Summary
Mating types are self-incompatibility systems that promote outcrossing in plants, fungi, and oomycetes. Mating-type genes have been widely studied in plants and fungi but have yet to be identified in oomycetes, eu-karyotic organisms closely related to brown algae that cause many destructive animal and plant diseases. We identified the mating-type locus of Plasmopara viticola, the oomycete responsible for grapevine downy mildew, one of the most damaging grapevine diseases worldwide. Using a genome-wide association approach, we identified a 570-kb repeat-rich non-recombining region controlling mating types, with two highly divergent alleles. We showed that one mating type was homozygous, whereas the other was heterozygous at this locus. The mating-type locus encompassed 40 genes, including one encoding a putative hormone receptor. Functional studies will, however, be required to validate the function of these genes and find the actual determinants of mating type. Our findings have fundamental implications for our understanding of the evolution of mating types, as they reveal a unique determinism involving an asymmetry of heterozygosity, as in sex chromosomes and unlike other mating-type systems. This identification of the mating-type locus in such an economically important crop pathogen also has applied implications, as outcrossing facilitates rapid evolution and resistance to harsh environmental conditions.
Introduction
Mating systems control the degree of outcrossing in natural populations, thus affecting adaptability [1–5]. Indeed, outcrossing promotes gene flow and, therefore, the rapid spread of beneficial alleles, the generation of new allelic combinations, and the purging of deleterious alleles. Various genetic systems have evolved across the tree of life, which enforce outcrossing, such as separate sexes or mating types [6]. The sex of many plants and animals is determined by sex chromosomes [6]. Mating types, preventing mating between individuals carrying the same alleles, have evolved independently in many lineages of the tree of life, including fungi, ciliates, green algae, and oomycetes [7–9]. Plants possess a molecular system called self-incompatibility, which enforces outcrossing and is analogous to mating type [10]. Given the fundamental importance of mating types in life cycles and evolution, their molecular determinism has been extensively studied, particularly in plants, fungi, and ciliates. Mating types are controlled by a number of different mechanisms, even within these groups [7, 8, 10].
Mating type systems enforcing outcrossing at the diploid stage, involving only two mating types, have been described in oomycetes on the basis of cross incompatibilities [11]. However, no genomic sequence for a mating-type locus has yet been identified in this lineage. Oomycetes are diploid eukaryotic organisms closely related to diatoms and brown algae [12]. This group includes a number of animal and plant pathogens causing significant environmental and economic damage. Oomycetes are responsible for damaging diseases, such as saprolegniosis, a lethal disease affecting wild and farmed fish [13]; sudden oak death; downy mildew in several crops (e.g., Bremia lactucae, responsible for lettuce downy mildew, and Plasmopara halstedii, responsible for sunflower downy mildew), and the infamous potato blight caused by Phytophthora infestans, responsible for the Irish potato famine in the 1840s.
Mating types are of the utmost importance in these organisms, as they control gamete compatibility, and sexual reproduction generates thick-walled spores called oospores that can resist harsh conditions and survive for several years. Outcrossing also produces recombinant genotypes, which can mediate faster adaptation to control measures. For example, invasive populations of Ph. infestans were not able to reproduce sexually for a long period, due to the lack of one mating type, but the recent introduction of the missing mating type has resulted in higher frequencies of recombinant genotypes in some areas [14] and the emergence of an aggressive lineage [15]. In oomycetes, the mating-type locus has been located on genetic linkage maps in Phytophthora species [16–19] and Bremia lactucae [20]. A recent study [21] identified several contigs associated with mating types on the Ph. capsici reference genome but could not precisely locate the locus controlling the phenotype. The only known mating-type factors are purified hormones in Phytophthora spp [22–24]. However, no genes determining mating type have yet been identified in oomycetes.
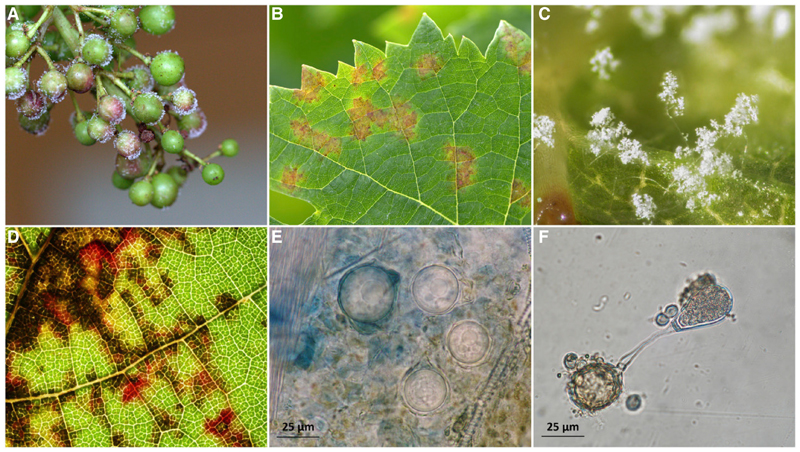
Plasmopara viticola, a pathogen causing grapevine downy mildew (Figure 1), one of the most devastating grapevine diseases worldwide, is an oomycete. Plasmopara viticola was introduced into European vineyards from North America in the 1870s [25] and rapidly spread, invading vineyards all over the world. The life cycle of Pl. viticola includes an asexual multiplication phase during the spring and summer (Figures 1A-1C) and a sexual reproduction event in the fall (Figure 1D), generating the thick-walled sexual spores (oospores; Figures 1E and 1F) required for overwintering [26]. In Pl. viticola, only two mating types have been observed, and mating can occur only between diploid individuals of different mating types [27]. This results in high rates of outcrossing, a key element explaining rapid adaptation to fungicides [28, 29] and to resistant cultivars [30, 31] in this species. We identified the first oomycete mating-type locus sequence, with an approach combining phenotypic analysis (mating types determined by crosses) with genome-wide single-nucleotide polymorphisms (SNPs) in Pl. viticola.
Figure 1. Symptoms and Asexual and Sexual Structures of the Grapevine Downy Mildew Pathogen, Plasmopara viticola .
(A) Diseased young grape berries (asexual reproduction).
(B) Typical pale yellow lesions (oil spots) on a diseased grape leaf at spring (asexual reproduction).
(C) Sporangiophores and sporangia (asexual structures) emerging through the stomata of diseased grape organs.
(D) Typical patchwork of angular yellow to brown red spots on the upper surface of a diseased leaf at fall, when sexual reproduction occurs.
(E) Oospores formed on leaf disks after sexual reproduction between two individuals of opposite mating types.
(F) Oospore (left) germinating to produce a macrosporangium (right) containing 4–6 asexual zoospores.
Results
We determined the mating type of 54 diploid individuals of Pl. viticola collected across Europe (Table S1), in experimental crosses against six testers, three for each of the two mating types, arbitrarily called P1 and P2. The P1 mating type was inferred for an individual if it produced oospores when inoculated on grapevine leaves with P2 testers, but not with P1 testers, and conversely for P2. We identified 26 individuals of the P1 mating type and 28 of the P2 mating type (Table S2). We sequenced the genomes of these 54 diploid individuals with short-read technology and mapped the reads onto a recent high-quality reference sequence obtained by long-read sequencing with high coverage of the INRA-PV221 individual [32]. Our mating-type phenotyping revealed that this reference INRA-PV221 individual had the P2 mating type. The reference genome covers around 81% of the estimated 115-Mb genome size of Pl. viticola and exhibits a high genome-wide heterozygosity of 0.8%. We retained 2.011 million SNPs after filtering. No genetic subdivision associated with mating types was detected in population structure analyses based on principal-component analysis or clustering analysis applied to a dataset filtered for SNPs in close linkage disequilibrium (LD) (Figure S1). Conditions were therefore favorable for genetic-phenotype association studies.
