Abstract
Chronic bone infection is considered as one of the most problematic biofilm-related infections. Its recurrent and resistant nature, high morbidity, prolonged hospitalization, and costly medical care expenses have driven the efforts of the scientific community to develop new therapies to improve the standards used today. There is great debate on the management of this kind of infection in order to establish consistent and agreed guidelines in national health systems. The scientific research is oriented toward the design of anti-infective biomaterials both for prevention and cure. The properties of these materials must be adapted to achieve better anti-infective performance and good compatibility, which allow a good integration of the implant with the surrounding tissue. The objective of this review is to study in-depth the antibacterial biomaterials and the strategies underlying them. In this sense, this manuscript focuses on antimicrobial coatings, including the new technological advances on surface modification; scaffolding design including multifunctional scaffolds with both antimicrobial and bone regeneration properties; and nanocarriers based on mesoporous silica nanoparticles with advanced properties (targeting and stimuli-response capabilities).
Keywords: bone infection, mesoporous silica nanoparticles, nanocarriers, scaffolds
1. Introduction
Orthopedic device-related infections, with over 1.5 million total hip and knee replacements performed each year, constitute one of the most serious and devastating risks facing society today.[1,2] As it is well known, the implant surface is an ideal environment for bacterial adhesion and colonization.[3] Besides, the micro-movement-induced wear of these orthopedic prostheses causes the release of debris that triggers local inflammation, providing an ideal niche for the onset of infection.[4] Currently, despite major advances in prophylactic measures and aseptic surgery techniques, which have significantly diminished the prevalence of orthopedic infection, infection rates have not fallen below 1–2%.[5] In addition to this fact, the rate of recurrent infection after revision surgery is very high (about 33%),[6] which significantly increases the cost per treatment, with a range that oscillates from $17 000 to $150 000 per patient.[7] These bone infections are generally triggered by pathogens of the genus Staphylococcus,[8] specifically Staphylococcus aureus.[9] This strain, present in the skin, is a very virulent opportunistic pathogen that has different resistance mechanisms, which make it so strong and unassailable.[10] Among them, biofilm formation is the most problematic one, as it provides bacteria with a long-term survival environment through different mechanisms. First, biofilms provide a physical and impenetrable barrier to antibiotics and immune cells, thus preventing the action of antibiotics, macrophages phagocytosis, and reactive oxygen species (ROS) destruction produced by the immune system cells. Second, the bacteria residing in the biofilm are particularly pathogenic due to their phenotypic diversity, which makes the biofilm bacteria highly resistant to antimicrobial treatment and can subsist drug doses up to 1000 times higher than in their planktonic phenotype.[11,12] Furthermore, the surrounding tissue suffers irreversible damage, causing bone resorption, both directly through the bacteria’s own virulence factors and indirectly through host inflammatory issues.[13,14]
When an infection is diagnosed in prosthesis, one of the challenges is its complete removal from the affected area (including the implant and the surrounding necrotic area). In general, the treatment consists of subjecting the patient to a massive administration of antibiotics during long hospital stays. In most cases, it is necessary to replace the prosthesis by a new surgical intervention that involves the implantation of poly(methyl methacrylate) beads loaded with gentamicin or vancomycin for local treatment.[15] However, success depends on the complete removal of infected tissue and implant, which is unfortunately very difficult to eradicate since latent bacteria are not entirely eliminated. The recurrent and resistant nature of bone infections as well as the associated high morbidity have driven the scientific community to dedicate much effort to develop new therapies aimed at improving the standards used today.[16] At present there is no effective therapy for this type of treatment, there is a great debate regarding the management of these infections in order to establish consistent and agreed guidelines in the national healthcare system.[17] Efforts are being directed toward the improvement of anti-infective biomaterials both from the prevention and cure points of view. In this respect, these biomaterials have to be adapted to the precise clinical application and all their properties have to be adjusted to accomplish the best anti-infective implementation, showing good biocompatibility[18–20] and appropriate tissue interactions. Advanced technologies are allowing the development of new biomaterials and surfaces capable of preventing bacterial adhesion, killing bacteria, and destroying the biofilm.[17,21] The purpose of this review is to emphasize the potential breakthroughs in bone infection treatment, which could be transformative for this damaging condition.
2. Strategies against Bone Infection
As it has been mentioned, there is a vital need to develop effective prevention and treatment approaches to minimize the risk of implant-related infection and its life-threatening complications.[12] Several reviews have shown many antibacterial biomaterials as well as different technological approaches to modify such biomaterials in order to prevent and combat such disease.[17,18] However, not all of them give an overview of the state of the art in all these new therapies combined. It is important to emphasize that the development of anti-infective biomaterials has to be adapted to the specific clinical application it is designed for. Therefore, the challenge is to achieve better anti-infective implementation together with safe biocompatibility and proper implant integration.[8] These efforts have mainly focused on designing biomaterial coatings that reduce bacterial adhesion and avoid the growth of the biofilm by constituting very promising prevention therapies. The most representative anti-infective coatings as well as the different technological approaches to achieve a better control in the bacteria adhesion without affecting to the implant integration will be discussed in Section 3.[22]
On the other hand, the development of tissue engineering and scaffolding processing techniques has enabled great advances in the treatment of the infections.[23] The “Holy grail” in this case is the design of 3D scaffolds which, while preventing the formation of a biofilm, are able to regenerate the bone tissue lost.[24] In this type of biomaterial, different strategies are proposed to combat and/or prevent the infection, which will be revised along the manuscript. An added value is the possibility to combine antibiotics with different delivery kinetics to afford a sustainable and enhanced therapeutic efficacy. Furthermore, the possibility to incorporate antimicrobial ions in addition to antibiotic therapy is emerging in recent years as a very effective alternative to avoid bacterial resistance (see Section 4).
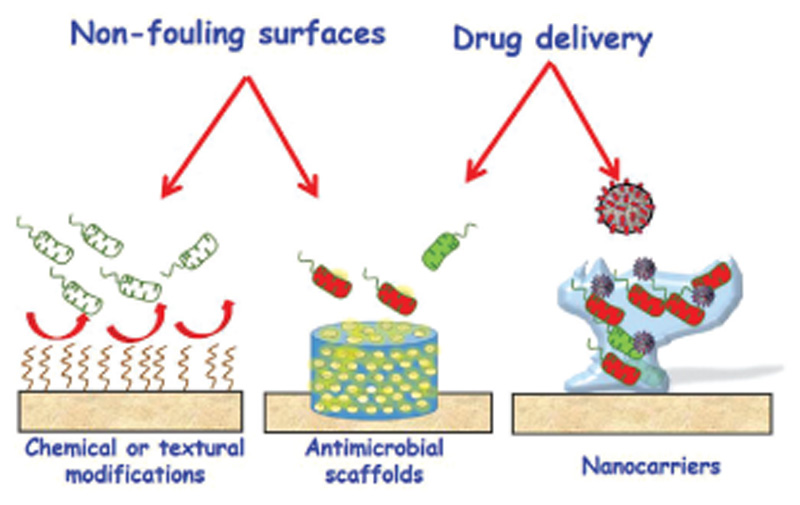
Finally, the irruption of nanotechnology in the treatment of infection offers promising alternatives for its treatment.[18] The possibility of release at the specific site and on demand offers great advantages over other therapies. Herein, we will focus on the design of nanocarriers with targeting and stimuli response capability, which will be discussed in Section 5.[25] Figure 1 schematizes the main strategies to be discussed in this review manuscript. In general, all these approaches to fight bone infection are critical to consistently accomplish favorable patient outcomes.
Figure 1.
Main strategies to combat bone infection, object of study in this review manuscript. In this sense, this manuscript addresses antimicrobial coating including the new technological advances on surface modification; scaffolding design including multifunctional scaffolds with both antimicrobial and bone regeneration properties; and nanocarriers with advanced properties (targeting and stimuli-response capabilities).
3. Antimicrobial Coatings on Implants
In this section, we focus on the prevention mechanisms underlying the development of implant-related infection.[26] These mechanisms can be divided into: i) manufacturing coatings with intrinsic antimicrobial properties such as silver and antibiotics and ii) using surface modification technologies to give them antimicrobial properties. These strategies incorporate surface coating by nanotopology and chemical modification. One of the advantages of these technological approaches is the use of drug-free surfaces that effectively inhibit the early adsorption of bacteria, which constitutes an added value in preventing bacterial resistance. One of the biggest challenges in the design of these structures is to design surfaces with an antagonistic behavior between bacteria and host cells.[26] Ideally, these surfaces should not only resist bacterial adhesion, but also induce rapid tissue integration and fast adhesion of host cells. Several reviews explain in detail the different adhesion mechanisms of the different cell-types in order to design these surfaces.[27,28]
3.1. Silver Coating
Silver has been widely used directly as coating onto orthopedic implants due to its broad activity against gram-positive and gram-negative bacteria.[12,17] Its mechanism of action is based on three main ways. First, the silver ions attached to the cell wall form morphological irregularities triggering cell lysis and intracellular contents release. Second, silver ions attach to sulfhydryl groups of proteins or DNA damage essential metabolic pathways and hinder DNA replication. And third, silver ions produce an excessive ROS provoking oxidative damage. Thus, the antimicrobial effect of silver ions prevents the biofilm formation, hindering the production of exopolysaccharides.
Therefore, silver ions or nanoparticles (NPs) have been used in the direct coating of orthopedic implants through different processes as galvanic deposition[29] or incorporated into biodegradable polymers.[30,31] Another strategies consist in the silver incorporation into bone cements by impregnation[32,33] or silver contained hydroxyapatite (HA) coatings by plasma spraying.[34–37] The clinical studies have shown that the silver efficacy substantially reduces implant-associated osteomyelitis showing a high antimicrobial activity against different bacterial strains (S. epidermidis, methicillin-resistant S. epidermis (MRSE), and methicillin-resistant S. aureus (MRSA)).[32] However, the therapeutic efficacy of its use in the clinic is being discussed in relation to its toxicity, which is depended on metal ions delivery to surrounding medium and therefore of the released doses as well as the delivery kinetics.[38–40] Moreover, it has been reported that the incorporation of silver into HA coatings promotes both good antimicrobial activity and osseointegration, which constitutes an added value. Recently, the inclusion of nanotechnology has meant a great advance in terms of efficiency and safety. In particular, silver NPs embedded onto titanium implants (Ag-NPs@Ti) through plasma ion immersion implantation (PIII)[41–43] have received much attention due to the broad antibacterial spectrum and the ability to modulate the surface morphology of biomaterials which enhance the osteogenesis properties. The bactericidal effect of AgNPs injected at the implant interface is related to the surface conductivity, indicating the importance of electron transport in their bactericidal effect. This type of technology has different limitations. Because although PIII-treated surfaces typically do not show toxicity, metal ion implantation above a certain threshold does cause release of the ions which is associated with a toxic effect. Therefore, using an appropriate concentration of injected metal is of vital importance in the application of the PIII-type technology onto implant surfaces. Also, these surfaces have a good antimicrobial action by direct contact, however, its effectiveness against planktonic bacteria is null and void. It shows the importance of combining with other antimicrobial agents to increase their effectiveness.
3.2. Antibiotic Coating
Antibiotics are considered the clinical standard for both local and systemic treatment of different infections caused by an extensive spectrum of pathogens and, unlike silver, are not considered highly cytotoxic. In this sense, a prophylactic coating of orthopedic implants with antibiotics should be a properly logical method to its prevention.[44,45] It is important to note that the local supply of antibiotics from a coating allows the use of high concentrations that would be systemically toxic.[21] Different antibiotic-loaded coating techniques have been considered to treat bone infections and are summarized in Table 1. The most usual approach for this coating is the attachment of biocompatible synthetic polymers loaded with antibiotics, such as poly(D,L-lactide) (PDLLA) and polylactic acid (PLA).[46,47] There are another strategies based on antibiotic incorporation by plasma chemical oxidized, nanofibers coated, or covalent bonding and multilayer (layer-by-layer deposition, LbL), which allow a sequential and sustained antibiotic release.[48–50] Gentamicin and vancomycin are the most studied antibiotics in implant coating due to their clinical applications. However, more recently, other types of antibiotics such as rifampicin are being successfully used in combination, in order to decrease or abolish the feared bacterial resistances.[8]
Table 1.
Most representative examples of antibiotic coatings on orthopedic implants.
| Implant | Antibiotic | Coating technology | Results | References |
|---|---|---|---|---|
| Tibia nails | Gentamicin | Poly(D, L-lactide) with “dipcoating process” | Clinical trial | [46,47] |
| (Palacos R+G; Heraeus Medical, Wehrheim, Germany) | Gentamicin | Antibiotic -impregnated bone cement | Clinical trial | [45] |
| Titanium | Vancomycin | Poly(ethylene glycol) (PEG)-based hydrogel covalently bound to Ti | Sustainable drug release (no initial burst release) In vivo rabbit model of S. aureus infection | [44] |
| Tialloy TiAl6V4 rods (Konigsee Implantate GmbH, Germany | Gentamicin | Plasma chemical oxidized | In vivo rat osteomyelitis model S. aureus inoculum High prophylactic effect on implant-related osteomyelitis | [48] |
| PEEK implants | Gentamicin | LbL self-assembled | Degradable multilayers that sequentially deliver the antibiotic and the osteoinductive growth factor (BMP-2). In vivo rat model infected with S. aureus | [50] |
| Locking peg titanium | Linezolid-rifampin | Nanofiber coating by electrospun | In vivo rabbit model of orthopedic implant-associated infection (OIAI) | [49] |
| Titanium | Antimicrobial peptide (Melamine) | Covalent bonding surface | In vivo females mice with S. aureus and P. aeruginosa | [56] |
| Tialloy | Gentamicin | Anodized Ti Nanotubes | Controlled-antibiotic release. In vitro studies against S. aureus and P. aeruginosa. In vivo osteomyelitis rabbit model infected with S. aureus | [51–53] |
One of the major risks associated with prophylactic antibiotic implant coatings is the antimicrobial-resistant strains. Attention should be paid to the development of new classes of antimicrobials to avoid the mechanisms of constantly evolving bacterial resistance mechanisms. An essential factor in the management of these infections is the control of drug release kinetics. However, it is difficult to achieve controlled release of the antibiotic from the implant coating. Ideally, the system should have a two-phase profile, consisting of a first stage of “burst effect” where high drug concentrations are achieved followed by sustained release above the minimum inhibitory concentration that will destroy any remaining bacteria.[50] Many research efforts have been invested in identifying and developing coatings that allow the controlled release of the drug. The different strategies are aimed at controlled delivery from nanostructured systems such as titania nanotubes[51–53] or mesoporous silica nanoparticles (MSNs)[54,55] that will be addressed in the following sections.
