Abstract
Background/Aim
The tetrazolium-based MTT cytotoxicity assay is well established for screening putative anti-cancer agents. However, it has limitations including lack of reproducibility with glioma cells treated with polyphenols. The aim of this study was to evaluate whether a flow cytometric assay with the anthraquinone, DRAQ7, was a better alternative than the colorimetric MTT assay for measuring cell viability.
Materials and Methods
Two glioma cell lines (IPSB-18, U373) and 1 pancreatic cancer cell line (AsPC-1) were treated with 4 polyphenols, namely red grape seed extract, red clover extract, anthocyanin-rich extract and curcumin. Cell viability was assessed using MTT assay and DRAQ7 staining.
Results
Limitations of MTT assay included lack of sensitivity and interference with the structure and absorbance spectra of polyphenols. Also, DMSO was toxic to glioma cells. Microscopic observations of cells treated with polyphenols confirmed the range of IC50 values evaluated by DRAQ7, but not by the MTT assay.
Conclusion
DRAQ7 is a better alternative than MTT for measuring viability of glioma cells treated with brightly coloured polyphenols.
Keywords: Glioma, viability, polyphenols, flow cytometry, DRAQ7, MTT
In vitro studies using cell cultures are valuable for screening putative anti-cancer agents, such as polyphenols, for cytotoxicity and cell survival. The most well established and versatile method for quantifying viable cells is the enzyme-based colorimetric MTT assay introduced by Mosmann (1). It determines mitochondrial dehydrogenase activity in living cells, which indirectly reflects viable cell numbers. This assay involves the ability of metabolically active cells to convert a soluble tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), into a formazan precipitate which is insoluble in water. The purple-coloured formazan crystals may be dissolved in a variety of organic solvents such as dimethyl sulfoxide (DMSO). Optical density of the resulting solution is determined spectrophotometrically by measuring changes in absorbance at 570nm using a microplate reader.
However, a number of factors (2–4) and cell types (5–9) that may affect the reliability of quantification of viable cell numbers have been identified. This suggests that the gold standard MTT assay has limitations. Additional issues of which to be cautious include lack of sensitivity of the assay, toxicity of organic solvents like dimethyl sulfoxide (DMSO) (10–13), and optical absorbance spectral properties of brightly coloured polyphenols. It has also been shown that there is chemical interference of polyphenolic compounds with MTT (14, 15).
Over the years, interest has increased in polyphenols which have been identified for having multiple therapeutic targets in cancer, generally (16–19) as well as in brain tumours (20–24). Our research on gliomas (25, 26) includes four such agents that are brightly coloured: red grape seed extract (RGSE) is rust in colour whereas red clover extract (RCE) from Trifolium pratense, anthocyanin-rich extract (ARE) from Aronia melanocarpa (black chokeberry) and curcumin (CUR) are dark green, purple and orange, respectively. When solubilised, these polyphenols display different absorbance spectra. This is an important consideration for determining which cell viability assay is suitable for use. Similar to other researchers, we have also experienced lack of reproducibility of MTT assay results with such polyphenols and possible misinterpretation of the data.
Tumour cell death serves as a useful endpoint in cytotoxicity studies. As an alternative and to minimise the limitation posed by MTT mentioned above, we chose a novel cell viability assay that uses the anthraquinone DRAQ7. This is a marker of apoptosis, necrosis and dead cells as it stains the nuclei in dead and permeabilized cells but not in intact live ones. The fluorescent properties of this novel non-invasive, far-red emitting (Exλmax 599/644, Emλmax 694) fluorescent DNA dye allow efficient differentiation of dead cells from live ones by real-time flow cytometry (27). In addition, it is possible to assess levels of background autofluorescence so that any contributions of the polyphenols can be taken into account.
The aim of this study was to evaluate whether DRAQ7 could be a better viability marker than tetrazolium bromide (MTT) for in vitro cytotoxicity studies. Two glioma cell lines (IPSB-18 and U373) were treated with 4 brightly coloured polyphenols: RGSE, RCE, ARE and CUR. Normal brain cells (MUAB-C) were used as controls and a pancreatic cancer cell (AsPC-1) was also studied for comparison.
Materials and Methods
Polyphenols
RCE, a dark green powder under the market name Red clover extract IFL 40 (UPS), was donated by Linnea SA, Lavertezzo Piano, Switzerland. RGSE, a rust-coloured powder sold under market name MegaNatural™-Gold, was donated by Canandaigua Concentrate and Polyphenolics, Divisions of Constellation Brands, Inc, Madera, California, USA. ARE is a dark purple coloured powder, donated by Artemis, International, Inc (Fort Wayne, Indiana, USA). It was processed with water and ethanol as an extract solvent. CUR is an orange-coloured powder, obtained from turmeric by a solvent extraction method (97% natural); it was supplied by Indus Biotech, Pune, Maharashtra, India.
