Abstract
Surface modification of orthopedic and dental implants has been demonstrated to be an effective strategy to accelerate bone healing at early implantation times. Among the different alternatives, coating implants with a layer of hydroxyapatite (HAp) is one of the most used techniques, due to its excellent biocompatibility and osteoconductive behavior. The composition and crystalline structure of HAp allow for numerous ionic substitutions that provide added value, such as antibiotic properties or osteoinduction. In this article, we will review and critically analyze the most important advances in the field of substituted hydroxyapatite coatings. In recent years substituted HAp coatings have been deposited not only on orthopedic prostheses and dental implants, but also on macroporous scaffolds, thus expanding their applications towards bone regeneration therapies. Besides, the capability of substituted HAps to immobilize proteins and growth factors by non-covalent interactions has opened new possibilities for preparing hybrid coatings that foster bone healing processes. Finally, the most important in vivo outcomes will be discussed to understand the perspectives of substituted HAp coatings from a clinical point of view.
1. Introduction
Orthopedic implants improve the quality of life of millions of patients every year. Due to clinical staff training, new prosthesis designs, control of sterility and protocols for antibiotic prophylaxis, the success rate of orthopedic prostheses has significantly increased in the last decades. For instance, the success rate of hip replacements 10 years after surgery is 90–95% and 80–85% at 20 years.1 In the case of titanium based dental implants, the success rate is very similar.2 Despite these clinical outcomes, thousands of prostheses and dental implants must be revised every year. The two most frequent causes for failure are insufficient bone formation around the implant immediately after implantation,3,4 especially in osteoporotic patients,5–8 and infection.9
Bone implants for load-bearing applications are generally made of metals such as titanium, Ti6Al4V alloys, stainless steel, etc., which exhibit appropriate mechanical properties to be used in the clinic as dental implants and stems of hip and knee prostheses, plates, screws and other fixation devices. In the case of endosseous metal components such as dental implant roots or joint prosthesis stems, the surfaces are commonly modified by roughening and/or coating with bioactive compounds to improve the osteoconductivity. HAp is a calcium phosphate (CaP) commonly used for the fabrication of coatings, layers or thin films on the surface of prosthetic devices10 to accelerate bone healing at early implantation times. For this aim, the coating must be biocompatible,11 bioactive12 and osteoconductive.13 Besides, ASTM and ISO standards require other properties, namely the presence of a dominant crystalline phase that prevents fast coating dissolution, the presence of an amorphous phase that promotes early osteointegration without losing stability, a programmed dissolution rate of the crystalline phase (commonly HAp), an elemental composition matching the mineral phase of bone and strong implant adhesion to prevent mechanical failures under load-bearing conditions.14,15 Currently, just a few coatings fulfil these requirements and only calcium phosphate bioceramics fabricated by plasma spraying are clinically approved for orthopedic coatings and commercially available.
Substituted HAp coatings have arisen as a very promising alternative to conventional CaP coatings. Ionic substitutions are aimed at providing additional properties to HAp such as osteoinduction or antibiotic activity. Therefore, substituted HAp coatings would be produced not only for accelerating biomechanical fixation but also for providing solutions in some pathological scenarios such as infection and osteoporosis. Currently, the field of substituted HAp coatings has become an ever-growing research field, mainly due to the variety of elements and ions with therapeutic effects discovered during the last 50 years.16 Moreover, HAp exhibits a crystalline structure that easily allows ion incorporation by substitutive and interstitial mechanisms. These facts have opened new scenarios where coatings play an active role in the treatment of pathologies, in addition to accelerating bone healing at early implantation times. In association with the recently developed additively manufactured metallic scaffolds, substituted HAp coatings are also expanding the clinical applications of metallic implants from their conventional substitutive functions towards bone regeneration purposes. Recently, the combination of substituted HAp with nanostructures has also opened new possibilities in the field of coatings for orthopedic purposes.17–19
This review is devoted to the performance of substituted HAp coatings for orthopedic and dental devices. Beginning from the bioinorganic characteristics of the substituents and the most frequently applied coating methods, the in vitro and in vivo biological performance are comprehensively reviewed and analyzed.
2. Crystalline structure of hydroxyapatite and ionic substitutions
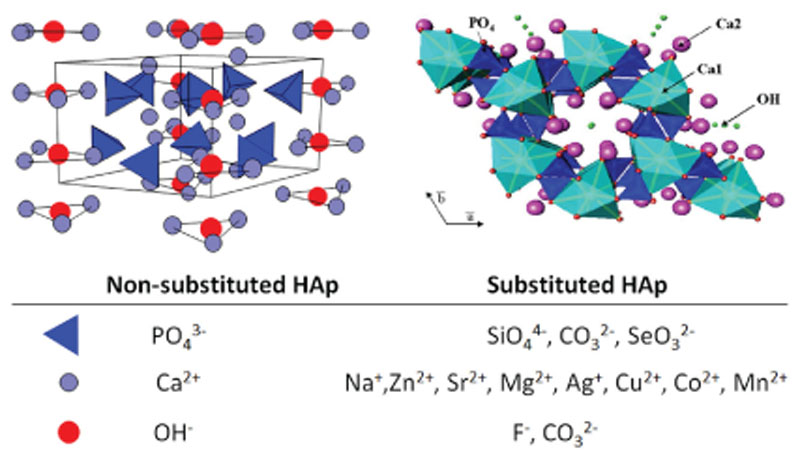
Stoichiometric HAp is one of the most important bioceramics used in dentistry and orthopedic surgery. Its composition, Ca10(PO4)6(OH)2, is like the mineral component of bone tissue although there exist important crystal-chemical and micro-structural differences, depending on the synthesis method. The structure of HAp can be described as a hexagonal unit cell with space group P63/m and lattice parameters a = 9.432 Å and c = 6.881 Å, having one formula Ca10(PO4)6(OH)2 per unit cell21 (Fig. 1). The OH ions and four Ca2+ ions at Ca(1) sites lie along columns parallel to the c axis. The OH– are sited along the c axis and the O–H bond direction is parallel to it, without straddling the mirror planes at z = 1/4 and 3/4. The remaining six Ca2+ ions, positioned at Ca(2) sites, are associated with the two OH– groups in the unit cell, where they form triangles perpendicular to the OH–. The phosphate tetrahedrons form the remaining basic structural unit of HAp.
Fig. 1.
The unit cell of hydroxyapatite projected along the a axis (left) and along the c axis (right) showing Ca1, Ca2, tetrahedral phosphates and hydroxyl sites. Ionic substitutions with potential therapeutic effects are indicated (bottom).
The bioactive behavior of stoichiometric HAp can be improved by introducing substitutions in both the cationic and anionic sublattices.22 The cations can exhibit the same oxidation state as Ca2+, such as Sr2+, Pb2+, Mg2+, etc.,23–26 and anions the same oxidation state as OH–, such as F– or C–.27,28 Ionic substitutions with different oxidation states are also very common29–31 and play an important role in the chemical, structural, and microstructural properties. For example in biological apatites CO3 2–, substitution for PO4 3– (type B) or OH– (type A) is a likely substitution.32,33 In the case of B-type carbonated HAp (CHAp), single valence cations (Na+ or K +) are often incorporated in the Ca2+ positions to keep the electrical balance.34,35
3. Production techniques of hydroxyapatite coatings
The production techniques of HAp coatings can by classified into two main groups: physical deposition techniques and wet chemical deposition techniques.36 Regardless of the coating technique used, the success or failure of the coating greatly depends on the previous preparation of the substrate. Surface cleaning is required to remove dirt, oils and other components coming from the machining of the prostheses. This cleaning commonly consists of an ultrasonic bath of ethanol or acetone and often includes a subsequent acid treatment (etching) and/ or sand or grit-blasting to facilitate the subsequent coating attachment and stability.
3.1. Physical deposition techniques
Thermal spraying techniques. The most important physical deposition techniques for the fabrication of HAp coatings are thermal spraying techniques. They are based on processes in which the coating materials (or precursors) are heated and sprayed on the substrate. Depending on the heating method, thermal spraying includes atmospheric plasma spraying,37 vacuum plasma spraying,38,39 suspension plasma spraying,40 liquid phase plasma spraying,41 high velocity oxy-fuel spraying42 and gas tunnel type plasma spraying.43
Due to the very high temperatures used in these techniques, differences between the feedstock material and the deposited coating are unavoidable. Deposited HAp and substituted HAp are dehydroxylated and partially decomposed into oxyapatite (OA) and other CaP phases. In fact, the so named HAp coatings are commonly multiphase coatings containing amorphous calcium phosphate (ACP), α or β tricalcium phosphate (α or β-TCP), OA, HAp and even CaO. In other words, the coating composition does not depend only on the feedstock material, but also on the heating and cooling processes undergone during their permanence within the plasma and cooling ratio. This multiphasic composition is decisive for the biocompatibility, since the content of more soluble phases such as ACP determines the stability of the coating. ISO rules determine that the HAp content shall be more than 95% by mass, whereas the content of α-TCP, β-TCP, TTCP and CaO shall be less than a 5% mass fraction. Regarding the coating crystallinity, the HAp phase shall have crystallinity values not less than 45% with respect to standard HAp (HAp after calcination at 1000 1C for 15 hours).14
Thermal spraying based techniques produce thick coatings of several tens of micrometers,44 although the use of a feedstock as a solution or suspension can result in thinner coatings (5 to 10 μm) in comparison with thermal spraying when using dry powders. For instance, high velocity oxy-fuel spraying, a thermal spray process developed in the 90s,45 allows the fabrication of thin films with low porosity and high adhesion, due to the velocity reached by the powder feedstock (around 800 m s–1) when injected into a gas stream produced by the combustion of a fuel with oxygen.
Electrophoretic deposition (EPD). EPD is a physical coating method that consists in depositing charged colloidal HAp particles onto a conductive substrate of opposite charge.46 The HAp particles are suspended in liquid media and subsequently deposited by the driving force of a DC electric field, the substrate being one of the electrodes. The coating thickness and morphology can be adjusted through the applied voltage, in such a way that the particle size and amount of deposited HAp increase with it. After deposition, the implant is commonly treated at 850 °C to 950 °C under high vacuum conditions.47 EPD is a very useful technique for the fabrication of coatings onto porous structures; however, the main drawback is the shrinkage and cracking that often appear during the sintering process.
Physical vapor deposition (PVD) techniques. PVD techniques, commonly referred to as sputtering techniques, are among the most widely used methods for the preparation of HAp coatings. PVD commonly involves the use of a highly energetic beam of ions or electrons projected onto a CaP target. Ca2+, PO4 3– and substitution ions are pulled out from the target surface and deposited onto the substrate. Depending on the technique used to knock the ions off the target, sputtering techniques are denoted as pulsed electron deposition (PED) if the ions are pulled out from the CaP target by means of collisions with electrons;48,49 pulsed laser deposition (PLD), or laser ablation deposition50,51 uses a high-power laser beam to hit a CaP target resulting in a gaseous phase made of atoms, ions, molecules and clusters, which is moved towards the substrate as a plasma plume. This approach leads to thin HAp coatings of 0.05 to 5 μm thickness52,53 and the heating of the substrate is required for crystallization. Radio-frequency magnetron sputtering (RFMS) produces coatings in large areas and with a large variety of morphologies. It is based on PVD in high vacuum conditions of CaP released into the sputtering chamber as a gas. The particles are ionized by powerful magnets and the charged material aligns on the substrate to form the coating.54,55 For the specific case of CaP coatings, Surmenev established the parameters that directly affect the quality of the coating, namely the discharge power, working pressure, substrate temperature, flow rate, deposition time and the subsequent thermal treatment.56 Since Cooley et al. used for the first time this approach to prepare CaP coatings,57 RFMS has been used to produce different biocompatible coatings on several substrates;58,59 matrix-assisted pulsed laser deposition (MAPLE) was introduced as an alternative to PLD for the synthesis of thermally unstable compounds, mainly organic coatings. This technique has also shown some advantages for the fabrication of inorganic coatings as well.60 In the case of CaP coatings, MAPLE has shown very interesting results for the fabrication of thermally unstable octacalcium phosphate coatings,61 organic–inorganic composites62 or even CaP coatings containing drugs.63
3.2. Wet chemical deposition techniques
The different chemical deposition approaches are characterized by occurring from solutions or suspensions. During the coating process, the chemical composition of the feedstock material changes by means of a chemical reaction, resulting in a different compound at the end of the coating production. Chemical deposition processes occur at moderate temperature and are mostly governed by the solution supersaturation, pH and temperature, although the addition of nucleators, inhibitors, etc. allows the control of the coating characteristics. In this approach, the previous conditioning of the substrate surface is compulsory. For instance, the coating of Ti implants commonly requires acid or alkaline pre-treatment to form a rich Ti–OH layer, which facilitates the subsequent calcium phosphate nucleation and growth.64
Chemical vapor deposition technique (CVD). CVD consists in the exposure of the substrate to volatile precursors that react or decompose on the surface. CVD has been used to prepare CaP-based coatings on metallic substrates65 demonstrating potential for controlling the crystal phase and microstructure and providing well-adhered coatings even on complex-shaped metal substrates.66
Biomimetic deposition techniques. Biomimetic deposition consists in mimicking natural manufacturing methods to generate artificial bone-like HAp, which can be used to improve the osteointegration of dental and orthopedic implants. The most common process consists in the crystallization of nonstoichiometric carbonate-substituted hydroxyapatite (C-HAp) from simulated physiological solutions at low temperature conditions.67 The biomimetic HAp crystallization takes place through the nucleation of ACP or OCP, which subsequently maturates into substituted and calcium-deficient hydroxyapatite (CDHAp). Like other chemical deposition techniques, biomimetic deposition commonly requires substrates with OH– containing surfaces such as Si–OH, Ti–OH, Zr–OH, Nb–OH etc., which can be easily obtained by acid or alkaline treatments. When these activated substrates are soaked in solutions where the ionic product overcomes the solubility constant (Kps) for HAp (for instance simulated body fluid, SBF), the hydroxyl groups promote CaP nucleation and subsequent crystallization into apatite like-phases. This technique allows for the co-deposition of other biological substances, since the coating process is carried out using mild conditions of pH and temperature.68,69
Sol–gel method. The sol–gel method consists in the preparation of a colloidal liquid suspension ‘sol’ by means of the hydrolysis and condensation of the CaP precursors (commonly phosphorous alkoxides and inorganic calcium salts).70 Aided by the presence of an acid or basic catalyst, the sol undergoes a transition into a solid ‘gel’ that is deposited on the substrate. Procedures such as dip-coating have proven to be very useful for the control of the thickness, morphology and homogeneity of the coatings.71,72 Dip-coating consists in the immersion of the substrate into a solution containing the coating precursors. In the case of HAp or substituted HAp coatings, the precursors are soluble salts of the cations (such as Ca(NO3)2·4H2O) and alkoxides of the anions, for instance triethyl phosphite P(OCH2CH3)3 (TIP) and tetraethyl orthosilicate Si(OCH2CH3)4 (TEOS) solutions in the case of silicon substituted hydroxyapatites (Si-HAp).73 The substrate is soaked at a constant speed and the coating is deposited during the substrate withdrawal. The pulling up speed determines the coating thickness: the faster the withdrawal the thinner the coating, although several immersions can be performed in order to obtain thicker coatings.
Electrochemical deposition (ECD). The ECD technique uses a supersaturated aqueous electrolyte solution of Ca2+ and PO4 3– ions in contact with platinum (anode) and metallic implant (cathode) electrodes connected to a current generator.74 The electrochemical reactions occurring around the cathode lead to a pH increase and subsequent calcium phosphate nucleation and growth occurs. This approach can be carried out under ambient conditions75 or higher temperatures in an autoclave76 and thin coatings of less than 1 μm with uniform structures can be obtained. The main drawback is the large volume of electrolyte solution required, the hydrogen gas produced, which can lead to inhomogeneities, and the limitation to coat only conductive materials.
