Abstract
Catadromous anguillid eels (Genus Anguilla) migrate from their freshwater or estuarine habitats to marine spawning areas. Evidence from satellite tagging studies indicates that tropical and temperate eel species exhibit pronounced diel vertical migrations between 150 to 300 m nighttime depths to 600 to 800 m during the day. Collections of eggs and larvae of Japanese eels (A. japonica) show they may spawn at these upper nighttime migration depths. How anguillid eels navigate through the ocean and find their spawning areas remains unknown, so this study describes the salinity, temperature and geostrophic currents between 0 and 800 m depths within two confirmed and three hypothetical anguillid spawning areas during likely spawning seasons. Within all four ocean gyres many eels would encounter subducted ‘Subtropical Underwater’ during their nighttime ascents that could provide odor plumes as signposts. Four spawning areas are located near the western margins of where subducted water masses form cores of elevated salinities (~35.0 to 36.8) around 150 m depths, while one is found near the center of subduction. Low salinity surface waters and fronts are present in some of the areas above the high-salinity cores. Spawning may occur at temperatures between 16 to 24°C where the thermocline locally deepens. At spawning depths, weak westward currents (~0 to 0.1 m s-1) prevail, and eastward surface countercurrents are present. Anguillid eels possess acute sensory capabilities to detect these hydrographic features as potential signposts guiding them to where they spawn.
Keywords: Anguilla, Diel vertical migration, Orientation, Satellite telemetry, Spawning
Introduction
How catadromous anguillid eels can migrate long distances from their freshwater or estuarine habitats through the seemingly featureless ocean and find their pelagic spawning areas has been one of the great mysteries in eel biology (Schmidt 1922, McCleave 1987, Tsukamoto 2009, Rigthon et al. 2012). After reproduction they die, and their larvae, called leptocephali, become widely distributed as they drift with currents toward recruitment areas (Schmidt 1922, Shinoda et al. 2011, Miller et al. 2015a). Among the 19 anguillid species or subspecies, the European eel (Anguilla anguilla) migrates the longest distances of up to 7000 km (Aoyama 2009) to reach their spawning area in the Sargasso Sea of the ‘western North Atlantic’ (WNA, Schmidt 1922). The western part of their spawning area is shared with American eel (A. rostrata, McCleave et al. 1987) that migrates up to about 3500 km. Similar distances are covered by A. japonica in the ‘western North Pacific’ (WNP, Aoyama 2009). These temperate anguillid eel migrations are among the longest one-way migrations known for any fish species (Alerstam et al. 2003). Even though some tropical species spawn offshore after only short migrations (Aoyama et al. 2003), all the known anguillid eel spawning areas are over deep water (>1000 m) in places with warm surface currents, probably because the genus is derived from an ancestral mesopelagic eel species (Inoue et al. 2010).
Besides the two confirmed spawning areas in the WNA and WNP, at least 3 more offshore spawning areas must exist in the other ocean gyres based on species ranges and current patterns, but they have not been confirmed yet by the collection of small larvae (see Materials and Methods). Relatively few leptocephali of the 6 species of anguillid eels present in the ‘western (WSP) and central (CSP) South Pacific’ have been collected and genetically identified (Kuroki et al. 2008; A. australis, A. dieffenbachii, A. marmorata, A. megastoma, A. obscura, A. reinhardtii) and the same is true for the 4 species in the ‘western Indian Ocean’ (WIO; Jespersen 1942, Miller et al. 2015b; A. bengalensis, A. bicolor, A. marmorata, A. mossambica). Considerably more leptocephali of some of those species were collected offshore of West Sumatra in the eastern Indian Ocean (Jespersen 1942, Aoyama et al. 2007). Catches of small leptocephali of A. celebesensis and A. borneensis in the central Indonesian Seas indicate those species can spawn after comparatively short migrations close to major landmasses (Aoyama et al. 2003).
It is still a mystery as to how silver eels navigate through the ocean to find their offshore spawning areas. No information is available to what extent eels might use “beaconing” (odor cues that build up a gradient), “trail following” (odor trails from conspecifics), “route reversal” (memory of signpost series), “path integration” (knowledge of own current position with respect to the goal in terms of distance and direction), “compass orientation” (e.g. sun, moon, magnetic compass; genetic and/or experience based components), “vector orientation” (genetic or acquired information about distance and direction of the goal), or “true navigation” (navigation, map and compass mechanism) as listed by Papi (2006) during different stages of their journey. They possess various sensory systems (vision, hearing, mechanoreception, pressure detection, chemo-, electro-, and magnetoreception; McCleave 1987, Tesch 2003, Tsukamoto 2009, Hunt et al. 2013), and orientation and navigation according to the earth’s magnetic field (see Nishi et al. 2004, Durif et al. 2011), temperature gradients, odor trails (Westin 1990, Van Ginneken and Maes 2005), or ocean currents (Rommel and McCleave 1973) may potentially be used.
Oceanographic fronts, which are narrow boundaries separating different water masses, have been hypothesized to provide hydrographic structures that define the spawning areas of anguillid eels. In the Sargasso Sea, two temperature fronts consistently form in the Subtropical Convergence Zone at about 22 and 24°C during the February to April spawning season (see Miller et al. 2015a) which gradually move northward with seasonal warming (Ullman et al. 2007). Leptocephali are consistently found south of the northern front (Kleckner & McCleave 1988, Munk et al. 2010). In the WNP A. japonica spawns within the westward flowing ‘North Equatorial Current’ (NEC) along the seamount chain of the West Mariana Ridge (Tsukamoto et al. 2011, Aoyama et al. 2014). Adult eels, their fertilized eggs, and recently hatched preleptocephali were collected exclusively along the seamount ridge (Chow et al. 2009, Kurogi et al. 2011, Tsukamoto et al. 2011, Aoyama et al. 2014), which seems to act as a longitudinal signpost (Tsukamoto et al. 2003, 2011). The latitude of spawning appears to be influenced by a shallow salinity front formed by rainfall that can move north or south, with spawning occurring on the south side of the front (Kimura & Tsukamoto 2006, Tsukamoto et al. 2011, Aoyama et al. 2014). Spawning can take place at a wider range of latitudes when the front is absent (Aoyama et al. 2014).
A new research approach of tagging migratory-stage silver eels with ‘pop-up satellite archival transmitters’ (PSAT) has revealed information about their unknown spawning areas and migration behavior. The pop-up locations of New Zealand longfin eels, A. diefenbachii, have pointed towards a possible spawning area east of New Caledonia in the WSP (Jellyman & Tsukamoto 2010) that is generally consistent with estimates from modelling of larval transport (Jellyman & Bowman 2009). Silver eels of two tropical anguillids, the giant mottled eel, A. marmorata, and the Polynesian longfin eel, A. megastoma, that were tagged within the archipelago of Vanuatu in the WSP, both had their tags pop-up in a potentially shared spawning area northwest of Fiji (Schabetsberger et al. 2015).
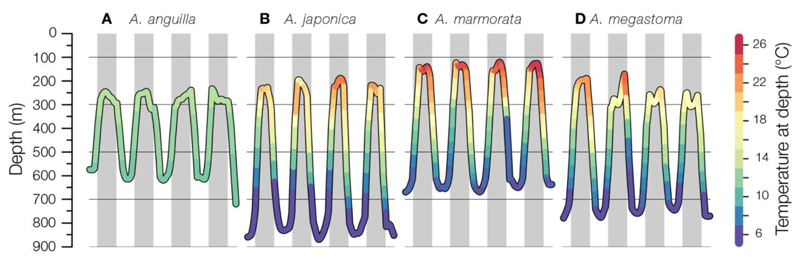
Tagged temperate and tropical anguillid eels show surprisingly similar oceanic diel vertical migration patterns (DVM, Fig. 1), although most of them were still far from their destinations. The eels predominantly migrate at 100 to 350 m depths during the night and then quickly descend to 600 to 800 m during dawn, remain there during the day and ascend again during dusk (Aarestrup et al. 2009, Jellyman and Tsukamoto 2010, Manabe et al. 2011, Wysujack et al. 2014, Schabetsberger et al. 2015, Béguer-Pon et al. 2015, Fig. 1). Some species such as A. rostrata (Béguer-Pon et al. 2015), A. japonica (Manabe et al. 2011), and A. dieffenbachii (Jellyman and Tsukamoto 2010) sometimes entered shallower water <100 m. The upper nighttime migration depths seem to be adjusted in response to the amount of moonlight, presumably to avoid epipelagic, nocturnally foraging predators (Schabetsberger et al. 2013, 2015, Chow et al. 2015). The subsurface upper nighttime migration depths of most of these studies raise the question about how migrating eels can detect the surface features of temperature or salinity fronts that are mostly only present in the upper 100 m (Kleckner and McCleave 1988, Aoyama et al. 2014) if they remain deeper when approaching the spawning area.
