Abstract
Seven South Pacific anguillid eel species live from New Guinea to French Polynesia, but their spawning areas and life histories are mostly unknown despite previous sampling surveys. A July–October 2016 research cruise was conducted to study the spawning areas and times, and larval distributions of South Pacific anguillid eels, which included a short 155°E station-line northeast of New Guinea and five long transects (5–25°S, 160°E–140°W) crossing the South Equatorial (SEC) and other currents. This survey collected nearly 4000 anguilliform leptocephali at 179 stations using an Isaacs-Kidd Midwater Trawl accompanied by 104 CTD casts. Based on mor-phometric observations and DNA sequencing, 74 anguillid leptocephali were collected, which in the southern areas included 29 larvae of six species: Anguilla bicolor pacifica, A. marmorata, A. australis, A. reinhardtii, A. megastoma, and A. obscura (all anguillid species of the region were caught except A. dieffenbachii). Small A. australis (9.0–16.8 mm) and A. reinhardtii (12.4, 12.5 mm) leptocephali were collected south of the Solomon Islands, other A. australis (10.8–12.0 mm) larvae were caught northwest of Fiji along with an A. obscura (20.0 mm) larva, and an A. marmorata (7.8 mm) larva was collected near Samoa. Considering collection sites, larval ages from otolith analysis, and westward SEC drift, multiple spawning locations occurred from south of the Solomon Islands and the Fiji area (16–20 days old larvae) to near Samoa (19 days old larva) during June and July in areas where high-salinity Subtropical Underwater (STUW, ~150 m depth) and the warm, low-salinity surface Fresh Pool were present. Five long hydrographic sections showed the strong Fresh Pool in the west and the STUW formation area in the east.
Keywords: Early life history, Freshwater eels, migration, Otolith, South Pacific, Spawning
1. Introduction
Catadromous freshwater eels of the genus Anguilla live in freshwater and estuarine habitats during their juvenile growth stage and then migrate into the ocean to reproduce. The 19 species and subspecies of anguillid eels live in areas adjacent to all major ocean gyres, except the South Atlantic and eastern Pacific (Ege, 1939; Watanabe, 2003; Watanabe et al., 2009). Many of these species have experienced population declines and there are concerns about their conservation status (Tsukamoto et al., 2009; Jacoby et al., 2015; Drouineau et al., 2018), so research efforts have increased in recent years to understand all aspects of their life histories to facilitate conservation efforts as many questions remain unanswered about these species (Righton et al., 2012).
A fundamental aspect of understanding the life history and population ecology of each anguillid species is where they spawn in the ocean and how their larvae are transported to their recruitment areas. The spawning areas of some species such as the European eel Anguilla anguilla, American eel A. rostrata, and Japanese eel A. japonica, were discovered by collecting smaller-and-smaller larvae (Schmidt, 1922; Tsukamoto, 1992), as reviewed in previous studies (McCleave, 2003; Tsukamoto et al., 2003; Shinoda et al., 2011; Miller et al., 2015, 2019). Their spawning areas were then evaluated from hydrographic perspectives in later years, which indicated that features such as oceanic fronts seem to help define where spawning is likely to occur (Kleckner and McCleave, 1988; Munk et al., 2010; Aoyama et al., 2014; Schabetsberger et al., 2016).
Efforts then shifted to discover where tropical species of anguillid eels in the Indo-Pacific spawn, after only one spawning area was found off Sumatra in the eastern Indian Ocean during the Danish Round the World expedition in 1928–1930 (Schmidt, 1935; Jespersen, 1942). Some anguillid leptocephali were collected in the South Pacific (Jespersen, 1942; Castle, 1963), but their body sizes were somewhat large to suggest the possible spawning area of the species.
Decades later, some small anguillid leptocephali were collected south of the Solomon Islands along the northeastern edge of the Coral Sea, and genetic identification, indicated that at least one of the subspecies of A. australis and A. reinhardtii, likely spawn in the South Equatorial Current (SEC) (Aoyama et al., 1999; Kuroki et al., 2008) and then recruit to either Australia or New Zealand (Shiao et al., 2001, 2002; Jellyman, 2003; Watanabe et al., 2006). It was also reported that some tropical eels such as A. borneensis and A. celebesensis spawn locally in the deep basins within the Indonesian Seas, without making long migrations to outside ocean areas (Aoyama et al., 2003, 2018). The North Pacific population of A. marmorata that recruits to the central Indonesian Seas as well as areas farther to the northwest was found to spawn in the North Equatorial Current (NEC) in an overlapping area with the Japanese eel (Kuroki et al., 2009). Efforts to understand the spawning areas and early life histories of anguillid eels in the Indian Ocean have produced some information about the leptocephali (Aoyama et al., 2007; Kuroki et al., 2007) and recruitment-stage glass eels (Robinet et al., 2003, 2008; Réveillac et al., 2009), but where the spawning areas are located are still only estimated from transport modeling studies (Pous et al., 2010).
