Abstract
The respiratory tract and its resident immune cells face daily exposure to stress, both from without and from within. Inhaled pathogens, including severe acute respiratory syndrome coronavirus 2, and toxins from pollution trigger a cellular defence system that reduces protein synthesis to minimise viral replication or the accumulation of misfolded proteins. Simultaneously, a gene expression programme enhances antioxidant and protein folding machineries in the lung. Four kinases (PERK, PKR, GCN2 and HRI) sense a diverse range of stresses to trigger this “integrated stress response”. Here we review recent advances identifying the integrated stress response as a critical pathway in the pathogenesis of pulmonary diseases, including pneumonias, thoracic malignancy, pulmonary fibrosis and pulmonary hypertension. Understanding the integrated stress response provides novel targets for the development of therapies.
Short abstract
By integrating signals from multiple stressors, the integrated stress response plays key roles in the pathophysiology of lung disease. Understanding the mechanisms involved will identify novel means of therapy. https://bit.ly/2Bg2kj4
The integrated stress response
The airway epithelium faces many stresses, both extrinsic, e.g. inhaled toxins, and intrinsic, e.g. protein misfolding [1, 2]. Stress-responsive pathways determine whether these stresses are withstood or if they cause disease. A variety of insults trigger the phosphorylation of eukaryotic initiation factor (eIF)2α [3]. Since eIF2α phosphorylation integrates signals from diverse sources, its downstream pathway has been named the “integrated stress response” (ISR) (figure 1). Four stress-sensing kinases trigger the ISR [4]: protein kinase R (PKR) responds to cytosolic double-stranded (ds)RNA [5]; PKR-like endoplasmic reticulum kinase (PERK) detects endoplasmic reticulum (ER) stress [6, 7]; heme-regulated inhibitor (HRI) responds to iron deficiency and oxidative stress [8]; while general control non-depressible (GCN)2) is activated during amino acid starvation [9, 10].
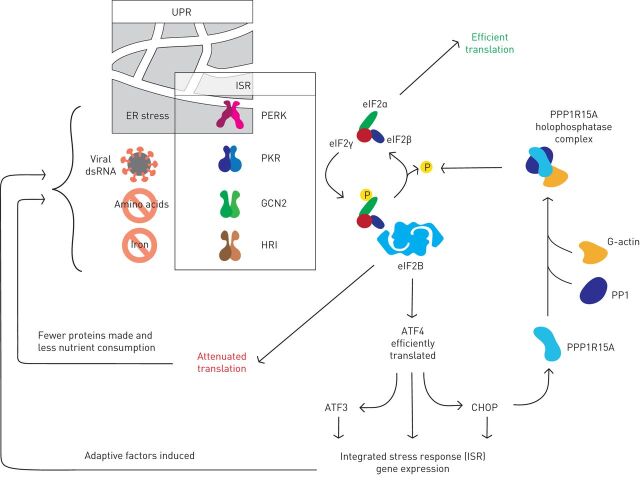
FIGURE 1.
The integrated stress response (ISR) is triggered by stress-sensing kinases that phosphorylate eukaryotic initiation factor (eIF)2α, a component of the eIF2 translation initiation complex. Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) responds to endoplasmic reticulum (ER) stress, and so the ISR overlaps with the unfolded protein response (UPR). PKR detects viral double-stranded (ds)RNA. General control non-depressible (GCN)2 is activated by amino acid deficiency. Heme-regulated inhibitor (HRI) responds to iron depletion. Phosphorylated eIF2α binds avidly to eIF2β to inhibit most translation, but some mRNAs including those encoding the transcription factors ATF4 and CHOP are translated more efficiently. The resulting gene expression restores homeostasis by enhancing oxidative protein folding in the ER; promoting amino-acyl transfer (t)RNA synthesis; and inducing antioxidant genes. PPP1R15A (also known as GADD34) is eventually induced and in complex with PP1 and G-actin dephosphorylates eIF2α to terminate the ISR.
The eIF2 complex recruits methionyl-transfer (t)RNA to the ribosome at the beginning of protein synthesis. During cycles of translation initiation, eIF2 hydrolyses its bound GTP, which is replenished by the guanine nucleotide exchange factor eIF2β. However, when eIF2α is phosphorylated it binds avidly to eIF2β inhibiting further GTP exchange. As a result, protein synthesis is attenuated, although a subset of transcripts is translated more efficiently thanks to regulatory elements within their 5′-untranslated regions [11, 12]. The transcription factors ATF4 and CHOP are induced in this way by the ISR and upregulate hundreds of ISR target genes. One of these encodes PPP1R15A (also known as GADD34), which forms a complex with protein phosphatase (PP)1 and G-actin to generate an eIF2α-specific phosphatase that terminates the ISR [13]. This negative feedback mechanism allows the ISR to be activated transiently, but premature inactivation of the ISR can cause cell death [14, 15].
Infection
The ISR can be activated by viral infection. During replication of RNA viruses and complex DNA viruses, dsRNA can be detected by PKR [16]. In addition, enveloped viruses require high levels of membrane protein synthesis, which can trigger PERK [17]. Phosphorylation of eIF2α then antagonises viral protein synthesis while triggering ISR gene expression [1, 17]. Innate antiviral pathways induce interferon (IFN)-dependent genes including PKR, thus enhancing ISR activation [16]. Simultaneously, phosphorylation of eIF2α causes formation of stress granules [18], membraneless organelles formed by a liquid–liquid phase separation of ribonuclear proteins, which sequester viral factors, block viral gene expression and recruit viral sensors such as PKR [19]. The importance of the ISR in defending against viral infection is illustrated by the wide variety of evasion mechanisms that viruses have evolved to overcome its effects, including decoy dsRNAs, PKR degradation, viral RNA sequestration, blockade of PKR dimerisation, pseudosubstrates that compete for PKR's activity and even expression of a viral eIF2α phosphatase (table 1) [5, 20–44]. In addition, some viruses inhibit IFN signalling to prevent induction of PKR [46].
TABLE 1.
Viral-mediated inhibition of protein kinase R (PKR) activity
| Virus(es) | Viral protein | Mode of PKR inhibition | References | |
| ssRNA | ||||
| Orthomyxoviridae | Influenza A | NS1 | Direct interaction/dsRNA-mediated interaction | [26, 45] |
| Influenza B | ||||
| Coronaviridae | Infectious bronchitis virus | NSp2 | Inhibition of phosphorylation | [21] |
| hCoV-229a | NSp15 | dsRNA sequestration | [22] | |
| MERS-CoV | NS4a | dsRNA sequestration | [23, 27] | |
| Bunyaviridae | Rift Valley fever virus | NSs | Proteasome-mediated degradation | [28] |
| Filoviridae | Ebolavirus | VP35 | Unknown/possible dsRNA sequestration | [29] |
| Retroviridae | HIV | Tat | Pseudosubstrate/direct interaction | [9] |
| TAR RNA | Decoy dsRNA | [10] | ||
| Flaviviridae | Hepatitis C virus | NS5a | Blocking of dimerisation/direct interaction | [32] |
| E2 | Pseudosubstrate/direct interaction | [33] | ||
| Reoviridae | Reoviruses | σ3/σ4 | dsRNA sequestration | [34] |
| dsRNA | ||||
| Herpesviridae | Human cytomegalovirus | pTRS1/pIRS1 | Interaction and relocalisation/dsRNA sequestration | [35] |
| dsDNA | ||||
| Herpesviridae | Herpes simplex virus | γ134.5 | Dephosphorylation of eIF2α substrate | [24, 36, 37] |
| US11 | dsRNA sequestration/direct interaction | |||
| US3/UL13 | Inhibition of activation | |||
| Kaposi's sarcoma herpes virus | vIRF2 | Direct interaction and inhibition of phosphorylation | [38] | |
| vIRF3 (LANA2) | Inhibition of PKR-induced apoptosis | [39] | ||
| Epstein–Barr virus | EBER RNAs | Decoy dsRNA | [40] | |
| SM | dsRNA sequestration/direct interaction | [41] | ||
| Poxiviridae | Vaccinia virus | E3L (p25/p20) | dsRNA sequestration/direct interaction | [42, 43] |
| K3L | Pseudosubstrate/direct interaction | [44] | ||
| Adenoviridae | Adenovirus | VAI RNA | Decoy dsRNA/pseudoactivator | [40] |
Summary of viral-encoded proteins that mediate evasion of PKR-mediated innate immune response. Viruses are classified by family and type of genome. MERS-CoV: Middle East respiratory syndrome-coronavirus; ss: single-stranded; ds: double-stranded; NSp: nonstructural protein; VP: viral protein; TAR: transactivation responsive; vIRF3: viral IRF3-like protein; EBER: Epstein–Barr virus encoded RNAs; VAI: adenovirus-associated RNA-I; eIF: eukaryotic initiation factor.
Influenza
Influenza A causes half a million deaths annually [47]. Two decades ago, it was discovered that influenza circumvents PKR by means of a nonstructural protein called NS1 [26] (table 1). NS1-deleted strains of influenza are enfeebled, but their virulence is restored in PKR-deficient cells [45]. NS1 was initially thought to enable influenza to evade PKR through activation of the co-chaperone p58IPK [48]. Indeed, p58IPK was named because of its putative role as a 58-kDa inhibitor of PKR [49]. However, subsequent work revealed that p58IPK resides exclusively in the ER lumen [50], while PKR is cytosolic, making a direct interaction impossible. By contrast, through its putative RNA-binding domain, cytosolic NS1 can bind to PKR, probably mediating its inhibition directly [45]. Supporting this, mutations of this NS1 domain restore PKR activation and stress granule formation, impairing influenza replication [51]. In addition, NS1 can inhibit PKR indirectly by promoting the expression of vtRNA [52], a form of long noncoding RNA found in ribonucleoprotein particles called vaults (vt) [53]. For example, vtRNA2-1 directly inhibits PKR activity [54]. Influenza A infection or isolated expression of NS1 enhances the production of vtRNAs in human and mouse cell lines [52]. By contrast, NS1-deleted viruses cause the production of little vtRNA, and depletion of host vtRNAs leads to PKR activation, which reduces influenza replication [52].
PKR activation may not always be beneficial. Different strains of influenza vary in their ability to activate or evade the ISR [55, 56]. Some highly pathogenic avian influenza viruses (e.g. H5N1, H7N7 and H7N9) induce severe pulmonary inflammation owing to excessive production of IFN-β, interleukin (IL)-6 and IL-8 [55]. This response appears to be mediated by PKR triggering a cascade that leads to phosphorylation of TRIM28, a regulator of many immunomodulatory genes. This raises the possibility that PKR-directed therapies might modify the immune response to highly pathogenic strains of influenza. The complex roles of PKR during inflammation are discussed in more detail later.
Coronaviruses
Coronaviruses primarily cause respiratory and enteric disease. Many human coronaviruses including hCoV-229E, OC43, NL63 and HKU-1 cause only mild respiratory symptoms [57], but the zoogenic coronaviruses severe acute respiratory syndrome (SARS)-CoV-1, SARS-CoV-2 and Middle East respiratory syndrome (MERS)-CoV cause severe damage to the respiratory epithelium, which can be fatal [58]. The coronavirus genome encodes a number of structural proteins including spike, envelope, membrane, nucleoprotein and hemagglutinin-esterase [59]. The expression of virus-specific accessory proteins modulates activation of the ISR during infection [6], and being enveloped, coronaviruses impose significant stress on the ER, triggering PERK [21, 60–62]. Of note, although PERK is activated by expression of SARS-CoV-1 spike protein and accessory protein 3a [61, 62], rather than promoting an antiviral ISR, it has been suggested that the accompanying unfolded protein response (UPR) favours viral protein synthesis through upregulation of ER chaperones.