In addition to silver and antibiotics, the anchoring of antibacterial peptides on the implant surface is emerging as a promising alternative due to its high efficacy, nonbacterial resistance, and biocompatibility.[56] Different reviews have explored several strategies to anchor these peptides to the surface, which escape the subject of this review.[57–59]
3.3. Surface Nanotopology
3.3.1. Ti Nanocolunnns
Currently, it has been suggested that some natural surface morphologies, as those seen on insect wings or in the lotus flower, have solid antimicrobial action.[60,61] The antibacterial action of these biological surfaces can mainly be ascribed to the mechanical interactions between the attaching bacteria and the nanoscale of such surfaces.[62–64] Although current studies also point to the production of bacterial matrix as the nanoscale directly affects to the bacterial mobility. There are many technologies to develop these “bioinspired” nanopatterned surfaces onto metallic implants.[65,66] Among the most environmentally friendly techniques are simple hydrothermal etching and magnetron sputtering (MS), respectively.[67] The studies reveal that obtaining a wide range of nanotopographies depending on the methodology is used. Our research has focused on the fabrication of titanium nanocolumnar coatings onto typical orthopedic device (Ti6Al4V) surfaces using glancing angle deposition technique by magnetron sputtering (MS-GLAD).[68] This technology allows fabricated nanostructured coatings with different nanotopographies depending on the oblique angle deposition with magnetron sputtering onto the surface of Ti-6Al-4 V disks.[68] Moreover, currently it has been demonstrated that its great versatility is able to cover real implants with very heterogenous morphologies.[69]
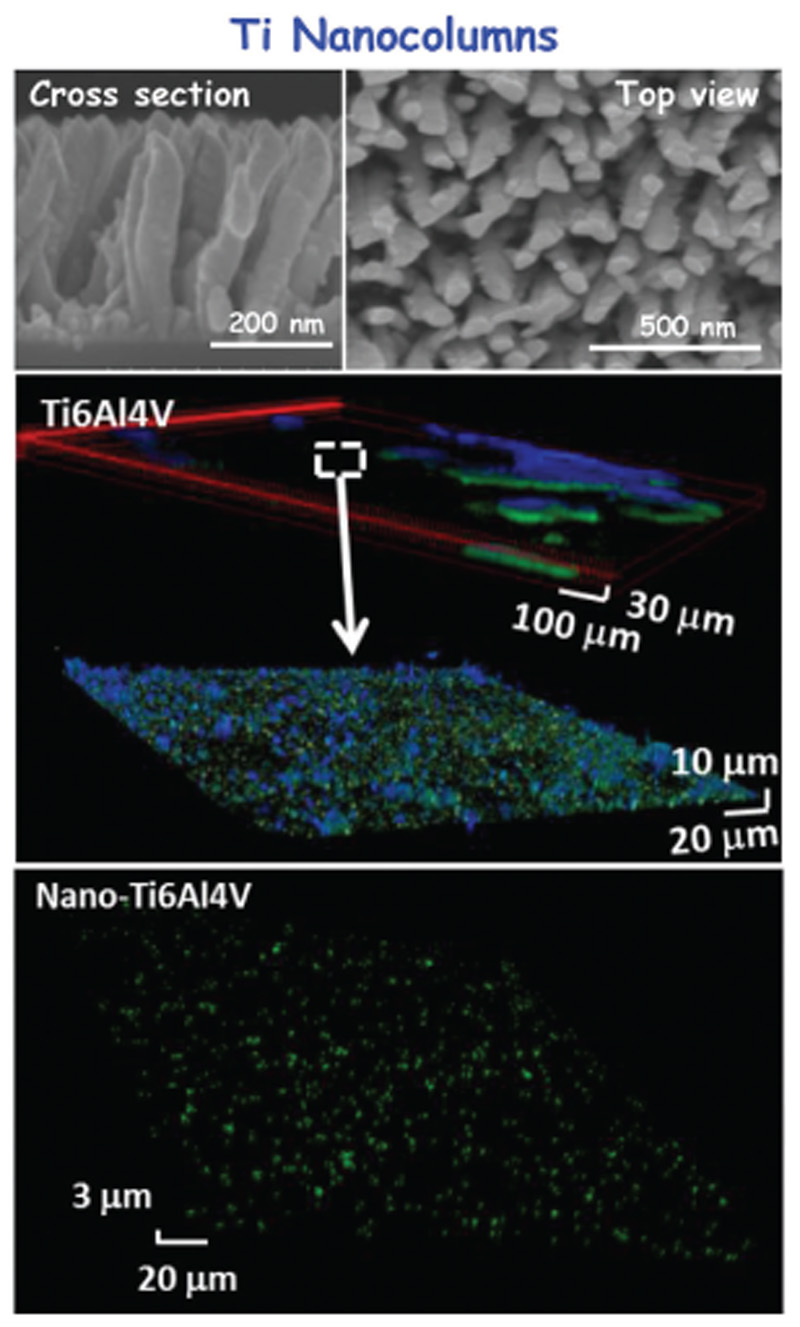
To date there is no consensus on the nanotopological characteristics and their bactericidal effect. Modaresifar et al.[67] have concluded that different types of nanopatterns with heights from 100 to > 900 nm, widths between 10 and 300 nm, and spacing of < 500 nm exhibit bactericidal properties. Most of these studies have also examined the impact on eukaryotic cells, determining that such surfaces exhibit adequate behavior with the exception of nanopatterns with extremely high aspect ratios. Figure 2 summarizes the main derived results of MS-GLAD technique onto Ti6Al4V disks. After MS-GLAD treatment, the Ti-based implant is fully coated with nanocolumns with lengths between 250 and 350 nm and diameters between 40 and 60 nm, separated by 100–200 nm. This nanotopography displays a superhydrophobic nature, showing an opposite behavior toward bacteria and osteoblast cells. While such structures prevent the adhesion of bacteria and the formation of biofilm, they simultaneously allow the colonization and proliferation of osteoblastic cells. Figure 2 depicts how the biofilm is formed on the bare Ti6Al4V surface, showing a green bacterial layer (living bacteria) covered with a blue layer corresponding to the mucopolysaccharide matrix of the biofilm. On the contrary, on the titanium covered with nanocolumns (Nano-Ti6Al4V), no biofilm appears, showing isolated bacteria on the surface of the implant. These findings represent a milestone in this field with important implications, not only for the quality of life of patients, but also for the promotion of a new generation of nanomaterial-based orthopedic implants.
Figure 2.
Results derived from the antimicrobial properties of Ti6Al4V implants by means of a nanopatterning coating enhanced by MDGLAD.[68] Top: SEM micrographs showed the full coating onto Ti-based implant by well-defined nanocolumns. Down: Confocal microscopy studies compared the surface of naked titanium, before and after coating, upon incubation with S. aureus bacteria. The reusable samples show that when the titanium implant is bare the biofilm is formed, while the nanocolumns coating inhibits the formation of the biofilm.
3.3.2. Ti Nanotubes
Another nanopatterning surface modification consists of the fabrication of titania nanotube coatings. In this case, the anodization technique is used to obtain this nanotube-type structure on the surface of metallic implants.[20] These nanotube coatings offer the advantage not only of inhibiting bacterial adhesion and promote bone formation but also the potential use as drug delivery systems.[70,71] In this sense, it has been demonstrated that specifically, titanium nanotubes increase osteoblasts adhesion to the surface, being inversely relative to the diameter of the nanotube. Moreover, titanium nanotube arrays have considerably reduced initial adhesion and colonization of Staphylococcus-type bacteria.[51,72] The greater the effect is the smaller the diameter of the nanotube will be. Therefore, drug-free nanotubes improve the adhesion of osteoblasts and reduce bacterial adhesion, being a promising technology for the manufacture of future implants.
In addition, this nanostructure has the property of being able to hold a large number of antimicrobial agents. Thus, this nanotube-type coating has been loaded with gentamicin or combined with vancomycin, showing a higher therapeutic efficacy due to the antimicrobial effect of antibiotic release as well as cytocompatibility.[51,73] These findings have been also confirmed in vivo, concluding that these gentamicin-loaded nanotubes can significantly reduce the growth of implant-associated infections in a rat model.[52]
3.4. Chemical Modification of the Surface
Wettability plays a key role in bacteria adhesion, with very hydrophilic surfaces being widely used to create nonfouling surfaces and able to inhibit the bacteria attachment. It is important to note that in Section 3.3, the nanotopological surfaces have super-hydrophobic nature, which also leads to nonbacterial adhesion surfaces. Thus, both ends, superhydrophobic and hydrophilic features, are associated with the inhibition of bacterial adhesion. In this section, the main strategies to create hydrophilic surfaces will be discussed.
3.4.1. Polyethylene Glycol (PEG) Coatings
One of the most commonly used approaches consists of grafting PEG polymer onto the surface to create an anti-infection effect.[74] Although PEG coating itself has no bactericidal nature, its intrinsic nonfouling features decrease the bacterial interaction with its surfaces. Different approaches can be followed in order to attach different hydrophilic polymers to the biomaterials surfaces, either physical or chemical modification has been used as covalent grafting or plasma polymerization as more representative.[75] In general, such approaches are used on metallic implants as titanium and stainless steel surfaces, which are superficially modified by synthetic polymer PEG in order to avoid the bacterial adhesion.[76,77] In vitro studies have shown low protein adhesion and inhibition of different bacterial strains in this type of PEG-modified metallic implants. However, PEG-based coatings do not only inhibit bacterial adhesion, but also eukaryotic cells. It has been demonstrated that these surfaces prevent the adhesion of mesenchymal cells and/or osteoblasts, compromising the integration of the implant with the native tissue.[78] In order to prevent this undesirable effect, it has been proposed that grafting biological active molecules as bone morphogenetic protein-2 (BMP-2) and RGD peptide (Arg-Gly-Asp) can enhance the integration processes.[79,80] Recently, it has been designed on titanium Kirschner wires, a smart nontoxic, biodegradable PEG-poly(propylene sulfide) (PEG-PPS) polymer coating as vehicle to release different antibiotics as vancomycin and tigecycline. Both in vitro and in vivo studies show that this smart polymers is able to release the antibiotic through a reactive oxygen cascade initiated by the presence of bacteria.[81]
3.4.2. Zwitterionic Surfaces
Another strategy to confer hydrophilic features is the creation of zwitterionic surfaces, which have an equal number of both positive and negative charged groups maintaining overall electrical neutrality.[82] The peculiarity of these surfaces is that the water molecules are more compactly distributed onto them, producing a hydration layer which forms a physical and energetic barrier that impedes the adsorption of nonspecific proteins or adhesion of bacteria and biofilm formation, respectively.[83] Moreover, one of the strengths of this process is that it allows the adhesion of native cells without affecting their cell proliferation or differentiation.[28,82] These findings justify the emergence of zwitterionization technology as a groundbreaking strategy to provide biomaterials with anti-infective surfaces while preserving the integration processes of the biomaterials themselves.
The zwitterionization process of biomaterials basically consists t in the functionalization of their surfaces with an exhaustive control in the atomic level.[80] There are multiple approaches used to confer bacterial-repelling surfaces. First, grafting with zwitterionic polymers, which bear varied positively and negatively charged moieties within the same biomolecule and have overall charge neutrality. In general, this methodology requires long synthesis processes in several stages, which can have an impact on homogeneity and with relatively expensive costs.[84] Second, grafting with small molecules as different amino acids such as cysteine and lysine,[85,86] sulfobetaine derivatives,[87] showing simultaneously positive and negative charges in the same molecule, depending on the pH of the environment. It has been shown that using simpler molecules reduces the complexity of the synthesis processes and the biocompatibility of the final material is not as compromised. An added value is to create a surface on/off switch depending on pH; in order to massively release an antibiotic load and have an anti-infective behavior by lowering the pH.[88,89] Another simple strategy to create zwitterionic surfaces is the direct and simultaneous grafting of different organosilanes, one bearing positive charge and another negative charge, respectively.[90] It is possible to tailor the zwitterionization grade by changing the proportions between both organosilanes. This bifunctionalization has allowed to create zwitterionic surfaces in different biomaterials such as silica-based NPs,[91] HA,[92] titanium implants,[93] and mesoporous bioactive glasses.[94,95] The strength of this type of surface is its ability to inhibit early bacterial adhesion and later biofilm formation without affecting the adhesion and biocompatibility of native cells. With this type of surfaces, it is possible to achieve up to 99% inhibition of bacterial adhesion which is increased when combined with certain antibiotics.[96]
4. 3D Antimicrobial Scaffolds
Nowadays, the development of 3D porous scaffolds is able to stimulate bone regeneration which makes the immediate upcoming of advanced therapies to regenerate bone possible.
Scaffolds for bone tissue engineering combine mechanical support requirements while serving as matrices for cell attachment and proliferation, therefore inducing bone regeneration at the same time. One of the main challenges after scaffold implantation is that they face bacterial colonization and resistance before completing bone repair. This issue can be overcome through the design of multifunctional scaffolds with both antimicrobial and bone regeneration properties to provide an aseptic environment needed for bone regeneration.[97–99]
Also, the possibility to combine two or more drugs in a single scaffold opens the possibility to treat more efficiently the infection and even to treat situations where two bone pathologies take place simultaneously. The tendency to multi-therapy or combined therapies comes true with dual scaffolds that, apart from the ability to regenerate the bone defect, can combine, e.g., proangiogenic or anticancer actions with the treatment or prevention of the infection. In the actual context of personalized medicine, this possibility would yield patient-specific scaffolds for advanced bone tissue engineering applications.
Furthermore, latest advances in the research area into staphylococcal-induced bone infection lead to the development of 3D scaffolds as models of infection. Recently, a collagen glycosaminoglycan scaffold, which represents the physiological bone microenvironment, has been seeded with both osteoblast and S. aureus and used as a representative 3D model of infection. The results of this study validate crucial events not observed before in 2D which are critical for understanding the advancement of bone infection using a 3D model.[100]
In this section, we offer a current overview on the recent development of scaffolds able to fight infection as a promising approach for bone repair. The classification is made according to the incorporated microbicidal agent on the scaffold.
4.1. Scaffolds with Intrinsic Antimicrobial Effect
This section describes scaffolds that possess antimicrobial activity due to the presence of molecules that themselves have antibacterial properties. The mechanism of action of these scaffolds is not based on the release of the antimicrobial agent, since it remains on the surface or in the own composition of the scaffold. Therefore, their mechanism is associated with the interaction of the bacteria with the scaffold surface where the antimicrobial agent is exposed producing an electrostatic interaction with the bacterial membrane or wall and destabilizing it (see Table 2).
Table 2.
3D antimicrobial scaffolds: scaffolds with intrinsic antimicrobial effect. Mechanism: presence of molecules with antimicrobial activity and interaction of bacteria with the scaffold surface.
| Scaffold composition | Antimicrobial agent | Fabrication technique | Results | Reference |
|---|---|---|---|---|
| PCL/HA composite surface modified with ϵ-poly-L-lysine | ϵ-Poly-L-lysine | 3D printing and fused deposition modeling technology | In vitro antibacterial activity against S. aureus, E. coli, and S. mutans | [102] |
| Chitosan/nanoHA/zoledronic acid | Chitosan | In situ precipitation | In vitro antibacterial activity against clinical pathogenic S. aureus and E. coli | [104] |
| Polylactide-co-glycolide/HA | Quaternized chitosan | 3D printing | In vivo infected bone defect models: femoral shaft defects in rats and femoral condyle defects in rabbits inoculated with S. aureus | [105] |
In this context, a polycaprolactone (PCL)/HA composite scaffold produced by 3D printing and the fused deposition modeling technology have been surface modified with ϵ-poly-L-lysine. ϵ-Poly-L-lysine is naturally occurring or biosynthesized homopolyamide. It is made up of 25–35 linear L-lysine residues which possess antibacterial activity and a wide antimicrobial spectrum as well as the advantage that microorganisms do not easily develop resistance to this antimicrobial polypeptide.[101] The composite scaffolds showed an exceptional broad-spectrum antimicrobial actions in vitro against S. aureus, E. coli, and S. mutans, and the antibacterial activity of EPL/PCL/HA scaffolds was retained for a prolonged time period.[102]
Another natural polymer with excellent antibacterial property is the polycationic linear polysaccharide chitosan.[103] Despite its numerous advantages, such as biocompatibility, biodegradability, antibacterial, and antioxidant activities, chitosan is not used alone in scaffold due to its poor mechanical strength and fast degradation rate. However, in combination with nanoHA scaffolds with mechanical properties, good porosity and bone regeneration ability can be processed. For example, a novel chitosan/nanoHA/zoledronic acid scaffold prepared with a straightforward method of in situ precipitation showed outstanding antimicrobial activity against clinical pathogenic S. aureus and E. coli. In addition to the osteoinductivity, the prepared CS/nHA/Zol scaffolds also had a multifunctional feature thanks to the zoledronic acid, which provides advantages in bone tumor therapy.[104] Similarly, the grafting of quaternized chitosan on a 3D-printed polylactide-co-glycolide/HA porous scaffold is endowed with antimicrobial functionality to the osteoconductive or osteogenic scaffold. A recent study has validated the improved anti-infection and bone regeneration properties of these quaternized chitosan-grafted scaffolds in different infected bone defect models in vivo.[105]
4.2. Scaffolds Loaded with Antibiotics
As explained above, a scaffold with both antibacterial and osteoinductive properties for the regeneration of infected bone defects is a current clinical need for bone repair. In this regard, a simple approach may be to incorporate antibiotics in the scaffold to achieve a sustained and local release for prevention or treatment of bacterial infection in combination with bone regeneration. There are several examples in the literature that we have classified attending to the composition of the scaffold, i.e., synthetic organic biopolymers, inorganic scaffolds, and composite polymer/bioceramic scaffolds. The examples shown in this section also take into account the processing method to fabricate the final device and the incorporated antibiotic (see Table 3).
