Abstract
High frequency Deep Brain Stimulation (DBS) targeting the motor thalamus is an effective therapy for essential tremor (ET). However, since tremor mainly affects periods of voluntary movements and sustained postures in ET, conventional continuous stimulation may deliver unnecessary current to the brain. Here we tried to decode movement states based on local field potentials (LFPs) recorded from motor thalamus and zona incerta in real-time to trigger the switching on and off of DBS in three patients with ET. Patient-specific models were first identified using thalamic LFPs recorded while the patient performed movements that tended to trigger tremor in everyday life. During the real-time test, LFPs were continuously recorded to decode movements and tremor, and the detection triggered stimulation. Results show that voluntary movements can be detected with a mean sensitivity ranging from 76.8% to 88.6% and a false positive rate ranging from 16.0% to 23.1% Postural tremor was detected with similar accuracy. The closed-loop DBS triggered by tremor detection suppressed intention tremor by 90.5% with a false positive rate of 20.3%.
Clinical Relevance
This is the first study on closed-loop DBS triggered by real-time movement and tremor decoding based solely on thalamic LFPs. The results suggest that responsive DBS based on movement and tremor detection can be achieved without any requirement for external sensors or additional electrocorticography strips.
I. Introduction
Essential tremor (ET), which is a progressive neurological disorder that causes involuntary and rhythmic shaking at 4-12 Hz, is one of the most common movement disorders [1]. Tremor in ET is typically intermittent, predominantly occurring during voluntary movement and/or while maintaining a certain posture[2]. Continuous deep brain stimulation (DBS) is an approved and effective therapy for ET [3]. However, due to disease progression or habituation to stimulation, many patients lose the benefit of DBS over time [4]. In these circumstances, an increased stimulation intensity is usually required in order to maintain the therapy effect, which may be associated with side effects including unpleasant sensations, slurred speech and unsteadiness walking [5]. Closed-loop or adaptive DBS, aiming to only deliver stimulation when necessary and thus reduce side effects and prolong clinical efficacy, is seen to be a promising innovative DBS treatment of ET [6]. Some existing studies have used wearable inertial sensors [7], surface electromyography (EMG) [8], or electrocorticography (ECoG) recorded from a Neurosciences strip of intracranial electrodes implanted over the surface of the motor cortex [9] to provide feedback for the control of the stimulator in closed-loop DBS systems. However, all these solutions require external sensors and/or additional invasive recording, which constrains their clinical application in the treatment of ET.
In our previous study, local field potentials (LFPs) recorded from ventral intermediate (VIM) thalamus were used to decode voluntary movement and postural tremor and achieved a promising offline decoding accuracy [10]. In the current study, we further evaluated the performance of online closed-loop DBS based on the real-time detection of voluntary movement and posture and any associated tremor using LFPs recorded from VIM thalamus and zona incerta (ZI) in three ET patients. Models for decoding voluntary movement with and without stimulation, and posture associated with any tremor with and without stimulation were trained separately, using features consisting of the average powers in several different frequency bands and some time-domain characteristics. Separate models were also trained for the conditions when the stimulation was switched on and switched off, due to the difference in the signal-to-noise ratios in these two states and potential differences in the brain activities induced by stimulation. Several commonly used classification methods including logistic regression (LR), linear discriminant analysis (LDA), support vector machine (SVM), naïve Bayes, decision tree (DT), and k-nearest neighbors (KNN) were tested. Our results with real-time decoding and stimulation suggest that both voluntary movement and posture and any associated tremor can be detected based on VIM thalamic and ZI LFPs recorded from the electrodes used for stimulation and used to close the loop in on-demand DBS for ET.