Micro-arc oxidation (MAO). MAO is a wet chemical technique that combines electrochemical oxidation with high voltage treatment in solutions containing calcium, phosphate and precursors of other ions as inorganic salts.77,78 MAO has been used to prepare substituted HAp coatings on metallic implants and is a suitable approach to obtain porous and rough ceramic surfaces.79
Electrospray deposition (ESD). ESD involves the formation of an aerosol from a solution of calcium and phosphorous solution in a volatile organic component. This aerosol is sprayed through a nozzle over the substrate under the presence of a high voltage.80 The coating characteristics can be tuned by controlling the precursor solution (mainly pH and concentration) and deposition parameters such as the temperature or the nozzle-to-substrate distance. In order to obtain highly dense and homogeneous surfaces, coatings produced by ESD require a further annealing stage.
Drop on demand microdispensing (DODMD). DODMD is an additive manufacturing process which has been successfully used for fabrication of substituted hydroxyapatite coatings.81 In this method, a suspension of a previously synthesized bioceramic is dispensed drop by drop by a micro-valve assisted by a pneumatic system. DODMD allows for depositing both organic and inorganic materials and living cells. Moreover, DODMD employs a layer-by-layer approach enabling the fabrication of multi-layered functionally graded coatings.
4. Substituted hydroxyapatite coatings
4.1. Cationic substitutions in hydroxyapatite coatings
Zinc-substituted hydroxyapatite (Zn-HAp) coatings. Zinc (Zn) is the second most abundant essential element in humans. In an average adult there are about 3 g of Zn and it is widely accepted that it is essential for all living beings. Zn enzymes catalyze the metabolic conversion (synthases, polymerases, ligases, and transferases) and the degradation (hydrolases) of proteins, nucleic acids, lipids, porphyrin precursors and other biologically important compounds. In addition to the catalytic function, Zn has a structural function in different proteins and participates in processes related to cell division, nucleic acid replication and gene transcription. Consequently, Zn is essential for growth, development and differentiation for all species, and particularly for humans. Zn deficiency leads to serious pathological effects, particularly a significant weakening of the immunological system.
Zn is only present in bone as a trace element. However, the incorporation of Zn2+ cations in HAp structure has attracted the interest of many researchers. Zn2+ isoelectrically substitutes for Ca2+, resulting in the decrease of the lattice parameters a and c of. This is due to the difference in ionic radius between Zn2+ (0.074 nm) and Ca2+ (0.099 nm),82 favoring the Ca(2) over the Ca(1) sites.83 Zn2+ cations can incorporate in the HAp structure in a limited amount (about 20 atom%).84
Several authors have stated that Zn stimulates bone formation by activating proliferation and differentiation of osteoblasts.85,86 Zn-HAp has also shown antibacterial properties against both Gram– and Gram+ bacteria. HAp doped with less than 1% of zinc ions has evidenced effective bioactivity and antibacterial properties.87,88
Zn-HAp coatings have been fabricated by different techniques including plasma spraying,89 EPD,90 sol–gel spin coating91 and magnetron sputtering.92 The fabrication method determines the Zn distribution within the coating. For instance, whereas the EPD method yields homogenous Zn-HAp coatings, magnetron sputtering produces Zn-HAp coatings with higher Zn concentration on the film surface.93 Zn-HAp coatings prepared by electrochemical deposition have been also proposed for enhancing the corrosion resistance of commercially pure titanium (CP-Ti) substrates.94 These coatings were deposited together with a calcium silicate by adding SiO2 nanoparticles to the electrolyte solution. Certainly, the Zn-HAp/calcium silicate coating enhanced the corrosion resistance of CP-Ti, but this effect could not be attributed exclusively to the presence of Zn within the HA structure due to the presence of a secondary phase in the coating.
The optimal amount of Zn to obtain appropriate biological outcomes has been considered by different authors. Webster et al. showed that Zn amounts as small as 1.3% cause an increase in osteoblast responses.95 This high response to small amounts of Zn2+ is very convenient, as the amount of Zn2+ that can be incorporated is limited, especially when high temperature methods are used. For instance, Zn-HAp coatings prepared by solution precursor plasma spraying easily decompose in the presence of Zn, resulting in the stabilization of α-TCP.96 Zn-HAp coatings exhibit antibacterial properties in a Zn-dose dependent manner, which has been attributed to Zn2+ release to the local environment.
The presence of Zn2+ cations partially inhibits the nucleation and growth of a newly formed apatite layer, commonly occurring when HAp is in contact with SBF. This fact could be explained in terms of inhibition of the Ca2+ and PO4 3– interactions due to the presence of Zn2+ on the surface.97 Zn-HAp coatings containing 7% Zn2+ substitution have been prepared by the hydrothermal method.98 Despite this decrease of in vitro bioactive behavior, the adhesion, proliferation and spreading studies evidenced that human osteoblast cells show better responses on Zn-HAp compared to pure HAp coatings. Since the hydrothermal method involves Zn-HAp precipitation from Zn2+ containing solutions, this coating is significantly thinner than pure HAp coatings (18 μm and 100 mm for Zn-HAp and HAp, respectively). However, scratch tests showed similar critical loads for both coatings, indicating that hydrothermally fabricated Zn-HAp prepared by this method presents good adhesion to titanium surfaces.
Despite the efforts for increasing the amount of Zn within HAp structure, there is not in vivo evidence of the positive effect of Zn-HAp coatings and the effects of Zn2+ cation release in bone marrow are controversial. Sogo et al. prepared Zn substituted β-TCP/HA composites and determined with ex-vivo studies an optimal Zn content of 0.316% in weight to promote bone formation.99 Subsequently, the same group carried out in vivo studies evidencing that incorporation of Zn in β-TCP resulted in both favorable and unfavorable results.100 Certainly, this material showed enhanced bone apposition to the implant surface. However, long-term studies also evidenced increased bone resorption in the medullar cavity area. These results indicate that Zn2+ release from CaP could be only clinically applied with small amounts of Zn2+ or carefully selecting implantation sites without exposure to bone marrow.
Increasing the bonding strength between HAp coatings and the substrate has been another topic of interest in the field of substituted HAp coatings. Certainly, the phase composition and the coating morphology play a fundamental role in the bone strength magnitude, and these features are strongly dependent on the coating method, especially at the substrate–coating interface. However, several studies have evidenced that the presence of certain ionic substitutions can enhance the bonding strength for certain coating–substrate pairs. Zn substitution modifies the interfacial properties between HAp coatings and Ti substrates, mainly due to the influence of Zn2+ ions on the preferential HAp crystal growth along the [0 0 2] and [2 1 1] directions.101
Copper-Substituted hydroxyapatite (Cu-HAp) coatings. Copper is an essential trace element for most living organisms. The amount of Cu in a 70 kg adult human is about 0.15 g. Cu is a fundamental component in the catalytic site of several redox enzymes and its presence is basic for the normal development of cellular respiration, defense against free radicals, synthesis of melanin, synthesis of conjunctive tissue and iron metabolism. Cu deficiency is a serious problem especially in newborns, resulting in anemia, bone anomalies and fractures in those cases with acute deficiency. On the other hand, an excess of copper results in toxicity by accumulation in the liver and brain. In those patients with Wilson syndrome, Cu accumulation can lead to the progressive destruction of liver and nervous tissue.
Cu2+ cations have been incorporated in HAp bone grafts to provide antimicrobial and bactericidal activity,102 angiogenic potential103 and the capability to stimulate the activity of osteoblastic cells.104 Several authors have proposed a Cu2+ for Ca2+ substitution mechanism for the incorporation into the HAp structure.105,106 However, recent studies have shown that an interstitial mechanism takes place during Cu2+ incorporation. For thermal treatment below 1100 °C, Ca10Cux(-PO4)6(OH)2–2xO2x is formed with x < 0.1 and Cu2+ cations occupying the interstitial 2b Wyckoff position. Above 1100 °C, Cu rich HAp can be synthesized with the presence of Cu+ and Cu2+ also following an interstitial mechanism.107 The potential advantages of Cu-rich HAp have been questioned due to the cytotoxicity of this cation. A high content of Cu precursors can result in the formation of CuO, which seriously compromises cell viability. There are very few studies of Cu-HAp coatings, as most of the prepared Cu-HAp have been obtained as powders or pieces. Cu-HAp coatings have been prepared by plasma spraying, but this coating did not show any advantage compared to non-substituted HAp.108 More recently, Cu-HAp coatings have been prepared by solution precursor plasma spraying on sandblasted stainless-steel substrates.109 However, even after optimizing the coating parameters, impure Cu-HAp coatings were obtained mixed with CaO and CuO. No biological evaluation was carried out in this study.
In Cu-HAp coatings prepared by PLD on Ti6Al4V substrates,110 Cu2+ for Ca2+ substitution mechanisms were described, although interstitial incorporation could not be discarded. Cu2+ cations were largely incorporated in the HAp structure and introduced a high degree of crystal-chemical modifications, including a reduction of the carbonate presence in the coatings. However, these differences were not reflected in the biological behavior, since Cu-HAp elicited almost identical behavior to Zn-HAp coatings with respect to pre-osteoblast proliferation and antimicrobial activity.
Silver-substituted hydroxyapatite (Ag-HAp) coatings. Silver is a metal element absent in the human body, unless accidental contamination occurs. Ag does not play any essential role for mammals since it is not found in the active site of any enzyme or exerting structural functions in cells or tissues. However, the incorporation of Ag+ in bioceramics has been widely studied in recent years. Ag+ is an effective antimicrobial agent and its inclusion in HAp coatings is used to prevent orthopedic and dental implant infections. The antimicrobial activity of Ag+ is related to its capability to bind to microbial DNA, preventing bacterial replication, and interacting with sulfhydryl groups of the metabolic enzymes of the bacterial electron transport chain.111
Ag-HAp coatings with proved antibacterial effects have been prepared by PLD,112 co-precipitation,113 plasma spraying,113–115 magnetron sputtering116 or sol–gel methods.117 In vivo studies have demonstrated the antibacterial properties of thermally sprayed Ag-HAp coatings.118 These authors proposed a subcutaneous model in rats to test the antimicrobial activity of thermally sprayed Ag-HAp coatings on titanium discs. The coating was evaluated against methicillin-resistant S. aureus isolated from the blood of a septic patient. Although this study could not evidence the intramedullary antibacterial effects in bone, as would be desirable for an orthopedic implant, it is one of the few in vivo studies developing an infection model to assess the antimicrobial activity of substituted HAp coatings. More specifically, it could be demonstrated that Ag-HAp coatings exhibit a high bactericidal effect even with a large number of bacteria inoculated (around 106 CFU) in the subcutaneous pocket. Plasma sprayed Ag-HAp coatings prepared on titanium substrates108 have also evidenced that these coatings are highly hydrophilic, show low resorbability in vitro and exhibit excellent antibacterial effects against S. aureus.
Ag+ ions also exert a significant influence on the bonding strength. In the case of Ag-HAp on Ti substrates, the presence of Ag seems to improve the adhesion strength for electrospray deposited coatings,119 although contradictory results were obtained by Yan et al., who observed a decrease of bonding strength for Ag-HAp coatings prepared by a similar method.120
From a theoretical point of view, Ag+ (and also Zn2+ ions) positively influences the adhesion between HAp and α-Ti substrates. First-principles electronic structure calculations121 predict that the work of adhesion at the interface between the (0001) planes of HA and α-Ti reaches larger values for Zn and Ag doped HAp than for stoichiometric HA/Ti interfaces. The analysis of the electronic structure of the calculated model indicates that doping with Ag or Zn increases the charge transfer between HAp and Ti slabs, reinforcing Ti–O bonds and driving the HA/Ti interface system to be more metallic.
Magnesium-substituted hydroxyapatite (Mg-HAp) coatings. Mg is an essential element with high presence in the human body. A 70 kg adult human contains about 30 g of Mg. The biological role of Mg in vertebrates is analogous to Ca in the formation of the skeleton and stabilization of cell membranes. Regarding the enzymatic activity, Mg is an essential factor for phosphate group transference reactions and in many non-oxidative nucleic acid cleavage reactions by nucleases. Mg deficiency has negative consequences on growth as well as on mental and physical capabilities, which is a consequence of insufficient energy production due to anomalies in phosphate transference reactions.
Mg is found in natural HAp partially replacing calcium. Mg2+ ions inhibit HAp crystallization, avoiding the formation of large crystals and promoting the formation of more apatite nuclei. This activity is very important as nanocrystalline bone apatites are required for the appropriate bone formation–resorption turnover carried out by bone cells. Mg2+ deficiency affects bone growth, reduces bone density and leads to bone fragility.122 These facts have encouraged different groups to carry out the artificial preparation of Mg-HAp in different forms, including coatings, where concentrations of Mg around 1% wt have shown optimal properties.123 Mg-HAp coatings have been prepared by thin film deposition techniques such as magnetron sputtering, PLD, MAPLE, PED,124 and plasma spraying,125 as well as by electrochemical deposition methods.126,127 Mg-HAp coatings prepared by both physical deposition and electrochemical methods show a crystallinity decrease and heterogeneous distribution of the Mg content, forming Mg-rich areas. In vitro cell culture tests have evidenced that osteoblasts preferentially concentrate, attach and grow in these areas, evidencing the positive effect of Mg on bone forming cells.
Electrochemical deposition of calcium orthophosphates commonly involves hydrogen gas production, which often leads to heterogeneous coatings with large pores caused by bubbles.128 Mg2+ incorporation as a soluble salt leads to a decrease in both the pore size and pore volume in Mg-HAp coatings, with preferential Mg accumulation in the regions outside. Although these features could provide better corrosion resistance, Mg-HAp coatings prepared by electrochemical deposition showed similar parameters to those observed for non-substituted HAp.127
Strontium-substituted hydroxyapatite (Sr-HAp) coatings. Sr is considered as a non-essential element that is present in the human body. About 0.14 g of Sr is contained in an average human adult. Sr is mainly found in the mineral phase of bones, especially in those regions where bone turnover is more active.129 The incorporation of Sr into CaPs has been motivated by its inhibitory effect on bone resorption and the improvement of bone formation in osteoporotic patients.130 Several studies have demonstrated that Sr2+ for Ca2+ substitution in CaPs increases the activity of osteoblasts and inhibits osteoclast proliferation.131,132
Contrarily to Mg2+ and Zn2+, Sr2+ has a larger ionic radius than Ca2+ (112 vs. 99 pm), which leads to an enlargement of the HAp unit cell and causes an increase of the cell volume. This crystalline distortion also favors the incorporation of carbonate and HPO4 2– anions when Sr-HAp is prepared by low temperature synthesis methods.133 Sr2+ can totally substitute for calcium in the HAp structure.134
Sr-HAp coatings have been fabricated on poly(etheretherketone) (PEEK) by PED.133 Amorphous Sr doped CaP was deposited at room temperature and a subsequent annealing treatment as low as 130 °C was enough to obtain homogenous and Sr-HAp coatings with a Sr/Ca ratio very similar to the target composition. Interestingly, coatings containing high amounts of Sr2+ exhibit lower wettability than non-substituted HAp coatings. This fact has been also observed for Sr-HAp coatings prepared by plasma electrolytic oxidation (PEO). Teng et al.135 observed that Sr-HAp coatings on Ti substrates increased the wettability for a Sr2+ : Ca2+ molar ratio of 0.12, whereas Sr2+ : Ca2+ of 0.25 led to a significant decrease of wettability. The low wettability of Sr-HAp with high Sr content would provide excellent protection against corrosion for metallic substrates.136 However osteointegration strongly depends on the protein–surface interactions, which are regulated by hydrophilicity. In a recent study, Wu et al.137 evidenced the positive effect of electrodeposited Sr-HAp coatings on the stability of Mg alloys, by means of protecting them from corrosion and enhancing cell proliferation. However, for those coatings with the highest Sr content, a lower expression of osteogenic markers Col-1, Runx2 and ALP was observed. These facts indicate that the Sr-HAp coating shows an optimal substitution degree that would result in the highest HAp content, hydrophilicity and cell viability. As expected, the coating procedure strongly influences the optimal Sr/Ca ratio for in vitro cell response. In this sense Sr-HAp coatings prepared by a pre-calcification method on an anodized titanium plate exhibited the best in vitro pre-osteoblast cell response for a Sr/Ca + Sr molar ratio of 0.5.138
Roy et al. prepared Sr and Mg doped HAp coatings (1 wt% in both cases) on Cp-Ti by inductively coupled radio frequency plasma spraying.139 The coatings exhibited adhesive bond strength similar to non-doped HAp (around 17 MPa). Despite the minimal effects that Sr and Mg had on the physical properties, the presence of these substituents led to significant improvements in cell–coating interactions. Sr-HAp coatings induced better cell attachment and proliferation of human fetal osteoblasts as well as higher expression of ALP. The incorporation of Mg also resulted in biocompatible coatings, albeit it did not lead to any improvement with respect to pure HAp coatings.