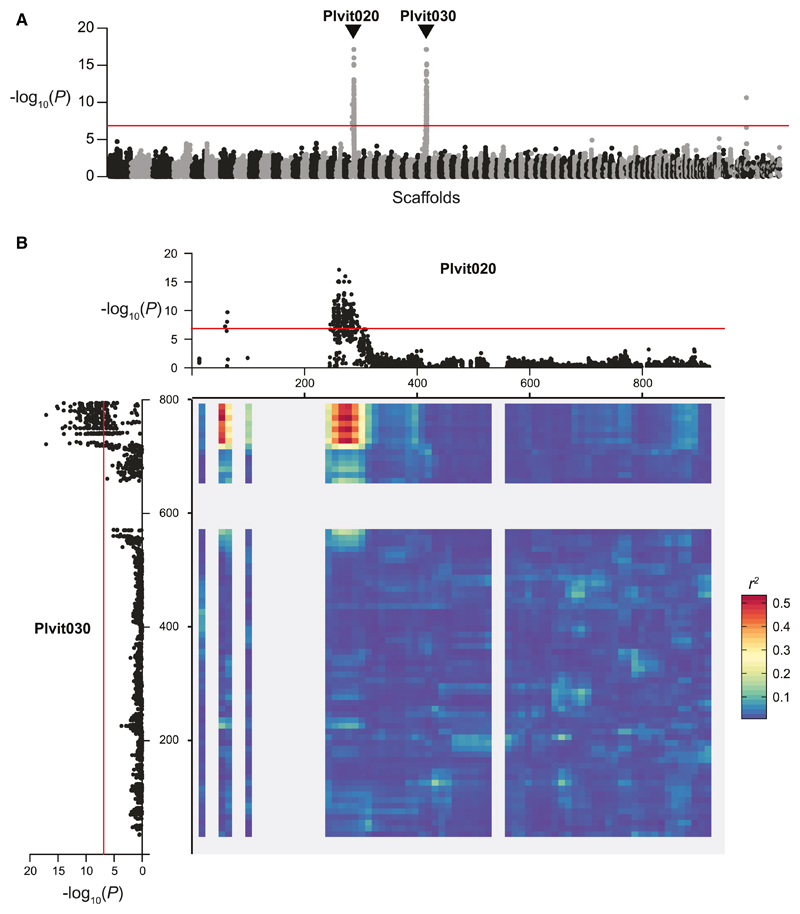
Using a genome-wide association approach, we identified two genomic regions with significant signals of association with the mating-type phenotype, located at the edges of the scaffolds Plvit020 and Plvit030 (Figure 2). SNPs at these two scaffold extremities were in very strong LD (Figure 2B), indicating that they were located in the same genomic region and that this region probably lacked recombination. The rate of LD decay was much lower in this region than in the rest of the genome (Figure S2), providing further support for the hypothesis of a lack of recombination. The incomplete assembly of this locus in the reference genome was probably due to its high repeat content. Indeed, we observed two large regions composed exclusively of tandem repeat arrays covering 327 kb (Figure S3).
Figure 2. Genome-wide Association Analysis for Identifying Mating-type Regions in Plasmopara viticola .
(A) Manhattan plot of the negative log10-transformed association p values between mating type and SNPs along the Pl. viticola genome. Alternating black and gray blocks of dots mark the limits between scaffolds. The two scaffolds with a significant association signal (Plvit020 and Plvit030) are indicated with arrows. Only 5% of the SNPs with a p > 0.1 are represented, to keep the number of plotted points manageable.
(B) Manhattan plots of the negative log10-transformed association p values between mating type and SNPs for Plvit020 and Plvit030. The significance threshold for the association analysis, computed with 10,000 permutations, is represented as a red line in both panels. Linkage disequilibrium between the two scaffolds is represented as a heatmap (80 bins), with redder colors representing higher r2 values (higher linkage disequilibrium) and therefore lower recombination rates. See also Figures S1–S4.
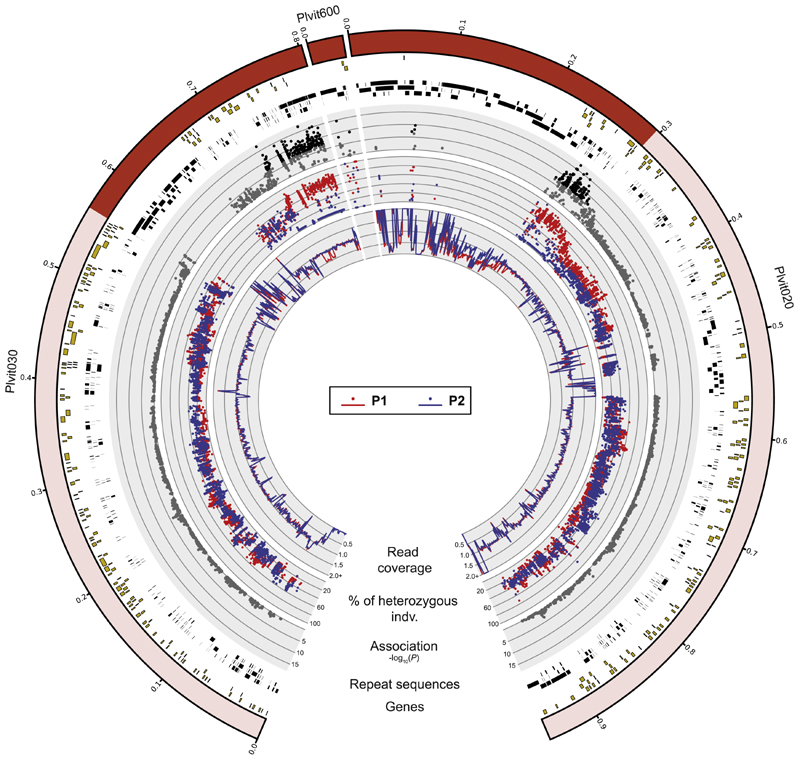
To identify potential missing sequences between the two scaffolds, we used a reference-free SNP calling method based on the analysis of the de Bruijn graph built from raw sequencing reads [33]. We detected short genomic sequences carrying SNPs with significant signals of association with the mating-type phenotype that were missing from the reference assembly (STAR Methods). We reassembled the PacBio reads corresponding to these short sequences and obtained a new contig, Plvit600 (32,220 bp), with SNPs significantly associated with the mating-type phenotype in strong linkage disequilibrium with SNPs located at the edges of Plvit020 and Plvit030 and therefore probably located between these two scaffolds (Figure S4). Plvit600 also included a large region entirely composed of tandem repeat arrays (Figure S3).We therefore concluded that the mating-type locus was at least 570 kb long (Figure 3). The mating-type locus might actually be larger given the difficulty of assembling and mapping reads in such a repeat-rich genomic region, but our use of a long-read reference assembly and the addition of a reference-free SNP calling method probably mitigated this issue.
Figure 3. Genomic Regions Associated with Mating Type in Plasmopara viticola .
The regions significantly associated with the mating-type phenotype are shown in dark red within the scaffold ideograms (outer track). Annotated genes are represented as yellow rectangles and repeated sequences as black rectangles (see Figure S3 for details about the repeat contentin the mating-typelocus region). Significant negative log10-transformed p values from the association analysis are represented by black dots, and other values are represented by gray dots. The percentage of heterozygous individuals for each SNP is shown for individuals of mating types P1 by red dots and P2 by blue dots. The mean normalized read coverage (for adjacent 1-kb windows) is shown by red lines for P1 individuals and blue lines for P2 individuals. The data were obtained with the reference-based SNP calling approach for the scaffolds Plvit020 and Plvit030 and the reference-free SNP calling approach for Plvit600 (STAR Methods). See also Figure S2 and Table S3.