Prior to use, each polyphenol was initially solubilised in DMSO 100 mg/ml (Sigma-Aldrich, Gillingham, UK) and filtered with a 0.22-μm syringe driven filter (Millipore, Watford, Hertfordshire, UK). This stock solution was then serially diluted to 1 mg/ml, in DMSO. This solution was further diluted in complete medium (CM) to attain a final working concentration of 300 μg/ml, reducing the total percentage of DMSO to less than or equal to 1%. Subsequently, concentrations ranging between 1 ng/ml and 250 μg/ml were prepared by diluting the working solution with clear DMEM, for brain tumour and normal brain cells, or with clear RPMI, for the pancreatic cancer cell line.
Cell culture and maintenance
An established glioma cell line, U373, at passage >100, was donated by Professor Rolf Bjerkvig (Bergen, Norway). IPSB-18 cell line was cultured and established from a glioma biopsy and used at passage >50. Normal human brain short-term cultures (MUAB-C) were derived from a biopsy obtained at temporal lobectomy from an epileptic patient. Both biopsies were obtained at the time of surgery (collected between 1985 and 2001), under local Ethical permission with written informed consent (LREC No 00-173) from the Neurosurgical staff at King’s College Hospital, London. The glioma was diagnosed by a neuropathologist, according to the World Health Organisation criteria (28). The human established pancreatic cancer cell line, AsPC-1, was purchased from the American Type Culture Collection, Manassas, Virginia, USA. All cultured cells used in this study were tested routinely for mycoplasma and confirmed to be of human origin.
Cells were cultured as monolayers in culture flasks, in a standard humidified incubator (37˚C, 5% CO2). Brain cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) whereas the pancreatic cancer cell line was cultured in RPMI-1640 medium (Sigma-Aldrich). Both media were supplemented with 10% v/v foetal calf serum (Sigma-Aldrich) and antibiotics (penicillin and streptomycin at final concentrations of 100 IU/ml and 100 μg/ml, respectively).
Determination of IC50 values using MTT viability assay
The MTT viability assay, optimised for gliomas, was used with some modifications (29). Briefly, cells were plated into 96-well plates (Fisher Scientific, Loughborough, UK), at a seeding concentration of 10,000 cells per well with 200 μl of CM (without phenol red). The cells were incubated for 24 h to allow adherence of cells to the plate. Then the CM was replaced with a wide range of concentrations (1 ng/ml – 250 μg/ml) of each of the four polyphenols (RCE, RGSE, ARE or CUR). Six wells were used for each polyphenol and the experiment was repeated three times. The cells were further incubated for 48 h at 37˚ C. Fresh CM and sterile distilled water were used as controls for normal growth and non-specific colour, respectively.
After 48 h of treatment, the solution from each well was discarded. Cells were washed three times in phosphate buffered saline, PBS (Sigma). Then freshly prepared MTT solution in clear medium (1 mg/ml) was added to each well and incubated at 37˚C for a further 4 h. MTT was removed and the formazan crystals were dissolved in 100 μl DMSO. The plates were then shaken and absorbance (optical density; OD), was read at 570nm, using a Microplate Autoreader (BIO-TEK Instruments, Winooski, VT, USA) as an indicator of cell viability.
In order to determine whether limitations of the MTT viability assay were due to DMSO toxicity, parallel experiments were conducted in which the brain tumour or pancreatic cancer cells were solubilised in clear medium instead of DMSO. In addition, the cells were treated with a wide range of DMSO concentrations instead of polyphenols for 48 h.
Absorption spectra of polyphenols
Since the polyphenols used in this study display dark colours even when solubilized, it is likely that their absorbance spectra overlap with that of the MTT solution. Hence, the optical density of the solutions was recorded using a spectrophotometer (BMG Labtech, Aylesbury, UK). Each polyphenolic compound was dissolved in clear media at concentrations ranging from 0.1 μg/ml to 200 μg/ml, as appropriate. For the base line, either clear (without phenol red indicator) complete DMEM was used for brain tumour cells or clear complete RPMI for pancreatic cancer cells. Even though the wavelength used to measure absorbance in the MTT assay is 570nm, the infrared and ultraviolet-visible spectroscopy range (190-1100nm wavelength) was included to study each sample. The means of duplicate readings were taken.
