Abstract
Cell-derived extracellular vesicles (EVs), present in synovial fluid and cartilage extracellular matrix (ECM), are involved in joint development and in the regulation of joint homeostasis. Although the exact function of EVs in these processes remains incompletely defined, the knowledge already acquired in this field suggests a role for these EVs as biomarkers of joint disease, and as a new tool to restore joint homeostasis and enhance articular tissue regeneration. In addition to direct injection of therapeutic EVs into the target site, surface coating of scaffolds and embedding of EVs in hydrogels might also lead to novel therapeutic possibilities. Based on the existing literature of EVs in synovial fluid and articular tissues, and investigation of the molecular factors (including microRNAs) active in joint homeostasis (or during its disturbance), we postulate novel perspectives for the implementation of EVs as a regenerative medicine approach in joint repair.
Extracellular vesicles (EVs) are small membrane-enclosed particles actively released by cells (BOX 1). These structures are found in all tissue types and body fluids studied to date, including synovial fluid and blood1,2. This heterogeneous group of particles (varying in size from ~40 nm to 5 μm) can be formed by direct budding off the cell membrane, or released after fusion of endosomal multivesicular bodies with the plasma membrane3. Among other functions, EVs act as protective carriers for biologically active signalling molecules (such as proteins, enzymes, mRNAs, microRNAs (miRNAs), DNA fragments and lipids) and contain ligands for receptors on the cell membrane of recipient cells. Upon binding to the target cell, EVs can signal from the plasma membrane or, alternatively, fuse with the cell membrane and deliver their cargo into the cytosol, thereby activating or inhibiting specific cellular processes. EVs can also be internalized via endocytosis, subsequently releasing their cargo in endosomal compartments4. Hence, EVs are efficient shuttling vehicles that participate in intercellular communication in a variety of biological processes in vivo 5,6, even over long distances7,8.
Box 1. EV types and nomenclature.
Extracellular vesicle (EV) is a generic name for all secreted lipid-bilayer-enclosed, cell-derived vesicles, defined in 2011 by the International Society for Extracellular Vesicles. However, other terms are often used to refer to EVs, including exosomes, microvesicles, apoptotic bodies, matrix vesicles, ectosomes, prostasomes, membrane particles and shedding vesicles. The different names are mainly based on particular research fields, physiological or pathological condition, vesicle morphology (mainly size), cell-type of origin and route of biogenesis (membrane-shed or endosomal-derived). As several different EV types share overlapping characteristics, and often no unique markers are available to clearly distinguish between EV types, the classification of EVs is difficult, and comparison of research data based on terminology alone can lead to inconsistencies and is not recommended81. In this Perspectives article, we use the generic term ‘extracellular vesicles’ for all EVs, with the exception of matrix vesicles, which are a well-defined EV type.
As early as 1969, matrix vesicles — a specialized type of EVs present in the growth plate of developing bone — were shown to initiate cartilage calcification during endochondral ossification9. During this process, matrix vesicles derived from chondroblasts and osteoblasts collect inorganic phosphate and calcium from the extracellular matrix (ECM), contributing to mineralization by the formation of hydroxyapatite crystals in the vesicle lumen. Eventually, the deposition of these crystals in the ECM results in further mineralization of cartilage10. With the discovery of a range of growth factors and proteins, including bone morphogenetic proteins (BMPs) and vascular endothelial growth factor (VEGF), in matrix vesicles, these structures were thought to be also involved in the regulation of neo-vascularization and the differentiation of chondrocytes and osteoblasts in the growth plate11. Despite the fact that these mineralizing matrix vesicles have not been directly implicated in joint repair and regeneration, they are an example of EVs that facilitate tissue development and regulate homeostasis.
Synovial-fluid-derived EVs, first isolated 2 decades ago12, were found in patients with rheumatoid arthritis (RA)2,12 and were suggested to play a part in this inflammation-driven disorder. Nowadays, EV-mediated communication in the joint has become a new field of interest, especially in the context of inflammatory joint diseases13.
Based on the existing knowledge of EVs derived from synovial fluid and articular tissue, as well as from in vitro studies, we evaluate the role of EVs in the maintenance and restoration of joint homeostasis, and discuss their potential for use in tissue regeneration. Taking into account findings concerning molecular factors (including miRNAs) in joint biology and pathology, we define a concept for clinical application of EVs in the field of joint repair and regeneration.
