Abstract
Background
Beta-based adaptive Deep Brain Stimulation (aDBS) is effective in Parkinson’s disease (PD), when assessed in the immediate post-implantation phase. However, the potential benefits of aDBS in patients with electrodes chronically implanted, in whom changes due to the microlesion effect have disappeared, are yet to be assessed.
Methods
To determine the acute effectiveness and side-effect profile of aDBS in PD compared to conventional continuous DBS (cDBS) and no stimulation (NoStim), years after DBS implantation, 13 PD patients undergoing battery replacement were pseudo-randomised in a crossover fashion, into three conditions (NoStim, aDBS or cDBS), with a 2-min interval between them. Patient videos were blindly evaluated using a short version of the Unified Parkinson’s Disease Rating Scale (subUPDRS), and the Speech Intelligibility Test (SIT).
Results
Mean disease duration was 16 years, and the mean time since DBS-implantation was 6.9 years. subUPDRS scores (11 patients tested) were significantly lower both in aDBS (p = <.001), and cDBS (p = .001), when compared to NoStim. Bradykinesia subscores were significantly lower in aDBS (p = .002), and did not achieve significance during cDBS (p = .08), when compared to NoStim. Two patients demonstrated re-emerging tremor during aDBS. SIT scores of patients who presented stimulation-induced dysarthria significantly worsened in cDBS (p = .009), but not in aDBS (p = .407), when compared to NoStim. Overall, stimulation was applied 48.8% of the time during aDBS.
Conclusion
Beta-based aDBS is effective in PD patients with bradykinetic phenotypes, delivers less stimulation than cDBS, and potentially has a more favourable speech side-effect profile. Patients with prominent tremor may require a modified adaptive strategy.
Keywords: Parkinson’s disease, Adaptive deep brain stimulation, Subthalamic nucleus, Local field potentials, Beta oscillations, Closed-loop, Dysarthria
Introduction
Parkinson’s disease (PD) patients in advanced stages of the disease usually present with motor symptoms which are not sufficiently suppressed by dopaminergic medication, together with intolerable medication-related side-effects. Deep brain stimulation (DBS) is a successful treatment option as the disease progresses [1]. Although DBS is highly effective, both in terms of motor improvement and improvement in quality of life, there are still limitations. These include incomplete symptom suppression and side effects, such as stimulation-induced dysarthria (SID), and dyskinesias. In addition to this, most of the studies have documented the benefits of DBS only up to three years after implantation [2]. A handful of studies that have followed DBS patients in the long term (5–10 years after implantation) have suggested that the efficacy of DBS may reduce over time, especially with bradykinesia components, narrowing the therapeutic window [3,4]. Recent developments in adaptive DBS (aDBS) systems may help overcome some of these drawbacks [5]. In aDBS, the amount of stimulation is modulated according to variations in the clinical state, or in neural activity patterns underpinning clinical state. Here the aim is to only deliver stimulation as necessary to improve motor impairment, thereby avoiding side-effects when stimulation can be spared or lowered. Up to now, the majority of clinical studies investigating aDBS in PD have applied stimulation based on the amount of beta activity (13–35 Hz) in the subthalamic nucleus (STN) local field potential (LFP), as this biomarker is correlated with contralateral bradykinesia and rigidity [6,7], and is suppressed by DBS [8].
Although studies have confirmed the efficacy of ‘beta-based’ aDBS [9–13], and suggested a superior side effect profile compared to continuous, conventional, DBS (cDBS) [14,15], these have been mostly performed in newly implanted patients. Such studies are potentially compromised by the microlesion effect [16], as this confounding factor provides temporary symptom relief, which can mask the true effects of stimulation. Consequently, bradykinesia or tremor may be suppressed by the microlesion effect, and the impact of intermittent OFF stimulation periods, caused by alternately ramping stimulation up and down, thereby masked. For this reason, it is important to assess the acute effects of aDBS in chronically implanted patients. Currently it is feasible to investigate the utility of aDBS in chronically implanted patients using externalised electrodes [17], and in patients implanted with bidirectional devices [18]. Nevertheless, whether there is additional benefit over cDBS, particularly with respect to side-effects, remains unclear. One such side-effect is SID, and another is motor impulsivity, as measured by tests of response inhibition [19]. Here, we compare the clinical effect, efficiency and side-effect profile of aDBS with both cDBS and no stimulation in PD patients with long implanted electrodes in the STN.
Materials and methods
Patients
We tested 13 patients with advanced idiopathic PD who were chronically treated with bilateral DBS of the STN and needed battery replacement surgery (Table 1). All patients gave written informed consent to the study protocol, which was approved by the local ethics committee. The study was registered in the Dutch Trial Register (trialregister.nl, trial # 5456) and the study protocol was published [20]. All patients stopped prolonged-release dopaminergic medication at least 24h prior to the measurements. In this group of advanced PD patients (mean disease duration: 16 years) the severity of OFF periods was considerably higher than in newly implanted patients. For that reason, we decided that patients skipped at least one dose of regular antiparkinsonian medication before the surgery, in order to achieve ≥6h of off-medication at the moment of the recordings. This washout period was enough to resemble the acute OFF periods that patients experience immediately after medication wears off. Battery replacement surgery was performed under local anaesthesia.
Table 1.
Clinical characteristics of the PD patients included in the study. Pt = patient, S = sex, A = age at the moment of the experiment, y = years, PD = Parkinson’s disease, M = male, F = female, LEDD = levodopa equivalent daily dose, L = left, R = right, C = case (used for monopolar stimulation).