Fig. 1.
Diel vertical migrations of individual migrating anguillid silver eels tagged with pop-up satellite transmitters in the WNA (A; Aarestrup et al., 2009), WNP (B; S. Watanabe Unpubl. Data), and in the in the WSP (C, D; Schabetsberger et al. 2013, 2015). Grey areas indicate night time periods.
Among all 19 Anguilla species, spawning-condition adult eels and eggs have only been collected for A. japonica and A. marmorata (adults only) and they were likely caught at depths between 150 and 300 m (Chow et al. 2009, Tsukamoto et al. 2011, Aoyama et al. 2014), which correspond to the upper nighttime migration depths of eels in the PSAT studies. This indicates that water masses at these depths should be evaluated for potential oceanographic structures that eels may use to locate their spawning areas. The most distinctive hydrographic feature at these depths is the high-salinity Subtropical Underwater (STUW) that is present in all the major ocean basins (Fig. 2A), which is formed by saltier water being subducted from the surface into the lower thermocline (Price 2001).
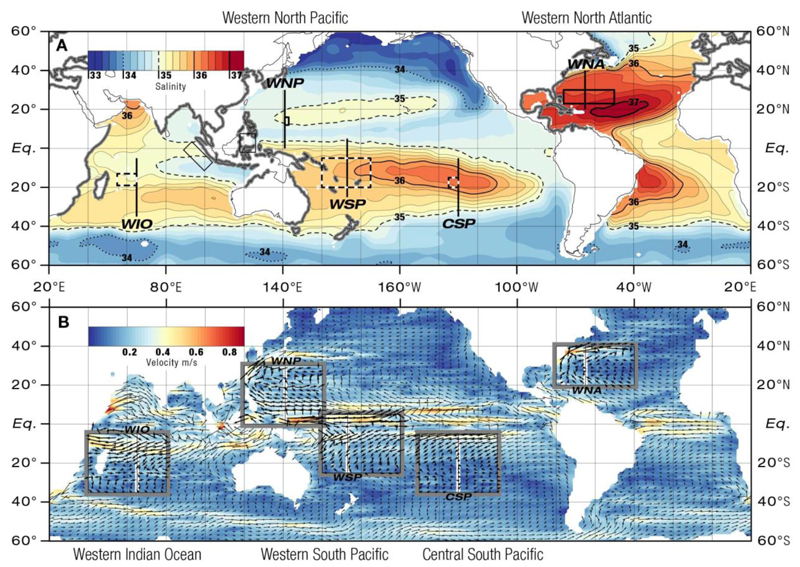
Fig. 2.
(A) Global map of salinity at 150 m depth from Argo float data for (long-term mean 2001- 2013) showing the elevated salinities of subducted Subtropical Underwater (STUW) within the subtropical gyres. Rectangles indicate the estimated spawning areas of anguillid eels (bold: confirmed, analysed; dotted: hypothetical, analysed; thin: confirmed, not analysed; see Table 1). Black transect lines indicate meridional sections shown in Fig. 3. Grey areas along coastlines indicate freshwater distribution of anguillid eels. (B) Global ocean surface currents derived from satellite altimeter and scatterometer data for July 2013. White transect lines and enclosing rectangles show locations of meridional sections and enlarged maps in Figs. 3 and 4, respectively, with current velocity arrows within the rectangles being in bold for emphasis.
These high-salinity waters are subducted from the surface mixed layer into the thermocline within in the centers of the wind-driven subtropical ocean gyres (Qui & Huang 1995, Qu et al. 2013). This process consists of downward pumping from Ekman convergence and horizontal advection by lateral geostrophic flow (Huang & Qui 1998 and references therein). When these water masses are transferred beneath the mixed layer (~100 to 350 m), they are shielded from the atmosphere and only slowly modify their properties through mixing in the ocean interior (Williams 2001). This type of water is found within the spawning areas of the Atlantic eels (Kleckner and McCleave 1988), A. japonica (Aoyama et al. 2014) and in the presumed spawning regions in the WIO (Pous et al. 2010). The STUW in the WSP (Qu et al. 2013) is a prominent feature at the pop- up locations of A. marmorata and A. megastoma and has been hypothesized to possibly help migrating eels locate this area (Schabetsberger et al. 2013, 2015).
The objective of the present study is to show the temperature and salinity structure and calculated geostrophic currents plotted from data obtained from autonomous drifting buoys (Argo floats) to interpretively evaluate the hydrographic structure and current flow patterns at the DVM migration depths of eels in each subtropical gyre where anguillid spawning occurs or may occur. The possible spawning depths are tentatively considered for inter-comparison of areas to be between 150 and 250 m in accordance with previous information from A. japonica (Tsukamoto et al. 2011, Aoyama et al. 2014). The potential significance of STUW and other features are discussed in relation to their vertical migration behavior and sensory capabilities.
Materials and Methods
Defining the spawning area locations
The Sargasso Sea spawning area of A. anguilla and A. rostrata in the WNA was discovered by the collection of recently hatched larvae by Schmidt (1922) and has been subsequently studied during many cruises, so the areas where spawning occurs have been defined by the presence of small leptocephali (see Miller et al. 2015a). We analysed data for March 2014 (Fig 2A, Table 1), because larvae were collected during surveys in March and April of that year (P. Munk and R. Hanel, personal communication).
Table 1.
Ocean gyres, eel species, the estimated spawning areas and seasons used in this study, the research status of each estimated spawning area, and hydrographic conditions of salinity, temperature, and geostrophic currents estimated from Argo float data at the presumed spawning depths of 150-250 m.
| Ocean Gyre | Species | Estimated spawning area | Analysis Time Period | Status of estimate | Salinity | Temp. (°C) | Westward currents at 150 m (m s-1) | Westward surface Currents | Eastward surface currents |
|---|---|---|---|---|---|---|---|---|---|
| Western North Atlantic WNA) | A. anguilla | 24–30°N, 50–73°W | March-April 2014 | Confirmed, with leptocephali <5 mm | 36.4-36.8 | 18-22 | 0.04-0.06 | Antilles Current (AC) | Gulf Stream (GS) |
| A. rostrata | 23–29°N, 60–76°W | Gulf Stream Recirculation (GSR) | |||||||
| Western Indian Ocean (WIO) | A. bicolor | West of 60.5°E, 13–19°S | October 2013 | Hypothetical, with leptocephali >40 mm, transport modelling | 35.2-35.4 | 16-22 | 0.04-0.06 | South Equatorial Current (SEC) | Subtropical Counter Current (SCC) |
| A. bengalensis | |||||||||
| A. marmorata | |||||||||
| A. mossambica | |||||||||
| Western North Pacific (WNP) | A. japonica | 12–16°N, 141–143°E | June 2011 | Confirmed, with eggs, preleptocephali, and spawning adults | 34.6-35.0 | 16-24 | 0.06-0.14 | North Equatorial Current (NEC) | Subtropical Counter Current (SCC) |
| A. luzonensis | |||||||||
| A. marmorata | |||||||||
| Western South Pacific (WSP) | A. australis | Larvae: 5–20°S, 160°E–175°W; Tagged eels from Vanuatu: 8–12°S, 170–175°E | July 2013 | Hypothetical, with leptocephali >15 mm, PSAT data | 35.0-36.0 | 16-24 | 0.00-0.08 | South Equatorial Current (SEC) | South Equatorial Counter Current (SECC) |
| A. dieffenbachii | Equatorial Counter Current (ECC) | Fiji Basin Counter Current (FBCC) | |||||||
| A. marmorata | |||||||||
| A. megastoma | |||||||||
| A. obscura | |||||||||
| A. reinhardtii | |||||||||
| Central South Pacific (CSP) | A. marmorata | 15–20°S, 130–135°W | July 2013 | Hypothetical, no larvae, based on species ranges and population structure | 35.4-36.2 | 18-24 | 0.00-0.06 | South Equatorial Current (SEC) | Subtropical Counter Current (SCC) |
| A. megastoma | |||||||||
| A. obscura |
Spawning areas in the WIO are not yet known because only a few large leptocephali of the 4 species in the region were collected during the Carlsberg Foundation’s Expedition Round the World (Jespersen 1942) at stations in the Mozambique Channel and north of Madagascar or during recent surveys in the area (Miller et al. 2015b). Based on otolith microstructure analyses of glass eels and elvers collected in the region, a spawning area near the Mascarene Plateau was predicted and evaluated by drift simulations (Robinet et al. 2008, Réveillac et al. 2009, Pous et al. 2010). We defined an estimated spawning area in this part of the WIO and analysed data for October 2013 (Figure 2A, Table 1) in accordance with information in Pous et al. (2010). Several species of tropical eels are known to spawn locally off West Sumatra in the eastern Indian Ocean (A. bicolor; Jespersen 1942, Aoyama et al. 2007) and within the Indonesian Seas (A. borneensis, A. celebesensis; Aoyama et al. 2003). However, these more local spawning areas (Fig. 2A) will not be examined in the present study because they are close to major landmasses and outside the major oceanic gyres and only require short migrations to spawn offshore over deep water. In addition, there is little or no coverage from the Argo floats to record the hydrographic conditions there.