EVs in the joint
Inflammatory joint disease
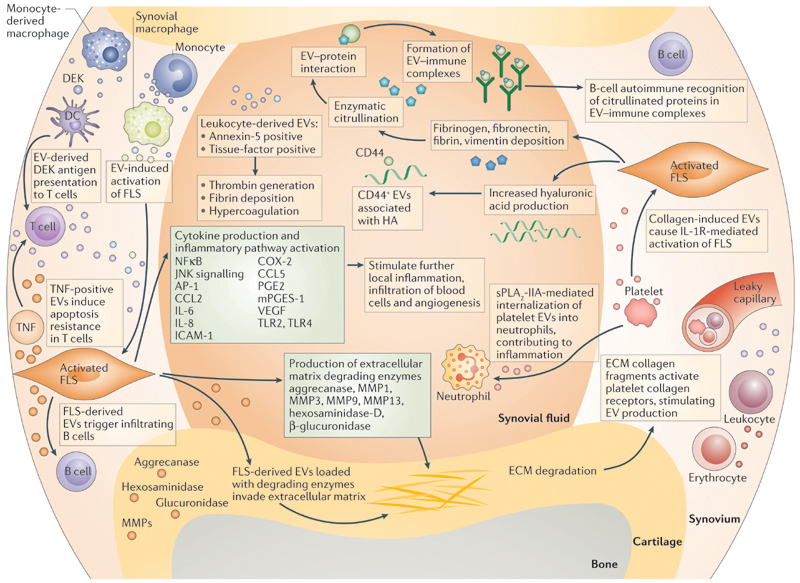
All joint diseases, including RA and osteoarthritis (OA) as the most prevalent, are characterized by a disturbance of the fine balance between anabolic and catabolic cues14. Any disturbance of joint homeostasis is reflected in the levels of soluble factors (such as cytokines, enzymes and growth factors) in the synovial fluid, and possibly also in the numbers and content of EVs. In theory, EVs in the synovial fluid can be derived from two sources: directly from cells in the synovial lining (fibroblast-like synoviocytes (FLSs), macrophage-like synoviocytes and leukocytes recruited from the circulation) or from resting chondrocytes in the cartilage; or indirectly from the blood plasma, of which synovial fluid is an ultrafiltrate. Therefore, various mechanisms of EV-mediated communication in action during deregulation of joint homeostasis could result in inflammatory joint disease (FIG. 1).
Figure 1. Proposed mechanisms of EV‑mediated communication in inflammatory joint disease.
During local inflammation, infiltrating leukocytes (T cells, B cells, monocytes, monocyte-derived macrophages) and resident synovial macrophages activate fibroblast-like synoviocytes (FLSs) in the synovial membrane by extracellular vesicle (EV)-mediated cell-to-cell communication. Activated FLSs further maintain inflammation by production of cytokines and enzymes. By the release of their own EVs, FLSs signal back to immune cells and enzyme-loaded, FLS-derived EVs can invade aggrecan-rich extracellular matrix (ECM). Leukocyte-derived EVs can carry factors that cause local hypercoagulation. Activated-platelet-derived EVs cause IL-1 receptor-mediated activation of FLSs and can be internalized by activated neutrophils, maintaining the inflammatory phenotype of the two cell types. B cells recog-nize citrullinated proteins in EV-immune complexes, which might be part of the autoimmune process in rheumatoid arthritis. AP1, activator protein 1; CCL2, C–C motif chemokine 2; CCL5, C–C motif chemokine 5; COX-2, cyclooxygenase 2 (prostaglandin G/H synthase 2); DC, dendritic cell; HA, hyaluronic acid; ICAM, intercellular adhesion molecule; JNK, c-Jun N-terminal kinase; MMP, matrix metalloproteinase; PGE, prosta-glandin E; mPGES-1, microsomal prostaglandin E synthase 1; sPLA2-IIA, secreted phospholipase A2-IIA; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor.
Evidence in support of a role for EVs during synovial inflammation is provided by studies showing that synovial-fluid- derived EVs from patients with RA or OA can induce cytokine and growth factor release by synoviocytes in vitro 15–17. Furthermore, the catabolic interaction of EVs, derived from IL-1β-stimulated synoviocytes, with chondrocytes in vitro also provides important insights into EV-mediated tissue degradation during joint inflammation18. Leukocyte and erythrocyte-derived EVs, found at high concentrations in the synovial fluid of patients with RA, expose platelet tissue-factor (also known as coagulation factor III or CD142) and support thrombin generation in vitro, suggesting their involvement in hypercoagulation and fibrin deposition2. Furthermore, EVs derived from T cells or monocytes increased the synthesis of matrix metalloproteinases (MMPs), prostaglandin G/H synthase 2 (PTGS2, also known as cyclooxygenase-2 (COX-2)), microsomal prostaglandin E synthase 1 (PTGES) and prostaglandin E2 (PGE2) in FLSs, and resulted in the activation of nuclear factor κB (NFκB), activator protein 1 (AP-1) and JNK (c-Jun N-terminal kinases) signalling pathways in these FLSs19,20. These findings demonstrate an EV-mediated, catabolic effect of immune cells on the cartilage and the synovial membrane.
