Abstract
Self-Incompatibility (SI) is a genetically controlled mechanism that prevents self-fertilisation and thus encourages outbreeding and genetic diversity. During pollination, most SI systems utilise cell-cell recognition to reject incompatible pollen. Mechanistically, one of the best-studied SI systems is that of Papaver rhoeas (poppy), which involves the interaction between the two S-determinants, a stigma-expressed secreted protein (PrsS) and a pollen-expressed plasma-membrane localised protein (PrpS). This interaction is the critical step in determining acceptance of compatible pollen or rejection of incompatible pollen. Cognate PrpS-PrsS interaction triggers a signalling network causing rapid growth arrest and eventually programmed cell death (PCD) in incompatible pollen. In this review, we provide an overview of recent advances in our understanding of the major components involved in the SI-induced PCD (SI-PCD). In particular, we focus on the importance of SI-induced intracellular acidification and consequences for protein function, and the regulation of soluble inorganic pyrophosphatase (Pr-p26.1) activity by post-translational modification. We also discuss attempts at the identification of protease(s) involved in the SI-PCD process. Finally, we outline future opportunities made possible by the functional transfer of the P. rhoeas SI system to Arabidopsis.
Keywords: Acidification, Arabidopsis, caspase-like activity, Papaver rhoeas, pH, pollen, programmed cell death (PCD), proteases, self-incompatibility (SI), signalling
Introduction
Programmed Cell Death (PCD) is essential for a range of developmental and defence-related processes in plants. Processes associated with the plant reproductive life cycle in flowering plants display a particularly rich collection of tightly controlled and executed PCD. This includes cell death-related events on the male side in the tapetum associated with microsporogenesis and male fertility, and in pollen tubes triggered by the self-incompatibility (SI) response in Papaver rhoeas. On the female side PCD is triggered in the embryo sac to ensure proper embryo development and in seeds to ensure their proper development and germination (Wu and Cheung, 2000; Domínguez and Cejudo, 2014; Van Hautegem et al., 2015). Despite the importance of PCD events for the reproductive success of plants, many of the underlying components and processes remain to be elucidated.
The interaction between the pollen and the pistil is one of the most important steps in the reproductive process of flowering plants, involving cell-cell recognition and signalling events (Dresselhaus and Franklin-Tong, 2013). Following penetration of the stigma, pollen tubes grow through the style towards the ovule, delivering the sperm cells to the female gametophyte. The communication and coordination between the pollen and the pistil establishes the limits of inbreeding and outbreeding of a species (Swanson et al., 2004). An estimated 40-50% of flowering plant species have developed a genetically controlled SI mechanism that prevents self-fertilisation and thus encourages outbreeding and genetic diversity (Darlington and Mather, 1949; Igic et al., 2008). For this reason, SI has made a significant contribution to the evolutionary success of flowering plants. During pollination, SI generally utilises cell-cell recognition to prevent self-fertilisation by rejection of “self” (incompatible) pollen. In many SI systems, this involves inhibition of pollen tube growth. In P. rhoeas, which represents one of the best understood SI systems at a mechanistic level, rapid growth arrest of incompatible pollen is followed by PCD.
The stigma of the Papaver pistil secretes a small polymorphic protein (PrsS) which acts as a signalling ligand. With a “self” pollination, PrsS interacts specifically with pollen expressing the male S-determinant, the plasma membrane-localised PrpS. A bioassay in which the SI response can be triggered in in vitro growing Papaver pollen tubes by the addition of recombinant PrsS proteins (Franklin-Tong et al., 1988; Foote et al., 1994) has allowed analysis of events triggered in incompatible pollen grains and tubes. Depending on the combination of S-haplotypes used, either an incompatible/SI response or a compatible situation can be achieved. Analysis can be carried out on either individual pollen tubes (fixed or live), using microscopy (e.g. Thomas and Franklin-Tong, 2006; Poulter et al., 2010; Wilkins et al., 2011), or on a larger scale, making extracts for biochemical or proteomic analysis (Rudd et al., 1996); see Franklin-Tong (2008) for more detail. This bioassay has been fundamental to achieve our current understanding of the mechanisms involved in Papaver SI. A cognate interaction between PrpS and PrsS triggers a signalling network in incompatible pollen, starting with an almost immediate increase in cytosolic free Ca2+ ([Ca2+]cyt) in incompatible pollen (Franklin-Tong et al., 1993, 1995, 1997), followed by transient increases in reactive oxygen species (ROS) and nitric oxide (Wilkins et al., 2011). These processes exhibit distinct temporal “signatures”. After SI induction, the cytoskeleton is rapidly depolymerised, and F-actin reorganises to form stable “punctate foci” that increase in size (Geitmann et al., 2000; Snowman et al., 2002; Poulter et al., 2010). These alterations in actin dynamics are integral to the network leading to PCD (Thomas et al., 2006). Cytoplasmic acidification is a more recently identified regulator of developmental PCD (Fendyrch et al., 2014) and SI in Papaver induces a substantial and rapid decrease of the cytosolic pH ([pH]cyt) in incompatible pollen tubes. The acidification is both necessary and sufficient for triggering several key hallmark features of the SI-PCD signalling network, including formation of punctate actin foci and generation of DEVDase/caspase-3-like activity (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). Other early targets of SI-induced signalling in incompatible pollen include the mitogen-activated protein kinase (MAPK) p56 (Rudd et al., 2003; Li et al., 2007) and soluble inorganic pyrophosphatase, Pr-p26.1 (Rudd et al., 1996; de Graaf et al., 2006), which are phosphorylated. Thus, a relatively well-integrated signalling network regulating Papaver SI is emerging (Wilkins et al., 2014; Figure 1).
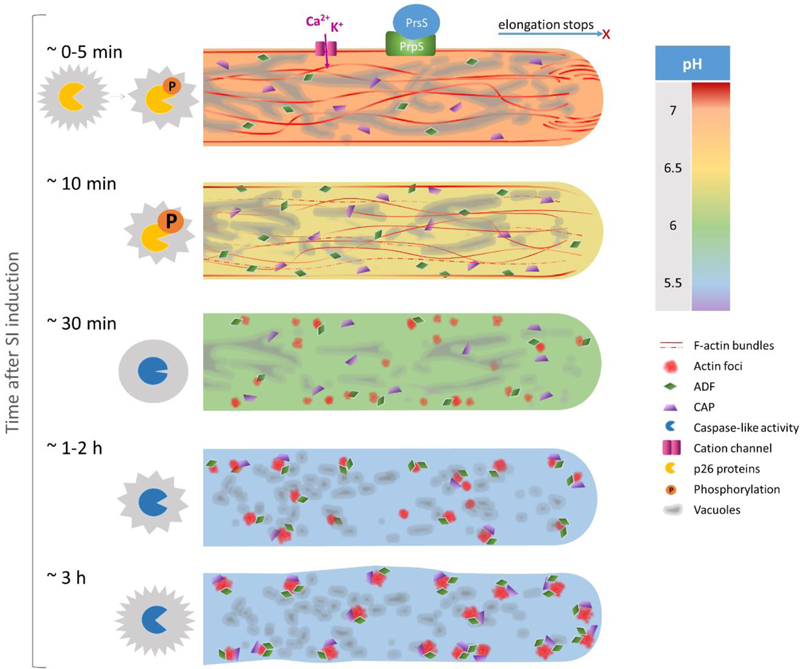
Fig. 1. A timeline showing targets of SI-induced cytosolic acidification in Papaver rhoeas pollen tubes.