It was reported that during infection with SARS-CoV-1, both PKR and PERK are phosphorylated [63]. In a kinome-wide short interfering (si)RNA screen of 293T cells expressing the SARS-CoV receptor angiotensin-converting enzyme-2, both PKR and PERK were necessary for cells to restrict SARS-CoV-1 replication. However, others have found that, although activated, PKR was unable to reduce viral titres, suggesting that SARS-CoV-1 can evade PKR's antiviral activity [64]. It is unclear whether the net effect of PKR is to limit infection or to exacerbate deleterious inflammation in vivo. Recent reports describe how the β-coronavirus endoribonuclease nonstructural protein (nsp)15 interferes with host RNA sensing [22, 65, 66] (table 1). Mutations of nsp15 in mouse hepatitis virus and HCoV-229E enhance the phosphorylation of PKR and eIF2α in infected macrophages [22, 65]. This increases the induction of type I IFN and reduces viral titres. Consistent with this, deletion of nsp15 renders the virus unable to replicate in cells except those lacking PKR. Conversely, MERS-CoV inhibits the formation of stress granules in a manner dependent upon another viral protein ns4a [23] (table 1). Ns4a contains a dsRNA-binding motif and can antagonise the activation of PKR, perhaps by preventing dsRNA sensing [23, 27].
Infectious bronchitis virus (IBV), a γ-coronavirus causing respiratory disease in birds, suppresses the ISR to enable viral protein synthesis [21]. In vitro, IBV infection of ISR-deficient eIF2αAA cells (in which a serine-51 to alanine mutation blocks phosphorylation) results in elevated viral replication confirming the importance of eIF2α phosphorylation for antiviral activity. During IBV infection, activation of PKR is suppressed by viral protein nsp2 (table 1). However, nsp2 is only a partial inhibitor of PKR and so, although it prevents a marked reduction of protein synthesis, there is sufficient residual ISR signalling to permit PPP1R15A expression, which then supports protein synthesis and therefore IBV replication. Similarly, depletion of CHOP, which induces PPP1R15A, suppresses viral replication [60]. Disappointingly, the putative PPP1R15A inhibitor, salubrinal, failed to block IBV replication. However, we have shown that salubrinal has little effect on PPP1R15A and most likely antagonises the UPR through other mechanisms [67] (table 2).
TABLE 2.
Integrated stress response (ISR)-modifying drugs
| Drug | Putative mode of action | Cautions | References | |
| ISR inhibitors | ||||
| PERK | GSK2656157 | Targets ATP binding site of PERK | Inhibits RIPK1 | [68, 69] |
| GSK2606414 | Targets ATP binding site of PERK | Inhibits RIPK1 and PKR Weakly activates GCN2 |
[69, 70] | |
| 4-PBA | Reduces ER stress by unclear mechanism | Affects all arms of the UPR | [71] | |
| TUDCA | Reduces ER stress by unclear mechanism | Affects all arms of the UPR | [71] | |
| HRI | Aminopyrazolindane | Not commercially available | [72] | |
| PKR | C16 | Targets ATP binding site of PKR | [73] | |
| C22 | Targets ATP binding site of PKR | [73] | ||
| 2-Aminopurine | Targets ATP binding site of PKR | [74] | ||
| GCN2 | 6D | Targets ATP binding site of GCN2 | Not commercially available | [75] |
| 6E (aka GCN2iA) | Targets ATP binding site of GCN2 | Not commercially available | [75, 76] | |
| eIF2β | ISRIB | Stablises eIF2β dimers | Cell lines can acquire ISRIB resistance mutations | [77–79] |
| Dibenzoylmethane | Cells insensitive to p-eIF2α | Mechanism of action unclear | [80] | |
| Trazodone | Cells insensitive to p-eIF2α | Mechanism of action unclear | [80] | |
| ISR activators | ||||
| PERK | CCT020312 | Enhances PERK activation | Mechanism of action unclear | [81] |
| Tunicamycin | Induces ER stress: inhibits N-glycosylation | Activates all arms of the UPR | [82] | |
| Bortezomib | Induces ER stress: inhibits the proteasome | Pleotropic effects of proteasome inhibition | [83] | |
| Montelukast | Enhances PERK signalling Mechanism unclear |
Leukotriene receptor antagonist | [84] | |
| HRI | BTdCPU | [85] | ||
| cHAUs | [86] | |||
| PKR | Interferon | Increases expression of PKR | Pleotropic effects of interferon signalling | [87] |
| poly I:C | RNA mimetic | Requires transfection to enter cell | [88] | |
| BEPP | Mechanism of action unclear | [89] | ||
| GCN2 | Histidinol | Inhibits histidinyl-tRNA synthetase | [90] | |
| Tryptophanol | Inhibits tryptophan-tRNA synthetase | [91] | ||
| Halofuginone | Inhibits prolyl-tRNA synthetase | [92] | ||
| L-asparaginase | Depletes extracellular asparagine | [93] | ||
| PPP1R15A | Salubrinal | Putative PPP1R15 inhibitor | Concerns that effects may be PPP1R15 independent | [67, 94] |
| Guanabenz | Putative PPP1R15 inhibitor | Concerns that effects may be PPP1R15 independent | [67, 95, 96] | |
| Sephrin1 | Putative PPP1R15 inhibitor | Concerns that effects may be PPP1R15 independent | [67, 97, 98] | |
| PPP1R15A and B | Jasplakinolide | Depletes G-actin required for PPP1R15 function | Pleotropic effects of actin stabilisation | [99] |
PERK: protein kinase R (PKR)-like endoplasmic reticulum kinase; HRI: heme-regulated inhibitor; GCN: general control nondepressible; eIF: eukaryotic initiation factor; 4-PBA: 4-phenylbutyric acid; ER: endoplasmic reticulum; UPR: unfolded protein response; TUDCA: tauroursodeoxycholic acid; C16: CAS 608512-97-6 [6,8-dihydro-8-(1H-imidazol-5-ylmethylene)-7H-pyrrolo[2,3-g]benzothiazol-7-one]; C22: CAS 852547-30-9 (5-chloro-3-[(3,5-dichloro-4-hydroxyphenyl)methylidene]-2,3-dihydro-1H-indol-2-one); ISRIB: integrated stress response inhibitor [trans-2-(4-chlorophenoxy)-N-(4-(2-(4-chlorophenoxy)acetylamino)cyclohexyl)acetamide]; CCT020312: [6-bromo-3-[5-(4-bromo-phenyl)-1-(3-diethylamino-propionyl)-4,5-dihydro-1H-pyrazol-3-yl]-4-phenyl-1H-quinolin-2-one]; cHAUs: [1-((1,4-trans)-4-arylox-ycyclohexyl)-3-arylureas]; polyI:C: polyinosinic-polycytidylic acid; BEPP: [1H-benzimidazole-1-ethanol, 2,3-dihydro-2-imino-α-(phenoxymethyl)-3-(phenylmethyl)-monohydrochloride].
Other respiratory viruses
Infection with respiratory syncytial virus (RSV) triggers formation of stress granules [100]. These are maintained throughout the infection via PKR-mediated eIF2α phosphorylation, and contain viral RNA. Similarly, human enterovirus (EV)-D68, which causes severe epidemic respiratory disease, triggers stress granules to form, although these are only transient and wane within 14 h [101]. This disaggregation of stress granules correlates with increased virus production and, since PKR remains active, appears to be mediated by cleavage of the stress granule protein G3BP1 by virally encoded factors.
Porcine reproductive and respiratory syndrome virus (PRRSV, also known as beta-arterivirus suid 1) infects pigs and inflicts a great economic strain on the farming industry, but frequent antigenic variations have hampered the development of a vaccine [102–105]. PRRSV primarily infects alveolar macrophages where it inhibits PKR to enable its own replication [102]. However, early pharmacological activation of PKR with polyinosinic-polycytidylic acid can block viral replication (table 2). Interestingly, UPR signalling by PERK and IRE1α appears to be more important than PKR in promoting stress granule formation during PRRSV infection [104, 105], but PERK may also play a role in severe pneumonias caused by co-infection with PRRSV and bacteria, since the resulting eIF2α phosphorylation inhibits IFN and tumour necrosis factor (TNF) production. Curiously, ISR activation during PRRSV infection might increase viral titres [103]. ATF4 is held in the cytosol by the viral proteins nsp2 and nsp3, preventing its migration to the nucleus. While impairing normal ISR signalling, this mislocalisation of ATF4 to sites of viral replication also benefits the virus through a poorly understood protein–RNA interaction.
Bacterial infection
We identified a role for the ISR during infections with Pseudomonas aeruginosa [106]. This opportunistic bacterium, which can cause morbidity in patients with bronchiectasis, secretes several virulence factors, among which pyocyanin and AprA can trigger the UPR and ISR [106]. Exposure of human bronchial epithelial cells to such factors caused a rapid induction of PPP1R15A, which protected against Pseudomonas-induced cytotoxicity. Surprisingly, the upstream eIF2α kinase involved proved to be the iron sensor, HRI. Iron availability is necessary for bacterial growth and its availability is limited by the host as a means to protect against bacterial infection [107]. It appears that in the airway, further depletion of iron from the microenvironment by P. aeruginosa is sensed by HRI to trigger a cytoprotective PPP1R15A response. Other studies have confirmed the phosphorylation of eIF2α during the exposure of human lung epithelial cells to pyocyanin [108, 109]. It has also been suggested that this P. aeruginosa-mediated activation of the ISR triggers a cytoprotective autophagic response [108].
Interventions against infection
The innate antiviral role of the ISR makes it an attractive target for the development of antiviral therapies. In a repurposing-screen of drugs approved for human use, an anti-asthma medication, montelukast, was found to inhibit influenza A replication (table 2) [84]. It appeared to exert a mild antiviral effect by enhancing PERK signalling. Accordingly, high concentrations of guanabenz, a putative inhibitor of eIF2α phosphatases, impaired viral protein synthesis [84] and a derivative of guanabenz called sephin1 blocked replication of RSV and EV-D68, but not influenza A in human cells [110]. However, the results could not be replicated in vivo owing to the toxicity of the high concentrations of sephin1 required, and there remains doubt as to whether the effects of guanabenz or sephin1 can be attributed to inhibition of PPP1R15A (table 2) [67, 97].
A challenge to the development of vaccines that impart cell-mediated immunity in the lung is the short lifespan of airway-resident memory T (TRM)-cells [111]. Unlike the long-lived TRM cells of other tissues, airway TRM cells are almost completely lost through apoptosis by 180 days following exposure to influenza or Sendai viruses [111]. For durable immunity, it is therefore important to understand what accounts for this short survival. Transcriptomic analysis revealed GCN2-dependent activation of the ISR as a unique signature in airway-resident TRM [111]. This appears to be a function of the nutrient-poor microenvironment of the airway but is reversible, since airway TRM cells cultured ex vivo in amino acid sufficient conditions are rescued. In theory, antagonism of GCN2 might prolong airway TRM cells, but the consequences of this for immunopathology and inflammation are unclear. The abnormal pulmonary vascular phenotypes of GCN2-deficient individuals, may serve as a warning in this regard (discussed later).