Different studies carried out with highly substituted Sr-HAp point out an undeniable inhibitory effect on osteoclastogenesis, but a limited benefit to the osteoblast function, which depends on the Sr amount. A very interesting alternative has been recently proposed by Boanini et al. consisting in the fabrication of gradient coatings of calcium phosphates substituted with different cations.140 By means of Combinatorial Matrix Assisted Pulsed Laser Evaporation (C-MAPLE), Sr-HA/Zinc β-TCP coatings were prepared with a homogeneous distribution of the two phosphates. This strategy consists in combinatorial coating fabrication aimed to obtain synergies from the osteogenic properties of Zn and the osteoclasts inhibitory effects of Sr. These authors could demonstrate that the cell response can be modulated as a function of compositional intermixing of both substituted calcium phosphates. The osteoblast activity improved as a function of the Zn β-TCP content, whereas a higher presence of Sr-HAp led to the inhibition of osteoclastogenesis without affecting the osteoblast biocompatibility.
Other substituted hydroxyapatite coatings (Co2+, Na+, Mn2+, and Ce3+). In addition to the above described examples, there are some other substituted HAp coatings that are mentioned in just a few papers, so that a detailed description and discussion are not always possible. However, there are some examples including substitution with essential cations or rare earths which have shown interesting results that deserve to be mentioned.
Cobalt is an essential trace element with a very specific function in humans. Among the elements of the first transition series, Co is the least abundant in the Earth’s crust and sea water. In fact, vitamin B12 and its derivatives are the only Co containing compounds with biological activity. A 70 kg adult human contains about 0.003 g of Co, being the second least abundant essential element after molybdenum. However, several studies involving Co-doped bioceramics141–143 point out an angiogenic effect of Co2+, which could favor the neovascularization of newly formed bone by inducing hypoxia conditions. These studies have been commonly done on bulk and powder materials, but studies on Co-HAp coatings are very scarce. Co-HAp coatings have been recently prepared by electrodeposition on Ti22Nb6Zr alloy.144 Although no biological effects were evaluated, this article evidences the improvement of corrosion resistance with the incorporation of Co2+. This effect is explained in terms of the higher particle aggregation undergone by HAp particles during electrodeposition in the presence of Co2+. Consequently, the electrodeposited Co-HAp coatings are denser and less porous than the undoped HAp coating, thus providing corrosion protection to the metal alloy.
Enhanced corrosion protection has been also obtained with sodium substituted hydroxyapatite (Na-HAp) coatings prepared by electrophoretic deposition.145 In this case, the coating is made of a composite containing Na-HAp and chitosan that is deposited on 316L stainless steel previously coated with poly(O-phenylenediamine). This coating showed in vitro bioactive behavior and high corrosion resistance in SBF. However, no conclusion could be obtained regarding the presence of Na+ in the ceramic component, since the required comparison with non-substituted HA was not carried out.
Manganese is an essential trace element that shows the most potent capacity for binding to integrins.146 Mn occupies the active site of several metalloenzymes and acts as a Lewis acid (as Mn2+) catalyzing hydrolytic reactions and as a redox catalyzer when Mn exhibits high oxidation states. A 70 kg adult human contains about 0.02 g of Mn. Several studies have endeavored to use Mn to enhance the osteoconductivity of Ti substrates by means of its potent cell adhesion-promoting effect.147–149 These coatings supported a better cell response with increased viability, proliferation and ALP activity in osteo-blastic cells compared with bare Ti or untreated β-TCP film. More recently, Mn-HAp coatings have been produced by elec-trodeposition on ZnO coated stainless steel.150 This bilayer coating improved the corrosion resistance, mechanical properties, and metal ion leach-out performance as well as the in vitro bioactivity and biocompatibility. However, Mn2+ does not always provide beneficial effects when it is associated with other coatings different from calcium phosphates. Park et al. studied the effects of Mn incorporation into a titanium oxide coating on titanium.151 These authors demonstrated that Mn incorporation, instead of providing biological benefits, impaired cell behavior by decreasing cellular attachment, spreading, proliferation, ALP activity, and osteoblast phenotype gene expression compared with the bare Ti surface. This apparently contradictory observation could be explained in terms of the different kinetic release of Mn ions from the CaP coating and Ti oxide layers, which would differ because of the differences in biodegradation rate.
Yttrium substituted HAp (Y-HAp) coatings have been proposed to improve osteoblast (or bone-forming cell) function over undoped HA.152 Based on the enhancement of the mechanical properties and conductivity in different ceramics, Y was incorporated in HAp powders and deposited on titanium substrates. Greater amounts of calcium deposition by osteo-blasts cultured on Y-HAp coatings were observed compared with undoped plasma-sprayed HA coatings.
HAp doped with rare earths has gained interest in recent years. For instance, Ce3+ or Ce4+ substitutions in HAp have shown antimicrobial activity.153 Recently, cerium-doped hydro-xyapatite (Ce-HAp)/collagen coatings have been prepared by means of a biomimetic process on Ti substrates.154 In this case, Ce4+ was added as Ce(SO4)2·4H2O with a final Ce4+ concentration as low as 0.5% in a supersaturated solution of CaCl2 and NaH2PO4, thus maintaining a cerium concentration within the therapeutic range.155 The Ce-HAp coatings so obtained showed 92.61 and 73.59% bactericidal ratios for E. coli and S. aureus, respectively, indicating a higher efficiency against Gram-negative bacterial strains. No cytotoxicity studies were carried out so the information about their potential application in biological systems is very restricted. Table 1 summarizes the most relevant studies carried out on cation-substituted HAp coatings and the enhanced biological function observed in vitro.
Table 1.
Cation-substituted HAp coatings and the enhanced biological function observed with in vitro studies
| Coating | Function | Substrate | Fabrication method | Ref. |
|---|---|---|---|---|
| Zn-HAp | Osteoblast function | Titanium, Ti6Al4V | Plasma spraying | 89 and 96 |
| Corrosion resistance | Ti-Coated silicone | Electrophoretic deposition | 90 | |
| Bactericidal | Silicon | Sol–gel spin coating | 91 | |
| Titanium; silicon | Magnetron sputtering | 92 and 93 | ||
| Titanium | Electrochemical deposition | 94 | ||
| Titanium | Hydrothermal coating | 98 | ||
| Ti6Al4V | Pulsed laser deposition | 110 | ||
| Cu-HAp | Bactericidal | Titanium | Plasma spraying | 108 |
| Angiogenesis | Stainless-steel | Plasma spraying | 109 | |
| Osteoblast function | Ti6Al4V | Pulsed laser deposition | 110 | |
| Ag-HAp | Bactericidal | Titanium, Ti6Al4V | Plasma spraying | 108 and 113–115 |
| Adhesion strength | Titanium | Electrospraying | 119 | |
| Mg-Hap | Osteoblast function | Titanium | Plasma spraying | 125 |
| Ti6Al4V | Electrochemical deposition | 126 | ||
| Sr-HAp | Osteoblast function | PEEK | Pulse electron deposition | 133 |
| Osteoclast inhibition | Titanium | Plasma electrolytic oxidation | 135 | |
| Corrosion resistance | Magnesium alloy | Hydrothermal, electrodeposition | 136 and 137 | |
| Titanium | Radio frequency plasma spraying | 139 | ||
| Co-HAp | Angiogenesis Corrosion resistance | Ti22Nb6Zr | Electrodeposition | 144 |
| Na-HAp | Corrosion resistance | 316L stainless steel | Electrophoretic deposition | 145 |
| Mn-HAp | Mechanical properties | Titanium | Pulsed laser deposition | 147–149 |
| Corrosion resistance | Stainless steel | Electrodeposition | 150 | |
| Y-HAp | Osteoblast function | Titanium | IonTitet | 152 |
| Ce-HAp | Bactericidal | Titanium | Biomimetic | 154 |
4.2. Anionic substitutions in hydroxyapatite coatings
Silicon-substituted hydroxyapatite (Si-HAp) coatings. Silicon is an essential trace element for most living organisms. A human adult of 70 kg contains about 1.4 g of silicon. The unique soluble Si form in physiological conditions is silicic acid, Si(OH)4, which can be dissolved up to 2 mM at pH = 7. The essentiality of Si for animal life was established by Carlisle157 by means of the determination of deficiency symptoms in rats and chickens fed with silicon depleted diets,156 demonstrating that Si deficiency led to serious effects on bone growth and defective formation of connective tissue. Silicon or silicates substitute for phosphorus, or phosphates, with subsequent charge imbalance.158 Although the amount of Si that can be incorporated into HAp ranges between 0.1 and 0.5 wt%,159–162 Si-HAps have evidenced higher bioactive behavior compared to non-substituted apatites. 163–167
Si-HAp coatings have been prepared by magnetron sputtering.168 Since the Si substitution is very limited, HAp and Si were sputtered from different targets instead of a single Si-HAp one, yielding a layer of Si-HAp of 0.7 μm in thickness and with a Si content of approximately 0.8 wt%. In vitro cell culture studies showed that Si-HAp thin coatings exhibited high bioactivity and biofunctionality. Attachment and growth of human osteoblast-like (HOB) cells was observed during the culture period, with more formation of extracellular matrix compared to the uncoated titanium substrate, although no comparison was established with pure HAp coatings. The same research group have carried out the preparation of Si-HAp coatings comparing the influence of the Si content: 0.8 wt%, 2.2 wt%, and 4.9 wt% on Ti to evaluate the long-term in vitro biocompatibility effects of these coatings in vitro.169,170 HOB cells showed improved adhesion on the coated surfaces with increasing Si content and developed mature cytoskeletons with well-defined actin stress fibers in the cell membranes. However, the reactivity provided by Si in the Si-HAp with the highest substitution resulted in fast dissolution that hindered the initial cell attachment, concluding that a Si content of 2.2 wt% would be the best substitution degree to improve the bioactive behavior of HAp thin films.
Surmeneva et al. have widely studied the microstructural characteristics of Si-HAp prepared by magnetron sputtering.171–173 The microstructure and coating composition can be controlled by changing the bias voltage from 0 V to –50 and –100 V.172 For instance, the coating thickness decreases with the magnitude of negative bias, whereas the Ca/P and Ca/P + Si ratios increase. Anyway, all the coatings exhibited good biocompatibility with respect to MG-63 osteoblast cells. Silicon incorporation also exerts an influence on the mechanical properties of magnetron-sputtered Si-HAp.173 The nanohardness and elastic modulus decrease as a function of Si content due to microstructural modifications derived from silicon incorporation. In addition, the adhesion behavior is also influenced by Si substitution. Whereas coating failure occurred due to low cohesion in non-substituted HAp coatings, Si-HAp with 1.2 at% deformed plastically without crack formation and a mixed elastic–plastic behavior was observed for the Si-HAp coating with 4.6 atom% of Si.
Si-HAp coatings have been also successfully prepared by PLD,174 sol–gel chemistry followed by dip coating,175 spin coating,176 electrochemical deposition using electrolytes containing Na2SiO3 as a silicon source177,178 and by means of hydrothermal treatment with metasilicic acid, H2SiO3, of a previously pure HA coating prepared by CVD methods,179 being one of the most widely investigated substituted HAp coatings.
Fluor-substituted hydroxyapatite (F-HAp) coatings. Fluor is an essential trace element with a presence of 2.6 g in a 70 kg adult human. Although essential, there is not much knowledge about the biological functions of F– and its biochemical activity is not well defined. Most of the F– content is in the skeleton and teeth where it isoelectronically substitutes for OH– in HAp. F– anions are also found in extracellular fluids at very low concentration (micromolar levels) and even lower in the intra-cellular compartment.
The incorporation of F– into the HAp structure reduces the dissolution rate and increases its hardness.180 Certainly, partial substitution of F– for OH– ions, which results in Ca10(PO4)6(OH)2–xFx, is a well-known strategy for enhancing the structural stability and resistance to dissolution with respect to non-substituted HAp. The lower solubility of F-HAp allows the preparation of very thin coatings (below 1 micrometer) that otherwise would be very unstable under in vivo conditions, and would result in implant failure by early degradation. F-HAp thin films have been recently prepared by PLD181 producing coatings of 1 μm in thickness, with higher resistance to dissolution and better cell attachment of human mesenchymal stem cells (HMSCs) compared to HAp coatings.
In vitro studies indicate that the F– for OH– substitution enhances cell proliferation and reduces bacterial activity.182 Antibacterial properties of F-HAp coatings on stainless steel substrates have been proposed for the prevention and treatment of peri-implantitis in dental implants.183 The antibacterial property against different pathogens was dependent on the coating crystallinity, which could be controlled with a post-hydrothermal treatment. In this sense both low crystalline and highly ordered crystalline F-HAp coatings reduced the bacterial viability, although the ordered one also reduced the bacterial adhesion. This study concludes that F– content, rather than release, is the most influential variable, although the surface characteristics also have a significant impact on the bacterial adhesion, as it has been recently demonstrated for nanopatterned Ti coatings on orthopedic devices.184
F-HAps coatings have been prepared by different methods including sol-gel185 or slip coating methods.186 There is some controversy regarding the positive or negative biological activity of fluoride with respect to osteoblast cells. Some studies have shown that osteoblast cells exhibit lower proliferation rates on fluoridated coatings compared with cells cultured on non-substituted HAp coatings, pointing out the potential toxicity of F– anions.187 However, the beneficial or detrimental effects of F– in HAp coatings seem to be dosage-dependent. For instance, electrodeposition can be used for preparing these coatings by adding NaF into the electrolyte.188 This method is appropriate to obtain coatings with a thickness of a few microns (around 5 micrometers) and F/Ca ratios as high as 0.125, i.e a nominal composition of Ca10(PO4)6(OH)0.75F1.25. However, the best bonding strength on titanium substrates, lower dissolution rate and most appropriate biological activity were obtained for those coatings with moderate F contents, with substitution for OH in the range Ca10(PO4)6(OH)0.75–1F1.25–1.
Carbonate-substituted hydroxyapatite (C-HAp) coatings. C-HAp has been synthesized as powders, grains, pieces and coatings for bone grafting applications. The interest in C-HAp arises from the chemical composition of biological apatites which are non-stoichiometric, Ca-deficient and carbonated. This ionic substitution contributes to the higher solubility of biological apatites, compared with the stoichiometric and highly crystalline synthetic ones. The presence of CO3 2– in the HAp structure helps to keep constant bone regeneration through dissolution–crystallization cycles.
C-HAp coatings have been prepared by RF-magnetron sputtering,189,190 electrophoretic deposition191–193 biomimetic deposition,194,195 electrochemical deposition,196 pulsed laser deposition,197 hydrothermal crystallization198 and even by a hybrid process of plasma spraying and hydrothermal synthesis.199 Since C-HAp coatings are more soluble than HAp, they are likely to provide a better osteogenic response, although the coating stability can be seriously compromised. Different strategies have been used to overcome this potential drawback. For instance, C-HAp coatings deposited on Ti substrates by RF-magnetron sputtering200,201 have been obtained as dense and well adhered films with controlled elemental composition. B-type C-HAp coatings have been fabricated by this method with a rough and homogeneous microstructure that facilitates the development of hMSC, differentiated cells and bone explanted osteoblasts. The coating adherence was improved by introducing a buffer layer of CHA1–xTix (x = 0–1) with a chemical gradient between the Ti substrate and the Ca10–2x/3(PO4)6–x(CO3)x(OH)2–x/3 coating. Another strategy for enhancing the stability of C-HAp coatings is the formation of composites with biocompatible polymers. Tang et al. prepared chitosan/C-HAp on Ti6Al4V substrates by electrophoretic deposition of CaCO3 and subsequent treatment in a phosphate buffer solution until transformation into C-HAp.192 However, the coating exhibited numerous cracks and macropores between C-HAp particles. This drawback was resolved by soaking the sample in a chitosan solution, which filled the cracks (linking the C-HAp particles) while keeping the pores, which could positively contribute to osteointegration after implantation.