Determination of IC50 values using DRAQ7 dye and flow cytometry
For the DRAQ7 assay, cells were grown in flasks and not 96-well plates, due to higher number of cells required (100,000 cells). Following 48 h of treatment with the appropriate concentrations of each polyphenol (range from 1 μg/ml to 250 μg/ml), cells were trypsinized and centrifuged at 300 ×g for 3 min. The harvested cells were suspended in 300 nM of the fluorescent dye DRAQ7 (Biostatus Ltd, Shepshed, Loughborough, UK) and incubated in the dark, at room temperature for 10 min. The samples were run on a FACSCalibur (BD Biosciences, San Jose, CA, USA) using 638 nm excitation for DRAQ7 with emitted fluorescence being collected using a 660/16 bandpass filter. DRAQ7 can also be excited using a 488 nm laser with emitted fluorescence being detected above 670 nm. The cell viability of the samples was analysed within 1 h from staining and a minimum of 10,000 events were collected.
Positive control cells (all dead) were treated with sterile distilled water, while negative controls were grown in fresh CM. To establish the position of DRAQ7– and DRAQ7+ gates, one of each negative and positive control was stained with DRAQ7 dye and the other with PBS alone.
Microscopic observations for viable cells
Cells from the established gliomas and pancreatic cancer cell lines were cultured in 6-well plates (Corning™ Costar, Hazlemere, UK) at a density of 100,000 cells per well in 2 ml DMEM. They were left overnight to adhere and then the medium was replaced with fresh containing a range of concentrations for each polyphenolic compound (1 ng/ml – 250 μg/ml). Two wells were used for each concentration and the experiment was repeated twice. The negative and positive controls contained CM and distilled water, respectively. After 48 h of incubation at 37˚C, cells were visualised under a phase contrast microscope (Olympus IX2, Watford, Hertfordshire, UK) at a magnification of 40× for cell viability. These observations were used as a rough guide only.
Statistical analysis
The absorbance data from MTT viability assay was analysed using Microsoft Excel (Office Excel 2016; Microsoft, Redmond, MA, USA) and StatsDirect software (StatsDirect Ltd, Cheshire, UK; http://www.statsdirect.com) to determine the mean and standard deviation values. For each parameter, an average of triplicate readings of 6 wells was taken. Cells treated with medium only were referred to as controls (equivalent to 100% viable cells) and the absorbance for the treated cells was expressed as percentage decreased viability compared to the controls. Graphs for determining IC50 values were produced using Origin 6.0 software (https://www.originlab.com/). Statistical significance of differences between treated cells and controls was determined by Student’s t-test. All p-values <0.001 were considered to be significant.
The DRAQ7 assay data was analysed using Microsoft Excel. An average value of each concentration was taken and compared with the negative control (untreated cell sample) which represents 100% cell viability. The dose response curve obtained gave an estimate of IC50 value for each of the cultured cells studied.
Results
Limitations of MTT viability assay
Over the years, we have routinely screened the in vitro therapeutic potential of various polyphenols for gliomas. All the cell lines in this study including the control (normal brain cell cultures, MUAB-C), glioma cell lines (IPSB-18 and U373) and pancreatic cancer cell line (AsPC 1) were treated with the 4 polyphenols (RGSE, RCE, ARE and CUR). However, only representative results were selected and presented for the purpose of specifically demonstrating limitations of MTT viability assay with brightly coloured polyphenols.
