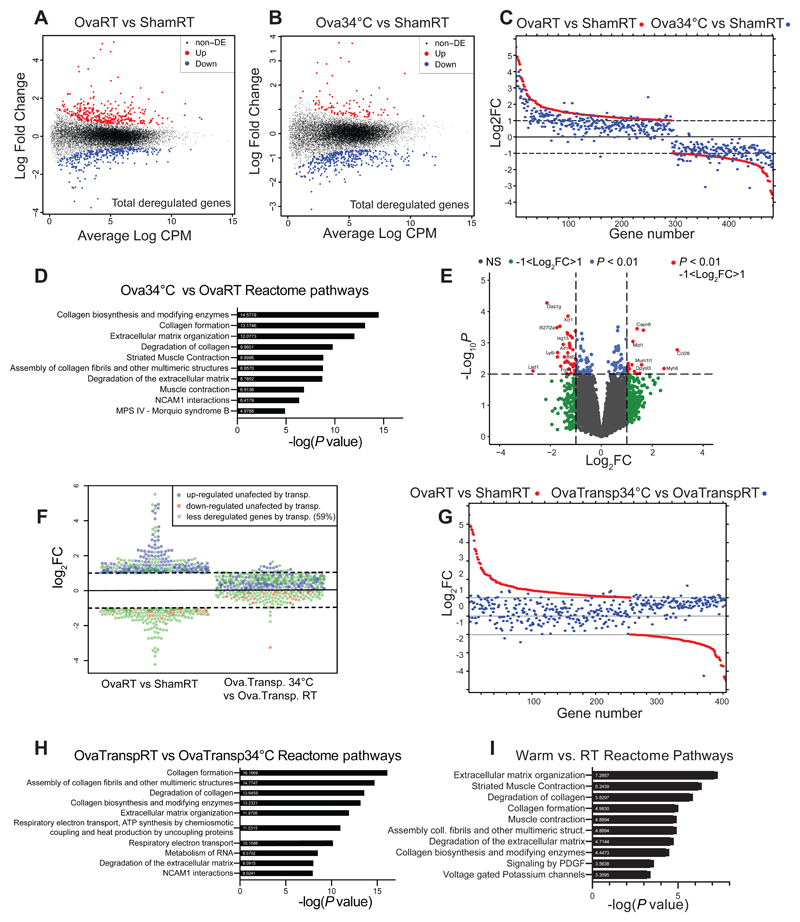
Aging leads to a decrease in bone strength and mass and alterations in the microarchitecture (Boskey, 2010; Demontiero, 2012; Mosekilde, 2000), which can increase the incidence of fracture. We, therefore, evaluated the effects of warm temperature exposure on mice after their development, using females exposed for 8 weeks to 34°C starting at 16 weeks of age. This treatment led to an increase in the tail length in the older female mice (Figure S1A), and the increase was even more pronounced following 34°C exposure for 1 month when started in 8 weeks-old male mice (Figure S1B). This was coupled to higher tail temperature as expected, without changing the overall body temperature (Figure S1C and S1D). Exposure to 34°C of the older females led to an increase in the trabecular bone volume vs total tibia volume ratio (BV/TV), the connectivity density of the tibias (Figure 1A-1F), and in the BV/TV of the caudal vertebra (Figure 1G), without affecting the cortical bone of the tibias (Figure 1H-1J), indicating a positive effect of warmth exposure on the trabecular bone.
Figure 1. Warmth Exposure Improves Bone Strength during Adulthood.
(A-F) Trabecular bone microarchitecture of tibias showing bone volume/total volume (BV/TV) (A), connectivity density (Conn. Dens) (B), number of trabeculae (Tb. N) (C), trabecular thickness (Tb.Th.) (D) and trabecular separation (Tb.Sp) (E) of 24 week-old female mice exposed to 34°C for 2 month prior sacrifice and their RT controls (n = 8 per group), all normalized to body weight.
(F) Representative reconstruction of trabecular bone used for the calculations in A-F. Scale = 100μm. Each trabecular reconstruction was done by scanning and compiling 262 sections from the beginning of the growth plate to the midshaft in each mouse using n = 8 per group.
(G-I) Cortical bone volume (G) and width (H) of mice as in (A), measured in midshaft of the tibias and normalized to body weight. (G right) Representative reconstruction (each consisting of 262 sections, n = 8 per group) of trabecular bone used for calculation. Scale = 100μm. (I) Representative cortical section (from 62 sections per bone of each mouse of n = 8 per group). Scale: 0.5mm.
(J) Bone volume/total volume (BV/TV) (left), measured in the caudal vertebra (CA2) (normalized to body weight) of mice as in (A).
(K-O) Biomechanical analysis of femur from mice as in (A) using a 3-point bending test. The parameters measured include the yield point (K), the ultimate force (L), the elastic energy (M), the energy to fracture (N) and the Young’s modulus (O) and normalized to their respected body weight.
Data are shown as mean ± SD (n = 8 per group). Significance (P value) is calculated using Mann-Whitney t-test *P < 0.05; **P < 0.01; ***P < 0.001.
These structural changes were also reflected at a biomechanical level. A three–point bending test in the femur highlighted the improvements in the yield point (Figure 1K) above which mechanical force causes permanent damage to the bone structure, and in the ultimate force (Figure 1L) that reflects the general integrity of the bone. No differences were detected in the elastic energy, energy to fracture or the Young modulus (Figures 1M-O). Exposure to 34°C reduced the food intake by 25% (Figure S1E), consistent with slowing of the overall metabolism and reduced activity at elevated temperatures (Kaiyala, 2012) (Figures S1E-F). As lowered food intake affects the bone mass (Devlin, 2010; Hamrick, 2008), and reduces body weight at room temperature (RT) (Fabbiano, 2016), but not during warmth exposure (Figure S1F), we compared the effects of warmth exposure to a pair-fed set of animals kept at RT. Warmth exposure led to elevated trabecular BV/TV (Figure S1G), and higher cortical bone volume and width (Figure S1H-J) compared to the pair–fed controls. This was coupled to a marked improvement of the biomechanical resistance that was independent of the food intake (Figures S1K-O). In addition, the warmth exposure also led to elongated femurs in comparison to the pair–fed RT–housed controls (Figure S1P). These data show that warmth exposure exerts beneficial effects on the biomechanical bone parameters in mice during adulthood, and that these effects are unrelated to the decreased food intake.
Warmth Exposure Correlates With Reduced Fracture Incidence in Humans and Prevents Experimentally-induced Bone Loss in Mice
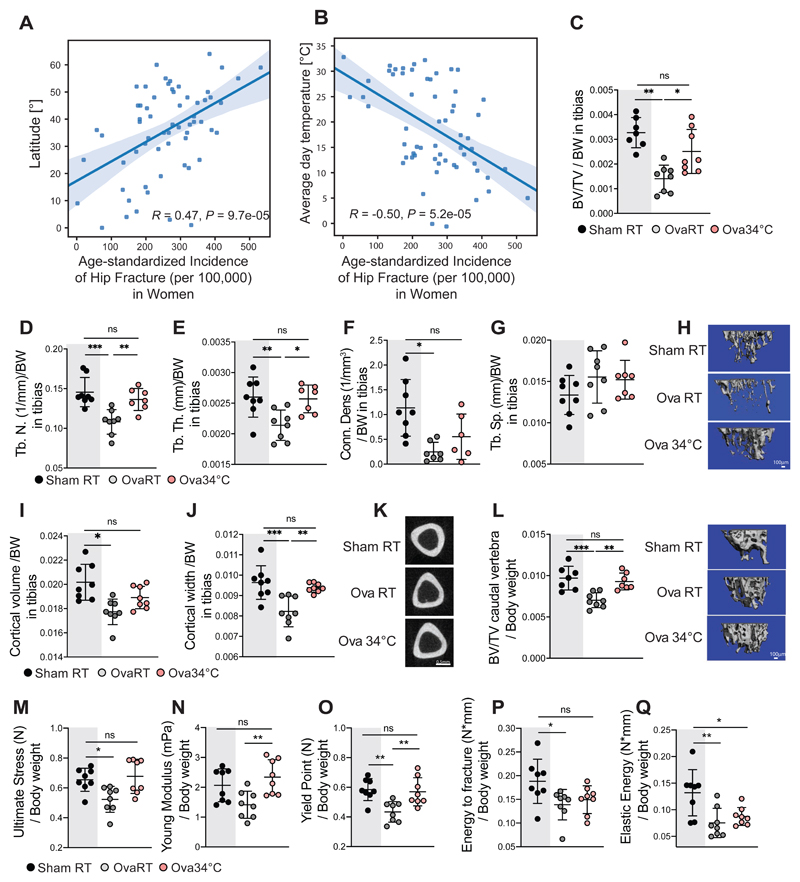
We next investigated if the warmth exposure could have protective effects on bone loss. To address the potential relevance of the temperature on the osteoporosis–related fractures in humans, we performed human metadata analysis on the incidence of hip fractures per capita and country worldwide (Cauley, 2014; Wahl, 2012; Balk, 2017), and found a positive correlation between the fractures and the latitude (Figures 2A and S2A). Conversely, there was a negative correlation between the average temperature and hip fracture incidence both in women and in the total population (Figures 2B and S2B). Partial correlation analysis (with the effect of the Vitamin D removed) showed that the temperature and latitude effect on the hip fracture incidence are independent of Vitamin D (Figures S2C-G). Similarly, correcting for the calcium intake did not influence the effect of the temperature and latitude on the hip fracture incidences (Figures S2H and S2I). Instead, when correcting for temperature, the association between latitude and the hip fracture was largely eliminated (Figure S2J).
Figure 2. Warmth Exposure Protects Against Osteoporosis.
(A and B) Metadata analysis showing age-standardized correlation between hip fracture incidence (per 100’000 inhabitants) in women per country, versus the latitude of the country’s capitals (A), or versus the country’s average day temperature (B).
(C-H) Trabecular bone microarchitecture of tibias in female mice that were ovariectomized, or sham-operated at 16 weeks of age, and then exposed to 34°C for 2 months (Ova34°C, or Sham34°C, respectively), or kept at RT (OvaRT, or ShamRT). Bone volume/total volume (BV/TV) (C), the number of trabeculae (Tb. N) (D), the trabecular thickness (Tb.Th.) (E), the connectivity density (Conn. Dens) (F), and the trabecular separation (Tb.Sp) (G) from the mice as in (C) at the end of the warm exposure, normalized to their respective body weight. (H) Representative reconstruction (each consisting of 262 sections, n = 8 per group) of trabecular bone used for calculations. Scale: 100μm.
(I-K) Cortical bone volume (I) and width (J) of mice as in (C) measured in the midshaft of the tibias and normalized to the body weight. (K) Representative cortical sections from each group (from 62 sections per bone of each mouse of n = 8 per group). Scale: 0.5mm.
(L) Trabecular bone volume/tissue volume (BV/TV), measured in the caudal vertebra (CA2) (normalized to body weight) of mice as in (C). Right, representative reconstruction (each consisting of 262 sections, n = 8 per group) of a vertebra used for calculation. Scale: 100μm (M-Q) Biomechanical analysis of femur from mice as in (C) showing ultimate stress (M), Young’s modulus (N), yield point (O), energy to fracture (P) and elastic energy (Q), all normalized to the body weight.
Data are shown as mean ± SD (n = 8 per group). Significance (P value) is calculated using Mann-Whitney t-test: *P < 0.05; **P < 0.01; ***P < 0.001. Sham RT mice (as shown in Figure 1) are shadowed in grey.
To directly test if warmer temperatures may exert protective effect on bone loss, we surgically ovariectomized 16 week-old mice, which is the most commonly used model for primary osteoporosis, and exposed them to 34°C or RT for 8 weeks (Ova34°C or OvaRT, respectively), using sham-operated mice as controls (Sham34°C, or ShamRT). Warmth exposure lowered the food intake as expected, and this was unaffected by the oophorectomy (Figure S2K). Strikingly, warmth exposure prevented the trabecular BV/TV loss caused by ovariectomy (Figure 2C), as assessed by computed-tomography (CT) and normalized to body weight (Figure S2L). This was consistent with the increase in the trabeculae number, the trabecular thickness, and the connectivity density (Figures 2D-H) in the Ova34°C mice, compared to the OvaRT controls. No differences were detected in the trabecular spacing and femur length (Figures 2G and S2M). The ovariectomy–induced decrease in the cortical bone volume and width of tibias was prevented in the Ova34°C mice to similar levels as the ShamRT controls (Figure 2I-K). This phenomenon was not restricted to long bones as we found that the decrease in the BV/TV of the caudal vertebra in the Ova34°C mice was ameliorated to the levels seen in the ShamRT controls (Figure 2L). These structural improvements were accompanied by a reduced fragility of the bones in the Ova34°C mice, as shown by the biomechanical measurements during the three-point bending test (Figures 2M-Q), as they showed similar reads for all the mechanical properties as the ShamRT controls, except for the elastic energy.