The biomimetic method is one of the easiest alternatives for the preparation of C-HAp coatings. By soaking preconditioned substrates in highly saturated SBF, C-HAp spontaneously nucleates and grows on the surface, with disregard of the substrate morphology, as biomimetic deposition is not a line-of-sight technique. Moreover, the coating roughness can be easily controlled by the solution concentration and time exposure of the substrate to the biomimetic solution. Costa et al.194 prepared C-HAp coatings biomimetically deposited on polycapro-lactone discs from SBFx7 and SBFx10 during periods of 24 and 48 hours. Whereas SBFx7 led to almost smooth C-HAp coatings, SBFx10 led to micro-rough topographies. All the coatings were biocompatible with respect to osteoblasts but, interestingly, different topographies elicited different responses with respect to osteoclast cells: smooth coatings allowed high osteoclast resorptive activity whereas micro-rough coatings partially hindered the formation of actine rings. In this way, the biomimetic technique would produce C-HAp coatings that improve osteo-conductivity while minimizing osteoclastic resorption.
Other anion-substituted hydroxyapatite coatings. Since the HAp structure allows for many different substitutions, some groups have explored other possibilities with less conventional anions than those reviewed in previous sections. Boron is considered an essential element for plants, but not for animals. Although some studies point out several benefits for osteogenic differentiation202 further research is required before B is accepted as an essential nutrient for humans. B is incorporated as borate ions by substitution of phosphate. B-HAp coatings have been fabricated on chitosan scaffolds203 and MC3T3-E1 cell cultures evidenced enhanced proliferation associated with B release, indicating the potential of these coatings for in vitro bone tissue engineering applications.
Selenium is a trace essential element for humans. Around 15 mg of Se is found in an average human. Selenium-doped hydroxyapatite (Se-HAp) coatings have been prepared by several methods such as PLD204 or biomimetic techniques including selenite ions in SBF.200 Rodríguez-Valencia et al.205 have hypothesized on the osteogenic, antitumoral and antibiotic activity of Se incorporated as SeO3 2– anions into the HAp structure. Coatings with 2.7 at% of Se resulted in significant osteogenic activity of MC3T3-E1 pre-osteoblasts, a significant antiproliferative effect on cancerous osteoblasts (MG63), and antibiofilm properties against S. epidermidis and S. aureus bacterial strains (Table 2).
Table 2.
Anion-substituted HAp coatings and the enhanced biological functions observed with in vitro studies
| Coating | Function | Substrate | Fabrication method | Ref. |
|---|---|---|---|---|
| Si-HAp | Osteoblast function | Titanium | Magnetron sputtering | 168–173 |
| Osteoinduction | Titanium | Pulsed laser deposition | 174 | |
| Mechanical properties | Ti6Al4V | Dip coating | 175 | |
| Bonding strength | Zirconia | Spin coating | 176 | |
| Mg5Zn0.3Ca, SiC | Electrochemical deposition | 177 and 178 | ||
| C/C composite | CLVD/hydrothermal | 179 | ||
| F-HAp | Structural stability | Titanium | Pulsed laser deposition | 181 |
| Osteoblast attachment | Stainless steel | Hydrothermal method | 183 | |
| Stainless steel | Sol–gel | 185 | ||
| Zirconia | Slip coating | 186 | ||
| Titanium | Electrochemical deposition | 188 | ||
| C-HAp | Osteoblast function | Titanium | RF-Magnetron sputtering | 189, 190, 200 and 201 |
| Ti6Al4V | Electrophoretic deposition | 191–193 | ||
| Polycaprolactone, Ti | Biomimetic deposition | 194 and 195 | ||
| TiO2 nanotubes | Electrochemical deposition | 196 | ||
| Titanium | Hydrothermal method | 198 | ||
| Ti6Al4V | Plasma spraying/hydrothermal | 199 | ||
| B-HAp | Osteoblast function | Chitosan | Microwave assisted precipitation | 203 |
| Se-Hap | Osteoblast function | Titanium | Pulsed laser deposition | 204 |
| Antitumoral | Ti6Al4V | Biomimetic | 205 | |
| Bactericidal |
4.3. Co-substituted hydroxyapatite coatings
Co-substitution is a very interesting strategy to optimize the biological performance of Hap coatings. Taking advantage of the different ions available for substitution and the variety of biological effects, the presence of two or more substituents can lead to synergistic, complementary or compensatory effects that provide added value to Hap coatings. Combination of cations with antimicrobial and osteogenic properties is one of the most attractive options. The antimicrobial efficiency of Ag+ is explained in terms of inhibition of the bacterial replication process against both Gram-negative and Gram-positive bacteria.206 However, Ag+ cations also exhibit toxicity to human cells above a certain concentration, even if they are incorporated in Hap coatings.207 This toxicity seems to be related to the affinity of Ag+ to the ALP high affinity metal site and the incorporation of a second cation able to alleviate the potential negative effect of Ag+ has been considered. Geng et al. evaluated the antibacterial effect and biocompatibility of Ag/Sr-Hap hydrothermally coated on Ti substrates.208,209 The addition of Sr2+ significantly decreases the Ag+ toxicity, in such a way that co-substituted coatings keep the antibacterial effect with low silver substitution, while maintaining the proliferation capability of pre-osteoblast cells. Sr2+ ions seem to counteract the silver toxicity by reducing the quantity of Ag+ that gets into the cells by competing for binding sites of specific cellular function and promoting cell differentiation.
The antibacterial effects of Cu2+ can be also limited due to the potential toxicity of this cation. Due to the small amount of Cu2+ that can be incorporated and the risk of segregation of cytotoxic phases,210 more attention has been paid to Cu containing co-substituted Hap coatings. For instance, co-substitution with Zn2+ provides much better results than single Cu2+ incorporation into Hap coatings. Cu/Zn-Hap coatings prepared by electrodeposition on pure titanium substrates exhibit antimicrobial activity associated with Cu2+ cations, whereas Zn2+ makes up for the cytotoxicity of Cu2+.211 Zn and Cu incorporation also led to an increase of corrosion resistance compared to non-substituted Hap coatings. This could be associated with the reduced grain size of Cu/Zn-Hap coatings, which plays an important role in elevating the electron activity at the grain boundaries and consequently improving the corrosion protection. Certainly, these coatings exhibited antimicrobial activity against E. coli, but the study could not clearly assign this effect to Cu2+ or to the concomitant effect of Zn2+ release.
Sr has been also proposed as a secondary substituent to alleviate the potential toxicity of Cu-Hap coatings.212 Cu/Sr-Hap coatings have been prepared by electrodepositon on CP-Ti and the antibacterial activity and cytocompatibility have been evaluated. The lattice parameters of Cu/Sr-Hap were enlarged with respect to pure Hap, evidencing that Sr2+, with a larger ionic radius than Ca2+, plays a major role in the crystal changes occurring in Hap. The coatings exhibited a bactericidal effect against E. coli (near to 91% inactivation), although the antimicrobial ratio did not reach the 99% inactivation required to be considered as antibacterial. Besides, the Cu/Sr-Hap coating increases the number of viable preosteoblast seeds compared to CP-Ti and stimulated the differentiation towards the osteoblast phenotype.
Mg/Sr-Hap coatings have been prepared by plasma spraying.213 This coating exhibited high bonding strength after subsequent thermal treatment at 500 1C and induced MC3T3-E1 preosteblast proliferation. Whereas Mg/Sr-Hap coatings prepared by plasma spraying do not show microstructural differences with respect to undoped HA, other attempts to prepare Mg/Sr-HAP by deposition methods yielded different outcomes. Mg2+ cations influence the microstructure of hydro-xyapatite coatings, when the fabrication method involves nucleation and crystallization from liquid solutions. For instance, Mg/Sr-Hap coatings prepared by electrochemical deposition indicate that the presence of Mg as a co-dopant led to less crystalline and irregular coatings, in agreement with the inhibitory effect of Hap crystallization attributed to Mg2+.214 This fact would question the convenience of incorporating Mg2+ in Hap when using wet route deposition-based techniques.
Substitution of Hap with rare earth elements has been a field of interest in recent decades, mainly due to the potential capability of Hap to retain radionuclides and be used for storage of nuclear waste.215 Recently, the use of substituted Hap with rare earths for biomedical purposes has been investigated and has opened new alternatives in the field of bone implants. For instance, samarium (Sm) substitution in Hap has demonstrated antibacterial activity, improvement of the osteoblast performance and also activity as a radiotherapeutic agent in preventing caries.216,217 On the other hand, certain rare earth cations such as Gd3+ improve the mechanical properties and corrosion resistance in metal alloys.218 Considering these antecedents, Sm/Gd-Hap coatings have been prepared by electro-deposition on stainless steel substrates.219 Different Sm/Gd ratios were incorporated in the Hap structure. Sm/Gd-Hap coatings containing a 1 : 1 Sm/Gd ratio exhibited an interconnected granular structure with uniform coverage, whereas 2 : 1 and 1 : 2 Sm/Gd ratios resulted in heterogeneous coatings made of agglomerated particles. The main consequence is that Sm/Gd-Hap coatings with similar contents of rare earths showed much better protection against corrosion. Besides, Sm provided antibacterial properties against E. coli and S. aureus together with high cytocompatibility with respect to MC3T3-E1 preosteoblastic cells.
Co-substituted Hap coatings have been also prepared by incorporating cations and anions simultaneously. These coatings are mainly prepared by introducing metal cations like Co2+, 220 Zn2+ 221 or Ag+ 222 in F-Hap, because F– ions exert a dose-dependent effect on osteoblast proliferation and osteogenic differentiation223 that compensates the toxic effects of transition metal cations. Birgani et al.220 incorporated F– and Co2+ ions in CaPs prepared by a biomimetic method on the surface of culture well plates. Although the crystalline phase formed seemed to correspond to OCP, the incorporation of F– rendered this CaP more apatitic. The incorporation of Co2+ into the CaP coating upregulates the expression of VEGF and CD31 angiogenic markers of hMSCs compared to CaP without Co2+. However, Co2+ suppresses the ALP activity and decreases the expression of bone sialoprotein (BSP), which results in reduced mineralization of hMSC. The incorporation of fluoride compensated the adverse effects of Co2+, favoring osteogenesis while keeping the angiogenic effect.
Zn/F-Hap coatings have been successfully fabricated by ED on cp-Ti, obtaining totally crack-free and dense layers, which led to a decrease in the corrosion current densities of Ti-cp in physiological solutions.221 Since ED is a low temperature process it allowed for obtaining nanostructured coatings. The nanopatterned surface together with the continuous Zn2+ release results in good osteoblast proliferation, and promoted ALP expression in MC3T3-E1 preosteoblast cells.
The incorporation of bactericidal species into F-Hap also provides interesting cationicanionic combinations. Ag+ has been incorporated into fluorapatite to fabricate hybrid coatings with TiO2 nanotubes on Ti substrates.222 The Ag/F-Hap coatings form rod shaped nanoparticles that get into the voids of TiO2 nanotubes, thus increasing the adhesion strength of the coatings. Fluoride incorporation also increases the dissolution resistance whereas the presence of Ag+ killed all viable S. aureus in the antibacterial tests. In addition, the hybrid coating increased the corrosion resistance by two orders of magnitude and showed high cell viability when MC3T3-E1 preosteoblasts were cultured on it.
Co-substitution can only occur to a limited extent as it causes crystal-chemical disorders that result in apatite decomposition and segregation of other phases. Research interest has been focused on preparing multimaterial coatings made of singly substituted Haps. Using production techniques that provide accurate control of the deposition pattern such as drop on demand microdispensing, the distribution of singly substituted HAPs can be designed in a homogeneous or controlled manner.224 Multimaterial coatings have been prepared by Lim et al.225 by means of depositing Si-Hap and Ag-Hap on glass substrates previously coated with a Hap layer. Interestingly the homogeneous distribution of Ag-Hap at every alternate position in the Si-Hap/Ag-Hap coating prevented S. aureus adhesion to the same extent as the pure Ag-Hap coating, even though 50% of Ag-Hap was replaced by Si-Hap. This result evidences the importance of the homogeneity and pattern deposition of Ag+ in the coating, although the total elimination of bacteria was not achieved in any case. On the other hand, the osteogenic response of the Si-Hap/Ag-Hap coating was significantly lower than the pure Si-Hap coating, indicating that the stimulatory effect of silicon is mainly dose-dependent (Table 3).
Table 3.
Co-substituted Hap coatings
| Coating | Function | Substrate | Fabrication method | Ref. |
|---|---|---|---|---|
| Ag/Sr-HAp | Antibacterial/osteoblast function | Titanium | Hydrothermal method | 208 and 209 |
| Cu/Zn-HAp | Antibacterial/biocompatibility/corrosion resistance | Titanium | Electrodeposition | 211 |
| Cu/Sr-HAp | Antibacterial/osteoblast function | Titanium | Electrodeposition | 212 |
| Mg/Sr-HAp | Mechanical properties/osteoblast function | Ti6Al4V | Plasma spraying | 213 |
| Ti40Nb | Electrochemical deposition | 214 | ||
| Sm/Gd-HAp | Antibacterial/corrosion resistance | Stainless steal | Electrodeposition | 219 |
| Co/F-HAp | Angiogenesis/osteogenesis | Culture plates | Biomimetic method | 220 |
| Zn/F-HAp | Corrosion resistance/osteogenesis | Titanium | Electrodeposition | 221 |
| Ag/F-HAp | Coating stability/adhesion strength/antibacterial | Titanium | Electrodeposition | 222 |
| Si-HAp/Ag-HAp | Osteogenesis/antibacterial | Glass | Drop on demand microdispensing | 225 |
5. In vivo studies of substituted hydroxyapatite coatings
In vivo animal models have been used to determine fundamental features of substituted HAp coatings such as bone regeneration at the peri-implant site, bone adhesion, angiogenesis, etc., which allows for determining the potential advantages provided by the ions incorporated in HAp. Table 4 shows the different ionic substitutions incorporated into HAp coatings, together with the expected biological outcomes and the animal models that they have been tested with. Zhao et al. studied the effects of Mg in Mg-HAp coatings on the osseointegration of dental implants.226 These authors observed enhanced in vitro cell proliferation, higher ALP activity and more osteocalcin production compared to pure HAp. However, only temporary effects could be identified in vivo. A slightly higher bone implant contact (BIC) for the Mg-HAp coatings was observed 2 weeks after implantation, whereas no significant differences were observed with respect to BIC or the amount of bone between threads after 4 and 8 weeks.
Table 4.