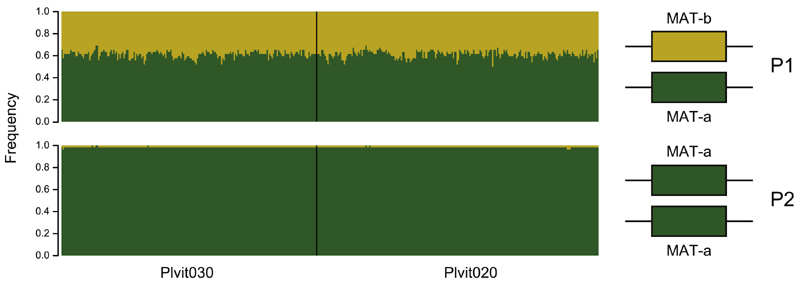
P2 individuals were homozygous for the reference allele (designated MAT-a) at the mating-type locus, whereas P1 individuals were heterozygous, carrying the MAT-a reference allele and a second allele, called MAT-b (Figure 4). We found no homozygous individual for the alternative MAT-b allele. This suggested that the MAT-b allele is dominant. The two alleles were highly differentiated along the 570 kb, as shown by the large difference of heterozygosity levels (Figure 3), again consistent with a lack of recombination in this region. Unfortunately, with only short-read data and given the richness in repeats in the mating-type locus region, we could not assess the degree of rearrangements between the MAT-a and MAT-b alleles. It is, however, likely that structural variation exists in such a non-recombining and repeat-rich region.
Figure 4. Mating Type Is Determined by a Two-Allele System in Plasmopara viticola .
Left panel: allele frequencies at SNPs associated with the mating-type phenotypein P1 individuals (top) and P2 individuals (bottom). Only results for the reference-based SNP calling approach are shown. Right panel: proposed model for mating-type determination is shown, with homozygosity in P2 individuals and het-erozygosity in P1 individuals. For both panels, the reference allele (i.e., the allele found in the reference genome) is shown in green and the alternative allele is shown in yellow.
The mating-type region included a total of 40 predicted coding sequences (Table S3), 26 of which had predicted functions and did not correspond to TEs (Table 1). Based on the predicted functions of these genes, the most promising candidate for involvement in mating-type determination was a gene encoding a transmembrane protein (PVIT_0010929.T1) with a sterol-sensing domain and lipid transport activity. This protein might act as a hormone receptor, and hormones have been identified as mating-type factors during initiation of sexual reproduction in Phytophthora spp [22–24]. Another two genes (PVIT_0010925.T1 and PVIT_0010927.T1) encoded proteins with lipid-binding domains that could potentially interact with mating hormones. We also found a putative mitogen-activated protein (MAP) kinase gene (PVIT_0027771.T1) in the identified mating-type region. MAP kinases play an important role in the mating pheromone response pathway in fungi [34], and genes encoding kinases are present at the mating-type loci of some fungi [35]. We also found genes encoding a protein with an HRCD (helicase and RNaseD C-terminal) (PVIT_0010933.T1) domain and a RecQ-mediated genome instability protein 2 (PVIT_0008617.T1). Proteins of this type can interact with DNA and are often associated with RecQ helicases and, thus, DNA repair. These proteins may also be involved in recombination suppression or gene regulation. This is potentially also the case for a Cdc48-like protein (PVIT_0027772.T1) encoded by a gene present at the mating-type locus. Cdc48 can interact with protein complexes on chromatin and is involved in gene regulation and cell cycle progression. Finally, we also found four genes (PVIT_0008616.T1, PVIT_0010916.T1, PVIT_0010917.T1, and PVIT_0010924.T1) encoding proteins with ubiquitin-like or ubiq-uitin-interacting domains, which could potentially be involved in addressing proteins to the proteasome and, thus, in protein degradation.
Table 1. Putative Function of Genes in the Mating-type Locus of Plasmopara viticola .
| Protein ID | Scaffold | Length | Putative Function | Non-synonymous Mutationsa |
|---|---|---|---|---|
| PVIT_0008600.T1 | Plvit020 | 795 | transferase activity, transferring acyl groups | |
| PVIT_0008605.T1 | Plvit020 | 752 | transferase activity, transferring acyl groups | |
| PVIT_0008608.T1 | Plvit020 | 713 | transferase activity, transferring acyl groups | I234T, Y239C |
| PVIT_0008616.T1 | Plvit020 | 1,375 | similar to peroxin-1, involved in protein targeting to peroxisome; contains a SEP and an UBX domains | P179S, D392G, E540D, R763K |
| PVIT_0008617.T1 | Plvit020 | 160 | similar to RecQ-mediated genome instability protein 2 | |
| PVIT_0008618.T1 | Plvit020 | 363 | disulfide isomerase, involved in cell redox homeostasis and protein folding in the endoplasmic reticulum | |
| PVIT_0008619.T1 | Plvit020 | 83 | molybdopterin synthase sulfur carrier subunit | |
| PVIT 0008620.T1 | Plvit020 | 303 | similar to peroxin-13 | V79G, L233S, E237D |
| PVIT 0010914.T1 | Plvit030 | 419 | glycosyltransferase GlcNAc | |
| PVIT_0010915.T1 | Plvit030 | 270 | protein N-terminal amidase, similar to NTA1 | |
| PVIT_0010916.T1 | Plvit030 | 218 | similar to sentrine-/SUMO-specific protease 8 (SENP8); contains an Ulp1 catalytic domain | |
| PVIT_0010917.T1 | Plvit030 | 449 | similar to 26S proteasome regulatory subunit N13; contains a ubiquitin interacting motif | |
| PVIT_0010918.T1 | Plvit030 | 765 | cation efflux protein/zinc transporter | |
| PVIT_0010919.T1 | Plvit030 | 137 | dynein light-chain roadblock type | |
| PVIT_0010922.T1 | Plvit030 | 203 | similar to protein N-lysine methyltransferase METTL21A | |
| PVIT_0010924.T1 | Plvit030 | 388 | thiol-dependent, ubiquitin-specific protease activity | |
| PVIT_0010925.T1 | Plvit030 | 1,071 | FYVE-finger-containing Rab5 effector protein Rabenosyn-5-related; contains a START-like domain | |
| PVIT 0010926.T1 | Plvit030 | 993 | calcium binding protein | |
| PVIT_0010927.T1 | Plvit030 | 1,230 | bactericidal permeability-increasing protein; lipid and protein binding | |
| PVIT_0010928.T1 | Plvit030 | 633 | similar to DNA polymerase eta; contains a DNA polymerase, Y-family, little finger domain; DNA repair | H163Y |
| PVIT_0010929.T1 | Plvit030 | 1,479 | transmembrane protein with sterol-sensing domain; involved in lipid transport, translational initiation, cell division | I297M, L402M, E1211D |
| PVIT_0010931T1 | Plvit030 | 417 | similar to serine-/arginine-rich splicing factor | D229G, L284R, S294G, S303G, T306S, G310S, G325V |
| PVIT_0010932.T1 | Plvit030 | 390 | ADP-ribosylation-factor-like-2-binding protein domain | G3D, L5F, S52T, H159Q, K219E, premature start |
| PVIT_0010933.T1 | Plvit030 | 479 | contains an HRDC domain | D130N, K176R, L165I, V249I, T250S |
| PVIT_0027771.T1 | Plvit600 | 444 | mitogen-activated protein kinase | |
| PVIT_0027772.T1 | Plvit600 | 842 | similar to transitional endoplasmic reticulum ATPase CDC48 |
See Table S3 for the detailed functional annotation for all genes in the region.