| Pt | S | A (y) | Time since PD start (y) | DBS use (y) | Most affected side | Tremora | Medication | Clinical setting | Experimental setting Contacts used Parameters | Freq used for filtering (±3Hz) | OFF Beta peak (Hz)b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contacts used | Parameters | Contacts used | Parameters | ||||||||||
| PD01c | M | 58 | 24 | 9 | Left | Levodopa, pramipexoleamantadine 1738 mg LEDD | L: 3/2+ R: 10/C+ |
2.9V 60μs 185Hz 0.8V 60μs 185Hz |
L: 2/C+ R: 10/C+ |
1.8V 60μs 130Hz 1.8V 60μs 130Hz |
15 | L: 15 R: 15 |
|
| PD02 | M | 47 | 16 | 12 | Right | No | Levodopa pramipexole 1538 mg LEDD | L: 3/2+ R: 11/C+ |
3.5V 60μs 135Hz 2.2V 60μs 135Hz |
L: 2/C+ R: 10/C+ | 2V 60μs 130Hz 2.2V 60μs 130Hz |
15 | L: 15 R: 13 |
| PD03 | M | 46 | 8 | 5 | Left | No | Levodopa duodopa 1582 mg LEDD | L: 1/2+ R: 11/10+ |
2.2V 90μs 180Hz 1.5V 90μs 180Hz |
L: 2/C+ R: 10/C+ |
1.86V 60μs 130Hz 1.86V 60μs 130Hz |
20 | L: 21 R: 21 |
| PD04 | F | 49 | 9 | 6 | Left | Yes | Levodopa rotigotine 1558 mg LEDD | L: 1/2+ R: 9/10+ |
2.8V 60μs 180Hz 1.2V 60μs 180Hz |
L: 1-/C+ R: 9/C+ |
2.8V 60μs 130Hz 1.2V 60μs 130Hz |
17 | L: 17 R: 19 |
| PD05 | M | 67 | 10 | 5 | Left | Yes | Levodopa 1500 mg LEDD | L: 2/C+ R: 11/C+ |
1.7V 60μs 180Hz 2.2V 60μs 180Hz |
L: 2/C+ R: 10/C+ |
1V 60μs 130Hz 1.3V 60μs 130Hz |
20 | L: 22 R: 21 |
| PD06d | F | 64 | 23 | 9 | Left | No | Duodopa amantadine 1759 mg LEDD | L: 2/3+ | 3V 60μs 130Hz | L: 1/C+ | 2.54V 60μs 130Hz |
22 | L:21 |
| PD07e | M | 59 | 19 | 9 | Right | Yes | Duodopa 2540 mg LEDD | L: 0/1 + R: 9/C+ |
2V 60μs 1.85Hz | L: 1/C+ | 2V 60μs 130Hz 2V 60μs 130Hz |
23 | L: 21 R: 21 |
| PD08 | M | 54 | 7 | 3 | Right | Levodopa pramipexole 725 mg LEDD | L: 0/-1/-2/3+ R: 9/10+ |
4.5V 90μs 190Hz 0.6V 60μs 190Hz |
L: 1/C+ R: 10/C+ |
2.5V 60μs 130Hz 1.5V 60μs 130Hz |
15 | L: 16 R: 16 |
|
| PD09 | M | 66 | 32 | 8 | Left | No | Levodopa, pramipexole amantadine 1125 mg LEDD | L: 1/2+ R: 10/11 + |
4.5V 90μs 135Hz 4.8V 90μs 135Hz |
L: 1/C+ R: 9/C+ |
2.05V 60μs 130Hz 2.05V 60μs 130Hz |
18 | L: 16 R: 15 |
| PD10 | M | 48 | 12 | 4 | Left | Yes | Levodopa, pramipexole amantadine 1081 mg LEDD | L: 1/C+ R: 9/C+ |
3.2V 120μs 130Hz 2.4V 120μs 130Hz |
L: 1/C+ R: 9/C+ |
3.2V 60μs 130Hz 2.4V 60μs 130Hz |
29 | L: 29 R: 27 |
| PD11 | M | 81 | 15 | 6 | Left | No | Levodopa, pramipexole amantadine 900 mg LEDD | L: 1/2+ R: 10/9+ |
2.1V 60μs 135Hz 2.5V 60μs 135Hz |
L: 1/C+ R: 10/C+ |
2.5V 60μs 130Hz 2.5V 60μs 130Hz |
17 | L: 17 R: 17 |
| PD12 | M | 71 | 12 | 7 | Right | No | 0 mg LEDD | L: 2/C+ R: 11/C+ |
3.5V 80μs 90Hz 3.6V 80μs 90Hz |
L: 2/C+ R: 10/C+ |
3V 60μs 130Hz 3V 60μs 130Hz |
18 | L: 18 R: 18 |
| PD13 | M | 72 | 21 | 7 | Right | Yes | Levodopa amantadine 1098 mg LEDD | L: 2/C+ R: 8/C+ |
2.3V 70μs 185Hz |
L: 2/C+ R: 10/C+ 2.9V 90μs 185Hz |
3V 60μs 130Hz 3V 60μs 130Hz |
26 | L: 26 R: 24 |
| Mean (±SE) | 60.1 (3.0) | 16 (2.0) | 6.9 (0.6) | 1318.7 (169.4) | 19.6 (1.2) | 19.2 (1.2) | |||||||
Tremor present at the moment of the trial.
Peaks reported here were calculated offline. Therefore, they might slightly differ with the frequencies used to filter the signal intraoperatively.
Due to a technical failure in one of the LFP channels, this patient was measured unilaterally in both hemispheres.
This patient had the right electrode implanted in the GPi. Therefore, only results from the right hand (left electrode) were included in the analysis.
This patient had the right electrode off as their standard clinical setting, as only the right hemibody required both stimulation and medication to supress clinical symptoms. However, both electrodes were tested and included in the analysis.
Recording procedure
In brief, the old battery was removed and a custom-made external stimulator-amplifier device (for details see Ref. [9]), which is able to record and stimulate simultaneously, was connected to the chronically implanted DBS electrodes (Fig. 1). From this point the protocol took about 25–30 min to be completed. Bipolar STN local field potentials (LFPs) were recorded in the resting state for 30–60 s, using contact pairs 02 and 13. Power spectral density estimates in the beta band range and their peak frequency were calculated. The contact (either 1 or 2) in the middle of the contact pair with the greatest beta activity on each side was selected for stimulation, and the beta activity in the bipolar channel bridging the stimulation contact used for the feedback control of aDBS. A common frequency range (±3Hz) that included the beta-peak frequencies of both hemispheres, was used in each patient to filter bilateral LFPs, based on the observation that beta peak frequencies are similar between sides [21,22]. Optimal stimulation voltages were determined before the experimental conditions, based on the response to monopolar cDBS, until either optimal symptom reduction (bradykinesia and/or tremor) was achieved, side-effects occurred or voltage-dependent artefacts interfered with the recordings. Stimulation was monopolar, with the return electrode being a large skin electrode over the neck or shoulder [9]. Bilateral aDBS, cDBS and no stimulation (NoStim) were applied in a pseudo-randomised order, with a short washout period (2 min) between them (Fig. 2). aDBS was triggered on and off when rectified beta power crossed a median power threshold up and then down, respectively. Supplementary video 1 shows examples of the signals recorded on-line during NoStim, cDBS and aDBS.
Fig. 1. Schematic representation of aDBS.
Upper part: During the operation, and after the old battery is exposed, the battery is detached from the DBS electrodes and explanted. At this moment, two temporary wires are attached to the DBS electrode extension cables at the level of the chest incision, and connected to a combined stimulator and amplifier (represented here).
Lower part: an aDBS recording is depicted. Left recordings are obtained from electrodes 0–2, while stimulation is provided from contact 1. Right recordings are obtained from electrodes 1–3, while stimulation is provided from contact 2. From top to bottom:
Original signal from left (blue) and right (red) electrodes, using a bandpass filter from 3 to 37 Hz. At this stage it is possible to appreciate artefacts caused by stimulation.