In vivo studies carried out with substituted HAp coatings
| Coating | Substrate | Fabrication method | In vivo animal model | Ref. |
|---|---|---|---|---|
| Ag-HAp | Titanium | Thermal spraying | Rat, subcutaneous | 117 |
| Mg-HAp | Ti, Ti6Al4V | Electrochemical deposition | Rabbit, bone | 226 |
| Ti6Al4V | Pulsed laser deposition | Rabbit, bone | 245 | |
| Titanium | Plasma spraying | Rat, bone | 227 | |
| Sr-HAp | Titanium | Electrochemical deposition | Rat, bone | 234 |
| Titanium (oxidized) | Biomimetic method | 235 | ||
| Si-HAp | Titanium (oxidized) | Biomimetic method | Rat, bone | 235 |
| Porous titanium | Biomimetic method | Rabbit, bone | 242 | |
| Porous titanium | Precipitation method | Rabbit, bone | 243 | |
| Porous Ti6l4V | Dip coating | Sheep, bone | 244 | |
| F-HAp | Ti6Al4V | Plasma spraying | Goat, bone | 230 and 231 |
| Ti6Al4V | Plasma spraying | Rabbit, bone | 232 | |
| Titanium | Plasma spraying | Goat, bone | 233 | |
| C-HAp | Ti6Al4V | Biomimetic method | Rat, subcutaneous | 229 |
| Sr/SiHAp | Porous titanium | Biomimetic method | Rat, bone | 236 |
| Zn/Si/Ag-HAp | Ti, Ti6Al4V | Plasma spraying | Rat, bone | 228 |
Ke et al. incorporated MgO and SiO2 within plasma sprayed hydroxyapatite coatings on titanium implants.227 The MgO/SiO2-HAp coatings exhibited increased osteogenesis, osteointegration and bone mineralization compared with non-substituted HAp after implantation in rats’ femurs. More interestingly, the pushout tests evidenced that the MgO/SiO2-HAp coatings exhibited a shear modulus much higher than uncoated and HAp coated implants (96% and 56.4%, respectively), demonstrating the better quality of the bone–implant interface for these coatings in a quantitative way. More recently, the same group coated Ti and Ti6Al4V implants with a ternary dopant system within HAp.228 This system was aimed at inducing osteogenesis, angiogenesis and infection control by means of ZnO, SiO2 and Ag2O incorporation, respectively. For this study, these authors used the same rat femur model, evidencing that the Zn/Si/Ag-HAp coating led to higher bone formation at the early stage as well as more mineralization compared with non-substituted HAp coatings. The Zn/Si/Ag-HAp coatings resulted in better osteointegration with higher shear modulus during the push out tests, although no evidence of angiogenesis or antibacterial properties was demonstrated in vivo.
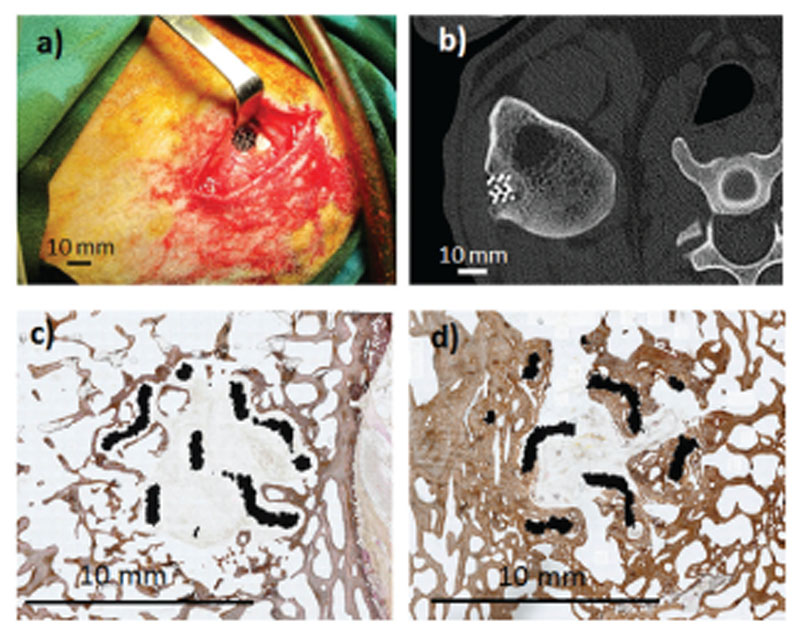
Early degradation of substituted HAp coatings prepared by low temperature methods is one of the most serious concerns for transferring these devices to clinical applications. This is the case of C-HAp coatings prepared by biomimetic methods. Measuring the coating degradation within bone involves certain difficulties that can be partially overcome using ectopic models. For instance, Barrère et al.229 implanted Ti6Al4V pieces biomimetically coated with C-HAp, using a subcutaneous rat model to determine the coating degradation and the biological behavior derived from the solubility of the coatings. Interestingly a dissolution–precipitation mechanism took place under in vitro conditions with immersion time in α-MEM. However, in vivo studies showed that no dissolution occurred of the CHAp coating. On the contrary, coating calcification could be observed on these implants.
F-HAp coatings can be considered as an alternative to HAp for avoiding early coating degradation. For instance, plasma sprayed F-HAp coatings on Ti6Al4V do not degrade after 12 and 25 weeks of being implanted into the femora and humeri of adult goats, whereas HAp coatings showed extensive degradation for the same period.230 Other studies confirmed these observations by evidencing an almost equal degree of bone apposition for plasma sprayed HAp and F-HAp coatings, but less F-HAp coating dissolution within the first 3 months of implantation.231,232 Studies on a goat maxilla model obtained similar results, as no significant differences were found in the histomorphometrical analysis between F-HAp and HAp coated implants, although higher coating thickness reduction was observed for pure HAp coatings.233
Osteoporotic animal models are very convienient for the evaluation of substituted HAp coatings aimed at treating patients having bones of low quality. Ovariectomized animal models are often used for these studies since they mimic the osteoporosis conditions in humans. Sr-HAp coatings have been proposed for application in osteoporotic conditions and implanted in the femur of ovariectomized rats.234 Different amounts of Sr were incorporated in HAp, but the Sr-HAp coatings with the highest Sr content led to the highest bone formation. Biomechanical tests demonstrated the beneficial effects of coatings with 20 mol% of Sr substitution on implant fixation with respect to 5%, 10% and 0% Sr-HAp coatings, evidencing the positive in vivo effect of this element under osteoporotic conditions.
Sr-HAp and Si-HAp coatings have been also deposited by using biomimetic methods on screw-shaped implants and placed in the tibia metaphysis of rats.235 The association of small amounts of Si and Sr with these coatings improves the bioactive behavior, especially at the very early stages after implantation. Si seems to stimulate bone apposition, whereas Sr fosters bone formation in the area within the threads. These positive effects of Sr and Si cosubstitution in CaP coatings have been recently confirmed by Bose et al.236 This group prepared Si–Sr CaP coatings on Ti cylinders previously coated with TiO2 nanotubes by electrochemical anodization, a technique previously developed by the same group.237 In agreement with previous work, these researchers could demonstrate that Sr and Si substitution in CaP coatings led to an increase of osteoid formation around the implant in the early stages (4 weeks) after implantation in the distal femur of rats.
6. In vivo studies of scaffolds coated with substituted-HAp
Traditionally, HAp based coatings have been aimed at improving the bone adhesion of solid implantable devices (prosthesis stems, dental implants, fixation screws, etc.) designed for bone substitution or fixation applications. An alternative to the current substitutive strategies in the treatment of bone defects is the concept of functionalized metallic macroporous scaffolds.238 These scaffolds must facilitate osteogenesis and new blood vessel formation within their macroporous structure, while exhibiting optimal mechanical behavior. Both aspects are mandatory for bone regeneration of critical defects, particularly in osteoporotic bones, where the implant integration with the hosting bone is seriously affected due to the low bone formation rate in the peri-implant region.239 The surface functionalization of these metal structures with a highly bioactive bioceramic has emerged as a very interesting alternative.240,241
The preparation of Si-HAp coatings by low temperature methods and their in vivo evaluation have been carried out by several research groups.242,243 For this aim Ti or Ti alloy macroporous scaffolds have been often chosen as substrates. Zhang et al.242 proposed Ti scaffolds prepared by fiber sintering coated with Si-HAp by a biomimetic method. The scaffolds exhibited a porosity of 67% with a pore size of 150–600 mm. This study compared uncoated Ti scaffolds with pure HAp coated Ti and Si-HAp coated scaffolds when implanted in New Zealand rabbits. Both the HAp and Si-HAp coatings led to a significant increase of the bone ingrowth rate compared to uncoated Ti, evidencing that both coatings enhanced the osteoconductive properties. Moreover, the Si-HAp coated scaffolds exhibited significantly higher bone ingrowth than the HAp coated scaffolds. After four weeks, 90% of the pore area was covered by new bone tissue, evidencing the positive role of Si substitution in this kind of coatings.
Recently, Si-HAp coatings prepared by a sol–gel route on Ti6Al4V macroporous scaffolds have demonstrated a synergistic effect when they are associated with vascular endothelial growth factor (VEGF) in osteoporotic sheep244 (Fig. 2). Ti6Al4V macroporous structures were fabricated by electron beam melting to obtain customized highly macroporous structures, which were coated with Si-HAp by the dip coating method. Subsequently, VEGF was immobilized on the coatings by soaking the specimens in a solution of VEGF in a phosphate buffered solution. In vitro studies demonstrated that the SiHAp coatings stimulated the proliferation of MC3T3-E1 pre-osteoblastic cells, whereas the adsorption of VEGF stimulates the proliferation of EC2 mature endothelial cells. When these scaffolds were implanted in osteoporotic sheep, only the simultaneous presence of the SiHAp coating and VEGF led to a significant increase of new tissue formation in osteoporotic bone, demonstrating that, under osteoporotic conditions, both osteogenesis and vascularization must be strengthened to reach bone regeneration within these metallic scaffolds.
Fig. 2.
Implantation of a macroporous Ti6Al4V scaffold coated with Si-HAp/VEGF in an osteoporotic sheep model (a). Computed tomography scan image of the implant within the bone defect (b). Histological overview of bones implanted with uncoated Ti6Al4V microporous implants (c) and SiHAp/VEGF coated implants (d).
Mg-HAp coatings on porous implants also enhance both early and long-term osteogenesis. Mróz et al.245 have prepared Mg-HAp and Mg-substituted octacalcium phosphate coatings by PLD on porous titanium-based implants. When these porous scaffolds were implanted in a rabbit femoral model for 6 months, the histopathological analysis revealed that all the implants, the coated ones and the uncoated reference, were biocompatible, exhibiting bone ingrowth within the pores. However, microCT analysis evidenced a significantly higher bone volume for implants coated with Mg-HAp compared to the uncoated and Mg-OCP coatings. Unfortunately, this work did not include a group of pure HAp coated implants to establish the positive role of Mg at long-term implantation stages.
7. Summary and future perspectives
Substituted HAp coatings provide a very interesting alternative to improve the performance of dental and orthopedic implants. The different ionic substitutions allowed by the HAp structure, together with the variety of fabrication methods, provide new characteristics to the commercially available plasma sprayed CaP coatings. For instance, Si-HAp coatings deposited on porous metallic implants have shown excellent bone regeneration capabilities through the synergy of osteoconductive HAp with the osteoinductive behavior of the soluble silica species released from the coatings. Other ionic substituents like Sr2+, Mg2+ or Zn2+ stimulate bone healing and are expected to provide relevant advances for the treatment of osteoporotic fractures.
Infection is also a serious cause of orthopedic implant failure. Thousands of metallic orthopedic prostheses are revised every year because of infections caused by S. aureus and S. epidermidis. In this sense, the incorporation of ions with antimicrobial properties to lower the risk of infection has become a priority research field for the development of new coatings. Substitutions with Ag+ and Cu2+ have evidenced positive in vitro results with bacterial strains. However, in vivo studies are very scarce, perhaps because of the difficulty in designing appropriate in vivo infection models in bone. The development of these models will be mandatory for the further development of these devices. Other biological activities such as angiogenesis or antitumoral properties can be obtained by the incorporation of Co2+ or SeO3 2–, although much more in vitro and in vivo evidence is required before being translated into clinical applications.
The association of drugs and osteogenic macromolecules with substituted HAp coatings has opened new possibilities for the treatment of bone pathologies. Some attempts have been carried out involving the antiosteoporotic activity of Sr2+ in combination with zoledronate acid246 or providing complementary therapeutic effects with the antibiotic activity of vancomycin.247 Substituted HAp coatings combined with biological entities provide great potential to improve new bone formation at the peri-implant site or bone regeneration in macroporous scaffolds. The combination of Mg substituted β-TCP and C-HAp with recombinant human bone morphogenetic protein-2 (rhBMP-2) has shown excellent behavior with respect to rhBMP-2 delivery, which resulted in superior bone formation within scaffolds.248 The increasing knowledge about the synergies of substituted HAp with proteins,249 growth factors,244 or microRNAs250 will eventually result in customized coatings for each specific clinical challenge.
The incorporation of rapid prototyping methods as a new strategy for coating fabrication is called to play a fundamental role in this field. Techniques such as drop on demand micro-dispensing allow coating customization, distributing at the micrometer scale different substituted HAp. These techniques also provide new tools for incorporation and controlled release of drugs from the coating to the local environment. However, all these possibilities will be carried out successfully only if appropriate in vivo animal models (osteoporotic, infection, tumoral, etc.) are previously developed.
Transferring new biomedical devices from the lab to clinical applications is a difficult challenge. Coatings are not an exception. As an estimative approximation, one technology project out of ten results in a biomedical device that goes to the clinical trial phase.251 Innovation in the biomaterials industry is commonly technology driven but always to satisfy unmet clinical needs. In this sense, the large amount of scientific literature evidences that research on new coatings is widely supported by technology development. There are many fabrication methods that allow for manufacturing substituted hydroxyapatite coatings, which have evidenced satisfactory in vitro behavior.
Certainly, improving coating adhesion and reducing the costs related to industrial upscaling can be considered as the critical issues for spreading the application of novel coatings.252,253 But accelerating early bone–implant integration and the treatment and/or prevention of prothesis infection remain as unmet clinical needs that must be also satisfied. The variety of production techniques and compositions should play a relevant role in transferring new substituted HAp coatings to clinical practice. However, the current scenario largely differs from this. Only a few CaP bioceramics fabricated by plasma spraying are commercially available. The causes of this lack of translation can be found in different aspects. Firstly, in vivo studies concerning substituted HAp coatings are very scarce, which is partially caused by the lack of appropriate animal models. One of the most relevant clinical needs is to avoid prosthesis infection and different cationic substitutions have been introduced in HAp coatings. However, there are not any in vivo studies including a bone infection model to test these devices, and only some subcutaneous models have been proposed.
The second hurdle is the regulatory processes toward market approval. In this sense new substituted HAp coatings fabricated with alternative technologies to plasma spraying are considered as devices that do not have any equivalent on the market. In these cases, pre-market approval (PMAs) is compulsory, which increases the financial and time costs compared to products having equivalent ones in the market, which just require 510k notification. This fact would make novel coatings less attractive to investors, especially because the biggest hurdle can come at the very end of the process with the clinical trials, when a large part of the investment has been already made.
In conclusion, commercial translation of substituted HAp coatings is not exclusively driven by the available technology. Evidence-based definition of the unmet clinical need and pre-clinical studies with appropriate animal models are mandatory to reduce the failure risk during clinical trials. In addition, regulatory filing and an effective marketing strategy would be required to allow novel HAp products to enter the market.
Supplementary Material
Acknowledgements
D. A. would like to thank the Ministerio de Economía y Competitividad for financial support (project MAT2016-75611-R AEI/FEDER, UE). M. V.-R. acknowledges funding from the European Research Council (Advanced Grant VERDI); ERC-2015-AdG Proposal 694160.
Biographies
 Daniel Arcos Daniel Arcos was born in Madrid, Spain, in 1971. He received his PhD at the University Complutense of Madrid (UCM) in 2002. He is Senior Lecturer of the Department of Chemistry in Pharmaceutical Sciences at the Faculty of Pharmacy (UCM), where he carries out his research in the field of bioceramics for bone regeneration. He has published more than 100 papers in the field of bioceramics for bone tissue applications and has led several research projects on this topic. Currently he is involved in the development of substituted hydroxyapatite coatings for the treatment of bone defects under osteoporotic conditions.
Daniel Arcos Daniel Arcos was born in Madrid, Spain, in 1971. He received his PhD at the University Complutense of Madrid (UCM) in 2002. He is Senior Lecturer of the Department of Chemistry in Pharmaceutical Sciences at the Faculty of Pharmacy (UCM), where he carries out his research in the field of bioceramics for bone regeneration. He has published more than 100 papers in the field of bioceramics for bone tissue applications and has led several research projects on this topic. Currently he is involved in the development of substituted hydroxyapatite coatings for the treatment of bone defects under osteoporotic conditions.