Studies have suggested the existence of specific EV types and functionalities in different joint diseases, and disease-dependent, morphological EV signatures have been found21. For example, Zhang and colleagues described a membrane-bound form of TNF in EVs shed by RA, but not OA, FLSs22. In addition, platelet-derived EVs were found in synovial fluid of patients with RA, but not in patients with OA23. In this study, Boilard and colleagues identified platelet glycoprotein VI, a collagen receptor, as a trigger for EV production in arthritis23, and found that collagen-induced EVs co-localized with leukocytes in the synovial fluid, where they were able to stimulate FLSs by activating the IL-1 receptor23. Importantly, this was the first observation that suggested a role for ECM molecules (specifically collagen) in cell-activation and EV production. The authors proposed that the synovial membrane would be the ideal location in the joint for interaction between ECM of the synovial membrane and cells from the circulation, theoretically enabling collagen-receptor-mediated platelet activation (and subsequent EV production) via fenestrations in the microvasculature23. In addition, a 2015 study has suggested that platelet-derived EVs in synovial fluid are internalized by neutrophils24, a process that has been suggested to enhance inflammation. Interestingly, conversion of arachidonic acid by EV-associated arachidonate 12(S)-lipoxygenase (12S-LOX) to 12(S)-hydroxyeicosatetraenoic acid (12[S]-HETE) was found to be necessary for platelet-derived EV internalization by immune cells. This conversion seems to be driven by the catalytic activity of secreted phospholipase A2 IIA (sPLA2-IIA) from the inflamed synovial fluid, which can release arachidonic acid from phospholipids in the EV membrane.
Several EV-related mechanisms have been suggested to act during inflammation, including recognition of pathogen-shed EVs by immune cells, EV-mediated shuttling of inflammation-related cytokines and lipid mediators, and the transportation by EVs of proteolytic enzymes that cause tissue destruction4,13. Immune complexes formed after recognition of EV-associated citrullinated proteins (such as vimentin and fibrinogen) by the immune system have also been found25; these processes might propagate inflammation in autoimmune diseases. Indeed, citrullinated proteins were detected in EVs from patients with RA, OA and reactive arthritis26, and EV-associated factors have been discovered that could trigger joint inflammation, including DEK, an autoantigen known to attract neutrophils, CD8+ T cells and natural killer (NK) cells27. EVs carrying these antigens have been proposed to facilitate their efficient uptake by dendritic cells (DCs) for antigen presentation27.
Finally, the expression of Toll-like receptors (TLRs) in RA and OA is upregulated in chondrocytes28 and FLSs29, resulting in increased cytokine, chemokine and enzyme production. Consequently, targeting of TLR signalling, specifically of TLR4, has been suggested as a therapy for these diseases30. The indication that EVs are able to activate monocytes in a TLR2 and TLR4-dependent manner31 — two TLR family members previously associated with inflammatory joint diseases32,33 — suggests that TLR activation in joint inflammation might be mediated by EVs.
ECM-EV interactions
EVs produced by RA FLSs might have the ability to infiltrate aggrecan-rich ECM, as was suggested by Lo Cicero and colleagues after finding aggrecanase activity in these EVs34. Similarly, hexosaminidase D and β-glucuronidase activity detected in EVs derived from synovial fluid and FLSs from patients with RA and OA was linked to cartilage degradation35,36. Although direct evidence is still lacking, EVs are now thought to have a role in the catabolic cascade during inflammatory joint diseases by contributing to the primary degradation of the ECM.
In the context of cancer, EVs positive for the hyaluronic acid (HA) receptor CD44 have been associated with binding of HA in the extracellular environment37. HA, a nonsulfated glycosaminoglycan, is also abundant in the joint, in both synovial fluid and cartilage ECM. Production of HA by FLSs and chondrocytes is regulated by the HA concentration in the synovial fluid, which is affected, in turn, by the disease state in the joint38. Interestingly, CD44 was detected on EVs isolated from healthy synovial fluid, and from chondrocyte or synoviocyte-derived culture media (J.B., unpublished data), indicating that HA can also serve as a matrix for EV binding in synovial fluid and cartilage ECM. Differences in HA concentration during inflammatory events might, therefore, be associated with changes in EV binding and diffusion in the joint.