Table 3.
3D antimicrobial scaffolds: scaffolds loaded with antibiotics. Mechanism: sustained and local release of antibiotics for prevention or treatment of bacterial infection.
| Scaffold composition | Antimicrobial agent | Fabrication technique | Results | References | |
|---|---|---|---|---|---|
| Synthetic organic biopolymers | Poly(ϵ-caprolactone)/polylactic acid | Tetracycline hyd rochloride | Thermally induced phase separation technique | In vitro and in vivo studies: rat femoral defect model for bone formation assessment | [106] |
| Polyethylene terephthalate fibrous matrix surface phosphorylated with poly(hydroxyethyl methacrylate) | Ciprofloxacin | Wet spinning for the preparation of the virgin PET fibrous matrix, then surface phosphorylation and in situ free radical-initiated polymerization of hydroxyethyl methacrylate | In vitro antibacterial activity against S. aureus and E. coli | [107] | |
| Electrospun PCL nanofibers decorated with PLGA) particles | Rifampicin | Electro-hyd rodynamic technique | In vitro antibacterial activity against S. aureus and E. coli | [108] | |
| Electrospun fibers of poly(L-lactide) aminolyzed and added to a hydrogel scaffold of silk fibroin/oxidized pectin | Vancomycin | Electro-hydrodynamic technique | In vitro antibacterial activity against methicillin-resistant S. aureus (MRSA) | [109] | |
| Poly(ϵ-caprolactone) and PEG | Roxithromycin | Melt electro-hydrodynamic 3D printing | In vitro antibacterial activity against S. aureus and E. coli | [110] | |
| Inorganic scaffolds | Nanocrystalline apatite uniformly embedded into mesostructured SiO2-CaO-P2O5 glass wall of hierarchical meso-macroporous 3D scaffolds (MGHA nanocomposite) | Levofloxacin | Rapid prototyping technique | In vitro antibacterial activity against S. aureus biofilm | [111] |
| Mg-Ca-TiO2 (MCT) composite scaffolds | Doxycycline | Space holder method | In vitro antibacterial activity against S. aureus and E. coli | [112] | |
| Bioactive monticellite scaffolds | Ciprofloxacin | Space holder method | In vitro antibacterial activity against S. aureus and E. coli | [113] | |
| Composites polymer/biocera mie scaffolds | Nanocomposite bioceramic formed by particles of nanocrystalline apatite embedded into amorphous mesoporous bioactive glass in the SiO2-P2O5-CaO system and polyvinyl alcohol. Hierarchical 3D scaffold coated with gelatin-glutaraldehyde | Rifampin, levofloxacin, and vancomycin | Rapid prototyping technique | In vitro antibacterial activity against S. aureus and E. coli biofilms | [114] |
| Gelatin/β-tricalcium phosphate (β-TCP) composite porous scaffolds | Vancomycin | Freeze-casting method | In vitro antibacterial activity against S. epidermidis
In vivo chronic osteomyelitis models of rabbits |
[115] | |
| Mesoporous bioactive glass combined with poly-(L-lactic-co-glycolic acid) | Vancomycin | Freeze-drying fabrication | In vitro antibacterial activity against S. aureus | [116] | |
| Polylactide and nanoHA-graft-polylactide | Vancomycin | Electrospinning | In vitro antibacterial activity against S. aureus | [117] | |
| Polyhyd roxybutyrate/poly(ϵ-caprolactone)/sol–gel-derived silica scaffolds | Levofloxacin | Electrospinning | In vitro antibacterial activity against S. aureus and E. coli | [118] | |
| Biphasic calcium phosphate/chitosan | Levofloxacin | Sintering-free robocasting deposition as additive manufacturing technique. The addition of levofloxacin to the extrudable inks is possible due to the nonexistence of a sintering step | In vitro antibacterial activity against methicillin susceptible S. aureus (MSSA) strain from culture collection and one clinical isolate methicillin-resistant S. aureus (MRSA) | [119] | |
| PCL/HA nanocrystals (composite slurry) and hyaluronic acid/gelatin (hydrogel-based bioink) | Rifampin, daptomycin, and viable macrophages | 3D bioprinting, which makes possible the incorporation of cells | In vivo mouse model of S. aureus craniotomy-associated biofilm infection | [120] |
4.2.1. Synthetic Organic Biopolymers
A number of biopolymers, such as PCL, poly(L-lactide), poly(lactic-co-glycolic acid) (PLGA), poly(hydroxyethyl methacrylate), and PEG have been studied for this purpose.
For example, a poly(ϵ-caprolactone)/polylactic acid scaffold incorporating tetracycline hydrochloride was prepared by a thermally induced phase separation technique and successfully tested in vivo.[106] The biodegradable polymer poly(hydroxyethyl methacrylate) impregnated with ciprofloxacin has been used for the surface phosphorylation of a polyethylene terephthalate fibrous matrix.[107] The electro-hydrodynamic technique has been used to fabricate polymeric electrospun scaffolds composed of PCL nanofibers decorated with PLGA particles loaded with rifampicin.[108] Also via this technique, electrospun fibers of poly(L-lactide) and vancomycin were aminolyzed and then added to a hydrogel scaffold of silk fibroin/oxidized pectin.[109] More recently, a melt electro-hydrodynamic 3D-printed poly(ϵ-caprolactone) and PEG scaffold has been loaded with roxithromycin as an anti-infective implant for bone tissue engineering.[110]
4.2.2. Inorganic Scaffolds
A pH-sensitive 3D hierarchical meso-macroporous 3D scaffold based on MGHA nanocomposite has been fabricated by rapid prototyping technique. In this scaffold, nanocrystalline apatite has been uniformly embedded into the mesostructured SiO2-CaO-P2O5 glass wall whose mesopores have been loaded with levofloxacin as antibacterial agent. Remarkably, these scaffolds showed a sustained levofloxacin release at physiological pH (pH 7.4), which significantly increases when pH decreases to specific values of bone infection processes (pH 6.7 and pH 5.5).[111] Other examples deal with Mg-Ca-TiO2 composite scaffolds fabricated via space holder method and coated with different concentrations of doxycycline[112] or with bioactive monticellite scaffolds containing ciprofloxacin.[113]
4.2.3. Composites Polymer/Bioceramic Scaffolds
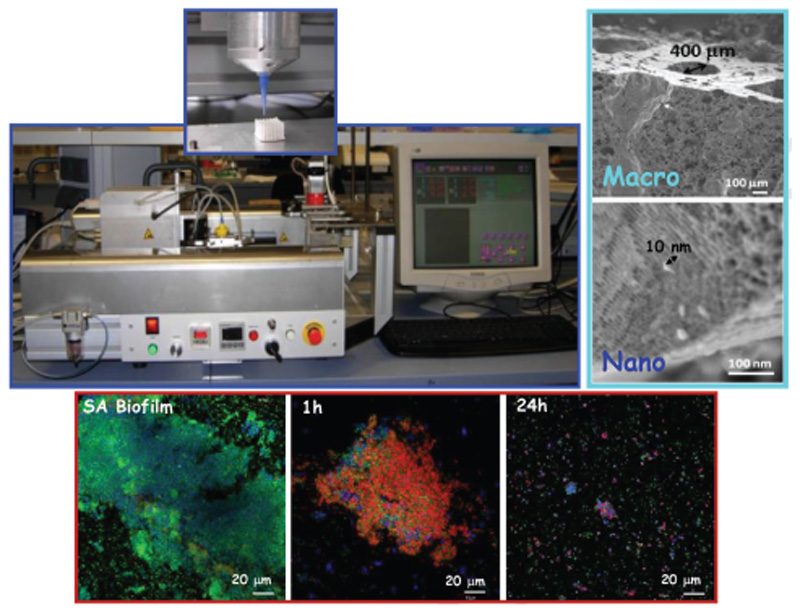
The rapid prototyping technique has been used to prepare hierarchical 3D multidrug scaffolds based on the nanocomposite bioceramic formed by particles of nanocrystalline apatite embedded into amorphous mesoporous bioactive glass in the SiO2-P2O5-CaO system and polyvinyl alcohol. These hierarchical 3D scaffolds have been provided with an external coating of gelatin-glutaraldehyde. These scaffolds contain three antimicrobial agents (rifampin, levofloxacin, and vancomycin), which have been confined in different compartments of the material to acquire different release kinetics and successful combined therapy. Levofloxacin was loaded into the mesopores of a nanocomposite bioceramic part, vancomycin was localized into polyvinyl alcohol biopolymer part, and rifampin was loaded in the external coating of gelatin-glutaraldehyde. Results confirm an early and fast release of rifampin followed by sustained and prolonged release of vancomycin and levofloxacin, respectively, which are mostly administered by the gradual in vitro degradability of these scaffolds. This dual treatment is able to abolish gram-positive and gram-negative bacteria biofilms as well as reduce the bacterial growth.[114] Figure 3 shows the fabrication of 3D multifunctional scaffolds by the rapid prototyping technique.
Figure 3.
Schematic representation of 3D multifunctional scaffolds development by Rapid Prototyping technique.[111,114] A photography of a 3D-bioplotter envisiontec device is shown in the upper image. On the right, SEM micrographs display the hierarchical macro-mesoporosity of 3D scaffolds derived of this technique. Confocal microscope study showing de antimicrobial effect of 3D scaffolds containing three different antibiotics (levofloxacin, vancomycin, and rifampicin) distributed in different compartments of the 3D scaffold is shown on the bottom.
Vancomycin has been loaded in several types of scaffolds such as a gelatin/β-tricalcium phosphate (β-TCP) composite porous scaffolds,[115] into mesoporous bioactive glass combined with poly-(L-lactic-co-glycolic acid) and prepared by freeze-drying fabrication,[116] and also in multifunctional electrospun composite scaffolds made of polylactide and nanoHA-graft-polylactide.[117]
Levofloxacin has also been incorporated in a variety of composite scaffolds. For instance, it has been loaded into electrospun hybrid scaffolds of polyhydroxybutyrate/poly(ϵ-caprolactone)/sol–gel-derived silica scaffolds.[118] Also, the development of sintering-free biphasic calcium phosphate/chitosane composite scaffolds using robocasting deposition as additive manufacturing technique provides the chance to obtain drug-loaded scaffolds by adding levofloxacin to the extrudable inks due to the nonexistence of a sintering step.[119]
As an interesting approach, the incorporation of cells is also possible in 3D bioprinted composite scaffolds. A 3D scaffold containing rifampin and daptomycin printed in a composite slurry of PCL and HA nanocrystals, with viable macrophages incorporated into a hyaluronic acid and gelatin-based hydrogel-based bioink, was fabricated through bioprinting to harness the potent antimicrobial action of macrophages together with antibiotics using a mouse S. aureus craniotomy-associated biofilm model.[120]
4.3. Scaffolds Doped with Metallic Ions
As it has been explained, antibiotic resistance is fast becoming a serious threat to public health. The increasing number of new cases of bacterial resistance to “last resort” antibiotics, together with the scarce new antibiotic approvals, makes necessary the development of free-antibiotic alternatives, such as the use of metallic ions which possess antibacterial properties (e.g., copper, silver). The bactericidal effect of metallic ions is due to several mechanisms of action against bacteria.[121] These mechanisms are explained below in Section 5. One strategy to locally deliver the metal ions at the defect site is to incorporate them in the scaffold itself (see Table 4).
Table 4.
3D antimicrobial scaffolds: scaffolds doped with metallic ions. Mechanism: free-antibiotic alternative: local deliver of metal ions which possess antibacterial properties.
| Scaffold composition | Antimicrobial agent | Fabrication technique | Results | References |
|---|---|---|---|---|
| Porous 3D collagen-based scaffold containing copper-doped bioactive glass | Cu2+ | Freeze drying a co-suspension of collagen and copper-doped bioactive glass particles | In vitro antibacterial activity against S. aureus
In vivo chick embryo model to assess biocompatibility, angiogenic, and osteogenic response |
[122] |
| Sr/Zn-codoped porous scaffolds of HA | Zn2+ antibacterial activity (Sr2+ promotion of new bone formation and inhibition of bone absorption) | Ion-exchange method to produce ion-doped HA NPs followed by a foaming method to produce porous HA scaffolds | In vitro antibacterial activity against S. epidermidis | [123] |
| Hierarchical meso-macroporous 3D scaffolds composed by 80% SiO2–15% CaO–5% P2O5 (mol%) mesoporous bioactive glass (MBG) scaffolds substituted with 4.0% and 7.0% of ZnO | Zn2+ | Rapid prototyping technique | In vitro antibacterial activity against S. aureus | [124] |
For example, a collagen-based scaffold containing copper-doped bioactive glass has shown potential as a one-step treatment for osteomyelitis. This porous 3D collagen scaffold contains a bioactive glass, an established osteoconductive material, as a delivery platform for copper ions. Scaffolds demonstrated antimicrobial activity, without the use of antibiotics, against S. aureus (up to 66% inhibition) while also increasing osteogenesis (up to 3.6-fold increase in calcium deposition) and angiogenesis both in vitro and in vivo.[122]
Incorporation of two trace essential ions such as Sr2+ and Zn2+ offers combined advantages to improve the osteoinductivity and antimicrobial activity of a porous scaffold of HA. On the one hand, Sr2+ can induce new bone formation and reduce bone absorption and, on the other hand, Zn2+ ions possess antibacterial activity. The Sr/Zn-codoped porous scaffolds of HA were developed by an ion-exchange method to produce ion-doped HA NPs followed by a foaming method to produce porous HA scaffolds. The structural properties, biocompatibility, osteoinductivity, and antimicrobial action against Staphylococcus epidermidis were systematically investigated, demonstrating that these scaffolds could successfully reduce bacterial infection and induce bone tissue regeneration.[123]
Moreover, the Zn2+ ions have also been incorporated on hierarchical meso-macroporous 3D scaffolds composed of mesoporous bioactive glasses, showing both osteogenic and antimicrobial effects. The bacterial inhibition capacity of the scaffolds was studied against S. aureus with different amounts of ZnO in mesoporous structure. While the antimicrobial capability is effective to all studied composition, the amount of Zn2+ delivered from the scaffold with 4.0% ZnO was more favorable for HOS cell growth than when the ZnO is higher (7%).[124]
4.4. Scaffolds Incorporating NPs
One approach to regulate the dosing of metal ions or drugs to fight bone infection is to release them locally at the defect site using a carrier nanomaterial embedded in the scaffold itself. Therefore, the nanocarrier can modulate the release profile with the advantage that the NPs are confined and are not able to target other cells or tissues (see Table 5).