II. Methods
A. Schematic
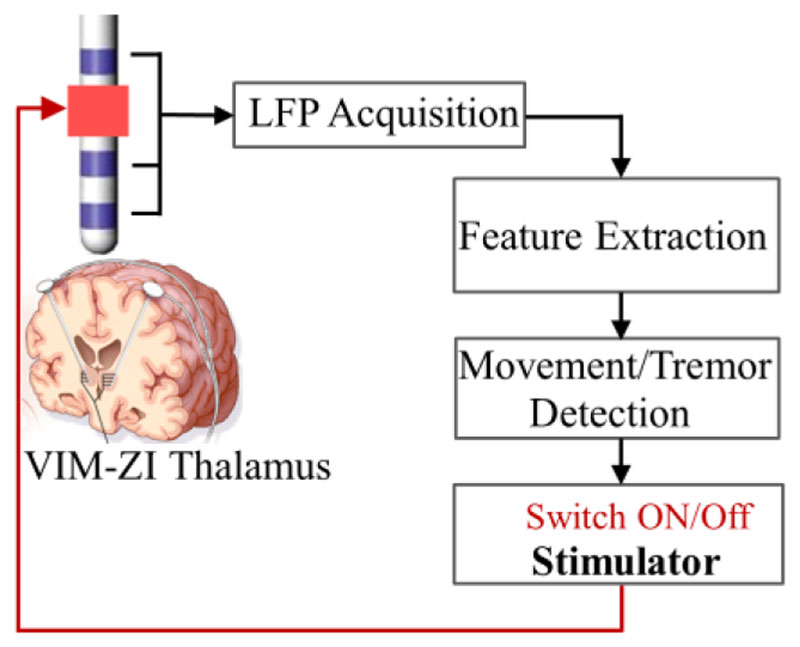
Fig. 1 shows the schematic of the closed-loop DBS system, including LFP signal acquisition from the DBS electrodes implanted in VIM thalamus, feature extraction, movement/tremor detection, i.e., classification based on the extracted features, and the switching on/off of the stimulator based on the detection. All these procedures were applied online in real-time.
Figure 1.
Schematic of the real-time closed-loop DBS based on VIM-ZI LFPs
B. Recording
Three ET patients who had undergone bilateral implantation of DBS electrodes (1.5mm spaced Abbott Infinity directional electrodes with upper electrode contacts in the VIM thalamus and lower contacts in the ZI) participated in our study. The study was approved by the local ethics committee and all patients gave informed written consent before the experiment. The recordings were undertaken 4-5 days after the first surgery for electrode implantation and prior to the second operation to connect the electrode to the subcutaneous pulse generator. Bilateral bipolar LFPs in ViM and EEG signals covering “Fz”, “′FCz”, “Cz”, “Oz”, “C3”, “C4”, “CP3”, and “CP4” in the standard 10-20 system were recorded using a TMSi Porti amplifier (TMS International, Netherlands) with a common average reference and a 2048-Hz sampling rate. A bipolar electromyography (EMG) channel and a 3D accelerometer were placed on each hand to monitor the presence of voluntary movement and/or tremor.
C. Stimulation
Stimulation was delivered using a neuroBi neurostimulator (Bionics Institute, Australia) with a fixed stimulation frequency of 130 Hz, a biphasic pulse width of 60 us, and an interphase gap of 20 us. Monopolar stimulation was delivered to one of the contacts in the middle of the electrode (seen in Fig. 1 with the reference connected to an electrode patch attached to the shoulder of the patient. When segmented leads were implanted, stimulation was delivered in the ring mode by connecting the three directional contacts together. Prior to the experiment, the contact to stimulate and the corresponding stimulation amplitude were selected based on tremor control assessed by a clinician. LFPs were recorded from the other contacts in bipolar modes. Simultaneous bilateral stimulation was delivered in two patients, and unilateral stimulation (to the right hemisphere) was delivered in one patient since tremor was unilateral in this patient. During the experiment, the closed-loop DBS algorithm only changed the stimulation amplitude between 0 to the previously selected amplitude.
D. Experimental design
The experiment consisted of a training session and a real-time test session. During the training session, the patients performed voluntary self-paced upper limb movements such as a pegboard movement or pouring rice from one cup to another using the worst affected hand. Data were also recorded while the participants maintained specific postures that provoked postural tremor. Each block of movements/posture lasted about 30 s, followed by a duration-matched period of resting. In total, LFPs were recorded from 6-8 blocks of voluntary upper limb movements and 8-10 blocks of sustained posture. The patients were asked to repeat these movements with and without stimulation separately, with around 20 minutes of data recorded from each stimulation state. Four models for movement and posture and any associated tremor decoding with-stimulation and without-stimulation were trained based on the recorded data. During the test session, the patients were asked to repeat the voluntary movements and to maintain the postures which tend to provoke tremor, while the trained models were applied to detect the movement and tremor in real-time in order to drive the switching on and off of the stimulation.