-Left and right LFPs filtered around the beta peak. Here it is possible to observe the individual beta bursts.
-Left and right envelopes of the beta-filtered and rectified LFPs. The dotted lines represent the thresholds selected to trigger stimulation.
-Stimulation bursts, with a ramping period when stimulation is switched on and off. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
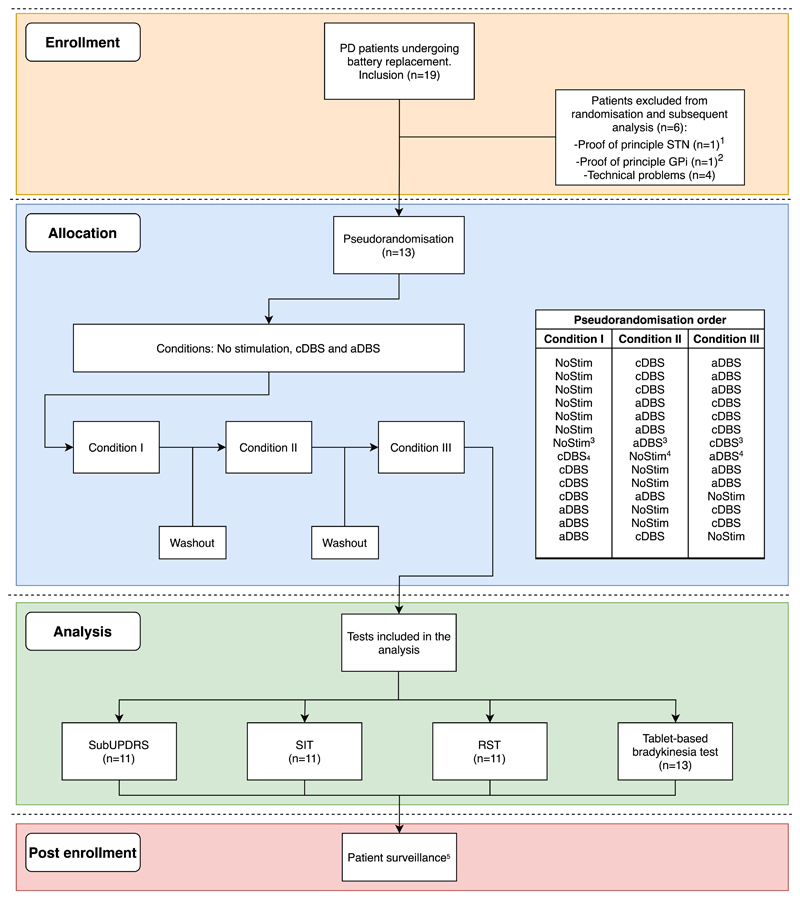
Fig. 2. Flowchart of the inclusion algorithm and pseudorandomisation order.
1The protocol and tests were modified after the inclusion of this patient, who was therefore excluded. Findings have been reported in Ref. [17].
2This patient was operated in the GPi, and therefore the results were reported separately in Ref. [54]
3,4These randomisation orders belong to the same patient (PD01). As this patient had to be measured unilaterally in both hemispheres, due to a defect in one of the aDBS box channels, each hemisphere was assigned to a different randomisation order.
5During patient surveillance, one patient presented a superficial infection on the surgical site, which was resolved completely after antibiotic treatment.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.brs.2020.07.016
Tests and data processing
During each of the three conditions, the following four tests were performed (See Ref. [20] for a more detailed description of the tests):
-
-
A short version of the motor Movement Disorders Society version of the Unified Parkinson’s Disease Rating Scale (sub-UPDRS) [23] was assessed and video recorded for subsequent blinded rating. Items tested bilaterally included were finger tapping, hand movements, hand pronation-supination, rest tremor and postural tremor. Videos were evaluated by three clinicians with expertise in movement disorders. Total scores were calculated for each rater/patient combination. Subgroup scores were calculated for bradykinesia and tremor items.
-
-
The speech intelligibility test (SIT) [14], in which each participant had to read out approximately 110 words divided into 18 sentences. Sentences were recorded, and the number of intelligible words of each SIT was blindly evaluated by a speech therapist. The total amount of intelligible words of each trial was determined. This score was normalised between 0 and 1, with 0 representing completely unintelligible speech.
-
-
Response inhibition was assessed with a tablet-based version of the reverse Stroop effect (RST) [24], in which a colour word is presented, and the patient has to select the colour named by the word, independently of which colour the word is printed. The paradigm included 5 congruent words and 15 incongruent words randomly presented on each trial. Reaction times were determined for each word, and the amount of correct words for congruent and incongruent conditions were calculated. Reaction times with duration ≤200 or ≥5000 ms were discarded. It has been postulated that any impaired Stroop-test responses in PD patients might be a consequence of deficits in attentional resources, rather than impairments in impulse control [25]. Accordingly we chose the RST over the Stroop test as the former arguably is less attention demanding [26].
-
-
A bradykinesia test was performed using a tablet-based version of a previously validated tapping test [27,28], in which patients had to press two separate squares in an alternating pattern (20 iterations). The first iteration of each trial was discarded, as it systematically differed from the rest, leaving 19 iterations for further analysis. Incorrect iterations were defined as each time a patient pressed the same square twice.
In the paradigm, the subUPDRS was the last test to be performed in each condition, in order to maximise the washout time between clinical scales. Hence, we use it as our benchmark measure of efficacy.
Statistical analysis
Statistical analysis were performed using R version 3.6.0, and the statistical packages lme4 [29] and lmerTest [30]. All items were assessed for normality using Q-Q plots. Iteration times of the tapping test, and RST reaction times were log-transformed prior to further analysis. subUPDRS scores, transformed iteration times, SIT scores and transformed RST reaction times were compared using a linear mixed-effects model. Condition was treated as a fixed effect in all tests and subject as a random effect. In addition, in the sub-UPDRS model, randomisation order was included as a fixed effect. Additionally, bradykinesia and tremor subscores were analysed using independent models. The intra-rater absolute agreement of UPDRS-subscores was analysed using 2-way random-effect intra-class correlation coefficient (ICC). In the SIT model, the presence of SID was included as a fixed effect, with SID defined as a decrement on the SIT score during cDBS, aDBS or both. In the RST analysis, congruency (congruent/incongruent) and accuracy (correct/incorrect) were added as fixed effects. In the tapping test, errors and their interaction with condition were added as fixed effects. Additionally, error counts of the tapping test and RST were analysed using a generalised linear mixed-effects model with a log-linear Poisson distribution model and an offset of 19 for each tapping-test trial, 5 for congruent words and 15 for incongruent words. In the RST error analysis, congruency was initially included as a fixed effect to test the presence of a reverse Stroop effect, and afterwards, its interaction with condition was added to the model, to test the selectivity of this effect to each condition. Models were compared using the Bayesian information criterion (BIC). The percentage time on stimulation was calculated for the aDBS condition. All results are expressed as mean ± standard error.