Inflammation
The airway epithelium has a role in regulating the immune microenvironment and the ISR seems to be important in this. Toll-like receptors (TLRs) enable bronchial epithelial cells to sense pathogen associated molecular patterns (PAMPS) such lipopolysaccharide (LPS), a component of bacterial cell walls (figure 2). TLR activation induces PKR via IFNs as part of the inflammatory response, but other ISR factors including ATF3 and PPP1R15A are upregulated through less well understood mechanisms [112].
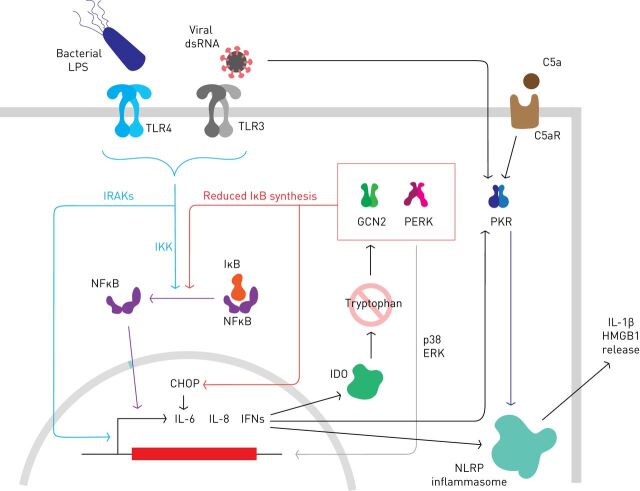
FIGURE 2.
The integrated stress response and inflammation. Pathogen-associated molecular patterns (PAMPs) recognised by Toll-like receptors (TLRs) can trigger the innate immune response. Phosphorylation of IκB by IKK promotes its destruction and releases nuclear factor (NF)-κB to transactive pro-inflammatory genes including interleukin (IL)-6, IL-8 and interferons (IFNs). Induction of indoleamine 2,3-dioxygenase (IDO) depletes cells of tryptophan (at least activated macrophages and dendritic cells) to activate general control nondepressible (GCN)2. Activation of GCN2 or protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) can trigger NFκB signalling by reducing the synthesis of IκB, while CHOP increases IL-6 expression directly. Some evidence suggests PERK-mediated p38/ERK signalling might also contribute to inflammatory gene expression. PKR is induced by IFN and further activated if viral double-stranded (ds)RNA is present in the cytoplasm. Inflammatory mediators including C5a fragment can activate PKR, e.g. via C5a receptor (C5aR) signalling. PKR contributes to inflammation by stimulating the NLRP3 inflammasome to generate pro-inflammatory mediators including IL-1β and high mobility group box (HMGB)1.
Early work showed that intense PERK activation can activate NFκB in vitro by inhibiting the synthesis of IκB, a short-lived negative-regulator of NFκB [113]. However, it is unclear if sufficiently high levels of phosphorylated eIF2α are achieved in vivo to induce this mechanism in response to ER stress. Nevertheless, PERK activation seems to enhance inflammatory signalling in vivo in response to PAMPs. For example, in bronchial epithelial cells treated with LPS, PERK activation increases proinflammatory IL-6 and IL-8 production via increased p38 and ERK signalling [114]. Furthermore, CHOP can dimerise with other members of the C/EBP transcription factor family to regulate the transcription of cytokines including IL-6 [115, 116]. Translational regulation of IκBα might nonetheless be important in activated macrophages and dendritic cells, where induction of indoleamine 2,3-dioxygenase, an enzyme that metabolises tryptophan, can trigger GCN2 and the ISR via amino acid sensing [117]. This enhances the secretion of IL-6 in response to LPS and is mediated, at least in part, by inhibition of IκB synthesis (figure 2).
LPS administration increases PKR phosphorylation in mouse lungs [118, 119]. In murine alveolar macrophages, PKR is critical in TLR-stimulated cytokine secretion and appears to contribute to lung damage in the context of sepsis [120]. A variety of PAMPS transactivate NOD-like receptor (NLR) inflammasomes [121]. During bacterial infection, complement activation generates a C5a fragment, which is chemotactic for neutrophils and enhances their capacity to phagocytose and kill bacteria [122]. One function of C5a appears to be the stimulation of PKR, which activates the NLRP3 inflammasome through direct interaction, promoting the release of HMGB1 (high mobility group box 1) (figure 2) [123, 124]. Conversely, HMGB1, which is a DNA binding protein with inflammatory cytokine activity, can itself activate PKR during macrophage M1 polarisation in acute lung injury, suggesting the possibility of a positive feedback loop [125]. Inhibition of PKR with C16 opposes both NLRP3 activation and HMGB1 release from LPS-challenged macrophages (table 2). Consequently, administration of C16 to mice prior to LPS challenge, inhibits PKR activation and so reduces the concentration of pro-inflammatory cytokines in lung lavage, limiting pulmonary damage [118, 119].
Barotrauma caused by mechanical ventilation can also induce pulmonary inflammation and acute lung injury [126]. In cell models of alveolar epithelial stretch, or if the lung is hyperinflated mechanically in vivo, PERK-dependent ISR signalling is triggered, possibly via stretch-induced release of calcium from the ER. This may mediate some of the toxicity of barotrauma, since pharmacological inhibition of PERK limits alveolar permeability and production of IL-8 during acute inflammation induced by cyclic strain [126, 127].
In the bronchoalveolar lavage fluid of asthmatic patients the levels of CHOP and the ER chaperone BiP are higher than healthy controls [128]. Ovalbumin (OVA) and LPS administration produces an asthma-like state in murine models in which lungs show evidence of ER stress with induction of the same proteins as seen in patients. OVA/LPS-sensitised mice, treated with 4-PBA, which ameliorates ER stress via poorly understood mechanisms, show less IκB degradation therefore reduced NFκB signalling (table 2). This also results in attenuated inflammatory signals such as IL-10 expression and reduces infiltration of neutrophils and dendritic cells into the lung [128, 129].
In other situations, the ISR and pro-inflammatory signalling pathways appear to be antagonistic, suggesting some of these effects might be tissue specific. For example, in the gut, eIF2α phosphorylation caused by GCN2 during acute amino acid starvation suppresses intestinal inflammation [130]. Conversely, TLR4 activation appears able to stimulate eIF2β to antagonise the inhibition of translation caused by eIF2α phosphorylation, thus suppressing the translation of ATF4 and CHOP [131, 132].
Smoke and exhaust fumes
Inhaled pollutants can trigger the UPR and ISR [133–138]. The resulting cytotoxicity is thought to impair epithelial integrity and lead to COPD [139]. The particulate matter found in diesel exhaust induces ER stress in mouse lung tissue [140] and increases the expression of CHOP and PPP1R15A in human airway epithelium [137]. This response appears to be accentuated in patients with COPD [138] and is even more marked if cells are co-exposed to bacterial components of nontypeable Haemophilus influenzae [141].
Lungs from patients with COPD have elevated levels of ISR markers [142]. Rats and mice chronically exposed to cigarette smoke also express increased levels of ISR markers, although there appears to be species-dependent differences with few genes modulated in a concordant manner between mice exposed to smoke and the lungs of humans with COPD [140, 143, 144]. It has long been known that primary bronchial epithelial cells exposed to cigarette smoke display a transient PERK-dependent phosphorylation of eIF2α followed by induction of ATF4 and PPP1R15A [134]. Indeed, PERK-dependent eIF2α phosphorylation is a consistent feature of cells exposed to cigarette smoke or smoke extract and at least some of the cytotoxicity is mediated by ER stress-induced cell death [135, 145]. For example, the aldehyde acrolein found in smoke induces ER stress-mediated cell death in A549 cells through multiple pathways including perturbed ER calcium homeostasis [146]. Acrolein is highly oxidising, as are numerous components of smoke, and this impairs the function of many macromolecules in cultured airway epithelial cells [147, 148] and in the lungs of humans and mice [149, 150]. Oxidative stress appears to be a key trigger for ISR activation by smoke, since treatment with the antioxidant N-acetylcysteine attenuates ISR induction [133, 135, 136, 151]. Cultured cells or mice treated with acrolein induce PPP1R15A, which appears to be a toxic phenomenon since Ppp1r15a−/− mice are more resistant to cell death and have better preserved lung architectures [152]. This is reminiscent of the increased tolerance of Ppp1r15a−/− mice to ER stress [14]. Interestingly, salubrinal, which increases eIF2α phosphorylation through poorly understood mechanisms also protects cells from smoke (table 2) [153].
As cigarette smoke is a potent oxidative stress, induction of the ISR is likely to be a protective response because ATF4 induces many antioxidant genes [1, 9]. It has been suggested that NRF2, another regulator of the antioxidant response, and ATF4 heterodimerise to regulate the expression of target genes [154, 155]. NRF2 can also induce ATF4 to amplify this response [156, 157]. It should be noted that although early reports suggested that PERK could activate NRF2 directly [158, 159], it was subsequently shown that PERK-dependent antioxidant gene expression is entirely determined by phosphorylation of eIF2α [160]. Interestingly, differences in the vigour of these antioxidant responses might contribute to individual differences in the susceptibility to smoke, since an ATF4 target gene signature distinguish the airway of smokers with COPD compared to smokers without COPD [161].
Pulmonary hypertension
Pulmonary arterial hypertension (PAH) is a rare, fatal condition primarily affecting young adults. It involves vascular remodelling causing increased pulmonary vascular resistance and right-sided heart failure [162]. In 70% of familial cases and 20% of sporadic cases, heterozygous germ line mutations are identified in the gene encoding the bone morphogenetic protein type 2 receptor (BMPR2). However, these mutations are variably penetrant, suggesting that additional modifying factors exist. Two subtypes of PAH, pulmonary veno-occlusive disease (PVOD) and pulmonary capillary haemangiomatosis (PCH) were recently shown to be caused by biallelic mutations of EIF2AK4, which encodes GCN2 (figure 3) [163–165]. The PVOD/PCH subtypes have an even worse prognosis than classical PAH and currently have no effective treatments apart from lung transplantation [166]. In families affected by PVOD, mutant alleles of EIF2AK4 segregate with the disease in a recessive manner, while in the general population such null alleles are vanishingly rare [163]. Remarkably, when histologically proven nonfamilial cases of PVOD were examined, 25% were also found to have pathogenic mutations of EIF2AK4 [163]. Subsequently, biallelic mutations of EIF2AK4 have been observed in some patients with classical PAH, suggesting that GCN2 may play a broader role in pulmonary vascular disease [167–171]. Individuals with PAH and biallelic mutations of EIF2AK4 tend to be younger (aged <50 years) with lower transfer coefficients of the lung for carbon monoxide (<50%) and normal spirometry, leading some to suggest that EIF2AK4 gene testing may be useful in the diagnosis and management of PAH [170, 172].
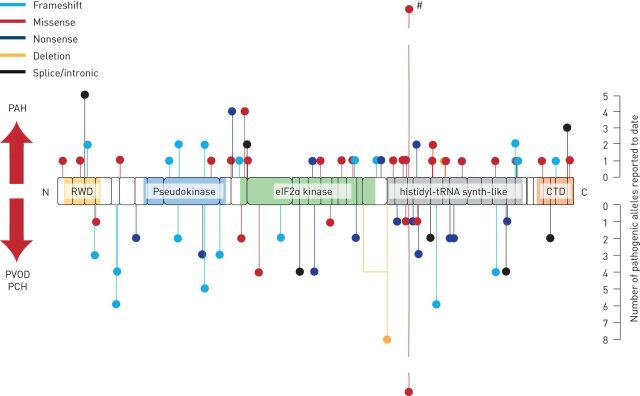
FIGURE 3.