E. Model training
As suggested in our previous study [10], oscillatory activities in the beta and theta frequency bands contributed most to the decoding of movements and postural tremor, respectively. In addition, due to stimulation artifact, the signal-to-noise levels in the recorded LFPs with and without stimulation were very different. Thus, four separate models were trained for the detection of movements with and without stimulation, posture and any associated tremor with and without stimulation, respectively. The training of each model consisted of the following four steps.
-
1)
Pre-processing: A forward 4-order Butterworth IIR band-pass filter with a pass band of 0.5-500 Hz was applied on each bipolar LFP for pre-processing. Only those algorithms that can be implemented in real-time were used during training, so a forward filter was used here.
-
2)
Feature extraction: The average powers in 8 different frequency bands, 1-3 Hz, 4-7 Hz, 8-12 Hz, 13-22 Hz, 23-34 Hz, 35-45 Hz, 56-95 Hz and 105-195 Hz, and 4 time-domain characteristics including the mean value and three Hjorth parameters (activity, mobility, and complexity) 11 were calculated based on the filtered bipolar LFP for each 250 ms moving window. These features were updated every 100 ms. Features from 10 previous windows from all bipolar LFPs recorded from both hemispheres were concatenated together into a long feature vector (12 features/window × 10 windows × n channels, here n equaled to the number of bipolar LFP recordings available) for each current sample. For the hemisphere to which monopolar stimulation was delivered, 2 bipolar LFP channels were recorded; for the hemisphere that was not simulated, 3 bipolar LFPs can be recorded. Thus n equaled to 4 or 5 for the patients with bilateral or unilateral stimulation, respectively.
-
3)
Labelling: The labels of ‘Movement’ vs ‘Rest’ and ‘Tremor’ vs ‘No-tremor’ for model training were determined based on the recorded accelerometer and EMG signals, respectively. The EMG or accelerometer signals were high pass filtered at 1 Hz using a forward-backward 4 order Butterworth IIR high pass filter, smoothed using a 50-ms time window, and rectified. We quantified the mean value across the recording session for the EMG (Temg) and accelerometer signals (Tacc). Lastly, the samples with the corresponding EMG/accelerometer values larger than αTemg or αTacc were labeled by 1, and the remainder labeled by -1. Here α was originally set to 0.8 but was manually adjusted according to the labelling performance.
-
4)
Model Training: Based on the extracted feature vectors and the corresponding labels, four binary classifiers were trained for the detection of movements with and without stimulation, as well as the detection of posture and any associated tremor with and without stimulation. Several commonly used classification methods including logistic regression (LR), linear discriminative analysis (LDA), a Support Vector Machine (SVM), naïve Bayes, Decision Tree Classification (DT), and the k-nearest neighbors (KNN) algorithm were tested. Five-fold cross-validation was used to evaluate the receiver operating characteristics (ROC) curves and reported the area under curves (AUCs) of different models derived from the training data.
F. Online testing
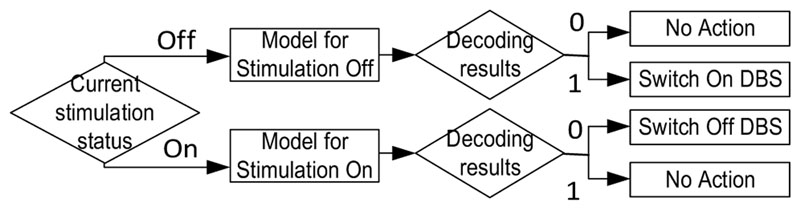
During online testing, the patients were asked to repeat the voluntary movements or to maintain the postures which provoked tremor. Meanwhile, the classification models showing the best performance during offline training were selected to detect movements or posture and any associated tremor based on bipolar LFPs measured in real-time. The decoding outputs from the model corresponding to the current stimulation status were calculated every 100 ms in order to drive the switching on/off of the stimulator, resulting in a 10Hz update rate for the DBS control. The decision process for each update is shown in Fig. 2 The LFPs, EMGs, and accelerometer signals were recorded for the evaluation of performance. The accuracy, true positive rate (TRP), and false positive rate (FPR) for the online detection of movements were compared against those detected from EMG and accelerometer measurements.
Figure 2.