Results
Patients had a mean disease duration of 16 ± 2 years, and the mean time since DBS implantation was 6.9 ± 0.6 years. The average levodopa equivalent daily dose used was 1318.7 ± 169.4 mg. Of the 13 patients included in this study, all patients completed the bradykinesia test. Due to time and technical constrains, the rest of the tests were completed only by 11 patients each. All patients tolerated the test procedure, and stimulation-induced transient paraesthesias were present only at stimulation voltages above those used for the experiment.
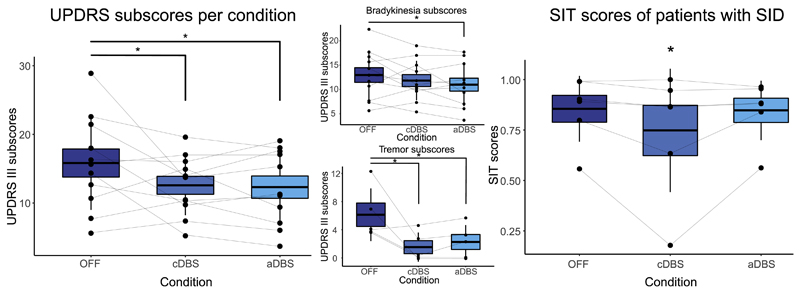
Bilateral subUPDRS: Whereas 7/11 patients were classified as tremor dominant based on the clinical records, only five presented evident tremor during the recordings. Independent raters showed an excellent degree of agreement in the scores, (ICC = 0.939, F(35,70) = 16.312, p = <.001). The linear mixed-effect model was significant for condition, (F(2,88) = 8.144, p = <.001) (Fig. 3). sub-UPDRS scores were significantly lower in aDBS (12.30 ± 0.85) when compared to NoStim (15.81 ± 0.75), (t(88) = -3.731, p = <.001), and in cDBS (12.57 ± 0.74) when compared to NoStim, (t(88) = -3.252, p = .001), but not between aDBS and cDBS, (t(88) = -0.443, p = .659). There was no significant effect of randomisation order, (F(2,88) = 2.62, p = .07). Bradykinesia subscores were significantly lower in aDBS (11.09 ± 0.58, t(88) = -3.096, p = .002), and did not achieve significance during cDBS (11.87 ± 0.58, t(88) = -1.754, p = .08), when compared to NoStim (12.90 ± 1.27). There was no difference in bradykinesia subscores between aDBS and cDBS (t(88) = 1.341, p = .18). Tremor subscores were reduced with both interventions (cDBS scores, 1.33 ± 0.73 vs NoStim 6.13 ± 0.92, t(40) = -6.572, p = <.001 and aDBS, 2.47 ± 0.73 vs No Stim, t(40) = -5.02, p = <.001). There was no difference in tremor subscores between aDBS and cDBS (t(88) = 1.189, p = .237). However, although most patients showed tremor suppression even when stimulation was triggered off during aDBS, two patients demonstrated re-emergence of tremor when stimulation triggered off during this condition.
Fig. 3. Scatter plots of the subUPDRS (means of the scores from the three independent raters per condition per patient) and SIT scores. Boxplot indicates the standard error of the mean, and upper and lower lines the standard deviation. Lines connect data from the same patient.
Left: total subUPDRS scores.
Upper center: subscores of bradykinesia items of the UPDRS III.
Lower center: subscores of tremor items of the UPDRS III.
Right: SIT scores of patients with SID.
SIT: the linear mixed-effect model showed a significant effect for the interaction of condition and SID (F(2,22) = 4.231, p = .027). SIT scores of patients with SID significantly worsened only in cDBS (0.74 ± 0.08) when compared to NoStim (0.85 ± 0.08, t(22) = -2.833, p = .009), but not in aDBS (0.84 ± 0.08) when compared to NoStim (t(22) = -0.845, p = .407).
RST: the simpler model revealed a significant effect of congruency, (Chi2(1) = 4.576, p = .032), where incongruent words had a significantly increased error rate, (z = 2.139, p = .032). No significant effect of condition was found, (Chi2(2) = 1.772, p = .41). A model with an interaction factor was not a better fit (BIC 146.09 vs 138.34 for the simpler model), and interaction was not significant, (Chi2(2) = 0.586, p = .745). No significant effect was found in reaction times for congruency, (F(2626.03) = 0.266, p = .766, condition, F(1, 625.99) = 0.310, p = .577), or their interaction, (F(1, 626.02) = 0.723, p = .485).
Tapping test: correct iteration times were reduced in cDBS (571.29 ± 9.83 ms) and aDBS (612.31 ± 10.14 ms) compared to NoStim (619.53 ± 10.35 ms). However, this reduction was only significant for cDBS, (t(1412.29) = -2.388, p = .017), and not for aDBS, (t(1412.24) = -0.776, p = .438). There was no significant difference between the two interventions, (t(1412.04) = -1.620, p = .105). No differences in errors were found between conditions, (Chi2(2) = 1.512, p = .469).
Overall, stimulation was applied 48.8% (SEM 3.8) of the time during aDBS.
Discussion
Our study provides the first pseudorandomised and blinded acute comparison of both UPDRS scores and side-effects in aDBS versus cDBS in the chronically implanted state. The results confirm previous findings that aDBS is well tolerated [9,11], performs equally well as cDBS, and does not compromise speech [14] in patients studied within days of electrode implantation. The effects in the chronically implanted state were seen despite stimulation being delivered about 50% of the time.
The patient cohort described in this study had DBS electrodes implanted for seven years on average (range 4–12 years), which provided the unique opportunity to observe the effects of aDBS in patients with advanced stages of the disease. It also confirms previous findings that beta oscillations remain informative of the clinical state of the patients [6,31], even several years after implantation.
There was significant improvement of the blindly evaluated subUPDRS during aDBS and cDBS. aDBS and cDBS did not differ significantly and improvements across both groups were relatively modest. However, this might reflect the reduction in the response of bradykinesia items to stimulation reported in some of the studies following DBS patients for more than 5 years [3,4]. This may also explain why the level of improvement in subUPDRS scores with both aDBS and cDBS (~20%) was at the lower limit of the range of improvements seen under similar blinded conditions when these interventions are contrasted acutely following electrode implantation [9,10,12,14,32]. Note too that scores from blinded video assessments are lower than those made during direct, unblinded clinical examination [9].