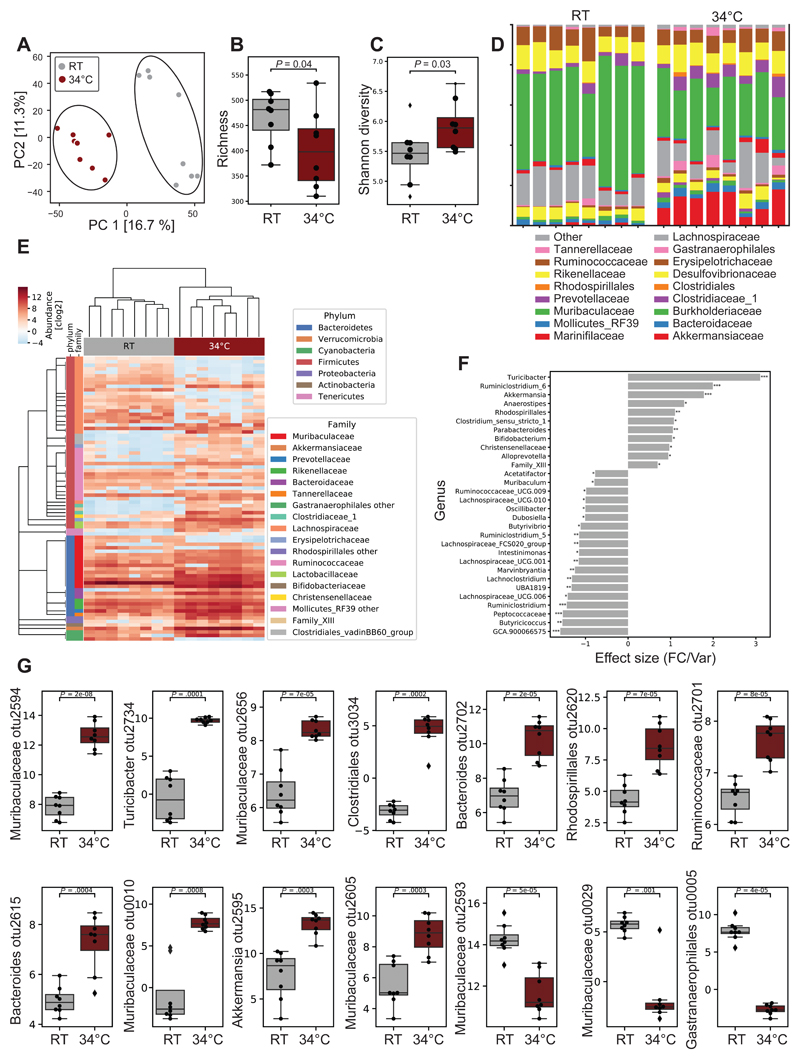
Warmth Exposure Alters the Microbiota Composition
Recent evidence suggest an interaction between the gut microbiota and bone metabolism (Li, 2019; Ohlsson, 2018; Hsu, 2018). To investigate whether warmth exposure can alter the microbiota composition, we performed 16S ribosomal DNA analysis of microbiota in cecum and feces from 24 week-old female mice that have been exposed to 34°C for 8 weeks. Among the 892 identified operational taxonomic units (OTUs), 81 were differently abundant (P ≤ 0.05) in warmth- versus RT-treated animals. Principal component analysis (PCA) of all the microbiomes showed segregation between the two groups (Figure 3A). Despite the reduced richness of the gut flora after warmth exposure, the Shannon diversity was higher (Figures 3B and 3C), indicating a more even distribution in abundance of the bacterial species after warmth exposure. This observation was further supported by the family relative abundance (Figure 3D), where the predominance of the Muribaculaceae family was dampened in the warm-adapted microbiota. The hierarchical clustering of the samples associated with a heatmap confirmed the clustering of the microbiota following warmth exposure, as well as the broad change in the microbial composition (Figure 3E). At a genus level, we observed a warm microbiota signature associated with an increase of the genera Turicibacter, Ruminiclostridium_6, Akkermansia, Rhodospirillales, Clostridium_sensus_stricto_1 and Parabacteroides; and a reduction of GCA.900066575, Butyricicoccus, Peptococcaceae, or Ruminiclostridium (Figures 3F and S3A). Curiously, despite the reduction of Muribaculum at the genus level, several of its OTUs were among the most elevated following warmth exposure, pointing to an extreme variability of the growth behavior within this genus (Figures 3G and S3B). Akkermansia muciniphila showed a strong increase in abundance after warmth exposure. Interestingly, this same species was strongly suppressed after cold exposure (Chevalier, 2015; Zietak, 2016), suggesting that Akkermansia muciniphila is consistently affected by the environmental temperature.
Figure 3. Warmth Exposure Changes the Gut Microbiota Composition.
(A) Principal component analysis (PCA) of 16S rDNA sequencing of fecal microbiota from 24 weeks old female mice exposed for 2 months to 34°C, or kept at RT. Each dot represents a fecal microbiota from one mouse. The analysis is based on the centric log2 ratio (CLR).
(B and C) Estimated richness (B) and Shannon diversity (C) of microbiota samples as in (A).
(D) Bar chart of the relative microbiome abundance at family level from mice as in (A).
(E) Hierarchical clustering associated with a heatmap comparing the CLRs of the OTUs selected for a P < 0.05 of mice as in (A). An idealized tree represents their taxonomic hierarchy down to genus level associated with bars that are color-coded for phylum and family. Each column represents one mouse.
(F) Effect size of all significantly changed genera calculated with aldex2 (FDR<0.05) in samples from mice as in (A).
(G) CLRs representing relative abundance of the most changed OTUs (FDR<0.01) by warm exposure in samples from mice as in (A). Boxplots represent median and quantiles; the whiskers show 1.5 inter quartile range and values outside the whisker’s box are represented as diamonds. Data are shown as mean ± SD (n = 8 per group). Significance in (F) and (G) is calculated using Welch t-test: *P < 0.05; **P < 0.01; ***P < 0.001.
To confirm the effect of the warm housing temperature in shaping the microbiota, we performed similar analysis in mice of different age and sex. Similar to the effect in the older females, 12 week-old male mice that were exposed to 34°C for 4 weeks showed altered microbiota composition as shown by PCA (Figure S3C), but without showing differences in the Shannon diversity or Richness (Figure S3D). The family abundance bar chart analysis revealed similar changes between the older females and the younger males despite the difference in the treatment length, with overall increase in the Akkermansiaceae and reduction of the Muribaculaceae family (Figure S3E). Alike the most altered OTUs in the older females, Muribaculaceae OTU2594, Clostridium_stricto_sensus_1_OTU2703 and Lactobacillus_otu2644 were more abundant (Figure S3F) in the microbiota of the warmth-exposed male mice compared to that of the RT controls. While the estrogen depletion caused changes in gut microbiota population (Figure S3G-H) (Markle, 2013; Cox-York, 2015; Kaliannan, 2018), the effects of warmth exposure on the microbiota composition were maintained after ovariectomy, with an increase in the Akkermansiaceae and reduction in the Muribaculaceae family. To further investigate the particular signature of the warmth exposure in changing the microbiota, we directly compared the warm-induced changes in the above three conditions: older female, young male, and ovariectomized mice. By plotting the PCAs, we observed a consistent shift in the microbiota composition selected by the PC2 (Figure S3H). Additional comparison of the 3 groups selected for consistent changes and a p-value<0.05, further supported these observations, and provided a signature of the warmth exposure on the microbiota composition with increase of Muribaculaceae otu2594, Muribaculaceae otu2618, Lactobacillus otu2644, Clotridium_stricto_sensu_1_otu2703 and Lachnospiraceae OTU2806 (Figure S3I). Accordingly, warmth exposure leads to robust and consistent changes in the gut microbiota composition that is independent of age, sex or hormonal status.
Transplantation of Warm–adapted Microbiota Prevents Bone Loss
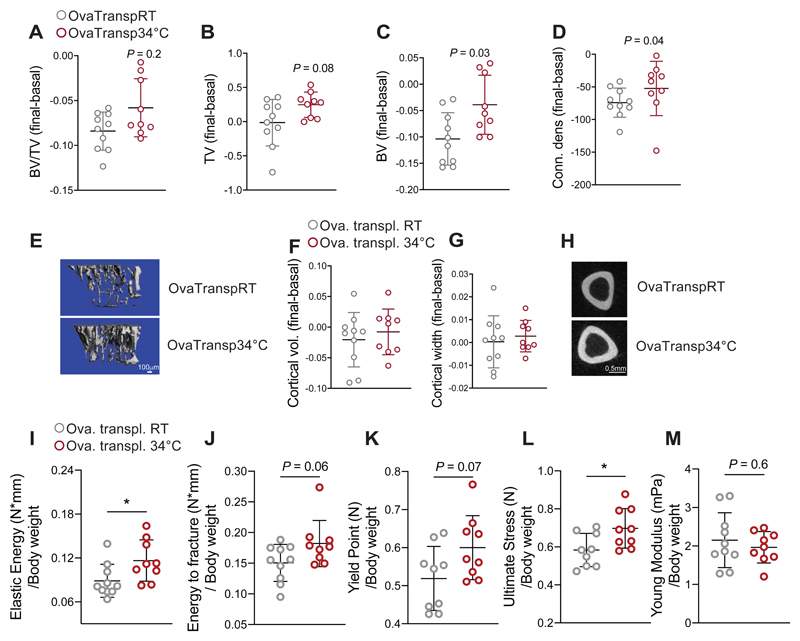
To uncover whether the microbiota impacts the bone parameters during warmth exposure, we first eliminated the microbiota using antibiotics. Microbiota depletion abolished the warmth-induced increase in femur strength in 23 week-old female mice (Figures S3J-N), and limited the warmth-mediated increase in trabecular bone volume and connectivity density of the tibia (Figure S3O-S). Similarly, warmth exposure did not alter the cortical bone in the microbiota-depleted mice (Figures S3T-V). To directly test the importance of the warm-adapted microbiota in a pathological context, we used mice that were ovariectomized at 16 weeks of age, and recurrently transplanted with fecal microbiota of either warmth-exposed or RT-kept mice (OvaTransp34°C or OvaTranspRT, Figure S4A). Both microbiota transplanted groups of mice were maintained at RT to isolate the microbiota effect. The PCA of the transplanted mice suggested microbiota similarities to the respective donors (Figure S4B). These observations were supported by the conserved microbial signature described earlier, including changes in the Lactobacillus otu2644 and Muribaculaceae otu2596, which were maintained in the transplanted groups (Figure S4C).
The recurrent microbiota transplantation did not affect the body weight gain, nor did it change the food intake (Figures S4D-E). However, tibia measurements before and after the transplantation showed higher bone volume (BV) in the OvaTransp34°C compared to the OvaTranspRT mice (Figures 4A-C and 4E). The increased tibia bone volume was associated with greater connectivity density delta before vs. after microbiota transplantation (Figure 4D) in the OvaTransp34°C compared to the delta in the OvaTranspRT without affecting the trabecular thickness, trabeculae space and number (Figure S4F), and the cortical bone volume and width (Figures 4F-H). The bone strength was improved at different levels during the three-point bending test on the femurs (Figures 4I-M), collectively indicating that the protective effect observed during warmth exposure on the bone loss outcome is in part phenocopied by the warm microbiota transplantation.
Figure 4. Warm–Microbiota Transplantation Prevents Bone Loss and Improves Bone Strength.