Variants of EIF2AK4 associated with pulmonary vascular disease. Schematic representation of the general control nondepressible (GCN)2 protein and its domains; boxes correspond to the 39 exons in EIF2AK4. Domains are highlighted: RWD (RING-finger proteins, WD repeat-containing proteins, yeast DEAD-like helicase), pseudokinase, eukaryotic initiation factor (eIF)2α kinase, histidyl-tRNA synthetase-like and carboxy-terminal domain (CTD). Predicted pathogenic variants are shown as lollipops: above the protein are likely pathogenic variants associated with pulmonary arterial hypertension (PAH), below are likely pathogenic variants associated with pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis (PCH). The lollipop length indicates the approximate number of such alleles reported in the literate allowing for incomplete reporting. Note, c.3344C>T (p.P1115L) in exon 23 in at the histidyl-tRNA synthetase-like domain has been reported in five families affected by PAH or PVOD (#). Potentially, 48 alleles have been described, but this may be confounded by overlaps between published reports.
GCN2 is normally detectable in pulmonary vessel smooth muscle, interstitial tissue and macrophages, but is absent from individuals homozygous for loss-of-function alleles of EIF2AK4 [163]. Interestingly, despite the loss of this kinase, lung tissue from patients with PVOD showed increased immunostaining for the downstream ISR targets CHOP and heme oxygenase (HO)-1, which are said to be absent from normal lung and the lungs of patients with classical PAH [173]. CHOP was increased in endothelial cells while HO-1 was found in the capillaries of haemangiomatosis foci. The mechanism by which loss of GCN2 enhances CHOP expression requires elucidation.
There is a suggestion of genotype–phenotype correlation in PVOD [174]. Individuals with PVOD due to mutations of EIF2AK4 appear to develop more severe intimal fibrosis and less severe medial hypertrophy of the pulmonary arteries. They show greater smooth muscle hyperplasia of interlobular septal veins compared with individuals without the mutation and have more foci of PCH, rather than the more diffuse haemangiomatous changes seen in mutation-negative cases.
Remarkably, while GCN2 protein is absent in those with biallelic EIF2AK4 mutations, even patients with PVOD and wildtype EIF2AK4 show reduced GCN2 expression. Moreover, patients with classical PAH can have reduced GCN2 protein expression suggesting that GCN2 might be relevant to many forms of pulmonary hypertension. Intriguingly, mitomycin C, which is a DNA alkylating agent that can induce PVOD in humans and rats [175], has been shown to reduce the expression of GCN2 in lung tissue [175]. This is associated with reduced phosphorylation of SMAD1/5/8, downstream mediators of BMP signalling, while depletion of GCN2 in pulmonary arterial endothelial cells also reduces the phosphorylation of SMAD1/5/9 in rats and human pulmonary arterial endothelial cells [173]. However, it has yet to be shown that restoration of GCN2 expression can rescue PVOD caused by mitomycin C.
The mechanistic links between loss of GCN2 and defects of the pulmonary vasculature are unclear, although we demonstrated recently that GCN2 expression can inhibit BMP signalling during development in Drosophila, in part by regulating the synthesis of components of the BMP signalling pathway [176]. Conversely, loss of GCN2 appears to impair the more complex BMP signalling found in mammals [173]. This was demonstrated both in Eif2ak4−/− rats and in pulmonary artery endothelial cells depleted of GCN2 by siRNA. Such an interaction between the ISR and BMP signalling might contribute to the incomplete penetrance of pathogenic BMPR2 mutations. This is supported by observations in a family with autosomal dominant PAH caused by a BMPR2 mutation, in which all living family members with diagnosed PAH also carried an EIF2AK4 mutation [167].
The relationship between the ISR and cell survival may be relevant to its role in pulmonary hypertension. Dysregulated pulmonary artery endothelial cell proliferation and apoptosis are believed to be important factors in the development of PAH [177]. It has been proposed that the initial apoptosis of pulmonary endothelial cells is followed by hyperproliferation of apoptosis-resistant cells with increased expression of anti-apoptotic factors including survivin [178]. Survivin is highly expressed in the pulmonary arteries of patients with PAH and in the monocrotaline-induced PAH rat model [179]. When a dominant negative mutant of survivin was expressed in monocrotaline-treated rats, established PAH was reversed resulting in prolonged survival [179]. In human pulmonary arterial endothelial cells, depletion of GCN2 by siRNA enhanced the proliferation and increased the expression of survivin, while inhibition of GCN2 with compound 2662034 also increased endothelial cell proliferation (table 2) [173]. This effect of GCN2 inhibition could be antagonised by exogenous BMP-9, which is known to reverse PAH in animal models [173, 180].
Inflammation is a recognised feature of PAH with increased circulating levels of IL-6, IL-8, IL-10 and IL-12p70 correlating with worse survival [181]. Inflammatory signalling appears to modify the penetrance of pathogenic alleles of BMPR2 in humans and the TLR4 ligand LPS worsens PAH in Bmpr2 mutant mice [182]. The ISR may play a role in the sensitisation of BMP-signalling-deficient animals to inflammation [183]. When pulmonary artery endothelial cells are treated with TNF, they produce the potent pro-inflammatory chemokine granulocyte–macrophage colony-stimulating factor (GM-CSF). Treatment with BMP2 suppresses GM-CSF production and so deficiency of BMPR2 results in enhanced TNF-mediated GM-CSF secretion. It appears that loss of BMPR2 activates p38MAPK to increase expression of PPP1R15A. The resultant dephosphorylation of eIF2α enhances translation of GM-CSF mRNA [183]. This suggests that ISR signalling normally limits inflammatory cytokine production in the pulmonary vasculature, and might explain why loss of an ISR kinase worsens PAH through enhanced translation of pro-inflammatory cytokines.
It appears that the ISR plays a role both to limit pulmonary endothelial cell proliferation and inflammatory cytokine production, while sharing a mutually regulating relationship with BMP signalling. It is therefore possible that ISR-directed therapies might have value in more common forms of PAH, not only in rarer genetic varieties.
Pulmonary fibrosis
The aetiology of pulmonary fibrosis is complex and is likely to involve many different triggers, including the ageing process. Prolonged inflammation or the expression of misfolded mutants of surfactant proteins can also lead to pulmonary fibrosis [2]. It has been noted that treating mice with tunicamycin, a toxin that causes ER stress, promotes lung fibrosis and causes mitochondrial dysfunction in primary type II alveolar epithelial cells (table 2) [82]. This is noteworthy because the type II alveolar epithelial cells from patients with idiopathic pulmonary fibrosis (IPF) also accumulate dysmorphic and dysfunctional mitochondria, as do other ageing animals. The integrity of a cell's mitochondria is maintained through the action of PINK1, the expression of which falls intriguingly with age and during ER stress. It is also downregulated in the lungs of patients with pulmonary fibrosis. PINK1-deficient mice are more susceptible to lung fibrosis induced by intratracheal instillation of bleomycin [82]. This drug drives epithelial to mesenchymal transition, fibroblast proliferation and subsequent extracellular matrix deposition in the lungs [184]. The repression of PINK1 seen during ER stress appears to be dependent upon ATF3, an ISR transcription factor, which binds directly to the PINK1 promoter [185]. Although circumstantial, it is interesting that the lungs of individuals with IPF show higher levels of ATF3 compared to age-matched controls [82]. In some stressful conditions, ATF3 is thought to contribute to CHOP expression [186]. CHOP, is also elevated in the lungs of patients with IPF and bleomycin-exposed mice, especially in areas affected by fibrosis that notably are also markedly hypoxic [187]. CHOP seems to play a crucial role in the development of these lesions since its deletion protects mice from fibrosis. Hypoxia can induce CHOP via ER stress and the UPR. Moreover, hypoxia (inspiratory oxygen fraction 14%) can worsen bleomycin-induced fibrosis, yet this is abrogated in Chop−/− mice. CHOP expression may have broader relevance in fibrosis, since CHOP is induced by silica, probably via ER stress, and inhibition of its induction by 4BPA ameliorates silicosis in rats [188, 189].
Thoracic malignancy
Having evolved from the ancestral general amino acid control pathway in yeast [190], the ISR in mammals plays a central role in regulating the availability of amino acids [9]. Cancers have a high demand for amino acids, both for new protein synthesis and to provide thiols for production of the antioxidant glutathione. As a tumour outgrows its vascular bed, both amino deprivation and ER stress trigger a cytoprotective ISR via GCN2 and PERK, respectively, elevating ATF4 and CHOP in its hypoxic core [191]. Consequently, the ability of cancer cells to phosphorylate eIF2α is critical for the growth of large solid tumours [191]. In hypoxic tumours, the ER generates increased levels of reactive oxygen species in part through CHOP-mediated induction of the ER oxidoreductase ERO1α [14, 192]. ERO1α expression enhances the capacity of a cell to generate disulphide bonds and so aids protein synthesis and tumour growth. Indeed, high ERO1α expression correlates with worse cancer prognosis and increased cancer cell growth [193, 194]. But excess generation of reactive oxygen species can be cytotoxic, and so one of the functions of the ISR is to defend against this.
In the lung, activated phospho-GCN2 is observed in carcinoma tissue, but less so normal tissues [195]. This is necessary for the expression of ATF4-target genes in lung cancer that promote the synthesis, import and mobilisation of amino acids. Increased ATF4-dependent production of glutathione may account for much of the resistance to cisplatin chemotherapy observed in multiple cell lines including those derived from lung cancers [196, 197]. PERK-mediated resistance to oxidative stress is also thought to be important in the resistance to radiotherapy [198, 199]. It has also been shown that drug-resistant KRAS-mutant lung cancers have elevated levels of ATF4 and are more susceptible than nonresistant lung cancer cells to inhibition of PERK [200]. PERK inhibition restores their sensitivity to the MEK inhibitor trametinib and raises to possibility of ISR-directed personalised therapies [200].
Analysis of The Cancer Genome Atlas reveals that advanced lung adenocarcinomas often exhibit an ATF4 target gene signature [201]. Correspondingly, in lung adenocarcinoma cell lines the drug ISRIB (integrated stress response inhibitor [trans-2-(4-chlorophenoxy)-N-(4-(2-(4-chlorophenoxy)acetylamino)cyclohexyl)acetamide]), which renders cells insensitive to eIF2α phosphorylation, blocked ATF4 expression and reduced both cancer cell proliferation and migration in amino acid deficient conditions (table 2) [201]. One of the mechanisms by which the ISR adapts cells to nutrient-poor environments is through enhanced import of amino acids. This too may produce specific vulnerabilities, since in nonsmall cell lung cancers the expression of the large neutral amino acid transporter LAT1 in vivo correlates with poor survival [202], while in vitro silencing of LAT1 in lung adenocarcinoma A549 cells can impair cancer cell growth [203]. The availability of amino acids can also be increased via activation of macro-autophagy, which liberates amino acids from long-lived proteins and damaged organelles. Accordingly, in hypoxic areas of tumour xenografts, increased expression of autophagy factors such as LC3 is observed, which is at least partially mediated by the GCN2/PERK–ATF4–CHOP axis [195, 199, 204].