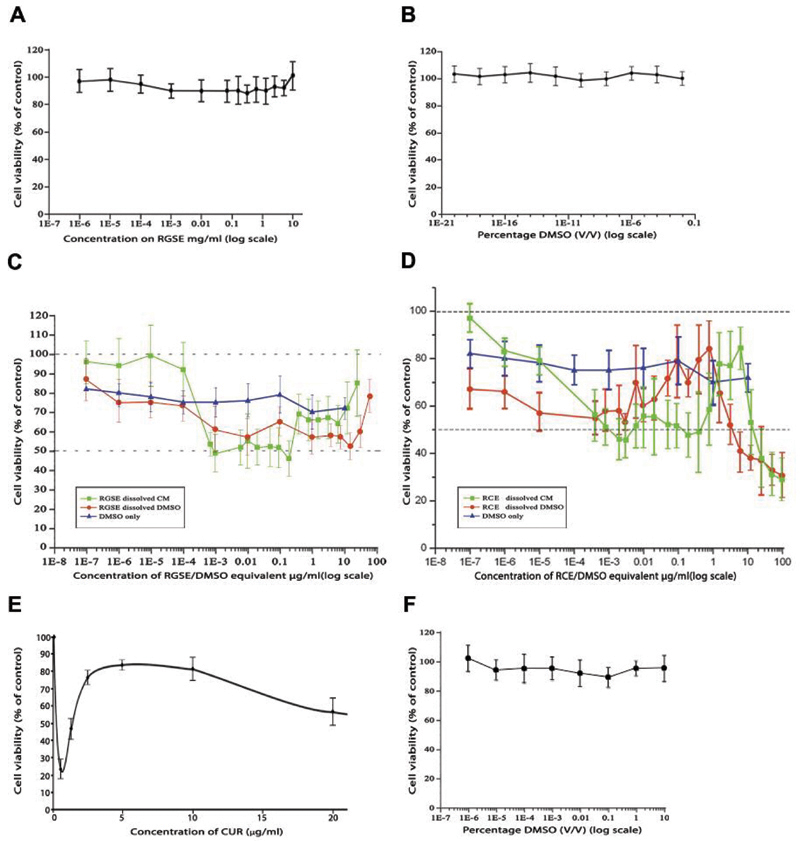
The viability of normal human brain cell cultures (MUAB-C) was unaffected by all 4 polyphenols. Representative data for MUAB-C cells treated with RGSE is demonstrated in Figure 1A as well as with DMSO only (Figure 1B). Generally, MTT assay results showed a biphasic response of glioma cells (IPSB-18) when treated with polyphenols, particularly with RGSE and RCE (Figure 1C and 1D), at low concentrations, whilst a variable and apparent increase in cell viability was observed at high concentrations. Interestingly, the solutions of the tested polyphenols (including RGSE and RCE) in DMSO were toxic, causing a 20% reduction in glioma cell (IPSB-18) viability when used at lower concentrations (up to 1×10–4 and 1×10–3 for RGSE and RCE respectively), compared to polyphenols dissolved in CM (paired t-test, p<0.05). However, this effect at higher concentrations of both RGSE and RCE was not statistically significant due to the cytotoxic effects of the polyphenols themselves. The IC50 value for RGSE and RCE appeared to be 10 μg/ml and 4 μg/ml, respectively. In Figure 1C and 1D, the large error bars also indicate lack of reproducibility of data. Representative data for CUR in the U373 glioma cells indicated three possible IC50 values including 0.5 μg/ml, 1.5 μg/ml, and 20 μg/ml (Figure 1E); the highest of these three values was more accurate. Unlike the cytotoxic effects of DMSO on glioma cells, the pancreatic cell line, AsPC 1, seemed to be unaffected (Figure 1F).
Figure 1.
In vitro cytotoxic effects of polyphenols on different cells lines determined by MTT assay. Cells were treated for 48 h. Normal astrocytic cell cultures (MUAB-C) were treated with concentrations of red grape seed extract (RGSE) (A) and dimethyl sulfoxide (DMSO) (B) on a log scale. (C) and (D) represent data for IPSB-18 cells treated with RGSE and red clover extract (RCE), respectively. Cells were treated with DMSO only (blue line) or with a wide range of concentrations of polyphenols, solubilised in clear DMEM (green line) or dimethyl sulfoxide (DMSO) (red line). A biphasic response was seen but the data lacked reproducibility. (E) A biphasic response was also seen when U373 cells were treated with curcumin (CUR). (F) Non-toxic effect of DMSO seen on AsPC-1 (Pancreatic cancer). Cell viability was calculated as a percentage of the positive control. The error bars represent standard deviation (n=3). Comparisons between cells treated with RGSE or RCE (C and D, respectively) dissolved in complete medium (CM) and RGSE or RCE dissolved in DMSO showed p>0.05 (not statistically significant).
Absorption spectra of polyphenols
Since inconsistency in viability at higher concentrations of all the polyphenols was observed, their absorption spectra were recorded. Absorption maxima for RGSE, RCE and ARE were seen between 200 and 400 nm (Figure 2A, 2B and 2C, respectively). However, absorption at 570 nm, which increased with higher concentrations of each polyphenol, suggested interference with absorption spectrum of MTT. In contrast, the spectrogram for CUR (10 μg/ml) (Figure 2D) showed a lower absorption maxima, below the 570 nm mark for MTT, suggesting negligible interference with the latter.
Figure 2.