Non-synonymous mutations caused by alternate alleles (i.e., present in the MAT-b allele) for SNPs associated with the mating-type phenotype
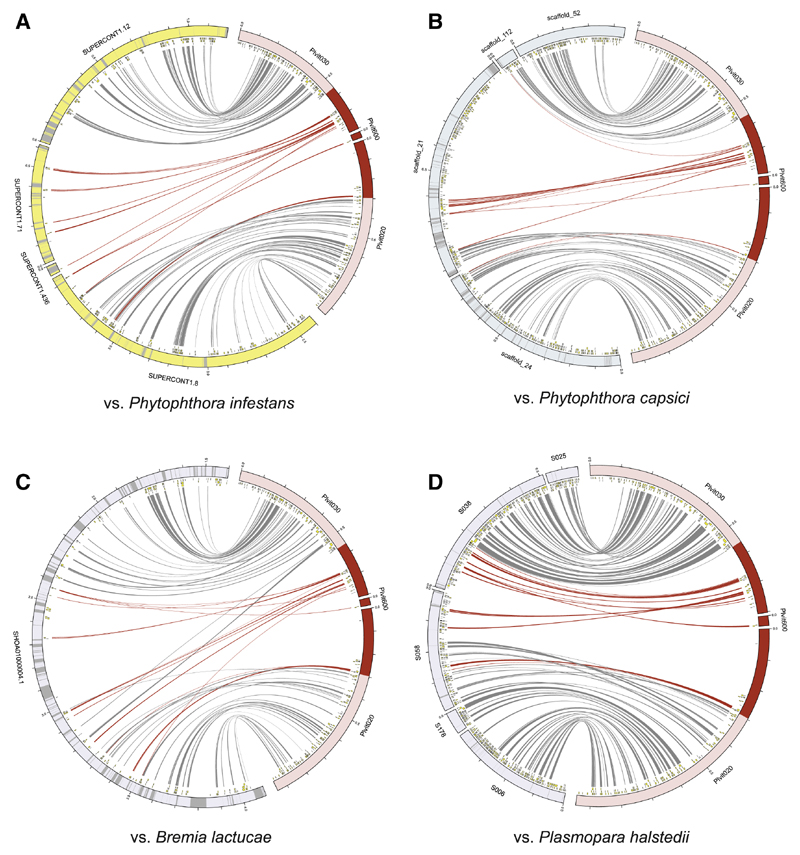
Using a reciprocal best-hit approach with whole proteomes, we located the regions orthologous to the Pl. viticola mating-type region in the available genomes of other oomycetes. The genes orthologous to putative Pl. viticola mating-type genes were found on three scaffolds for Ph. infestans and Ph. capsici: one for Br. lactucae and two for Pl. halstedii (Figure 5; Table S4). Orthologs of the genes from the Plvit600 contig were found in these four species close to the other candidate gene orthologs, confirming that this contig most probably belonged to the mating-type locus in Pl. viticola. A previous study in Ph. capsici based on genotyping by sequencing (GBS) (sequencing only a fraction of the genomes) identified SNPs associated with mating types in five different scaffolds [21]. We found that none of these scaffolds corresponded to the regions orthologous to the Pl. viticola mating-type locus. Association or functional studies are required to check whether the regions orthologous to the Pl. viticola mating-type locus also control mating type in other oomycete species.
Figure 5. Orthologs of the Plasmopara viticola Mating-type Locus Genes in the Genomes of Phytophthora infestans, Phytophthora capsici, Bremia lactucae, and Plasmopara halstedii .
Orthology relationships among protein-coding sequences between Pl. viticola and (A) Ph. in-festans, (B) Ph. capsici, (C) Br. lactucae, and (D) Pl. halstedii were determined with reciprocal best hits and are represented by links between ideograms. The regions significantly associated with the mating-type phenotype in Pl. viticola and the links corresponding to genes in these regions are shown in dark red. Predicted genes are represented by yellow boxes, and the grayed-out regions in ideograms represent gaps in the assemblies. See Table S4 for the corresponding genomic coordinates in the mating-type region.
Discussion
We report here the first identification of an oomycete mating-type locus sequence, in the genome of Pl. viticola. The strong signal of association with mating type constitutes compelling evidence that the identified region is involved in controlling mating types. Functional studies deleting this region would, however, be required to provide definitive evidence for its role in mating-type determinism and identify the precise mating-type genes, but such functional studies are currently not possible in Pl. viticola. The Pl. viticola mating-type locus was found in a 570-kb non-re-combining, repeat-rich region of the genome encompassing 40 genes. The region was heterozygous (MAT-a/MAT-b) in the P1 mating type and homozygous (MAT-a/MAT-a) in the P2 mating type, indicating dominance of the MAT-b allele. These findings are consistent with previous findings for marker segregation analyses in Phytophthora species and Br. lactucae [16, 19–21, 36]. This genetic determinism of mating type is unique in the tree of life and resembles determinism based on sex chromosomes in plants and animals [6], with one sex typically being homozygous and the other heterozygous. By contrast, fungal mating types are determined at the haploid stage, with mating occurring between gametes carrying different mating-type al-leles and many species harboring multiple alleles. Diploid or di-karyotic fungal individuals are necessarily heterozygous at the mating-type locus in heterothallic species and can thus undergo selfing [37]. Mating types in fungi therefore do not prevent diploid selfing; they only prevent same-clone mating [7], although mating types in oomycetes and plants do prevent diploid selfing.
As expected for non-recombining regions under long-term balancing selection, we found the two Pl. viticola mating-type al-leles to be highly divergent. The mating-type locus in Ph. infestans is also heteromorphic, with highly differentiated alleles, hemizygous fragments, and genomic translocations, and it is associated with deleterious recessive alleles [18, 36, 38]. The high degree of divergence between alleles, the number of rearrangements, the high repeat content, and the sheltering of deleterious alleles by permanent heterozygosity are typical of sex and mating-type chromosomes in plants, animals, and fungi [39–42].
The identification of regions orthologous to the Pl. viticola mating-type locus in other oomycete species paves the way for comparative genomic studies. Further association studies are, however, required to test whether these regions also control mating-type determination in other oomycetes. Only in Ph. capsici were enough genomic data available for association studies with mating type: a previous study identified scaffolds with different allelic frequencies between mating types [21], but these scaffolds did not include any of the genes orthologous to the putative Pl. viticola mating-type genes. Mating-type genes may have moved into different genomic backgrounds during the Ph. capsici and Pl. viticola divergence, as is often observed for animal sex-determining genes, with a high rate of sex chromosome turnover [43]. Alternatively, the mating-type determinism itself may have changed, as it occurred in fungi: mating type is determined by pheromone and pheromone receptor genes in basidiomycetes, although it is determined by transcription factors controlling the expression of these genes in ascomycetes [44]. Ph. capsici and Pl. viticola are very distantly related [45], leaving time for genetic changes in mating-type locus or mating-type determinism. Additional association studies in other oomycetes species and functional validation are consequently required for drawing inferences on mating-type gene evolution in oomycetes.
In conclusion, our study identifies the localization of the mating-type genomic sequence in the oomycete pathogen Pl. viti-cola responsible for the devastating downy mildew grapevine disease and elucidates the determinism of mating type (homozygous versus heterozygous). We identified possible mating-type-determining genes, including one encoding a putative hormone receptor. This may guide the development of innovative control methods based on disruption of the sexual cycle of the pathogen. The pathogen indeed overwinters as oospores, which play a major role during grapevine downy mildew epidemics [46]. Furthermore, the identification of the mating-type locus in such an economically important crop pathogen may improve our understanding of pathogen adaptation, as sex and outcrossing promote rapid evolution and sexual structures confer resistance to harsh conditions.