Nutrient and oxygen deficiency both cause tissues to release signals that induce angiogenesis. It is well known that hypoxia leads to the stabilisation of the transcription factor HIF1α to promote angiogenesis [205]. However, the ISR also contributes to angiogenesis because ATF4 directly upregulates vascular endothelial growth factor (VEGF)A and downregulates inhibitors of angiogenesis [206–208]. Consequently, depletion of PERK prevents VEGFA secretion in response to glucose deprivation independent of HIF1α, while inhibition of PERK reduces xenograft vascularity and perfusion in mice [208]. The PERK inhibitor GSK2656157 reduces cancer growth in vivo, most likely via reducing angiogenesis and impairing amino-acid metabolism (table 2) [209].
Cancers are often associated with local immunosuppression [210]. Expression of the inflammatory ISR kinase PKR is reduced in many non-small cell lung cancers with lower expression correlating with poorer prognosis [211]. Conversely, pharmacological activation of PKR or its overexpression kills lung cancer cells [211, 212]. Recently, it was discovered that many lung cancer cell lines are dependent on ADAR1, an RNA deaminase [213]. Loss of ADAR1 leads to PKR activation and cell death, while loss of PKR rescues the lethality of ADAR1 deletion. This suggests that PKR detects an RNA signal in lung cancer cells that is suppressed by ADAR1. It is unclear why lung cancer cells behave in this manner, but this raises the possibility of targeted therapies, for example ADAR1 inhibition, to trigger PKR-mediated lung cancer cell killing [213].
It is often stated that CHOP triggers cell death, but the reality is more nuanced. For example, expression of CHOP is an independent predictor of a poor prognosis in malignant pleural mesothelioma, suggesting CHOP expression is associated with increased tumour growth [214]. However, in chemoresistant mesothelioma, activation of the PERK–ATF4–CHOP pathway by bortezomib reverses the chemoresistance (table 2) [215]. Very few target genes of CHOP are prodeath factors, but rather are involved in oxidative protein folding, protein secretion and autophagy [14, 199, 216]. In fact, the recovery of protein synthesis and oxidative protein folding mediated by CHOP, may explain much of CHOP's relationship with ER stress induced cell death [14, 216]. The recovery of translation at later time points during the ISR is mediated by the CHOP target PPP1R15A, and consequently Ppp1r15a−/− mice are resistant to ER stress induced tissue damage, as they are protected from the toxicity of excessive protein synthesis [14]. In a murine model of medulloblastoma, loss of PPP1R15A increased phospo-eIF2α and promoted tumour growth, invasiveness and angiogenesis perhaps via the increased expression of VEGFA [217]. Although mutations of CHOP or PPP1R15A are uncommon in human cancers, their expression might be suppressed by other means. For example, in breast carcinoma, PERK-induced expression of microRNA mir211 promotes tumour cell survival in part by repressing CHOP expression [118]. Loss of PPP1R15A is also observed in the more aggressive sarcomatoid subtype of malignant pleural mesothelioma, and so low PPP1R15A expression correlates with a worse prognosis [218]. The mechanism by which PPP1R15A expression is suppressed in sarcomatoid mesothelioma remains unclear.
Conclusion
The ISR is central in responding to infection, oxidative stress and nutrient deprivation. Studies of human lung disease have revealed its importance in normal pulmonary physiology, perhaps relating to the uniquely oxidising and nutrient poor environment of the airway. The role of the ISR in regulating cell survival and inflammation make it an attractive target for the development of immunomodulators and anticancer therapies alike. More recent discoveries of the importance of GCN2 in maintaining pulmonary vascular health also raise the possibility that new treatments for pulmonary hypertension might be developed by targeting the ISR.
Footnotes
Provenance: Commissioned article, peer reviewed
Conflict of interest: G. Emanuelli has nothing to disclose.
Conflict of interest: N. Nassehzadeh-Tabriz has nothing to disclose.
Conflict of interest: N.W. Morrell has nothing to disclose.
Conflict of interest: S.J. Marciniak has nothing to disclose.
Support statement: Funding was received from the Alpha-1 foundation (pC ID 614939), the British Lung Foundation, and Research Councils UK Medical Research Council (MCMB MR/R009120/1). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.van ’t Wout EFA, Hiemstra PS, Marciniak SJ. The integrated stress response in lung disease. Am J Respir Cell Mol Biol 2014; 50: 1005–1009. doi: 10.1165/rcmb.2014-0019TR [DOI] [PubMed] [Google Scholar]
- 2.Dickens JA, Malzer E, Chambers JE, et al. Pulmonary endoplasmic reticulum stress – scars, smoke, and suffocation. FEBS J 2019; 286: 322–341. doi: 10.1111/febs.14381 [DOI] [PubMed] [Google Scholar]
- 3.Pakos-Zebrucka K, Koryga I, Mnich K, et al. The integrated stress response. EMBO Rep 2016; 17: 1374–1395. doi: 10.15252/embr.201642195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalton LE, Healey E, Irving J, et al. Phosphoproteins in stress-induced disease. In: Shenolikar S, ed. Protein Phosphorylation in Health and Disease. 1st Edn. Amsterdam, Elsevier, 2012; 106: pp. 189–221. [DOI] [PubMed] [Google Scholar]
- 5.Dauber B, Wolff T. Activation of the antiviral kinase PKR and viral countermeasures. Viruses 2009; 1: 523–544. doi: 10.3390/v1030523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers JE, Marciniak SJ. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress. Am J Physiol Cell Physiol 2014; 307: C657–C670. doi: 10.1152/ajpcell.00183.2014 [DOI] [PubMed] [Google Scholar]
- 7.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397: 271–274. doi: 10.1038/16729 [DOI] [PubMed] [Google Scholar]
- 8.Suragani RNVS, Zachariah RS, Velazquez JG, et al. Heme-regulated eIF2α kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood 2012; 119: 5276–5284. doi: 10.1182/blood-2011-10-388132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11: 619–633. doi: 10.1016/S1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- 10.Masson GR. Towards a model of GCN2 activation. Biochem Soc Trans 2019; 47: 1481–1488. doi: 10.1042/BST20190331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 2004; 167: 27–33. doi: 10.1083/jcb.200408003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young SK, Wek RC. Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J Biol Chem 2016; 291: 16927–16935. doi: 10.1074/jbc.R116.733899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novoa I, Zeng H, Harding HP, et al. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J Cell Biol 2001; 153: 1011–1022. doi: 10.1083/jcb.153.5.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004; 18: 3066–3077. doi: 10.1101/gad.1250704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheuner D, Patel R, Wang F, et al. Double-stranded RNA-dependent protein kinase phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J Biol Chem 2006; 281: 21458–21468. doi: 10.1074/jbc.M603784200 [DOI] [PubMed] [Google Scholar]
- 16.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev 2001; 14: 778–809. doi: 10.1128/CMR.14.4.778-809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 2006; 13: 393–403. doi: 10.1038/sj.cdd.4401833 [DOI] [PubMed] [Google Scholar]
- 18.McInerney GM, Kedersha NL, Kaufman RJ, et al. Importance of eIF2α phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell 2005; 16: 3753–3763. doi: 10.1091/mbc.e05-02-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick C, Khaperskyy DA. Translation inhibition and stress granules in the antiviral immune response. Nat Rev Immunol 2017; 17: 647–660. doi: 10.1038/nri.2017.63 [DOI] [PubMed] [Google Scholar]
- 20.García MA, Gil J, Ventoso I, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 2006; 70: 1032–1060. doi: 10.1128/MMBR.00027-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Liao Y, Yap PL, et al. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J Virol 2009; 83: 12462–12472. doi: 10.1128/JVI.01546-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng X, Hackbart M, Mettelman RC, et al. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc Natl Acad Sci USA 2017; 114: E4251–E4260. doi: 10.1073/pnas.1618310114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabouw HH, Langereis MA, Knaap RC, et al. Middle East respiratory coronavirus accessory protein 4a inhibits PKR-mediated antiviral stress responses. PLoS Pathog 2016; 12: E1005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennisi R, Musarra-Pizzo M, Lei Z, et al. VHS, US3 and UL13 viral tegument proteins are required for herpes simplex virus-induced modification of protein kinase R. Sci Rep 2020; 10: 5580. doi: 10.1038/s41598-020-62619-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman DE, Pretto CD, Krepostman TA, et al. Enhanced replication of mouse adenovirus type 1 following virus-induced degradation of protein kinase R (PKR). mBio 2019; 10: e00668-19. doi: 10.1128/mBio.00668-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Wambach M, Katze MG, et al. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 1995; 214: 222–228. doi: 10.1006/viro.1995.9937 [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa K, Narayanan K, Wada M, et al. Inhibition of stress granule formation by Middle East respiratory syndrome coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J Virol 2018; 92: e00902-18. 10.1128/jvi.00902-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habjan M, Pichlmair A, Elliott RM, et al. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J Virol 2009; 83: 4365–4375. doi: 10.1128/JVI.02148-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schümann M, Gantke T, Mühlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol 2009; 83: 8993–8997. doi: 10.1128/JVI.00523-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand SR, Kobayashi R, Mathews MB. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem 1997; 272: 8388–8395. doi: 10.1074/jbc.272.13.8388 [DOI] [PubMed] [Google Scholar]
- 31.Endo-Munoz L, Warby T, Harrich D, et al. Phosphorylation of HIV Tat by PKR increases interaction with TAR RNA and enhances transcription. Virol J 2005; 2: 17. doi: 10.1186/1743-422X-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gale M, Kwieciszewski B, Dossett M, et al. Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J Virol 1999; 73: 6506–6516. doi: 10.1128/JVI.73.8.6506-6516.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor DR, Shi ST, Romano PR, et al. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 Protein. Science 1999; 285: 107–110. doi: 10.1126/science.285.5424.107 [DOI] [PubMed] [Google Scholar]
- 34.Yue Z, Shatkin AJ. Double-Stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 1997; 234: 364–371. doi: 10.1006/viro.1997.8664 [DOI] [PubMed] [Google Scholar]
- 35.Child SJ, Hakki M, De Niro KL, et al. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J Virol 2004; 78: 197–205. doi: 10.1128/JVI.78.1.197-205.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He B, Gross M, Roizman B. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA 1997; 94: 843–848. doi: 10.1073/pnas.94.3.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoo D, Perez C, Mohr I. Characterization of RNA determinants recognized by the arginine- and proline-rich region of Us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J Virol 2002; 76: 11971–11981. doi: 10.1128/JVI.76.23.11971-11981.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burýsek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J Virol 2001; 75: 2345–2352. doi: 10.1128/JVI.75.5.2345-2352.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteban M, García MA, Domingo-Gil E, et al. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J Gen Virol 2003; 84: 1463–1470. doi: 10.1099/vir.0.19014-0 [DOI] [PubMed] [Google Scholar]
- 40.Sharp TV, Schwemmle M, Jeffrey I, et al. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein–Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res 1993; 21: 4483–4490. doi: 10.1093/nar/21.19.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poppers J, Mulvey M, Perez C, et al. Identification of a lytic-cycle Epstein–Barr virus gene product that can regulate PKR activation. J Virol 2003; 77: 228–236. doi: 10.1128/JVI.77.1.228-236.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano PR, Zhang F, Tan SL, et al. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol Cell Biol 1998; 18: 7304–7316. doi: 10.1128/MCB.18.12.7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp TV, Moonan F, Romashko A, et al. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 1998; 250: 302–315. doi: 10.1006/viro.1998.9365 [DOI] [PubMed] [Google Scholar]