Supplementary video related to this article can be found at https://doi.org/10.1016/j.brs.2020.07.016
Regarding tremor subscores, both cDBS and aDBS significantly suppressed tremor compared to NoStim (Supplementary video 2). Although differences in clinical scores between cDBS and aDBS were not significant, two patients demonstrated re-emergence of tremor when stimulation was off during aDBS (Supplementary video 3). This phenomenon was previously described in a similar proportion (2/12) of cases undergoing aDBS [18], and might be related to the fact that beta oscillations are only correlated with bradykinesia symptoms, but not tremor [6,7]. It remains unclear which factors determine the re-emergence of tremor. As observed in Supplementary video 3, one clue may be re-emergence of tremor even during cDBS. It has been shown that tremor is associated with a reduction in beta oscillations [33], so potentially when tremor is not successfully suppressed by stimulation, it may attenuate ongoing beta activity and temporally cause stimulation to turn off, until pathological beta resynchronisation overcomes tremor and triggers stimulation again. This ‘flip-flopping’ might cause the ‘erratic’ tremor suppression during aDBS. Therefore, patients with stimulation-resistant tremor could require a lower threshold to trigger stimulation than their rigid-bradykinetic counterparts. Advances in tremor detection using LFPs [34] may provide additional biomarkers to control tremor in patients in which tremor cannot be effectively suppressed using only beta-based algorithms. Meanwhile in the minority of patients in whom tremor breakthrough does occur during aDBS, trials should assess the efficacy of control algorithms that have a minimum which is a low, rather than zero, stimulation intensity. Alternatively, the period between triggered stimulation bouts could be reduced in the aDBS condition, or the threshold dynamically changed to take account of medication state, which is linked to tremor.
A large number of patients classified as tremulous PD patients in the clinical records were included in this study. This could be explained by our inclusion criteria, which required patients with chronically implanted electrodes who were cognitively preserved. It has been shown that tremor-dominant PD patients usually present a more favourable cognitive prognosis [35]. However, it has also been observed that the presence of tremor in PD patients is not constant, and some patients who start with tremor as the main symptom may switch to a bradykinetic phenotype as disease progresses [36].
The potential benefits of aDBS regarding dysarthria are promising. SID is one of the most common side effects of cDBS, with a prevalence of around 10% in treated patients [37]. This usually adds to speech problems which are already present as consequence of the disease [38]. The pathophysiology of SID is up to now unclear. Stimulation spread to tracts adjacent to the STN has been proposed as a factor that contributes to the onset of SID [39]. New technologies, such as directional DBS [40] can improve the spatial accuracy of DBS, in order to avoid stimulation of unwanted tracts. However, increasing evidence indicates that the sensorimotor part of the STN itself is involved in speech production [41]. Therefore, the uninterrupted stimulation of the motor networks that are meant to be targeted with cDBS can also lead to SID. aDBS, by delivering stimulation only when it is necessary, reduces the overall electrical energy delivered and in this way limits speech-related side-effects. In this study, the benefits of aDBS were only seen when patients did present SID during cDBS. Nevertheless, the test used in our protocol only provides a general overview of the patient’s speech. Studies using more sophisticated methods, such as articulography [42] or speech signal processing [43], might reveal even more insights about the potential benefits of aDBS. In addition to this, other activities in the STN LFP or biomarkers from the motor cortex could be used to monitor speech articulation, in order to further improve the efficacy of aDBS [44]. However, this is yet to be tested.
We found non-significant differences between cDBS and aDBS in the tapping test. Iteration times were shorter with cDBS, even though bradykinesia UPDRS subscores were lower with aDBS. The contrast might be explained by the effects of break-through tremor in the minority of patients on aDBS. In the RST, an interference effect was found, but this was not affected by stimulation (either continuous or adaptive).
Limitations
Some limitations of this study have already been highlighted, particularly the short duration of the stimulation conditions, but others remain. Because of the intra-operative nature of this study, not all the UPDRS items could be assessed. As operations were performed under local anaesthesia, the time available to complete the protocol was limited. Therefore, the results may potentially be affected by incomplete washouts between stimulation conditions. Pseudo-randomisation should have helped limit the impact of incomplete washout and condition order was not found to be a significant factor upon analysis. Moreover, wash-out has been considered a two-step process, consisting of an initial fast decrease in stimulation’s therapeutic effect, followed by a further, slow decline. Our wash-out interval was sufficient for the former fast process [45,46]. Similar arguments may apply to the wash-in of effects, given that stimulation was only applied for about 5 min in each condition. The brief wash-out and wash-in periods necessitated by our intra-operative constraints should have served to reduce the differences between conditions, but significant differences were still detected. In a similar way, our baseline recording was limited to 30–60 s. However, beta oscillations in ‘off’ STN LFP-recordings are similar whether measured 1 min or 1 h later [47], and show relatively little variation over time [48]. Another possible confound in the present methodology is that both aDBS and cDBS were limited to monopolar stimulation at either contact 1 or 2, and higher voltages sometimes interfered with the recordings through stimulation-related artefacts, which led to the use of submaximal voltages in some instances. The resultant stimulation choices may therefore have differed from the chronically optimised contact selection and stimulation voltage prior to battery change (see Table 1). Still, voltages, pulse width and stimulation frequency were kept fixed between the cDBS and aDBS conditions, and the optimal voltages were estimated based on the response to cDBS during the assessment.
Nevertheless, the selection of cDBS contacts was more constrained than usual and bipolar stimulation was avoided so as to keep the two stimulation conditions as similar to one another as possible. Additionally, due to the bipolar recording configuration focused on the middle contacts, the beta activity in outer contacts was not explored. Future aDBS experiments using non-segmented or segmented octopolar DBS electrodes may allow for more versatile stimulation montages [49].
Furthermore, the effects of movement on beta oscillations were not directly assessed in this trial. It has been observed that the average STN beta power is modulated during voluntary movements [50] and walking [51]. However, we and others have not found a negative impact of these beta-effects on clinical scores during aDBS [9–11,18,32,52–54]. This may be due to the fact that while average beta power is reduced during voluntary movements, pathological beta bursts still occur on some trials [55,56]. Therefore, aDBS will continue to be triggered, as needed, by these bursts [57]. Although beta-based aDBS studies on non-human primates [58] and PD patients [12] have shown that aDBS improves overall bradykinesia and rigidity symptoms, more subtle elements of motor behaviour can be compromised, such as the speed or accuracy of the return phase during reaching tasks, when beta rebounds. On the other hand, these effects will also be shared by cDBS, which involves stimulation during all beta rebounds. The impact of these more subtle motor effects in real-life conditions will need to be further explored. Finally, it is still possible that the effects demonstrated here do not persist in the chronic setting. Ultimately, chronic trials are necessary that contrast the best possible cDBS parameters established during chronic therapy with aDBS.