Star★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| CTAB | Sigma-Aldrich | CAT#H6269-250 g |
| Chloroform-Isoamyl alcohol | Sigma-Aldrich | CAT#C0549-1PJ |
| RNase A | QIAGEN | CAT#19101 |
| Sodium Acetate (3 M), pH 5.5, RNase-free | Invitrogen | CAT#AM9740 |
| TE Buffer | Invitrogen | CAT#12090015 |
| Tris (1 M), pH 7.0, RNase-free | Invitrogen | CAT#AM9851 |
| UltraPure 0.5M EDTA, pH 8.0 | Invitrogen | CAT#15575020 |
| Sodium dodecyl sulfate | Sigma-Aldrich | CAT#436143-25G |
| Agar | SETEXAM | CAT#FAL2 |
| Critical Commercial Assays | ||
| DNeasy Plant Mini Kit | QIAGEN | CAT#69104 |
| Deposited Data | ||
| Plasmopara viticola INRA-PV221 reference genome & sequencing reads | [32] | PRJNA329579 |
| Plasmopara viticola sequencing reads | This paper | PRJNA644583 |
| Phytophthora infestans reference genome | [47] | GCA_000142945.1 |
| Phytophthora capsici reference genome | [19] | GCA_000325885.1 |
| Bremia lactucae reference genome | [48] | GCA_004359215.1 |
| Plasmopara halstedii reference genome | [49] | GCA_003724065.1 |
| Experimental Models: Organisms/Strains | ||
| Plasmopara viticola | See Table S1 | See Table S1 |
| Software and Algorithms | ||
| Glint v1.0.rc12.826:833 | N/A | https://forge-dga.jouy.inra.fr/projects/glint |
| SAMtools 1.3.1 | [50] | http://www.htslib.org/download/ |
| VARSCAN 2.4.3 | [51] | http://varscan.sourceforge.net/ |
| BCFtools 1.1-60-g3d5d3d9 | [52] | http://www.htslib.org/download/ |
| snpEff 4.3 | [53] | http://snpeff.sourceforge.net/ |
| VCFtools | [54] | https://vcftools.github.io/ |
| PopLDdecay 3.28 | [55] | https://github.com/BGI-shenzhen/PopLDdecay |
| PLINK 1.07 | [56] | http://zzz.bwh.harvard.edu/plink/ |
| PCAdapt R package | [57] | https://cran.r-project.org/web/packages/pcadapt/ |
| R | [58] | https://www.R-project.org/ |
| sNMF | [59] | N/A |
| LEA R package | [60] | https://www.bioconductor.org/packages/release/bioc/html/LEA.html |
| TASSEL 5.2.41 | [61] | https://www.maizegenetics.net/tassel |
| discoSnp++ | [33] | https://github.com/GATB/DiscoSnp |
| BLAST+ 2.4.0 | [62] | ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/ |
| SMARTdenovo | N/A | https://github.com/ruanjue/smartdenovo |
| MUMmer 3.23 | [63] | http://mummer.sourceforge.net/ |
| BWA 0.7.12 | [64] | https://github.com/lh3/bwa |
| NGMLR | [65] | https://github.com/philres/ngmlr |
| Pilon 1.22 | [66] | https://github.com/broadinstitute/pilon |
| REPET 2.2 | [67, 68] | https://urgi.versailles.inra.fr/Tools/REPET |
| STAR 2.4 | [69] | https://github.com/alexdobin/STAR |
| AUGUSTUS 3.1 | [70] | https://github.com/Gaius-Augustus/Augustus |
| InterProScan 5 | [71] | https://www.ebi.ac.uk/interpro/download/ |
| TransposonPSI | N/A | http://transposonpsi.sourceforge.net |
| Blast2GO 4.1.9 | [72] | https://www.blast2go.com/blast2go-pro/download-b2g |
| CENSOR 4.2.29 | [73] | https://www.girinst.org/censor/ |
| Circos | [74] | http://circos.ca/software/download/circos/ |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yann Dussert (dussert.yann@gmail.com).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Raw reads for each individual have been deposited in GenBank: PRJNA644583 and analysis files have been deposited in Dataverse: http://doi.org/10.15454/ILQ12T.
Experimental Model and Subject Details
Grapevine leaves infected with Pl. viticola were collected across Europe (Table S1). Diploid oomycete individuals were isolated from diseased leaves by dilution to isolate single spores, as follows: isolates of Pl. viticola were first propagated on detached leaves of Vitis vinifera cv. Cabernet-Sauvignon. Sporangia were collected and suspended in water at a concentration of 104 sporangia/mL (estimated using Malassez cells) and serial dilutions were performed to obtain a concentration of 0.1 sporangia/10 μL. We applied 10 μL of this inoculum on 18-mm-diameter leaf disks, excised using a cork borer from V. vinifera cv. Cabernet-Sauvignon leaves, which were placed on filter paper soaked with deionized water in Petri dishes. After seven days in a growth chamber at 22°C (12 h light/12 h dark photoperiod), we retained a single sporulating disk per isolate, which was, thus, derived from a single diploid sporangium.
Method Details
DNA extraction and whole-genome resequencing
Detached leaves from V. vinifera cv. Cabernet-Sauvignon or Muscat Ottonel plants were sterilized with bleach, washed with sterilized water and infected with fresh Pl. viticola inoculum. Leaves were placed in a growth chamber for 4-7 days at 21-22°C (12 h/12 h or 16 h/8 h light/dark photoperiod). Sporangiophores and sporangia were then collected in distilled water. The suspension was centrifuged at maximum speed, the supernatant was removed and the pellet was stored at –80°C. DNA was extracted with a modified CTAB procedure [32] or with the QIAGEN DNeasy Plant Mini Kit.
Genomic DNA was sequenced by Beckman Coulter Genomics (Grenoble, France) or at the GeT-PlaGe facility (Toulouse, France), on an Illumina HiSeq 2000 sequencer (2×100 bp paired-end reads) or an Illumina HiSeq 3000 sequencer (2×150 bp), except for the PV221 individual, for which sequencing reads were already available [32] (SRA accession numbers: SRX1970160, SRX1970161, SRX1970162). Read lengths and mean genome coverage are provided in Table S1.
Mating-type determination
Six Pl. viticola individuals (Tables S1 and S2) were chosen as mating-type testers: three individuals of each mating type. These testers were first crossed with each other in all possible pairings, for separation of the six individuals into two opposite mating-type groups, P1 and P2 (three individuals each). We then crossed 54 individuals with these six testers to determine their mating type. In all experiments, 18-mm-diameter leaf disks, excised from V. vinifera cv. Cabernet-Sauvignon leaves, were placed on 20 g/L agar in Petri dishes and co-inoculated with 15 μL droplets containing 4×104sporangia/mLfrom each individual (three droplets per disk, six replicate disks per pairing). After four to five days in a growth chamber at 22°C (12 h light/12 h dark photoperiod), disks were examined for sporulation, to check for successful inoculation. The leaf disks were then transferred to a growth chamber at 12°C (12 h light/12 h dark photoperiod) for about three weeks [75]. Each leaf disk was then observed with a binocular microscope, to check for the presence of oospores, indicative of successful mating.
Quantification and Statistical Analysis
Read mapping and single-nucleotide polymorphism (SNP) calling
Reads from each of the 54 sequenced individuals were mapped onto the Pl. viticola PacBio reference genome [32] with the Glint aligner v1.0.rc12.826:833, allowing a maximum of 15% mismatches, to handle genomic regions with high heterozygosity (other parameters: mappe mode,-no-lc-filtering-best-score-lrmin 80), and filtering out reads with a mapping quality below 3 and reads that were not properly paired.
A pileup file was generated from the resulting alignment files with SAMtools 1.3.1 [50] mpileup without probabilistic alignment quality computation (-B parameter). SNPs and short insertions and deletions (indels) were then called with the multi-sample procedure (mpileup2snp and mpileup2indel) of VARSCAN 2.4.3 [51] (–min-coverage 10–min-reads2 5–min-avg-qual 15–min-var-freq 0.2-min-freq-for-hom 0.75–p value 0.001-strand-filter 1). For each individual, sites with a read coverage more than 1.5 standard deviations on either side of the mean value for genome coverage were discarded with BCFtools 1.1-60-g3d5d3d9 [52]. SNPs located within 2 bp of a detected indel were also filtered out. Finally, only sites with less than 10% missing data were retained. After filtering, the dataset contained 2,011 million SNPs. Variant annotation was carried out using snpEff 4.3 [53] (parameters:-no-downstream -no-upstream).