- 44.Sharp TV, Witzel JE, Jagus R. Homologous regions of the α subunit of eukaryotic translational initiation factor 2 (eIF2α) and the vaccinia virus K3L gene product interact with the same domain within the dsRNA-activated protein kinase (PKR). Eur J Biochem 1997; 250: 85–91. doi: 10.1111/j.1432-1033.1997.00085.x [DOI] [PubMed] [Google Scholar]
- 45.Schierhorn KL, Jolmes F, Bespalowa J, et al. Influenza A virus virulence depends on two amino acids in the N-terminal domain of Its NS1 protein to facilitate inhibition of the RNA-dependent protein kinase PKR. J Virol 2017; 91: e00198-1. doi: 10.1128/JVI.00198-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kindler E, Thiel V, Weber F, Ziebuhr J, ed. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. 1st Edn. Amsterdam, Elsevier, 2016; Vol. 96: pp. 219–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391: 1285–1300. doi: 10.1016/S0140-6736(17)33293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan S-L, Gale MJ, Katze MG. Double-stranded RNA-independent dimerization of interferon-induced protein kinase PKR and inhibition of dimerization by the cellular P58IPK inhibitor. Mol Cell Biol 1998; 18: 2431–2443. doi: 10.1128/MCB.18.5.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gale M, Tan SL, Wambach M, et al. Interaction of the interferon-induced PKR protein kinase with inhibitory proteins P58IPK and vaccinia virus K3L is mediated by unique domains: implications for kinase regulation. Mol Cell Biol 1996; 16: 4172–4181. doi: 10.1128/MCB.16.8.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutkowski DT, Kang SW, Goodman AG, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell 2007; 18: 3681–3691. doi: 10.1091/mbc.e07-03-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khaperskyy DA, Hatchette TF, McCormick C. Influenza A virus inhibits cytoplasmic stress granule formation. FASEB J 2012; 26: 1629–1639. doi: 10.1096/fj.11-196915 [DOI] [PubMed] [Google Scholar]
- 52.Li F, Chen Y, Zhang Z, et al. Robust expression of vault RNAs induced by influenza A virus plays a critical role in suppression of PKR-mediated innate immunity. Nucleic Acids Res 2015; 43: 10321–10337. doi: 10.1093/nar/gkv1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nandy C, Mrázek J, Stoiber H, et al. Epstein–Barr virus-induced expression of a novel human vault RNA. J Mol Biol 2009; 388: 776–784. doi: 10.1016/j.jmb.2009.03.031 [DOI] [PubMed] [Google Scholar]
- 54.Lee K, Kunkeaw N, Jeon SH, et al. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011; 17: 1076–1089. doi: 10.1261/rna.2701111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krischuns T, Günl F, Henschel L, et al. Phosphorylation of TRIM28 enhances the expression of IFN-β and proinflammatory cytokines during HPAIV infection of human lung epithelial cells. Front Immunol 2018; 9: 2229. doi: 10.3389/fimmu.2018.02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok BWY, Liu H, Chen P, et al. The role of nuclear NS1 protein in highly pathogenic H5N1 influenza viruses. Microbes Infect 2017; 19: 587–596. doi: 10.1016/j.micinf.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 57.Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol 2014; 5: 296. doi: 10.3389/fmicb.2014.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siu KL, Chan CP, Kok KH, et al. Comparative analysis of the activation of unfolded protein response by spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus HKU1. Cell Biosci 2014; 4: 3. doi: 10.1186/2045-3701-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masters VR. The molecular biology of coronaviruses. Adv Virus Res 2006; 66: 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao Y, Fung TS, Huang M, et al. Upregulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. J Virol 2013; 87: 8124–8134. doi: 10.1128/JVI.00626-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan C-P, Siu K-L, Chin K-T, et al. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2006; 80: 9279–9287. doi: 10.1128/JVI.00659-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minakshi R, Padhan K, Rani M, et al. The SARS coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS One 2009; 4: e8342. doi: 10.1371/journal.pone.0008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Wilde AH, Wannee KF, Scholte FE, et al. A kinome-wide small interfering RNA screen identifies proviral and antiviral host factors in severe acute respiratory syndrome coronavirus replication, including double-stranded RNA-activated protein kinase and early secretory pathway proteins. J Virol 2015; 89: 8318–8333. doi: 10.1128/JVI.01029-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krähling V, Stein DA, Spiegel M, et al. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J Virol 2009; 83: 2298–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kindler E, Gil-Cruz C, Spanier J, et al. Early endonuclease-mediated evasion of RNA sensing ensures efficient coronavirus replication. PLoS Pathog 2017; 13: e1006195. doi: 10.1371/journal.ppat.1006195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volk A, Hackbart M, Deng X, et al. Coronavirus endoribonuclease and deubiquitinating interferon antagonists differentially modulate the host response during replication in macrophages. J Virol 2020; 94: e00178-20. doi: 10.1128/JVI.00178-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespillo-Casado A, Chambers JE, Fischer PM, et al. PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. Elife 2017; 6: e26109. doi: 10.7554/eLife.26109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Axten JM, Romeril SP, Shu A, et al. Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med Chem Lett 2013; 4: 964–968. doi: 10.1021/ml400228e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojas-Rivera D, Delvaeye T, Roelandt R, et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ 2017; 24: 1100–1110. doi: 10.1038/cdd.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Axten JM, Medina JR, Feng Y, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem 2012; 55: 7193–7207. doi: 10.1021/jm300713s [DOI] [PubMed] [Google Scholar]
- 71.Özcan U, Yilmaz E, Özcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313: 1137–1140. doi: 10.1126/science.1128294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosen MD, Woods CR, Goldberg SD, et al. Discovery of the first known small-molecule inhibitors of heme-regulated eukaryotic initiation factor 2α (HRI) kinase. Bioorg Med Chem Lett 2009; 19: 6548–6551. doi: 10.1016/j.bmcl.2009.10.033 [DOI] [PubMed] [Google Scholar]
- 73.Jammi NV, Whitby LR, Beal PA. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun 2003; 308: 50–57. doi: 10.1016/S0006-291X(03)01318-4 [DOI] [PubMed] [Google Scholar]
- 74.Hu Y, Conway TW. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J Interferon Res 1993; 13: 323–328. doi: 10.1089/jir.1993.13.323 [DOI] [PubMed] [Google Scholar]
- 75.Fujimoto J, Kurasawa O, Takagi T, et al. Identification of novel, potent, and orally available GCN2 inhibitors with type I half binding mode. ACS Med Chem Lett 2019; 10: 1498–1503. doi: 10.1021/acsmedchemlett.9b00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura A, Nambu T, Ebara S, et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci USA 2018; 115: E7776–E7785. doi: 10.1073/pnas.1805523115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sidrauski C, Acosta-Alvear D, Khoutorsky A, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2013; 2: e00498. doi: 10.7554/eLife.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sekine Y, Zyryanova A, Crespillo-Casado A, et al. Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 2015; 348: 1027–1030. doi: 10.1126/science.aaa6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sidrauski C, Tsai JC, Kampmann M, et al. Pharmacological dimerization and activation of the exchange factor eIF2β antagonizes the integrated stress response. Elife 2015; 4: e07314. doi: 10.7554/eLife.07314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halliday M, Radford H, Zents KAM, et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 2017; 140: 1768–1783. doi: 10.1093/brain/awx074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stockwell SR, Platt G, Barrie SE, et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One 2012; 7: e28568. doi: 10.1371/journal.pone.0028568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bueno M, Lai YC, Romero Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 2015; 125: 521–538. doi: 10.1172/JCI74942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006; 107: 4907–4916. doi: 10.1182/blood-2005-08-3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landeras-Bueno S, Fernández Y, Falcón A, et al. Chemical genomics identifies the PERK-mediated unfolded protein stress response as a cellular target for influenza virus inhibition. mBio 2016; 7: e000085-16. doi: 10.1128/mBio.00085-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burwick N, Zhang MY, de la Puente P, et al. The eIF2-alpha kinase HRI is a novel therapeutic target in multiple myeloma. Leuk Res 2017; 55: 23–32. doi: 10.1016/j.leukres.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yefidoff-Freedman R, Fan J, Yan L, et al. Development of 1-((1,4-trans)-4-aryloxycyclohexyl)-3-arylurea activators of heme-regulated inhibitor as selective activators of the eukaryotic initiation factor 2 alpha (eIF2α) phosphorylation arm of the integrated endoplasmic reticulum stress response. J Med Chem 2017; 60: 5392–5406. doi: 10.1021/acs.jmedchem.7b00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomis DC, Samuel CE. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2α protein kinase (PKR) expression in transfected mammalian cells. Proc Natl Acad Sci USA 1992; 89: 10837–10841. doi: 10.1073/pnas.89.22.10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oganesyan G, Saha SK, Guo B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 2006; 439: 208–211. doi: 10.1038/nature04374 [DOI] [PubMed] [Google Scholar]
- 89.Hu W, Hofstetter W, Wei X, et al. Double-stranded RNA-dependent protein kinase-dependent apoptosis induction by a novel small compound. J Pharmacol Exp Ther 2009; 328: 866–872. doi: 10.1124/jpet.108.141754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang P, McGrath BC, Reinert J, et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 2002; 22: 6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnes NA, Stephenson SJ, Tooze RM, et al. Amino acid deprivation links BLIMP-1 to the immunomodulatory enzyme indoleamine 2,3-dioxygenase. J Immunol 2009; 183: 5768–5777. doi: 10.4049/jimmunol.0803480 [DOI] [PubMed] [Google Scholar]
- 92.Sundrud MS, Koralov SB, Feuerer M, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 2009; 324: 1334–1338. doi: 10.1126/science.1172638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reinert RB, Oberle LM, Wek SA, et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J Biol Chem 2006; 281: 31222–31233. doi: 10.1074/jbc.M604511200 [DOI] [PubMed] [Google Scholar]
- 94.Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of elF2α dephosphorylation protects cells from ER stress. Science 2005; 307: 935–939. doi: 10.1126/science.1101902 [DOI] [PubMed] [Google Scholar]
- 95.Tsaytler P, Harding HP, Ron D, et al. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 2011; 332: 91–94. doi: 10.1126/science.1201396 [DOI] [PubMed] [Google Scholar]
- 96.Perego J, Mendes A, Bourbon C, et al. Guanabenz inhibits TLR9 signaling through a pathway that is independent of eIF2α dephosphorylation by the GADD34/PP1c complex. Sci Signal 2018; 11: eaam8104. doi: 10.1126/scisignal.aam8104 [DOI] [PubMed] [Google Scholar]