 María Vallet-Regí María Vallet-Regí (Las Palmas, 1946) studied Chemistry at the Complutense University of Madrid (UCM) and received a doctorate from the same university in 1974. Currently, she is Emeritus Professor of Inorganic Chemistry and Director of the Intelligent Biomaterials Research Group of the Department of Chemistry in Pharmaceutical Sciences of the Faculty of Pharmacy of UCM. She is the author of more than 700 scientific articles and has received numerous awards and distinctions throughout her extensive scientific career.
María Vallet-Regí María Vallet-Regí (Las Palmas, 1946) studied Chemistry at the Complutense University of Madrid (UCM) and received a doctorate from the same university in 1974. Currently, she is Emeritus Professor of Inorganic Chemistry and Director of the Intelligent Biomaterials Research Group of the Department of Chemistry in Pharmaceutical Sciences of the Faculty of Pharmacy of UCM. She is the author of more than 700 scientific articles and has received numerous awards and distinctions throughout her extensive scientific career.
Footnotes
Conflicts of interest
References
- 1.Crawford RW, Murray DW. Ann Rheum Dis. 1997;56:455. doi: 10.1136/ard.56.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pye AD, Lockhart DEA, Dawson MP, Murray CA, Smith AJ. J Hosp Infect. 2009;72:104. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Tomsia AP, Launey ME, Lee JS, Mankani MH, Wegst UGK, Saiz E. Int J Oral Maxillofac Implants. 2011;26:25. [PMC free article] [PubMed] [Google Scholar]
- 4.Christenson EM, Anseth KS, van der Beucken LJJP, Chan CK, Ercn B, Jansen JA, et al. J Orthop Res. 2007;25:11. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 5.Arcos D, Boccaccini AR, Bohner M, Díez-Pérez A, Epple M, Gómez-Barrena E, Herrera A, Planell JA, Rodríguez-Mañas L, Vallet-Regí M. Acta Biomater. 2014;10:1793. doi: 10.1016/j.actbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fini M, Giavaresi G, Torricelli P, Krajewski A, Ravaglioli A, Belmonte MM, Biagini G, Giardino R. J Bone Jt Surg. 2001;83:139. doi: 10.1302/0301-620x.83b1.10162. [DOI] [PubMed] [Google Scholar]
- 7.Fini M, Giavaresi G, Torricelli P, Borsari V, Giardino R, Nicolini A, Carpi A. Biomed Pharmacother. 2004;58:487. doi: 10.1016/j.biopha.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Franchi M, Fini M, Giavaresi G, Ottani V. Micron. 2005;36:630. doi: 10.1016/j.micron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Campoccia D, Montanaro L, Ariola CR. Biomaterials. 2006;27:2331. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 10.Vallet-Regí M, Arcos D. RSC Nanoscience and Nano-technology, RSC; Cambridge: 2016. Nanoceramics in clinical use. From materials to applications. [Google Scholar]
- 11.Williams DF. The Williams dictionary of Biomaterials. Liverpool University Press; Liverpool: 1999. [Google Scholar]
- 12.Hench LL. J Am Ceram Soc. 1991;74:1487. [Google Scholar]
- 13.Goosen JHM, Kums AJ, Kolln BJ, Verheyen CCPM. Arch Orthop Trauma Surg. 2009;129:1165. doi: 10.1007/s00402-008-0749-9. [DOI] [PubMed] [Google Scholar]
- 14.ISO. Implants for surgery-hydroxyapatite-Part 3: Chemical analysis and characterization of crystallinity and phase purity. 13779-3:2008. [Google Scholar]
- 15.ISO. Implants for surgery-hydroxypatite- Part 2: Coatings of hydroxyapatite. 13779-2:2008 [Google Scholar]
- 16.Chellan P, Sadler PJ. Philos Trans R Soc, A. 2015;373 doi: 10.1098/rsta.2014.0182. 20140182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao H, Song G, Huang Y, Yang H, Han S, Zhang X, Wang Z, Ma J, Bu X, Fu L. RSC Adv. 2019;9 doi: 10.1039/c9ra01168d. 13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao H, Zou Q, Yuan C, Zhang X, Han S, Wang Z, Bu X, Tang H, Huang Y. Ceram Int. 2018;44 16632. [Google Scholar]
- 19.Huang Y, Wang W, Zhang X, Liu X, Xu Z, Han S, Su Z, Liu H, Gao Y, Yang H. Ceram Int. 2018;44:5528. [Google Scholar]
- 20.Elliott JC. Studies in Inorganic Chemistry. Vol. 18 Elsevier; Amsterdam: 1999. Structure and Chemistry of the Apatites and Other Calcium orthophosphates. [Google Scholar]
- 21.Kay MI, Young RA, Posner AS. Nature. 1964;204:1050. doi: 10.1038/2041050a0. [DOI] [PubMed] [Google Scholar]
- 22.Ratnayake JTB, Mucalo M, Dias GJ. J Biomed Mater Res, Part B. 2017;105:1285–1299. doi: 10.1002/jbm.b.33651. [DOI] [PubMed] [Google Scholar]
- 23.Bigi A, Ripamonti A, Brückner S, Gazzano M, Roveri N, Thomas SA. Acta Crystallogr, Sect B: Struct Sci. 1989;45:247. [Google Scholar]
- 24.Bigi A, Falini E, Foresti M, Gazzano M, Ripamonti A, Roveri N. Acta Crystallogr, Sect B: Struct Sci. 1996;52:879–812. [Google Scholar]
- 25.Ergun C, Webster TJ, Bizios R, Doremus RH. J Biomed Mater Res. 2002;59:305. doi: 10.1002/jbm.1246. [DOI] [PubMed] [Google Scholar]
- 26.Webster TJ, Ergun C, Doremus RH, Bizios R. J Biomed Mater Res. 2002;5:312. doi: 10.1002/jbm.1247. [DOI] [PubMed] [Google Scholar]
- 27.Zapanta LeGeros R. Arch Oral Biol. 1974;20:6313. [Google Scholar]
- 28.Mackie PE, Elliott JC, Young RA. Acta Crystallogr, Sect B: Struct Crystallogr Cryst Chem. 1972;28:1840. [Google Scholar]
- 29.Elliott JC, Bonel G, Trombe JC. J Appl Crystallogr. 1980;13:618. [Google Scholar]
- 30.DeBoer BG. Acta Crystallogr, Sect B: Struct Sci. 1991;47:683. [Google Scholar]
- 31.Serret A, Cabañas MV, Vallet-Regí M. Chem Mater. 2000;12:3836. [Google Scholar]
- 32.Young RA, Mackie PE. Mater Res Bull. 1980;15:17. [Google Scholar]
- 33.Wilson RM, Elliott JC, Dowker SEP. Am Mineral. 1999;84:1406. [Google Scholar]
- 34.De Maeyer EAP, Verbeeck RMH, Naessens DE. Inorg Chem. 1993;32:5709. [Google Scholar]
- 35.Verbeeck RMH, De Maeyer EAP, Driessens CM. Inorg Chem. 1995;34:2084. [Google Scholar]
- 36.Surmenev RA, Surmeneva MA, Ivanova AA. Acta Biomater. 2014;10:557. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Heiman RB. Surf Coat Technol. 2006;201:2012. [Google Scholar]
- 38.Gledhill HC, Turner IG, Doyle C. Biomaterials. 1999;20:315. doi: 10.1016/s0142-9612(98)00166-5. [DOI] [PubMed] [Google Scholar]
- 39.Gledhill HC, Turner IG, Doyle C. Biomaterials. 2001;22:1233. doi: 10.1016/s0142-9612(00)00273-8. [DOI] [PubMed] [Google Scholar]
- 40.Podlesak H, Pawlowski L, D’Haese R, Laurenys J, Lampke T, Bellayer S. J Therm Spray Technol. 2010;19:657. [Google Scholar]
- 41.Huang Y, Song L, liu X, Xiao Y, Wu Y, Chen J, Wu F, Gu Z. Biofabrication. 2010;2 doi: 10.1088/1758-5082/2/4/045003. 045003. [DOI] [PubMed] [Google Scholar]
- 42.Hasan S, Stokes J. J Therm Spray Technol. 2011;20:186. [Google Scholar]
- 43.Morks MF, Kobayashi A. Appl Surf Sci. 2007;253:7136. [Google Scholar]
- 44.Dorozhkin S. Prog Biomater. 2012;1:1. doi: 10.1186/2194-0517-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oguchi H, Ishikawa K, Ojima S, Hirayaa Y, Seto K, Eguchi G. Biomaterials. 1992;13:471. doi: 10.1016/0142-9612(92)90169-o. [DOI] [PubMed] [Google Scholar]
- 46.Boccaccini AR, Keim S, Ma R, Li Y, Zhitomirsky I. J R Soc, Interface. 2010;7 doi: 10.1098/rsif.2010.0156.focus. S581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besra L, Liu M. Prog Mater Sci. 2007;52:1. [Google Scholar]
- 48.Choi JM, Kim HE, Lee IS. Biomaterials. 2000;21:469. doi: 10.1016/s0142-9612(99)00186-6. [DOI] [PubMed] [Google Scholar]
- 49.Coelho PG, Lemons JE. J Biomed Mater Res. 2009;90:351. doi: 10.1002/jbm.a.32097. [DOI] [PubMed] [Google Scholar]
- 50.Rajesh P, Muraleedharan CV, Komath M, Varma H. J Mater Sci: Mater Med. 2011;22:497. doi: 10.1007/s10856-011-4230-x. [DOI] [PubMed] [Google Scholar]
- 51.Jelinek M, Weiserova M, Kocourek T, Zezulova M, Strnad J. Laser Phys. 2011;21:1265. [Google Scholar]
- 52.Tri LQ, Chua DHC. Appl Surf Sci. 2009;256:76. [Google Scholar]
- 53.Sygnatowich M, Tiwari A. Mater Sci Eng, C. 2009;29:1071. [Google Scholar]
- 54.Wolke JGC, van Dijk K, Schaeken HG, de Groot K, Jansen JA. J Biomed Mater Res. 1994;28:1477. doi: 10.1002/jbm.820281213. [DOI] [PubMed] [Google Scholar]
- 55.van Dijk K, Schaeken HG, Wolke JCG, Jansen JA. Biomaterials. 1996;17:405. doi: 10.1016/0142-9612(96)89656-6. [DOI] [PubMed] [Google Scholar]
- 56.Surmenev RAA. Surf Coat Technol. 2012;206:2035. [Google Scholar]
- 57.Cooley DR, van Dellen AF, Burgess JO, Windeler S. J Prosthet Dent. 1992;67:93. doi: 10.1016/0022-3913(92)90057-h. [DOI] [PubMed] [Google Scholar]
- 58.Snysers R, Bousser E, Music D, Jensen J, Hocquet S, Schneider JM. Plasma Processes Polym. 2008;5:168. [Google Scholar]
- 59.Ievlev VM, Domashevskaya EP, Putlaiev VI, Tretyakov YD, Barinov SM, Belonogov EK, Kostyuchenko AV, Petrzhik MI, Kiryukhantsev-Korneev FV. Glass Phys Chem. 2008;34:608. [Google Scholar]
- 60.Bigi A, Boanini E, Capuccini C, Fini M, Mihailescu IN, Ristoscu C, Sima F, Torricelli P. Biomaterials. 2009;30:6168. doi: 10.1016/j.biomaterials.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 61.Boanini E, Torricelli P, Fini M, Sima F, Servan F, Mihailescu IN, Bigi A. J Inorg Biochem. 2012;107:65. doi: 10.1016/j.jinorgbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Negroiu G, Piticescu RM, Chitanu GC, Mihailescu IN, Zdrentu L, Miroiu M. J Mater Sci: Mater Med. 2008;18:1537. doi: 10.1007/s10856-007-3300-6. [DOI] [PubMed] [Google Scholar]
- 63.Bigi A, Boanini E, Capuccini C, Fini M, Mihailescu IN, Ristoscu C, Sima F, Torricelli P. Biomaterials. 2009;30:6168. doi: 10.1016/j.biomaterials.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 64.Wang XX, Hayakawa S, Tsuru K, Osaka A. J Biomed Mater Res. 2000;52:172. doi: 10.1002/1097-4636(200010)52:1<171::aid-jbm22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 65.Cabañas MV, Vallet-Regí M. J Mater Chem. 2003;3:1104. [Google Scholar]
- 66.Goto T, Katsui H. Chemical Vapor Deposition of Ca– P–O Film Coating. In: Sasaki K, Suzuki O, Takahashi N, editors. Interface Oral Health Science. Vol. 2014 Springer; Tokyo: 2015. [Google Scholar]
- 67.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. J Biomed Mater Res. 1990;24:721. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 68.Escada ALA, Machado JPB, Shneider SG, Alves Rezende MCR, Alves Claro APR. J Mater Sci: Mater Med. 2011;22:2457. doi: 10.1007/s10856-011-4434-0. [DOI] [PubMed] [Google Scholar]
- 69.Aparecida AH, Fook MVL, Guastaldi AC. J Mater Sci: Mater Med. 2009;20:1215. doi: 10.1007/s10856-008-3682-0. [DOI] [PubMed] [Google Scholar]
- 70.Hench LL, West JK. Chem Rev. 1990;90:33. [Google Scholar]
- 71.Izquierdo-Barba I, Vallet-Regí M, Rojo JM, Blanco E, Esquivias L. J Sol-Gel Sci Technol. 2003;26:1179. [Google Scholar]
- 72.Izquierdo-Barba I, Asenjo A, Esquivias L, Vallet-Regí M. Eur J Inorg Chem. 2003;1608 [Google Scholar]
- 73.Hijón N, Cabañas MV, Peña J, Vallet-Regí M. Acta Biomater. 2006;2:567. doi: 10.1016/j.actbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Qiu D, Yang L, Yin Y, Wang A. Surf Coat Technol. 2011;205:3280. [Google Scholar]
- 75.Lin S, Legeros RZ, Legeros JP. J Biomed Mater Res, Part A. 2003;66:819. doi: 10.1002/jbm.a.10072. [DOI] [PubMed] [Google Scholar]
- 76.Ban S, Maruno S. J Biomed Mater Res. 1998;42:387. doi: 10.1002/(sici)1097-4636(19981205)42:3<387::aid-jbm6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 77.Cimenoglu H, Gunyuz M, Kose GT, Baydogan M, Ugurlu F, Sener C. Mater Charact. 2011;62:304. [Google Scholar]
- 78.Luo R, Liu Z, Yan F, Kong Y, Zhang Y. Appl Surf Sci. 2013;266:57. [Google Scholar]
- 79.Liu F, Song Y, Wang F, Shimizu T, Igarashi K, Zhao L. J Biosci Bioeng. 2005;100:100. doi: 10.1263/jbb.100.100. [DOI] [PubMed] [Google Scholar]
- 80.Bosco R, Edreira REU, Wolke JGC, Leeuwenburgh SCG, van den Beucken JJJP. Surf Coat Technol. 2013;233:91. [Google Scholar]
- 81.Thian ES, Chang L, Lim PN, Gurucharan B, Sun J, Fuh JYH. Surf Coat Technol. 2012;231:29. [Google Scholar]
- 82.Miyaji F, Kono Y, Suyama Y. Mater Res Bull. 2005;40:209. [Google Scholar]
- 83.Tang Y, Chappell HF, Dove MT, Reeder RJ, Lee YJ. Biomaterials. 2009;30:2864. doi: 10.1016/j.biomaterials.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 84.Ren F, Xin R, Ge X, Leng Y. Acta Biomater. 2009;5:3141. doi: 10.1016/j.actbio.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi M, Oishi H, Suketa Y. Biochem Pharmacol. 1987;36:4007. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi M. J Trace Elem Exp Med. 1998;11:119. [Google Scholar]