EV‑associated miRNAs
EV-mediated transfer of miRNAs — silencing molecules that target specific mRNAs during post-transcriptional regulation of gene expression — has been described in the past decade5. Nowadays, EVs are acknowledged as vehicles that efficiently protect fast-degrading RNA molecules, enabling their safe transport within the extracellular space. Given the crucial role of miRNAs in cell function, the transport of these molecules might be one the most important mechanisms by which EVs facilitate intercellular communication.
Two interesting examples of this functional link between EVs and miRNAs involve miR-140 and miR-146a. Although these miRNAs have not been associated with EVs in the joint, they are involved in joint homeostasis and disease, and have been associated with EVs in other tissues39–41. MiRNA-140, one of the few miRNAs highly expressed in chondrocytes, is an important regulator of cartilage homeostasis42. Expression levels of miR-140 were found to be lower in human articular cartilage from patients with OA than from healthy controls, and IL-1β could suppress miR-140 expression in articular chondrocytes43. In addition, mRNA expression of a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) and aggrecan core protein were regulated by miR-140, suggesting a role for this miRNA in regulating the balance between ECM formation and degradation43. These effects were only present in IL-1β-stimulated cells and, therefore, seem to be specific for catabolic processes — an important observation in relation to potential treatment options. In addition to its function as a general immune-regulatory factor, miRNA-146a, differentially expressed in both RA and OA44, also has a role during osteoclastogenesis in RA45. Given these observations, EV-mediated transfer of miRNAs is likely to be a mechanism in pathological processes in the joint and, hence, a promising target deserving future research.
EV applications in the joint
Biomarkers
The special features of EVs, including their particular composition, mechanisms of regulated release and cargo-protective properties, have led to hope that these structures can be used as biomarkers to monitor physiological and pathological processes46–48. For example, in cancer research, the potential of circulating tumour-derived EVs as biomarkers in liquid biopsies is currently being investigated for use in early tumour detection, in defining disease stage and in prediction of treatment outcome49. Joint disease-specific EV signatures might also be identified in the circulation or synovial fluid.
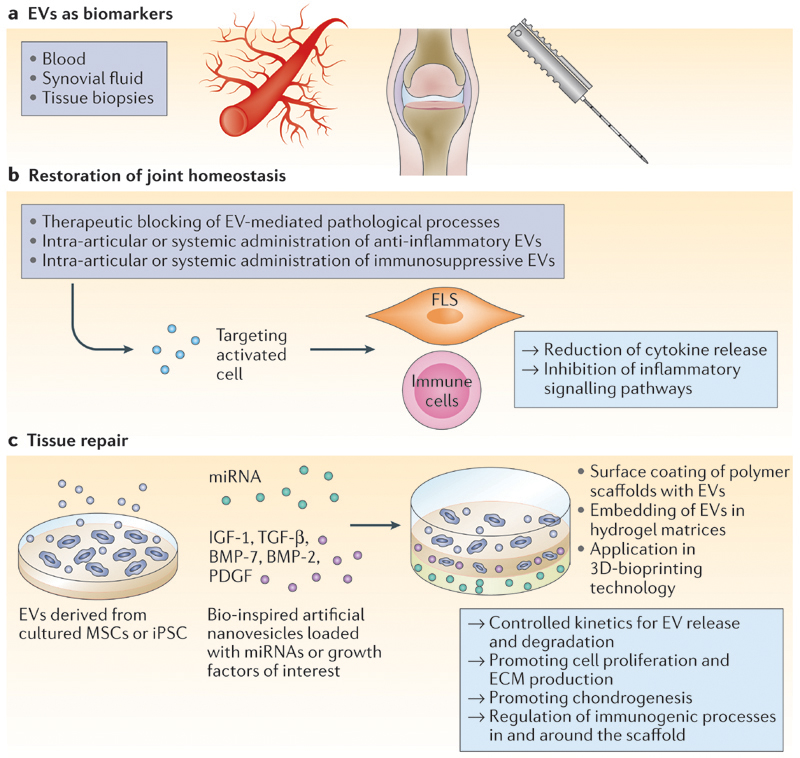
Based on the reports of EVs from synovial fluid and blood of patients with RA and OA discussed in this Perspectives, we hypothesize that at least three possible EV biomarker types could be defined for joint diseases (FIG. 2): immune-cell-derived inflammatory EVs in the circulation could be a sign of early-onset joint disease; EVs in the synovial fluid of patients could provide information about inflammation type, disease state and progression; and EVs derived from FLSs and chondrocytes, isolated from healthy individuals, could provide information about predisposition for autoimmune disease or cartilage disorders. Furthermore, considering the possible role of EV-associated miRNAs in inflammatory joint disease, evaluation of miRNA levels in synovial-fluid-derived EVs from patients with RA or OA would further substantiate the involvement of EVs in deregulated joint homeostasis, and might lead to the development of novel diagnostic tools.