Future perspectives
Several clinical trials [9–11,18,32,52–54], including the present study, have demonstrated that beta oscillations in the STN correlate with bradykinesia and rigidity in PD patients [59]. For that reason, beta oscillations are likely to play a central role in future aDBS trials [60,61]. With the advent of new stimulation devices that are able to perform chronic LFP-recordings, the next step towards implementing beta-based aDBS in the clinical practice will be to explore the clinical benefits and limitations of aDBS in real-life situations. One important aspect to take into account in the design of these trials is the interaction of beta oscillations with medication. Beta oscillations in the STN are reduced after levodopa administration [50]. For that reason, beta-based aDBS can avoid the delivery of excessive stimulation when the effect of levodopa is present. Preliminary evidence has shown that this could help reduce medication-induced dyskinesias in patients treated with aDBS [15,52,62]. Both low-frequency oscillations (4–12 Hz) [63] and gamma oscillations (35–80 Hz) [64] in the STN, and gamma oscillations in the motor cortex [65], are increased during the presence of levodopa-induced dyskinesias. These biomarkers could also be incorporated into aDBS [66]. In addition, the stimulation technique could potentially be improved, so that the targeting of specific phases of synchronization, or plasticity-modulating patterns could replace regular, high frequency stimulation in aDBS [67,68]. However, the potential benefits of any prospective biomarker need first to be clinically contrasted with cDBS, as this is the present gold standard. Lastly, aDBS will need to continue working during sleep, in order to prevent problems related to turning in bed [69]. Algorithms that can recognise sleep, or that switch to cDBS over night, can be designed [70].
Conclusion
The acute therapeutic effects of beta-based aDBS are as great as conventional DBS in chronically implanted patients, just as immediately after electrode implantation [9]. This is in line with the evidence that beta oscillations remain present and informative over time [71]. The present findings are important, as the assessments in chronically implanted patients are not confounded by microlesion effects. Furthermore, comparisons were performed with cDBS, using stimulation parameters that were optimised for cDBS, with the exception of stimulation contacts. We found that SID was reduced with aDBS, most likely because stimulation was applied only as necessary. However, it remains to be seen whether aDBS remains effective with prolonged use, and whether stimulation algorithms have to be adjusted in some patients with tremor.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.07.016.
Acknowledgements
We would like to thank all the patients for their participation in this study. We would also like to thank Renske de Vries for the evaluation of the voice recordings, Jasper Peeters for his assistance during the measurements, Alek Pogosyan for the facilitation of custom-made scripts, Cor Kliphuis for his assistance with the patient assessments, and the OR staff for their help during the recordings.
Funding
This study is publicly funded by grants received from the Dutch Brain Foundation (‘Hersenstichting Nederland’, grant F2015(1)-04), the National Mexican Council of Science and Technology (CON-ACYT) grant number CVU 652927, and the University of Groningen/ University Medical Center Groningen (RuG/UMCG). SL is personally supported by a Wellcome trust post-doctoral clinical research training grant (105804/Z/14/Z). PB is supported by the Medical Research Council [MC_UU_12024/1], National Institute of Health Research Oxford Biomedical Research Centre and Rosetrees Trust.
Footnotes
CRediT authorship contribution statement
Dan Piña-Fuentes: Patient inclusion, Data collection, Formal analysis, Data analysis-execution, Writing - original draft, Writing - review & editing, Writing of manuscript, Review and approval of the manuscript. J. Marc C. van Dijk: Surgical assistance during recordings, Supervision, Project supervision, Writing - original draft, Writing - review & editing, Writing of manuscript, Review and approval of the manuscript. Jonathan C. van Zijl: Blinded ratings, Writing - review & editing, Review and approval of the manuscript. Harmen R. Moes: Blinded ratings, Writing - review & editing, Review and approval of the manuscript. Teus van Laar: Writing -review & editing, Critical advic, Review and approval of the manuscript. D.L.Marinus Oterdoom: Surgical assistance during recordings, Writing - review & editing, Review and approval of the manuscript. Simon Little: Writing - review & editing, Critical advic, Review and approval of the manuscript. Peter Brown: Formal analysis, Supervision, Data analysis-Supervision, Project supervision, Writing - original draft, Writing - review & editing, Writing of manuscript, Review and approval of the manuscript. Martijn Beudel: Conceptualization, Conceptualization and design, Blinded ratings, Patient inclusion, Formal analysis, Supervision, Data analysis-Supervision, Project supervision, Writing - original draft, Writing - review & editing, Writing of manuscript, Review and approval of the manuscript.
Declaration of competing interest
PB is a consultant with Medtronic.
References
- [1].Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- [2].Mansouri A, Taslimi S, Badhiwala JH, Witiw CD, Nassiri F, Odekerken VJJ, et al. Deep brain stimulation for Parkinson’s disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. 2018;128:1199–213. doi: 10.3171/2016.11.JNS16715. [DOI] [PubMed] [Google Scholar]
- [3].Aviles-Olmos I, Kefalopoulou Z, Tripoliti E, Candelario J, Akram H, Martinez-Torres I, et al. Long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson’s disease using an MRI-guided and MRI-verified approach. J Neurol Neurosurg Psychiatry. 2014;85:1419–25. doi: 10.1136/jnnp-2013-306907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Castrioto A, Lozano AM, Poon Y-Y, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–6. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- [5].Krack P, Volkmann J, Tinkhauser G, Deuschl G. Deep brain stimulation in movement Disorders: from experimental surgery to evidence-based therapy. Mov Disord. 2019;34(12):1795–810. doi: 10.1002/mds.27860. [DOI] [PubMed] [Google Scholar]
- [6].Neumann W-J, Staub-Bartelt F, Horn A, Schanda J, Schneider G-H, Brown P, et al. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s disease. Clin Neurophysiol. 2017;128:2286–91. doi: 10.1016/j.clinph.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beudel M, Oswal A, Jha A, Foltynie T, Zrinzo L, Hariz M, et al. Oscillatory beta power correlates with akinesia-rigidity in the parkinsonian subthalamic nucleus. Mov Disord. 2017;32:174–5. doi: 10.1002/mds.26860. [DOI] [PubMed] [Google Scholar]