At 0 to 5 min after SI induction triggered by the interaction of cognate S-determinants, pollen tube growth is rapidly inhibited. SI activates nonspecific cation conductance involved in mediating Ca2+ and K+ influx. At this early stage the vacuolar network appears as a highly dynamic reticulate structure but the [pH]cyt already starts to drop from its normal physiological pH of around 7. F-actin bundles in the shank and an F-actin ‘reverse fountain’ in the apical area can still be observed. The two p26 proteins are rapidly phosphorylated after SI induction. At about 10 min after SI induction the [pH]cyt has dropped to around pH 6.4. Much of the F-actin is depolymerised with only filaments adjacent to the plasma membrane remaining. ADF predominantly localises at cortical regions and starts to partly colocalise with F-actin. Vacuolar reorganisation with small aggregations can be observed within 15 min after SI induction. At about 30 min after SI induction, the [pH]cyt drops to around pH 6 and the typical reticulate structure of vacuoles cannot be observed any more. No F-actin bundles can be observed and small punctate foci start to form. ADF and CAP form small speckles and both colocalise with F-actin foci. DEVDase activity is not detected at this time point. At 1 to 2 hours after SI induction, the cytosolic pH reaches an equilibrium of pH 5.5. The diameter of F-actin foci increases with their total number decreasing. The level of colocalisation of ADF and CAP with F-actin significantly increases until 1 hour after SI induction. After 1 hour post SI, nearly all of the F-actin foci colocalise with ADF and CAP. Increase in DEVDase activity is rarely observed during this period of time. At about 3 hours after SI induction, F-actin and both ABPs form larger punctate foci and colocalise. Significantly increased activity of DEVDase is detected at this time point and peaks at 5 hours after SI induction.
The first indication suggesting the involvement of PCD in the SI response came from evidence that DNA fragmentation, generally considered a hallmark of late PCD, was specifically triggered in incompatible, and not compatible, pollen with Ca2+ signalling implicated in this process (Jordan et al., 2000). Dramatic alterations in the morphology of cellular organelles, including mitochondria, Golgi bodies and ER within 1 h of SI induction and condensation of the vegetative and generative nuclei further implicated the involvement of PCD in the SI response of P. rhoeas pollen (Geitmann et al., 2004). Conclusive evidence establishing that SI triggers PCD came from a study showing the involvement of a DEVDase/caspase-3-like activity (Thomas and Franklin-Tong, 2004); see later for details. Hallmark features associated with the execution of PCD (e.g. the detection of caspase-like activities and DNA-fragmentation), were only detected several hours after SI-induction.
In this review, we will provide an overview of several recent advances in our understanding of the signalling components involved in Papaver SI-PCD and how these integrate in the signalling cascade leading to PCD. A particular focus is the SI-induced protein phosphorylation, and intracellular acidification and consequences for protein function. We will also discuss attempts to identify the protease(s) responsible for the DEVDase/caspase-3-like activity involved in the PCD process. Lastly, sparked by the successful functional transfer of the Papaver SI system to Arabidopsis thaliana (de Graaf et al., 2012; Lin et al., 2015), we will discuss new opportunities that have emerged because of this functional transfer of SI to further elucidate and dissect key mechanisms and components involved in SI-PCD.
Cytosolic pH alterations involved in SI-PCD
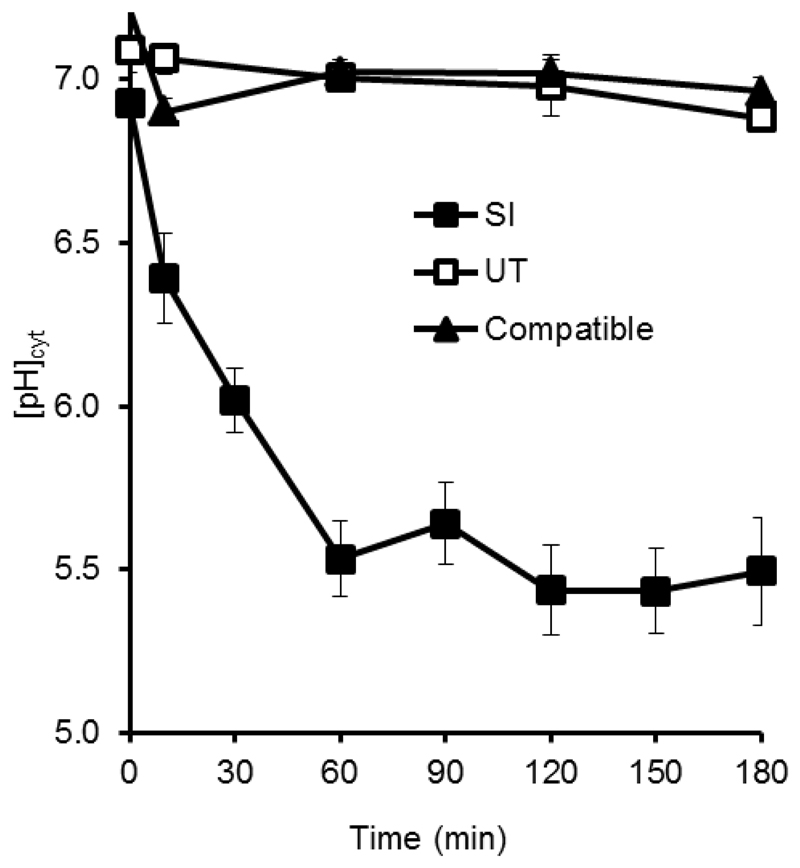
Although cytosolic acidification is not considered a marker for PCD, it has been observed in several developmentally regulated PCD systems in plants (Bosch and Franklin-Tong, 2007; Young et al., 2010; Fendrych et al., 2014; Wilkins et al., 2015). Using the ratiometric pH indicator 2’,7’-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein (BCECF), dramatic and rapid acidification during the SI-response was observed in the pollen cytosol of Papaver (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). Within 10 min of SI induction, the [pH]cyt was found to decrease from pH ~7 to a pH of approximately 6.4, followed by a continuous pH drop to reach levels as low as pH 5.5 one hour after SI induction (Wilkins et al., 2015; Figure 2). By manipulating the pH of the pollen tube cytosol in vivo using propionic acid, cytosolic acidification was found to be both sufficient and necessary for SI-PCD (Wilkins et al., 2015). Here, we review a series of events relevant to SI-PCD signalling that have been established as targets of this physiological alteration and discuss potential mechanisms involved in generating the SI-induced acidification of the pollen tube cytosol.
Fig. 2. SI-induced pollen tubes undergo rapid cytosolic acidification.
Pollen tubes labelled with the pH indicator BECEF-AM were treated with either PrsS to provide an incompatible response (SI) or a compatible response (Compatible), or untreated (UT). Reproduced from Wilkins KA, Bosch M, Haque T, Teng N, Poulter NS, Franklin-Tong VE. 2015. Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiology 167, 766-779, by kind permission of the American Society of Plant Biologists (www.plantphysiol.org).
Targets of SI-induced cytosolic acidification
The actin cytoskeleton plays important roles in regulating pollen tube growth (Gibbon et al., 1999; Vidali et al., 2001; Qu et al., 2017). In the Papaver SI response, the usual actin filament bundles largely disappear and the level of filamentous actin (F-actin) in pollen tubes undergoes a rapid and dramatic reduction (Geitmann et al., 2000; Snowman et al., 2002). Rapid actin depolymerisation was demonstrated after SI induction (Snowman et al., 2002), followed by the formation of highly stable F-actin foci (Geitmann et al., 2000; Snowman et al., 2002; Poulter et al., 2010). By manipulating the [pH]cyt of pollen tubes with propionic acid buffered at pH 5.5, it was established that the formation of F-actin foci can be triggered. Importantly, pre-treatment of pollen tubes with propionic acid buffered at pH 7 followed by treatment with recombinant PrsS to induce the SI response, prevented SI-induced cytosolic acidification and the formation of F-actin foci (Wilkins et al., 2015). These observations demonstrated that the actin cytoskeleton is a major target of pollen tube cytosolic acidification during SI (see also Figure 1) and provided evidence that the drop of [pH]cyt is required for the dramatic changes in actin cytoskeleton configuration/organisation.