The spawning area in the WNP has been studied extensively since its discovery in 1991 and its location has been confirmed (Tsukamoto 1992, Tsukamoto et al. 2003, 2011, Shinoda et al. 2011, Aoyama et al. 2014). In 2008 the first spawning adults of A. japonica and A. marmorata were caught along the ridge at depths above 350 m (Chow et al. 2009). Eggs of A. japonica were first collected in 2009 (Tsukamoto et al. 2011), and then again during cruises in 2011 and 2012 (Aoyama et al. 2014). Spawning occurs during new moon periods based on both backcalculated hatching dates of leptocephali and when the eggs and preleptocephali have been collected. The eels spawn somewhere below the thermocline because the eggs and preleptocephali appear to accumulate at about 150 m depths (Tsukamoto et al. 2011, Aoyama et al. 2014). The spawning area of A. marmorata overlaps with A. japonica (Kuroki et al. 2009), and the newly discovered anguillid species A. luzonensis may also spawn offshore in the NEC (Kuroki et al. 2012). We defined the spawning area as extending across most of the latitudes where eggs and preleptocephali have been collected and analysed a time period that corresponds to the June 2011 egg collections (Figure 2A, Table 1; Aoyama et al. 2014).
In the WSP a few leptocephali of 5 of the 6 species in the region were collected (Jespersen 1942, Miller et al. 2006, Kuroki et al. 2008) indicating that spawning likely occurs somewhere within the westward flowing South Equatorial Current (SEC). Presently, no leptocephali of A. dieffenbachii have been found (Jellyman and Bowen 2009). The smallest leptocephali of A. marmorata (Kuroki et al. 2008) were found close to the pop-up locations of PSAT tags attached to adult A. marmorata and A. megastoma released in Vanuatu, which pointed to a potential shared spawning area (Schabetsberger et al. 2015). Therefore, we defined a large spawning area in the WSP that is consistent with the small leptocephalus catches and encompasses the narrower area where satellite tags surfaced. Although tropical eels may spawn throughout various times of the year (Jellyman 2003), we analysed data for July 2013, as tagged eels from Vanuatu presumably reached their spawning area between June and September of that year (Fig. 2A, Table 1; Schabetsberger et al. 2015).
In the CSP separate spawning populations of A. marmorata and A. megastoma seem to exist based on morphometric (Ege 1939, Watanabe et al. 2008, 2011) and genetic analyses (Minegishi et al. 2008) of adult eels. From the arrival of glass eels, Marquet (1992) hypothesized that a relatively narrow eastern spawning area is located west of the Tuamotu Archipelago (also see Jellyman 2003), but no larvae have been caught in the region yet. Due to the uncertainty associated with the location and timing of spawning in this region, we used this proposed spawning area that is within the representative water mass of the region and the same time period as for the WSP. In Vanuatu silver eels seem to predominantly migrate during the rainy season between January and April and reach the spawning area approximately 3 months later (Schabetsberger et al. 2013). Weather conditions and migration distances seem to be similar in the CSP.
Hydrographic analysis
The hydrographic structures of the 4 subtropical gyres where anguillid eels are present were examined graphically (WNA, WIO, WNP, WSP and CSP) in areas shown in Fig. 2 and defined in Table 1. Patterns of salinity, temperature, and currents at the two confirmed offshore spawning areas in the Atlantic and North Pacific and within presumed spawning areas in the Indian and the South Pacific Ocean were plotted in both vertical sections from 0 to 800 m and horizontal sections at 150 m, which corresponds to the upper DVM swimming depths of migrating eels and the depths where eggs and newly hatched larvae may accumulate after spawning. Current systems are defined in Table 1. The common patterns of hydrographic conditions among all spawning areas are qualitatively evaluated for similarities and differences.
The Argo project has deployed a global array of about 3900 profiling floats that drift freely in the ocean while they measure temperature and salinity from 0 to 2000 m every 10 days and then transmit the data to satellites (www-argo.ucsd.edu/). Gridded temperature and salinity fields from Argo floats with a spatial resolution of 1 degree, a temporal resolution of 1 month, and 25 vertical levels from the surface to 2000 dbars were used in the analysis (Hosoda et al. 2008). Geostrophic currents were calculated with respect to a reference depth of 2000 m. Bathymetry data were gathered from the ETOPO 1-minute dataset (www.ngdc.noaa.gov/mgg/global/global.html). Global ocean surface currents were plotted from data derived from satellite altimeter and scatterometer data (Ocean Surface Currents Analysis Realtime, www.oscar.noaa.gov).
Results
Salinity
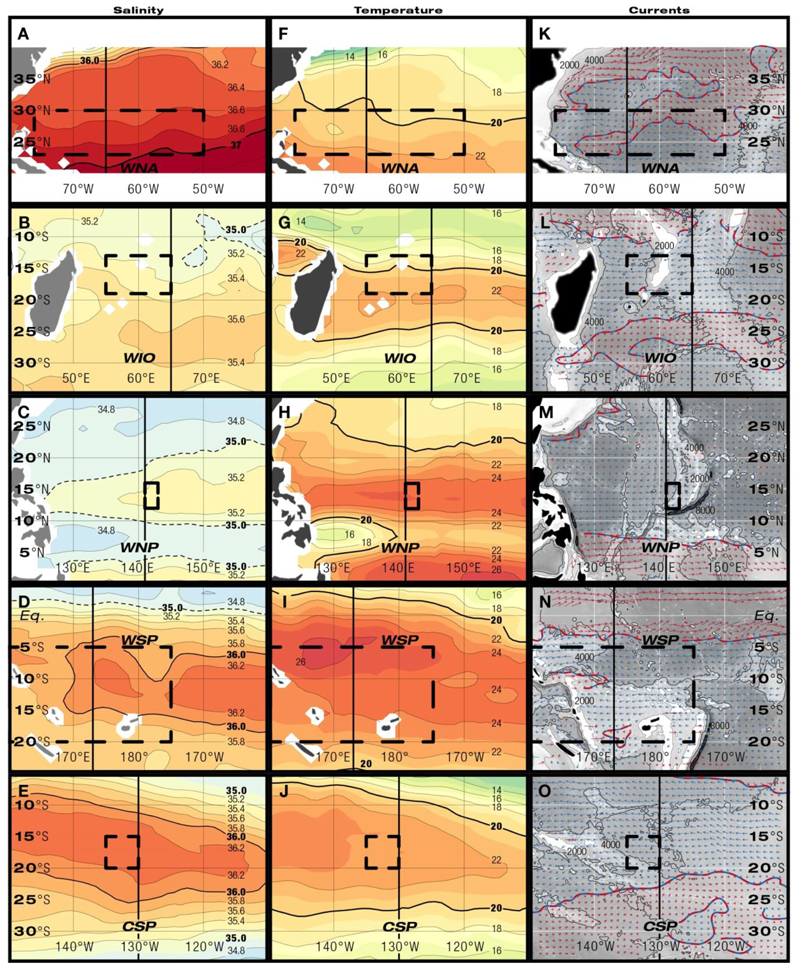
Within all four investigated subtropical gyres tongues of high-salinity subducted STUW were present at the upper nighttime migration depths of eels between 100 to 350 m (Figs. 1, 2A). The areas of formation of the STUW indicated by high surface salinity occur in the eastern parts of the gyres (Fig. 2A). The STUW extends obliquely towards the equator while being carried by horizontal circulation. Four oceanic spawning areas of Anguilla species are located near the western margins (Fig. 2A) of where subducted water masses form either cores of higher salinities (WNA 36.8, WNP 35.0, WSP 36.0) or inclined layers of subducted water masses (WIO 35.4, CSP 36.2) stretch down from the surface and bend equatorward into the thermocline (Fig. 3A-D, 4A-D). The hypothetical spawning area in the CSP, where no leptocephali have ever been caught, is within the formation area of STUW (Figs. 2A, 3E, 4E). In the Pacific Ocean, the spawning areas are more or less congruent with the latitudinal extension of high-salinity waters while in the Indian Ocean and the Atlantic they are located northwest of them (Fig. 2A). At the presumed spawning depths around 150 to 250 m salinities ranged from 34.6 to 36.8 and were highest in the WNA and lowest in the WNP (Fig. 3A-E, 4A-E, Table 1). In three areas shallow lenses (<100 m) of lower salinity water masses were present (WIO, WNP, WSP) with salinities ranging from 34.0 to 35.0. Differences were also observed among the 5 areas in the structure and upper depths of the lower salinity water below the STUW (Fig. 3A-E).