- [8].Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord. 2015;30:1750–8. doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- [9].Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74:449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Little S, Beudel M, Zrinzo L, Foltynie T, Limousin P, Hariz M, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:717–21. doi: 10.1136/jnnp-2015-310972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology. 2018;90:e971–6. doi: 10.1212/WNL.0000000000005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iturrate I, Martin S, Chavarriaga R, Orset B, Leeb R, Sobolewski A, et al. Beta-driven closed-loop deep brain stimulation can compromise human motor behavior in Parkinson’s Disease. BioRxiv. 2019 doi: 10.1101/696385. 696385. [DOI] [Google Scholar]
- [13].Rosa M, Arlotti M, Ardolino G, Cogiamanian F, Marceglia S, Di Fonzo A, et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord. 2015;30:1003–5. doi: 10.1002/mds.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Little S, Tripoliti E, Beudel M, Pogosyan A, Cagnan H, Herz D, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016;87:1388–9. doi: 10.1136/jnnp-2016-313518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosa M, Arlotti M, Marceglia S, Cogiamanian F, Ardolino G, Fonzo A Di, et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov Disord. 2017;32:628–9. doi: 10.1002/mds.26953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen CC, Pogosyan A, Zrinzo LU, Tisch S, Limousin P, Ashkan K, et al. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’s disease surgery. Exp Neurol. 2006;198:214–21. doi: 10.1016/j.expneurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- [17].Piña-Fuentes D, Little S, Oterdoom M, Neal S, Pogosyan A, Tijssen MAJ, et al. Adaptive DBS in a Parkinson’s patient with chronically implanted DBS: a proof of principle. Mov Disord. 2017;32 doi: 10.1002/mds.26959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Velisar A, Syrkin-Nikolau J, Blumenfeld Z, Trager MH, Afzal MF, Prabhakar V, et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019;12:868–76. doi: 10.1016/j.brs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- [19].Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]
- [20].Pina-Fuentes D, Beudel M, Little S, Brown P, Oterdoom DLM, van Dijk JMC. Adaptive deep brain stimulation as advanced Parkinson’s disease treatment (ADAPT study): protocol for a pseudo-randomised clinical study. BMJ Open. 2019;9:e029652. doi: 10.1136/bmjopen-2019-029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Little S, Tan H, Anzak A, Pogosyan A, Kuhn A, Brown P. Bilateral functional connectivity of the basal ganglia in patients with Parkinson’s disease and its modulation by dopaminergic treatment. PloS One. 2013;8:e82762. doi: 10.1371/journal.pone.0082762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de Solages C, Hill BC, Koop MM, Henderson JM, Bronte-Stewart H. Bilateral symmetry and coherence of subthalamic nuclei beta band activity in Parkinson’s disease. Exp Neurol. 2010;221:260–6. doi: 10.1016/j.expneurol.2009.11.012. [DOI] [PubMed] [Google Scholar]
- [23].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- [24].Blais C, Besner D. Reverse stroop effects with untranslated responses. J Exp Psychol Hum Percept Perform. 2006;32:1345–53. doi: 10.1037/0096-1523.32.6.1345. [DOI] [PubMed] [Google Scholar]
- [25].Woodward TS, Bub DN, Hunter MA. Task switching deficits associated with Parkinson’s disease reflect depleted attentional resources. Neuropsychologia. 2002;40:1948–55. doi: 10.1016/S0028-3932(02)00068-4. [DOI] [PubMed] [Google Scholar]
- [26].Melara RD, Mounts JRW. Selective attention to Stroop dimensions: effects of baseline discriminability, response mode, and practice. Mem Cognit. 1993;21:627–45. doi: 10.3758/BF03197195. [DOI] [PubMed] [Google Scholar]
- [27].Bronte-Stewart HM, Ding L, Alexander C, Zhou Y, Moore GP. Quantitative digitography (QDG): a sensitive measure of digital motor control in idiopathic Parkinson’s disease. Mov Disord. 2000;15:36–47. doi: 10.1002/1531-8257(200001)15:1<36::aid-mds1008>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [28].Noyce AJ, Treacy C, Budu C, Fearnley J, Lees AJ, Giovannoni G. The new Bra-dykinesia Akinesia Incoordination (BRAIN) test: preliminary data from an online test of upper limb movement. Mov Disord. 2012;27:157–8. doi: 10.1002/mds.23947. [DOI] [PubMed] [Google Scholar]
- [29].Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software. 2015;1(1) [Google Scholar]
- [30].Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Software. 2017;1(13) [Google Scholar]
- [31].Arlotti M, Palmisano C, Minafra B, Todisco M, Pacchetti C, Canessa A, et al. Monitoring subthalamic oscillations for 24 hours in a freely moving Parkinson’s disease patient. Mov Disord. 2019;34:757–9. doi: 10.1002/mds.27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Herz DM, Little S, Pedrosa DJ, Tinkhauser G, Cheeran B, Foltynie T, et al. Mechanisms underlying decision-making as revealed by deep-brain stimulation in patients with Parkinson’s disease. Curr Biol. 2018;28:1169–1178.e6. doi: 10.1016/j.cub.2018.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qasim SE, de Hemptinne C, Swann NC, Miocinovic S, Ostrem JL, Starr PA. Electrocorticography reveals beta desynchronization in the basal ganglia-cortical loop during rest tremor in Parkinson’s disease. Neurobiol Dis. 2016;86:177–86. doi: 10.1016/j.nbd.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tan H, Debarros J, He S, Pogosyan A, Aziz TZ, Huang Y, et al. Decoding voluntary movements and postural tremor based on thalamic LFPs as a basis for closed-loop stimulation for essential tremor. Brain Stimul. 2019;12:858–67. doi: 10.1016/j.brs.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wojtala J, Heber IA, Neuser P, Heller J, Kalbe E, Rehberg SP, et al. Cognitive decline in Parkinson’s disease: the impact of the motor phenotype on cognition. J Neurol Neurosurg & Psychiatr. 2019;90:171 LP–179. doi: 10.1136/jnnp-2018-319008. [DOI] [PubMed] [Google Scholar]
- [36].Eisinger RS, Martinez-Ramirez D, Ramirez-Zamora A, Hess CW, Almeida L, Okun MS, et al. Parkinson’s disease motor subtype changes during 20 years of follow-up. Park Relat Disord. 2019 doi: 10.1016/j.parkrel-dis.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21:S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- [38].Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1999;11:131–7. [PubMed] [Google Scholar]
- [39].Fenoy AJ, McHenry MA, Schiess MC. Speech changes induced by deep brain stimulation of the subthalamic nucleus in Parkinson disease: involvement of the dentatorubrothalamic tract. J Neurosurg. 2017;126:2017–27. doi: 10.3171/2016.5.JNS16243. [DOI] [PubMed] [Google Scholar]
- [40].Dembek TA, Reker P, Visser-Vandewalle V, Wirths J, Treuer H, Klehr M, et al. Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord. 2017;32:1380–8. doi: 10.1002/mds.27093. [DOI] [PubMed] [Google Scholar]