- 87.Staníc V, Dimitrijevíc S, Antíc-Stankovíc J, Mitríc M, Jokíc B, Plecas IB, Raicevic S. Appl Surf Sci. 2010;256:6083. [Google Scholar]
- 88.Thian ES, Konishi T, Kawanobe Y, Lim PN, Choong C, Ho B, Aizawa M. J Mater Sci: Mater Med. 2013;24:437. doi: 10.1007/s10856-012-4817-x. [DOI] [PubMed] [Google Scholar]
- 89.Lyasnikova AV, Dudareva OA, Lyasnikov VN, Markelova OA, Grishina IP. Glass Ceram. 2018;75:163. [Google Scholar]
- 90.Peñaflor Galindo TG, Kataoka T, Fujii S, Okuda M, Tagaya M. Colloid Interface Sci Commun. 2016;15:10–11. [Google Scholar]
- 91.Predoi D, Iconaru SL, Predoi MV, Buton N, Motelica-Heino M. Coatings. 2019;9:256. doi: 10.3390/nano9040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prosolov KA, Belyavskaya OA, Linders J, Loza K, Prymak O, Mayer C, Rau JV, Epple M, Sharkeev YP. Coatings. 2019;9:220. [Google Scholar]
- 93.López EO, Rossi AL, Bernardo PL, Freitas RO, Mello A, Rossi AM. Ceram Int. 2019;45:793. [Google Scholar]
- 94.Huang Y, Zhang H, Qiao H, Nian X, Zhang X, Wang W, Zhang X, Chang X, Han S, Pang X. Appl Surf Sci. 2015;357:1776. [Google Scholar]
- 95.Webster TJ, Massa-Schlueter EA, Smith JL, Slamovich EB. Biomaterials. 2004;25:2111. doi: 10.1016/j.biomaterials.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Sergi R, Bellucci D, Candidato RT, Jr, Lusvarghi L, Bolelli G, Pawlowski L, Candiani G, Atomare L, De Nardo L, Cannillo V. Surf Coat Technol. 2018;352:84. [Google Scholar]
- 97.Candidato RT, Thouzellier C, Pawlowski L. J Biomed Mater Res, Part B. 2018;106:2101. doi: 10.1002/jbm.b.34014. [DOI] [PubMed] [Google Scholar]
- 98.Ortiz IY, dos Santos AR, Costa AM, Mavropoulos E, Tanaka MN, Prado da Silva MH, de Souza Camargo S., Jr Ceram Int. 2016;42 15502. [Google Scholar]
- 99.Sogo Y, Sakurai T, Onuma K, Ito A. J Biomed Mater Res, Part A. 2002;62:457. doi: 10.1002/jbm.10200. [DOI] [PubMed] [Google Scholar]
- 100.Kawamura H, Ito A, Muramatsu T, Miyakawa S, Ochiai N, Tateishi T. J Biomed Mater Res, Part A. 2003;65:468. doi: 10.1002/jbm.a.10524. [DOI] [PubMed] [Google Scholar]
- 101.Ma J, Qin J. Cryst Growth Des. 2015;15:1273. [Google Scholar]
- 102.Borkow G, Gabbay J. Curr Med Chem. 2005;12:2163. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 103.Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y. Biomaterials. 2013;34:422. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 104.Ewald A, Kappel C, Vorndran E, Moseke C, Gelinsky M, Gbureck U. J Biomed Mater Res, Part A. 2012;100:2392. doi: 10.1002/jbm.a.34184. [DOI] [PubMed] [Google Scholar]
- 105.Pujari M, Patel PN. J Solid State Chem. 1989;83:100. [Google Scholar]
- 106.Shanmucham S, Gopal B. Ceram Int. 2014;40 15655. [Google Scholar]
- 107.Gomes S, Vichery C, Descamps S, Martinez H, Kaur A, Jacobs A, Nedelec J-M, Renaudin G. Acta Biomater. 2018;65:462. doi: 10.1016/j.actbio.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 108.Lyasnikova AV, Markelova OA, Dudareva OA, Lyasnikov VN, Barabash AP, Shpinyak SP. Powder Metall Met Ceram. 2016;55:97. [Google Scholar]
- 109.Unabia RB, Bonebeau S, Candidato RT, Jr, Pawlowski L. Surf Coat Technol. 2018;353:370. [Google Scholar]
- 110.Hidalgo-Robatto BM, Álvarez M López, Azevedo AS, Dorado J, Serra J, Azevedo NF. Surf Coat Technol. 2018;333:168. [Google Scholar]
- 111.Darouiche RO. Clin Infect Dis. 1999;29:137. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 112.Jelinek M, Kocourek T, Jurek K, Remsa J, Miksovsky J, Weisasrova´ M. Appl Phys A: Mater Sci Process. 2010;101:615. [Google Scholar]
- 113.Chen YK, Zheng XB, Xie YT, Ji H, Ding CX, Li HW, et al. Surf Coat Technol. 2010;205:1892. [Google Scholar]
- 114.Chen Y, Zheng Z, Xie Y, Ding C, Ruan H, Fan C. J Mater Sci: Mater Med. 2008;19:3603. doi: 10.1007/s10856-008-3529-8. [DOI] [PubMed] [Google Scholar]
- 115.Fielding GA, Roy M, Bandyopadhyay A, Bose S. Acta Biomater. 2012;8:3144. doi: 10.1016/j.actbio.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, et al. Biomaterials. 2006;27:5512. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Chen W, Oh S, Ong AP, Oh N, Liu Y, Courtney HS, Appleford M, Ong JL. J Biomed Mater Res, Part A. 2007;82:899. doi: 10.1002/jbm.a.31197. [DOI] [PubMed] [Google Scholar]
- 118.Shimazaki T, Miyamoto H, Ando Y, Noda I, Yonekura Y, Kawano S. J Biomed Mater Res, Part B. 2010;92:386. doi: 10.1002/jbm.b.31526. [DOI] [PubMed] [Google Scholar]
- 119.Gokcekaya O, Webster TJ, Ueda K, Narushima T, Ergun C. Mater Sci Eng, C. 2017;77:555. doi: 10.1016/j.msec.2017.03.233. [DOI] [PubMed] [Google Scholar]
- 120.Yan Y, Zhang X, Huang Y, Ding Q, Pang X. Appl Surf Sci. 2014;314:348. [Google Scholar]
- 121.Sun JP, Song Y. ACS Appl Nano Mater. 2018;9:4940. doi: 10.1021/acsanm.8b01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rude RK, Singer FR, Gruber HE. J Am Coll Nutr. 2009;28:131. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 123.Banyopadhyay A, Bernard S, Xue W, Bose S. J Am Ceram Soc. 2006;89:2675. [Google Scholar]
- 124.Graziani G, Boi M, Bianchi M. Coatings. 2018;8:269. [Google Scholar]
- 125.Bose S, Vu A, Emshadi K, Bandyopadhyay A. Mater Sci Eng, C. 2018;88:166. doi: 10.1016/j.msec.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Min J, Choe H-C. Appl Surf Sci. 2018;432:294. [Google Scholar]
- 127.Bakin B, Koc Delice T, Tiric U, Birlik I, Ak Azem A. Surf Coat Technol. 2016;301:29. [Google Scholar]
- 128.Layrolle P. Calcium phosphate coatings. In: Ducheyne P, Healy K, Hutmacher DW, Grainger DW, Kirkpatric CJ, editors. Comprehensive Biomaterials. Vol. 1. Elsevier; Amsterdam, Netherlands: pp. 223–229. [Google Scholar]
- 129.Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, Tsouderos Y, Delmas PD, Christiansen C. Bone. 2001;28:446. doi: 10.1016/s8756-3282(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 130.Marie PJ. Curr Opin Pharmacol. 2005;5:633. doi: 10.1016/j.coph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 131.Ravi ND, Balu R, Sampath Kumar TS. J Am Ceram Soc. 2012;95:2700. [Google Scholar]
- 132.Capuccini C, Torricelli P, Boanini E, Gazzano M, Giardino R, Bigi A. J Biomed Mater Res, Part A. 2009;89:594. doi: 10.1002/jbm.a.31975. [DOI] [PubMed] [Google Scholar]
- 133.Bianchi M, Degli Esposti L, Ballardini A, Liscio F, Berni M, Gambardella A, Leeuwenburgh SCG, Sprio S, Tampieri A, Iafisco M. Surf Coat Technol. 2017;319:191. [Google Scholar]
- 134.Bigi A, Boanini E, Capuccini C, Gazzano M. Inorg Chim Acta. 2007;360:1009. [Google Scholar]
- 135.Teng H-P, Lin H-Y, Huang Y-H, Lu F-H. Surf Coat Technol. 2018;350:1112. [Google Scholar]
- 136.Li Y, Shen S, Zhu L, Cai S, Jiang Y, Ling R, Jiang S, Lin Y, Hua S, Xu G. J Ceram Soc Jpn. 2019;127:158. [Google Scholar]
- 137.Wu X, Lin W, Li D, Guo H, Li P, Fan Y. RSC Adv. 2019;9 doi: 10.1039/c9ra02210d. 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nguyen T-DT, Jang Y-S, Lee M-H, Bae T-S. J Appl Biomater Funct Mater. 2019;17 doi: 10.1177/2280800019826517. [DOI] [PubMed] [Google Scholar]
- 139.Roy M, Bandyopadhyay A, Bose S. J Biomed Mater Res, Part B. 2011;99:258. doi: 10.1002/jbm.b.31893. [DOI] [PubMed] [Google Scholar]
- 140.Boanini E, Torricelli P, Sima F, Axente E, Fini M, Mihailescu IN, Bigi A. J Inorg Biochem. 2018;183:1. doi: 10.1016/j.jinorgbio.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 141.Birgani ZT, Fennema E, Gijbels MJ, de Boer J, van Blitterswijk CA, Habibovic P. Acta Biomater. 2016;36:267. doi: 10.1016/j.actbio.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 142.Birgani ZT, Gharraee N, Malhotra A, van Blitters-wijk CA, Habibovic P. Biomed Mater. 2016 doi: 10.1088/1748-6041/11/1/015020. 015020. [DOI] [PubMed] [Google Scholar]
- 143.Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, Ahang M, Xiao Y. Biomaterials. 2012;33:2076. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 144.Drevet R, Zhukova Y, Dubinskiy S, Kazakbiev A, Naumenko V, Abakumov M, Fauré J, Benhayoune H, Prokoshkin S. J Alloys Compd. 2019;793:576. [Google Scholar]
- 145.Prem Ananth K, Sun J, Bai J. Mater Today Chem. 2018;10:153. [Google Scholar]
- 146.Mould AP, Akiyama SK, Humphries MJ. J Biol Chem. 1995;270 doi: 10.1074/jbc.270.44.26270. 26270. [DOI] [PubMed] [Google Scholar]
- 147.Gyorgy E, Toricelli P, Socol G, Iliescu M, Mayer I, Mihailescu IN, Bigi A, Werckman J. J Biomed Mater Res, Part A. 2004;71:353. doi: 10.1002/jbm.a.30172. [DOI] [PubMed] [Google Scholar]
- 148.Bigi A, Bracci B, Cuisinier F, Elkaim R, Fini M, Mayer I, Mihailescu IN, Socol G, Sturba L, Torricelli P. Biomaterials. 2005;26:2381. doi: 10.1016/j.biomaterials.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 149.Sima F, Socol G, Axente E, Mihailescu IN, Zdrentu L, Petrescu SM, Mayer I. Appl Surf Sci. 2007;254:1155. [Google Scholar]
- 150.Prem Ananth K, Sun J, Bai J. Int J Mol Sci. 2018;19:2340. doi: 10.3390/ijms19082340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park JW, Kim YJ, Jang JH. Appl Surf Sci. 2011;258:977. [Google Scholar]
- 152.Sato M, Sambito MA, Aslani A, Kalkhoran NM, Slamovich EB, Webster TJ. Biomaterials. 2006;27:2358. doi: 10.1016/j.biomaterials.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 153.Lin Y, Yang Z, Cheng J. J Rare Earths. 2007;25:452. [Google Scholar]
- 154.Ciobani G, Harja M. Ceram Int. 2019;45:2852. [Google Scholar]
- 155.Jakupec MA, Unfried P, Keppler BK. Rev Physiol, Biochem Pharmacol. 2005;153:101. doi: 10.1007/s10254-004-0024-6. [DOI] [PubMed] [Google Scholar]
- 156.Carlisle EM. Science. 1970;167:179. doi: 10.1126/science.167.3916.279. [DOI] [PubMed] [Google Scholar]
- 157.Carlisle EM. Calcif Tissue Int. 1981;33:27. doi: 10.1007/BF02409409. [DOI] [PubMed] [Google Scholar]
- 158.Gibson IR, Best SM, Bonfield W. J Biomed Mater Res. 1999;44:422. doi: 10.1002/(sici)1097-4636(19990315)44:4<422::aid-jbm8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 159.Gibson IR, Best SM, Bonfield W. J Am Ceram Soc. 2002;85:2771. [Google Scholar]
- 160.Arcos D, Rodríguez-Carvajal J, Vallet-Regí M. Chem Mater. 2004;16:2300. [Google Scholar]
- 161.Arcos D, Rodríguez-Carvajal J, Vallet-Regí M. Solid State Sci. 2004;6:987. [Google Scholar]
- 162.Arcos D, Sánchez-Salcedo S, Izquierdo-Barba I, Ruiz L, González-Calbet J, Vallet-Regí M. J Biomed Mater Res. 2006;78:762. doi: 10.1002/jbm.a.30790. [DOI] [PubMed] [Google Scholar]
- 163.Matesanz MC, Linares J, Oñaderra M, Feito MJ, Martínez-Vázquez FJ, Sánchez-Salcedo S, Arcos D, Teresa Portolés M, Vallet-Regí M. Colloids Surf, B. 2015;133:304. doi: 10.1016/j.colsurfb.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 164.Matesanz MC, Linares J, Lilue I, Sánchez-Salcedo S, Feito MJ, Arcos D, Vallet-Regí M, Portolés MT. J Mater Chem B. 2014;2:2910. doi: 10.1039/c3tb21697g. [DOI] [PubMed] [Google Scholar]
- 165.Vallet-Regí M, Arcos D. J Mater Chem. 2005;15:1509. [Google Scholar]
- 166.Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E, Revell PA. J Mater Sci: Mater Med. 2002;13:1199. doi: 10.1023/a:1021114710076. [DOI] [PubMed] [Google Scholar]
- 167.Porter AE, Botelho CM, Lopes MA, Santos JD, Best SM, Bonfield W. J Biomed Mater Res, Part A. 2004;69:670. doi: 10.1002/jbm.a.30035. [DOI] [PubMed] [Google Scholar]
- 168.Thian ES, Huang J, Best SM, Barber ZH, Bonfield W. Biomaterials. 2005;26:2947. doi: 10.1016/j.biomaterials.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 169.Thian ES, Huang J, Best SM, Barber ZH, Brooks RA, Rushton N, Bonfield W. Biomaterials. 2006;27:2692. doi: 10.1016/j.biomaterials.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 170.Thian ES, Huang J, Vickers ME, Best SM, Barber ZH, Bonfield W. J Mater Sci. 2006;41:709. [Google Scholar]
- 171.Surmeneva MA, Chaikina MV, Zaikovskiy VI, Pichugin VF, Prymak O, Epple M, et al. Surf Coat Technol. 2013;218:39. [Google Scholar]
- 172.Surmeneva MA, Kovtum A, Peetsch A, Goroja SN, Sharonova A, Picugin VF, et al. RSC Adv. 2013;3 11240. [Google Scholar]
- 173.Surmeneva MA, Mukhametkaliyev TM, Tyurin AI, Teresov AD, Koval NN, Pirozhkova TS, Shuvarin IA, Shuklinov AV, Zhigachev AO, Oehr C, Surmenev RA. Surf Coat Technol. 2015;275:176. [Google Scholar]