Table 5.
3D antimicrobial scaffolds. Scaffolds incorporating NPs. Mechanism: use of nanocarriers embedded in the scaffold to control the dosing of metal ions or antibiotic/drugs delivered locally at the defect site. Advantage: the NP carries are confined and are not able to target other cells or tissues.
| Scaffold composition | Antimicrobial agent | Fabrication technique | Results | References | |
|---|---|---|---|---|---|
| Antimicrobial metallic NPs embedded in the scaffold | β-tricalcium phosphate bioceramic scaffolds modified with silver NPs-graphene oxide nanocomposite | AgNPs | 3D printing followed by a soaking method | In vitro antibacterial activity against E. coli
In vitro osteogenic differentiation and proliferation of rabbit bone marrow stromal cells cultured in the scaffolds |
[125] |
| MgSrFe-layered double hydroxide/chitosan composite scaffold loaded with uniformly dispersed Ag NPs on the scaffold surfaces | AgNPs | Freeze-drying preparation of the precursor scaffold followed by deposition of AgNPs on the MgSrFe/CS composite | In vitro antibacterial activity against S. aureus biofilm | [126] | |
| In vitro osteogenetic differentiation of human bone marrow-derived mesenchymal stem cells cultured in the scaffolds | |||||
| Scaffolds containing chitosan and carboxymethyl cellulose decorated with AgNPs | AgNPs and chitosan | Freeze-drying method | In vitro antibacterial activity against E. coli and Enterococcus hirae (E. hirae) | [127] | |
| Chitosan, HA, and silver nanowires composite scaffold | Silver nanowires and chitosan | Freeze drying of the thermosensitive hydrogels after sol–gel transition | In vitro antibacterial activity against E. coli and S. aureus, methicillin-resistant S. aureus and S. saprophyticus | [128] | |
| Porous titanium scaffold possessing a micro/nanostructured titanate layer and provided with nanosilver encapsulated in physically cross-linked silk fibrin | Nanosilver | Metallic powder 3D printing followed by in situ hydrothermal growth of a metal oxide and cross-linking of silk fibrin to physically encapsulate nanosilver | In vitro antibacterial activity against clinically relevant pathogenic S. aureus bacteria biofilm | [129] | |
| Zinc oxide NPs nanocrystalline HA scaffolds coated by gelatine | ZnO NPs | Space holder technique | In vitro | [130] | |
| Cu NPs on carboxymethyl chitosan and alginate | CuNPs | Cross-linking of the polymer mixtures followed by freeze drying | In vivo rat ectopic osteogenesis and infection model inoculated with S. aureus clinically collected S. aureus | [131] | |
| Antibiotic-loaded NPs embedded in the scaffold | MSNs/gelatin matrix composite scaffold | Vancomycin-loaded MSNs | Freeze-drying method | In vitro antibacterial activity against S. aureus | [133] |
| MSNs immobilized on the surface of a nanoHA/polyurethane bioactive composite scaffold | Levofloxacin-loaded MSNs | Immobilization of the MSNs by pretreatment of scaffold with chitosan and subsequent cross-linking by vanillin | In vitro antibacterial activity against E. coli and S. aureus | [134] | |
| ZIF8 nanocrystals (metal-organic frameworks) embedded in chitosan scaffolds | Vancomycin-loaded ZIF8 | Wet spinning | In vitro antibacterial activity against S. aureus | [135] | |
| BMP2 encapsulated into gelatin microspheres and vancomycin encapsulated into gelatin microspheres drug-contained gelatin microspheres assembled on GO-functionalized Ti porous scaffold | Vancomycin encapsulated into gelatin microspheres | Deposition of GO nanosheets onto mussel-inspired polydopamine-modified Ti scaffolds prepared via a powder sintering method | In vitro antibacterial activity against S. epidermidis
In vivo ectopic bone regeneration in subcutaneous rat model to assess bone regeneration ability of the scaffolds |
[136] | |
| Incorporation of NPs in the scaffold for a dual effect | Chitosan/nanoHA scaffold doped with OD carbon dots | Chitosan combined with the photothermal effect of carbon dots | Physical mixing and freeze-drying method | In vitro antibacterial activity against clinically relevant S. aureus and E. coli
In vivo model in muscle pouches of rats inoculated with clinically collected S. aureus and E. coli |
[139] |
| Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold | Rifampin combined with the magnetic hyperthermia effect | Polymer sponge and sintering technique to fabricate 3D porous scaffolds from Mg2SiO4-CoFe2O4 nanocomposite | In vitro antibacterial activity against S. aureus | [140] | |
| Silk fibroin NPs loaded with vascular endothelial growth factor embedded in a silk scaffold containing vancomycin | Vancomycin combined with the delivery of angiogenic factors | Freeze drying followed by cross-linking | In vitro antibacterial against methicillin-resistant S. aureus (MRSA) | [141] |
4.4.1. Antimicrobial Metallic NPs Embedded in the Scaffold
In this sense, the sustained release of antimicrobial ions can be achieved from metallic nanostructures embedded in the scaffold which slowly lixiviate the antimicrobial ions. Silver is the most common metallic nanostructure used in this context, and it has been incorporated in a variety of organic and inorganic supports to form composite scaffolds with antibacterial activity.
For example, silver NPs synthesized via chemical reduction and uniformly dispersed on graphene oxide form a homogenous nanocomposite that was successfully modified on 3D-printed β-tricalcium phosphate bioceramic scaffolds by a simple soaking method to achieve bifunctional implants with antimicrobial and osteogenic properties. The antimicrobial action of the composite scaffolds was evaluated with E. coli.[125] Similarly, a MgSrFe-layered double hydroxide/chitosan composite scaffold was loaded with uniformly dispersed Ag NPs on the scaffold surfaces. In addition to the osteogenic effect achieved from the released Sr ions, the Ag NPs in the composite scaffold successfully avoid biofilm formation against S. aureus.[126]
A nanocomposite of cellulose nanowhiskers decorated with AgNPs was used to fabricate scaffolds containing chitosan and carboxymethyl cellulose using a freeze-drying method. The highly antimicrobial efficiency of the scaffold against both gram-positive and -negative bacteria may be ascribed to the synergistic effect of chitosan and AgNPs.[127] Synergistic effect has also been achieved in a scaffold prepared from chitosan, HA, and silver nanowires. Antimicrobial studies indicated that the composite formulation was able to inhibit bacterial growth in suspension, and able to totally inhibit biofilm formation on the scaffold in the presence of resistant bacterial strains such as MRSA.[128]
A fully porous titanium scaffold tailor-made by metallic powder 3D printing and subjected to in situ hydrothermal growth of a micro/nanostructured titanate layer, has been provided with nanosilver encapsulated in physically cross-linked silk fibrin. This silver-immobilized scaffold was tested employing the clinically relevant pathogenic S. aureus bacteria, showing decreased adherence of bacteria on the surface and active killing of those planktonic preserving from biofilm colonization.[129]
In addition to silver nanostructures, further studies deal with other metal elements incorporated into scaffolds in the form of NPs. For example, zinc oxide NPs impart antibacterial behavior on nanocrystalline HA scaffolds coated by gelatine.[130] Also, Cu NPs added to a mixture of anionic carboxymethyl chitosan and alginate led to the gradual cross-linking of the polymer mixtures further turned into a scaffold with an interconnected porous structure by freeze drying. Also, the CuNPs impart the scaffold the ability of killing clinical bacteria.[131]
4.4.2. Antibiotic-Loaded NPs Embedded in the Scaffold
Advanced nanocarriers such as MSNs have also been incorporated in scaffolds. From the firsts designs of “gated scaffolds” based on the combination of capped MSNs with porous biomaterials,[132] some examples for the treatment of infected bone defects have been reported taking into account the protection of the antibiotic in a drug-delivery nanocarrier.
For instance, vancomycin has been loaded in MSNs to form a composite scaffold in a gelatin matrix that effectively inhibits the growth of S. aureus.[133] As well, levofloxacin-loaded MSNs have been immobilized on the surface of a nanoHA/polyurethane bioactive composite scaffold. This scaffold offers satisfactory antibacterial activity against both gram-positive S. aureus and gram-negative E. coli bacteria.[134]
Other kinds of inorganic nanostructures such as metal-organic frameworks have also been used to be loaded with an antibiotic and then embedded in a scaffold for the potential treatment of serious bone infections like osteomyelitis. Recently, vancomycin was loaded into ZIF8 nanocrystals for a pH-responsive controlled release. Chitosan scaffolds coated with ZIF8/vancomycin were developed by wet-spinning to obtain 3D biocompatible scaffolds. These scaffolds induced a significant decrease of S. aureus activity.[135]
In addition to the inorganic nanostructures, organic-based drug-delivery systems have been combined in scaffold composite systems. BMP2 and vancomycin were separately encapsulated into gelatin microspheres and these drug-contained gelatin microspheres were assembled on GO-functionalized Ti porous scaffold. These scaffolds independently released multiple biomolecules with diverse physiochemical characteristics, without interfering with each other, therefore inducing bone regeneration and preventing bacterial infection.[136] Remarkably, the incorporation of antibiotic-loaded liposomes into scaffolds takes advantages of both liposomes as drug-delivery systems and scaffolds’ strength to provide a novel platform that is more appropriate for clinical applications.[137]
4.4.3. Incorporation of NPs in the Scaffold for a Dual Effect
More complicated scenarios can be fought by providing the scaffolds with NPs or nanosystems able to have an effect over additional pathologies to the infection, so that two issues present on the same tissue can be treated simultaneously. As an example, mesoporous bioactive glasses as an inorganic support with bone regenerative properties have been implemented with molecular gates demonstrating a useful approach for bone cancer and bone infection treatments.[138]
This dual effect or combined therapies for bone cancer and bone infection treatments has been reported for composite scaffolds able to yield a hyperthermia effect. A chitosan/nanoHA scaffold doped with 0D carbon dots was fabricated by a facile freeze-drying method to promote bone regeneration. Inspired by the outstanding photothermal effect of carbon dots, the scaffolds were applied in tumor photothermal therapy under near-infrared (NIR) irradiation. Moreover, the scaffolds showed different antimicrobial properties toward clinically collected S. aureus and E. coli, and their antimicrobial action was further improved under NIR irradiation.[139] In the same line, a magnetic nanocomposite was developed through a two-step synthesis approach in which CoFe2O4 NPs are prepared via sol–gel combustion method and then coated by sol–gel method with Mg2SiO4. This core@shell structure was used for the fabrication of 3D scaffolds through polymer sponge technique, resulting in a magnetic porous scaffold which is biodegradable and bioactive. Furthermore, the scaffold shows controlled release of rifampin having antibacterial effect against S. aureus and the exposure to different magnetic fields produces heat for several kinds of hyperthermia-based therapies.[140]
Silk fibroin NPs loaded with vascular endothelial growth factor were embedded in a silk scaffold loading with vancomycin. This scaffold represents a dual drug release platform with potential use for the treatment of contaminated bone injuries because it is able to simultaneously deliver antibiotic and angiogenic factors. The bactericidal effect against methicillin-resistant S. aureus, as well as the expression of the endothelial markers and matrix mineral production was confirmed for this dual effect scaffold.[141]
5. Mesoporous Silica NPs as Nanocarriers
NPs are used to target bacteria as an alternative to conventional antimicrobials, due to their specific physicochemical properties.[18,142,143] As it has been mentioned in the introduction, biofilm formation, the absence of novel molecules or drugs, and the conventional fail of antibiotics (because of AMR) are the main reasons for the increasing use of NPs for infection treatment.[18,144] Several metals, metallic salts, and metallic oxides have demonstrated to induce intrinsic antibacterial effects.[18,145,146] Silver NPs are the most promising of inorganic NPs in the treatment of bacterial infections but not the unique. Other metal NPs such as Au or Mg, and metal oxide NPs such as titanium dioxide, zinc oxide, or copper oxide, among others, are widely used in different studies to fight bacterial infection.[144,147–151]
The mechanism of action of the antibacterial effects of these metals remained unclear.[152,153] Recent studies have indicated that these metals decrease the bacterial activity as a result of several mechanisms as oxidative stress, formation of ROS, gene expression alterations, and DNA and membrane damage.[141,150] Bacteria can be found in a dormant or active state that makes it very difficult for antibiotics to act properly. As previously mentioned, NPs have several advantages to solve the classic antibiotic resistance problem due to their multiple mechanisms of action which are different to classical antibiotic mechanisms.[18,140–142] The higher the surface area, the smaller the size of these NPs, and the possibility to modify their shape, are very useful properties to prevent and destroy the biofilm.
Oxygen is a strong oxidant promoter that can be lethal for bacteria.[154] These metal NPs induce themselves an increase of oxygen-free radicals and ROS that produces oxidative stress damage in the structure of proteins, DNA, and different cell components, including membranes.[124,135] In addition, nonoxidative mechanisms are implicated in the intimate interaction between these types of metal NPs and bacteria.[155] There are different methods to modify the NPs with positive charges that interact electrostatically with the negative charges of bacterial walls. This union is critical to the antibacterial effect of metal-based NPs inducing the rupture of the membrane and accumulating by modifying the metabolic processes of the bacteria.[153] Furthermore, several metal NPs release metal toxic ions that react with the intracellular compartment and with several functional groups of nucleic acids and protein.[153] Ag+ is one of the main cations that trigger mechanisms against bacterial walls, especially gram-negative. These mechanisms affect the bacterial metabolism, inhibiting DNA replication and cellular respiration.[151,156] Cd2+ and Zn2+ ions bind to proteins present in sulfur-containing cell membranes by altering their metabolism.[140]
NPs not only have inherent antibacterial properties, but also can operate as nanocarriers of different drugs and antibiotics[18] by multiple drug-delivery mechanisms. With this purpose, these nanosystems must preserve the active compound from degradation and improve its bioavailability in the infection treatment. These systems have the possibility of being able to control the sustained release, very useful when an optimal dose release in a required time is pursued. In addition, these systems offer the possibility to use a combined therapy, using different antibiotics or drugs to respond to different stimuli, or even can provide a theranostic strategy, e.g., with high-resolution bimodal imaging and antibiotic/photodynamic combined therapy of osteomyelitis.[157] We can find different types of NPs with these properties: inorganic nanocarriers as mesoporous silica NPs–MSNs,[158,159] magnetic NPs, carbon nanodevices, and quantum dots,[126] polymer-based NPs and polymer micelles,[160] dendrimers,[161] liposomal NPs, or solid lipid NPs.[124] In this manuscript, we focused on MSNs as nanocarriers to prevent and/or kill bacterial infection.