Genetic diversity and association of SNPs with mating types
We used SAMtools to assess the normalized read coverage for 1 kb windows for each individual. Using only SNPs with a minor allele frequency (MAF) below 0.1, the percentage of heterozygous individuals for each site along the genome was calculated separately for P1 and P2 individuals with VCFtools [54]. Linkage disequilibrium between sites, as assessed with the r2 statistic, was also computed with VCFtools. Finally, linkage disequilibrium decay along scaffolds was analyzed with PopLDdecay 3.28 [55].
SNP thinning was performed by clumping [76] with PLINK 1.07 [56], with MAF as the statistic of importance and a LD threshold (r2) of 0.2. Population genetic structure was investigated by principal component analysis (PCA) with PCAdapt [57] in R [58]. We also investigated population clustering by computing ancestry coefficients for individuals (Q-matrix) with the non-negative matrix factorization algorithm sNMF [59] implemented in the LEA package [60] in R. We set the number of groups K between 1 and 10, with 50 runs for each value of K. The optimal number of groups was chosen using the minimum cross-entropy criterion.
For identification of the genomic region controlling mating types, we analyzed the association between genotype and mating type (genome-wide association approach) with TASSEL 5.2.41 [61] on SNPs with no missing data and a MAF above 0.1, using a GLM model with the Q-matrix estimated by sNMF. P-values were corrected with 10,000 permutations (threshold: 0.05). We filtered out the small number of association signals found in short scaffolds, corresponding to isolated SNPs in repeat regions.
Identification of genomic regions associated with mating type and absent from the reference genome
The discovery of SNPs associated with mating type in the edges of two scaffolds suggested that the mating-type locus had been only partially assembled in the reference assembly. We therefore used discoSnp++ [33], a reference-free method for identifying SNPs. Briefly, SNPs are detected from bubbles found in a de Bruijn graph built from raw sequencing reads, and short contigs are reconstructed around the SNPs. This analysis was carried out with a subset of 30 individuals (Table S1), as computing resource issues arose if the analysis was extended to a larger number of individuals. We used a k-mer size of 31, the smart branching strategy (-b 1), a maximum of five SNPs in unique bubbles (-P 5), and polymorphisms were extended with left and right contigs (-T). Only sites with less than 10% missing data and a MAF below 0.1 were retained. Contigs were mapped onto the reference genome with the VCF_creator module of discoSnp++ and an association analysis was carried out with TASSEL, as described above, to check that our results were robust to the SNP calling method.
Contigs from discoSnp++ including SNPs significantly associated with mating type were aligned with the self-corrected PacBio reads used for reference genome assembly [32] with BLASTN+ [62], retaining hits displaying at least 95% identity and 95% query coverage. PacBio reads corresponding to these hits were assembled with SMARTdenovo (available from https://github.com/ruanjue/smartdenovo) using default parameters. Assembled contigs were aligned against the reference scaffolds including SNPs with a significant association signal, with NUCmer from the MUMmer 3.23 package [63] using default parameters, and only regions absent from the reference assembly were retained. Available paired-end reads and 3 kb mate-pair reads [77] and the self-corrected PacBio reads were aligned against the reference genome and the new mating-type contigs, with BWA 0.7.12 [64] for short reads and NGMLR [65] for long reads. Aligned reads were used to polish the new mating-type contigs with Pilon 1.22 [66].
After sequence polishing, contigs from the discoSNP analysis were again mapped, but this time to the reference assembly and the newly assembled contigs, and a final association analysis was carried out. Newly assembled contigs with no SNP significantly associated with the matin type were discarded. For the remaining contigs, repeat sequences were annotated with TEannot from REPET 2.2 [67, 68], using the Pl. viticola repeat library [32]. Available RNA-seq data [32, 78] were aligned against the reference assembly and the new contigs with STAR 2.4 [69], and were used as hints for gene prediction in the new contigs with AUGUSTUS 3.1 [70], together with the training files produced by the previous annotation [32]. Proteins for which functional domains of transposable elements were detected with InterProScan 5 [71] or for which hits were obtained with TransposonPSI (http://transposonpsi.sourceforge.net; 30% coverage, e-value < 1e-10) were filtered out. The remaining proteins were aligned against the NCBI nr database with BLASTP+ (-evalue 1e-5 -max_hsp 20 -num_alignment 20), and functionally annotated with Blast2GO 4.1.9 [72] using the BLASTP+ and Inter-ProScan results for gene ontology (GO) term mapping.
For all candidate genes within the mating-type locus (i.e., from the scaffolds in the reference assembly and the new contigs), we further checked whether coding sequences corresponded to transposable elements: protein sequences for those genes were masked with CENSOR 4.2.29 [73] using RepBase 23.08, which allowed us to compute for each protein a percentage of coverage from known transposable elements.
Comparative genomics with Phytophthora infestans, Phytophthora capsici, Bremia lactucae and Plasmopara halstedii
We identified the orthologs of the mating-type candidate genes in the annotated proteomes of Ph. infestans [47], Ph. capsici [19], Br. lactucae [48] and Pl. halstedii [49], with the reciprocal best hits (RBH) method using BLASTP+ (-evalue 1e-6 -qcov_hsp_perc 50–use_sw_tback, min. identity 40%). Results were visualized with Circos [74].
Supplementary Material
Supplemental Information can be found online at https://doi.org/10.1016/j.cub.2020.07.057.
Highlights.
Identification of the first oomycete mating-type locus using a GWAS approach
This 570-kb repeat-rich non-recombining region showed two highly divergent alleles
One mating type was heterozygous and the other homozygous, as in XY sex chromosomes
Acknowledgements
We thank Vincent Thomas for helping with the production of material and Pal Kozma (University of Pécs, Hungary), Mauro Jermini (Agroscope, Switzerland), Sara Legler (UCSC, Italy), Hervé Steva (Bordeaux, France), and Atanas Atanassov (JGC, Bulgaria) for providing Pl. viticola isolates. We are grateful to Jéroôme Gouzy (INRAE, Toulouse) for his help and suggestions concerning data analysis. We thank the Genotoul bioinformatics platform Toulouse Midi-Pyrenees (Bioinfo Genotoul) for providing help and computing resources. This work was performed in collaboration with the GeT core facility, Toulouse, France (http://get.genotoul.fr). This study was supported by the European Commission (INNOVINE, FP7-KBBE-311775) and the French National Research Agency (GANDALF project, ANR-12-ADAP-0009; EFFECTOORES project, ANR-13-ADAP-0003; Investments for the Future Program in the Cluster of Excellence COTE, ANR-10-LABX-45; Investments for the Future Program France Génomique national infrastructure, ANR-10-INBS-09). T.G. acknowledges receipt of the ERC advanced grant EvolSexChrom no. 832252.