Two actin binding proteins (ABPs), cyclase-associated protein (CAP) and actin-depolymerising factor (ADF)/cofilin, were found to be colocalised with large F-actin foci that are formed after SI induction (Poulter et al., 2010), suggesting that they may play crucial roles in the formation of F-actin foci. Later studies showed that in Papaver pollen tubes the colocalisation of CAP and ADF with F-actin is triggered by the acidification of the pollen tube cytosol (Wilkins et al., 2015; Figure 1). In this study, pollen tubes treated with propionic acid (pH 5.5) for three hours had ADF and CAP colocalising with F-actin foci, which resembled the phenomenon observed in the SI-induced pollen tubes (Poulter et al., 2010). Critically, pollen tubes buffered by pre-treating with propionic acid (pH 7) before SI induction showed a significantly lower level of F-actin colocalisation with either ADF or CAP (Wilkins et al., 2015), showing that acidification was required for these alterations. It has been established that the activities of most ADFs in plants are sensitive to cytosolic pH (Carlier et al., 1997; Gungabissoon et al., 1998; Allwood et al., 2002; Chen et al., 2002; Lovy-Wheeler et al., 2006). Studies have shown that at normal [pH]cyt (approximately seven), ADF possesses the ability to sever F-actin filaments, while at acidic pHs the function of ADF is altered to bind and stabilise F-actin (Carlier et al., 1997; Bamburg et al., 1999; Allwood et al., 2002; Wilkins et al., 2015). This suggests that in SI pollen tubes with acidified [pH]cyt the depolymerising activity of ADF is altered and ADF binds and stabilises actin; this could account for the formation and remarkable stability of F-actin foci decorated by ADF in incompatible pollen. Mammalian CAP1 can sever actin filaments at basic pH, but not at neutral and acid pH (Normoyle and Brieher, 2012). However, no such pH dependency has yet been reported in plants and other actin-regulatory proteins may affect how CAP controls actin dynamics (Ono, 2013). As CAP is associated with stable F-actin foci in SI pollen, this suggests that its severing activity is lost and that this may be due to acidic [pH]cyt conditions. Wilkins et al. (2015) suggested that under the acidic condition induced by SI, CAP might act to produce filament ends to facilitate the assembly of actin by ADF. The observation that both artificial cytosolic acidification and SI induction result in the colocalisation of ADF and CAP with F-actin suggests that these two ABPs are targets of SI-induced [pH]cyt acidification, an intriguing hypothesis to explore in future studies.
Another target of the cytosolic acidification triggered by SI is the activity of two pollen-expressed soluble inorganic pyrophosphatases (sPPases) in P. rhoeas, Pr-p26.1a and Pr-p26.1b. The phosphate-metabolising activity of sPPases generates the thermodynamic driving force for many metabolic reactions, including protein, polysaccharide and nucleotide biosynthesis. A screen for SI-induced phosphorylated proteins showed that the two Papaver pollen sPPases are rapidly phosphorylated in a Ca2+-dependent manner after SI induction (Rudd et al., 1996) with increases in cytosolic Ca2+ and phosphorylation leading to a reduction in their activity (de Graaf et al., 2006; Figure 1). Notably, sPPase activity assays using recombinant Pr-p26.1a and Pr-p26.1b proteins showed that the sPPase activity is pH dependent with activities being dramatically reduced in acidic conditions when compared to those at the normal physiological pH 7 (Wilkins et al., 2015). The phosphorylation sites for Pr-p26.1a and Pr-p26.1b have recently been mapped and, importantly, the sPPase activities of the p26 phosphomimic mutants were more sensitive to a low pH environment (6.8 to 5.5) compared to the wild-type enzymes or the corresponding phosphonull mutants (Eaves et al., 2017). Additionally, when combined with the presence of Ca2+ and H2O2, the activities of the phosphomimic and phosphonull forms of both sPPases were further reduced at pH 7. The same activity tests using low pH levels related to SI (pH 6.8 to 5.5) demonstrated that, in addition to low pH, both Ca2+ and H2O2 contribute to the inhibition of p26 activity (Eaves et al., 2017). Considering the rapid stimulation of Ca2+ and ROS, as well as the later pH drop induced by SI, these observations suggest that SI not only triggers the Ca2+-dependent phosphorylation of Pr-p26 but also contributes to the reduction of sPPase activity by stimulating several intracellular events that cumulatively contribute to changes in intracellular conditions that inhibit sPPase activity.
A key enzyme activity identified as being involved in SI-induced PCD is a DEVDase/caspase-3-like activity that has been characterised by its ability to cleave the Ac-DEVD-AMC substrate over a range of different pH values relevant to SI (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). It was revealed that the SI-induced DEVDase activity is optimal at unusually acidic pH, with a narrow pH optimum between pH 4.5 and pH 5.5 (Bosch and Franklin-Tong, 2007), almost exactly matching the [pH]cyt from one hour onwards after SI-induction (Figure 1 and 2). The results of an in vivo test utilising the live-cell caspase-3 probe carboxyfluorescein-DEVD-fluoromethylketone Fluorescent-Labelled Inhibitor of Caspases (FAM-DEVD-FMK FLICA) showed that the addition of propionic acid (pH 5.5) to pollen tubes was sufficient for the induction of DEVDase activity (Wilkins et al., 2015), mimicking the SI-induced samples (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). Pre-treatment with propionic acid (pH 7) before SI-induction prevented DEVDase activation (Wilkins et al., 2015). Thus, the drop of [pH]cyt is a pivotal event in SI-PCD, acting as a gateway to PCD, by providing the acidic environment that matches the pH optimum for the SI-induced DEVDase activity (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). Although it has been shown that lowering the pH of the germination medium is sufficient to trigger DEVDase activity in pollen tubes (Wilkins et al., 2015), further studies are required to determine the exact role of the acidification for the execution phase of this PCD. Crucially, it remains to be established if the SI-induced cytosolic acidification activates the DEVDase by altering cellular conditions to create an optimal pH for DEVDase activity (i.e. directly stimulating DEVDase activation), or whether other cellular components are required to mediate the activation of the caspase-like activities.
Although alterations in [pH]cyt have been reported in many other biological processes in plants (reviewed in Felle, 2001) and animal PCD systems (Park et al., 1999; Matsuyama et al., 2000; Sergeeva et al., 2017), the [pH]cyt changes observed in these studies were transient and mild (with small scale changes of < 1.0 pH unit). This makes the dramatic [pH]cyt shift triggered by SI in Papaver pollen tubes a (to date) rare example of PCD-associated cytosolic acidification in plants (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015). However, a previous study demonstrated the reduction in the fluorescence of a pH-sensitive yellow fluorescent protein (YFP) probe during BAX-induced PCD in onion cells, indicating a large pH drop (Young et al., 2010). Utilising a pH-sensitive green fluorescent protein (GFP) variant pHGFP (Moseyko and Feldman, 2001), a sharp drop of [pH]cyt was also observed during PCD controlling root cap development in A. thaliana (Fendrych et al., 2014), but measurements were not calibrated to establish the absolute change in [pH]cyt. Together, these findings of significant [pH]cyt drops related to PCD suggest that this may be a rather more widespread phenomenon than generally thought and is worth further investigation in other plant PCD systems to examine if they also exhibit cytosolic acidification.
Apart from the ABPs, DEVDase, and sPPases mentioned above, the functional properties of most proteins, including their activity, stability, associations, and subcellular translocation are greatly affected by pH (Talley and Alexon, 2010). It is therefore likely that the SI-induced acidification contributes to the termination of incompatible pollen tube growth by altering the properties of many pollen tube proteins responsible for regulating growth. Importantly, by activating a DEVDase activity, the SI-induced cytosolic acidification plays a pivotal role in triggering incompatible pollen tubes to undergo PCD (Bosch and Franklin-Tong, 2007; Wilkins et al., 2015).
How is the SI-induced cytosolic acidification achieved?