Figure 2. Proposed applications of EVs in joint disease.
a | Extracelluar vesicles(EVs) have bio-marker potential for joint diseases, both to predict disease development in healthy individuals and to monitor disease progression in patients. In the blood, circulating inflammatory EVs can be an alert for early onset of inflammatory joint diseases. In synovial fluid, EVs from patients can provide information about inflammation type, disease state and disease progression. In the context of tissue biopsies, EVs derived from cultured synovial tissue could indicate predisposition for development of autoimmune disease and cartilage disorders. b,c | Regulatory EVs can be exploited for intra-articular injection or for application in biomaterials designed for implantation purposes to restore joint homeostasis and improve tissue repair in patients. BMP, bone morphogenetic protein;|GF-1, insulin-like growth factor 1; iPSC, induced pluripotent stem cell; miRNA, microRNA; MSC, mesenchymal stem/stromal cell; PDGF, platelet derived growth factor; TGF-β, transforming growth factor-β.
Restoration of joint homeostasis
In addition to containing information related to the disease state of an individual, EVs could also be applied as therapy to counteract inflammatory events at the tissue level (FIG. 2). Previous successful attempts to target synovial inflammation in experimental arthritis with liposomes loaded with glucocorticoids or with an immuno-suppressive peptide showed the potential of targeted therapies50,51. Furthermore, EVs derived from IL-10-stimulated or IL-10-expressing DCs have shown their potential as modulators of the inflammatory immune response during experimentally induced joint disease52. Interestingly, besides EV periarticular injection, systemic injection also had a therapeutic effect in the same study52.
EVs derived from multipotent mesenchymal stem/stromal cells (MSCs) have also been demonstrated to exert immunosuppressive and anti-inflammatory effects53; for example, in a clinical trial using MSC-derived EVs as treatment, symptoms were considerably mitigated in a patient with therapy-refractory graft-versus-host disease54. Therefore, the role of MSCs as modulators of joint homeostasis, which includes previously described anti-inflammatory properties55, suggests these cells as interesting therapeutic alternatives in the restoration of homeostasis in the inflamed joint (relevant, for example, for patients with RA). Based on the current knowledge of EV-mediated immune regulation56, MSC-derived EVs could become a part of these therapeutic efforts for joint diseases53.
Tissue repair
EVs also hold potential as inducers of tissue regeneration, given that they affect regulatory processes including cell recruitment, proliferation and differentiation57. Current regenerative treatments for articular cartilage often rely on the use of biomaterials combined with autologous chondrocytes expanded in vitro 58. In this context, the use of MSCs as a cellular therapy is increasingly gaining attention despite difficulties in controlling undesired ossification of the newly formed tissue59. New advances in cocultures of primary chondrocytes with allogeneic MSCs have simplified the treatment60, and resulted in a shift from in vitro expansion and subsequent reimplantation to a single-stage procedure61. As MSCs establish a regenerative microenvironment by secreting bioactive molecules62, the supposed underlying mechanism by which these cells enhance chondrogenic differentiation is thought to be based on trophic factors. Consequently, soluble biomolecules — and, possibly, EVs — are likely to be the main effectors in the MSC-driven regenerative pathway53. In line with this assumption, in a scenario of tissue injury, mRNA and miRNA-laden EVs from local MSCs in the tissue could lead to genetic reprogramming and induction of cell dedifferentiation, instructing a cell-cycle ‘reboot’ and starting the regeneration process63,64. These regenerative approaches could be simplified, potentially, by the replacement of cultured MSCs with the MSC secretome, or even by specific MSC-derived EVs. This is an exciting prospect and might lead to an off-the-shelf regenerative treatment with fewer regulatory constraints than the present strategies, as no living cells would be injected into patients65. Moreover, the efficacy of MSC-derived EVs (or of EVs derived from other cell types, such as induced pluripotent stem cells) might be further enhanced by the incorporation of selected biological molecules, such as miRNAs and proteins, which support chondrogenesis66,67(FIG. 2).