Comparative spectrograms of polyphenols. Selective absorption spectra over a wavelength range of 190-1100 nm for (A) red grape seed extract (RGSE) at 1 μg/ml (green), 1 μg/ml (red), 10 μg/ml (blue), (B) red clover extract (RCE) at 1μg/ml (green), 1 μg/ml (red), 10 μg/ml (blue), (C) anthocyanin-rich extract (ARE) at 100 μg/ml (green), 200 μg/ml (red), (D) curcumin (CUR) at 100 μg/ml (blue). Optical density readings were means of duplicates. The absorption spectrum reference for MTT is marked by a line at 570 nm.
Determination of IC50 values using DRAQ7 dye and flow cytometry
Typical scatter plots for the DRAQ7 assay used to estimate IC50 values are shown in Figure 3. When U373 cells were treated with 100 μg/ml RGSE, 30.8% of the cells were dead (Figure 3D). Representative data derived from such scatter plots for the DRAQ7 flow cytometry assay are presented for glioma cells (IPSB-18 and U373) treated with RGSE and RCE in Figure 4A-4D, U373 treated with CUR and the pancreatic cell line (AsPC-1) treated with ARE in Figure 4E and 4F, respectively. In contrast to the MTT assay, the IC50 values for DRAQ7 were higher and showed consistent dose-dependent toxicity in every cell line treated with each polyphenolic compound.
Figure 3.
Representative scatter plots for glioma cells treated with RGSE for DRAQ7 viability assay. U373 cells either untreated (A and B) or treated with 100 μg/ml red grape seed extract (RGSE) for 48 h (C and D). Panels A and C represent scatter plots of harvested cells with whole cells being selected via the region. Plots B and D show cells within each region and dead cells are defined by DRAQ7 positivity.
Figure 4.
In vitro cytotoxic effects of different concentrations of polyphenols on different cell lines determined by DRAQ7 viability assay. The IC50 values for IPSB-18 cells (A) and U373 cells (B) treated with grape seed extract (RGSE) were 38 μg/ml and 130 μg/ml, respectively. Similarly for IPSB-18 cells (C) and U373 cells (D) treated with red clover extract (RCE), the IC50 values were 16 μg/ml and 10.5 μg/ml, respectively. For U373 cells (E) treated with curcumin (CUR) and AsPC-1 cells (F) treated with anthocyanin-rich extract (ARE), the IC50 values were 13 μg/ml and 132 μg/ml, respectively. The fluorescent marker, DRAQ7 was used as a marker of viability. This data gave reproducible IC50 values. The error bars represent standard deviation (n=3).
Microscopic observations of viable cells
Representative micrographs for the glioma cell line IPSB-18, illustrate a direct dose dependent relationship seen between RGSE concentration and cell viability (Figures 5A-D). The microscopic observations indicated that IC25 value for RGSE was between 30 μg/ml and 40 μg/ml, whereas IC75 was between 100 μg/ml and 120 μg/ml. Most importantly, the IC50 value was estimated to be between 40 μg/ml and 50 μg/ml. Complete cell death was observed when the cells were treated with RGSE at concentrations equal to or higher than 150 μg/ml (Figure 5D). Microscopic observations were consistent with the viability results derived from DRAQ7 flow cytometry and not MTT assay, for all cell lines and polyphenols tested.
Figure 5.
Microscopic observations of effect of RGSE on IPSB-18 cell line. Micrographs of monolayers of cultured cells treated with different concentrations of red grape seed (RGSE) for 48 h. Magnification of 40× by phase contrast microscopy. Representative data of concentrations used was a rough guide for viability: (A) 1 ng/ml, (B) 30 μg/ml, (C) 100 μg/ml, (D) 150 μg/ml. Scale bars=250 μm.
Discussion
Considerable evidence has been documented over the years in support of therapeutic potential of polyphenolic compounds in different cancers including our own research on gliomas. Cell-based assays are frequently used for pre-clinical screening of anti-tumour agents in order to determine if they show direct cytotoxic effects that lead to cell death. The MTT enzyme-based, colorimetric assay is a commonly used and well-established method of indirectly determining the number of viable cells in cytotoxicity studies.
Although there have been modifications to this assay, other new assays have been developed, which measure different end points and are considered to be better alternatives (30). In addition, limitations and pitfalls have been reported by other workers (31) and some have suggested that the MTT assay may not be the best assay of choice for certain therapeutic agents (32). The present study demonstrated the limitations of MTT assay with respect to lack of reproducibility, interaction of polyphenolic compounds with MTT, interference of absorbance spectrum of MTT at 570 nm with that of these compounds and toxicity of the solubilisation solution DMSO.