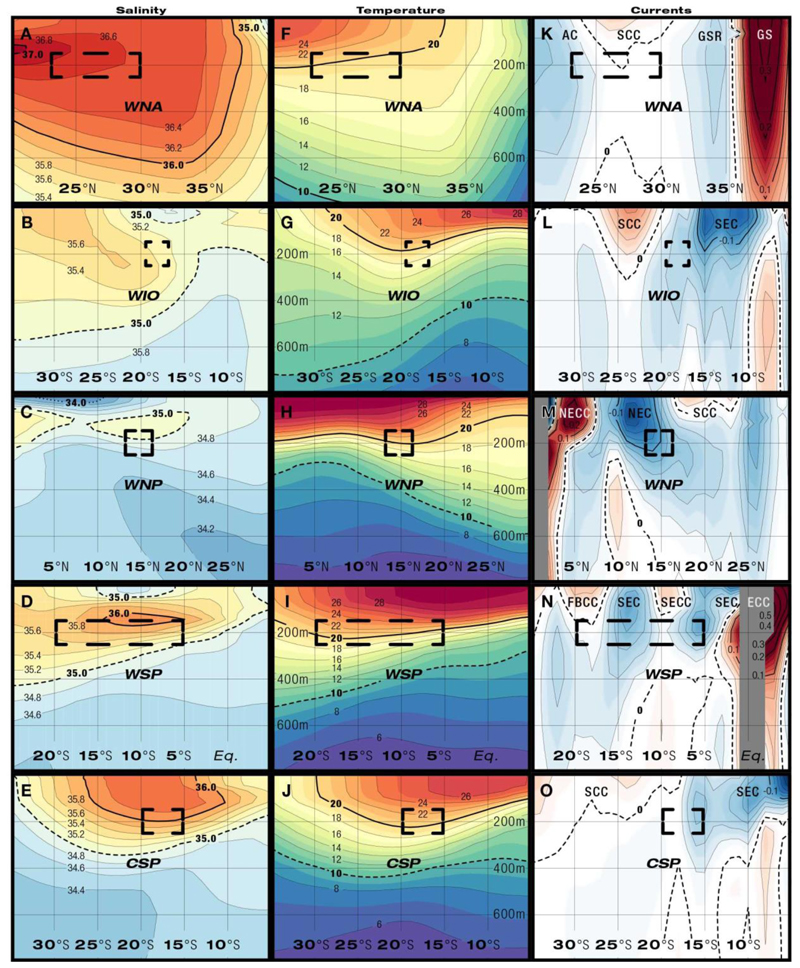
Fig. 3.
Meridional sections through spawning areas between 0–800 m depths (y-axis) of salinity (A- E), temperature (°C, F-J), and geostrophic currents (K-O, U, m s-1, red: eastward currents, blue: westwards currents) during known or presumed spawning times. Dashed rectangles indicate assumed latitudinal and vertical extensions of spawning areas. The major west- and eastward currents are identified (see Table 1).
Fig. 4.
Maps of horizontal sections of salinity (A-E), temperature (°C, F-J), and geostrophic currents (U, m s-1, K-O, red: eastward currents, blue: westwards currents) at a depth of 150 m during known and presumed spawning times. Dashed rectangles indicate latitudinal and longitudinal extensions of spawning areas. Black vertical lines show the positions of meridional sections in Fig. 3.
Temperature
Within the regions of the spawning areas, surface temperatures (22 to 29°C) increased towards lower latitudes, with a more gradual shoaling of isotherms along higher latitudes in the WNP compared to the other areas (Fig. 3F-J). Within these broader latitudinal gradients shallow temperature fronts may form locally, for example in areas where different water masses meet (Fig. 3K-O), but they are too narrow to be visible in the temperature fields interpolated from Argo data. Temperatures at estimated spawning depths within or near the thermocline ranged from 16 to 24°C. The vertical structure of the thermocline was congruent with the extension of the high-salinity STUW in all areas (Fig. 3A-J). At the presumed spawning latitudes the thermocline locally deepens (Fig. 3F-J) and tongues of warmer water stretching east to west at 150 m are bordered by colder water to the north and south (Fig. 4F-J). Only in the WNA (Fig. 4F) and the WIO (Fig. 4G) spawning seems to occur just north of these elevated temperatures at spawning depths. Differences in the temperature structure were also seen at deeper depths, with the 10°C isotherm reaching near or deeper than 800 m in the WNA, but mostly remaining from 350 to 600 m in the other areas (Fig. 3F-J).
Currents
Predominantly westward surface currents were present in the anguillid spawning areas (Fig 2B, Table 1, Fig. 3K-O). However, eastward countercurrents occurred. In the WNA the Subtropical Counter Current (SCC) was indicated to be above presumed spawning depths (Fig. 3K), because fronts with eastward currents are known to exist in this area. In the WIO (Fig. 3L) the spawning area was located just north of the eastward SCC. In the WNP (Fig. 3M) the spawning area extended within the North Equatorial Current (NEC), south of the SCC and north of the North Equatorial Counter Current (NECC). The WSP had the most complex current structure with 4 bands of alternating east-west current flows being present (Fig. 3N). Spawning locations within this area could include both branches of the SEC and the South Equatorial Counter Current (SECC) or the Fiji Basin Counter Current (FBCC). In the CSP the spawning area extended across the border of the eastward SCC and the westward SEC (Fig. 3O). Weak westward currents prevailed at the presumed spawning depths of the 5 areas that ranged from 0 to .14 m s-1 (Fig. 4K-O, Table 1). The strongest currents occurred outside the spawning areas either to the north in the WNA (Gulf Stream, GS) and the WSP (Equatorial Counter Current, ECC), or to the south in the WNP (NECC).
Discussion
Hydrographic features of spawning areas
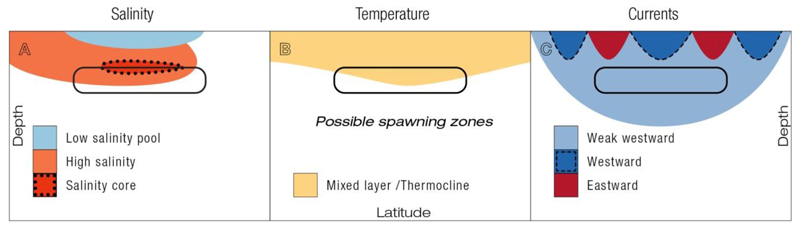
Eels have been hypothesized to use hydrographic features like temperature and salinity fronts or major current patterns to help decide where to spawn (reviewed in Tsukamoto 2009), but understanding of the importance of these and other oceanic signposts and the sensory capabilities of eels to detect them are still at a very early stage. In the present study we compared salinity, temperature and current patterns on a global scale and at a fine-scale within two confirmed and three hypothetical spawning areas in four different subtropical ocean gyres. Spawning probably occurs within subducted high-salinity water masses (STUW), where the thermocline locally deepens, and within weak westward currents. Low salinity pools and stronger west- or eastward currents are present above, that potentially cause the formation of oceanographic fronts (Fig. 5).