(A-E) Delta between two Micro-CT measurements (day 0 and day 32 after starting the microbiota transplantation) of proximal tibias at trabecular level in 21 weeks old ovariectomized, microbiota recipient female mice. The recipient mice were ovariectomized at week 16, and repetitivelly transplanted with fecal microbiota from 34°C exposed, or RT-kept donors (OvaTransp34°C, or OvaTranspRT, respectively). The 34°C treatment of the 16 weeks old female donor mice was initiated one month before the starting the transplantations, and lasted for the whole length of the experiment. Bone volume/tissue volume (BV/TV) (A), bone volume (BV), (B) total volume (TV) (C), and connectivity density (D). (E) Representative reconstruction (each consisting of 262 sections, n = 10 per group) of trabecular bone use for the calculations. Scale: 100μm.
(F-H) Cortical bone volume (F) and width (G) measured in midshaft of tibias from mice as in (A). (H) representative cortical section (from 62 sections per bone of each mouse of n = 10 per group), scale: 0.5mm.
(I-M) Biomechanical analysis of tibias from mice as in (A) showing elastic energy (I), energy to fracture (J), yield point (K), ultimate stress (L) and Young’s modulus (M), all normalized to their body weights. Data are shown as mean ±SD (n = 10 per group). Significance is calculated using Mann-Whitney t-test *P < 0.05; **P < 0.01; ***P < 0.001.
We also investigated whether microbiota transplantation could have an effect in nonpathological conditions, using male germ-free (GF) mice transplanted with microbiota from male donors that were exposed to warmth for 4 weeks, or kept at RT. The PCA of the microbiota from the transplanted GF mice revealed differences between the groups (Figures S4G and S4H and not shown). Fecal warm microbiota transplantation of the young male GF mice led to higher cortical bone volume and width (Figures S4I and S4J), despite these measurements being done only 20 days after the transplantation, without affecting the femur length (Figure S4L). Warm microbiota transplantation in the GF mice led to a slight improvement in the bone strength parameters compared to the RT microbiota transplanted controls (Figure S4K). These data show that warm-adapted microbiota transplantation improves bone strength and physiology, with the effect being more pronounced in pathological conditions.
Warmth Exposure and Warm–microbiota Ameliorate the Ovariectomy–induced Transcriptional Bone Remodeling
To gain insights into the magnitude and mechanisms of the bone remodeling, we performed RNA sequencing on tibias from ovariectomized and sham-operated mice exposed to 34°C and their RT controls, as well as from the ovariectomized microbiota-transplanted mice. Ovariectomy induced severe transcriptional alterations, which were markedly reduced when the ovariectomized mice were kept at 34°C (Figures 5A-B). Specifically, to dissect the effect of warmth on the ovariectomy–induced transcriptional alterations, we selected the deregulated genes (|log2FC| > 1 in OvaRT versus ShamRT, and logCPM>0), and compared them to the changes in Ova34°C versus ShamRT. Strikingly, warmth exposure reduced the ovariectomy–induced transcriptomics changes in 90.5% of the genes partially or completely (93.2% from the upregulated, and 86.4% from the downregulated) (Figure 5C), demonstrating that warmth exposure exerts a major protective effect on the transcriptional bone remodeling induced by estrogen deficiency. Reactome pathway analysis between ovariectomized mice at RT or at 34°C indicated differences in the collagen biosynthesis and degradation, associated with extracellular matrix reorganization, suggesting that warmth exposure leads to alterations in the bone remodeling pathways at the transcriptional level (Figure 5D). Comparative analysis suggested overlap between the reactomes of ovariectomized and control non-ovariectomized mice kept at 34°C when compared to their respective RT controls (Figures 5I, S5A-C). Specifically, out of 21 deregulated pathways between ShamRT vs 34°C, 20 pathways (95%) were found altered by warmth exposure in the ovariectomized mice (OvaRT vs. 34°C).
Figure 5. Warmth and Warm–Microbiota Transplantation Ameliorate Ovariectomy–Induced Transcriptional Deregulation.
(A) Mean-difference plot (MD-plot) of the log fold change gene expression between tibias of 24 weeks old female mice that were ovariectomized or sham-operated at 16 weeks of age, and then kept at RT for two months (OvaRT vs ShamRT, respectively) shown as average count per million (CPM). Red dots show the increased and blue show the decreased genes selected for FDR<0.05.
(B) MD-plot of the log fold change of gene expression between tibias of 24 weeks old female mice that were ovariectomized at 16 weeks of age and then kept at 34°C for two months (Ova34), versus ShamRT, shown as average count per million (CPM). Red dots show increased and blue show decreased genes selected for FDR<0.05.
(C) Comparison between the log fold change of genes deregulated by ovariectomy at RT shown in red (|log2FC| > 1; OVART vs ShamRT) and the same genes when exposing the ovariectomized-mice at 34°C shown in blue (|log2FC| > 1; Ova34°C vs ShamRT).
(D) Top 10 most deregulated Reactome pathways between tibias of Ova RT and Ova34°C mice.
(E) Volcano-plot comparing the p-value and the log fold change of gene expression between tibias of 21 weeks old ovariectomized, microbiota recipient female mice (OvaTransp34°C, or OvaTranspRT) as in Figure 4A-E. Green dots: (|log2FC| > 1; blue dots: P < 0.01; red dots: P <0.01 and (|log2FC| > 1.
(F and G) Expression analysis of the ovariectomy-altered genes ((|log2FC| > 1) at RT (OvaRT vs shamRT)) in tibia from OvaTransp34°C mice compared to the OvaTranspRT controls (OvaTransp34°C vs. OvaTranspRT). In (F) blue and red show genes (up- or downregulated, respectively) unaltered by microbiota transplantation. Green show genes with reduced or reverted expression when mice are transplanted with warm-microbiota. (G) Comparison between the log fold changes of genes (|log2FC| > 1) as in (C) using the groups of mice from
(F): OvaRT vs ShamRT (red) and OvaTransp34°C vs. OvaTranspRT (blue).
(H) Top 10 most deregulated reactome pathways between tibias from OvaTransp34°C and OvaTranspRT mice.
(I) Top 10 most deregulated reactome pathways between tibias from 34°C and RT mice.
Transplantation of the ovariectomized mice with the warm–adapted microbiota (OvaTransp34°C) also induced transcriptional alterations (FDR<0.01 and (|log2FC| > 1) in bone when compared to the RT–microbiota transplanted controls (OvaTranspRT) (Figure 5E). To investigate if the warm–adapted microbiota could exert similar effect as the warm exposure on mitigating the ovariectomy–induced transcriptional deregulation, we specifically analyzed the altered genes (shown in Figure 5C) in the OvaTransp34°C mice compared to the OvaTranspRT controls. Interestingly, the ovariectomy–induced transcriptional changes were reduced or reverted in 59% of the total deregulated, and in 94% of the reduced genes (shown in green in Figure 5F), when the ovariectomized mice were transplanted with warm–microbiota, similar to the direct effect of warmth exposure (Figures 5F-G). In the top 10 deregulated Reactome pathways of the microbiota–transplanted ovariectomized mice, we observed a similar pattern to the warmth exposure-induced changes (Figure 5H). These data indicate that warmth exposure and warm–microbiota transplantation suppress the ovariectomy–induced transcriptional alterations.
Warmth and Warm-Microbiota Enhance Periosteal Bone Formation
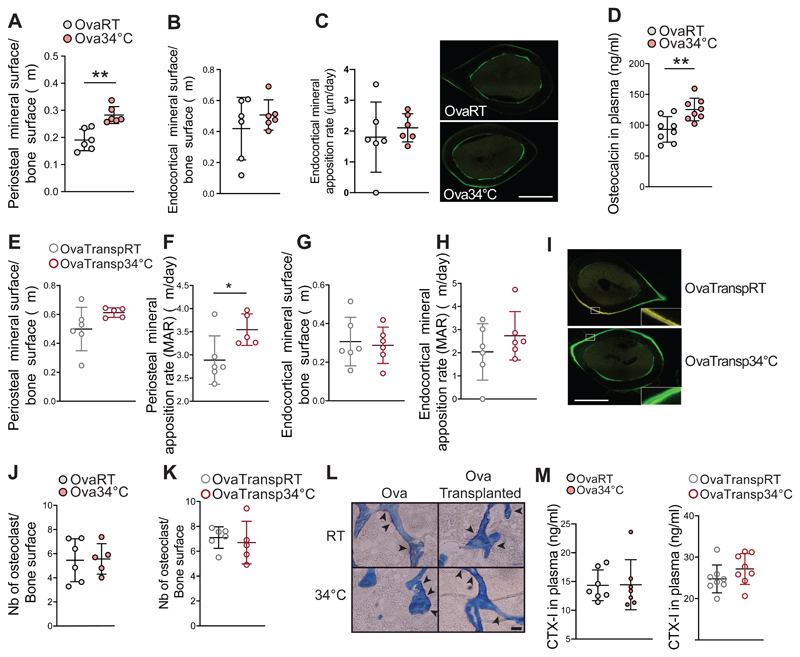
To gain further insights into the mechanisms by which warmth exposure increases bone strength, we investigated the number and activity of the cells responsible for the bone remodeling. During homeostasis, the activity of osteoblast and osteoclasts is at equilibrium. In the context of osteoporosis, the overall bone remodeling is increased, where the activity of the osteoclasts is superior to the one of the osteoblasts, leading to bone loss. We quantified the osteoblast activity by measuring the fluorescent calcein deposits within the bone during its formation between 2 injections, 7 days apart. The cortical bone in the midshaft of the femur showed a specific increase of the periosteal mineralized surface in the ovariectomized mice exposed to warmth compared to the RT-housed controls, without the endocortical surface and the trabecular bone mineralization (measured in the head of the femur) being affected (Figures 6A-C and S5D). The increased osteoblast activity was confirmed by the higher levels of circulating osteocalcin in the warmth-exposed ovariectomized mice, but not in the shamoperated controls (Figures 6D, S5E). Consistent with warmth exposure, the specific increase in the periosteal mineral apposition rate (MAR) was observed in the ovariectomized-mice receiving the warm–adapted microbiota (Figures 6E-I and S5F), suggesting that similar mechanisms mediate the effects of warmth exposure and warm-microbiota transplantation. The active osteoclast number was then quantified using Tartrate-resistant acid phosphatase (TRAP) staining on femur trabecular histological sections. No differences were observed after warmth exposure, or warm-microbiota transplantation in any of the groups (Figure 6J-L and not shown). This was supported by the circulating CTX-1 levels as marker of bone resorption, which was neither changed by warmth in both ovariectomized and non-ovariectomized mice, nor in the warm microbiota-transplanted animals (Figure 6M and Figure S5G). Notably, the bone remodeling effects were independent of the collagen deposition, bone mineral content and circulating vitamin D levels, as we did not detect differences for these parameters between any of the groups (Figures S5H-K and not shown). These data indicate that warmth exposure or warm-microbiota transplantation shift the balance between osteoblast activity and the number of active osteoclasts towards bone formation.
Figure 6. Warmth and Warm–Microbiota Transplantation Increase Periosteal Bone Formation.
(A-C) Periosteal (A) and endocortical (B) mineralized surface after calcein injection in 24 weeks old female mice that were ovariectomized at 16 weeks of age and exposed for 2 months to 34°C, or kept at RT. (C) Representative images (of n = 6) of fluorescent calcein in femur midshaft used for the quantifications.
(D) Osteocalcin levels in plasma of mice as in (A).
(E-I) Periosteal mineralized surface (E), periosteal mineral apposition rate (MAR) (F), endocortical mineralized surface (G), and endocortical MAR (H) in tibias of 21 weeks old ovariectomized, microbiota recipient female mice (OvaTransp34°C, or OvaTranspRT) as in Figure 4A-E (I) Representative images (of n = 6) of fluorescent calcein in femur midshaft used for the quantifications. Scale: 0.5mm.
(J-L) Tartrate-resistant acid phosphatase (TRAP) staining quantification of osteoclast number in femur trabeculae of mice as in (A) shown in panel (J), and of mice as in (E) shown in panel (K). (L) Representative images (of n = 6) of the quantifications shown in (J) and (K). Arrowheads correspond to the TRAP signal. Scale: 50μm.
(M) CTX-1 levels in plasma of mice as in (J) and (K).