5.1. MSNs
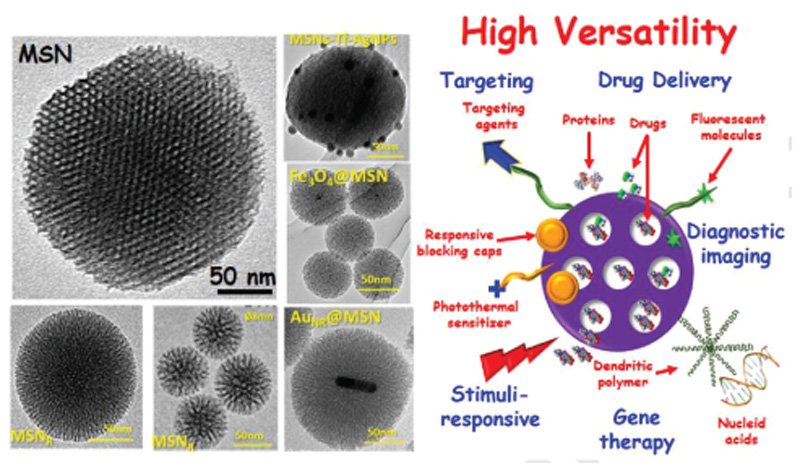
MSNs represent highly developed inorganic nanocarriers with an increasing interest in the field of drug-delivery systems[155,156] (Figure 4). In addition, MSNs have high versatility to create high-performing hybrid materials.[156] The main features of interest of these nanocarriers are the following: 1) porous and robust structure with high pore volume (> 0.6 cm3 g–1) and large surface area (> 700 m2 g–1), with maximal drug-loading capacities;[162] 2) variable mesopore size (2–10 nm), pore shape, and connectivity;[163] 3) regarding morphology, MSNs with 100–150 nm avoid fast clearance and acute toxicity;[164] 4) their surface can be chemically modified with different moieties;[155] 5) high biocompatibility both in vitro and in vivo;[160,165] 6) manageable degradability under living conditions;[166,167] 7) and high level of excretion.[168]
Figure 4.
Schematic representation of the MSNs versatility and their functionalization properties. Left: TEM micrographs showed different MSN-based NPs with different size, mesoporous arrangement, and core@shell type. Right: Depiction of the high MSNs versatility with different capabilities (targeting, drug delivery, stimuli-response, gene therapy, and diagnostic imaging).
Therefore, different types of MSNs, with several morphologies, sizes, mesopore sizes, and connectivity, can be simply produced in a relatively large-scale synthesis following a modification of the Stöber method.[160,169] In Figure 4, different types of transmission electron microscopy micrographs of MSN are represented: NPs with radial pore arrangement (MSNR) with 3D structure and greatly accessible mesopores, and MSNs coated with different inorganic NPs such as gold nanorods (MSN-AuNR), silver NPs (MSN-AgNPs), and magnetite NPs (Fe3O4) as core.
As we previously mentioned, modifications in surface materials can increase the targeting properties of NPs to develop stimuli-responsive nanosystems.[155] Although most studies or applications of MSN are in the field of cancer treatment,[155,160] several studies indicate the usefulness of these nanocarriers in the treatment of infection[18,25,170] and osteoporosis.[171,172] Herein, it will be only focused on the most recent reported advances for target, control release and drug delivery by MSNs in bone infection.
5.2. Selective Targeting
As it is well known, the NPs, and among them the MSNs, can be recognized by the immune system in tissues where macrophages are present.[155,160] MSNs can accumulate at nonrelevant tissues, leading to deficient accumulation in the target tissues where they must perform their function.[155,160] Consequently, it is necessary to improve the specificity to the target cell/tissue in order to improve their therapeutic efficiency, also reducing their possible secondary effects.
In case of cancer treatment, nanosystems are administered through the blood stream and tend to collect at the tumor areas, due to blood vessel structure with the presence of fenestrations in the diseased tissues.[25,160,161] This is due to the enhanced permeation and retention effect which works as passive targeting.[155,160,161] In the case of infection treatment, the aim is to develop MSNs with active targeting related to receptor-ligand affinities with the bacteria or with the biofilm, where the therapeutic effect would be produced specifically, enhancing antimicrobial effectiveness (Figure 5).
Figure 5.
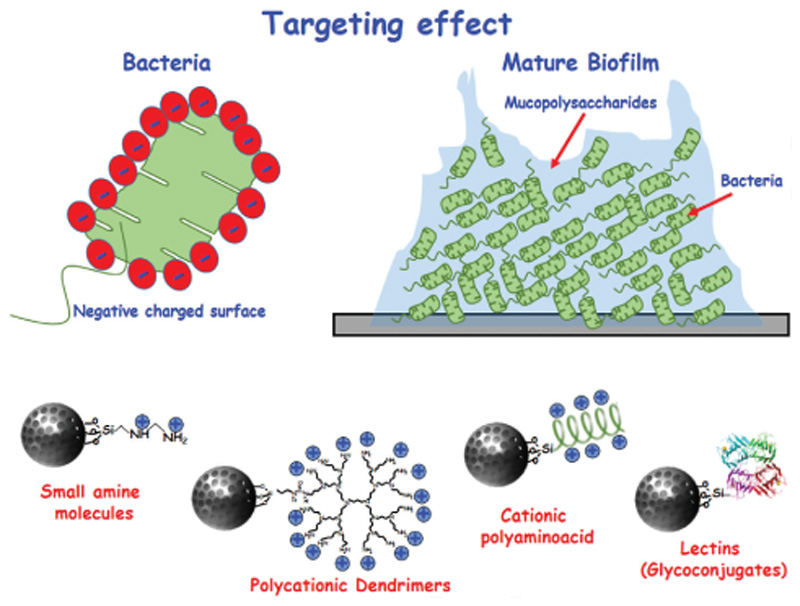
Schematic representation of MSNs with targeting capability to both bacteria and biofilm. Because of the negative charges in the surface of the bacteria wall, one of the strategies to target bacteria is to functionalize the surface with positive charges. In this sense, several small molecules and biomacromolecules have been used: amines, polycationic dendrimers, and cationic polyaminoacid (among others). On the other hand, the biofilm is formed by a protective external layer of mucopolysaccharide, which has high affinity properties. In this case, this glycoconjugate has been used successfully as targeting agent.
To destroy the bacteria (Figures 5 and 6), we can take advantage of the negative charge of the bacterial wall, to modify with positive charges the surface of the MSNs for a specific electrostatic interaction between them. Macromolecules such as cationic polypeptides or dendrimers[18,25] and small amine molecules,[18,25,173] can be used for this purpose (Figure 5). MSNs are then internalized by bacteria and able to kill them. To penetrate and destroy the bacterial biofilm (Figure 5), MSNs can be loaded with antibiotics into their pores and functionalized with lectins on their surface.[18,25,168,174] Lectins are molecules that specifically recognize the mucopolysaccharides present in the biofilm.[172,175] When the biofilm is specifically recognized by the lectins present in the surface of MSNs, the antibiotic is released removing the infection.
Figure 6.
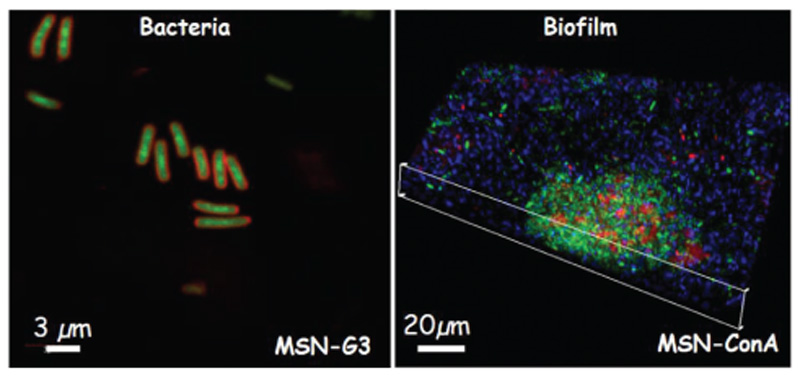
Confocal microscopy images of (left) bacteria-targeting capability of MSN-G3 materials and (right) biofilm-targeting capability of MSN-ConA, respectively. The external functionalization with a polycationic dendrimer (G3) favors the internalization ofthese modified nanosystems (MSN-G3) within the E. coli bacteria. The left image shows the bacterial wall in red and MSN-G3 nanosystem in green.[177] In the case of the external functionalization with the lectin (ConA), such modification enhances the internalization of the MSN-ConA nanosystem into the biofilm, even penetrating its innermost parts. The right image shows the biofilm composed by living bacteria in green and the biofilm matrix in blue, in this case the NPs are stained in red.[174]
At this respect, Yang et al.[176] designed and evaluated an effective alternative to traditional antibiotic nanocarrier, based on dendritic MSNs with controllable particle sizes and antibacterial enzyme delivery performance. This nanosystem is loaded with lysozyme, an antimicrobial enzyme, and presents great pore size of 22.4 nm and a small particle size of 79 nm, showing an optimal antimicrobial activity with a total inhibition of E. coli during at least 5 days. In another study by Vallet-Regí et al.,[177] MSNs as “nanoantibiotics” were loaded with levofloxacin (LEVO) inside the mesopores and functionalized by active targeting to bacterial membrane by polycationic poly[propyleneimine] dendrimer of third generation (G3), covalently grafted to the external surface of this nanocarrier. This nanosystem showed good internalization by gram-negative bacterial membranes (E. coli) with a positive antimicrobial effect because of the combination of polycationic dendrimers and LEVO (Figures 6 and 7). In a study by Pedraza et al.,[173] MSNs nanocarriers loaded with LEVO were functionalized by adding to their surface N-(2-aminoethyl)-3-aminopropyltrimethoxysilane, providing positive charges that increased their affinity with negatively charged bacterial wall. This nanocarrier completely destroyed S. aureus biofilm evaluated in the mentioned study.
Figure 7.
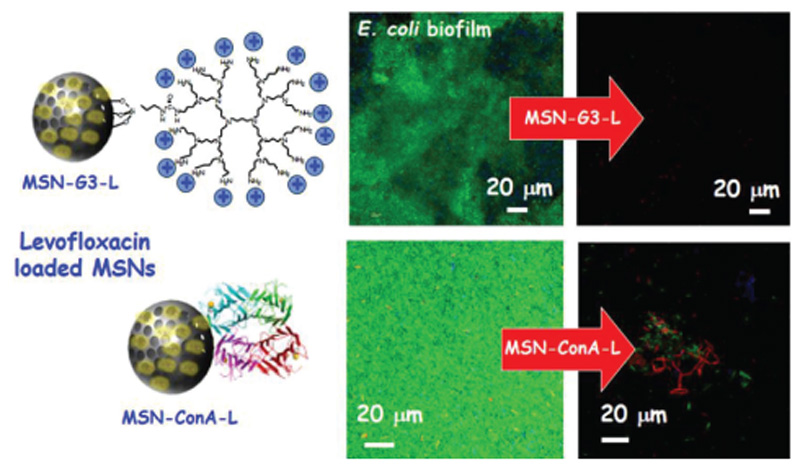
Schematic representation of the antimicrobial efficacy of (top) levofloxacin-loaded MSN-G3 and (down) levofloxacin-containing MSN-ConA. Both cases reveal that the targeting effect enhances significantly the antimicrobial effect of loaded antibiotic. These results are derived from refs. [177] and [174], respectively.
Of special interest is the study of Martinez-Camona et al.,[174] where the authors designed a novel nanocarrier based on MSNs loaded with LEVO covalently grafted with the lectin concanavalin A (ConA). ConA are glycoproteins isolated from plants, able to selectively identify, bind, and uptake to certain cell-surface glycans. These glycans are also exist in the bacterial biofilm.[172] This nanocarrier showed good internalization rates in gram-negative bacteria biofilm, increasing the antibacterial efficiency of LEVO loaded in the mesopores (Figures 6 and 7).
5.3. Drug Release at the Infection Focus
MSNs can hold different drugs or antibiotic combinations in their mesopores that act synergistically at the focus of the infection, resulting in a combination therapy. In this regard, an ideal nanocarrier must protect the therapeutic agent, transport them to the target tissue or cell and, finally, release high local concentrations of this agent keeping the control of the drug release. With this purpose, there are several approaches to cap the pore and avoid premature release of the cargo.[155,160,161] Those caps grafted strategies should respond to the application of several stimuli, triggering the antimicrobial cargo release into the site of infection, as pH and temperature variations, light, redox processes, or enzymes, among others.
A decrease has been observed in pH values after surgery, infection, or implant loosening.[178–180] This reducing environment in the area of bone infection induces a pH < 7 compared to pH 7.35–7.45 in noninfected tissues. These changes in the pH values induce some variations in the capacity of bacterial adhesion and in the isoelectric point and surface charge of biomaterials, increasing bacterial infections.[178–180] In this regard, it has been demonstrated that S. aureus and S. epidermidis, typical orthopedic implant-related infection bacteria, increased their penetration and adhesion to ceramic pore materials in the presence of lower pH values,[178,180] related to a decrease in the repulsive forces between material and bacteria. Conversely, some authors showed an unexpected reduced adherence of both S. aureus and S. epidermidis in HA and biphasic calcium phosphate materials,[178,180] probably because of these materials did not have pores large enough to allow the internalization of staphylococci. These results indicated the possibly that the decreased pH values in bone infection is a consequence of active bacterial growth and function. The observed decrease in the pH values near of loosening implants induced an increase in the production and function of cathepsin K,[179] a highly strong collagenase and papain-like cysteine protease expressed in osteoclasts. This protease, which is present in pseudosynovial fluid, can destroy bone in the phagolysosomes, Howship lacunae, and in the acidic extracellular space closed to acidic loosening implants interfaces.[179] In this sense, cathepsin K activation induced an inhibition of type I bone collagen, leading to demineralization in peri-implant bone.
In this sense, a pH stimuli-responsive nanocarrier for detection and eradication of bacteria was developed by Yan and co-workers.[181] MSNs were loaded with vancomycin and the external surface was modified with fluorescein isothiocyanate with the aim to add a “sense-and-treat” hydrogel. Poly(N-isopropyl acrylamide-co-acrylic acid) is a pH-sensitive polymer (that acts as a gatekeeper) that was copolymerized with a derivative of rhodamine B, functionalized with an acrylamide moiety (RhBAM), and grafted onto MSNs. RhBAM moiety is present in the spirolactam form at basic pH values (no fluorescence), whereas at acidic pH values it converts into the open form and emits strong fluorescence. MSNs were immobilized in an agarose matrix layer for sensing and destroying bacteria. The protons produced by bacteria, triggered an antibacterial vancomycin to inhibit bacterial growth. In addition, vancomycin showed higher release at acidic pH from the MSNs with RhBAM and MS-hydrogel showing an ideal antimicrobial action in the presence of E. coli.
Considering a temperature stimuli-responsive nanocarriers to trigger the antibacterial cargo release to infection site, Yu et al.[182] prepared a nanosystem consistent in NPs containing a Fe3O4 core and MSN layer capped with the poly(N-isopropylacrylamide) (PNIPAM) (thermoresponsive), loaded with the enzyme lysozyme (antibacterial). Rapid drug release was observed at 37 °C by both capped-core-shell NPs, because the pore opening the PNIPAM polymer was in the collapsed form at this temperature, whereas no drug released was induced at 25 °C. Moreover, lysozyme delivery at 37 °C induced a significantly inhibition of Bacillus cereus and Micrococcus luteus growth. In a study by Sun et al.,[183] a self-enriched MSN composite membrane with photodynamic stimuli-responsive antimicrobial properties was developed by facile electrospinning method. A polymeric matrix was produced with PCL and zein, and MSNs were loaded with methylene blue and modified by trichloro silane, acting as ROS generator to induce antibacterial actions. These fluorinated MSNs significantly enhanced ROS generation, and the composite membrane presented bacterial repellency. The composite membrane showed photodynamic antimicrobial effects against gram-negative E. coli and gram-positive S. aureus under visible light (660 nm) irradiation.