- 97.Crespillo-Casado A, Claes Z, Choy MS, et al. A Sephin1-insensitive tripartite holophosphatase dephosphorylates translation initiation factor 2α. J Biol Chem 2018; 293: 7766–7776. doi: 10.1074/jbc.RA118.002325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das I, Krzyzosiak A, Schneider K, et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015; 348: 239–242. doi: 10.1126/science.aaa4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chambers JE, Dalton LE, Clarke HJ, et al. Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2α dephosphorylation. Elife 2015; 4: e04872. doi: 10.7554/eLife.04872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindquist ME, Lifland AW, Utley TJ, et al. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J Virol 2010; 84: 12274–12284. doi: 10.1128/JVI.00260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng J, Gao S, Zhu C, et al. Typical stress granule proteins interact with the 3′ untranslated region of enterovirus D68 to inhibit viral replication. J Virol 2020; 94: e02041-19. doi: 10.1128/JVI.02041-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao Y, Ma Z, Wang R, et al. Downregulation of protein kinase PKR activation by porcine reproductive and respiratory syndrome virus at its early stage infection. Vet Microbiol 2016; 187: 1–7. doi: 10.1016/j.vetmic.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 103.Gao P, Chai Y, Song J, et al. Reprogramming the unfolded protein response for replication by porcine reproductive and respiratory syndrome virus. PLoS Pathog 2019; 15: e1008169. doi: 10.1371/journal.ppat.1008169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y, Fang L, Wang D, et al. Porcine reproductive and respiratory syndrome virus infection induces stress granule formation depending on protein kinase R-like endoplasmic reticulum kinase (PERK) in MARC-145 cells. Front Cell Infect Microbiol 2017; 7: 111. doi: 10.3389/fcimb.2017.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W-Y, Schniztlein WM, Calzada-Nova G, et al. Genotype 2 strains of porcine reproductive and respiratory syndrome virus dysregulate alveolar macrophage cytokine production via the unfolded protein response. J Virol 2017; 92: e01251-17. doi: 10.1128/jvi.01251-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van ‘t Wout EFA, van Schadewijk A, van Boxtel R, et al. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog 2015; 11: e1004946. doi: 10.1371/journal.ppat.1004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol 1999; 34: 399–413. doi: 10.1046/j.1365-2958.1999.01586.x [DOI] [PubMed] [Google Scholar]
- 108.Yang ZS, Ma LQ, Zhu K, et al. Pseudomonas toxin pyocyanin triggers autophagy: implications for pathoadaptive mutations. Autophagy 2016; 12: 1015–1028. doi: 10.1080/15548627.2016.1170256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perra L, Balloy V, Foussignière T, et al. CHAC1 is differentially expressed in normal and cystic fibrosis bronchial epithelial cells and regulates the inflammatory response induced by Pseudomonas aeruginosa. Front Immunol 2018; 9: 2823. doi: 10.3389/fimmu.2018.02823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fusade-Boyer M, Dupré G, Bessière P, et al. Evaluation of the antiviral activity of Sephin1 treatment and its consequences on eIF2α phosphorylation in response to viral infections. Front Immunol 2019; 10: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayward SL, Scharer CD, Cartwright EK, et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8+ T cells. Nat Immunol 2020; 21: 309–320. doi: 10.1038/s41590-019-0584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cláudio N, Dalet A, Gatti E, et al. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J 2013; 32: 1214–1224. doi: 10.1038/emboj.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng J, Lu PD, Zhang Y, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 2004; 24: 10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mijošek V, Lasitschka F, Warth A, et al. Endoplasmic reticulum stress is a danger signal promoting innate inflammatory responses in bronchial epithelial cells. J Innate Immun 2016; 8: 464–478. doi: 10.1159/000447668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hattori T, Ohoka N, Hayashi H, et al. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett 2003; 541: 33–39. doi: 10.1016/S0014-5793(03)00283-7 [DOI] [PubMed] [Google Scholar]
- 116.Caristi S, Piraino G, Cucinotta M, et al. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J Biol Chem 2005; 280: 14433–14442. doi: 10.1074/jbc.M410725200 [DOI] [PubMed] [Google Scholar]
- 117.Liu H, Huang L, Bradley J, et al. GCN2-dependent metabolic stress is essential for endotoxemic cytokine induction and pathology. Mol Cell Biol 2014; 34: 428–438. doi: 10.1128/MCB.00946-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Xiao J, Tan Y, et al. Inhibition of PKR ameliorates lipopolysaccharide-induced acute lung injury by suppressing NF-κB pathway in mice. Immunopharmacol Immunotoxicol 2017; 39: 165–172. doi: 10.1080/08923973.2017.1303839 [DOI] [PubMed] [Google Scholar]
- 119.Zeng Y, Qin Q, Li K, et al. PKR suppress NLRP3-pyroptosis pathway in lipopolysaccharide-induced acute lung injury model of mice. Biochem Biophys Res Commun 2019; 519: 8–14. doi: 10.1016/j.bbrc.2019.08.054 [DOI] [PubMed] [Google Scholar]
- 120.Cabanski M, Steinmüller M, Marsh LM, et al. PKR regulates TLR2/TLR4-dependent signaling in murine alveolar macrophages. Am J Respir Cell Mol Biol 2008; 38: 26–31. doi: 10.1165/rcmb.2007-0010OC [DOI] [PubMed] [Google Scholar]
- 121.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016; 16: 407–420. doi: 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 122.Denk S, Neher MD, Messerer DAC, et al. Complement C5a functions as a master switch for the pH balance in neutrophils exerting fundamental immunometabolic effects. J Immunol 2017; 198: 4846–4854. doi: 10.4049/jimmunol.1700393 [DOI] [PubMed] [Google Scholar]
- 123.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 2012; 488: 670–674. doi: 10.1038/nature11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu S, Wang D, Huang L, et al. The complement receptor C5aR2 promotes protein kinase R expression and contributes to NLRP3 inflammasome activation and HMGB1 release from macrophages. J Biol Chem 2019; 294: 8384–8394. doi: 10.1074/jbc.RA118.006508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li R, Shang Y, Yu Y, et al. High-mobility group box 1 protein participates in acute lung injury by activating protein kinase R and inducing M1 polarization. Life Sci 2020; 246: 117415. doi: 10.1016/j.lfs.2020.117415 [DOI] [PubMed] [Google Scholar]
- 126.Dolinay T, Himes BE, Shumyatcher M, et al. Integrated stress response mediates epithelial injury in mechanical ventilation. Am J Respir Cell Mol Biol 2017; 57: 193–203. doi: 10.1165/rcmb.2016-0404OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dolinay T, Aongbangken C, Zacharias W, et al. Protein kinase R-like endoplasmatic reticulum kinase is a mediator of stretch in ventilator-induced lung injury. Respir Res 2018; 19: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim SR, Kim DI, Kang MR, et al. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor κB activation. J Allergy Clin Immunol 2013; 132: 1397–1408. doi: 10.1016/j.jaci.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 129.Yuan X, Wang J, Li Y, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25-induced endoplasmic reticulum stress in asthma. Sci Rep 2018; 8: 7950. 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ravindran R, Loebbermann J, Nakaya HI, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 2016; 531: 523–527. doi: 10.1038/nature17186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woo CW, Cui D, Arellano J, et al. Adaptive suppression of the ATF4–CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 2009; 11: 1473–1480. doi: 10.1038/ncb1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woo CW, Kutzler L, Kimball SR, et al. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2β. Nat Cell Biol 2012; 14: 192–200. doi: 10.1038/ncb2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hengstermann A, Müller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic Biol Med 2008; 44: 1097–1107. doi: 10.1016/j.freeradbiomed.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 134.Jorgensen E, Stinson A, Shan L, et al. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 2008; 8: 229. doi: 10.1186/1471-2407-8-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tagawa Y, Hiramatsu N, Kasai A, et al. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic Biol Med 2008; 45: 50–59. doi: 10.1016/j.freeradbiomed.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 136.Tagawa Y, Hiramatsu N, Kato H, et al. Induction of CCAAT/enhancer-binding protein–homologous protein by cigarette smoke through the superoxide anion-triggered PERK–eIF2α pathway. Toxicology 2011; 287: 105–112. doi: 10.1016/j.tox.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 137.Zarcone MC, Duistermaat E, van Schadewijk A, et al. Cellular response of mucociliary differentiated primary bronchial epithelial cells to diesel exhaust. Am J Physiol Lung Cell Mol Physiol 2016; 311: L111–L123. doi: 10.1152/ajplung.00064.2016 [DOI] [PubMed] [Google Scholar]
- 138.Zarcone MC, van Schadewijk A, Duistermaat E, et al. Diesel exhaust alters the response of cultured primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD) to non-typeable Haemophilus influenzae. Respir Res 2017; 18: 27. doi: 10.1186/s12931-017-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ni L, Chuang C-C, Zuo L. Fine particulate matter in acute exacerbation of COPD. Front Physiol 2015; 6: 294. doi: 10.3389/fphys.2015.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laing S, Wang G, Briazova T, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 2010; 299: 736–749. doi: 10.1152/ajpcell.00529.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zarcone MC, Duistermaat E, Alblas MJ, et al. Effect of diesel exhaust generated by a city bus engine on stress responses and innate immunity in primary bronchial epithelial cell cultures. Toxicol in Vitro 2018; 48: 221–231. doi: 10.1016/j.tiv.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 142.Min T, Bodas M, Mazur S, et al. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med 2011; 89: 577–593. doi: 10.1007/s00109-011-0732-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gan G, Hu R, Dai A, et al. The role of endoplasmic reticulum stress in emphysema results from cigarette smoke exposure. Cell Physiol Biochem 2011; 28: 725–732. doi: 10.1159/000335766 [DOI] [PubMed] [Google Scholar]
- 144.Yun JH, Morrow J, Owen CA, et al. Transcriptomic analysis of lung tissue from cigarette smoke-induced emphysema murine models and human chronic obstructive pulmonary disease show shared and distinct pathways. Am J Respir Cell Mol Biol 2017; 57: 47–58. doi: 10.1165/rcmb.2016-0328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan SX, Jiang DX, Hu RC, et al. Endoplasmic reticulum stress induces HRD1 to protect alveolar type II epithelial cells from apoptosis induced by cigarette smoke extract. Cell Physiol Biochem 2017; 43: 1337–1345. doi: 10.1159/000481845 [DOI] [PubMed] [Google Scholar]
- 146.Tanel A, Pallepati P, Bettaieb A, et al. Acrolein activates cell survival and apoptotic death responses involving the endoplasmic reticulum in A549 lung cells. Biochim Biophys Acta 2014; 1843: 827–835. doi: 10.1016/j.bbamcr.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 147.Taylor M, Vella L, Carr T. Assessment of an in vitro model of lung epithelial cell stress responses. Free Radic Biol Med 2014; 75: Suppl. 1, S51. doi: 10.1016/j.freeradbiomed.2014.10.821 [DOI] [PubMed] [Google Scholar]
- 148.van Rijt SH, Keller IE, John G, et al. Acute cigarette smoke exposure impairs proteasome function in the lung. Am J Physiol Cell Mol Physiol 2012; 303: L814–L823. doi: 10.1152/ajplung.00128.2012 [DOI] [PubMed] [Google Scholar]
- 149.Kenche H, Ye ZW, Vedagiri K, et al. Adverse outcomes associated with cigarette smoke radicals related to damage to protein-disulfide isomerase. J Biol Chem 2016; 291: 4763–4778. doi: 10.1074/jbc.M115.712331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Z, Wang D, Liu X, et al. Oxidative DNA damage is involved in cigarette smoke-induced lung injury in rats. Environ Health Prev Med 2015; 20: 318–324. doi: 10.1007/s12199-015-0469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kenche H, Baty CJ, Vedagiri K, et al. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. FASEB J 2013; 27: 965–977. doi: 10.1096/fj.12-216234 [DOI] [PubMed] [Google Scholar]