Hormesis is an interesting phenomenon also referred to as biphasic response, characterised by a low dose stimulation and high dose inhibition (33). The hormetic curve can be either U-shaped or inverted U-shaped, depending on the end point. The biphasic dose response seen with the MTT assays confirms the findings of others who have contributed it to various factors, such as the polyphenols epigallocatechin (EGCG also found in red grape seed extract), quercetin (found in ARE) and curcumin (34). These agents have been reported to enhance the proliferation of cancer cells at low concentrations but show toxic effects or inhibitory effects at higher concentrations.
One possible reason why the MTT assay results in this study are unreliable and not reproducible is that the absorbance spectra of RGSE, RCE and ARE overlap the spectrum of the MTT solution at 570 nm. MTT is converted by viable cells with active metabolism to a purple color formazan product with an absorbance maximum at 570 nm. Ultraviolet and visible spectroscopy of various polyphenolic compounds has been documented distinguishing the different subgroups of flavonoid based on their structure (35).
We have shown that the very dark color solutions of RGSE, RCE and ARE (rust, green and purple, respectively) have a more profound effect in interference with formazan’s absorbance spectrum including in the range of expected IC50 values. Indeed, the resulting under-estimation of the IC50 values may reflect upon the colour intensity of the formazan dye not correlating directly with the number of viable cells. The residual colour from the extracts (ARE, RGSE and RCE) was probably not completely removed, despite copious washing. In contrast, as the peak of CUR’s absorbance spectrum is around 400nm, any spectral interference with that of MTT (570nm) is less compared to that seen with ARE, RGSE and RCE. The former is consistent with the findings of Cai et al. (36) who have also recently reported CUR’s peak absorbance (420nm) and spectral interference with another colorimetric viability assay, the cell counting kit-8 (CCK-8) assay.
A variety of chemical compounds have been shown to interfere with the MTT assay. They usually lead to either increased activity of succinate dehydrogenase activity, as reported for epigallocatechin-3-gallate (32), or non-enzymatic reduction of MTT to formazan, as reported for quercetin (37). A small study evaluated the limitations of MTT assay with 15 polyphenols from green tea. These included chlorogenic acid, epicatechin, catechin, quercetin which are all present in our extracts: aronia melanocarpa, red grape seed and red clover (25). They suggested that the hydroxyl groups in the polyphenols were responsible for the reduction of MTT to formazan, thereby giving a false increased MTT reduction (38).
An important factor to consider with the MTT assay is the use of DMSO to solubilize the formazan crystals. Indeed, it is an important and widely used solvent for various compounds which are not water soluble such as the polyphenols of interest to our research. Nevertheless, when possible toxic effects were investigated on the glioma cells lines (U373 and IPSB-18), there was a 20% reduction in viability over a wide range of concentrations, from 1×10–8 μg/ml to 10 μg/ml. Cytotoxicity effects were, however, not seen with either the malignant pancreatic cancer, AsPC-1 or normal human brain cells. Thus, selective toxicity of solvent to brain tumour cells but not pancreatic cancer cells is enigmatic. Furthermore, cytotoxicity of DMSO at a low-dose has been reported in a retinal neuronal cell line unexpectedly (12) but not in colon tumour cell cultures even at higher concentration of 10% (39).
It is worth noting that when comparing the 2 viability assays using MTT tetrazolium compound and the anthracycline derivative, DRAQ7, the latter was preferred as it gave more reliable and reproducible IC50 values for the glioma cell lines (U373 and IPSB-18), and the pancreatic cancer cell line (AsPC-1). We have shown that the IC50 values calculated are generally underestimated with the MTT assay for IPSB-18 and U373, but not necessarily for AsPC-1. This confirms its limitations and pitfalls suggested above and also the finding that DMSO is not toxic to AsPC-1 cell line.
In contrast to MTT, the anthraquinone, DRAQ7, is a novel far-red emitting (Exλmax at 599/644nm) viability dye for in vitro cytotoxicity studies for detection by flow cytometry. The latter assay has no ultra violet excitation and has advantages over the MTT colorimetric assay since it does not cross the membranes of viable (or intact) cells but instead it enters leaky cells and labels the nuclear DNA. This makes it an excellent marker for cell membrane permeabilization and dead cells; apoptotic or necrotic. Another advantage of using the DRAQ7 fluorescent dye is that it does not require any washing steps thereby preventing loss of cells.
It can be concluded that although the detection method for the MTT assay has been well established and popular, it has limitations for use with brightly coloured polyphenolic compounds and glioma cell lines. Hence it is not suitable for our research as a viability assay but instead of this colorimetric assay, the DRAQ7 flow cytometry method is preferred and recommended as an alternative.