Decision process for DBS control based on decoding in each update
III. Results
A. Offline decoding accuracy based on training data
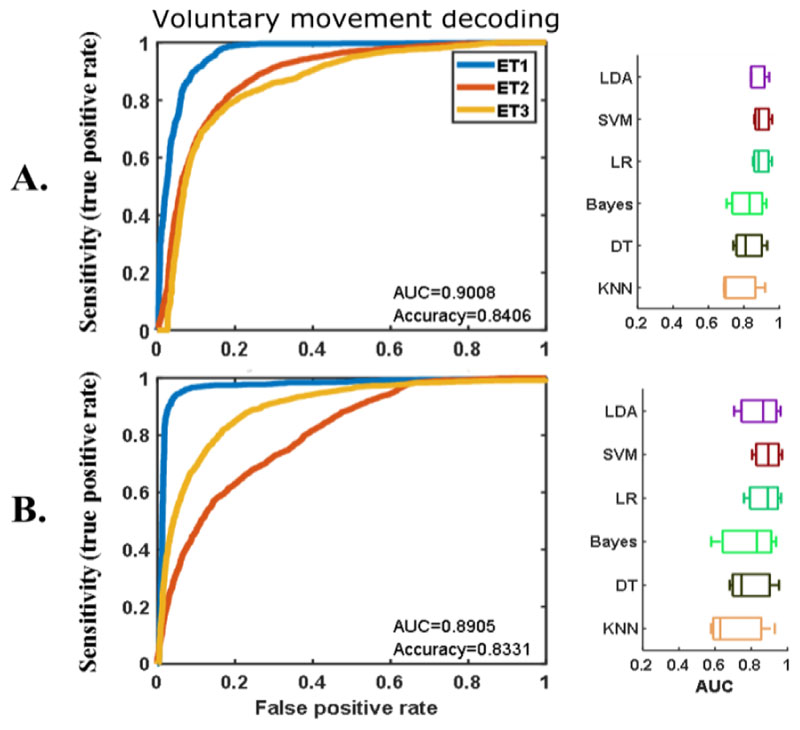
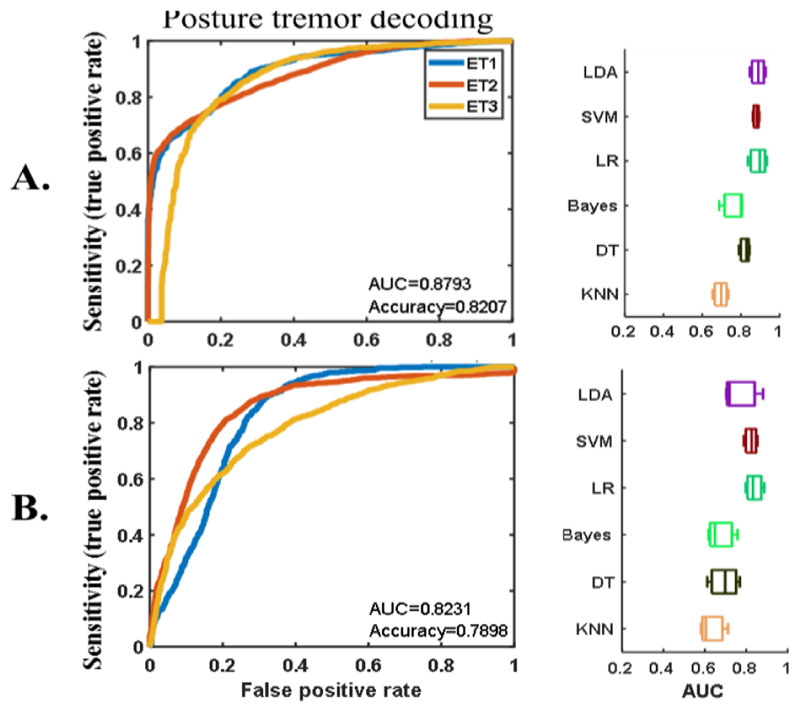
The offline movement decoding when the stimulation was off had similar accuracy to that shown in our previous study [10]. The AUCs of the five-fold cross validation using different classification methods are shown in Fig. 3A. Similar decoding accuracy was achieved even when the stimulation was switched on (Fig. 3B), with stimulation artefacts that significantly lowered the signal-to-noise ratio in the LFP recording. SVM provided the best decoding across all participants no matter whether stimulation was switched on or off. This achieved an averaged AUC of about 0.9 for voluntary movement decoding either with (Fig. 3B) or without (Fig. 3A) stimulation. We were also able to decode posture and any associated tremor with an average AUC of 0.88 without stimulation or 0.82 with stimulation using SVM (shown in Fig. 4). Similar results were also achieved using other classification methods, suggesting that it is possible to have a sensitivity of 0.8 with a false positive rate of 0.2-0.3 in voluntary movement and posture and any associated tremor decoding based on VIM-ZI LFPs either with or without stimulation.