- 152.Sun Y, Ito S, Nishio N, et al. Enhancement of the acrolein-induced production of reactive oxygen species and lung injury by GADD34. Oxid Med Cell Longev 2015; 2015: 170309. doi: 10.1155/2015/170309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yuan T, Luo B, Wei T, et al. Salubrinal protects against cigarette smoke extract-induced HBEpC apoptosis likely via regulating the activity of PERK-eIF2α signaling pathway. Arch Med Res 2012; 43: 522–529. doi: 10.1016/j.arcmed.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 154.Ye P, Mimura J, Okada T, et al. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol 2014; 34: 3421–3434. doi: 10.1128/MCB.00221-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He CH, Gong P, Hu B, et al. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem 2001; 276: 20858–20865. doi: 10.1074/jbc.M101198200 [DOI] [PubMed] [Google Scholar]
- 156.DeNicola GM, Chen PH, Mullarky E, et al. NRF2 regulates serine biosynthesis in non–small cell lung cancer. Nat Genet 2015; 47: 1475–1481. doi: 10.1038/ng.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Afonyushkin T, Oskolkova OV, Philippova M, et al. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 2010; 30: 1007–1013. doi: 10.1161/ATVBAHA.110.204354 [DOI] [PubMed] [Google Scholar]
- 158.Cullinan SB, Zhang D, Hannink M, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 2003; 23: 7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 2004; 279: 20108–20117. doi: 10.1074/jbc.M314219200 [DOI] [PubMed] [Google Scholar]
- 160.Lu PD, Jousse C, Marciniak SJ, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 2004; 23: 169–179. doi: 10.1038/sj.emboj.7600030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steiling K, van den Berge M, Hijazi K, et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med 2013; 187: 933–942. doi: 10.1164/rccm.201208-1449OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018; 360: j5492. doi: 10.1136/bmj.j5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46: 65–69. doi: 10.1038/ng.2844 [DOI] [PubMed] [Google Scholar]
- 164.Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145: 231–236. doi: 10.1378/chest.13-2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abou Hassan OK, Haidar W, Arabi M, et al. Novel EIF2AK4 mutations in histologically proven pulmonary capillary hemangiomatosis and hereditary pulmonary arterial hypertension. BMC Med Genet 2019; 20: 176. doi: 10.1186/s12881-019-0915-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Montani D, Price LC, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2009; 33: 189–200. doi: 10.1183/09031936.00090608 [DOI] [PubMed] [Google Scholar]
- 167.Eichstaedt CA, Song J, Benjamin N, et al. EIF2AK4 mutation as “second hit” in hereditary pulmonary arterial hypertension. Respir Res 2016; 17: 141. doi: 10.1186/s12931-016-0457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gómez J, Reguero JR, Alvarez C, et al. A semiconductor chip-based next generation sequencing procedure for the main pulmonary hypertension genes. Lung 2015; 193: 571–574. doi: 10.1007/s00408-015-9736-4 [DOI] [PubMed] [Google Scholar]
- 169.Tenorio J, Navas P, Barrios E, et al. A founder EIF2AK4 mutation causes an aggressive form of pulmonary arterial hypertension in Iberian Gypsies. Clin Genet 2015; 88: 579–583. doi: 10.1111/cge.12549 [DOI] [PubMed] [Google Scholar]
- 170.Hadinnapola C, Bleda M, Haimel M, et al. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136: 2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Best DH, Sumner KL, Smith BP, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 2017; 151: 821–828. doi: 10.1016/j.chest.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 172.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 173.Manaud G, Nossent EJ, Lambert M, et al. Comparison of human and experimental pulmonary veno-occlusive disease. Am J Respir Cell Mol Biol 2020; 63: 118–131. doi: 10.1165/rcmb.2019-0015OC [DOI] [PubMed] [Google Scholar]
- 174.Nossent EJ, Antigny F, Montani D, et al. Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Heart Lung Transplant 2018; 37: 647–655. doi: 10.1016/j.healun.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 175.Perros F, Günther S, Ranchoux B, et al. Mitomycin-induced pulmonary veno-occlusive disease: evidence from human disease and animal models. Circulation 2015; 132: 834–847. doi: 10.1161/CIRCULATIONAHA.115.014207 [DOI] [PubMed] [Google Scholar]
- 176.Malzer E, Dominicus CS, Chambers JE, et al. The integrated stress response regulates BMP signalling through effects on translation. BMC Biol 2018; 16: 34. doi: 10.1186/s12915-018-0503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001; 15: 427–438. doi: 10.1096/fj.00-0343com [DOI] [PubMed] [Google Scholar]
- 178.Sakao S, Taraseviciene-Stewart L, Lee JD, et al. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 2005; 19: 1178–1180. doi: 10.1096/fj.04-3261fje [DOI] [PubMed] [Google Scholar]
- 179.McMurtry MS, Archer S, Altieri DC, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005; 115: 1479–1491. doi: 10.1172/JCI23203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Long L, Ormiston ML, Yang X, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 2015; 21: 777–785. doi: 10.1038/nm.3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. doi: 10.1161/CIRCULATIONAHA.109.933762 [DOI] [PubMed] [Google Scholar]
- 182.Song Y, Jones JE, Beppu H, et al. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 2005; 112: 553–562. doi: 10.1161/CIRCULATIONAHA.104.492488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sawada H, Saito T, Nickel NP, et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med 2014; 211: 263–280. doi: 10.1084/jem.20111741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Cell Mol Physiol 2008; 294: L152–L160. doi: 10.1152/ajplung.00313.2007 [DOI] [PubMed] [Google Scholar]
- 185.Bueno M, Brands J, Voltz L, et al. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell 2018; 17: e12720. doi: 10.1111/acel.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang HY, Wek SA, McGrath BC, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 2004; 24: 1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burman A, Kropski JA, Calvi CL, et al. Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein. JCI Insight 2018; 3: e99543. doi: 10.1172/jci.insight.99543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang L, Xu D, Li Q, et al. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) attenuates silicotic fibrosis by suppressing apoptosis of alveolar type II epithelial cells via mediation of endoplasmic reticulum stress. Toxicol Appl Pharmacol 2018; 350: 1–10. doi: 10.1016/j.taap.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 189.Xiaojun W, Yan L, Hong X, et al. Acetylated α-tubulin regulated by N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) exerts the anti-fibrotic effect in rat lung fibrosis induced by silica. Sci Rep 2016; 6: 32257. doi: 10.1038/srep32257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 2005; 59: 407–450. doi: 10.1146/annurev.micro.59.031805.133833 [DOI] [PubMed] [Google Scholar]
- 191.Bi M, Naczki C, Koritzinsky M, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 2005; 24: 3470–3481. doi: 10.1038/sj.emboj.7600777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song B, Scheuner D, Ron D, et al. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008; 118: 3378–3389. doi: 10.1172/JCI34587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 2010; 29: 3881–3895. doi: 10.1038/onc.2010.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kutomi G, Tamura Y, Tanaka T, et al. Human endoplasmic reticulum oxidoreductin 1-α is a novel predictor for poor prognosis of breast cancer. Cancer Sci 2013; 104: 1091–1096. doi: 10.1111/cas.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ye J, Kumanova M, Hart LS, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J 2010; 29: 2082–2096. doi: 10.1038/emboj.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Igarashi T, Izumi H, Uchiumi T, et al. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene 2007; 26: 4749–4760. doi: 10.1038/sj.onc.1210289 [DOI] [PubMed] [Google Scholar]
- 197.Tanabe M, Izumi H, Ise T, et al. Activating transcription factor 4 increases the cisplatin resistance of human cancer cell lines. Cancer Res 2003; 63: 8592–8595. [PubMed] [Google Scholar]
- 198.Rouschop KM, Dubois LJ, Keulers TG, et al. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci USA 2013; 110: 4622–4627. doi: 10.1073/pnas.1210633110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rouschop KMA, van den Beucken T, Dubois L, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 2010; 120: 127–141. doi: 10.1172/JCI40027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang H, Liang SQ, Xu D, et al. HSP90/AXL/eIF4E-regulated unfolded protein response as an acquired vulnerability in drug-resistant KRAS-mutant lung cancer. Oncogenesis 2019; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Albert AE, Adua SJ, Cai WL, et al. Adaptive protein translation by the integrated stress response maintains the proliferative and migratory capacity of lung adenocarcinoma cells. Mol Cancer Res 2019; 17: 2343–2355. doi: 10.1158/1541-7786.MCR-19-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kaira K, Oriuchi N, Imai H, et al. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I–III nonsmall cell lung cancer. Br J Cancer 2008; 98: 742–748. doi: 10.1038/sj.bjc.6604235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cormerais Y, Giuliano S, LeFloch R, et al. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res 2016; 76: 4481–4492. doi: 10.1158/0008-5472.CAN-15-3376 [DOI] [PubMed] [Google Scholar]
- 204.Dey S, Sayers CM, Verginadis II, et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest 2015; 125: 2592–2608. doi: 10.1172/JCI78031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16: 4604–4613. doi: 10.1128/MCB.16.9.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Blais JD, Addison CL, Edge R, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol 2006; 26: 9517–9532. doi: 10.1128/MCB.01145-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ghosh R, Lipson KL, Sargent KE, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 2010; 5: e9575. doi: 10.1371/journal.pone.0009575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y, Alam GN, Ning Y, et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res 2012; 72: 5396–5406. doi: 10.1158/0008-5472.CAN-12-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Atkins C, Liu Q, Minthorn E, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 2013; 73: 1993–2002. doi: 10.1158/0008-5472.CAN-12-3109 [DOI] [PubMed] [Google Scholar]
- 210.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013; 73: 2381–2388. doi: 10.1158/0008-5472.CAN-12-3932 [DOI] [PubMed] [Google Scholar]
- 211.Pataer A, Raso MG, Correa AM, et al. Prognostic significance of RNA-dependent protein kinase on non-small cell lung cancer patients. Clin Cancer Res 2010; 16: 5522–5528. doi: 10.1158/1078-0432.CCR-10-0753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pataer A, Vorburger SA, Barber GN, et al. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res 2002; 62: 2239–2243. [PubMed] [Google Scholar]
- 213.Gannon HS, Zou T, Kiessling MK, et al. Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nat Commun 2018; 9: 5450. doi: 10.1038/s41467-018-07824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dalton LE, Clarke HJ, Knight J, et al. The endoplasmic reticulum stress marker CHOP predicts survival in malignant mesothelioma. Br J Cancer 2013; 108: 1340–1347. doi: 10.1038/bjc.2013.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu D, Liang SQ, Yang H, et al. Increased sensitivity to apoptosis upon endoplasmic reticulum stress-induced activation of the unfolded protein response in chemotherapy-resistant malignant pleural mesothelioma. Br J Cancer 2018; 119: 65–75. doi: 10.1038/s41416-018-0145-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013; 15: 481–490. doi: 10.1038/ncb2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lin W, Lin Y, Li J, et al. A deregulated integrated stress response promotes interferon-γ-induced medulloblastoma. J Neurosci Res 2011; 89: 1586–1595. doi: 10.1002/jnr.22693 [DOI] [PubMed] [Google Scholar]
- 218.Chitnis NS, Pytel D, Bobrovnikova-Marjon E, et al. miR-211 is a prosurvival microRNA that regulates CHOP expression in a PERK-dependent manner. Mol Cell 2012; 48: 353–364. doi: 10.1016/j.molcel.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]