Challenges and translation
EV preparation and selection
Although ultracentrifugation and density gradient flotation are recommended techniques for EV isolation for analytical and experimental purposes, these procedures are not feasible for clinical applications (BOX 2). One of the biggest challenges for the future is the large-scale production and isolation of (engineered) EVs for clinical application. Therefore, the development of robust, scalable biological production procedures and of appropriate EV isolation methods is urgently needed. Furthermore, detailed knowledge of the complex lipid, protein and nucleic acid composition of EVs could be used to design bio-inspired synthetic carriers with lower variability than that of EVs produced biologically. For example, EV lipid membrane selection or modification could be used to improve the delivery of EVs and their interaction with target cells. Some EV lipids can interact with specific lipid receptor proteins on target cells (for instance, phosphatidylserine binding to TIMD-4, or glycosphingo-lipids binding to sialoadhesin), and EVs have been shown to carry bioactive lipids (such as eicosanoids and lysophospholipids) and enzymes that convert structural lipids into bioactive molecules (examples of these enzymes include sPLA2-IIA, 12S-LOX and prostaglandin synthases)24,68. Hence, targeted delivery of these bioactive compounds to particular cells might enhance their effect and specificity of this therapeutic approach.
Box 2. Isolation and purification of EVs.
Isolation of extracellular vesicles (EVs), particularly from body fluids, is challenging, and the quality of the EV isolation procedure determines the validity of the claim of EV-mediated functional effects1,81. Lack of sufficient information about EV isolation procedures and EV characteristics complicates the interpretation of data and hampers the comparability of studies. Moreover, limited centrifugation steps, omission of density gradient centrifugation and lack of EV-depleted controls preclude conclusions that observed functional effects are caused solely via EVs81. Skriner and colleagues26 were among the first to isolate EVs from synovial fluid using a multistep centrifugation protocol followed by sucrose density gradient flotation and size determination by electron microscopy. These steps justify their conclusion that the effects observed can be fully attributed to small EVs (<300 nm). In previous reports of EVs derived from synovial fluid, EV characteristics were not specified and the EV fraction isolated most likely contained several EV subtypes as well as nonvesicular particulates. Immune complexes are also known to be present in synovial fluid and can hamper EV analysis, providing an additional reason to purify EVs using ultracentrifugation and density gradient flotation82. Finally, to provide researchers with guidelines, the International Society for Extracellular Vesicles published the minimal experimental requirements for definition of EVs and their function in 2014 (REF. 81).
EV delivery
Achieving effective and controlled delivery of EVs to disease sites is challenging, but is paramount to the efficient restoration of joint homeostasis and sustained positive effect on the regenerative process. Different modes of EV delivery could include intravenous (systemic) EV injection, intra-articular EV injection, and surface coating of scaffolds or embedding in hydrogel matrices57. Systemic EV injection can result in passive targeting to inflamed joints owing to increased synovial vascular permeability, as has been observed in arthritic joints51,69. Conversely, intra-articular injection of EVs, possibly in combination with other therapeutics or regenerative cells, is a direct application at the affected site.
The use of EV-mediated delivery is expected to be more efficient than using soluble proteins or RNA molecules, which are usually prone to fast degradation after injection. However, in view of the gradual clearance of EVs from the joint space, a more sustained effect might be achieved by temporarily immobilizing EVs on or within a biomaterial scaffold. A wide range of scaffolds for musculoskeletal regenerative applications is currently being evaluated, generally in combination with cells and bioactive molecules70. Coating with, or incorporation of, EVs is an additional opportunity to optimize the functionality of these scaffolds (FIG. 2). More specifically, EVs could be bound on the surface of polymer scaffolds by specific linkers, antibodies or ECM components such as HA37.
Alternatively, EVs could also be incorporated within a hydrogel matrix. Hydrogels recapitulate several features of the natural ECM and enable the encapsulation of cells and EVs. Polymer network density and degradation kinetics of hydrogels can be adjusted and tailored to the specific release kinetics of EVs. For this purpose, the field can take particular advantage of the extensive experience available in the embedding of liposomes for drug delivery applications71. Moreover, it has been suggested that EV surface modification with functional polymers can be used to modulate their release kinetics further72. Nevertheless, how specific biomaterials interact with embedded EVs and preserve their activity needs further research. For example, combinatorial use of different hydrogels loaded with distinct EV populations within a single construct could further facilitate the controlled and sequential release of the different fractions of embedded EVs. 3D printing technologies now have the ability to generate these multimaterial constructs with precisely defined anatomical architectures73,74, and cell or EV-laden hydrogels can be used as potential building blocks for the production of tissue analogues.