Lee et al.[184] designed a nanosystem based on MSNs loaded with moxifloxacin and coated with a redox-sensitive disulfide snap-tops. This nanosystem was functionalized with adamantanethiol forming a disulfide bond and with (3-mercaptopropyl) trimethoxysilane. This disulfide bond can be cleaved in reducing environments and the moxifloxacin delivery is produced intracellularly, staining the nuclei of macrophages thanks to glutathione presence, and inhibiting Francisella tularensis. In vivo, moxifloxacin-loaded MSNs prevented premature death and significantly decreased the presence of F. tularensis in the spleen, lung, and liver. In addition, Ding et al.[185] prepared a dual-functional nanocarrier with an enzyme-responsive action for tissue regeneration and bacterial infection application. Ag-NPs were pre-encapsulated in MSNs (MSN-Ag) and poly-L-glutamic acid (PG) and polyallylamine hydrochloride (PAH) were added by the LBL assembly technique on MSN-Ag (LBL@MSN-Ag). Glutamyl endonuclease secreted by Staphylococcus aureus can degrade the amide linkage present into the polyamide PG. These nanosystems were placed on the surface of polydopamine-improved Ti substrates. These substrates with an LBL@MSN-Ag nanocoating induced a significantly antimicrobial effect in vitro and showed excellent results in a rat bone defect infection treatment, promoting new bone formation.
6. Outlook
Finding novel and alternative strategies to fight biomaterials-associated infections is a major challenge for the biomedical scientific community. Conventionally, several approaches have been developed to design anti-infective biomaterials, most of them focused on prevention (i.e., inhibiting the early stages of bacterial adhesion) or treatment (once the biofilm has been formed) of the infection process. This review provides an overview of the state of the art of those different approaches.
Conventional biomaterials try to achieve great antibacterial effect while preserving their biocompatibility. These strategies are based on: i) implant surface coatings that involve new technological advances in the field of surface modification, ii) design of different scaffolds, including multifunctional scaffolds with both antimicrobial and bone regeneration properties, and iii) nanocarriers based on MSNs with advanced properties (targeting and stimuli-response capabilities).
Novel technological advances in surface modification technologies, as nanostructured coatings or zwitterionization of biomaterials surfaces, are emerging as very powerful tools to avoid bacterial adhesion and biofilm formation without the use of antibiotics while keeping great biocompatibility rates. Additionally, their great biocompatibility has raised this technology as a new concept of implant fabrication.
In order to address infection issues and bone defects regeneration simultaneously, scaffolds offer great advantages both in prevention and cure. In this sense, there are many different possibilities of multitherapy or combined therapies to regenerate bone tissue, which are very promising for clinical use. Furthermore, the design of scaffolds as 3D models of bone infection is currently being demanded as a need for a better understanding of cellular responses.
MSNs exhibit promising features since they can load and release antibiotics locally and in a controlled manner. Among them, MSNs can be functionalized with targeting agents, and gives the possibility to design these materials as stimuli-responsive systems releasing the loaded charge on demand. This is the starting point to achieve a remarkable improvement of conventional treatments, where the main point is to combine different elements to abolish infections.
All of them represent promising and powerful tools that could replace conventional therapies. However, their potential has only been proven in vitro and, in the lesser case, in preclinical models. At this point, one of the weaknesses is the lack of clinical experimentation. It is important to design multicenter clinical trials, well-structured international antibiotic register, and mutant causative strains. Only with evidence-based data, will be a real progress in this field. In this sense, futures therapies are aimed at implementing personalize treatments, which adjust more effectively to the clinical needs of each patient with fewer side effects than conventional therapies.
Acknowledgements
This work was supported by the European Research Council, ERC-2015-AdG (VERDI), Proposal no. 694160.
Biographies
 María Vallet-Regí is a chemist, scientist, and professor at Universidad Complutensede Madrid, Spain. She is recognized as a pioneer in the field of ceramic materials applied to medicine. Shewasthe pioneer who suggested introducing drugs into the pores of MSNs, which inspired thousands of publications worldwide involving MSNsfordrugdelivery. Her current research interests are focused on developing nanomedicines for treating different diseases, such as bone infection, osteoporosis, or bone cancer.
María Vallet-Regí is a chemist, scientist, and professor at Universidad Complutensede Madrid, Spain. She is recognized as a pioneer in the field of ceramic materials applied to medicine. Shewasthe pioneer who suggested introducing drugs into the pores of MSNs, which inspired thousands of publications worldwide involving MSNsfordrugdelivery. Her current research interests are focused on developing nanomedicines for treating different diseases, such as bone infection, osteoporosis, or bone cancer.
 Blanca González wasbornin Madrid, Spain,in1974. She graduatedinchemistry from Universidad Autónomade Madrid (1998) and received her Ph.D.degree fromthesame university (2003)inthefield of electroactive organometallic macromolecules. In 2006, she moved to the Faculty of Pharmacyat Universidad Complutensede Madrid, where she currently holds an associate professor position at the Chemistry in Pharmaceutical Sciences (Inorganic Chemistry, Bioinorganic and Biomaterials Unit). Her research interests are focused on organic–inorganic hybrid materials, dendritic macromolecules, bioceramics, nanoparticles, and stimuli-responsive nanosystems for biomedical applications, in particular bone pathologies such as cancer and infection.
Blanca González wasbornin Madrid, Spain,in1974. She graduatedinchemistry from Universidad Autónomade Madrid (1998) and received her Ph.D.degree fromthesame university (2003)inthefield of electroactive organometallic macromolecules. In 2006, she moved to the Faculty of Pharmacyat Universidad Complutensede Madrid, where she currently holds an associate professor position at the Chemistry in Pharmaceutical Sciences (Inorganic Chemistry, Bioinorganic and Biomaterials Unit). Her research interests are focused on organic–inorganic hybrid materials, dendritic macromolecules, bioceramics, nanoparticles, and stimuli-responsive nanosystems for biomedical applications, in particular bone pathologies such as cancer and infection.
 Isabel Izquierdo-Barba was bornin Socuéllamos (C.Real), Spain, in 1974. She graduated in pharmacy chemistry at Universidad Complutensede Madrid (1997) and received her Ph.D. degree (2002) in the field of bioactive glasses. She has carried out different research stays in the Department of Condensed Matter Physics at the University of Cadiz, Laboratorie Chimiedela Matiére Condensée Pierreat the Marie Curie University (CNRS, France), Stockholm University (Sweden), and Jiao Tong University in Shanghai (China). Since 2012, she has held a senior lecturer position at the Chemistry in Pharmaceutical Sciences (Inorganic Chemistry, Bioinorganic and Biomaterials Unit). Her research interests are focused on bone tissue regeneration and design of nanomaterials for bone infection.
Isabel Izquierdo-Barba was bornin Socuéllamos (C.Real), Spain, in 1974. She graduated in pharmacy chemistry at Universidad Complutensede Madrid (1997) and received her Ph.D. degree (2002) in the field of bioactive glasses. She has carried out different research stays in the Department of Condensed Matter Physics at the University of Cadiz, Laboratorie Chimiedela Matiére Condensée Pierreat the Marie Curie University (CNRS, France), Stockholm University (Sweden), and Jiao Tong University in Shanghai (China). Since 2012, she has held a senior lecturer position at the Chemistry in Pharmaceutical Sciences (Inorganic Chemistry, Bioinorganic and Biomaterials Unit). Her research interests are focused on bone tissue regeneration and design of nanomaterials for bone infection.
Footnotes
Conflict of I nterest
The authors declare no conflict of interest.
Contributor Information
Prof. María Vallet-Regí, Departamento de Química en Ciencias Farmacéuticas Facultad de Farmacia Universidad Complutense de Madrid Instituto de Investigación Sanitaria Hospital 12 de Octubre i+12 Plaza Ramón y Cajal s/n, Madrid 28040, Spain; CIBER de Bioingeniería Biomateriales y Nanomedicina CIBER-BBN C/Monforte de Lemos, 3–5 Madrid 28029, Spain.
Dr. Daniel Lozano, Departamento de Química en Ciencias Farmacéuticas Facultad de Farmacia Universidad Complutense de Madrid Instituto de Investigación Sanitaria Hospital 12 de Octubre i+12 Plaza Ramón y Cajal s/n, Madrid 28040, Spain; CIBER de Bioingeniería Biomateriales y Nanomedicina CIBER-BBN C/Monforte de Lemos, 3–5 Madrid 28029, Spain.
Dr. Blanca González, Departamento de Química en Ciencias Farmacéuticas Facultad de Farmacia Universidad Complutense de Madrid Instituto de Investigación Sanitaria Hospital 12 de Octubre i+12 Plaza Ramón y Cajal s/n, Madrid 28040, Spain; CIBER de Bioingeniería Biomateriales y Nanomedicina CIBER-BBN C/Monforte de Lemos, 3–5 Madrid 28029, Spain
Dr. Isabel Izquierdo-Barba, Departamento de Química en Ciencias Farmacéuticas Facultad de Farmacia Universidad Complutense de Madrid Instituto de Investigación Sanitaria Hospital 12 de Octubre i+12 Plaza Ramón y Cajal s/n, Madrid 28040, Spain; CIBER de Bioingeniería Biomateriales y Nanomedicina CIBER-BBN C/Monforte de Lemos, 3–5 Madrid 28029, Spain
References
- [1].Kremers HM, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ. J Bone Jt Surg, Am Vol. 2015;97:1386. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vallet-Regí M. Biomaterials against bone infection. [accessed: February 2020]; doi: 10.1002/adhm.202000310. https://www.youtube.com/watch?v=II3nFxEUnUo. [DOI] [PMC free article] [PubMed]
- [3].WHO. High Levels of Antibiotic Resistance Found Worldwide, New Data Shows. WHO, News Release. [accessed: January 2018]; https://www.who.int/mediacentre/news/releases/2018/antibiotic-resistancefound/en/
- [4].Saeed K, McLaren AC, Schwarz EM, Antoci V, Arnold WV, Chen AF, Clauss M, Esteban J, Gant V, Hendershot E, Hickok N, et al. J Orthop Res. 2019;37:1007. doi: 10.1002/jor.24229. [DOI] [PubMed] [Google Scholar]
- [5].Schwarz EM, Parvizi J, Gehrke T, Aiyer A, Battenberg A, Brown SA, Callaghan JJ, Citak M, Egol K, Garrigues GE, Ghert M, et al. J Orthop Res. 2019;37:997. doi: 10.1002/jor.24293. [DOI] [PubMed] [Google Scholar]
- [6].Rosas S, Ong AC, Buller LT, Sabeh KG, Law TY, Roche MW, Hernandez VH. World J Orthop. 2017;8:895. doi: 10.5312/wjo.v8.i12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. J Arthroplasty. 2012;27:61. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- [8].Arciola CR, An Y, Campoccia D, Donati M, Montanaro L. Int J Artif Organs. 2005;28:1091. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- [9].Kaplan SL. J Infect. 2014;68:S51. doi: 10.1016/j.jinf.2013.09.014. [DOI] [PubMed] [Google Scholar]
- [10].Hall-Stoodley L, Costerton JW, Stoodley P. Nat Rev Microbiol. 2004;2:95. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- [11].Davies D. Nat Rev Drug Discovery. 2003;2:114. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- [12].Masters EA, Trombetta RP, de Mesy Bentley KL, Boyce BF, Lindley Gill A, Gill SR, Nishitani K, Ishikawa M, Morita Y, Ito H, Bello-lrizarry SN, et al. Bone Res. 2019;7:20. doi: 10.1038/s41413-019-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Redlich K, Smolen JS. Nat Rev Drug Discovery. 2012;11:234. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- [14].Putnam NE, Fulbright LE, Curry JM, Ford CA, Petronglo JR, Hendrix AS, Cassat JE. PLoS Pathog. 2019;15:e1007744. doi: 10.1371/journal.ppat.1007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arciola CR, Campoccia D, Montanaro L. Nat Rev Microbiol. 2018;16:397. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- [16].O’Neill J. Rev Antimicrob Resist. 2014;1:1. [Google Scholar]
- [17].Campoccia D, Montanaro L, Arciola CR. Biomaterials. 2013;34:8533. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- [18].Vallet-Regi M, Gonzalez B, Izquierdo-Barba I. IntJ Mol Sci. 2019;20:3806. doi: 10.3390/ijms20153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Campoccia D, Montanaro L, Renata Arciola C. Biomaterials. 2013;34:8018. doi: 10.1016/j.biomaterials.2013.07.048. [DOI] [PubMed] [Google Scholar]
- [20].Wang M, Tang TG. J Orthop Transl. 2019;17:42. doi: 10.1016/j.jot.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gisbert-Garzarán M, Manzano M, Vallet-Regí M. Pharmaceutics. 2020;12:83. doi: 10.3390/pharmaceutics12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sánchez-Salcedo S, Colilla M, Izquierdo-Barba I, Vallet-Regí M. IntJ Bioprinting. 2015;2:20. [Google Scholar]