Data are shown as mean ± SD (n = 6 per group). Significance (p-value) is calculated using Mann-Whitney t-test: *P < 0.05; **P < 0.01; ***P < 0.001.
Warmth Exposure Increases Production of Polyamines that Affect Osteoblast Activity and Decrease Osteoclasts Differentiation
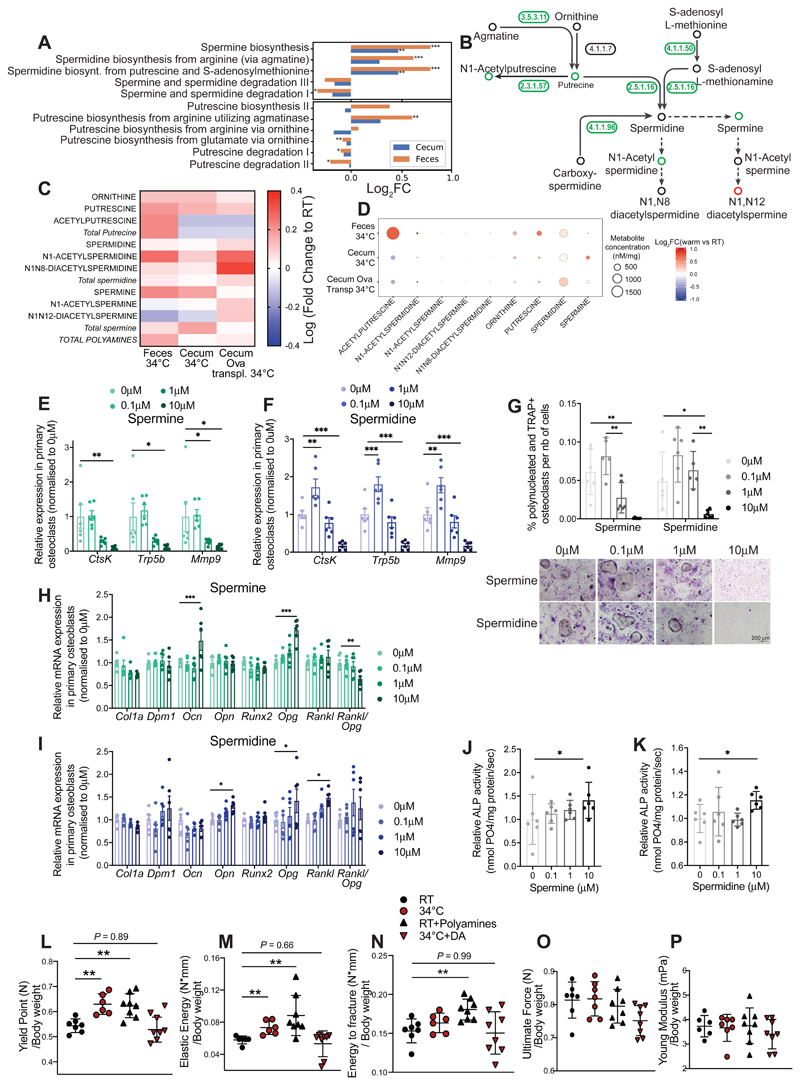
To investigate the link between microbiota and bone during warmth, we performed a metagenomic analysis of the gut microbiota from 24 week-old female mice exposed for 8 weeks to 34°C, and from the RT-housed controls. Among 536 identified pathways, 134 were differentially abundant in the feces of warmth-exposed animals compared to RT controls. Within the top-ten regulated pathways was the polyamine synthesis showing higher levels for the key genes responsible for spermine and spermidine production, and lower levels for those involved in the polyamine degradation processes (Figures 7A-B and S6A). Deeper analysis of the metagenomic data by genus suggested that expansion of Akkermansia muciniphila during warmth exposure, as well as the genera Bacteroides and Alitsipes, may be the main contributors to the polyamine biosynthesis (Figure S6B). Conversely, the decreased propensity of the microbiota to degrade spermine and spermidine could be attributed to the decline of the bacteria from the Muribaculaceae or Lachnospirae genera (Figures S6C). The polyamines have fast plasma turnover and rapidly reach their target tissues (Pegg, 2009). To directly test whether the metagenomic data would correlate with an actual increase in the respective polyamine metabolites, we developed an isotope dilution–based, hydrophilic interaction chromatography coupled to targeted tandem mass spectrometry (HILIC - MS/MS) method for absolute quantification of the polyamine metabolites. In agreement with the metagenomics data, the concentrations of putrescine, N1-acetylputrecine, N1-acetylspermidine and spermine were higher in feces of the warmth-exposed animals (Figures 7C-D and S6D). This was coupled to higher spermine levels in the cecum of warmth–exposed mice, or elevated N1-acetylspermidine and N1N12-acetylspermidine in the warm microbiota-transplanted mice (Figures 7C, S6E-F).
Figure 7. Microbial Production of Polyamines Mediate the Warmth Effects on the Bone.
(A) Bar chart representing metagenomics analysis of the bacterial polyamine biosynthetic and degradation pathways in feces and cecum samples from 24 weeks old female mice that were exposed to 34°C for 2 months, or kept at RT. Significance shows false discovery rate (FDR): *P < 0.05; **P < 0.01; ***P < 0.001.
(B) Graphical representation of the polyamine biosynthetic pathway where numbers (enzymes from keg nomenclature) represent the level of respective genes present in the gut microbiota from fecal samples of mice as in (A). Green–coloured circles show increase, red–coloured show decrease, and black–coloured indicate unchanged concentrations after warm exposure. 3.5.3.11: agmatinase, 4.1.1.7: benzoylformate decarboxylase, 2.3.1.57: putrescine acetyltransferase / spermine-spermidine N1-acetyltransferase, 4.1.1.50: adenosylmethionine decarboxylase, 2.5.1.16: spermidine synthase, 4.1.1.96: carboxynorspermidine decarboxylase.
(C and D) Heatmap (C), or heatmap associated with absolute polyamine levels (D), showing fold change of polyamines measured using hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry (HILIC - MS/MS) in feces or cecum samples from 24 weeks old female mice that were exposed to 34°C for 2 months versus RT controls (34°C vs. RT; Feces 34°C and Cecum 34°C); or cecum of 21 weeks old ovariectomized, microbiota recipient female mice (Cecum Ova transpl34°C).
(E and F) Relative mRNA expression levels in cultured primary osteoclasts subjected to different spermine (E) or spermidine (F) concentrations, measured by qPCR.
(G) Quantification of the polynucleated and TRAP+ differentiated osteoclasts normalized to the total number of cells in presence of spermine or spermidine. (Below) Representative images (from 6 wells per condition) from TRAP staining of osteoclasts differentiated in presence of spermine or spermidine. Scale: 200 μm.
(H and I) Relative mRNA expression levels in cultured primary osteoblasts subjected to different spermine (H) or spermidine (I) concentrations, measured by qPCR.
(J and K) Relative alkaline phosphatase (ALP) activity in osteoblast culture after spermine (J) or spermidine (K) supplementation at different concentrations.
Significance (p-value) in all panels except (A and L-P) is calculated using Mann-Whitney t-test: *P < 0.05; **P < 0.01; ***P < 0.001.
(L-P) Biomechanical analysis using 3–point bending test of femur from 23 weeks old female mice that were either RT kept (RT); warm exposed (34°C); supplemented with freshly prepared polyamine mix and RT kept (RT-Polyamines); or provided with 50μm Diaminazene Acetureate (DA) and kept at 34°C (34°C-DA), starting at 16 weeks of age until sacrifice. Polyamines and DA were supplemented in drinking water every second day. The panels show yield point (L), elastic energy (M), energy to fracture (N), ultimate force (O), and Young’s modulus (O) that are normalized to their bodyweight values at sacrifice.
Data are shown as mean ±SD (n = 8 per group). Significance is calculated based on One-Way ANOVA: *P < 0.05; **P < 0.01; ***P < 0.001.
To test the effect of polyamines on bone cellular activity, we performed an ex vivo experiment with spermine and spermidine supplementation in primary osteoblast or osteoclast cultures during differentiation. Since it is difficult to correlate the in vivo levels with those in vitro, we used different concentrations in the primary cultures, and assessed the outcome in a dose-response manner. Supplementation of spermine and spermidine at high (but not low) doses in osteoclasts during differentiation caused downregulation of cathepsin K (CtsK), Tartrate resistant acid phosphatase 5 (Trap5b) and Matrix metalloprotease 9 (Mmp9), which are markers of osteoclast activity and differentiation (Figures 7E-F and S6G). The decrease in expression of the osteoclast differentiation markers was associated with a reduced number of differentiated osteoclasts measured by TRAP staining (Figures 7G and S6H). These findings are in agreement with the studies in mice, were oral supplementation of spermine and spermidine in an ovariectomy-induced model of osteoporosis was sufficient to prevent bone loss, measured by %BV/TV in vertebra (Yamamoto, 2012). The anti-osteoclast effects of spermidine, but not spermine, were observed even when these polyamines were added after differentiation, where 24h of spermidine supplementation was sufficient to reduce the expression level of Ctsk and Trap5b (Figures S6I and S6J). Spermine increased osteoblast expression of osteocalcin (Ocn) and osteoprotegerine (Opg) in a dose–dependent manner (Figure 7H). Similarly, elevated spermidine levels augmented osteopontine (Opn), osteoprotegerin (Opg) and receptor activator of nuclear factor kappa-B ligand (Rankl) expression, indicating an increased osteoblast function (Figure 7I), coupled to a decrease in the Rankl/Opg ratio that could explain the decreased osteoclastogenesis. These observations were supported by an enhanced activity of alkaline phosphatase (ALP) when spermine and spermidine were added to the osteoblasts (Figures 7J-K). These data support the observations regarding the osteoblast function in vivo during warmth exposure and warm-microbiota transplantation, and they demonstrate that polyamines could mediate the enhanced osteoblast activity. This increase was observed despite the slight reduction of the cell viability (lower total protein and RNA) upon spermine supplementation (Figures S6K-L), which may result from interaction of spermine with serum from the media, producing H2O2 radicals by oxidative degradation of the polyamines (Wang, 2018).
Finally, we directly tested the necessity of the polyamine biosynthesis in mediating the warmth-induced effects on the bone in vivo. Polyamine supplementation in older female mice increased the yield point, elastic energy and energy to fracture of the femur to similar values as the warmth-exposed mice (Figures 7L-P). Conversely, we used a polyamine biosynthesis pathway inhibitor, diaminazene acetureate (DA), which prevents formation of decarboxy-S-adenosinemethionine, a metabolite that turns into spermine and spermidine (Karvonen, Kauppinen, Partanen, & Poso, 1985). DA also blocks spermidine and spermine acetyltransferase activity (Libby & Porter, 1992; Neidhart, Karouzakis, Jüngel, Gay, & Gay, 2014), thus inhibiting the back conversion of spermine and spermidine towards putrescine, leading to reduced acetylspermine and acetylspermidine formation. Treating warmth-exposed older female mice with the polyamine inhibitor abolished the warmth-induced increase in the yield point and elastic energy during the three-point binding test in femur, revealing similar biomechanical parameters between the warmth-exposed, DA-treated mice and the RT-housed controls (Figures 7L-P). Moreover, the warmth-induced increase in the trabecular bone volume and connectivity density of tibia were reduced in the polyamine inhibitor-treated mice despite the warmth exposure, and there were no differences between RT and warmth-exposed, inhibitor-treated mice in any of the trabecular and cortical parameters (Figures S7A-F).