Acknowledgements
The Authors are grateful to Mandeep Singh Rooprai for his assistance with formatting the figures and to Noor A. Thani and Satinder Kaur Lall for their technical assistance in the lab.
Funding
The work was supported by the Francis Crick Institute which receives core funding from Cancer Research UK (FC001999), the UK Medical Research Council (FC001999) and the Wellcome Trust (FC001999). The late Dennis Roth (Have a Chance Inc, USA) is also acknowledged for financial support
Footnotes
Conflicts of Interest
None declared.
Author’s Contributions
DD and HKR designed the research, PL, SK and PY performed the research, RWG and RPS provided the surgical material for the study and contributed to review of the manuscript, DD and HKR analysed the data; and HKR wrote the paper.
References
- 1.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 2011;716:157–168. doi: 10.1007/978-1-61779-012-6-9. [DOI] [PubMed] [Google Scholar]
- 3.Mello DF, Trevisan R, Rivera N, Geitner NK, Di Giulio RT, Wiesner MR, Hsu-Kim H, Meyer JN. Caveats to the use of MTT, Neutral Red, Hoescst and Resazurin to measure silver nanoparticle cytotoxicity. Chem Biol Interact. 2020;315 doi: 10.1016/j.cbi.2019.108868. 108868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi S, Abe T, Gotoh J, Fukuuchi Y. Substrate-dependence of reduction of MTT: a tetrazolium dye differs in cultured astroglia and neurons. Neurochem Int. 2002;40(5):441–448. doi: 10.1016/s0197-0186(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 6.Holst CM, Oredsson SM. Comparison of three cytotoxicity tests in the evaluation of the cytotoxicity of a spermine analogue on human breast cancer cell lines. Toxicol In Vitro. 2005;19(3):379–387. doi: 10.1016/j.tiv.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Young FM, Phungtamdet W, Sanderson BJS. Modification of MTT assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, WIL2NS. Toxicol In Vitro. 2005;19(8):1051–1059. doi: 10.1016/j.tiv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Bigl K, Schmitt A, Meiners I, Münch G, Arendt T. Comparison of results of the CellTiter Blue, the tetrazolium (3-[4,5-dimethylthioazol-2-yl]-2,5-diphenyl tetrazolium bromide), and the lactate dehydrogenase assay applied in brain cells after exposure to advanced glycation end products. Toxicol In Vitro. 2007;21(5):962–971. doi: 10.1016/j.tiv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Schröterová L, Králová V, Vorácová A, Hasková P, Rudolf E, Cervinka M. Antiproliferative effects of selenium compounds in colon cancer cells: comparison of different cytotoxicity assays. Toxicol In Vitro. 2009;23(7):1406–1411. doi: 10.1016/j.tiv.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi AB, Kitabatake N, Doi E. Toxicity of dimethyl sulfoxide as a solvent in bioassay system with HeLa cells evaluated colorimetrically with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide. Agric Biol Chem. 1990;54(11):2961–2966. [PubMed] [Google Scholar]
- 11.Öz ES, Aydemir E, Fişkin K. DMSO exhibits similar cytotoxicity effects to thalidomide in mouse breast cancer cells. Oncol Lett. 2012;3(4):927–929. doi: 10.3892/ol.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014;28(3):1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 13.Joshi DR, Adhikari N. An overview on common organic solvents and their toxicity. J Pharm Res Int. 2019;28(3):1–18. doi: 10.9734/jpri/2019/v28i330203. [DOI] [Google Scholar]
- 14.Talorete TPN, Bouaziz M, Sayadi S, Isoda H. Influence of medium type and serum on MTT reduction by flavonoids in the absence of cells. Cytotechnology. 2006;52(3):189–198. doi: 10.1007/s10616-007-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maioli E, Torricelli C, Fortino V, Carlucci F, Tommassini V, Pacin A. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol Proced Online. 2009;11:227–240. doi: 10.1007/s12575-009-9020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carocho M, Ferreira ICFR. The role of phenolic compounds in the fight against cancer–A review. Anticancer Agents Med Chem. 2013;13(8):1236–1258. doi: 10.2174/18715206113139990301. [DOI] [PubMed] [Google Scholar]
- 17.Asensi M, Ortega A, Mena S, Feddi F, Estrela JM. Natural polyphenols in cancer therapy. Crit Rev Clin Lab Sci. 2011;48(5–6):197–216. doi: 10.3109/10408363.2011.631268. [DOI] [PubMed] [Google Scholar]