Despite several independent reports showing that cytosolic acidification is a pivotal event for the cell to enter PCD (Fendrych et al., 2014; Wilkins et al., 2015), to date, there is no clear mechanism that has been demonstrated to be responsible for this pH shift. Since the vacuole is one of the most acidic organelles, with a pH 5.2-6 (Martinière et al., 2013; Shen et al., 2013), and is involved in one of the PCD classes in plants (van Doorn et al., 2011), one obvious mechanism for the acidification would be that the drop in [pH]cyt is caused by vacuolar rupture, as observed in PCD triggering xylem differentiation (Groover and Jones, 1999; Obara et al., 2001). Pollen tube vacuoles exhibit a highly dynamic reticulate structure (Hicks et al., 2004) and SI-induction triggers a rapid vacuolar reorganisation, with the typical reticulate structure being lost within 15 minutes (Wilkins et al., 2015; see Figure 1). However, at this early stage, the vacuolar membrane system (tonoplast) appears to be intact, with apparent extensive vacuolar permeabilisation only observed after 30 minutes and complete permeabilisation and possibly breakdown detected one hour after SI-induction, well after the initial cytosolic acidification (Wilkins et al., 2015). A similar observation was reported by Fendrych et al. (2014) during developmental PCD in Arabidopsis lateral root cap cells; [pH]cyt dramatically decreased prior to the permeabilisation of the tonoplast for proteins. Wilkins et al. (2015) showed that the artificial acidification of the cytosol in Papaver pollen tubes triggered something that resembled the reorganisation and collapse of vacuoles after SI induction. Pre-treatment of pollen tubes with propionic acid (pH 7) prior to SI-induction largely reduced vacuolar reorganisation but did not completely prevent it (Wilkins et al., 2015). These observations suggest that the cytosolic acidification, at least in the systems of SI-PCD and lateral root cap developmental PCD, are unlikely to be caused by vacuolar collapse or breakdown. Instead, the initial cytosolic acidification induced by SI appears to occur earlier and upstream of vacuolar permeabilisation and this acidification somehow affects the integrity of the tonoplast. At this stage it can however not be ruled out that the initial cytosolic acidification may result from protons entering the cytosol through channels or other gateways from the vacuole before general permeabilisation of the vacuoles becomes visible using fluorescent probes.
Since the apoplastic pH is acidic, with values between pH 5 and 6 (Felle, 2001), a possibility is that H+ influx is triggered by the very first SI events, as upon cognate interaction of PrsS with PrpS, Ca2+ and K+ influx are the earliest events observed (Franklin-Tong et al., 1993; Franklin-Tong et al., 1995; Wu et al., 2011). The Ca2+ and K+ influx observed at the shank of incompatible pollen tubes (Wu et al., 2011) provides evidence for the activation of Ca2+- conducting/non-specific cation channels, which could also play a key role in triggering the drop of [pH]cyt if they also allowed protons through. It should be noted that treatment of pollen tubes with the calcium ionophore A23187, which also increases the permeability of the plasma membrane, mimicked the increase of [Ca2+]cyt exhibited in SI and could also trigger rapid cytosolic acidification (Wilkins et al., 2015). In mammalian systems, an increase in [Ca2+]cyt can lead to intracellular acidification through the Ca2+/H+ exchange activity of a plasma membrane Ca2+/ATPase (Hwang et al., 2011). In Arabidopsis roots, the elevation of [Ca2+]cyt induced by mechanical stimulation showed very similar kinetics to changes in pH and ROS triggered by mechanical stimulation (Monshausen et al., 2009). Treatment of the root with A23187 resulted in an increase in ROS and decrease in [pH]cyt (Monshausen et al., 2009). These observations suggest a strong link between elevated [Ca2+]cyt levels and decreased [pH]cyt with changes in both Ca2+ and H+ ions potentially mediated by the same channels or channels acting together.
Regardless of the identity/nature of the channels involved, it seems likely that pollen plasma membrane-localised PrpS proteins are in some way associated with such channels, or themselves form channels or pores. Structural predictions indicate that PrpS proteins might comprise four transmembrane regions with a relatively short (~34 aa) extracellular loop containing several hypervariable residues between different alleles (Wheeler et al., 2009). Although PrpS has no significant sequence homology to any protein in existing databases, it exhibits weak structural homology to a transport protein called Flower, a Drosophila protein that multimerises to function as a Ca2+-permeable channel involved in presynaptic vesicle endocytosis (Yao et al., 2009; Wheeler et al., 2010). Whether PrpS forms a channel/pore at the plasma membrane or whether it is involved in the SI-induced acidification through proton transport remains to be determined.
Investigating the identity of proteases involved in SI-PCD
In SI-induced pollen, besides a caspase-3-like/DEVDase, a VEIDase and later a LEVDase activity was detected; all three exhibiting optimal activity at an acidic pH. The DEVDase and VEIDase were activated within 1–2 h after SI, with peak activity around 5 h after SI induction (Bosch and Franklin-Tong, 2007). SI-induced DNA fragmentation was significantly inhibited following pretreatment with Ac-VEID-CHO but not with Ac-LEVD-CHO, suggesting that the VEIDase activity, but not the LEVDase, is functionally involved in SI-mediated PCD (Bosch and Franklin-Tong, 2007). Since no SI-induced cleavage of the GRR substrate (diagnostic for metacaspases) was observed, the evidence from substrate cleavage assays (Bosch and Franklin-Tong, 2007) indicate that metacaspases are not involved in the execution of PCD in Papaver pollen. As mentioned earlier, SI-induced DEVDase activity has been demonstrated to play a crucial role in several physiological hallmarks of SI-PCD. Pre-treatment with the DEVDase inhibitor, Ac-DEVD-CHO, prior to SI induction, markedly alleviated pollen tube growth arrest stimulated by SI (Thomas and Franklin-Tong, 2004), rescued SI-induced loss of pollen tube viability (de Graaf et al., 2012; Li et al., 2007) and nuclear DNA fragmentation (Thomas and Franklin-Tong, 2004) in incompatible pollen. In addition, it was shown that PARP, a classic substrate for caspase-3 activity in animal cells, was cleaved in an S-specific manner (Thomas and Franklin-Tong, 2004). Detailed characterisation of the temporal and spatial activation of plant caspase-like enzymes revealed a cytosolic and later nuclear localisation of the SI-induced DEVDase activity (Bosch and Franklin-Tong, 2007). In mammalian systems, caspases are synthesised as precursors (pro-caspases) and often translocated from the cytoplasm into the nucleus after induction of apoptosis (Zhivotovsky et al., 1999; Kamada et al., 2005). The change in localisation of the SI-induced DEVDase activity suggested that a similar strategy may be employed in the SI-induced PCD in Papaver pollen. However, to date, the identity of the SI-induced DEVDase remains a mystery.
Because plants have no caspase homologues, the nature and identity of their caspase-like proteases is of key importance to our understanding of PCD. Although plants contain metacaspases they are unable to cleave synthetic caspase substrates (Vercammen et al.,2007) so are not responsible for the caspase-like activities observed during SI-PCD. The identities of several plant proteases that exhibit caspase-like activities involved in PCD have now been revealed. Examples include phytaspases predominantly exhibiting a VEIDase/caspase-6-like activity (Chichkova et al., 2010) and vacuolar processing enzymes (VPEs), exhibiting YVADase/caspase-1-like activity (Hatsugai et al., 2004). Regarding the identity of DEVDases, two distinct proteases have been confirmed to cleave substrates diagnostic of caspase-3-like activity. Arabidopsis cathepsin B3 has been identified as a DEVDase/caspase-3 involved in UV stress (Ge et al., 2016) while the 20S proteasome β subunit 1 (PBA1) has been demonstrated to act as a plant caspase-3-like enzyme in the regulation of pathogen-induced PCD (Hatsugai et al., 2009). The 20S proteasome has also shown to be responsible for the caspase-3-like activity during PCD in xylem development (Han et al., 2012).
In eukaryotes, proteolysis is mainly carried out by the ubiquitin-proteasome system (UPS) and is crucial to diverse plant physiological events, such as growth and development (Moon et al., 2004), responses to abiotic stresses (Stone, 2014), and the SI response in Solanaceae and Brassicaceae (Entani et al., 2014; Indriolo et al., 2012, 2014). In mammalian systems, the UPS plays an important role in regulating apoptosis by targeting key cell death proteins, including caspases (Bader and Steller, 2009). Interference with the UPS, such as with the use of proteasome inhibitors, can either trigger apoptosis or protect the cell from apoptosis in mammalian cell lines (Orlowski, 1999), illustrating the complex role of UPS in regulating apoptosis. Likewise in plants, disruption of proteasome function by silencing has been shown to activate PCD in Nicotiana benthamiana (Kim et al., 2003) while the proteasome inhibitor MG132 prevented heat shock-induced PCD in Nicotiana tabacum Bright-Yellow 2 cells (Vacca et al., 2007).