Fig. 5.
Schematic representation of generalized salinity (A), temperature (B), and current patterns (C), which may provide signposts for migrating eels to detect their presumed spawning areas and depths. Spawning likely occurs in high-salinity waters, near the thermocline, and within weak westward drift. Not all spawning areas show the same patterns of hydrographic structures shown in Fig. 3.
One interesting observation is that the STUW water mass is present at the upper nighttime migration depths of eels in all of the spawning areas. This water is subducted from the mixed layer into the stratified thermocline and spreads horizontally over large areas of all 4 subtropical gyres. However, except for the estimated spawning location in the CSP, the analysed spawning areas are found along the western or northwestern edges of these tongues of higher salinity water (Fig. 2). Vertically, the spawning areas appear to be located at the lower edges of the cores or within the STUW and near the thermocline as previously suggested in the WNP based on the distributions of egg and preleptocephalus catches (150 to 250 m, Aoyama et al. 2014). The cores of the STUW water masses are centered at about 150 m depths as seen in individual surveys (Kleckner and McCleave 1988, Roden 1998, Miller et al. 2006, Aoyama et al. 2014). Due to the subduction process the STUW gets injected into intermediate depths and results in a deepening of the thermocline (Price 2001, Williams 2001).
Above these subducted water masses, pronounced temperature and weaker salinity gradients exist. Our analysis showed there are areas of low-salinity water in the upper 100 m at the spawning areas in the WIO, WNP, and WSP. These lenses of low-salinity water are caused by tropical rainfall (Kimura & Tsukamoto 2006). Oceanographic fronts not shown in this large scale analyses are known to form locally in the WNA (Kleckner and McCleave 1988, Munk et al. 2010, Miller et al. 2015a) and the WNP (Kimura & Tsukamoto 2006, Tsukamoto et al. 2011, Aoyama et al. 2014). Similarly, fronts may also develop in the WIO at the edges of the shallow layer (~50 m) of low- salinity surface water (New et al. 2006) and in the WSP just north of Fiji where small anguillid larvae have been collected previously at the edges of the so called “western Pacific fresh pool” (Roden 1998, Miller et al. 2006, 2009).
The transport of larvae to the major recruitment areas is ensured by spawning predominantly within westward surface currents (NEC, SEC) and at depths where weak but consistent westward flows were observed. However, eastward flowing countercurrents were also present within or near the spawning areas. In the WNA eastward transport within the SCC has been proposed as a supplementary drift route to the Gulf Stream for A. anguilla larvae (McCleave 1993, Munk et al. 2010; Miller et al. 2015a). Dynamic seasonal alterations of westward and eastward flow patterns exist in the WIO (Schott et al. 2008), the WSP (Chen and Qiu 2004), and the CSP (Martinez et al. 2009) that are strongly influenced by Indian Ocean Dipole and El Niño-Southern Oscillation events, respectively. This may result in occasional eastward transport of leptocephali (Watanabe et al. 2014, Schabetsberger et al. 2015), explaining for example the presence of Anguilla spp. as far east as Pitcairn Island or the Galapagos Archipelago.
Hydrographic signposts for eel orientation
Our global hydrographic analyses show that eels swimming at the observed nighttime migration depths would encounter various types of hydrographic features that characterize their spawning areas. They would either start migrating within the STUW or encounter it near the spawning area as weak zonal gradients in salinity when moving towards the STUW cores. The salinity discontinuities are crossed twice a day during DVM based on pop-up satellite archival transmitter (PSAT) tagging studies (e.g. Aarestrup et al. 2009, Jellyman and Tsukamoto 2010, Schabetsberger et al. 2015), with the eels migrating below the STUW during the day and within it at night. The spawning areas seem to be located where the thermocline is weakening and extending deeper, with westward currents being stronger than at deeper depths. The eels would therefore experience different types of vertical gradients on either side of these areas during their DVMs. The salinity and temperature structures at deeper daytime depths do not seem to provide markers of spawning locations though. It may be unlikely that migrating eels can detect very gradual horizontal gradients of salinity, temperature or currents especially in the context of their vertical migrations, but subducted STUW water masses may contain specific olfactory cues. The renewal time for STUW is estimated to be 10 to 15 years (Price 2001) so compared with the surface mixed layer they may provide stable water mass signatures that can be detected by migrating eels.
If eels recurrently ascend to shallower water to search for more ephemeral oceanographic features above the STUW to locate their spawning areas remains unknown until more telemetric data become available. For example, two A. marmorata and one A. megastoma tagged with PSATs in Vanuatu that may have reached their spawning area northwest of Fiji almost never entered waters above 90 m throughout their entire journey and only once did an eel come up to 75 m (Schabetsberger et al, 2013, 2015). There is evidence that A. rostrata (Béguer-Pon et al. 2015), A. japonica (Manabe et al. 2011), and A. dieffenbachii (Jellyman & Tsukamoto 2010) more frequently ascended to shallower water above 75 m depth. However, these eels may have been more severely affected by longer holding before release and more invasive attachment or implantation techniques (see Økland et al. 2011), as tagged fish seem to exhibit irregular vertical migrations in shallower water when they are exhausted (Schabetsberger et al. 2015).
Using shallow oceanographic fronts, spawning area landmarks would seemingly require the eels to enter the upper 100 m at night to detect them, unless the fronts are linked to deeper features. In the Sargasso Sea (Kleckner and McCleave 1988) and the WSP (Roden 1998, Miller et al. 2006) the edges of the STUW cores correspond to where shallow temperature/density fronts occur, but it is unknown if these features move latitudinally in synchrony. Both the salinity front within the A. japonica spawning area (Kimura & Tsukamoto 2006, Aoyama et al. 2014) and the temperature fronts in the Sargasso Sea (Kleckner & McCleave 1988, Munk et al. 2010) are most prominent above 100 m. Therefore, unless the eels can perceive altered patterns of sound or light transmission below fronts, or chemical components of different water masses that sink downward on either side, they may not be able to detect these dynamic features without entering shallow water. However, swimming at the base of these hydrographic structures may provide sufficient sensory input to know their position in relation to the different water masses above. Some eel species such as A. rostrata (Béguer-Pon et al. 2015) might be adapted to search for shallow features, but it remains to be determined how important fronts are as signposts, because A. japonica must have used other cues when the salinity front was absent (Aoyama et al. 2014).
Sensory ecology of finding spawning areas
Eels have a variety of sensory systems that would enable them to detect hydrographic properties as they move vertically through about half a kilometre of water column every day while migrating toward the spawning area, but the relative importance of these sensory cues for navigation remains unknown. They might be able to perceive strong horizontal and vertical salinity gradients with sensory cells in their highly sensitive nares, gills, esophagus, oral cavity, and gastrointestinal epithelia (Tesch 2003, Evans et al. 2005, Kültz 2012). In addition they have a complex set of osmosensors in their brain, pituitary gland, and vasculature (Kültz 2012). Eels might also back- track to follow imprinted odor trails from specific biological communities within certain water masses (McCleave 1987, Westin 1990, Tsukamoto et al. 2003, van Ginneken & Maes 2005). To locate mates, they could follow odors from other eels, as released mucus, urine, and/or bile salts, are potential pheromones (Huertas et al. 2008).
Once within the spawning areas, there are vertical gradients of salinity and temperature that eels could use to detect their preferred spawning depths. For example, within the high-salinity cores of STUW, an eel ascending or descending at a speed of 5 m min-1 would experience salinity changes of more than 1.0 within an hour. Concurrently, eels should be able to recognize the thermocline during their DVM, assuming their sensitivity is similar to some freshwater fish that can detect rapid temperature changes down to 0.05°C (Bardach & Bjorklund 1957). Additionally, there is evidence that fish can accurately sense their depth with the swimbladder acting as a pressure receptor organ (Holbrook & Burt de Perera 2011). Eels may also perceive the hydrodynamic field around them, although in the open ocean they are immersed within the moving currents where there is a lack of stationary reference points (Montgomery et al. 2000). They may not feel the current itself, but sense infrasound and weak electric/magnetic fields induced by ocean currents (Rommel and McCleave 1973, Sand & Karlsen 2000, Manoj et al. 2006).