- [41].Phokaewvarangkul O, Boonpang K, Bhidayasiri R. Subthalamic deep brain stimulation aggravates speech problems in Parkinson’s disease: objective and subjective analysis of the influence of stimulation frequency and electrode contact location. Park Relat Disord. 2019;66:110–6. doi: 10.1016/j.parkreldis.2019.07.020. [DOI] [PubMed] [Google Scholar]
- [42].Wong MN, Murdoch BE, Whelan B-M. Kinematic analysis of lingual function in dysarthric speakers with Parkinson’s disease: an electromagnetic articulo-graph study. Int J Speech Lang Pathol. 2010;12:414–25. doi: 10.3109/17549507.2010.495784. [DOI] [PubMed] [Google Scholar]
- [43].Sakar CO, Serbes G, Gunduz A, Tunc HC, Nizam H, Sakar BE, et al. A comparative analysis of speech signal processing algorithms for Parkinson’s disease classification and the use of the tunable Q-factor wavelet transform. Appl Soft Comput. 2019;74:255–63. doi: 10.1016/j.asoc.2018.10.022. [DOI] [Google Scholar]
- [44].Chrabaszcz A, Neumann W-J, Stretcu O, Lipski WJ, Bush A, Dastolfo-Hromack CA, et al. Subthalamic nucleus and sensorimotor cortex activity during speech production. J Neurosci. 2019;39:2698–708. doi: 10.1523/JNEUROSCI.2842-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Keresztenyi Z, Valkovic P, Eggert T, Steude U, Hermsdorfer J, Laczko J, et al. The time course of the return of upper limb bradykinesia after cessation of sub-thalamic stimulation in Parkinson’s disease. Park Relat Disord. 2007;13:438–42. doi: 10.1016/j.parkreldis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [46].Cooper SE, Noecker AM, Abboud H, Vitek JL, McIntyre CC. Return of brady-kinesia after subthalamic stimulation ceases: relationship to electrode location. Exp Neurol. 2011;231:207–13. doi: 10.1016/j.expneurol.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Trager MH, Koop MM, Velisar A, Blumenfeld Z, Nikolau JS, Quinn EJ, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol Dis. 2016;96:22–30. doi: 10.1016/j.nbd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- [48].Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215:20–8. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [49].Tinkhauser G, Pogosyan A, Debove I, Nowacki A, Shah SA, Seidel K, et al. Directional local field potentials: a tool to optimize deep brain stimulation. Mov Disord. 2018;33:159–64. doi: 10.1002/mds.27215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002;125:1196–209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- [51].Fischer P, Chen CC, Chang Y-J, Yeh C-H, Pogosyan A, Herz DM, et al. Alternating modulation of subthalamic nucleus beta oscillations during stepping. J Neurosci. 2018;38:5111–21. doi: 10.1523/JNEUROSCI.3596-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Arlotti M, Rossi L, Rosa M, Marceglia S, Priori A. An external portable device for adaptive deep brain stimulation (aDBS) clinical research in advanced Parkinson’s Disease. Med Eng Phys. 2016;38:498–505. doi: 10.1016/j.medengphy.2016.02.007. [DOI] [PubMed] [Google Scholar]
- [53].Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol. 2013;245:77–86. doi: 10.1016/J.EXPNEUROL.2012.09.013. [DOI] [PubMed] [Google Scholar]
- [54].Pina-Fuentes D, van Zijl JC, van Dijk JMC, Little S, Tinkhauser G, Oterdoom DLM, et al. The characteristics of pallidal low-frequency and beta bursts could help implementing adaptive brain stimulation in the parkinso-nian and dystonic internal globus pallidus. Neurobiol Dis. 2019;121:47–57. doi: 10.1016/j.nbd.2018.09.014. [DOI] [PubMed] [Google Scholar]
- [55].Lofredi R, Tan H, Neumann W-J, Yeh C-H, Schneider G-H, Kuhn AA, et al. Beta bursts during continuous movements accompany the velocity decrement in Parkinson’s disease patients. Neurobiol Dis. 2019;127:462–71. doi: 10.1016/j.nbd.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Anidi C, O’Day JJ, Anderson RW, Afzal MF, Syrkin-Nikolau J, Velisar A, et al. Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease. Neurobiol Dis. 2018;120:107–17. doi: 10.1016/j.nbd.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tinkhauser G, Pogosyan A, Little S, Beudel M, Herz DM, Tan H, et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain. 2017;140:1053–67. doi: 10.1093/brain/awx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB, Vitek JL. Closed-Loop deep brain stimulation effects on parkinsonian motor symptoms in a non-human primate - is beta enough? Brain Stimul. 2016;9:892–6. doi: 10.1016/j.brs.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- [60].Little S, Brown P. Debugging adaptive deep brain stimulation for Parkinson’s disease. Mov Disord. 2020;35(4):555–61. doi: 10.1002/mds.27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pina-Fuentes D, van Dijk JMC, Beudel M. Adaptive DBS in Parkinson’s disease: headlines, perspectives and challenges. Brain Stimul. 2019;12:1091–2. doi: 10.1016/j.brs.2019.04.014. [DOI] [PubMed] [Google Scholar]
- [62].Bocci T, Arlotti M, Marceglia S, Prenassi M, Ardolino G, Cogiamanian F, et al. Adaptive deep brain stimulation for Parkinson’s disease: safety and effectiveness. Clin Neurophysiol. 2019;130:e17. doi: 10.1016/j.clinph.2018.09.098. [DOI] [Google Scholar]
- [63].Alonso-Frech F, Zamarbide I, Alegre M, Rodriguez-Oroz MC, Guridi J, Manrique M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–57. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- [64].Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Lazzaro V Di. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson ’ s. Diseases. 2001;21:1033–8. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018;15 doi: 10.1088/1741-2552/aabc9b. 46006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hell F, Palleis C, Mehrkens JH, Koeglsperger T, Bötzel K. Deep brain stimulation programming 2.0: future perspectives for target identification and adaptive closed loop stimulation. Front Neurol. 2019;10:314. doi: 10.3389/fneur.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Popovych OV, Tass PA. Adaptive delivery of continuous and delayed feedback deep brain stimulation - a computational study. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47036-4. 10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, et al. Coordinated reset neuromodulation for Parkinson’s disease: proof-of-concept study. Mov Disord. 2014;29:1679–84. doi: 10.1002/mds.25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sringean J, Taechalertpaisarn P, Thanawattano C, Bhidayasiri R. How well do Parkinson’s disease patients turn in bed? Quantitative analysis of nocturnal hypokinesia using multisite wearable inertial sensors. Park Relat Disord. 2016;23:10–6. doi: 10.1016/j.parkreldis.2015.11.003. [DOI] [PubMed] [Google Scholar]
- [70].Urrestarazu E, Iriarte J, Alegre M, Clavero P, Rodríguez-Oroz MC, Guridi J, et al. Beta activity in the subthalamic nucleus during sleep in patients with Parkinson’s disease. Mov Disord. 2009;24:254–60. doi: 10.1002/mds.22351. [DOI] [PubMed] [Google Scholar]
- [71].Neumann WJ, Staub F, Horn A, Schanda J, Mueller J, Schneider GH, et al. Deep brain recordings using an implanted pulse generator in Parkinson’s disease. Neuromodulation. 2016;19:20–3. doi: 10.1111/ner.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.