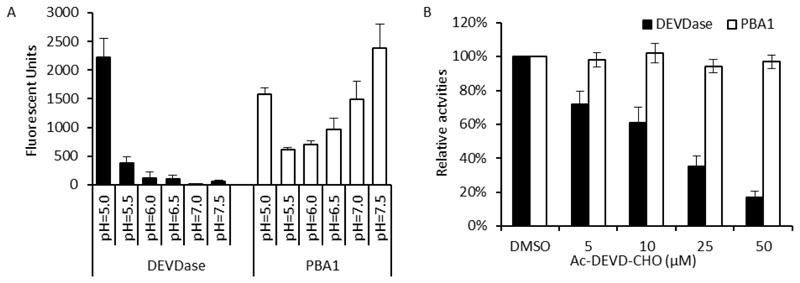
As several studies had identified caspase-3-like proteases involved in plant PCD as proteasomal proteins, we investigated whether the proteasome might be involved in the Papaver SI-PCD response. A pharmacological approach was used to test if inhibiting the proteasome might affect the SI response: pollen was pre-treated with MG132, epoxomicin or β-lactone, which are all potent proteasome inhibitors widely used in proteasome-related studies (Kisselev and Goldberg, 2001). However, viability assays using fluorescein diacetate staining showed no alleviation of death of pollen after SI in the presence of MG132 and TUNEL assays revealed that there was no significant alleviation in SI-induced pollen nuclear DNA fragmentation in SI-induced pollen pre-treated with several different proteasome inhibitors compared with non-treated SI pollen (Lin, 2015). Thus, these data suggest that the proteasome is not involved in the SI-PCD signalling pathway. Moreover, in vitro activity assays showed that the pH optimum for the Papaver pollen proteasome activity was neutral/basic, which contrasts with the acidic pH optimum for the SI-induced DEVDase activity in pollen (Figure 3A). Lastly, the DEVDase inhibitor, Ac-DEVD-CHO, which inhibits the DEVDase/caspase-3-like activity induced by SI, had no inhibitory effect on the PBA1 proteasomal activity (Figure 3B). Together, these data show quite clearly that the identity of the Papaver pollen DEVDase is not a proteasomal protein (Lin, 2015). Thus, the identity of this DEVDase remains to be established.
Fig. 3. The proteasome is not involved in the SI-PCD response.
(A) Papaver pollen proteasome and DEVDase activities have different pH-optima. Papaver pollen DEVDase and proteasome activities were profiled in different pHs using fluorogenic substrate-based activity assay. Note that the observed increase in PBA1 activity at pH 5.0 is caused by non-specific cleavage of the fluorogenic substrate Ac-nLPnLD-amc by DEVDase activity present in the pollen protein extract. Black bars: DEVDase activities profiled using Ac-DEVD-amc. White bars: PBA1 activities profiled using Ac-nLPnLD-amc. mean ±SD, n=3. (B) Proteasome activities are not inhibited by a DEVDase inhibitor. The effects of the DEVDase inhibitor, Ac-DEVD-CHO, on Papaver pollen PBA1 activity was tested in vitro. Pollen protein extracts were incubated with different concentrations (0-50 µM) of the inhibitor for 0.5 h before being subjected to activity measurement. DEVDase activity measurements were included as controls. DEVDase (black bars) and PBA1 (white bars) activities were measured by monitoring the hydrolysis of fluorogenic substrates Ac-DEVD-amc and Ac-nLPnLD-amc, respectively. Ac-DEVD-CHO did not significantly affect PBA1 activity, but dramatically inhibited DEVDase activity. Mean ±SD, n=6.
DEVD pull-downs identified a Papaver pollen VPE
In an attempt to identify proteins interacting with the DEVDase/caspase-3-like protein, pull-downs of SI-induced pollen protein extracts using a DEVD-biotin probe identified peptides corresponding to a VPE (Bosch et al., 2010). VPEs are vacuolar localised cysteine proteases involved in many plant cell death programmes, ranging from developmental PCD to PCD induced by abiotic and biotic stresses (reviewed in Hatsugai et al., 2015). VPE silencing suppresses the disintegration of the vacuolar membranes in leaves infected with tobacco mosaic virus, suggesting that vacuolar collapse is VPE-mediated (Hatsugai et al., 2004). Although the mechanism of this is unclear, it is thought that this is a key step, initiating the proteolytic cascade leading to PCD (Fukuda, 2000; Jones, 2001; Hara-Nishimura and Hatsugai, 2011; Hatsugai et al., 2015).
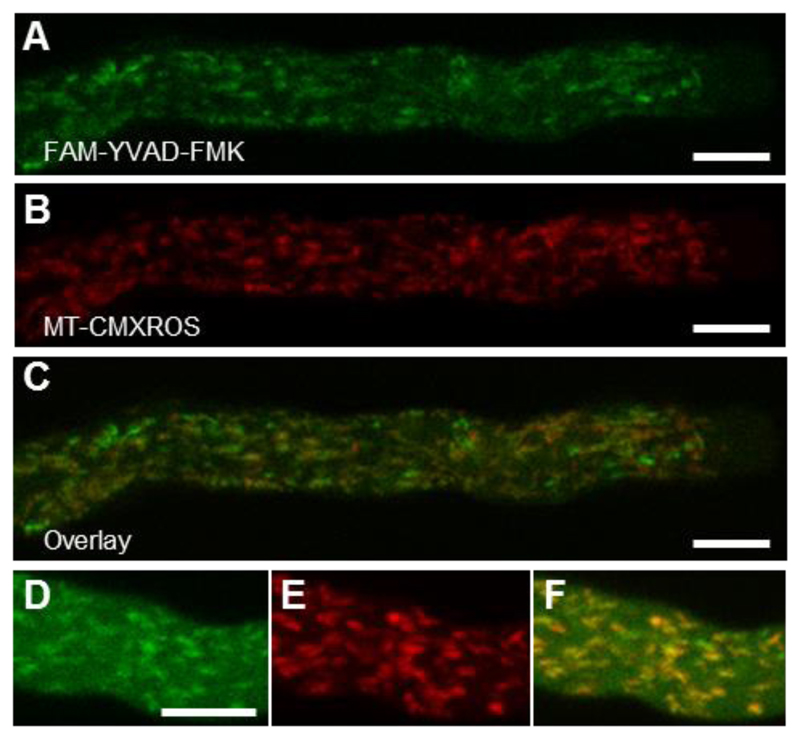
Based on this apparent interaction, which suggested that this VPE might be part of the SI-PCD network, a P. rhoeas pollen expressed VPE (PrVPE1) was cloned and characterised. Although recombinant PrVPE1 indeed exhibited some DEVDase activity, its main activity was YVADase with optimum substrate cleavage at acidic pHs. PrVPE1 localised to the vacuolar compartment, like other VPEs. Since a YVADase activity is not required for SI-mediated PCD, it is unlikely that PrVPE1 is directly involved in the SI-PCD response and that the SI-induced vacuolar breakdown is not mediated by the activity of VPEs. However, it is worth mentioning that the characterisation of PrVPE1 revealed several features distinct from other characterised VPEs (Bosch et al., 2010). The unprocessed recombinant VPE pre-pro-protein, which is expected to be inactive, displayed YVADase activity while no activity could be detected for the mature (and normally active) form. Moreover, there was no evidence of the pre-pro-protein being processed under acidic conditions, which is unusual. Interestingly, localisation of the YVADase activity using live-cell imaging with a caspase-1 fluorescent probe FAM-YVAD-FMK showed labelling in mitochondria of untreated growing pollen tubes (Figure 4), while >1 h after SI induction this changed to a diffuse cytosolic signal (Bosch et al., 2010). This suggests mitochondrial permeabilisation, perhaps releasing YVADase, is triggered by SI. However, since PrVPE1 is not localised to mitochondria, this implies that a different protease is responsible for the observed mitochondrial YVADase activity.
Fig. 4. A mitochondrial localisation for YVADase activity.