In addition to the perception of salinity/odor, temperature and current patterns, several other cues might also be used. Eels might be sensitive to large scale gradients in the inclination and the intensity of the earth’ magnetic field (Durif et al. 2013), and potentially even to the fine scale- mosaic of geomagnetic anomalies in the ocean floor (Walker et al. 2002, Lohmann et al. 2008), but it is still unknown if they rely on a large-scale geomagnetic map and compass for true navigation. This sense is important for orientation in sea turtles and salmon (Walker et al. 2002, Papi 2006, Lohmann et al. 2008, Putman et al. 2014). Polarized light provides an azimuth bearing for the sun down to several hundreds of meters (Waterman 2006), but navigation according to a direct sun- or moon compass is unlikely at the depths eels are migrating, as these celestial bodies would only be visible down to about 50 m in clear and calm ocean water (Partridge 1990). Sharp descents and ascents (50 to 605 m) during dawn and dusk, so called spike dives, seem to provide polarized light and/or magnetic field intensity cues for orientation in bluefin tuna (Thunnus maccoyi, Willis et al. 2009), so it should be explored if eels might also obtain similar cues during their vertical migrations.
Concluding remarks
The present study briefly evaluated the hydrographic structures associated with 2 confirmed and 3 hypothetical spawning areas of anguillid eels and discussed these features in relation to what is known about the oceanic migratory behavior and sensory systems of eels. Although it is clear that the mystery remains about how they can find their spawning areas during such long migrations, our study suggests some hypotheses about various features and senses that may be involved during the different stages of their migration. All spawning areas include STUW and probably shallower oceanographic fronts, and the properties of the water masses associated with either one or both features could be imprinted on by the larvae and later used to return as adults. However, the relative importance of these hydrographic features for navigation in relation to the possible use of magnetic cues remains to be determined.
It is also not yet known if the hydrographic conditions within the STUW are in some way advantageous for spawning eels to enhance egg and larval survival. At least salinities and temperatures at these depths roughly correspond to the optimum conditions for egg development (salinity~35, 20 to 25°C) in A. anguilla (Sørensen et al. 2016) and A. japonica (Okamoto et al. 2009, Ahn et al. 2012, Unuma et al. 2012) determined under laboratory conditions. Additionally, salinity is a primary determinant of the buoyancy of marine fish eggs that influences their vertical distributions and dispersal (Sundby & Kristiansen 2015). Eel eggs seem to be positively buoyant (Tsukamoto et al. 2009, Sørensen et al. 2016), so spawning in elevated salinities would accelerate uplift into warmer water.
For effective protection and management of eels, more information is urgently needed on the marine part of their life cycle (Jacoby et al. 2015). Important steps are to locate more of the spawning areas in the Indo-Pacific to determine how the eels find their spawning areas, and if changes in ocean-atmosphere conditions may affect that ability or the survival of their larvae (Knights 2003, Tsukamoto 2009, Miller et al. 2009, Righton et al. 2011). So far, the oceanic spawning areas of four species (A. anguilla, A. rostrata, A. japonica, A. marmorata) have been found through research cruises targeting the collection of smaller and smaller leptocephali over several years or decades. Satellite tags now provide a comparatively cheap way to narrow down the search areas. They will also provide information on the behavior of eels that can then be related to environmental conditions observed with remote sensing technologies, especially when extra or improved sensors (e.g. salinity, low light conditions, magnetic properties) will hopefully become available in the future. Numerical models used to simulate migration path, duration, diel vertical migration behavior, energy expenditure, and ambient oceanographic conditions (Béguer-Pon et al. 2016, Chang et al. 2016) could then be better calibrated against actual measurements. Additionally, the sensitivity of eels to magnetism, odors, infrasound, and polarised light could be further evaluated in laboratory experiments.
Acknowledgements
Funding for this study was provided by the Austrian Science Fund (P28381-B29). These data were collected and made freely available by the International Argo Project and the national programmes that contribute to it (http://www.argo.ucsd.edu, http://argo.jcommops.org) and by Ocean Surface Currents Analysis Realtime (OSCAR, www.oscar.noaa.gov). We thank 5 anonymous reviewers for their critical comments.
References
- Aarestrup K, Økland F, Hansen MM, Righton D. Oceanic spawning migration of the European eel (Anguilla anguilla) Science. 2009;325:1660. doi: 10.1126/science.1178120. [DOI] [PubMed] [Google Scholar]
- Ahn H, Yamada Y, Okamura A, Horie N, Mikawa N, Tanaka S, Tsukamoto K. Effects of water temperature on embryonic development and hatching time of the Japanese eel Anguilla japonica . Aquaculture. 2012;330-333:100–105. [Google Scholar]
- Alerstam T, Hedenström A, Åkesson S. Long distance migration: evolution and determinants. Oikos. 2003;103:247–260. [Google Scholar]
- Aoyama J. Life history and evolution of migration in catadromous eels (Genus Anguilla) Aqua BioSci Monogr (ABSM) 2009;2:1–42. [Google Scholar]
- Aoyama J, Wouthuyzen S, Miller MJ, Inagaki T, Tsukamoto K. Short-distance spawning migration of tropical freshwater eels. Biol Bull. 2003;204:104–108. doi: 10.2307/1543500. [DOI] [PubMed] [Google Scholar]
- Aoyama J, Wouthuyzen S, Miller MJ, Minegishi Y. Distribution of leptocephali of the freshwater eels, genus Anguilla, in the waters off west Sumatra in the Indian Ocean. Env Biol Fish. 2007;80:445–452. [Google Scholar]
- Aoyama J, Watanabe S, Miller MJ, Mochioka N, Otake T, Yoshinaga T, Tsukamoto K. Spawning sites of the Japanese eel in relation to oceanographic structure and the West Mariana Ridge. PLoS ONE. 2014;9(2):e88759. doi: 10.1371/journal.pone.0088759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardach JE, Bjorklund RG. The temperature sensitivity of some American freshwater fishes. Am Nat. 1957;91:233–251. [Google Scholar]
- Béguer-Pon M, Castonguay M, Shan S, Benchetrit J, Dodson JJ. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat Commun. 2015;6 doi: 10.1038/ncomms9705. 8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguer-Pon M, Shan S, Thompson KR, Castonguay M, Sheng J, Dodson JJ. Exploring the role of physical marine environment in silver eel migrations by using a biophysical particle tracking model. ICES J Mar Sci. 2016;73:57–74. [Google Scholar]
- Chang Y-L, Miyazawa Y, Béguer-Pon M. Simulating the Oceanic Migration of Silver Japanese Eels. PLoS ONE. 2016;11(3):e0150187. doi: 10.1371/journal.pone.0150187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Qiu B. Seasonal variability of the South Equatorial Countercurrent. J Geophys Res. 2004;109 doi: 10.1029/2003JC002243. C08003. [DOI] [Google Scholar]
- Chow S, Kurogi H, Mochioka N, Kaji S, Okazaki M, Tsukamoto K. Discovery of mature freshwater eels in the open ocean. Fish Sci. 2009;75:257–259. [Google Scholar]
- Chow S, Okazaki M, Watanabe T, Segawa K. Light-sensitive vertical migration of the Japanese eel Anguilla japonica revealed by real-time tracking and its use for geolocation. PloS ONE. 2015;10(4):e121801. doi: 10.1371/journal.pone.0121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durif CMF, Browman HI, Phillips JB, Skiftesvik AB, Vøllestad LA, Stockhausen HH. Magnetic compass orientation in the European eel. PLoS ONE. 2013;8(3):e59212. doi: 10.1371/journal.pone.0059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege V. A revision of the Genus Anguilla Shaw. Dana Reports. 1939;16:8–256. [Google Scholar]