Discussion
Over a century ago, Joel Asaph Allen proposed an ecogeographical model of adaptation to temperature differences for the homeothermic animals, in which the body surface area-to-volume ratio varies with the average temperature of the habitat, where higher temperatures would favor the higher ratios and heat dissipation, called the Allen’s rule (Allen, 1877). In growing long bones, a cartilaginous disk called the growth plate separates the epiphysis from the metaphysis and diaphysis. A new cartilage is produced at the epiphyseal side of the growth plate, while the previously made cartilage at the metaphyseal side is replaced by new bone leading to bone elongation. With the onset of puberty in humans, the deposition of cartilage ceases and the metaphysis fuses with the epiphysis, leading to ceased longitudinal appositional and disappearance of the growth plate (Jilka, 2013; Pines, 1991). During adulthood, bones undergo permanent remodeling that enables adaptation of the skeleton to the environment and in response to the continuous microfractures. This remodeling is kept in balance by precise coupling between the osteoclast and osteoblast activities. During aging, this exquisite balance is often disrupted, progressively leading to osteopathy. Our data show that the bone remodeling is largely affected by the environmental temperature, and that warmth exposure leads to improved trabecular and cortical bone structure and strength. Interestingly, in mice while warmth exposure during development can indeed lead to lengthening of the bones (Racine, 2018; Serrat, 2013; Serrat, 2014; Serrat, 2008; Serrat, 2015), in our experiments applying the heat post-developmentally and during middle adulthood did not change the bone length (and thus surface area-to-volume ratio), but still increased the bone strength and mass. Accordingly, transplantation of the warm-adapted microbiota in older female, and younger male mice increased the bone strength and density, but did not change the bone length. These data may therefore imply an extension to Allen’s rule, suggesting elongation-independent effects of warmth exposure, which predominantly favors increased bone density and strength through microbiota alterations.
The frequency of major osteoporotic fractures varies substantially depending on the country, being highest in Scandinavia and lowest in Africa. While this may partly reflect ethnic influences, it is also observed in Europe where the hip fracture rate in northern Europe is 11 times higher than in the Mediterranean area (Cheng, 2011; Cauley, 2014; Johnell, 1992; Eastell, 2016). There could be several explanations regarding the origin of these geographical differences, and it’s been proposed that levels of vitamin D, calcium consumption, as well as diet and genetics may be causal factors (Prentice, 2004; Yeum, 2016; Zengin, 2015; Prentice, 2004). Without excluding any of the above mentioned causes, our human metadata analysis supports a geographical gradient, and shows that the osteoporosis–related hip fractures inversely correlate with the average temperatures, and positively with latitude independently of vitamin D and calcium consumption, suggesting that the results we observe in mice could be translated to humans.
Our work shows that more than 90% of the ovariectomy-induced transcriptional changes in the bone are dampened by exposure to warmth, and 59% by transplanting the ovariectomized mice with warm-adapted microbiota. These results suggest a major protective effect of these treatments on the overall bone alterations that underlie bone loss. The findings that the warmth exposure affects the polyamine biosynthetic pathway in the microbiota suggest a possible link between these changes and the effects on bone mass and strength, and presumably other tissues that are affected by the polyamine levels. Aging is associated with decline in the polyamine levels (Scalabrino, 1984; Pucciarelli, 2012), and polyamine supplementation protects against several age-related diseases (Ramos-Molina, 2019; Tofalo, 2019), including memory impairment (Gupta, 2013; Fruhauf, 2015), cardiovascular disease (Eisenberg, 2016), cancer (Yue, 2017), while extending the lifespan of various organisms (Soda, 2009; Kiechl, 2018). Warmth-induced microbiota-mediated increases in polyamine biosynthesis may therefore be of general physiological importance that could extend well beyond bone–related research, impacting several age–related diseases and prolonging healthspan.
Limitations of Study
Our study does not address the upstream mechanisms by which warmth exposure alters the microbiota composition. These changes are unlikely due to a direct temperature effect as the internal body temperature of the mice was unaffected when we exposed them to 34°C. It is likely that the decreased food intake and movement during warmth exposure will jointly impact the microbiota composition to a certain extent; however, follow up work is needed to clarify the initial triggers and pathways by which the gut microbiota responds to the increased environmental temperature. Similarly, while the study suggests a critical direct or indirect contribution of the polyamines and the gut microbiota, it does not exclude that there could be additional microbiota-related, or -unrelated, mechanisms by which warmth exposure increases bone strength.
Star Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Mirko Trajkovski (Mirko.Trajkovski@unige.ch)
Materials Availability
All materials used in this study are either commercially available or obtained through collaboration, as indicated.
Data and Code Availability
All sequencing data generated in this study is deposited at the Sequence Read Archive (SRA) of the National Center for Biotechnology Information. The 16S rDNA sequencing of the female and the male mice have accessions PRJNA647833 and PRJNA648020, respectively. Shotgun metagenomics data is available under the id PRJNA647832 and the RNA seq data under PRJNA648022. The data for targeted metabolomics and the correlation analysis in human are deposited in the open access repository figshare with the doi: 10.6084/m9.figshare.12696419 and 10.6084/m9.figshare.12696407, respectively. The scripts used for the analysis of the 16S rDNA, metagenomc and human epidemiology data are available at github.com/SilasK/WarmMicrobiota.
Experimental Model and Subject Details
Mouse Models
All C57BL/6J mice were purchased from Janvier Labs and were kept in a specific pathogen-free facility (SPF) in individually ventilated cages, 2 mice per cage. All mice were in a 12h day/night cycle and fed a standard chow diet (SDS RN3). Upon arrival and before the start of the experiment, mice were allowed to acclimatize in the new environment for 1 week. Germ-free (GF) mice on C57BL/6J background were obtained from the GF clean animal facility of the University of Bern (in collaboration with A.M.) and transplanted with donor microbiota immediately upon arrival. At the start of the experiments, the male mice were at 8 weeks, and females were at 16 weeks of age. Animals were equally allocated into groups based on their body weight to ensure equal starting points, and otherwise randomly. Warmth exposure was done at 34°C in a light and humidity-controlled climatic chamber (TSE, Germany) in SPF conditions using individually ventilated cages. The 34°C-like pair-fed animals were kept at room temperature (RT) and fed an equal amount to the warm exposed animals. This was equivalent to ~25% less than RT ad libitum fed, and the food was provided each day at 6pm. No signs of stress or suffering we detected in any of the mice. All animal experiments were approved by the Swiss federal and Geneva cantonal authorities for animal experimentation (Office Vétérinaire Fédéral and Commission Cantonale pour les Expériences sur les animaux de Gèneve).
Primary Cell Culture
Three male C57Bl/6J mice of 3 weeks old were sacrificed and their tibias, femurs and humeri were collected. After epiphyses excisions, the bone marrow was flushed, collected in αMEM 10% FBS and pooled from each bone for osteoclasts differentiation. The rest of the bone was kept for the osteoclast isolation. For the osteoclasts, after filtration through a 70 μm filter, the cells were plated in T75 in αMEM 10% FBS + 10ng/ml MCFS. After incubation for 24h at 37°C and 5% CO2, the supernatant was collected and the cells were seeded as 200 000 cells/ml in αMEM + 10% FBS + 30ng/ml MCFS. After 48h, the differentiation was initiated by adding 100ng/ml RANKL to the medium as well as spermine or spermidine at the indicated concentration (0-0.1-1-10 μM). After 14 days, RNA was extracted and cells were fixed for 1h at RT in 3.7% formaldehyde for TRAP staining. Alternatively, cells were kept in differentiating medium for 14 days and spermine and spermidine were added for 24h before RNA extraction and TRAP staining. For the osteoblasts, the bones were cut in small pieces and incubated in αMEM 10% FBS + 1 mg/ml Collagenase II for 90°C at 37°C with shaking. The cells were rinsed and then incubated in aMEM10%FBS medium at 37°C with 5%CO2, and split a couple of times for proliferation before seeding them to 70 000cells/ml in differentiating medium (αMEM 10%FBS + 50μg/ml ascorbic acid + 10mM β-glycerophospate) supplemented with spermine or spermidine (at 0; 0.1; 1 and 10 μM). After 7 days of treatment, cells were harvested for RNA extraction and ALP measurements, and after 25 days of treatment for Sirius red or Alizarin red measures. The following chemical compounds were used: aMEM medium (bioconcept ref 1-23P10-M supplemented with 25mM NaHCO3, 100X Penicillin Streptomycin (Gibco Ref: 15140), 100x glutamine (Gibco Ref: 25030) and 3.75 ml amino-acids mix (BioConcept ref: 5-12K01-H)), recombinant murine M-CFS (peprotech ref 31502), recombinant murine sRANK Ligand (Peprotech ref 315-11C), Spermine (Sigma ref S4264), Spermidine (Sigma Ref: S0266).
Method Details
Ovariectomy
Mice were anesthetised with Xylazine/Ketamine (mixture of 100 mg/kg ketamine and 16 mg/kg xylazine) and shaved below the ribs on the back side. Betadine was applied on the area for appropriate disinfection. After a 1-2 cm incision through the skin and the muscle layer just below the ribs, the ovary was localized, the fallopian tube ligated with dissolving suture and the ovary removed. The muscle layer was sutured with dissolving suture, the wound closed with staples and disinfected. The same procedure was performed on the other side. A dose of Tamgesic was administered 4 hours after the surgery, and the staples were removed 7 days after the surgery under isoflurane anaesthesia. The sham-operated animals underwent the same procedure, without ligating the fallopian tube and the ovary excision.
Sample Collection at Sacrifice
To measure the dynamic indices of bone formation, mice received subcutaneous injections of calcein in saline solution at 9 and 2 days before euthanasia. 500 μl of blood was taken from terminally anesthetized mice in tubes with 10 μl of 0.5 mM EDTA, 4 μl of aprotinin (1.3%) and 4 μl of DPP-IV (10mM) and plasma stored at -80 °C. Samples for RNA isolation were stored in RNAlater solution (Invitrogen ref AM7020). Bone samples for the CT-scan analysis were stored in a humid package at -20°C, and the samples for histology in 3.8% formaldehyde. All other samples snap frozen in liquid nitrogen. The tail length was measured with a ruler from the tip of the tail to the border between the fur and the skin.
Microbiota Transplantation
Upon arrival, 8 weeks old male GF mice were handled in aseptic conditions and immediately colonized by gavaging them with cecal content of the appropriate donor. The donors were male C57Bl/6J mice that were exposed to 34°C, or kept at RT for 4 weeks starting at 8 weeks of age. 500 μl of freshly collected cecal contents from donors were pooled and suspended in 5 ml of anaerobic PBS, to make a gavage mixture for each group of colonized mice. Each mouse was orally gavaged with 100 ul of the solution upon arrival and 2 days later. Animals were kept for 7 days in dirty cages from the respective donors and then switched to sterilized cages.
For microbiota transplantation of the ovariectomized mice, we used female C67Bl/6J recipients with conventional microbiota already present, and the ovariectomy was done at 16 weeks of age. The donors for these experiments were female mice that were exposed to 34°C for 4 weeks starting at 16 weeks of age. Fresh fecal pellets from the donors were freshly collected every 2 days and immediately homogenized in 1 ml of anaerobic PBS. After a short centrifugation (300g, 30sec), the supernatant was then immediately gavaged to the respective recipient. In this condition, one cage of donors (1 pellet per mouse from both mice) was used to repopulate 1 cage of recipients. Each recipient received 200 μl of the donor mixture every 2 days during 4 weeks.
Antibiotic treatment
16-weeks old female mice were treated with fresh antibiotics and kept at either room temperature or 34°C for 7 weeks (RT-Abx and 34°C-Abx respectively). Antibiotics cocktail was composed of 100 μg/ml neomycin, 50 μg/ml streptomycin, 100 U/ml penicillin, 50 μg/ml vancomycin, 100 μg/ml metronidazole, 100 μg/ml CEFAZ, 125 μg/ml Ciprofloxine hydrocholoride, 1 mg/ml bacitracin provided in the drinking water changed twice per week (Chevalier, 2015; Suarez-Zamorano, 2015)
Polyamines supplementation and inhibitor treatment
6-weeks old C57BL/6J female mice were given a mixture of Spermine (Sigma-Aldrich) and Spermidine (Sigma-Aldrich) freshly dissolved in drinking water at concentration of 0,5mM from each compound every second day during additional 45 days at room temperature. Diaminazene Acetureate (Sigma-Aldrich) was supplemented in drinking water at a concentration of 50μM every second day during 45 days to the 16-weeks old C57BL/6J female mice that are kept at 34°C with temperature-controlled chamber in conventional facility. Food and water were given to the mice in ad libitum.