- [23].Zhang K, Wang S, Zhou C, Cheng L, Gao X, Xie X, Sun J, Wang H, Weir MD, Reynolds MA, Zhang N, et al. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vallet-Regí M, Izquierdo-Barba I, Colilla M. Philos Trans R Soc, A. 2012;370:1400. doi: 10.1098/rsta.2011.0258. [DOI] [PubMed] [Google Scholar]
- [25].Vallet-Regí M, Colilla M, Izquierdo-Barba I. Enzymes. 2018;44:35. doi: 10.1016/bs.enz.2018.08.001. [DOI] [PubMed] [Google Scholar]
- [26].Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudré C. Ada Biomater. 2019;83:37. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- [27].Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. Ada Biomater. 2010;6:3824. doi: 10.1016/j.actbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [28].Colilla M, Izquierdo-Barba I, Vallet-Regí M. Medicines. 2018;5:125. doi: 10.3390/medicines5040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hardesa J, Ahrensa H, Geberta C, Streitbuergera A, Buergerb H, Errenc M, Gunseld A, Wedemeyere C, Saxlere G, Winkelmanna W, Goshegera G. Biomaterials. 2007;28:2869. doi: 10.1016/j.biomaterials.2007.02.033. [DOI] [PubMed] [Google Scholar]
- [30].Szaraniec B, Pielichowska K, Pac E, Menaszek E. Mater Sci Eng, C. 2018;93:950. doi: 10.1016/j.msec.2018.08.065. [DOI] [PubMed] [Google Scholar]
- [31].Feng K, Li Z, Cai X, Chu PK. Mater Chem Phys. 2011;126:6. [Google Scholar]
- [32].Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. Biomaterials. 2004;25:4383. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- [33].Prokopovich P, Leech R, Carmalt CJ, Parkin IP, Perni S. IntJ Nanomed. 2013;8:2227. doi: 10.2147/IJN.S42822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, Ong JL. Biomaterials. 2006;27:5512. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [35].Noda I, Miyaji F, Ando Y, Miyamoto H, Shimazaki T, Yonekura Y, Miyazaki M, Mawatari M, Hotokebuchi T. J Biomed Mater Res, Part B. 2009;89B:456. doi: 10.1002/jbm.b.31235. [DOI] [PubMed] [Google Scholar]
- [36].Liu X, Mou Y, Wu S, Man HC. Appl Surf Sci. 2013;273:748. [Google Scholar]
- [37].Akiyama T, Miyamoto H, Yonekura Y, Tsukamoto M, Ando Y, Noda I, Sonohata M, Mawatari M. J Orthop Res. 2013;31:1195. doi: 10.1002/jor.22357. [DOI] [PubMed] [Google Scholar]
- [38].Alt V. Injury. 2017;48:599. doi: 10.1016/j.injury.2016.12.011. [DOI] [PubMed] [Google Scholar]
- [39].Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. J Orthop Res. 2004;22:39. doi: 10.1016/S0736-0266(03)00152-9. [DOI] [PubMed] [Google Scholar]
- [40].Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB. Nanotoxicology. 2010;4:319. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- [41].Wang G, Jin W, Qasim AM, Gao A, Peng X, Li W, Feng H, Chu PK. Biomaterials. 2017;124:25. doi: 10.1016/j.biomaterials.2017.01.028. [DOI] [PubMed] [Google Scholar]
- [42].Braem A, De Brucker K, Delattin N, Killian MS, Roeffaers MBJ, Yoshioka T, Hayakawa S, Schmuki P, Cammue BPA, Virtanen S, Thevissen K, Neirinek B. ACS Appl Mater Interfaces. 2017;9:8533. doi: 10.1021/acsami.6b16433. [DOI] [PubMed] [Google Scholar]
- [43].Qiao S, Cao H, Zhao X, Lo H, Zhuang L, Gu Y, Shi J, Liu X, Lai H. Int J Nanomed. 2015;10:653. doi: 10.2147/IJN.S73467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li D, Lv P, Fan L, Huang Y, Yang F, Mei X, Wu D. Biomater Sci. 2017;5:2337. doi: 10.1039/c7bm00693d. [DOI] [PubMed] [Google Scholar]
- [45].Vester H, Wildemann B, Schmidmaier G, Stockle U, Lucke M. Injury. 2010;41:1053. doi: 10.1016/j.injury.2010.05.010. [DOI] [PubMed] [Google Scholar]
- [46].Fuchs T, Stange R, Schmidmaier G, Raschke MJ. Arch Orthop Trauma Surg. 2011;131:1419. doi: 10.1007/s00402-011-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Metsemakers WJ, Reul M, Nijs S. Injury. 2015;46:2433. doi: 10.1016/j.injury.2015.09.028. [DOI] [PubMed] [Google Scholar]
- [48].Diefenbeck M, Schrader C, Gras F, Mückley T, Schmidt J, Zankovych S, Bossert J, Jandt KD, Völpel A, Sigusch BW, Schubert H, Bischoff S, Pfister W, Edel B, Faucon M, Finger U. Biomaterials. 2016;101:156. doi: 10.1016/j.biomaterials.2016.05.039. [DOI] [PubMed] [Google Scholar]
- [49].Miller RJ, Thompson JM, Zheng J, Marchitto MC, Archer NK, Pinsker BL, Ortines RV, Jiang X, Martin RA, Brown ID, Wang Y, et al. J BoneJt Surg, Am Vol. 2019;101:e12. doi: 10.2106/JBJS.18.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Min J, Choi KY, Dreaden EC, Padera RF, Braatz RD, Spec-tor M, Hammond PT. ACS Nano. 2016;10:4441. doi: 10.1021/acsnano.6b00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aunon A, Esteban J, Doadrio AL, Boiza-Sanchez M, Mediero A, Eguibar-Blazquez D, Cordero-Ampuero J, Conde A, Arenas MA, de-Damborenea JJ, Aguilera-Correa JJ. J Orthop Res. 2020;38:588. doi: 10.1002/jor.24496. [DOI] [PubMed] [Google Scholar]
- [52].Yang Y, Ao HY, Yang SB, Wang YG, Lin WT, Yu ZF, Tang TT. IntJ Nanomed. 2016;11:2223. doi: 10.2147/IJN.S102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu D, He C, Liu Z, Xu W. Int J Nanomed. 2017;12:5461. doi: 10.2147/IJN.S137137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vallet-Regí M, Rámila A, Del Real RP, Pérez-Pariente J. Chem Mater. 2001;13:308. [Google Scholar]
- [55].Braem A, De Cremer K, Delattin N, De Brucker K, Neirinck B, Vandamme K, Martens JA, Michiels J, Vleugels J, Cammue BP, Thevissen K. Colloids Surf, B. 2015;126:481. doi: 10.1016/j.colsurfb.2014.12.054. [DOI] [PubMed] [Google Scholar]
- [56].Chen R, Willcox MDP, Ho KKK, Smyth D, Kumar N. Biomaterials. 2016;85:142. doi: 10.1016/j.biomaterials.2016.01.063. [DOI] [PubMed] [Google Scholar]
- [57].Moriarty TF, Kuehl R, Coenye T, Metsemakers WJ, Morgen-stern M, Schwarz EM, Riool M, Zaat SAJ, Khana N, Kates SL, Richards RG. EFORT Open Rev. 2016;1:89. doi: 10.1302/2058-5241.1.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudre C. Ada Biomater. 2019;83:37. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- [59].Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. J Am Chem Soc. 2010;132:5761. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- [60].Ivanova EP, Hasan J, Webb HK, Truong VK, Watson GS, Watson JA, Baulin VA, Pogodin S, Wang JY, Tobin MJ, Löbbe C, et al. Small. 2012;8:2489. doi: 10.1002/smll.201200528. [DOI] [PubMed] [Google Scholar]
- [61].Guo Z, Liu W, Su BL. J Colloid Interface Sci. 2011;353:335. doi: 10.1016/j.jcis.2010.08.047. [DOI] [PubMed] [Google Scholar]
- [62].Ross AM, Jiang Z, Bastmeyer M, Lahann J. Small. 2012;8:336. doi: 10.1002/smll.201100934. [DOI] [PubMed] [Google Scholar]
- [63].Bagherifard S, Hickey DJ, De Luca AC, Malheiro VN, Markaki AE, Guagliano M, Webster TW. Biomaterials. 2015;73:185. doi: 10.1016/j.biomaterials.2015.09.019. [DOI] [PubMed] [Google Scholar]
- [64].Bassous NJ, Jones CL, Webster TJ. Ada Biomater. 2019;96:662. doi: 10.1016/j.actbio.2019.06.055. [DOI] [PubMed] [Google Scholar]
- [65].Bhadra CM, Truong VK, Pham VTH, Al Kobaisi M, Seniuti-nas G, Wang JY, Juodkazis S, Crawford RJ, Ivanova EP. Sci Rep. 2015;5 doi: 10.1038/srep16817. 16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cruz DM, González MU, Tien-Street W, Castro MF, Crua AV, Fernández-Martínez I, Martínez L, Huttel Y, Webster TJ, García-Martín JM. Nanomed: Nanotechnoi, Biol Med. 2019;17:36. doi: 10.1016/j.nano.2018.12.009. [DOI] [PubMed] [Google Scholar]
- [67].Modaresifar K, Azizian S, Ganjian M, Fratila-Apachitei LE, Zadpoor AA. Ada Biomater. 2019;83:29. doi: 10.1016/j.actbio.2018.09.059. [DOI] [PubMed] [Google Scholar]
- [68].Izquierdo-Barba I, García-Martín JM, Alvarez R, Palmero A, Esteban J, Pérez-Jorge C, Arcos D, Vallet-Regí M. Ada Biomater. 2015;15:20. doi: 10.1016/j.actbio.2014.12.023. [DOI] [PubMed] [Google Scholar]
- [69].Alvarez R, Muñoz-Piña S, González MU, Izquierdo-Barba I, Fernández-Martínez I, Rico V, Arcos D, García-Valenzuela A, Palmero A, Vallet-Regi M, González-Elipe AR, García-Martín JM. Nanomaterials. 2019;9:1217. doi: 10.3390/nano9091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Peng Z, Ni J, Zheng K, Shen Y, Wang X, He G, Sungho J, Tang T. IntJ Nanomed. 2013;8:3093. doi: 10.2147/IJN.S48084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang L, Liao X, Fok A, Ning C, Ng P, Wang Y. Mater Sci Eng, C. 2018;82:91. doi: 10.1016/j.msec.2017.08.024. [DOI] [PubMed] [Google Scholar]
- [72].Aguilera-Correa JJ, Doadrio AL, Conde A, Arenas MA, de-Damborenea JJ, Vallet-Regí M, Esteban J. J Mater Sci: Mater Med. 2018;29:118. doi: 10.1007/s10856-018-6119-4. [DOI] [PubMed] [Google Scholar]
- [73].Lin W, Tan H, Duan Z, Yue B, Ma R, He G, Tang TT. IntJ Nanomed. 2014;9:1215. doi: 10.2147/IJN.S57875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pranjali P, Meher MK, Raj R, Prasad N, Poluri KM, Kumar D, Guleria A. ACS Omega. 2019;4:19255. doi: 10.1021/acsomega.9b02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Park JY, Kim JS, Nam YS. Carbohydr Polym. 2013;97:753. doi: 10.1016/j.carbpol.2013.05.064. [DOI] [PubMed] [Google Scholar]
- [76].Caro A, Humblot V, Meʹthivier C, Minier M, Salmain M, Pradier CM. J Phys Chem B. 2009;113:2101. doi: 10.1021/jp805284s. [DOI] [PubMed] [Google Scholar]
- [77].Buxadera-Palomero J, Canal C, Torrent-Camarero S, Garrido B, Gil FJ, Rodríguez D. Biointerphases. 2015;10 doi: 10.1116/1.4913376. 029505. [DOI] [PubMed] [Google Scholar]
- [78].Harris LG, Tosatti S, Wieland M, Textor M, Richards RG. Bio-materials. 2004;25:4135. doi: 10.1016/j.biomaterials.2003.11.033. [DOI] [PubMed] [Google Scholar]
- [79].Shi Z, Neoh KG, Kang ET, Poh C, Wang W. Tissue Eng, Part A. 2009;15:417. doi: 10.1089/ten.tea.2007.0415. [DOI] [PubMed] [Google Scholar]
- [80].Muszanska AK, Rochford ET, Gruszka A, Bastian AA, Busscher HJ, Norde W, van der Mei HC, Herrmann A. Biomacromolecules. 2019;2014:15. doi: 10.1021/bm500168s. [DOI] [PubMed] [Google Scholar]
- [81].Stavrakis AI, Zhu S, Hegde V, Loftin AH, Ashbaugh AG, Niska JA, Miller LS, Segura T, Bernthal NM. J Bonejt Surg. 2016;98:1183. doi: 10.2106/JBJS.15.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Izquierdo-Barba I, Colilla M, Vallet-Regí M. Ada Biomater. 2016;40:201. doi: 10.1016/j.actbio.2016.02.027. [DOI] [PubMed] [Google Scholar]
- [83].Chen S, Li L, Zhao C, Zheng J. Polymer. 2010;51:5283. [Google Scholar]
- [84].Suzuki H, Murou M, Kitano H, Ohno K, Saruwatari Y. Colloids Surf, B. 2011;84:111. doi: 10.1016/j.colsurfb.2010.12.023. [DOI] [PubMed] [Google Scholar]
- [85].Rosen JE, Gu FX. Langmuir. 2011;27:10507. doi: 10.1021/la201940r. [DOI] [PubMed] [Google Scholar]
- [86].Villegas F, Garcia-Uriostegui L, Rodríguez O, Izquierdo-Barba I, Salinas AJ, Toriz G, Vallet-Regí M, Delgado E. Bioengineering. 2017;4:80. doi: 10.3390/bioengineering4040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sun JT, Yu ZQ, Hong CY, Pan CY. Macromol Rapid Commun. 2012;33:811. doi: 10.1002/marc.201100876. [DOI] [PubMed] [Google Scholar]
- [88].Cheng G, Xue GH, Zhang Z, Chen S, Jiang S. Angew Chem, Int Ed. 2008;47:8831. doi: 10.1002/anie.200803570. [DOI] [PubMed] [Google Scholar]
- [89].Izquierdo-Barba I, Sánchez-Salcedo S, Colilla M, Feito MJ, Ramírez-Santillán C, Portolés MT, Vallet-Regí M. Ada Biomater. 2011;7:2977. doi: 10.1016/j.actbio.2011.03.005. [DOI] [PubMed] [Google Scholar]
- [90].Colilla M, Izquierdo-Barba I, Sánchez-Salcedo S, Fierro JLG, Hueso JL, Vallet-Regí M. Chem Mater. 2010;22:6459. [Google Scholar]
- [91].Encinas N, Angulo M, Astorga C, Colilla M, Izquierdo-Barba I, Vallet-Regí M. Ada Biomater. 2019;84:317. doi: 10.1016/j.actbio.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sánchez-Salcedo S, Colilla M, Izquierdo-Barba I, Vallet-Regí M. J Mater Chem B. 2013;1:1595. doi: 10.1039/c3tb00122a. [DOI] [PubMed] [Google Scholar]
- [93].Rodríguez-Palomo A, Monopoli D, Afonso H, Izquierdo-Barba I, Vallet-Regí M. J Mater Chem B. 2016;4:4356. doi: 10.1039/c6tb00675b. [DOI] [PubMed] [Google Scholar]
- [94].Sánchez-Salcedo S, García A, Vallet-Regí M. Ada Biomater. 2017;57:472. doi: 10.1016/j.actbio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [95].Pontremoli C, Izquierdo-Barba I, Montalbano G, Vallet-Regi M, Vitale-Brovarone C, Fiorilli S. J Colloid Interface Sci. 2020;563:92. doi: 10.1016/j.jcis.2019.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Colilla M, Martínez-Carmona M, Sánchez-Salcedo S, Ruiz-González ML, González-Calbet JM, Vallet-Regí M. J Mater Chem B. 2014;2:5639. doi: 10.1039/c4tb00690a. [DOI] [PubMed] [Google Scholar]
- [97].Mourino V, Cattalini JP, Roether JA, Dubey P, Roy I, Boccaccini AR. Expert Opin Drug Delivery. 2013;10:1353. doi: 10.1517/17425247.2013.808183. [DOI] [PubMed] [Google Scholar]
- [98].Johnson CT, García AJ. Ann Biomed Eng. 2015;43:515. doi: 10.1007/s10439-014-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dorati R, DeTrizio A, Modena T, Conti B, Benazzo F, Gastaldi G, Genta I. Pharmaceuticals. 2017;10:96. doi: 10.3390/ph10040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kavanagh N, O’Brien FJ, Kerrigan SW. PLoS One. 2018;13:e0198837/1. doi: 10.1371/journal.pone.0198837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gao H, Luo S-Z. RSCAdv. 2016;6:58521. [Google Scholar]