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85:97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- Holbrook RI, Burt de Perera T. Fish navigation in the vertical dimension: can fish use hydrostatic pressure to determine depth? Fish Fish. 2011;12:370–379. [Google Scholar]
- Hosoda S, Ohira T, Nakamura T. A monthly mean dataset of global oceanic temperature and salinity derived from Argo float observations. JAMSTEC Rep Res Dev. 2008;8:47–59. [Google Scholar]
- Huang RX, Qui B. The structure of the wind-driven circulation in the subtropical South Pacific Ocean. J Phys Oceanogr. 1998;28:1173–1186. [Google Scholar]
- Huertas M, Canário AVM, Hubbard PC. Chemical communication in the Genus Anguilla: a minireview. Behaviour. 2008;145:1389–1407. [Google Scholar]
- Hunt DM, Hart NS, Collin SP. Sensory Systems. In: Trischitta F, Takei Y, Sébert P, editors. Eel Physiology. CRC Press; Boca Raton: 2013. pp. 119–159. [Google Scholar]
- Inoue JG, Miya M, Miller MJ, Sado T. Deep-ocean origin of the freshwater eels. Biol Lett. 2010;6:363–366. doi: 10.1098/rsbl.2009.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DMP, Casselman JM, Crook V, DeLucia M-B. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob Ecol Conserv. 2015;4:321–333. [Google Scholar]
- Jellyman DJ. The distribution and biology of the South Pacific Species of Anguilla. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel Biology. Springer; Tokyo: 2003. pp. 275–292. [Google Scholar]
- Jellyman DJ, Bowen MM. Modelling larval migration routes and spawning areas of anguillid eels of New Zealand and Australia. Am Fish Soc Symp. 2009;69:255–274. [Google Scholar]
- Jellyman DJ, Tsukamoto K. Vertical migrations may control maturation in migrating female Anguilla dieffenbachii . Mar Ecol Prog Ser. 2010;404:21–247. [Google Scholar]
- Jespersen P. Systematic and biological studies. Vol. 22. Carlsberg Foundation; 1942. Indo-Pacific leptocephalids of the genus Anguilla . Dana-Rep. [Google Scholar]
- Kimura S, Tsukamoto K. The salinity front in the North Equatorial Current: a landmark for the spawning migration of the Japanese eel (Anguilla japonica) related to the stock recruitment. Deep-Sea Res II. 2006;53:315–325. [Google Scholar]
- Kleckner RC, McCleave JD. The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to thermal fronts and surface water masses. J Mar Res. 1988;46:647–667. [Google Scholar]
- Knights B. A review of the possible impacts of long-term oceanic and climate changes and fishing mortality on recruitment of anguillid eels of the Northern Hemisphere. Sci Total Environ. 2003;310:237–244. doi: 10.1016/S0048-9697(02)00644-7. [DOI] [PubMed] [Google Scholar]
- Kültz D. The combinatorial nature of osmosensing in fishes. Physiology (Bethesda) 2012;27:259–275. doi: 10.1152/physiol.00014.2012. [DOI] [PubMed] [Google Scholar]
- Kurogi H, Okazaki M, Mochioka N, Jinbo T. First capture of post-spawning female of the Japanese eel Anguilla japonica at the southern West Mariana Ridge. Fish Sci. 2011;77:199–205. [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Watanabe S. Distribution and early life-history characteristics of anguillid leptocephali in the western South Pacific. Aust J Mar Freshwat Res. 2008;59:1035–1047. [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Yoshinaga T, Shinoda A, Hagihara S, Tsukamoto K. Sympatric spawning of Anguilla marmorata and Anguilla japonica in the western North Pacific Ocean. J Fish Biol. 2009;78:1853–1865. doi: 10.1111/j.1095-8649.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Yoshinaga T, Watanabe S, Tsukamoto K. Offshore spawning of the newly discovered anguillid species Anguilla luzonensis (Teleostei: Anguillidae) in the western North Pacific. Pac Sci. 2012;66:497–507. [Google Scholar]
- Lam CH, Nielsen A, Sibert JR. Improving light and temperature based geolocation by unscented Kalman filtering. Fish Res. 2008;91:15–25. [Google Scholar]
- Lohmann KJ, Lohmann CM, Endres CS. The sensory ecology of ocean navigation. J Exp Biol. 2008;211:1719–1728. doi: 10.1242/jeb.015792. [DOI] [PubMed] [Google Scholar]
- Manabe R, Aoyama J, Watanabe K, Kawai M, Miller MJ, Tuskamoto K. First observations of the oceanic migration of Japanese eel, from pop-up archival transmitting tags. Mar Eol Prog Ser. 2011;437:229–240. [Google Scholar]
- Manoj C, Kuvshinov A, Maus S, Lühr H. Ocean circulation generated magnetic signals. Earth Planets Space. 2006;58:429–437. [Google Scholar]
- Marquet G. L’étude du recrutement et de la physiologie des anguilles de Polynésie française permet-elle de cerner leur aire de ponte? Bull Inst Oceanogr Monaco. 1992;10:129–147. [Google Scholar]
- Martinez E, Ganachaud A, Lefevre J, Maamaatuaiahutapu K. Central South Pacific thermocline water circulation from a high-resolution ocean model validated against satellite data: Seasonal variability and El Niño 1997-1998 influence. J Geophys Res. 2009;114 doi: 10.1029/2008JC004842. C05012. [DOI] [Google Scholar]
- McCleave JD. Migration of Anguilla in the ocean: Signposts for adults! Signposts for leptocephali? Signposts-in-the-Sea. In: Hernnkind WF, Thistle AB, editors. Proceedings of a multidisciplinary workshop on marine animal orientation and migration. Florida State University; Tallahassee: 1987. pp. 102–117. [Google Scholar]
- McCleave JD. Physical and behavioural controls on the oceanic distribution and migration of leptocephali. J Fish Biol. 1993;43:243–273. [Google Scholar]
- McCleave JD, Kleckner RC, Castonguay M. Reproductive sympatry of American and European eels and implications for migration and taxonomy. In: Dadswell MJ, Klauda RJ, Moffitt C, Saunders RL, Rulifson RA, Cooper JE, editors. Common strategies of anadromous and catadromous fishes. American Fisheries Society; Massachusetts: 1987. pp. 186–297. [Google Scholar]
- Miller MJ, Aoyama J, Mochioka N, Otake T. Geographic variation in the assemblages of leptocephali in the western South Pacific. Deep-Sea Res I. 2006;53:776–794. [Google Scholar]
- Miller MJ, Kimura S, Friedland KD, Knights B, Kim H, Jellyman JD, Tsukamoto K. Review of ocean-atmospheric factors in the Atlantic and Pacific oceans influencing spawning and recruitment of anguillid eels. Am Fish Soc Symp. 2009;69:231–249. [Google Scholar]
- Miller MJ, Bonhommeau S, Munk P, Castonguay M, Hanel R, McCleave JD. A century of research on the larval distributions of the Atlantic eels: a re-examination of the data. Biol Rev. 2015a;90:1035–1064. doi: 10.1111/brv.12144. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Feunteun E, Aoyama J, Watanabe S. Biodiversity and distribution of leptocephali west of the Mascarene Plateau in the southwestern Indian Ocean. Prog Oceanogr. 2015b;137:84–102. [Google Scholar]
- Minegishi Y, Aoyama J, Tsukamoto K. Multiple population structure of the giant mottled eel Anguilla marmorata . Mol Ecol. 2008;17:3109–3122. doi: 10.1111/j.1365-294X.2008.03822.x. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Carton G, Voigt R, Baker C, Diebel C. Sensory processing of water currents by fishes. Philos T Roy Soc B. 2000;355:1325–1327. doi: 10.1098/rstb.2000.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk P, Hansen MM, Maes GE, Nielsen TG. Oceanic fronts in the Sargasso Sea control the early life and drift of Atlantic eels. Proc Roy Soc B. 2010;277:3593–3599. doi: 10.1098/rspb.2010.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AL, Alderson SG, Smeed DA, Stansfield KL. On the circulation of the water masses across the Mascarene Plateau in the Southern Indian Ocean. Deep-Sea Res I. 2006;54:42–74. [Google Scholar]
- Nishi T, Kawamura G, Matsumoto K. Magnetic sense in the Japanese eel, Anguilla japonica, as determined by conditioning and electrocardiography. J Exp Biol. 2004;207:2965–2970. doi: 10.1242/jeb.01131. [DOI] [PubMed] [Google Scholar]