Micro-CT Analysis
The limbs were scanned in vivo before the ovariectomy to determine the basal state using a micro-CT (VivaCT40/Scanco system; Zurich, Switzerland). After Xylazine/Ketamine anaesthesia, limb were scanned for 18 min. Final scans were performed post mortem on isolated bones. Subsequent analysis was done using micro-CT software. For the femoral and tibial trabecular region, we analyzed one hundred slices starting from 50 slices below the distal growth plate. Femoral and tibial cortical structure was assessed through 60 continuous CT slides (600 μM) from the bone midshaft. Images were segmented using an adaptative-iterative threshold approach, rather than a fixed threshold. Morphometric variables were computed from binarized images using direct 3D technique that does not rely on prior assumptions about the underlying structure (5). For trabecular bone regions, we assessed the bone volume/total volume (BV/TV). For cortical bone at the femoral and tibial midshaft, we measured the cortical bone volume (mm3) and the average cortical thickness named cortical width (μM). The lengths of the femurs were also measured from the CT-scans.
Biomechanical Analysis of the Bone
For the 3-points bending test to address the biomechanical parameters, tibias were placed on two supports separated by a distance of 9.9 mm and load was applied to the midpoint of the shaft (creating a 3-points bending). Mechanical resistance to failure (displacement and load applied) was measured using a servo-controlled electromechanical system (Instron 1114, Instron corp., High Wycombe, UK) with actuator displaced at 2 mm/minute. Ultimate force (maximal load, measured in Newtons [N]), Yield point (N), stiffness (elastic energy, N/mm), and energy to fracture (surface under the curve of the plastic region, N*mm) were calculated. Young’s modulus (MPa) was determined by the previously described equation (Mcmillan, 1989).
Human Metadata Analysis
We correlated the age-standardized incidence of hip fracture (per 100’000 inhabitants) per country using the data obtained from (Cauley, 2014) with the country’s average day temperature (1961-1990, Climate Change Data, World Bank Group), or the distance from the equator (latitude) of their capitals. We accounted for the effect of calcium intake and Vitamin D serum levels (Wahl, 2012; Balk, 2017) using partial-correlation analysis. This dataset contains age-standardized incidence of hip fracture and latitude for 62 countries, temperature for 60 countries, Vitamin D serum level for 38 countries and the calcium levels for 49 countries. The regression was performed using statsmodels (Skipper, 2010). All code is available as specified in the Key Resource Table.
Key Resources Table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N/A | ||
| Bacterial and Virus Strains | ||
| N/A | ||
| Biological Samples | ||
| N/A | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Calcein | Sigma-Aldrich | C0875 |
| RNA-later | Invitrogen | AM7020 |
| Neomycin-streptomycin-penicillin | Sigma-Aldrich | P4083 |
| vancomycin | Sigma-Aldrich | V2002 |
| metronidazole | Sigma-Aldrich | M3761 |
| bacitracin | Sigma-Aldrich | 11702 |
| Ciprofloxacine HCL | Alkaloid | 2000314 |
| CEFAZ | Alkaloid | 1046371 |
| Spermine for in-vivo | Sigma-Aldrich | 85590 |
| Spermidine for in-vivo | Sigma-Aldrich | S2626 |
| Diaminazene Acetureate | Sigma-Aldrich: | D7770 |
| Trizol | Thermo Fisher Scientific | 15596018 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | 4368813 |
| Power-up SYBR Green | Applied Biosystems | A25742 |
| aMEM medium | bioconcept | 1-23P10-M |
| 100X Penicillin Streptomycin | Gibco | 15140 |
| 100x glutamine | Gibco | 25030 |
| amino-acids mix | BioConcept | 5-12K01-H |
| Recombinant murine M-CFS | Peprotech | 31502 |
| Recombinant murine sRANK Ligand | Peprotech | 315-11C |
| Spermine for cell culture | Sigma-Aldrich | S4264 |
| Spermidine for cell culture | Sigma-Aldrich | S0266 |
| Fast violet B salt | ChemCruz | sc-215029B |
| Sirius red dye reagent: Direct red 80 | Sigma-Aldrich | P744 |
| EDTA 0,5M ph=8.0, Molecular Biology Grade | Promega | V4231 |
| Aprotinin | PanReac AppliChem | A2132,0025 |
| KR-62436 hydrate (DPPIV) | Sigma-Aldrich | K4264 |
| Ketasol 100 | Graeub | Swissmedic 50’375 |
| Rompun 2% | Bayer | Swissmedic 35’464 |
| Temgesic Buprenorphinum | Reckitt Benckiser | Swissmedic 419310 |
| Critical Commercial Assays | ||
| PowerFecal DNA Kit | Qiagen | 12830-50 |
| 5Prime HotMaster mix | Quantabio | 2200400 |
| MiSeq reagent kit V2 | Illumina | MS-102-2003 |
| TruSeq Nano DNA Library Prep Kit | Illumina | 20015964 |
| TruSeq RNA Library Prep Kit v2 | Illumina | RS-122-2001 |
| Calcitriol (INN) Elisa kit | Abbexa | abx 513030 |
| Osteocalcin Elisa Kit | Immutopics (quidel) | 60-1305 |
| RatLaps CTX-I EIA | Immunodiagnostic Systems | AC-06F1 |
| Pierce BCA Protein assay kit | Thermo Scientific | 23225 |
| Deposited Data | ||
| Raw shotgun-metagenome data of female mice | This study | PRJNA647832 |
| Raw amplicon data of male mice | This study | PRJNA648020 |
| Raw amplicon data of female mice | This study | PRJNA647833 |
| Raw RNA seq data | This study | PRJNA648022 |
| SILVA database v132 | (Quast et al., 2013) | |
| Reactome pathways database | (Fabregat et al., 2017) | |
| Resources for the metagenomic analysis | This study | github.com/SilasK/WarmMicrobiota |
| Resources for the 16SrDNA analysis | This study | github.com/SilasK/WarmMicrobiota |
| Resources for the human correlation analysis | This study | 10.6084/m9.figshare.12696407 |
| Targeted Metabolomic data | This study | 10.6084/m9.figshare.12696419 |
| Experimental Models: Cell Lines | ||
| N/A | ||
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Janvier Labs | SC-C57J-M |
| Germ-free mice on C57BL/6J background | Germ-free Clean Animal Facility, University of Bern, Switzerland | N/A |
| Oligonucleotides | ||
| Primer for 16S rDNA library preparation: 806 Reverse Primer GGACTACNVGGGTWTCTAAT - 515 Forward Primer GTGYCAGCMGCCGCGGTAA. |
515F-806R barcoded primers Integrated DNA Technologies (IDT) |
10776320 (plate5) and 10776323 (plate6) |
| Primer for 16S rDNA library sequencing: read1, 5’-TATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3’, read2, 5’-AGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3’ and index read, 5’-ATTAGAWACCCBDGTAGTCCGGCTGACTGACT-3’ |
Illumina | N/A |
| Primer used for the cell culture characterization: See Supplemental Item Table 1 | This study | N/A |
| Recombinant DNA | ||
| N/A | ||
| Software and Algorithms | ||
| VivaCT40 associated software | Scanco system; Zurich, Switzerland | N/A |
| Instron 1114 associated software | Instron corp., High Wycombe, UK | N/A |
| Amplicon sequencing plipline | github.com/SilasK/amplicon-seq-dada2 | |
| Statistical 16S data analysis | github.com/SilasK/microbiome-analysis | |
| Sci-kit bio, compositional data analysis | http://scikit-bio.org | N/A |
| Metagenomic reads analysis: metagenome-atlas v2 | (Kieser et al.) | N/A |
| MetaCyc Pathways | (Caspi et al., 2016) | N/A |
| TopHat v.2 software | John Hopkins university | N/A |
| RSeQC V2.3.3 | http://rseqc.sourceforge.net/ | |
| PicardTools1.92 | https://broadinstitute.github.io/picard | |
| R/Bioconductor package EdgeR v.3.4.2 | https://www.bioconductor.org/packages/release/bioc/html/edgeR.html | |
| Raw LC-MS/MS data was processed using the Agilent Quantitative analysis software (version B.07.00 | MassHunter Agilent technologies | N/A |
| Metaexpress (5.1.41) software | Molecular devices | N/A |
| Leica Q image analyzer | Leica Corp | N/A |
| Bioquant osteo software | Bioquant | N/A |
16S Gut Microbiota Profiling
At the end of the experiment fecal samples were collected in sterile tubes and immediately frozen and kept at -80°C. Cecal samples were collected after sacrifice of the mice, snap frozen and conserved at -80°C. Fecal and cecal bacterial DNA was extracted using the PowerFecal DNA Kit (Qiagen, Ref. 12830-50) and the 16SrDNA library was built following the standardized protocol from the earth microbiome project (Caporaso, 2012; Caporaso, 2011). DNA was amplified with QuantaBio 5Prime HotMasterMix using barcoded universal bacterial primers targeting variable region V4 of 16SrRNA gene (515F-806R barcoded primers, Illumina): 806 Reverse Primer GGACTACNVGGGTWTCTAAT - 515 Forward Primer GTGYCAGCMGCCGCGGTAA. 2 ng of template was used and the PCR conditions included an initial denaturation at 94°C for 3’, followed by 35 cycles of denaturation at 94°C for 45”, annealing at 50°C for 1’, and extension at 72°C for 90”, with a final extension at 72°C for 10’. Each PCR was done in triplicates and later combined and quality checked on an agarose gel. Each PCR amplification was then quantified with Quant-iT PicoGreen dsDNA Assay with SpectraMax Gemini XPS microplate reader and pooled to an equal amount of 200 ng per sample to form the library. The library was purified using QIAquick PCR purification Kit (Qiagen, Ref. 28104), and sequenced from both ends on Illumina MiSeq (kit v2) to generate 2x250bp paired-end reads (Illumina, San Diego, CA, USA). Eighteen picometers of the library were mixed with PhiX DNA (10%) and were loaded on a MiSeq Reagent kit V2 (500 cycles) together with customized sequencing primers; read1, 5’-TATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3’, read2, 5’-AGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3’ and index read, 5’-ATTAGAWACCCBDGTAGTCCGGCTGACTGACT-3’. 250 bp paired-end sequencing was performed on the MiSeq platform (Illumina, USA) in the iGE3, Institute of Genetics and Genomics in Geneva, CMU, University of Geneva. Sequencing results were obtained and de-multiplexed using standard method supplied by the MiSeq, Illumina.
Reads were processed using a pipeline based on dada2 v 1.8.0 (Callahan, 2016) (code accessible here : github.com/SilasK/amplicon-seq-dada2). In short, the reads were quality filtered with the parameters (truncLen=”180,100”, and maxEE=2), dereplicated, merged, and chimeras removed. sequences with length outside of 253+-7 were removed. The resulting operational taxonomic units were given numbers and were annotated with the SILVA database v132 (Quast, 2013). Richness and Shannon diversity were calculated after rarefaction. Compositional data analysis was performed on OTUs with at least 1 count on average using aldex2 (Fernandes, 2013) for the OTU, genus and family level. The reported P values are calculated using the welch test within aldex2 and were corrected for multiple testing with the benjamini-hochberg procedure. Principal component analysis was performed on the centered log2 ratios after multiplicative replacement of the zero values (Martin-Fernandez, 2003).
Metagenomics Sequencing
Paired-end metagenomic libraries were prepared from 100 ngDNA using TruSeq Nano DNA Library Prep Kit (Illumina) and size selected at about 350 bp. The pooled indexed library was sequenced in a HiSeq4000 instrument at the iGE3 facility (University of Geneva).Metagenomics reads were processed using atlas v2 (Kieser et al., 2019, 2020). In short, using tools from the BBmap suite v37.78 (Bushnell), reads were quality trimmed, and contaminations from the mouse genome were filtered out. Reads were error corrected and merged before assembly with metaSpades v.1.13 (Nurk). Contigs were binned using metabat2 (Kang, 2019) and maxbin2 (Wu, 2016) and their predictions were combined using DAS Tool (Sieber, 2018). The predicted metagenome assembled genomes (MAGs), which had at least 50% completeness and < 10% contamination based on the estimation by checkM (Ferris; Kasper; Parks) were clustered (95% average nucleotide identity) resulting in 147 representative genomes (referred later as genomes). The genes of each genome were predicted using prodigal (Hyatt, 2010) and clustered using linclust (Steinegger) to a nonredundant gene catalog. The 2.3M genes were annotated using KofamScan (Aramaki, 2019) and InterproScan 5 (Madeira, 2019). Using pygenomeprop (https://github.com/Micromeda/pygenprop) the presence of genomes were annotated to contain complete or partial MetaCyc Pathways (Caspi, 2016). The genomes were quantified by the median of coverage in 1kb windows along the genome. MetaCyc pathways and Kegg orthologs (KO) were quantified as the sum of the relative abundance of the genomes containing them. Welch test was used for testing significant differences in pathway abundances between the groups.