- 18.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel) 2019;11(1):28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, Novellino E, Santini A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, Rodriguez AI, Koubi D, Hunter TJ, Corcoran GB, et al. Resveratrol-induced apoptosis death in human U251 glioma cells. Mol Cancer Ther. 2005;4(4):554–561. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, Fried A, Hussaini R, White R, Baidoo J, Yalamanchi S, Banerjee P. Phytosomal curcumin causes natural killer cell-dependent repolarization of glioblastoma (GBM) tumor-associated microglia/macrophages and elimination. J Exp Clin Cancer Res. 2018;37(1):168–185. doi: 10.1186/s13046-018-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A. Quercetin promotes degradation of surviving and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. 2009;11(2):122–131. doi: 10.1215/15228517-2008-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98g and U87MG cells but not in human normal astrocytes. Cancer. 2010;116(1):164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng S, Zhao X, Xu LS, Yang D, Chen L, Xu MH. Apoptosis induction effect of Apocynum venetum polyphenol on human U87 glioma cells via NF-κB pathway. Future Oncol. 2019;15(32):3723–3738. doi: 10.2217/fon-2019-0381. [DOI] [PubMed] [Google Scholar]
- 25.Rooprai HK, Christidou M, Pilkington GJ. The potential for strategies using micronutrients and heterocyclic drugs to treat invasive gliomas. Acta Neurochir (Wien) 2003;145(8):683–690. doi: 10.1007/s00701-003-0073-7. [DOI] [PubMed] [Google Scholar]
- 26.Abdullah Thani NA, Sallis B, Nuttall R, Schubert FR, Ahsan M, Davies D, Purewal S, Cooper A, Rooprai HK. Induction of apoptosis and reduction of MMP gene expression in the U373 cell line by polyphenolics in Aronia melanocarpa and by curcumin. Oncol Rep. 2012;28(4):1435–1442. doi: 10.3892/or.2012.1941. [DOI] [PubMed] [Google Scholar]
- 27.Akagi J, Kordon M, Zhao H, Matuszek A, Dobrucki J, Errington R, Smith PJ, Takeda K, Darzynkiewicz Z, Wlodkowic D. Realtime cell viability assays using a new anthracycline derivative DRAQ7® . Cytometry A. 2013;83(2):227–234. doi: 10.1002/cyto.a.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 29.Nikkhah G, Tonn JC, Hoffmann O, Kraemer HP, Darling JL, Schachenmayr W, Schönmayr R. The MTT assay for chemosensitivity testing of human tumors of the central nervous system. Part II: Evaluation of patient- and drug-specific variables. J Neurooncol. 1992;13(1):13–24. doi: 10.1007/bf00172942. [DOI] [PubMed] [Google Scholar]
- 30.Derenne A, Van Hemelryck V, Lamoral-Theys D, Kiss R, Goormaghtigh E. FTIR spectroscopy: a new valuable tool to classify the effects of polyphenolic compounds on cancer cells. Biochim Biophys Acta. 2013;1832(1):46–56. doi: 10.1016/j.bbadis.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Stepanenko AA, Dmitrenko VV. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene. 2015;574(2):193–203. doi: 10.1016/j.gene.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One. 2010;5(4):e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabrese EJ. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35(6):463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- 34.Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010;68(7):418–428. doi: 10.1111/j.1753-4887.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 35.Harnly JM, Bhagwat S, Lin L-Z. Profiling methods for the determination of phenolic compounds in foods and dietary supplements. Anal Bioanal Chem. 2007;389(1):47–61. doi: 10.1007/s00216-007-1424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai L, Qin X, Xu Z, Song Y, Jiang H, Wu Y, Ruan H, Chen J. Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega. 2019;4(7):12036–12042. doi: 10.1021/acsomega.9b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng L, Wang B, Ren P. Reduction of MTT by flavonoids in the absence of cells. Colloids Surf B Biointerfaces. 2005;45(2):108–111. doi: 10.1016/j.colsurfb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Han M, Li J-F, Tan Q, Sun Y-Y, Wang Y-Y. Limitations of the use of MTT assay for screening in drug discovery. J Chinese Pharmaceutical Sci. 2010;19:195–200. doi: 10.5246/jcps.2010.03.027. [DOI] [Google Scholar]
- 39.Da Violante G, Zerrouk N, Richard I, Provot G, Chaumeil JC, Arnaud P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol Pharm Bull. 2002;25(12):1600–1603. doi: 10.1248/bpb.25.1600. [DOI] [PubMed] [Google Scholar]