(A) FAM-YVAD-FMK provides localisation of YVADase activity in Papaver pollen tubes. (B) MT-CMXRos mitochondrial signal. (C) MT-CMXRos and FAM-YVAD-FMK merge shows considerable co-localisation. Detail of (D) FAM-YVAD-FMK, (E) MT-CMXRos and (F) co-localisation. Scale bar, 10 µm. Reproduced from Bosch M, Poulter NS, Perry RM, Wilkins KA, Franklin-Tong VE. 2010. Characterization of a legumain/vacuolar processing enzyme and YVADase activity in Papaver pollen. Plant Molecular Biology 74, 381-393, by kind permission of Springer Nature (www.springernature.com).
The detection of a caspase-like activity localised to mitochondria in plants is of interest and warrants further investigation, as in mammalian cells, it is known that pro-caspases (inactive precursors of caspases) can localise to the mitochondria, and active caspases have also been found in the mitochondrial fraction (Zhivotovsky et al., 1999; Chandra and Tang, 2003). It is well established that mitochondria play key roles in activating apoptosis in mammalian cells, with permeabilisation of the mitochondrial outer membrane and subsequent release of proapoptotic molecules such as cytochrome c into the cytoplasm being involved in the activation of caspases (Wang and Youle, 2009). In Papaver pollen tubes, release of cytochrome c from the mitochondria into the cytosol has been detected as early as 10 minutes after SI induction, with levels of cytosolic cytochrome c continuing to increase up to 2 h after SI induction (Thomas and Franklin-Tong, 2004). It has been observed that mitochondria undergo significant structural changes within 1 h of SI induction, including swelling, loss of cristae and blebbing, similar to PCD/apoptosis-associated changes described for mammalian systems (Geitmann et al., 2004). These findings, together with the observation that SI-induces hot spots of ROS production localised to organelles resembling mitochondria (Wilkins et al., 2011), suggest a possible critical role for mitochondria in the SI-PCD process that requires further exploration. This is corroborated by recent findings supporting a central role for mitochondria in several plant PCD systems, involving inhibition of the mitochondrial electron transport chain and ROS production (Van Aken and Van Breusegem, 2015; Zhao et al., 2018).
Conclusion and outlook
SI in P. rhoeas triggers an intricate signalling network leading to PCD of incompatible pollen. Here we have discussed the importance of cytosolic acidification as a physiological regulator of SI-PCD. Up to this date, cytosolic acidification involved in signalling to PCD has been mostly found in animals, with relatively small drop in pH of approximately 0.3–0.4 pH units (Furlong et al., 1997; Matsuyama et al., 2000; Roy et al., 2001; Shin and Loewen, 2011). However, in addition to the Papaver SI response, several studies have reported changes in [pH]cyt associated with PCD in plants (Moseyko and Feldman, 2001; Young et al., 2010; Fendrych et al., 2014). Together, these findings suggest that acidification, perhaps on a larger scale than seen in animal cells, may be a more general phenomenon in plant PCD that requires further characterisation to determine its extent, role and origin.
While Papaver SI has provided an excellent model system to investigate the molecular basis of cell-cell recognition and intracellular signalling in plant cells, its limited genetic resources represent a bottleneck to advance the field further. In an attempt to overcome this limitation, it was demonstrated that PrpS expressed in pollen of A. thaliana is functional (De Graaf et al., 2012). Transgenic A. thaliana pollen expressing PrpS undergoes a SI response with key features of Papaver SI when challenged with recombinant Papaver PrsS proteins, including pollen tube inhibition, actin alterations and PCD involving DEVDase activities (De Graaf et al., 2012). More recently, both the Papaver SI S-determinants have been functionally transferred to A. thaliana. Plants expressing PrsS in the stigma and PrpS-GFP in the pollen exhibit robust self-incompatibility and do not set seed (Lin et al., 2015). This finding demonstrates that the two components PrpS and PrsS are sufficient to elicit an SI response in another plant species. The successful transfer, despite the substantial evolutionary distance between Papaver and Arabidopsis of some 140 million years, suggests that the network of signalling factors mediating SI-PCD is highly conserved and likely to be ancient, as they could be recruited from a distantly related species (Lin et al., 2015). The simplest explanation for why this works in Arabidopsis is that all the components downstream of the “receptor-ligand” interaction of PrpS and PrsS are common and universal (e.g. actin, sPPase etc.) and so when cognate S-determinants interact, all the components are in place to specify the network of events that characterise the SI-PCD response.
The availability of Arabidopsis plants expressing an SI response with all the key features of Papaver SI, opens up exciting opportunities to genetically dissect SI-induced signalling networks leading to PCD. Such Arabidopsis plants carrying the Papaver SI system allow the exploitation of the full forward and reverse genetics toolbox available for this model plant. For instance, crosses to CRISPR- or T-DNA mutant lines or gene-silencing/overexpression lines and lines with fluorescent markers of interest can further dissect the molecular mechanisms involved in SI-PCD. Forward genetic approaches could be employed to identify new genes involved in SI-PCD.
Likewise, the heterologous Arabidopsis SI system provides opportunities for identifying components involved in the later “execution” phase of the SI-induced PCD process. Although affinity-based approaches using pull-down assays of Papaver pollen extracts have identified various proteins that interact with the DEVD tetrapeptide, including the VPE discussed in this review, they have so far not led to the identification of the protease(s) responsible for the SI-induced caspase-3-like/DEVDase activity involved in the execution of PCD. Thus far, annotating peptides following pull-down assays and mass-spectrometry analyses required searches against the “whole green plant” database as the genome of P. rhoeas has not been sequenced. The drawback of this approach is that only Papaver peptides identical to those present in the “whole green plant” database can be identified. Utilisation of Arabidopsis lines expressing the Papaver SI system for pull-down assays with DEVD-based probes would alleviate this drawback, therefore providing an opportunity, together with the availability of T-DNA lines and/or generation of knockouts/overexpression lines to validate candidates, to identify proteases involved in the execution of PCD in Papaver SI.
In summary, the availability of the heterologous Arabidopsis “SI” system should provide a powerful genetic tool for testing new hypotheses about SI-PCD in Papaver and to increase our understanding of the cellular mechanisms and genetic components involved in the SI-PCD response.
Acknowledgements
We gratefully acknowledge funding by the Biotechnology and Biological Sciences Research Council (grant numbers BB/P005489/1) to VEF-T and MB, the ERC StG PROCELLDEATH (Project Number: 749 639234) to MKN, and funding by the FWO (project numbers G011215N and 12I7417N) to MT and ZL, respectively. We would like to thank Renier van der Hoorn for his advice regarding the proteasome studies.