- Økland F, Thorstad EB, Westerberg H, Aarestrup K, Metcalfe JD. Development and testing of attachment methods for pop-up satellite archival transmitters in European eel. Animal Biotelemetry. 2013;1:3. [Google Scholar]
- Okamoto T, Kurokawa T, Gen K, Murashita K. Influence of salinity on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Aquaculture. 2009;293:113–118. [Google Scholar]
- Papi F. Navigation of marine, freshwater and coastal animals: concepts and current problems. Mar Freshw Behav Phy. 2006;39:3–12. [Google Scholar]
- Partridge J. The color sensitivity and vision of fishes. In: Herring PJ, Campbell AK, Whitefield M, Maddock L, editors. Light and life in the sea. Cambridge University Press; Cambridge: 1990. pp. 127–148. [Google Scholar]
- Pous S, Feunteun E, Ellien C. Investigation of tropical eel spawning area in the South Western Indian Ocean: influence of the oceanic circulation. Prog Oceanogr. 2010;86:396–413. [Google Scholar]
- Price JF. Subduction. In: Siedler G, Church J, Gould J, editors. Ocean circulation and climate –Observing and modelling the global ocean. Academic Press; San Diego: 2001. pp. 357–371. [Google Scholar]
- Putman NF, Scanlan MM, Billman EJ. An inherited magnetic map guides ocean navigation in juvenile Pacific salmon. Curr Biol. 2014;24:446–450. doi: 10.1016/j.cub.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Qiu B, Huang RX. Ventilation of the North Atlantic and North Pacific: subduction versus obduction. J Phys Oceanogr. 1995;25:2374–2390. [Google Scholar]
- Qu T, Gao S, Fine RA. Subduction of South Pacific tropical water and its equatorward pathways as shown by simulated passive tracer. J Phys Oceanogr. 2013;43:1551–1565. [Google Scholar]
- Réveillac E, Robinet T, Rabenevanana M-W, Valade P, Feunteun E. Clues to the location of the spawning area and larval migration characteristics of Anguilla mossambica as inferred from otolith microstructural analyses. J Fish Biol. 2009;74:1866–1877. doi: 10.1111/j.1095-8649.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Righton D, Aarestrup K, Jellyman D, Sébert P, van den Thillart G, Tsukamoto K. The Anguilla spp. migration problem: 40 million years of evolution and two millennia of speculation. J Fish Biol. 2012;81:365–386. doi: 10.1111/j.1095-8649.2012.03373.x. [DOI] [PubMed] [Google Scholar]
- Robinet T, Réveillac E, Kuroki M, Aoyama J. New clues for freshwater eels (Anguilla spp.) migration routes to eastern Madagascar and surrounding islands. Mar Biol. 2008;154:453–463. [Google Scholar]
- Roden G. Upper ocean thermohaline, oxygen, nutrient, and flow structure near the date line in the summer of 1993. J Geophys Res. 1998;103:12919–12939. [Google Scholar]
- Rommel SA, Jr, McCleave JD. Sensitivity of American eels (Anguilla rostrata) and Atlantic salmon (Salmo salar) to weak electric and magnetic fields. J Fish Res Board Can. 1973;30:657–663. [Google Scholar]
- Sand O, Karlsen HE. Detection of infrasound and linear acceleration in fishes. Phil Trans Roy Soc Lond B. 2000;355:1295–1298. doi: 10.1098/rstb.2000.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabetsberger R, Økland F, Aarestrup K, Sichrowsky U. Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 2013;475:177–190. [Google Scholar]
- Schabetsberger R, Økland F, Kalfatak D, Sichrowsky U. Sympatric spawning of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 2015;521:171–187. [Google Scholar]
- Schmidt J. The breeding places of the eel. Phil Trans R Soc Lond. 1922;211:179–208. [Google Scholar]
- Schott FA, Xie S-P, McCreary JP., Jr Indian Ocean circulation and climate variability. Rev Geophys. 2008;47 doi: 10.1029/2007RG000245. RG1002. [DOI] [Google Scholar]
- Shinoda A, Aoyama J, Miller MJ, Otake T. Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev Fish Biol Fisheries. 2011;21:591–611. [Google Scholar]
- Sørensen SR, Butts IAE, Munk P, Tomkiewicz J. Effects of salinity and sea salt type on egg activation, fertilization, buoyancy, and early embryology of European eel, Anguilla anguilla . Zygote. 2016;24:121–138. doi: 10.1017/S0967199414000811. [DOI] [PubMed] [Google Scholar]
- Sundby S, Kristiansen T. The Principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PloS ONE. 2015;10(10):e0138821. doi: 10.1371/journal.pone.0138821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch F-W. The eel. 3ed. Blackwell; Oxford: 2003. [Google Scholar]
- Tsukamoto K. Discovery of the spawning area for the Japanese eel. Nature. 1992;356:789–791. [Google Scholar]
- Tsukamoto K. Oceanic migration and spawning of anguillid eels. J Fish Biol. 2009;74:1833–1852. doi: 10.1111/j.1095-8649.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Otake T, Mochioka N, Lee TW. Seamounts, new moon and eel spawning: The search for the spawning site of the Japanese eel. Environ Biol Fish. 2003;66:221–229. [Google Scholar]
- Tsukamoto K, Yamada Y, Okamura A, Kaneko T. Positive buoyancy in eel leptocephali: an adaption for life in the ocean surface layer. Mar Biol. 2009;156:835–846. [Google Scholar]
- Tsukamoto K, Chow S, Otake T, Kurogi H. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun. 2011;2 doi: 10.1038/ncomms1174. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman DS, Cornillon PC, Shan Z. On the characteristics of subtropical fronts in the North Atlantic. J Geophys Res. 2007 doi: 10.1029/2006JC003601. [DOI] [Google Scholar]
- Unuma T, Sawaguchi S, Hasegawa N, Tsuda N, Tanaka T, Nomura K, Tanaka H. Optimum temperature of rearing water during artificial induction of ovulation in Japanese eel. Aquaculture. 2012;358-359:216–223. [Google Scholar]
- Van Ginneken VJT, Maes GE. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev Fish Biol Fisher. 2005;15:367–398. [Google Scholar]
- Walker MM, Dennis TE, Kirschvink JL. The magnetic sense and its use in long distance navigation by animals. Curr Opin Neurobiol. 2002;12:735–744. doi: 10.1016/s0959-4388(02)00389-6. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Aoyama J, Miller MJ, Ishikawa S, Feunteun E, Tsukamoto K. Evidence of population structure in the giant mottled eel, Anguilla marmorata, using total number of vertebrae. Copeia. 2008;2008:680–688. [Google Scholar]
- Watanabe S, Miller MJ, Aoyama J, Tsukamoto K. Analysis of vertebral counts of the tropical anguillids, Anguilla megastoma A. obscura, and A. reinhardtii, in the western South Pacific in relation to their possible population structure and phylogeny. Env Biol Fish. 2011;91:353–360. [Google Scholar]
- Watanabe S, Miller MJ, Aoyama J, Tsukamoto K. Evaluation of the population structure of Anguilla bicolor and A. bengalensis using total number of vertebrae and consideration of the subspecies concept for the genus Anguilla . Ecol Freshw Fish. 2014;23:77–85. [Google Scholar]
- Waterman TH. Reviving a neglected celestial underwater polarization compass for aquatic animals. Biol Rev. 2006;81:111–115. doi: 10.1017/S1464793105006883. [DOI] [PubMed] [Google Scholar]
- Westerberg H. Marine migratory behavior of the European silver eel. In: Ueda H, Tsukamoto K, editors. Physiology and Ecology of Fish Migration. CRC Press; Boca Raton: 2014. pp. 81–104. [Google Scholar]
- Westin L. Orientation mechanism in migrating European silver eel (Anguilla anguilla): Temperature and olfaction. Mar Biol. 1990;106:175–179. [Google Scholar]
- Williams RG. Ocean subduction. In: Steel JH, Turekian KK, Thorpe SA, editors. Encyclopedia of Ocean Sciences. Academic Press; San Diego: 2001. pp. 1982–1993. [Google Scholar]
- Willis J, Phillips J, Muheim R, Diego-Rasilla FJ, Hobday AJ. Spike dives of juvenile southern Bluefin tuna (Thunnus maccoyii): a navigational role? Behav Ecol Sociobiol. 2009;64:57–68. [Google Scholar]
- Wysujack K, Westerberg H, Aarestrup K, Trautner J, Kurwie T, Nagel F, Hanel R. The migration behaviour of European silver eels (Anguilla anguilla) released in open ocean conditions. Mar Freshw Res. 2014;66:145–157. [Google Scholar]