Figure 3.
Offline results of voluntary movement decoding when there was no stimulation (A) and when high frequency stimulation was switched on (B). Plots on the left show the ROCs of the cross-validation with SVM in different patients (different colors show results from different participants). Plots on the right show the cross-validation AUCs of different classification methods.
Figure 4.
Offline results of posture tremor decoding when there was no stimulation (A) and when high frequency stimulation was switched on (B). Plots on the left show the ROCs of the cross-validation with SVM in different patients (different colors show results from different participants). Plots on the right show the cross-validation AUCs of different classification methods.
B. Online decoding and stimulating results
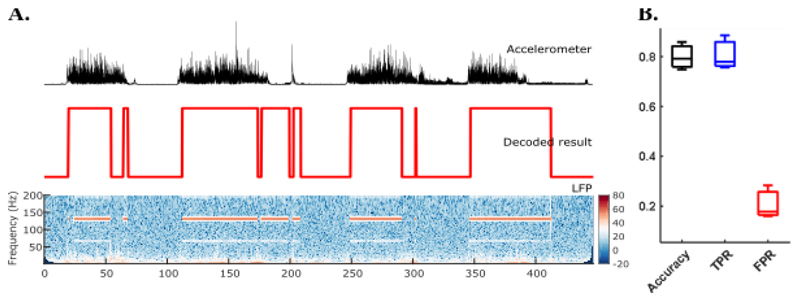
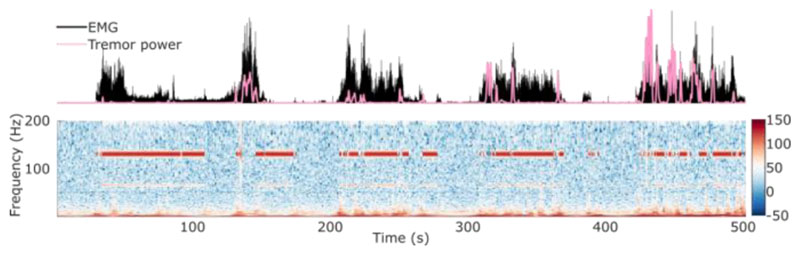
The models identified by SVM were used for online decoding, and the outputs of the model were used to drive the DBS as shown in Fig. 2. For the voluntary movements, we compared the time when the stimulation was switched on and the time when movements and/or tremor could be detected based on EMG or accelerometer measurements, to quantify the averaged accuracy, TPR, and FPR of the real-time LFP based decoding. On average, we achieved an accuracy and TPR of about 80%, with an FPR of about 20% (Fig. 5). This means the stimulation was on average switched on for 80% of time when the participants were making any voluntary movements and for 20% of time when the participants were at rest. Real-time tremor provoking posture decoding to drive the DBS was also tested when the patients changed posture from resting to the posture which tended to trigger tremor when there was no stimulation. The closed loop DBS based on tremor detection suppressed postural tremor by 90.5% with a false detection rate of 20.3% when the participant was at rest (Fig. 6).
Figure 5.
Online results of voluntary movement decoding and DBS control. (A) An example of voluntary movement decoding results in ET2. The upper plot shows the acceleration signal with increased value indicating voluntary movements. The middle plot shows online decoding results with 1 and 0 for with and without movement, respectively. The bottom plot shows the power spectra of one bipolar VIM-ZI thalamic LFP signal. The red areas on the figure indicate stimulation artefacts at 130 Hz and sub-harmonics when stimulation was switched on. (B) Averaged accuracy, true positive rate (TPR), and false positive rate (FPR) of voluntary movement decoding across three patients
Figure 6.
Online results of tremor provoking posture decoding and DBS control. EMG and tremor power calculated from accelerometer measurements indicate postures. The power spectra of the ViM thalmic LFP indicates when the stimulation was ON.