Experimental considerations
In previous clinical trials of EV therapy, these structures were shown to be generally well-tolerated and to have a low risk of immunogenicity and toxicity75. However, important questions remain about the kinetics and biodistribution of EVs. The distribution of systemically administered EVs has been demonstrated to be dependent on dose, route of administration and cellular origin of the EVs76. No biodistribution data exists for articular tissues yet, and detailed investigations of EV distribution and clearance from the synovial fluid and articular tissues will be necessary for each EV therapy. Directing EVs to the target site and decreasing the risk of fast clearance from the tissues of interest, as has been achieved in cancer research77, might be a way to increase treatment efficiency.
The future development of EV-mediated therapeutic approaches for joint diseases requires further elucidation of the potential roles of EVs in joint homeostasis and regeneration. Advanced bioreactor systems and ex vivo culture models can, to a certain extent, be used for this purpose, but animal models will be indispensable for translation to the clinic. For translational studies in musculoskeletal diseases, the horse is generally seen as the best animal model for joint disease, whereas the dog is better suited for intervertebral disk disease (IVDD) studies78,79. The prevalence of OA and IVDD in horses and dogs, respectively, is considerable, enabling the design of clinical trials that use them as experimental animals and therapeutic target animals simultaneously, an important factor from an ethical perspective. A practical advantage of the equine model is the large size of the joints, which enables sequential sampling of relevant quantities of synovial fluid for the monitoring of EV profiles. Furthermore, comparative studies of human and equine articular cartilage have shown great similarities in thickness and composition between the two species80.
Conclusions
EVs have a prominent role in joint development and in the regulation of intra-articular homeostasis. Although their composition and function in the joint are not yet clearly understood, EVs are envisaged as next-generation biomarkers of the pathophysiological state of the joint. Further, an important role for EVs in future therapies for the treatment of joint disorders is also foreseen, in particular as they provide a simpler and safer alternative to current cell-based therapeutic options. EVs can also be combined with scaffolds, either bound on their surface or embedded within scaffold matrices, enabling the controlled release of specific populations of EVs. Bioengineering approaches could further assist in defining the location of EV delivery by 3D generation of organized tissue constructs. Research on biomaterial-based release of well-defined EV populations is still in its infancy, but holds considerable promise. However, the therapeutic use of EVs demands high-quality standardization of isolation and analysis techniques to yield reproducible EV preparations. In this regard, synthetic alternatives to cell-derived EVs might simplify production and scalability of EV-based therapeutic delivery systems, further enhancing their therapeutic potential.
Acknowledgements
The authors’ research work is supported by the Dutch Arthritis Foundation (grant numbers LLP-12 and LLP-22; J.M., P.R.W.), the EU Seventh Framework Programme (FP7/2007–2013, grant agreement 309962 [HydroZONES]) (J.M.), the European Research Council (grant agreement 647426 [3D-JOINT]) (J.M., P.R.W.), and a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant number FES0908) (J.B.).
Footnotes
Author contributions
J.M. and J.B. contributed equally to researching data for the article and writing the manuscript. All authors made substantial contributions to discussion of content and to review and edit the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Contributor Information
Jos Malda, Department of Orthopaedics, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, Netherlands.
Janneke Boere, Department of Equine Sciences, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 112, 3584 CM, Utrecht, Netherlands.
Chris H.A. van de Lest, Department of Biochemistry & Cell Biology, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 2, 3584 CM, Utrecht, Netherlands
P.René van Weeren, Department of Equine Sciences, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 112, 3584 CM, Utrecht, Netherlands.