Footnotes
Author Contributions
Conceptualization, F.D.; Methodology, Y.D. and F.D.; Formal Analysis, Y.D. and L.L.; Investigation, I.D.M., C.C., M.-C.P., R.-F.S., O.B., P.M., F.D., and S.L.T.; Writing - Original Draft, Y.D. and T.G.; Writing - Review & Editing, Y.D., L.L., I.D.M., C.C., M.-C.P., R.-F.S., O.B., P.M., S.L.T., T.G., and F.D.; Supervision, F.D. and T.G.; Funding Acquisition, P.M. and F.D.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:237–268. [Google Scholar]
- 2.Charlesworth D, Morgan MT, Charlesworth B. Inbreeding depression, genetic load, and the evolution of outcrossing rates in a multi-locus system with no linkage. Evolution. 1990;44:1469–1489. doi: 10.1111/j.1558-5646.1990.tb03839.x. [DOI] [PubMed] [Google Scholar]
- 3.Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 4.Igic B, Lande R, Kohn JR. Loss of self-incompatibility and its evolutionary consequences. Int J Plant Sci. 2008;169:93–104. [Google Scholar]
- 5.Lande R, Porcher E. Maintenance of quantitative genetic variance under partial self-fertilization, with implications for evolution of selfing. Genetics. 2015;200:891–906. doi: 10.1534/genetics.115.176693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beukeboom LW, Perrin N. The Evolution of Sex Determination (Oxford University) 2014 [Google Scholar]
- 7.Billiard S, López-Villavicencio M, Hood ME, Giraud T. Sex, outcrossing and mating types: unsolved questions in fungi and beyond. J Evol Biol. 2012;25:1020–1038. doi: 10.1111/j.1420-9101.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 8.Orias E, Singh DP, Meyer E. Genetics and epigenetics of mating type determination in Paramecium and Tetrahymena . Annu Rev Microbiol. 2017;71:133–156. doi: 10.1146/annurev-micro-090816-093342. [DOI] [PubMed] [Google Scholar]
- 9.Sekimoto H. Sexual reproduction and sex determination in green algae. J Plant Res. 2017;130:423–431. doi: 10.1007/s10265-017-0908-6. [DOI] [PubMed] [Google Scholar]
- 10.Fujii S, Kubo K, Takayama S. Non-self-and self-recognition models in plant self-incompatibility. Nat Plants. 2016;2:16130. doi: 10.1038/nplants.2016.130. [DOI] [PubMed] [Google Scholar]
- 11.Judelson HS. Sexual reproduction in oomycetes: biology, diversity and contributions to fitness. In: Lamour K, Kamoun S, editors. Oomycete Genetics and Genomics: Diversity, Interactions and Research Tools. John Wiley &Sons; 2009. pp. 121–138. [Google Scholar]
- 12.Levesque CA. Fifty years of oomycetes—from consolidation to evolutionary and genomic exploration. Fungal Divers. 2011;50:35. [Google Scholar]
- 13.van West P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist. 2006;20:99–104. [Google Scholar]
- 14.Brurberg MB, Elameen A, Le VH, Naerstad R, Hermansen A, Lehtinen A, Hannukkala A, Nielsen B, Hansen J, Andersson B, Yuen J. Genetic analysis of Phytophthora infestans populations in the Nordic European countries reveals high genetic variability. Fungal Biol. 2011;115:335–342. doi: 10.1016/j.funbio.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Gavino PD, Smart CD, Sandrock RW, Miller JS, Hamm PB, Lee TY, Davis RM, Fry WE. Implications of sexual reproduction for Phytophthora infestans in the United States: generation of an aggressive lineage. Plant Dis. 2000;84:731–735. doi: 10.1094/PDIS.2000.84.7.731. [DOI] [PubMed] [Google Scholar]
- 16.Fabritius AL, Judelson HS. Mating-type loci segregate aberrantly in Phytophthora infestans but normally in Phytophthora parasitica: implications for models of mating-type determination. Curr Genet. 1997;32:60–65. doi: 10.1007/s002940050248. [DOI] [PubMed] [Google Scholar]
- 17.Judelson HS, Spielman LJ, Shattock RC. Genetic mapping and non-Mendelian segregation of mating type loci in the oomycete, Phytophthora infestans . Genetics. 1995;141:503–512. doi: 10.1093/genetics/141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Lee T, Testa A, Robold A, van ’t Klooster J, Govers F. High-density genetic linkage maps of Phytophthora infestans reveal trisomic progeny and chromosomal rearrangements. Genetics. 2004;167:1643–1661. doi: 10.1534/genetics.104.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamour KH, Mudge J, Gobena D, Hurtado-Gonzales OP, Schmutz J, Kuo A, Miller NA, Rice BJ, Raffaele S, Cano LM, et al. Genome sequencing and mapping reveal loss of heterozygosity as a mechanism for rapid adaptation in the vegetable pathogen Phytophthora capsici . Mol Plant Microbe Interact. 2012;25:1350–1360. doi: 10.1094/MPMI-02-12-0028-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sicard D, Legg E, Brown S, Babu NK, Ochoa O, Sudarshana P, Michelmore RW. A genetic map of the lettuce downy mildew pathogen, Bremia lactucae, constructed from molecular markers and avirulence genes. Fungal Genet Biol. 2003;39:16–30. doi: 10.1016/s1087-1845(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 21.Carlson MO, Gazave E, Gore MA, Smart CD. Temporal genetic dynamics of an experimental, biparental field population of Phytophthora capsici . Front Genet. 2017;8:26. doi: 10.3389/fgene.2017.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harutyunyan SR, Zhao Z, Hartog Td, Bouwmeester K, Minnaard AJ, Feringa BL, Govers F. Biologically active Phytophthora mating hormone prepared by catalytic asymmetric total synthesis. Proc Natl Acad Sci USA. 2008;105:8507–8512. doi: 10.1073/pnas.0709289105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojika M, Molli SD, Kanazawa H, Yajima A, Toda K, Nukada T, Mao H, Murata R, Asano T, Qi J, Sakagami Y. The second Phytophthora mating hormone defines interspecies biosynthetic crosstalk. Nat Chem Biol. 2011;7:591–593. doi: 10.1038/nchembio.617. [DOI] [PubMed] [Google Scholar]
- 24.Qi J, Asano T, Jinno M, Matsui K, Atsumi K, Sakagami Y, Ojika M. Characterization of a Phytophthora mating hormone. Science. 2005;309:1828. doi: 10.1126/science.1114756. [DOI] [PubMed] [Google Scholar]
- 25.Fontaine MC, Austerlitz F, Giraud T, Labbé F, Papura D, Richard-Cervera S, Delmotte F. Genetic signature of a range expansion and leap-frog event after the recent invasion of Europe by the grapevine downy mildew pathogen Plasmopara viticola . Mol Ecol. 2013;22:2771–2786. doi: 10.1111/mec.12293. [DOI] [PubMed] [Google Scholar]
- 26.Vercesi A, Toffolatti SL, Zocchi G, Guglielmann R, Ironi L. A new approach to modelling the dynamics of oospore germination in Plasmopara viticola. . Eur J Plant Pathol. 2010;128:113–126. [Google Scholar]
- 27.Wong FP, Burr HN, Wilcox WF. Heterothallism in Plasmopara viticola . Plant Pathol. 2001;50:427–432. [Google Scholar]
- 28.Delmas CEL, Dussert Y, Delière L, Couture C, Mazet ID, Richart Cervera S, Delmotte F. Soft selective sweeps in fungicide resistance evolution: recurrent mutations without fitness costs in grapevine downy mildew. Mol Ecol. 2017;26:1936–1951. doi: 10.1111/mec.14006. [DOI] [PubMed] [Google Scholar]
- 29.Toffolatti SL, Prandato M, Serrati L, Sierotzki H, Gisi U, Vercesi A. Evolution of Qol resistance in Plasmopara viticola oospores. Eur J Plant Pathol. 2011;129:331–338. [Google Scholar]
- 30.Peressotti E, Wiedemann-Merdinoglu S, Delmotte F, Bellin D, Di Gaspero G, Testolin R, Merdinoglu D, Mestre P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010;10:147. doi: 10.1186/1471-2229-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delmas CEL, Fabre F, Jolivet J, Mazet ID, Richart Cervera S, Deliére L, Delmotte F. Adaptation of a plant pathogen to partial host resistance: selection for greater aggressiveness in grapevine downy mildew. Evol Appl. 2016;9:709–725. doi: 10.1111/eva.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dussert Y, Mazet ID, Couture C, Gouzy J, Piron M-C, Kuchly C, Bouchez O, Rispe C, Mestre P, Delmotte F. A high-quality grapevine downy mildew genome assembly reveals rapidly evolving and lineage-specific putative host adaptation genes. Genome Biol Evol. 2019;11:954–969. doi: 10.1093/gbe/evz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterlongo P, Riou C, Drezen E, Lemaitre C. DiscoSnp++: de novo detection of small variants from raw unassembled read set(s) bioRxiv. 2017 doi: 10.1101/209965. [DOI] [Google Scholar]