- [102].Tian L, Zhang Z, Tian B, Zhang X, Wang N. RSC Adv. 2020;10:4805. doi: 10.1039/c9ra10275b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kong M, Chen XG, Xing K, Park HJ. IntJ Food Microbiol. 2010;144:51. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- [104].Lu Y, Li M, Li L, Wei S, Hu X, Wang X, Shan G, Zhang Y, Xia H, Yin Q. Mater Sci Eng, C. 2018;82:225. doi: 10.1016/j.msec.2017.08.043. [DOI] [PubMed] [Google Scholar]
- [105].Yang Y, Chu L, Yang S, Zhang H, Qin L, Guillaume O, Eglin D, Richards RG, Tang T. Ada Biomater. 2018;79:265. doi: 10.1016/j.actbio.2018.08.015. [DOI] [PubMed] [Google Scholar]
- [106].Farzamfar S, Naseri-Nosar M, Sahrapeyma H, Ehterami A, Goodarzi A, Rahmati M, Ahmadi Lakalayeh G, Ghorbani S, Vaez A, Salehi M. IntJ Polym Mater Polym Biomater. 2019;68:472. [Google Scholar]
- [107].Sreeja S, Muraleedharan CV, Varma PRH, Sailaja GS. Mater Sci Eng, C. 2020;109:110491. doi: 10.1016/j.msec.2019.110491. [DOI] [PubMed] [Google Scholar]
- [108].Aragon J, Feoli S, Irusta S, Mendoza G. IntJ Pharm. 2019;557:162. doi: 10.1016/j.ijpharm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- [109].Ahadi F, Khorshidi S, Karkhaneh A. Eur Polym J. 2019;118:265. [Google Scholar]
- [110].Bai J, Wang H, Gao W, Liang F, Wang Z, Zhou Y, Lan X, Chen X, Cai N, Huang W, Tang Y. IntJ Pharm. 2020;576 doi: 10.1016/j.ijpharm.2019.118941. 118941. [DOI] [PubMed] [Google Scholar]
- [111].Cicuéndez M, Doadrio JC, Hernández A, Portolés MT, Izquierdo-Barba I, Vallet-Regí M. Ada Biomater. 2018;65:450. doi: 10.1016/j.actbio.2017.11.009. [DOI] [PubMed] [Google Scholar]
- [112].Bakhsheshi-Rad HR, Hamzah E, Staiger MP, Dias JG, Hadisi Z, Saheban M, Kashefian M. Mater Des. 2018;139:212. [Google Scholar]
- [113].Bakhsheshi-Rad HR, Chen XB, Ismail AF, Aziz M, Hamzah E, Najafinezhad A. Mater Chem Phys. 2019;222:118. [Google Scholar]
- [114].García-Álvarez R, Izquierdo-Barba I, Vallet-Regí M. Ada Biomater. 2017;49:113. doi: 10.1016/j.actbio.2016.11.028. [DOI] [PubMed] [Google Scholar]
- [115].Zhou J, Zhou XG, Wang JW, Zhou H, Dong J. Bonejt Res. 2018;7:46. doi: 10.1302/2046-3758.71.BJR-2017-0129.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cheng T, Qu H, Zhang G, Zhang X. Artif Cells, Nanomed, Biotechnol. 2018;46:1935. doi: 10.1080/21691401.2017.1396997. [DOI] [PubMed] [Google Scholar]
- [117].Zhao X, Han Y, Zhu T, Feng N, Sun Y, Song Z, Li S, Liu J, Ding J. J Biomed Nanotechnol. 2019;15:1213. doi: 10.1166/jbn.2019.2773. [DOI] [PubMed] [Google Scholar]
- [118].Ding Y, Li W, Correia A, Yang Y, Zheng K, Liu D, Schubert DW, Boccaccini AR, Santos HA, Roether JA. ACSAppl Mater Interfaces. 2018;10:14540. doi: 10.1021/acsami.8b02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Marques CF, Olhero SM, Torres PMC, Abrantes JCC, Fateixa S, Nogueira HIS, Ribeiro IAC, Bettencourt A, Sousa A, Granja PL, Ferreira JMF. Mater Sci Eng, C. 2019;94:426. doi: 10.1016/j.msec.2018.09.050. [DOI] [PubMed] [Google Scholar]
- [120].Aldrich A, Kuss MA, Duan B, Kielian T. ACS Appl Mater Interfaces. 2019;11:12298. doi: 10.1021/acsami.9b00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Vallet-Regí M, González B, Izquierdo-Barba I. IntJ Mol Sci. 2019;20:3806. doi: 10.3390/ijms20153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ryan EJ, Ryan AJ, Gonzalez-Vazquez A, Philippart A, Ciraldo FE, Hobbs C, Nicolosi V, Boccaccini AR, Kearney CJ, O’Brien FJ. Biomaterials. 2019;197:405. doi: 10.1016/j.biomaterials.2019.01.031. [DOI] [PubMed] [Google Scholar]
- [123].Wang Q, Tang P, Ge X, Li P, Lv C, Wang M, Wang K, Fang L, Lu X. Appl Surf Sci. 2018;462:118. [Google Scholar]
- [124].Sánchez-Salcedo S, Shruti S, Salinas AJ, Malavasi G, Menabue L, Vallet-Regí M. J Mater Chem B. 2014;2:4836. doi: 10.1039/c4tb00403e. [DOI] [PubMed] [Google Scholar]
- [125].Zhang Y, Zhai D, Xu M, Yao Q, Zhu H, Chang J, Wu C. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6ed6. 025037. [DOI] [PubMed] [Google Scholar]
- [126].Cao D, Xu Z, Chen Y, Ke Q, Zhang C, Guo Y. J Biomed Mater Res, Part B. 2018;106:863. doi: 10.1002/jbm.b.33900. [DOI] [PubMed] [Google Scholar]
- [127].Hasan A, Waibhaw G, Saxena V, Pandey LM. IntJ Biol Macromol. 2018;111:923. doi: 10.1016/j.ijbiomac.2018.01.089. [DOI] [PubMed] [Google Scholar]
- [128].De Mori A, Hafidh M, Mele N, Yusuf R, Cerri G, Gavini E, Tozzi G, Barbu E, Conconi M, Draheim RR, Roldo M. Pharmaceutics. 2019;11:116. doi: 10.3390/pharmaceutics11030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jia Z, Zhou W, Yan J, Xiong P, Guo H, Cheng Y, Zheng Y. ACS Biomater Sci Eng. 2019;5:244. doi: 10.1021/acsbiomaterials.8b00857. [DOI] [PubMed] [Google Scholar]
- [130].Sahmani S, Saber-Samandari S, Shahali M, Joneidi Yekta H, Aghadavoudi F, Montazeran AH, Aghdam MM, Khandan A. J Mech Behav Biomed Mater. 2018;88:238. doi: 10.1016/j.jmbbm.2018.08.030. [DOI] [PubMed] [Google Scholar]
- [131].Lu Y, Li L, Zhu Y, Wang X, Li M, Lin Z, Hu X, Zhang Y, Yin Q, Xia H, Mao C. ACS Appl Mater Interfaces. 2018;10:127. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mas N, Arcos D, Polo L, Aznar E, Sánchez-Salcedo S, Sancenón F, García A, Marcos MD, Baeza A, Vallet-Regí M, Martínez-Máñez R. Small. 2014;10:4859. doi: 10.1002/smll.201401227. [DOI] [PubMed] [Google Scholar]
- [133].Zhou X, Weng W, Chen B, Feng W, Wang W, Nie W, Chen L, Mo X, Su J, He C. J Mater Chem B. 2018;6:740. doi: 10.1039/c7tb01246b. [DOI] [PubMed] [Google Scholar]
- [134].Wei J, Wang Y, Jiang J, Yan Y, Fan D, Yang X, Zuo Y, Li Y, Gu H, Li J. J Biomed Nanotechnol. 2019;15:1097. doi: 10.1166/jbn.2019.2743. [DOI] [PubMed] [Google Scholar]
- [135].Karakecili A, Topuz B, Korpayev S, Erdek M. Mater Sci Eng, C. 2019;105 doi: 10.1016/j.msec.2019.110098. 110098. [DOI] [PubMed] [Google Scholar]
- [136].Han L, Sun H, Tang P, Li P, Xie C, Wang M, Wang K, Weng J, Tan H, Ren F, Lu X. Biomater Sci. 2018;6:538. doi: 10.1039/c7bm01060e. [DOI] [PubMed] [Google Scholar]
- [137].Cheng R, Liu L, Xiang Y, Lu Y, Deng L, Zhang H, Santos HA, Cui W. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119706. 119706. [DOI] [PubMed] [Google Scholar]
- [138].Polo L, Gómez-Cerezo N, Aznar E, Vivancos JL, Sancenón F, Arcos D, Vallet-Regí M, Martínez-Máñez R. Acta Biomater. 2017;50:114. doi: 10.1016/j.actbio.2016.12.025. [DOI] [PubMed] [Google Scholar]
- [139].Lu Y, Li L, Li M, Lin Z, Wang L, Zhang Y, Yin Q, Xia H, Han G. Bioconjugate Chem. 2018;29:2982. doi: 10.1021/acs.bioconjchem.8b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bigham A, Aghajanian AH, Behzadzadeh S, Sokhani Z, Shojaei S, Kaviani Y, Hassanzadeh-Tabrizi SA. Mater Sci Eng, C. 2019;99:83. doi: 10.1016/j.msec.2019.01.096. [DOI] [PubMed] [Google Scholar]
- [141].Hassani Besheli N, Damoogh S, Zafar B, Mottaghitalab F, Motasadizadeh H, Rezaei F, Shokrgozar MA, Farokhi M. ACS Biomater Sci Eng. 2018;4:2836. doi: 10.1021/acsbiomaterials.8b00149. [DOI] [PubMed] [Google Scholar]
- [142].Farouk SN, Muhammad A, Aminu MA. Arch Nanomed: Open Access J. 2018;1:59. [Google Scholar]
- [143].Hemeg HA. IntJ Nanomed. 2017;12:8211. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Molecules. 2018;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gold K, Slay B, Knackstedt M, Gaharwar AK. Adv Ther. 2018;1 1700033. [Google Scholar]
- [146].Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, De Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M. Trends Biotechnol. 2012;30:499. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [147].Markowska K, Grudniak AM, Wolska KI. Acta Biochim Pol. 2013;60:530. [PubMed] [Google Scholar]
- [148].Yu Q, Li J, Zhang Y, Wang Y, Liu L, Li M. Sci Rep. 2016;6 doi: 10.1038/srep26667. 26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Miao L, Wang C, Hou J, Wang P, Ao Y, Li Y, Geng N, Yao Y, Lv B, Yang Y, You G, Xu Y. Bioresour Technol. 2016;216:537. doi: 10.1016/j.biortech.2016.05.082. [DOI] [PubMed] [Google Scholar]
- [150].Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH. ACS Appl Mater Interfaces. 2013;5:9322. doi: 10.1021/am402618w. [DOI] [PubMed] [Google Scholar]
- [151].Lellouche J, Friedman A, Gedanken A, Banin E. IntJ Nanomed. 2012;7:5611. doi: 10.2147/IJN.S37075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Turner RJ. Microb Biotechnol. 2017;10:1062. doi: 10.1111/1751-7915.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Slavin YN, Asnis J, Häfeli UO, Bach H. J Nanobiotechnol. 2017;15:65. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Imlay JA. Curr Opin Microbiol. 2015;24:124. doi: 10.1016/j.mib.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Shaikh S, Nazam N, Mohd S, Rizvi D, Ahmad K, Baig MH, Lee EJ, Choi I. IntJ Mol Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Niño-Martínez N, Salas-Orozco MF, Martínez-Castañón GA, Torres Méndez F, Ruiz F. IntJ Mol Sci. 2019;20:2808. doi: 10.3390/ijms20112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lu X, Chen R, Lv J, Xu W, Chen H, Ma Z, Huang S, Li S, Liu H, Hu J, Nie L. Acta Biomater. 2019;99:363. doi: 10.1016/j.actbio.2019.08.043. [DOI] [PubMed] [Google Scholar]
- [158].Castillo RR, Lozano D, González B, Manzano M, Izquierdo-Barba I, Vallet-Regí M. Expert Opin Drug Delivery. 2019;16:415. doi: 10.1080/17425247.2019.1598375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Vallet-Regí M, Balas F, Arcos D. Angew Chem, Int Ed. 2007;46:7548. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- [160].Álvarez-Paino M, Muñoz-Bonilla A, Fernández-García M. Nanomaterials. 2017;7:48. doi: 10.3390/nano7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chauhan AS. Molecules. 2018;23:938. [Google Scholar]
- [162].Baeza A, Colilla M, Vallet-Regí M. Expert Opin Drug Delivery. 2015;12:319. doi: 10.1517/17425247.2014.953051. [DOI] [PubMed] [Google Scholar]
- [163].Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Molecules. 2017;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Croissant JG, Fatieiev Y, Khashab NM. Adv Mater. 2017;29 doi: 10.1002/adma.201604634. 1604634. [DOI] [PubMed] [Google Scholar]
- [165].Lu J, Liong M, Li Z Z, Tamanoi F. Small. 2010;6:1794. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Paris JL, Colilla M, Izquierdo-Barba I, Manzano M, Vallet-Regí M. J Mater Sci. 2017;52:8761. [Google Scholar]
- [167].Li L, Liu T, Fu C, Tan L, Meng X, Liu H. Nanomed: Nanotechnol, Biol Med. 2015;11:1915. [Google Scholar]
- [168].Lindén M. Enzymes. 2018;43:155. doi: 10.1016/bs.enz.2018.07.007. [DOI] [PubMed] [Google Scholar]
- [169].Rahikkala A, Pereira SAP, Figueiredo P, Passos MLC, Araújo ARTS, Saraiva MMFS, Santos HA. Adv Biosyst. 2018;2 1800020. [Google Scholar]
- [170].Martínez-Carmona M, Gun’ko YK, Vallet-Regí M. Pharmaceutics. 2018;10:279. doi: 10.3390/pharmaceutics10040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mora-Raimundo P, Lozano D, Manzano M, Vallet-Regí M. ACS Nano. 2019;13:5451. doi: 10.1021/acsnano.9b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Mora-Raimundo P, Manzano M, Vallet-Regí M. AIMS Bioeng. 2017;4:259. [Google Scholar]
- [173].Pedraza D, Díez J, Colilla M, Vallet-Regí M. Biomed Glasses. 2017;3:111. [Google Scholar]
- [174].Martínez-Carmona M, Izquierdo-Barba I, Colilla M, Vallet-Regí M. Acta Biomater. 2019;96:547. doi: 10.1016/j.actbio.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Wagner S, Hauck D, Hoffmann M, Sommer R, Joachim I, Müller R, Imberty A, Varrot A, Titz A. Angew Chem, Int Ed. 2017;56:16559. doi: 10.1002/anie.201709368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wang JY, Nor YA, Song H, Yang Y, Xu C, Yu M, Yu C. J Mater Chem B. 2016;4:2646. doi: 10.1039/c6tb00053c. [DOI] [PubMed] [Google Scholar]
- [177].González B, Colilla M, Díez J, Pedraza D, Guembe M, Izquierdo-Barba I, Vallet-Regí M. Acta Biomater. 2018;68:261. doi: 10.1016/j.actbio.2017.12.041. [DOI] [PubMed] [Google Scholar]
- [178].Kinnari TJ, Esteban J, Martin-de-Hijas NZ, Sánchez-Muñoz O, Sánchez-Salcedo S, Colilla M, Vallet-Regí M, Gomez-Barrena E. J Med Microbiol. 2009;58:132. doi: 10.1099/jmm.0.002758-0. [DOI] [PubMed] [Google Scholar]
- [179].Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, Li T-F, Virtanen I, Liljestro M, Sakai H, Kobayashi Y, et al. J Bone Miner Res. 2001;16:1780. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- [180].Ribeiro M, Monteiro FJ, Ferraz MP. Biomatter. 2012;2:176. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Yan Z, Shi P, Ren J, Qu X. Small. 2015;11:5540. doi: 10.1002/smll.201501958. [DOI] [PubMed] [Google Scholar]
- [182].Yu E, Galiana I, Martinez-Manez R, Stroeve P, Marcos MD, Aznar E, Sancenon F, Murguia JR, Amoros P. Colloids Surf, B. 2015;135:652. doi: 10.1016/j.colsurfb.2015.06.048. [DOI] [PubMed] [Google Scholar]
- [183].Sun J, Fan Y, Zhang P, Zhang X, Zhou Q, Zhao J, Ren L. J Colloid Interface Sci. 2020;559:197. doi: 10.1016/j.jcis.2019.10.021. [DOI] [PubMed] [Google Scholar]
- [184].Lee BY, Li Z, Clemens DL, Dillon BJ, Hwang AA, Zink JI, Horwitz MA. Small. 2016;12:3690. doi: 10.1002/smll.201600892. [DOI] [PubMed] [Google Scholar]
- [185].Ding Y, Hao Y, Yuan Z, Tao B, Chen M, Chuanchuan L, Liu P, Cai K. Biomater Sci. 2020;8:1840. doi: 10.1039/c9bm01924c. [DOI] [PubMed] [Google Scholar]