- 174.Rau JV, Cacciotti I, Laureti S, Fosca M, Varvaro G, Latini A. J Biomed Mater Res, Part B. 2015;103:1621. doi: 10.1002/jbm.b.33344. [DOI] [PubMed] [Google Scholar]
- 175.Hijon N, Cabañas MV, Peña J, Vallet-Regí M. Acta Biomater. 2006;2:567. doi: 10.1016/j.actbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 176.Cha J-Y, Lim C-H, Kim Y-J. J Ceram Soc Jpn. 2018;126:940. [Google Scholar]
- 177.Dehghanian C, Aboudzadeh N, Shokrgozr MA. Mater Chem Phys. 2018;203:27. [Google Scholar]
- 178.Kezhi L, Qian G, Leilei Z, Yulei Z, Shoujie L, Kebing G, Shaoxian L. Ceram Int. 2017;43:1410. [Google Scholar]
- 179.Xin-Bo X, Xin-Ye N, Ya-Yun L, Cen-Cen C, Ji-Zhao Z, Xie-Rong Z. Sci Rep. 2016;6 doi: 10.1038/srep31309. 31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.López EO, Rossi AL, Archanjo BS, Ospina RO, Mello A, Rossi AM. Surf Coat Technol. 2015;264:163. [Google Scholar]
- 181.Hashimoti Y, Ueda M, Kohiga Y, Imura K, Hontsu S. Dent Mater J. 2018;37:408. doi: 10.4012/dmj.2017-122. [DOI] [PubMed] [Google Scholar]
- 182.Marquis RE. Can J Microbiol. 1995;41:955. doi: 10.1139/m95-133. [DOI] [PubMed] [Google Scholar]
- 183.Alhilou A, Do T, Mizban L, Clarkson BH, Wood DJ, Katsikogianni MG. ACS Omega. 2016;1:264. doi: 10.1021/acsomega.6b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alvarez R, Muñoz-Piña S, González MU, Izquierdo-Barba I, Fernández-Martínez I, Rico V, Arcos D, García-Valenzuela A, Palmero A, Vallet-Regí M, González-Elipe AR, García-Martín JM. Nanomaterials. 2019;9:1217. doi: 10.3390/nano9091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Caligari Conti M, Xerri G, Peyrouzet F, Schembri Wismayer P, Sionagra E, Matovani D, Vella D, Buhagiar J. Surf Eng. 2019;35:255. [Google Scholar]
- 186.León B, Albano M, Garrido L, Ferraz E, Rosa A, Oliveira PT. Int J Appl Ceram Technol. 2018;15:1415. [Google Scholar]
- 187.Lee EJ, Lee SH, Kim HW, Kong YM, Kim HE. Biomaterials. 2005;26:3843. doi: 10.1016/j.biomaterials.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 188.Wang J, Chao Y, Wan Q, Zhu Z, Yu H. Acta Biomater. 2009;5:1798. doi: 10.1016/j.actbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 189.Sima LE, Stan GE, Morosanu CO, Melinescu A, Melinte R, et al. J Biomed Mater Res. 2010;95:1203. doi: 10.1002/jbm.a.32947. [DOI] [PubMed] [Google Scholar]
- 190.Winning L, Robinson L, Boyd AR, El Karim IA, Lundy FT, Meenan BJ. J Biomed Mater Res, Part A. 2017;105:1692. doi: 10.1002/jbm.a.36044. [DOI] [PubMed] [Google Scholar]
- 191.Guo Y-P, Yao Y-B, Ning C-Q, Chu L-F, Guo Y-J. Mater Lett. 2011;65:1007. [Google Scholar]
- 192.Tang S, Tian B, Guo Y-J, Zhu Z-A, Guo Y-P. Surf Coat Technol. 2014;251:210. [Google Scholar]
- 193.Tang S, Tian B, Ke Q-F, Zhu Z-A, Guo Y-P. RSC Adv. 2014;4 41500. [Google Scholar]
- 194.Costa DO, Prowse PDH, Chrones T, Sims SM, Hamilton DW, Rizkalla AS, Dixon SJ. Biomaterials. 2013;34:7215. doi: 10.1016/j.biomaterials.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 195.Stigter M, Bezemer J, de Groot K, Layrolle P. J Controlled Release. 2004;99:127. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 196.Utku FS, Seckin E, Goller G, Tamerler C, Urgen M. Ceram Int. 2014;40 15479. [Google Scholar]
- 197.Rau JV, Generosi A, Laureti S, Komlev VS, Ferro D, Nunziante Cesaro S, Paci B, Rossi Albertini V, Agostinelli E, Barinov SM. ACS Appl Mater Interfaces. 2009;1:1813. doi: 10.1021/am900356e. [DOI] [PubMed] [Google Scholar]
- 198.Wei X, Fu C, Savino K, Yates MZ. Cryst Growth Des. 2012;12:3474. [Google Scholar]
- 199.Han Y, Xu K, Montay G, Fu T, Lu J. J Biomed Mater Res. 2002;60:511. doi: 10.1002/jbm.10097. [DOI] [PubMed] [Google Scholar]
- 200.Sima LE, Stan GE, Morosanu CO, Melinescu A, Melinte R, et al. J Biomed Mater Res. 2010;95:1203. doi: 10.1002/jbm.a.32947. [DOI] [PubMed] [Google Scholar]
- 201.Winning L, Robinson L, Boyd AR, El Karim IA, Lundy FT, Meenan BJ. J Biomed Mater Res, Part A. 2017;105:1692. doi: 10.1002/jbm.a.36044. [DOI] [PubMed] [Google Scholar]
- 202.Gumusderelioglu M, Tuncay EO, Kaynak G, Demirtas TT, Aydin Tigli RS, Hakki SS. J Trace Elem Med Biol. 2015;31:120. doi: 10.1016/j.jtemb.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 203.Tunçay EÖ, Demirtaş TT, Gümüş-derelioğlu M. J Trace Elem Med Biol. 2017;40:72. doi: 10.1016/j.jtemb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 204.Rodríguez-Valencia C, Freixeiro P, Serra J, Ferreirós CM, González P, López-Álvarez M. Biomed Mater. 2017;12 doi: 10.1088/1748-605X/aa5a6f. 015028. [DOI] [PubMed] [Google Scholar]
- 205.Yilmaz B, Evis Z, Tezcaner A, Banerjee S. Int J Appl Ceram Technol. 2016;13:1059. [Google Scholar]
- 206.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. J Biomed Mater Res. 2000;52:662. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 207.Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S. ACS Appl Mater Interfaces. 2012;4:1341. doi: 10.1021/am201610q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Geng Z, Cui Z, Li Z, Zhu S, Liang Y, Liu Y, Li X, He X, Yu X, Wang R, Yang X. Mater Sci Eng, C. 2016;58:467. doi: 10.1016/j.msec.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 209.Geng Z, Wang R, Zhuo X, Li Z, Huang Y, Ma L, Cui Z, Zhu S, Liang Y, Liu Y, Bao H, Li X, Huo Q, Liu Z, Yang X. Mater Sci Eng, C. 2017;71:852. doi: 10.1016/j.msec.2016.10.079. [DOI] [PubMed] [Google Scholar]
- 210.Yang L, Perez-Amodio S, Barrere-de Groot FYF, Everts V, van Blitterswijk CA, Habibovic P. Biomaterials. 2006;31:2976. doi: 10.1016/j.biomaterials.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 211.Huang Y, Zhang X, Mao H, Li T, Zhao R, Yan Y, Pang X. RSC Adv. 2015;5 17076. [Google Scholar]
- 212.Huang Y, Hao M, Nian X, Qiao H, Zhang X, Zhan X, Song G, Guo J, Pang X, Zhang H. Ceram Int. 2016;42 11876. [Google Scholar]
- 213.Cao L, Ullah I, Li N, Niu S, Sun R, Xia D, Yang R, Zhang X. J Mater Sci Technol. 2019;35:719. [Google Scholar]
- 214.Morejón-Alonso L, Mochales C, Nascimento L, Müller W-D. Mater Lett. 2019;248:65. [Google Scholar]
- 215.Arcos D, Rodríguez-Carvajal J, Vallet-Regí M. Chem Mater. 2005;17:57. [Google Scholar]
- 216.Morais SD, Coelho J, Ferraz MP, Gomes PS, Fernandes MH, Hussain NS, Santos JD, Lopes MA. J Mater Chem B. 2014;2:5872. doi: 10.1039/c4tb00484a. [DOI] [PubMed] [Google Scholar]
- 217.Ciobanu CS, Popa CL, Predoi D. J Nanomater. 2014 780686. [Google Scholar]
- 218.Yang M, Qin C, Pan F, Zhou T. J Rare Earths. 2011;29:550. [Google Scholar]
- 219.Sathiskumar S, Louis K, Shinyjoy E, Gopi D. Ind Eng Chem Res. 2016;55:6331. [Google Scholar]
- 220.Birgani ZT, Gharraee N, Malhotra A, van Blitters-wijk CA, Habibovic P. Biomed Mater. 2016;11 doi: 10.1088/1748-6041/11/1/015020. 015020. [DOI] [PubMed] [Google Scholar]
- 221.Huang Y, Zhang X, Qiao H, Hao M, Zhang H, Xua Z, Zhang X, Pang X, Lin H. Ceram Int. 2016;42:1903. [Google Scholar]
- 222.Huang Y, Song G, Chang X, Wang Z, Zhang X, Han S, Su Z, Yang H, Yang D, Zhang X. Int J Nanomed. 2018;13:2665. doi: 10.2147/IJN.S162558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Qu W, Zhong D, Wu P, Wang J, Han B. J Bone Miner Metab. 2008;26:328. doi: 10.1007/s00774-007-0832-2. [DOI] [PubMed] [Google Scholar]
- 224.Qiu X, Lim PN, Tong SY, Thian ES. Mater Technol. 2018;33:406. [Google Scholar]
- 225.Lim PN, Wang Z, Cahng L, Konishi T, Choong C, Ho B, Thian ES. J Mater Sci: Mater Med. 2017;28:3. doi: 10.1007/s10856-016-5812-4. [DOI] [PubMed] [Google Scholar]
- 226.Zhao S-F, Jiang Q-H, Peel S, Wang X-X, He F-M. Clin Oral Implants Res. 2013;24:34. doi: 10.1111/j.1600-0501.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 227.Ke D, Robertson SF, Dernell WS, Bandyopadhyay A, Bose S. ACS Appl Mater Interfaces. 2017;9 doi: 10.1021/acsami.7b05574. 25731. [DOI] [PubMed] [Google Scholar]
- 228.Vu AA, Robertson SF, Ke D, Bandyopadhyay A, Bose S. Acta Biomater. 2019;92:325. doi: 10.1016/j.actbio.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 229.Barrère F, van der Valk CM, Dalmeijer RAJ, van Blitterswijk CA, de Groot K, Layrolle P. J Biomed Mater Res, Part A. 2003;64:378. doi: 10.1002/jbm.a.10291. [DOI] [PubMed] [Google Scholar]
- 230.Dhert WJ, Klein CPAT, Wolke JG, Van der Velde EA, de Groot K, Rozing PM. J Biomed Mater Res. 1991;25:1183. doi: 10.1002/jbm.820251002. [DOI] [PubMed] [Google Scholar]
- 231.Dhert WJ, Klein CPAT, Jansen JA, van der Velde EA, Vrieste RC, Rozing PM, de Groot K. J Biomed Mater Res. 1993;27:127. doi: 10.1002/jbm.820270116. [DOI] [PubMed] [Google Scholar]
- 232.Dhert WJ, Thompson P, Klein CPAT, de Groot K, Rozing PM, Ericson LE. J Mater Sci: Mater Med. 1994;5:59. [Google Scholar]
- 233.Caulier H, Vercaigne S, Naert I, van der Waerden JPCM, Wolke JGC, Kalk W, Jansen JA. J Biomed Mater Res. 1997;34:121. doi: 10.1002/(sici)1097-4636(199701)34:1<121::aid-jbm16>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 234.Tao ZS, Bai B-L, He XW, Liu W, Li H, Zhou Q, Sun T, Huang Z-L, Tu K-K, Lv Y-X, Cui W, Yang L. Med Biol Eng Comput. 2016;54:1959. doi: 10.1007/s11517-016-1494-9. [DOI] [PubMed] [Google Scholar]
- 235.Ballo AM, Xia W, Palmquist A, Lindahl C, Emanuelsson L, Lausma J, Engqvist H, Thompsen P. J R Soc, Interface. 2012;9:1615. doi: 10.1098/rsif.2011.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bose S, Banerjee D, Shivaram A, Tarafder S, Bandyopadhyay A. Mater Des. 2018;151:102. doi: 10.1016/j.matdes.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Das K, Bose S, Bandyopadhyay A. J Biomed Mater Res, Part A. 2009;90:225. doi: 10.1002/jbm.a.32088. [DOI] [PubMed] [Google Scholar]
- 238.Heinl P, Muller L, Korner C, Singer RF, Muller FA. Acta Biomater. 2008;4:1536. doi: 10.1016/j.actbio.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 239.Franchi M, Fini M, Giavaresi G, Ottani V. Micron. 2005;36:630. doi: 10.1016/j.micron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 240.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Biomaterials. 2000;21:1803. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 241.Izquierdo-Barba I, Asenjo A, Esquivias L, Vallet-Regí M. Eur J Inorg Chem. 2003:1608. [Google Scholar]
- 242.Zhang E, Zou C. Acta Biomater. 2009;5:1732. doi: 10.1016/j.actbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 243.Ilea A, Vrabie O-G, Babtan A-M, Miclaus V, Ruxanda F, Sarkozi M, Barbu-Tudoran L, Mager V, Berce C, Bosca BA, Petrescu NB, et al. J Mater Sci: Mater Med. 2019;30:26. doi: 10.1007/s10856-019-6227-9. [DOI] [PubMed] [Google Scholar]
- 244.Izquierdo-Barba I, Santos-Ruiz L, Becerra J, Feito MJ, Fernández-Villa D, Serrano MC, Díaz-Güemes I, Fernández-Tomé B, Enciso S, Sánchez-Margallo FM, Monopoli D, et al. Acta Biomater. 2019;83:456. doi: 10.1016/j.actbio.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 245.Mróz W, Budner B, Syroka R, Golansky Niedzielski G, Slósarczyk A, Schwarze D, Douglas TEL. J Biomed Mater Res, Part B. 2015;103:151. doi: 10.1002/jbm.b.33170. [DOI] [PubMed] [Google Scholar]
- 246.Boanini E, Torricelli P, Sima F, Axente E, Fini M, Mihailescu IN, Bigi A. J Colloid Interface Sci. 2015;448:1. doi: 10.1016/j.jcis.2015.01.088. [DOI] [PubMed] [Google Scholar]
- 247.Nancy D, Rajendran N. Int J Biol Macromol. 2018;110:197. doi: 10.1016/j.ijbiomac.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 248.Dadsetan M, Guda T, Runge MB, Mijares D, LeGeros RZ, LeGeros JP, Silliman DT, Lu L, wenke JC, Brown Baer PR, Yaszemski MJ. Acta Biomater. 2015;18:9. doi: 10.1016/j.actbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 249.Huang B, Yuan Y, Li T, Ding S, Zhang W, Gu Y, Liu C. Sci Rep. 2016;6 doi: 10.1038/srep24323. 24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Geng Z, Wang X, Zhao J, Li Z, Ma L, Zhu S, Liang Y, Cui Z, He H, Yang X. Biomater Sci. 2018;6:2654. doi: 10.1039/c8bm00716k. [DOI] [PubMed] [Google Scholar]
- 251.Bayon Y, Bohner M, Eglin D, Procter P, Richards RG, Weber J, Zeugolis DI. J Mater Sci: Mater Med. 2016;27:144. doi: 10.1007/s10856-016-5759-5. [DOI] [PubMed] [Google Scholar]
- 252.Bosco R, Van Den Beucken J, Leeuwenburgh S, Jansen J. Coatings. 2012;2:95. [Google Scholar]
- 253.Surmenev RA. Surf Coat Technol. 2012;206:2035. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.