RNA Extraction, Reverse Transcription and Real-time qPCR
Upon collection, tissues were stored in 1ml RNAlater and immediately processed for RNA extraction for the bone tissues or stored at -80°C. For RNA extraction, tissues were placed in 2 ml Eppendorf tubes containing 1 ml Trizol (Thermo Fisher Scientific) and mechanically disaggregated using the bead-based TissueLyser equipment (Qiagen) by shaking for 40 seconds at 30 Hz in presence of a silicate bead for the bone and a metal bead for the other tissues. After brief centrifugation to remove tissue debris (3 minutes at 12000g at 4°C), 200 μl chloroform was added, samples were shaken and centrifuged for 15 minutes at 12000g at 4°C. The chloroform phase was collected, mixed with 500 μl isopropanol and centrifuged again as before. The pellet obtained was washed twice with 70% ethanol and ultimately resuspended in 50 μl PCR-grade water. RNA from cell culture was extracted using 1ml of Trizol using the standard protocol. For retro-transcription we used High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with 1 μg RNA per sample. qPCR were done on a LightCycler 480 machine (Roche) with SYBR Green-based detection (Applied Biosystem, Power-up). The primer sequences are shown in the Supplementary Data, as Supplemental Item Table 1.
Results were calculated using standard curve method and normalized to the TATA Box binding Protein (TBP) housekeeping gene, and shown as fold change relative to the control group.
RNA Sequencing
Next Gen Sequencing of mRNA transcripts was done on Ilumina MiSeq 2500 platform at the sequencing facility of the Institute of Genetics and Genomics of Geneva (iGE3), University of Geneva. RNA was isolated from entire tibias. For the experiment comparing the ovariectomised mice transplanted with warm- or RT-microbiota (Ova. Transp. RT and Ova transp. 34°C), each group contained 4 samples where each sample correspond to one mice. For the experiment comparing Ovarectomised and sham operated mice exposed to 34°C or RT (OvaRT, Ova 34°C, shamRT and sham34°C), each group was a sample pooled from 5 mice. Libraries for the sequencing were prepared with poly-A selection according to Ilumina TrueSeq protocol. The reads were mapped with the TopHat v.2 software to the UCSC mm10 reference on new junctions and known junctions’ annotations. Biological quality control and summarization were done with RSeQC-2.3.3 and PicardTools1.92.
The differential expression analysis was performed with the statistical analysis R/Bioconductor package EdgeR v.3.4.2 for the genes annotated in mm10. Briefly, the counts were normalized according to the library size and filtered. The genes above 1 count per million reads (cpm) (in experiment with replicate, in at least 3 samples) were kept for the further analysis. After normalization of the counts, transcript abundances were compared in pairwise condition in a modified Fischer exact test (as implemented in edgeR). p-values of the differentially expressed genes were corrected for multiple testing error with a 5% FDR (false discovery rate), using the Benjamini-Hochberg (BH) correction. Genes were called differentially expressed between any given two conditions when their false-discovery rate was <0.05 and their fold-change >2. Transcripts with log(cpm)>0 and p≤0.05 were subsequently subjected to pathway analysis using Reactome pathways database (Fabregat, 2017), reporting the enrichment ratio (# DEGs/Total Genes in dataset) and FDR-adjusted Pvalue computed by Fisher exact test.
Cecal and Fecal Concentration of Polyamines
Feces and cecum content samples were pre-weighed directly in the lysis tubes (soft tissue homogenizing CK 14 tubes, Bertin Technologies, Rockville, MD, US) and extracted (using ceramic beads) by adding ice-cold MeOH:H2O (4:1; v:v) spiked with internal standards in the Cryolys Precellys 24 sample Homogenizer (2 × 20 seconds at 10000 rpm, Bertin Technologies, Rockville, MD, US). Homogenized tissue extracts were centrifuged for 15 minutes at 21000 g at 4°C and the resulting supernatant was transferred to LC-MS vials for the injection into the LC-MS system. For feces, the sample amount normalization was based on weight whereas the cecum content was extracted entirely (whole cecum per specimen). Thirteen-point calibration curves were generated by the addition of IS mixture (25 μL) to each calibrator (i.e. standard mixture) (75 μL), vortexed and transferred to LC-MS vials for the injection.
Extracted samples were analyzed by Hydrophilic Interaction Liquid Chromatography coupled to tandem mass spectrometry (HILIC - MS/MS) in positive mode using a 6495 triple quadrupole system (QqQ) interfaced with 1290 UHPLC system (Agilent Technologies). The chromatographic separation was carried out in an Acquity BEH Amide, 1.7 μm, 100 mm × 2.1 mm I.D. column (Waters, Massachusetts, US). Mobile phase was composed of A = 50 mM ammonium formate and 0.1 % FA in water and B = 50 mM ammonium formate, 0.1 % formic acid in ACN/H2O (8:2; v:v). The linear gradient elution from 100% B (0-1.5 min) down to 60% B was applied (1.5 min - 12 min) and these conditions were held for 4 min, followed by the initial chromatographic conditioning during the 5 min post-run for column re-equilibration. The flow rate was 400 μL/min, column temperature 40 °C and sample injection volume 2μl. ESI source conditions were set as follows: dry gas temperature 230 °C, nebulizer 35 psi and flow 14 L/min, sheath gas temperature 400 °C and flow 12 L/min, nozzle voltage 500 V, and capillary voltage 4000 V. Dynamic Multiple Reaction Monitoring (dMRM) was used as acquisition mode with a total cycle time of 500 ms. Optimized collision energies for each metabolite were applied.
Raw LC-MS/MS data was processed using the Agilent Quantitative analysis software (version B.07.00, MassHunter Agilent technologies). For absolute quantification, calibration curves and the stable isotope-labeled internal standards (IS) were used to determine the response factor. Linearity of the standard curves was evaluated for each metabolite using a thirteen-point range; in addition, peak area integration was manually curated and corrected where necessary.
Elisa
1,25-dihydroxycholecalciferol was measured with Abbexa Calcitriol (INN) Elisa kit (ref: abx 513030) in 1:10 diluted plasma samples. Osteocalcin was measured with Immutopics (quidel) Elisa KIT ref 60-1305 in 1:11 diluted plasma samples. CTX-I was measured with RatLaps CTX-I EIA Immunodiagnostic Systems (ref: AC-06F1) from undiluted plasma samples. All measures were done according to manufacturers’ instructions.
Bone Mineral Content
Tibias were dried in oven for 3 days at 60°C. The dry weight of each bone was recorded (wi) and the tibias were burned and reduced to ashes with a furnace set at 800°C for 1h. The weight of the remaining ashes was measured (wf), and the ratio between the weight of the bone ash and the dry weight (wf/wi) was calculated to determine the bone mineral content (Tsai, 2017)
TRAP Staining
Cells were fixed with 3.7% Formol (formaldehyde) for 1h at RT and rinsed with water. The staining solution was freshly prepared by mixing equal amount of Fast violet B salt (ChemCruz ref sc-215029B) solution in acetate buffer (7mg/ml), and Naphtol AS-TR phosphate disodium salt (Sigma ref: N6125) solution in acetate buffer (2mg/ml, 225mM sodium acetate, 75mM acetic acid and 4mM NaOH). Cells were incubated overnight at 4°C with the staining solution, rinsed with water, incubated 30 min at RT in Sodium Fluorine (4.2g/L H2O). and rinsed with water once again before being processed for imaging and analysis. Images were acquired with ZeissAxio Observer ZI and ImagexpressXL (Molecular devices). The total cells per well were quantified with Metaexpress (5.1.41) software, and the number of differentiated osteoclasts manually (defined as multinucleated and TRAP+ cells).
Alkaline Phosphatase (ALP) Activity Measurement
Cells were rinsed with cold PBS and scraped in 500μl H2O. After short sonication (2x15sec-10kHtz), samples were centrifuged at 10 000rpm for 15min at 4°C. 100μl of the supernatant was collected and incubated for 10min at 37°C, while the rest of the supernatant was kept for measuring the protein concentration. The reactive solution was as the following: 10mM p-nitrophenylphosphate, 560mM 2-amino-2-methyl-1-propanol, 1mM MgCl2, pH 10.5. 900μl of the reactive solution was added to the pre-heated 100ul sample and time monitored until sample turned yellow (approx. 7min). The reaction was stopped with 200ul of 1M NaOH and the time recorded. Absorbance of the sample was measured at 405nm, and the concentration of the remaining protein was measured using Pierce BCA Protein assay kit (Thermo scientific ref 23225). The ALP activity was obtained with the following formula: ALP activity (nmol PO4/mg of prot/sec) = [(OD × 1000)/time(sec)]/protein (mg/ml).
Bone Histomorphometric Analysis
After 24 hours of fixation in 3.8% Formol, bones were dehydrated in absolute ethanol for 3 days followed by overnight incubation in acetone at -20°C before being embedded in methylmethacrylate (Merck). 8μm thick transversal sections of the midshaft, and sagittal section of the proximal femur were cut with Leica RM 22 65 microtome (Leica Corp Microsystems AG) and mounted unstained for the evaluation of fluorescence from the calcein deposition. Histomorphometric measurements were performed on the secondary spongiosa of the proximal tibia metaphysis and on the endocortical and periosteal bone surfaces in the middle of the tibia using Leica Q image analyzer (Leica Corp) at 40x magnification, and Bioquant osteo software. All parameters were calculated and expressed according to standard formulas and nomenclatures (Parfitt, 1988): mineral apposition rate (micrometers per day), mineralizing surface per bone surface (percentage) and bone formation rate (cubic micrometers per square micrometer per day). Osteoclast surface per bone surface and numbers were evaluated after TRAP staining performed following the same protocol as for the cell culture staining on free floating slides and followed by a Methylene blue counterstaining and mounted with Permount (fisher chemical ref: SP15-100). Sirius red staining was performed with Sirius red dye reagent (Direct red 80 Sigma #P744, in saturated aqueous picric acid (1.3% in H2O) at concentration of 0.1% w/v).
Quantification and Statistical Analysis
Data Representation and Statistical Analysis
Plots include each datapoint, mean and ± Standard deviation (SD). All P values, n (number of animals) and each applied statistical test is specified in the figure legends. To compare two different groups we applied Mann-Whitney t-test. To compare more than two groups we applied One-Way ANOVA with Tukey’s multiple comparison test. All experiments were reproduced at least twice, and shown are the representative data. In vivo measurements were done without blinding, while the histomorphometric and biomechanical measurements were done with blinding. No data were excluded from the analysis. Where available, group sizes were calculated based on power calculations of 0.8. Results were considered significant when P < 0.05 in the respected statistical test and represented significance as *P <0.05; **P <0.01; ***P <0.001.
We analyzed the data using Prizm Version 8.4.3., and assembled the figures in Adobe Illustrator. We generated the graphical abstract in Powerpoint, using some of the illustrations available at Servier Medical Art.
Additional Resources
N/A
Supplementary Material
Acknowledgements
We thank Claes Wollheim and the members of our lab for discussions and critical reading of the manuscript; Serge Ferrari, Tony Teav and Hector Gallart-Ayalla for technical expertise and advice. This work is part of a project that has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC Consolidator Grant agreement No. 815962, Healthybiota), and from the Clayton Foundation for biomedical research.
Footnotes
Author Contributions
Conceptualization, C.C. and M.T.; Methodology, C.C., S.K., M.Ç., N.H., B.B., N.B and M.T.; Investigation, C.C., S.K., M.Ç., N.H., J.B., N.S.Z., M.S., S.F. and N.B.; Writing, C.C., and M.T.; Resources, N.B., A.M., M.T.; Funding Acquisition, M.T.; Supervision, M.T.
Declaration of Interests
M.T. and C.C. disclose that they are inventors of a submitted patent application for treatment of bone diseases. All other authors do not have competing interests.