References
- 1.Witwer KW, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berckmans RJ, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857–2866. doi: 10.1002/art.10587. [DOI] [PubMed] [Google Scholar]
- 3.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 4.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 5.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Yanez-Mo M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.27066. 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zomer A, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridder K, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 11.Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab. 2008;26:514–519. doi: 10.1007/s00774-008-0859-z. [DOI] [PubMed] [Google Scholar]
- 12.Fourcade O, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 13.Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 14.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berckmans RJ, et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther. 2005;7:R536–R544. doi: 10.1186/ar1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messer L, et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res Ther. 2009;11:R40. doi: 10.1186/ar2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reich N, et al. Microparticles stimulate angiogenesis by inducing ELR+ CXC-chemokines in synovial fibroblasts. J Cell Mol Med. 2011;15:756–762. doi: 10.1111/j.1582-4934.2010.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato T, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16:R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungel A, et al. Microparticles stimulate the synthesis of prostaglandin E2 via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007;56:3564–3574. doi: 10.1002/art.22980. [DOI] [PubMed] [Google Scholar]
- 20.Distler JH, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102:2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyorgy B, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE. 2012;7:e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, et al. A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 23.Boilard E, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchez AC, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc Natl Acad Sci USA. 2015;112:E3564–E3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloutier N, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5:235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54:3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 27.Mor-Vaknin N, et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006;26:9484–9496. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sillat T, et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013;84:585–592. doi: 10.3109/17453674.2013.854666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu F, et al. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and Th17 cell responses in rheumatoid arthritis. PLoS ONE. 2014;9:e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis — finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 31.Bretz NP, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HA, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 33.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62:2004–2012. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo Cicero A, Majkowska I, Nagase H, Di Liegro I, Troeberg L. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 2012;31:229–233. doi: 10.1016/j.matbio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasztoi M, et al. The recently identified hexosaminidase D enzyme substantially contributes to the elevated hexosaminidase activity in rheumatoid arthritis. Immunol Lett. 2013;149:71–76. doi: 10.1016/j.imlet.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Pasztoi M, et al. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res Ther. 2009;11:R68. doi: 10.1186/ar2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–122. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- 39.Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937–10949. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014;86:433–444. doi: 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- 41.Gernapudi R, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150:685–695. doi: 10.1007/s10549-015-3326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong E, Reddi AH. MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering. Tissue Eng Part B Rev. 2012;18:445–453. doi: 10.1089/ten.TEB.2012.0116. [DOI] [PubMed] [Google Scholar]
- 43.Miyaki S, et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki K, et al. Expression of microRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 2009;60:1035–1041. doi: 10.1002/art.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011;63:1582–1590. doi: 10.1002/art.30321. [DOI] [PubMed] [Google Scholar]
- 46.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23743. 23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julich H, Willms A, Lukacs-Kornek V, Kornek M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front Immunol. 2014;5:413. doi: 10.3389/fimmu.2014.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanniasinghe AS, et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin Immunol. 2014;151:43–54. doi: 10.1016/j.clim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Metselaar JM, et al. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann Rheum Dis. 2004;63:348–353. doi: 10.1136/ard.2003.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 53.Heldring N, Mager I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 54.Kordelas L, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald GI, Augello A, De Bari C. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–2557. doi: 10.1002/art.30474. [DOI] [PubMed] [Google Scholar]
- 56.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grande DA, Schwartz JA, Brandel E, Chahine NO, Sgaglione N. Articular cartilage repair: where we have been, where we are now, and where we are headed. Cartilage. 2013;4:281–285. doi: 10.1177/1947603513494402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savkovic V, et al. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9:469–488. doi: 10.2174/1574888x09666140709111444. [DOI] [PubMed] [Google Scholar]
- 60.De Windt TS, et al. Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Transl Med. 2014;3:723–733. doi: 10.5966/sctm.2013-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US National Library of Medicine. ClinicalTrials gov. 2014 [online], https://clinicaltrials.gov/ct2/show/NCT02037204.
- 62.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 64.Bruno S, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamichhane TN, et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21:45–54. doi: 10.1089/ten.teb.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vonk LA, Kragten AH, Dhert WJ, Saris DB, Creemers LB. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthr Cartil. 2014;22:145–153. doi: 10.1016/j.joca.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Cloutier N, et al. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 70.Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol. 2015;11:213–222. doi: 10.1038/nrrheum.2015.27. [DOI] [PubMed] [Google Scholar]
- 71.Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 72.Sawada S, et al. Functional polymer gel–exosomes hybrids for drug delivery system and tissue engineering. Presented at the 2014 ISEV Annual Meeting; 2014. [abstract P4C-192] [Google Scholar]
- 73.Malda J, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 74.Visser J, et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5 doi: 10.1088/1758-5082/5/3/035007. 035007. [DOI] [PubMed] [Google Scholar]
- 75.Dai S, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiklander OP, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.26316. 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohno S, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hurtig MB, et al. Preclinical studies for cartilage repair: recommendations from the International Cartilage Repair Society. Cartilage. 2011;2:137–152. doi: 10.1177/1947603511401905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergknut N, et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila. Pa 1976) 2012;37:351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 80.Malda J, et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr Cartil. 2012;20:1147–1151. doi: 10.1016/j.joca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Lotvall J, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.26913. 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gyorgy B, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]