The South Pacific region extending from Australia to French Polynesia in the central Pacific is inhabited by seven species and subspecies of anguillid eels (Jellyman, 1987, 2003), but many aspects of their life histories remain poorly understood. The geographic distribution ranges of each species extend across the boundaries and jurisdictions of many different countries in the region and require international cooperation for their management and conservation. Only A. dieffenbachii that is endemic in New Zealand is exclusively present in only one area, whereas other species (A. marmorata, A. megastoma, and A. obscura) are widely distributed across the South Pacific (Ege, 1939). Anguilla reinhardtii is distributed in northeastern Australia and nearby islands, and A. australis is separated into two subspecies that recruit to either Australia (A. australis australis) or New Zealand (A. australis schmidtii).
The recruitment patterns of glass eels to tropical islands such as French Polynesia and Fiji have begun to be studied (Helme et al., 2018; Hewavitharane et al., 2019). Other efforts to find the spawning areas of tropical eel species in the South Pacific have been conducted using popup satellite archival tags (PSATs) on adult silver eels (e.g., Jellyman and Tsukamoto, 2002, 2010; Schabetsberger et al., 2013, 2019). However, there are major gaps in understanding most aspects of the life histories of all anguillid eel species in the South Pacific. Therefore, a wide range of research is needed to learn about when and where each species or population spawns, how their larvae are distributed, and what their recruitment patterns are. It has been considered that at least some eel species in the western South Pacific likely use the westward flow of the SEC to transport their larvae towards their recruitment areas in Australia and New Zealand (Jellyman, 1987, 2003; Shiao et al., 2001, 2002; Kuroki et al., 2008; Jellyman and Bowen, 2009). However, for the other species that live on the many offshore islands in the South Pacific, it is less clear where they spawn and how their larvae use current systems for transport.
Other aspects of the hydrographic structure of the western South Pacific that have been considered to possibly help define the spawning areas of anguillid eels in the region (Schabetsberger et al., 2016) include the presence of the “Pacific Warm Pool” that forms from about 20°N and 15°S (Delcroix, 1998; Delcroix et al., 2000) and extends into the northern area, with decreasing water temperatures to the south, as well as some clear salinity structures. The shallow surface layer of low-salinity water (e.g. 34.4–34.7) referred to as the Equatorial Pacific “Fresh Pool” extends into the region north of Fiji, but may not extend much farther east (Delcroix and Picaut, 1998; Hénin et al., 1998). These features can be seen clearly in various types of hydrographic sections (Roden, 1998; Schabetsberger et al., 2016). Below the Fresh Pool in the western South Pacific is a layer of high-salinity water (e.g. 35.7–36.0) that has been referred to by names such as the southern Tropical Water (Gouriou and Toole, 1993), but is now realized to occur in specific regions throughout the world and is referred to as Subtropical Underwater (STUW) (see Schabetsberger et al., 2016). This water forms at the surface in specific areas where there is excess evaporation compared to precipitation, which results in surface salinity maximum areas forming in each ocean gyre (Gordon et al., 2015), including in the central South Pacific (Hasson et al., 2013), where it is subducted into the thermocline (Qu et al., 2008, 2013; Zhang and Qu, 2014).
The objective of the present study was to learn about the spawning areas, early life histories and possible patterns of larval transport of anguillid eel species in the South Pacific in relation to the hydrographic features of the region by conducting a sampling survey in 2016. The survey sampled for eel larvae in five long transects across the SEC, the collected larvae were aged using their otolith microstructure, and extensive hydrographic observations were made, which provided new information about the possible spawning areas of anguillid eels in the South Pacific.