References
- Alhilli F, Wright EA. The Effects of Changes in the Environmental-Temperature on the Growth of Tail Bones in the Mouse. Brit J Exp Pathol. 1983;64:34–42. [PMC free article] [PubMed] [Google Scholar]
- Allen J. The influence of Physical conditions in the genesis of species. Radical Review. 1877;1:108–140. [Google Scholar]
- Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, Ogata H. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoub MA. Effect of two extreme temperatures on growth and tail-length of mice. Nature. 1958;181:284. doi: 10.1038/181284a0. [DOI] [PubMed] [Google Scholar]
- Balk EM, Adam GP, Langberg VN, Earley A, Clark P, Ebeling PR, Mithal A, Rizzoli R, Zerbini CAF, Pierroz DD, et al. Global dietary calcium intake among adults: a systematic review. Osteoporos Int. 2017;28:3315–3324. doi: 10.1007/s00198-017-4230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89:1333–1348. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell B. BBmap [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme Journal. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. P Natl Acad Sci USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Mueller LA, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44:D471–480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Chalhoub D, Kassem AM, El-Hajj Fuleihan G. Geographic and ethnic disparities in osteoporotic fractures. Nature Reviews Endocrinology. 2014a;10:338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014b;10:338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Levy AR, Lefaivre KA, Guy P, Kuramoto L, Sobolev B. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporosis Int. 2011;22:2575–2586. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanovic A, Hagemann S, et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Cox-York KA, Sheflin AM, Foster MT, Gentile CL, Kahl A, Koch LG, Britton SL, Weir TL. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol Rep. 2015;3 doi: 10.14814/phy2.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4:61–76. doi: 10.1177/1759720X11430858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric Restriction Leads to High Marrow Adiposity and Low Bone Mass in Growing Mice. Journal of Bone and Mineral Research. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.69. 16069. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbiano S, Suarez-Zamorano N, Rigo D, Veyrat-Durebex C, Dokic AS, Colin DJ, Trajkovski M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metabolism. 2016;24:434–446. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Fabregat A, Sidiropoulos K, Viteri G, Forner O, Marin-Garcia P, Arnau V, D’Eustachio P, Stein L, Hermjakob H. Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinformatics. 2017;18:142. doi: 10.1186/s12859-017-1559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067019. e67019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein GR, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Two-year Update From CheckMate 141: Outcomes With Nivolumab (Nivo) vs Investigator’s Choice (IC) in Recurrent or Metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN) in the Overall Population and PD-L1 Subgroups. Int J Radiat Oncol. 2018;100:1317–1317. [Google Scholar]
- Fruhauf PK, Ineu RP, Tomazi L, Duarte T, Mello CF, Rubin MA. Spermine reverses lipopolysaccharide-induced memory deficit in mice. J Neuroinflammation. 2015;12:3. doi: 10.1186/s12974-014-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KSY, Schroeder S, Stunnenberg HG, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nature Neuroscience. 2013;16 doi: 10.1038/nn.3512. 1453-+ [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: Implications for the regulation of bone mass by body weight. Journal of Bone and Mineral Research. 2008;23:870–878. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- Harland SC. Effect of Temperature on Growth in Weight and Tail-Length of Inbred and Hybrid Mice. Nature. 1960;186:446–446. [Google Scholar]
- Hsu E, Pacifici R. From Osteoimmunology to Osteomicrobiology: How the Microbiota and the Immune System Regulate Bone. Calcified Tissue Int. 2018;102:512–521. doi: 10.1007/s00223-017-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Philbrick KA, Wong CP, Gordon JL, Kahler-Quesada AM, Olson DA, Branscum AJ, Sargent JL, DeMambro VE, Rosen CJ, et al. Room temperature housing results in premature cancellous bone loss in growing female mice: implications for the mouse as a preclinical model for age-related bone loss. Osteoporos Int. 2016;27:3091–3101. doi: 10.1007/s00198-016-3634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O, Gullberg B, Allander E, Kanis JA, Dilzen G, Gennari C, Lopezvaz AA, Lyritis G, Mazzuoli GF, Miravet L, et al. The Apparent Incidence of Hip Fracture in Europe - a Study of National Register Sources. Osteoporosis Int. 1992;2:298–302. doi: 10.1007/BF01623186. [DOI] [PubMed] [Google Scholar]
- Jones RM, Mulle JG, Pacifici R. Osteomicrobiology: The influence of gut microbiota on bone in health and disease. Bone. 2017 doi: 10.1016/j.bone.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Morton GJ, Thaler JP, Meek TH, Tylee T, Ogimoto K, Wisse BE. Acutely Decreased Thermoregulatory Energy Expenditure or Decreased Activity Energy Expenditure Both Acutely Reduce Food Intake in Mice. Plos One. 2012;7 doi: 10.1371/journal.pone.0041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Li F, Kirton ES, Thomas A, Egan RS, An H, Wang Z. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. 2019:0–10. doi: 10.7717/peerj.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Gillison ML, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Vokes EE, Even C, et al. Nivolumab (Nivo) vs Investigator’s Choice (IC) for Platinum-Refractory (PR) Recurrent or Metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN; CheckMate 141): Outcomes in First-line (1L) R/M Patients (Pts) and Updated Safety and Efficacy. Oncol Res Treat. 2018;41:96–96. [Google Scholar]
- Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C, Iglseder B, Weger S, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108:371–380. doi: 10.1093/ajcn/nqy102. [DOI] [PubMed] [Google Scholar]
- Kieser S, Brown J, Zdobnov EM, Trajkovski M, McCue LA. ATLAS: a Snakemake workflow for assembly, annotation, and genomic binning of metagenome sequence data. bioRxiv. 2019:737528–737528. doi: 10.1186/s12859-020-03585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu N, Zhang H, Yang L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen. 2019a;8 doi: 10.1002/mbo3.810. e00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LS, Rao ST, Cheng YZ, Zhuo XY, Deng CH, Xu NN, Zhang H, Yang L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen. 2019b;8 doi: 10.1002/mbo3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez JA, Barcelo-Vidal C, Pawlowsky-Glahn V. Dealing with zeros and missing values in compositional data sets using nonparametric imputation. Math Geol. 2003;35:253–278. [Google Scholar]
- Mcmillan PJ, Dewri RA, Joseph EE, Schultz RL, Deftos LJ. Rapid Changes of Light Microscopic Indexes of Osteoclast-Bone Relationships Correlated with Electron-Microscopy. Calcified Tissue Int. 1989;44:399–405. doi: 10.1007/BF02555968. [DOI] [PubMed] [Google Scholar]
- Meyer CW, Ootsuka Y, Romanovsky AA. Body Temperature Measurements for Metabolic Phenotyping in Mice. Frontiers in Physiology. 2017;8 doi: 10.3389/fphys.2017.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosekilde L. Age-related changes in bone mass, structure, and strength - effects of loading. Z Rheumatol. 2000;59:1–9. doi: 10.1007/s003930070031. [DOI] [PubMed] [Google Scholar]
- Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Research. 2017;27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Sjogren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26:69–74. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Sjogren K. Osteomicrobiology: A New Cross-Disciplinary Research Field. Calcif Tissue Int. 2018;102:426–432. doi: 10.1007/s00223-017-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM. Bone histomorphometry: proposed system for standardization of nomenclature, symbols, and units. Calcif Tissue Int. 1988;42:284–286. doi: 10.1007/BF02556360. [DOI] [PubMed] [Google Scholar]
- Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome research. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/595962. 595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M, Hurwitz S. The role of the growth plate in longitudinal bone growth. Poult Sci. 1991;70:1806–1814. doi: 10.3382/ps.0701806. [DOI] [PubMed] [Google Scholar]
- Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004;7:227–243. doi: 10.1079/phn2003590. [DOI] [PubMed] [Google Scholar]
- Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine HL, Meadows CA, Ion G, Serrat MA. Heat-Induced Limb Length Asymmetry Has Functional Impact on Weight Bearing in Mouse Hindlimbs. Front Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Molina B, Queipo-Ortuno MI, Lambertos A, Tinahones FJ, Penafiel R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Frontiers in Nutrition. 2019;6 doi: 10.3389/fnut.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster JY, Burlet N. Osteoporosis: A still increasing prevalence. Bone. 2006;38:4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Romsos DR, Ferguson D, Vandertuig JG. Effects of a Warm Environment on Energy-Balance in Obese (Ob/Ob) Mice. Metabolism-Clinical and Experimental. 1985;34:931–937. doi: 10.1016/0026-0495(85)90141-6. [DOI] [PubMed] [Google Scholar]
- Scalabrino G, Ferioli ME. Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26:149–164. doi: 10.1016/0047-6374(84)90090-3. [DOI] [PubMed] [Google Scholar]
- Serrat MA. Allen’s Rule Revisited: Temperature Influences Bone Elongation During a Critical Period of Postnatal Development. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2013;296:1534–1545. doi: 10.1002/ar.22763. [DOI] [PubMed] [Google Scholar]
- Serrat MA. Environmental Temperature Impact on Bone and Cartilage Growth. Compr Physiol. 2014;4:621–655. doi: 10.1002/cphy.c130023. [DOI] [PubMed] [Google Scholar]
- Serrat MA, King D, Lovejoy CO. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. P Natl Acad Sci USA. 2008;105:19348–19353. doi: 10.1073/pnas.0803319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrat MA, Schlierf TJ, Efaw ML, Shuler FD, Godby J, Stanko LM, Tamski HL. Unilateral Heat Accelerates Bone Elongation and Lengthens Extremities of Growing Mice. J Orthop Res. 2015;33:692–698. doi: 10.1002/jor.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, Banfield JF. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nature Microbiology. 2018;3:836–843. doi: 10.1038/s41564-018-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper S, Perktold J. statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference; 2010. [Google Scholar]
- Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinegger M, Söding J. Clustering huge protein sequence sets in linear time. Nature Communications. 2018;9:2542–2542. doi: 10.1038/s41467-018-04964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofalo R, Cocchi S, Suzzi G. Polyamines and Gut Microbiota. Frontiers in Nutrition. 2019;6 doi: 10.3389/fnut.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YF, Hsu LH, Wu CC, Cai WH, Yang KC, Fan FY. Long-Term Oral Toxicity and Anti-osteoporotic Effect of Sintered Dicalcium Pyrophosphate in Rat Model of Postmenopausal Osteoporosis. J Med Biol Eng. 2017;37:181–190. doi: 10.1007/s40846-016-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, Josse R, Kanis JA, Mithal A, Pierroz DD, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–172. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- Wang LL, Liu Y, Qi C, Shen LY, Wang JY, Liu XJ, Zhang N, Bing T, Shangguan DH. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci Rep-Uk. 2018;8 doi: 10.1038/s41598-018-28648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–607. doi: 10.1093/bioinformatics/btv638. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hinoi E, Fujita H, Iezaki T, Takahata Y, Takamori M, Yoneda Y. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br J Pharmacol. 2012;166:1084–1096. doi: 10.1111/j.1476-5381.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeum KJ, Song BC, Joo NS. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. Int J Environ Res Public Health. 2016;13:184. doi: 10.3390/ijerph13020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Li W, Zou J, Jiang X, Xu G, Huang H, Liu L. Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res. 2017;77:2938–2951. doi: 10.1158/0008-5472.CAN-16-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin A, Prentice A, Ward KA. Ethnic differences in bone health. Front Endocrinol (Lausanne) 2015;6:24. doi: 10.3389/fendo.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietak M, Kovatcheva-Datchary P, Markiewicz LH, Stahlman M, Kozak LP, Backhed F. Altered Microbiota Contributes to Reduced Diet-Induced Obesity upon Cold Exposure. Cell Metabolism. 2016;23:1216–1223. doi: 10.1016/j.cmet.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data generated in this study is deposited at the Sequence Read Archive (SRA) of the National Center for Biotechnology Information. The 16S rDNA sequencing of the female and the male mice have accessions PRJNA647833 and PRJNA648020, respectively. Shotgun metagenomics data is available under the id PRJNA647832 and the RNA seq data under PRJNA648022. The data for targeted metabolomics and the correlation analysis in human are deposited in the open access repository figshare with the doi: 10.6084/m9.figshare.12696419 and 10.6084/m9.figshare.12696407, respectively. The scripts used for the analysis of the 16S rDNA, metagenomc and human epidemiology data are available at github.com/SilasK/WarmMicrobiota.