Abbreviations
- [Ca2+]cyt
Cytosolic free calcium
- [pH]cyt
Cytosolic pH
- ABPs
Actin-binding proteins
- ADF
Actin-depolymerising factor
- CAP
Cyclase-associated protein
- F-actin
Filamentous actin
- GFP
Green fluorescent protein
- PBA1
Proteasome β subunit 1
- PCD
Programmed cell death
- PrpS
Papaver rhoeas pollen S
- PrsS
Papaver rhoeas stigma S
- PrVPE1
Papaver rhoeas vacuolar processing enzyme1
- ROS
Reactive oxygen species
- SI
Self-incompatibility or Self-incompatible
- SI-PCD
Self-incompatibility induced programmed cell death
- sPPases
Soluble inorganic pyrophosphatases
- UPS
Ubiquitin-proteasome system
- VPEs
Vacuolar processing enzymes
References
- Allwood EG, Anthony RG, Smertenko AP, Reichelt S, Drobak BK, Doonan JH, Weeds AG, Hussey PJ. Regulation of the pollen-specific actin-depolymerizing factor LlADF1. The Plant Cell. 2002;14:2915–2927. doi: 10.1105/tpc.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Steller H. Regulation of cell death by the ubiquitin-proteasome system. Current Opinion in Cell Biology. 2009;21:878–884. doi: 10.1016/j.ceb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends in Cell Biology. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- Bosch M, Franklin-Tong VE. Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen. Proceedings of the National Academy of Sciences, USA. 2007;104:18327–18332. doi: 10.1073/pnas.0705826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Poulter NS, Perry RM, Wilkins KA, Franklin-Tong VE. Characterization of a legumain/vacuolar processing enzyme and YVADase activity in Papaver pollen. Plant Molecular Biology. 2010;74:381–393. doi: 10.1007/s11103-010-9681-9. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. Journal of Cell Biology. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Tang DG. Mitochondrially localized active caspase-9 and caspase-3 result mostly from translocation from the cytosol and partly from caspase-mediated activation in the organelle. Lack of evidence for Apaf-1-mediated procaspase-9 activation in the mitochondria. Journal of Biological Chemistry. 2003;278:17408–17420. doi: 10.1074/jbc.M300750200. [DOI] [PubMed] [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. The Plant Cell. 2002;14:2175–2190. doi: 10.1105/tpc.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichkova NV, Shaw J, Galiullina RA, et al. Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO Journal. 2010;29:1149–1161. doi: 10.1038/emboj.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CD, Mather K. The elements of genetics. New York: Macmillan; 1949. [Google Scholar]
- de Graaf BH, Rudd JJ, Wheeler MJ, Perry RM, Bell EM, Osman K, Franklin FC, Franklin-Tong VE. Self-incompatibility in Papaver targets soluble inorganic pyrophosphatases in pollen. Nature. 2006;444:490–493. doi: 10.1038/nature05311. [DOI] [PubMed] [Google Scholar]
- de Graaf BH, Vatovec S, Juarez-Diaz JA, Chai L, Kooblall K, Wilkins KA, Zou H, Forbes T, Franklin FC, Franklin-Tong VE. The Papaver self-incompatibility pollen S-determinant, PrpS, functions in Arabidopsis thaliana . Current Biology. 2012;22:154–159. doi: 10.1016/j.cub.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez F, Cejudo FJ. Programmed cell death (PCD): an essential process of cereal seed development and germination. Frontiers in Plant Science. 2014;5:366. doi: 10.3389/fpls.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Molecular Plant. 2013;6:1018–1036. doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- Eaves DJ, Haque T, Tudor RL, et al. Identification of phosphorylation sites altering pollen soluble inorganic pyrophosphatase activity. Plant Physiology. 2017;173:1606–1616. doi: 10.1104/pp.16.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entani T, Kubo K, Isogai S, Fukao Y, Shirakawa M, Isogai A, Takayama S. Ubiquitin-proteasome-mediated degradation of S-RNase in a solanaceous cross-compatibility reaction. The Plant Journal. 2014;78:1014–1021. doi: 10.1111/tpj.12528. [DOI] [PubMed] [Google Scholar]
- Felle HH. pH: signal and messenger in plant cells. Plant Biology. 2001;3:577–591. [Google Scholar]
- Fendrych M, Van Hautegem T, Van Durme M, et al. Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Current Biology. 2014;24:931–940. doi: 10.1016/j.cub.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Foote HCC, Ride JP, Franklin-Tong VE, Walker EA, Lawrence MJ, Franklin FCH. Cloning and expression of a distinctive class of self-incompatibility (S) gene from Papaver rhoeas L. Proceedings of the National Academy of Sciences, USA. 1994;91:2265–2269. doi: 10.1073/pnas.91.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE. Self-Incompatibility in Papaver rhoeas: progress in understanding mechanisms involved in regulating self-incompatibility in Papaver. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants. Springer, Berlin, Heidelberg: 2008. [Google Scholar]
- Franklin-Tong VE, Hackett G, Hepler PK. Ratio-imaging of Ca2+ i in the self-incompatibility response in pollen tubes of Papaver rhoeas . The Plant Journal. 1997;12:1375–1386. [Google Scholar]
- Franklin-Tong VE, Lawrence MJ, Franklin FCH. An in vitro bioassay for the stigmatic product of the self-incompatibility gene in Papaver rhoeas L. New Phytologist. 1988;110:109–118. [Google Scholar]
- Franklin-Tong VE, Ride JP, Franklin FCH. Recombinant stigmatic self-incompatibility (S-) protein elicits a Ca2+ transient in pollen of Papaver rhoeas . The Plant Journal. 1995;8:299–307. [Google Scholar]
- Franklin-Tong VE, Ride JP, Read ND, Trewavas AJ, Franklin FCH. The self-incompatibility response in Papaver rhoeas is mediated by cytosolic free calcium. The Plant Journal. 1993;4:163–177. [Google Scholar]
- Fukuda H. Programmed cell death of tracheary elements as a paradigm in plants. Plant Molecular Biology. 2000;44:245–253. doi: 10.1023/a:1026532223173. [DOI] [PubMed] [Google Scholar]
- Furlong IJ, Ascaso R, Lopez Rivas A, Collins MK. Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. Journal of Cell Science. 1997;110:653–661. doi: 10.1242/jcs.110.5.653. [DOI] [PubMed] [Google Scholar]
- Ge Y, Cai YM, Bonneau L, Rotari V, Danon A, McKenzie EA, McLellan H, Mach L, Gallois P. Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death and Differentiation. 2016;23:1493–1501. doi: 10.1038/cdd.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A, Franklin-Tong VE, Emons AC. The self-incompatibility response in Papaver rhoeas pollen causes early and striking alterations to organelles. Cell Death and Differentiation. 2004;11:812–822. doi: 10.1038/sj.cdd.4401424. [DOI] [PubMed] [Google Scholar]
- Geitmann A, Snowman BN, Emons AM, Franklin-Tong VE. Alterations in the actin cytoskeleton of pollen tubes are induced by the self-incompatibility reaction in Papaver rhoeas . The Plant Cell. 2000;12:1239–1251. doi: 10.1105/tpc.12.7.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. The Plant Cell. 1999;11:2349–2363. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungabissoon RA, Jiang C-J, Drøbak BK, Maciver SK, Hussey PJ. Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. The Plant Journal. 1998;16:689–696. [Google Scholar]
- Han JJ, Lin W, Oda Y, Cui KM, Fukuda H, He XQ. The proteasome is responsible for caspase-3-like activity during xylem development. The Plant Journal. 2012;72:129–141. doi: 10.1111/j.1365-313X.2012.05070.x. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Hatsugai N. The role of vacuole in plant cell death. Cell Death and Differentiation. 2011;18:1298–1304. doi: 10.1038/cdd.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, Hara-Nishimura I. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes and Development. 2009;23:2496–2506. doi: 10.1101/gad.1825209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Yamada K, Goto-Yamada S, Hara-Nishimura I. Vacuolar processing enzyme in plant programmed cell death. Frontiers in Plant Science. 2015;6:234. doi: 10.3389/fpls.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV. Geminating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiology. 2004;134:1227–1239. doi: 10.1104/pp.103.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Koo NY, Jin M, Davies AJ, Chun GS, Choi SY, Kim JS, Park K. Intracellular acidification is associated with changes in free cytosolic calcium and inhibition of action potentials in rat trigeminal ganglion. Journal of Biological Chemistry. 2011;286:1719–1729. doi: 10.1074/jbc.M109.090951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B, Lande R, Kohn JR. Loss of self-incompatibility and its evolutionary consequences. International Journal of Plant Sciences. 2008;169:93–104. [Google Scholar]
- Indriolo E, Safavian D, Goring DR. The ARC1 E3 ligase promotes two different self-pollen avoidance traits in Arabidopsis . The Plant Cell. 2014;26:1525–1543. doi: 10.1105/tpc.114.122879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E, Tharmapalan P, Wright SI, Goring DR. The ARC1 E3 ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. The Plant Cell. 2012;24:4607–4620. doi: 10.1105/tpc.112.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. Programmed cell death in development and defense. Plant Physiology. 2001;125:94–97. doi: 10.1104/pp.125.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan ND, Franklin FC, Franklin-Tong VE. Evidence for DNA fragmentation triggered in the self-incompatibility response in pollen of Papaver rhoeas . The Plant Journal. 2000;23:471–479. doi: 10.1046/j.1365-313x.2000.00811.x. [DOI] [PubMed] [Google Scholar]
- Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s) Journal of Biological Chemistry. 2005;280:857–860. doi: 10.1074/jbc.C400538200. [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn JW, Jin UH, Choi D, Paek KH, Pai HS. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. Journal of Biological Chemistry. 2003;278:19406–19415. doi: 10.1074/jbc.M210539200. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chemistry and Biology. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Li S, Samaj J, Franklin-Tong VE. A mitogen-activated protein kinase signals to programmed cell death induced by self-incompatibility in Papaver pollen. Plant Physiology. 2007;145:236–245. doi: 10.1104/pp.107.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Functional transfer of the Papaver SI system into self-compatible A. thaliana and investigating the role of the proteasome in the Papaver SI response. PhD thesis; University of Birmingham, Birmingham: 2015. [Google Scholar]
- Lin Z, Eaves DJ, Sanchez-Moran E, Franklin FC, Franklin-Tong VE. The Papaver rhoeas S determinants confer self-incompatibility to Arabidopsis thaliana in planta. Science. 2015;350:684–687. doi: 10.1126/science.aad2983. [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK. Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of Lily. The Plant Cell. 2006;18:2182–2193. doi: 10.1105/tpc.106.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N. In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. The Plant Cell. 2013;25:4028–4043. doi: 10.1105/tpc.113.116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nature Cell Biology. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. The Plant Cell. 2009;21:2341–2356. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. The Plant Cell. 2004;16:3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseyko N, Feldman LJ. Expression of pH-sensitive green fluorescent protein in Arabidopsis thaliana . Plant Cell and Environment. 2001;24:557–563. doi: 10.1046/j.1365-3040.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- Normoyle KP, Brieher WM. Cyclase-associated protein (CAP) acts directly on F-actin to accelerate cofilin-mediated actin severing across the range of physiological pH. Journal of Biological Chemistry. 2012;287:35722–35732. doi: 10.1074/jbc.M112.396051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Kuriyama H, Fukuda H. Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiology. 2001;125:615–626. doi: 10.1104/pp.125.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. The role of cyclase-associated protein in regulating actin filament dynamics - more than a monomer-sequestration factor. Journal of Cell Science. 2013;126:3249–3258. doi: 10.1242/jcs.128231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death and Differentiation. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lyons JC, Ohtsubo T, Song CW. Acidic environment causes apoptosis by increasing caspase activity. British Journal of Cancer. 1999;80:1892–1897. doi: 10.1038/sj.bjc.6690617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter NS, Staiger CJ, Rappoport JZ, Franklin-Tong VE. Actin-binding proteins implicated in the formation of the punctate actin foci stimulated by the self-incompatibility response in Papaver . Plant Physiology. 2010;152:1274–1283. doi: 10.1104/pp.109.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhang R, Zhang M, Diao M, Xue Y, Huang S. Organizational innovation of apical actin filaments drives rapid pollen tube growth and turning. Molecular Plant. 2017;10:930–947. doi: 10.1016/j.molp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW. Maintenance of caspase-3 proenzyme dormancy by an intrinsic "safety catch" regulatory tripeptide. Proceedings of the National Academy of Sciences, USA. 2001;98:6132–6137. doi: 10.1073/pnas.111085198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JJ, Franklin F, Lord JM, Franklin-Tong VE. Increased phosphorylation of a 26-kD pollen protein is induced by the self-incompatibility response in Papaver rhoeas . The Plant Cell. 1996;8:713–724. doi: 10.1105/tpc.8.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JJ, Osman K, Franklin FC, Franklin-Tong VE. Activation of a putative MAP kinase in pollen is stimulated by the self-incompatibility (SI) response. FEBS Letters. 2003;547:223–227. doi: 10.1016/s0014-5793(03)00710-5. [DOI] [PubMed] [Google Scholar]
- Sergeeva TF, Shirmanova MV, Zlobovskaya OA, Gavrina AI, Dudenkova VV, Lukina MM, Lukyanov KA, Zagaynova EV. Relationship between intracellular pH, metabolic co-factors and caspase-3 activation in cancer cells during apoptosis. Biochimica et Biophysica Acta. 2017;1864:604–611. doi: 10.1016/j.bbamcr.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L. Organelle pH in the Arabidopsis endomembrane system. Molecular Plant. 2013;6:1419–1437. doi: 10.1093/mp/sst079. [DOI] [PubMed] [Google Scholar]
- Shin JJ, Loewen CJ. Putting the pH into phosphatidic acid signaling. BMC Biology. 2011;9:85. doi: 10.1186/1741-7007-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ. Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. The Plant Cell. 2002;14:2613–2626. doi: 10.1105/tpc.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Frontiers in Plant Science. 2014;5:135. doi: 10.3389/fpls.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Edlund AF, Preuss D. Species specificity in pollen-pistil interactions. Annual Review Genetics. 2004;38:793–818. doi: 10.1146/annurev.genet.38.072902.092356. [DOI] [PubMed] [Google Scholar]
- Talley K, Alexov E. On the pH-optimum of activity and stability of proteins. Proteins. 2010;78:2699–2706. doi: 10.1002/prot.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature. 2004;429:305–309. doi: 10.1038/nature02540. [DOI] [PubMed] [Google Scholar]
- Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. Journal of Cell Biology. 2006;174:221–229. doi: 10.1083/jcb.200604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca RA, Valenti D, Bobba A, de Pinto MC, Merafina RS, De Gara L, Passarella S, Marra E. Proteasome function is required for activation of programmed cell death in heat shocked tobacco Bright-Yellow 2 cells. FEBS Letters. 2007;581:917–922. doi: 10.1016/j.febslet.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Van Aken O, Van Breusegem F. Licensed to kill: mitochondria, chloroplasts, and cell death. Trends in Plant Science. 2015;20:754–766. doi: 10.1016/j.tplants.2015.08.002. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. Morphological classification of plant cell deaths. Cell Death and Differentiation. 2011;18:1241–1246. doi: 10.1038/cdd.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hautegem T, Waters AJ, Goodrich J, Nowack MK. Only in dying, life: programmed cell death during plant development. Trends in Plant Science. 2015;20:102–113. doi: 10.1016/j.tplants.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Declercq W, Vandenabeele P, Van Breusegem F. Are metacaspases caspases? 2007;179:375–380. doi: 10.1083/jcb.200705193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Molecular Biology of the Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annual Review of Genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MJ, de Graaf BH, Hadjiosif N, Perry RM, Poulter NS, Osman K, Vatovec S, Harper A, Franklin FC, Franklin-Tong VE. Identification of the pollen self-incompatibility determinant in Papaver rhoeas . Nature. 2009;459:992–995. doi: 10.1038/nature08027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MJ, Vatovec S, Franklin-Tong VE. The pollen S-determinant in Papaver: comparisons with known plant receptors and protein ligand partners. Journal of Experimental Botany. 2010;61:2015–2025. doi: 10.1093/jxb/erp383. [DOI] [PubMed] [Google Scholar]
- Wilkins KA, Bancroft J, Bosch M, Ings J, Smirnoff N, Franklin-Tong VE. Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of Papaver . Plant Physiology. 2011;156:404–416. doi: 10.1104/pp.110.167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KA, Bosch M, Haque T, Teng N, Poulter NS, Franklin-Tong VE. Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiology. 2015;167:766–779. doi: 10.1104/pp.114.252742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KA, Poulter NS, Franklin-Tong VE. Taking one for the team: self-recognition and cell suicide in pollen. Journal of Experimental Botany. 2014;65:1331–1342. doi: 10.1093/jxb/ert468. [DOI] [PubMed] [Google Scholar]
- Wu HM, Cheung AY. Programmed cell death in plant reproduction. Plant Molecular Biology. 2000;44:267–281. doi: 10.1023/a:1026536324081. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang S, Gu Y, Zhang S, Publicover SJ, Franklin-Tong VE. Self-incompatibility in Papaver rhoeas activates nonspecific cation conductance permeable to Ca2+ and K+ . Plant Physiology. 2011;155:963–973. doi: 10.1104/pp.110.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B, Wightman R, Blanvillain R, Purcel SB, Gallois P. pH-sensitivity of YFP provides an intracellular indicator of programmed cell death. Plant Methods. 2010;6:27. doi: 10.1186/1746-4811-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Luo L, Xu J, et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana . Cell Research. 2018;28:448–461. doi: 10.1038/s41422-018-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death and Differentiation. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]