- 34.Zhao X, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell. 2007;6:1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karos M, Chang YC, McClelland CM, Clarke DL, Fu J, Wickes BL, Kwon-Chung KJ. Mapping of the Cryptococcus neoformans MATalpha locus: presence of mating type-specific mitogen-activated protein kinase cascade homologs. J Bacteriol. 2000;182:6222–6227. doi: 10.1128/jb.182.21.6222-6227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Judelson HS. Chromosomal heteromorphism linked to the mating type locus of the oomycete Phytophthora infestans . Mol Gen Genet. 1996;252:155–161. doi: 10.1007/BF02173215. [DOI] [PubMed] [Google Scholar]
- 37.Giraud T, Yockteng R, López-Villavicencio M, Refrégier G, Hood ME. Mating system of the anther smut fungus Microbotryum violaceum: selfing under heterothallism. Eukaryot Cell. 2008;7:765–775. doi: 10.1128/EC.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randall TA, Ah Fong A, Judelson HS. Chromosomal heteromorphism and an apparent translocation detected using a BAC contig spanning the mating type locus of Phytophthora infestans . Fungal Genet Biol. 2003;38:75–84. doi: 10.1016/s1087-1845(02)00512-1. [DOI] [PubMed] [Google Scholar]
- 39.Bachtrog D. Sex chromosome evolution: molecular aspects of Y-chromosome degeneration in Drosophila . Genome Res. 2005;15:1393–1401. doi: 10.1101/gr.3543605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlesworth D. Plant contributions to our understanding of sex chromosome evolution. New Phytol. 2015;208:52–65. doi: 10.1111/nph.13497. [DOI] [PubMed] [Google Scholar]
- 41.Badouin H, Hood ME, Gouzy J, Aguileta G, Siguenza S, Perlin MH, Cuomo CA, Fairhead C, Branca A, Giraud T. Chaos of rearrangements in the mating-type chromosomes of the anther-smut fungus Microbotryum lychnidis-dioicae . Genetics. 2015;200:1275–1284. doi: 10.1534/genetics.115.177709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 43.Jeffries DL, Lavanchy G, Sermier R, Sredl MJ, Miura I, Borzeé A, Barrow LN, Canestrelli D, Crochet PA, Dufresnes C, et al. A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat Commun. 2018;9:4088. doi: 10.1038/s41467-018-06517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heitman J, Kronstad JW, Taylor JW, Casselton LA. Sex in Fungi: Molecular Determination and Evolutionary Implications (ASM) 2007 [Google Scholar]
- 45.Bourret TB, Choudhury RA, Mehl HK, Blomquist CL, McRoberts N, Rizzo DM. Multiple origins of downy mildews and mito-nuclear discordance within the paraphyletic genus Phytophthora . PLoS ONE. 2018;13:e0192502. doi: 10.1371/journal.pone.0192502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gobbin D, Jermini M, Loskill B, Pertot I, Raynal M, Gessler C. Importance of secondary inoculum of Plasmopara viticola to epidemics of grapevine downy mildew. Plant Pathol. 2005;54:522–534. [Google Scholar]
- 47.Haas BJ, Kamoun S, Zody MC, Jiang RHY, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature. 2009;461:393–398. doi: 10.1038/nature08358. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher K, Gil J, Bertier LD, Kenefick A, Wood KJ, Zhang L, Reyes-Chin-Wo S, Cavanaugh K, Tsuchida C, Wong J, Michelmore R. Genomic signatures of heterokaryosis in the oomycete pathogen Bremia lactucae . Nat Commun. 2019;10:2645. doi: 10.1038/s41467-019-10550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pecrix Y, Buendia L, Penouilh-Suzette C, Maréchaux M, Legrand L, Bouchez O, Rengel D, Gouzy J, Cottret L, Vear F, Godiard L. Sunflower resistance to multiple downy mildew pathotypes revealed by recognition of conserved effectors of the oomycete Plasmopara halstedii . Plant J. 2019;97:730–748. doi: 10.1111/tpj.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cingolani P, Platts A, Wang L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Dong S-S, Xu J-Y, He W-M, Yang T-L. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics. 2019;35:1786–1788. doi: 10.1093/bioinformatics/bty875. [DOI] [PubMed] [Google Scholar]
- 56.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luu K, Bazin E, Blum MGB. pcadapt: an R package to perform genome scans for selection based on principal component analysis. Mol Ecol Resour. 2017;17:67–77. doi: 10.1111/1755-0998.12592. [DOI] [PubMed] [Google Scholar]
- 58.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2017. http://www.R-project.org/ [Google Scholar]
- 59.Frichot E, Mathieu F, Trouillon T, Bouchard G, François O. Fast and efficient estimation of individual ancestry coefficients. Genetics. 2014;196:973–983. doi: 10.1534/genetics.113.160572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frichot E, François O. LEA: an R package for landscape and ecological association studies. Methods Ecol Evol. 2015;6:925–929. [Google Scholar]
- 61.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- 62.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sedlazeck FJ, Rescheneder P, Smolka M, Fang H, Nattestad M, von Haeseler A, Schatz MC. Accurate detection of complex structural variations using single-molecule sequencing. Nat Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quesneville H, Bergman CM, Andrieu O, Autard D, Nouaud D, Ashburner M, Anxolabehere D. Combined evidence annotation of transposable elements in genome sequences. PLoS Comput Biol. 2005;1:166–175. doi: 10.1371/journal.pcbi.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flutre T, Duprat E, Feuillet C, Quesneville H. Considering transposable element diversification in de novo annotation approaches. PLoS ONE. 2011;6:e16526. doi: 10.1371/journal.pone.0016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–644. doi: 10.1093/bioinformatics/btn013. [DOI] [PubMed] [Google Scholar]
- 71.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Götz S, Garía-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohany O, Gentles AJ, Hankus L, Jurka J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics. 2006;7:474. doi: 10.1186/1471-2105-7-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung CR-TM, Clerjeau M. Techniques for formation, maturation, and germination of Plasmopara viticola oospores under controlled conditions. Plant Dis. 1988;72:938–941. [Google Scholar]
- 76.Privé F, Aschard H, Ziyatdinov A, Blum MGB. Efficient analysis of large-scale genome-wide data with two R packages: bigstatsr and bigsnpr. Bioinformatics. 2018;34:2781–2787. doi: 10.1093/bioinformatics/bty185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dussert Y, Gouzy J, Richart-Cervera S, Mazet ID, Delière L, Couture C, Legrand L, Piron M-C, Mestre P, Delmotte F. Draft genome sequence of Plasmopara viticola, the grapevine downy mildew pathogen. Genome Announc. 2016;4:e009877–16. doi: 10.1128/genomeA.00987-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mestre P, Carrere S, Gouzy J, Piron M-C, Tourvieille de Labrouhe D, Vincourt P, Delmotte F, Godiard L. Comparative analysis of expressed CRN and RXLR effectors from two Plasmopara species causing grapevine and sunflower downy mildew. Plant Pathol. 2016;65:767–781. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw reads for each individual have been deposited in GenBank: PRJNA644583 and analysis files have been deposited in Dataverse: http://doi.org/10.15454/ILQ12T.