IV. Discussions
In the current study, we tested an adaptive DBS system for ET based on the LFPs recorded from the same electrodes in VIM thalamus and ZI used for DBS. Our results suggest that both voluntary movements and posture and any associated tremor could be detected based on VIM-ZI LFPs in real time for triggering DBS. Four decoding models may be necessary. These were determined by our paradigm in the current study, but clinical application may need state identification tools to allow for the selection of the context-appropriate decoding models.
1. Decoding with and without stimulation
Different models were trained and used in this study for decoding with and without ongoing stimulation. This is necessary due to changes in the feature weights, and our results also demonstrated that even with the stimulation artifact, VIM-ZI LFPs still contain enough information for the detection of voluntary movements and posture and any associated tremor, and thus are suitable as the feedback signals for adaptive DBS for ET.
2. Detection of voluntary movements and posture and any associated tremor
In the current study, separate models were trained for the detection of voluntary movements and posture and any associated tremor. This is based on our previous study showing that different features contributed to the detection of postural tremor and voluntary movements, suggesting different models would be required to detect these two different states [10]. Detecting both states helps ensure that stimulation will be switched on wherever needed.
3. Detection algorithms
Several commonly used machine learning methods were tested in the current study. Although these methods gave similar results using the extracted features in the time and frequency domains, it would be interesting to see whether the decoding performance could be further improved using more advanced methods, such as convolutional neural networks.
4. Decoding based on thalamic LFPs
Compared with other adaptive DBS systems developed based on external sensors or cortical strip electrodes in [7]-[9], the advantage of the method proposed here is it doesn't require any external sensor or additional invasive electrode. As a next step, we will also investigate if we can predict the onset of voluntary movements and postural tremor based on VIM-ZI LFPs before movements can be measured based on external sensors, so that the DBS can be switched on before the presence of tremor. This will further improve the clinical effect of the adaptive DBS system.
Conclusion
In this preliminary study, we tested a closed-loop DBS approach for ET based on the LFPs recorded from the same DBS electrodes implanted for stimulation. Results from three participants showed that both voluntary movements and posture and any associated tremor can be detected with a sensitivity around 80 % and a false positive rate of around 20%. These results suggest that responsive DBS for ET can be achieved without the requirement of external sensors or additional electrocorticography strips.
References
- [1].Louis ED, Ottman R. Genetics of Movement Disorders. Academic Press; 2003. Essential tremor; pp. 353–363. [Google Scholar]
- [2].Baizabal-Carvallo JF, et al. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry. 2014;85:567–572. doi: 10.1136/jnnp-2013-304943. [DOI] [PubMed] [Google Scholar]
- [3].Louis ED. Treatment of medically refractory essential tremor. N Engl J Med. 2016;375:792–793. doi: 10.1056/NEJMe1606517. [DOI] [PubMed] [Google Scholar]
- [4].Shih LC, et al. Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor (ET): how prevalent is it? Park Relat Disord. 2013;19:676–679. doi: 10.1016/j.parkreldis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- [5].Hedera P. Emerging strategies in the management of essential tremor. Ther Adv Neurol Disord. 2017;10:137–148. doi: 10.1177/1756285616679123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ramirez-Zamora A, et al. Proceedings of the sixth deep brain stimulation think tank modulation of brain networks and application of advanced neuroimaging, neurophysiology, and optogenetics. Frot Neurosci. 2019;13:936. doi: 10.3389/fnins.2019.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cagnan H, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain. 2017;140:132–145. doi: 10.1093/brain/aww286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamamoto T, et al. On-demand control system for deep brain stimulation for treatment of intention tremor. Neuromodulation J Int Neuromodulation Soc. 2013;16:230–235. doi: 10.1111/j.1525-1403.2012.00521.x. [DOI] [PubMed] [Google Scholar]
- [9].Herron JA, et al. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg. 2017;127:580–587. doi: 10.3171/2016.8.JNS16536. [DOI] [PubMed] [Google Scholar]
- [10].Tan H, et al. Decoding voluntary movements and postural tremor based on thalamic LFPs as a basis for closed-loop stimulation for essential tremor. Brain stimulation. 2019;12(4):858–867. doi: 10.1016/j.brs.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hjorth B, Elema-Schönander AB. EEG analysis based on time domain properties. Electroencephalography and Clinical Neurophysiology. 1970;29:306–310. doi: 10.1016/0013-4694(70)90143-4. [DOI] [PubMed] [Google Scholar]