2. Materials and methods
2.1. Sampling and oceanographic observations
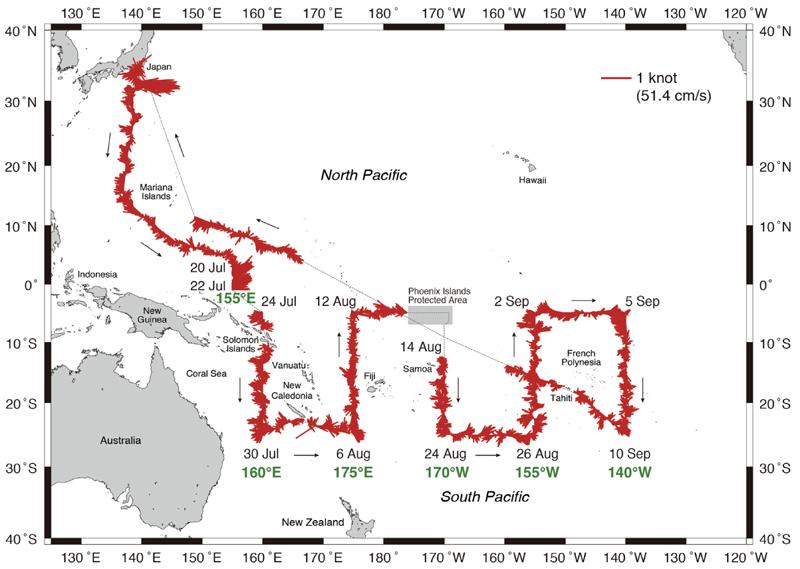
The KH-16-4 research cruise from 10 July to 3 October 2016 by the R/V Hakuho Maru (JAMSTEC) was conducted mainly in the South Pacific (Fig. 1). Two stations (14°N and 16°N, 137°E) where anguillid leptocephali including A. japonica and A. marmorata were collected, and six stations (5.5–11.5°N, 149–164°E) where no anguillid leptocephali were collected in the North Pacific were excluded from the present study, because this study focuses on sampling in the southern areas that was conducted to continue the exploration of the spawning areas of anguillid eels by sampling across the South Pacific. These southern stations were sampled from 21 July to 11 September and included a short transect along 155°E from 5°N to 1°S and five long transects from 5°S to 25°S along 160°E, 175°E, 170°W, 155°W, and 140°W (Fig. 1). Small islands and some EEZ (Exclusive Economic Zones) were avoided along the transects.
Fig. 1.
Track chart of the R/V Hakuho Maru KH-16–4 cruise and Acoustic Doppler Current Profiler (ADCP) vectors that were measured at 100 m depth along the ship track during the cruise from July to October 2016. The Phoenix Islands Protected Area (PIPA) in the Republic of Kiribati was excluded from the ADCP observations.
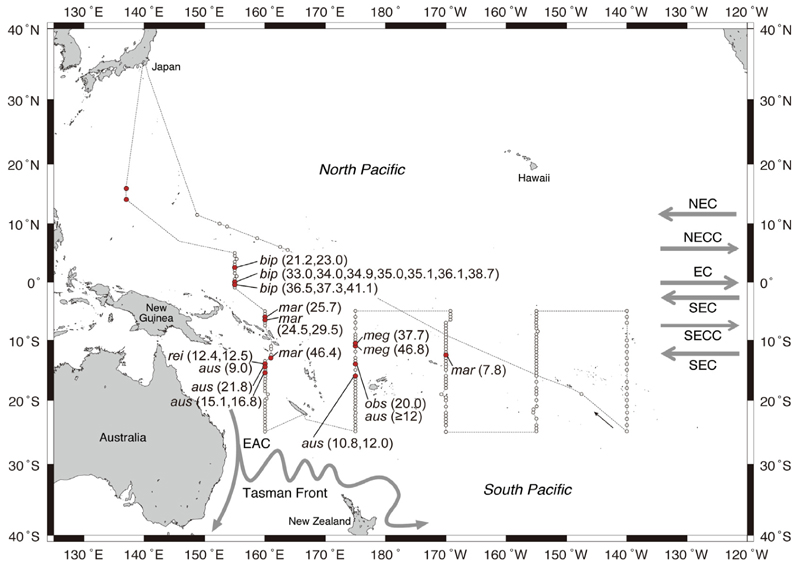
A total of 179 stations were sampled during the whole cruise, with the present study analyzing data from the 171 stations in the southern areas, which included 13 stations within the short transect and 158 stations in the five long latitudinal transects (Fig. 2). All net sampling was conducted using an 8.7 m2 mouth opening Isaacs-Kidd Midwater Trawl (IKMT) with 0.5 mm mesh. The IKMT was fished using step tows at three depth layers from the surface to 200 m, with the depths of the towing layers being adjusted slightly according to the depth of thermocline or acoustic scatting layers using CTD and Acoustic Biomass Investigation System (ABIS, Furuno Electric Co., Ltd.) information. Each towing time was about 1–1.5 h and the ship speed during the tows was 2–3 knots.
Fig. 2.
Map of the sampling stations (open circles), major current latitudes or locations in the western Pacific, and collection locations (red circles) and total lengths (in parentheses) of anguillid eel leptocephali, which included Anguilla bicolor pacifica (bip), A. marmorata (mar), A. megastoma (meg), A. obscura (obs), A. reinhardtii (rei), and A. australis (aus). The latitudes of the North Equatorial Current (NEC), North Equatorial Countercurrent (NECC), Equatorial Current (EC), South Equatorial Current (SEC), South Equatorial Countercurrent (SECC) (arrows on right side of map), and the locations of East Australian Current (EAC) and Tasman Front are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The 104 CTD (Conductivity, Temperature, and Depth) casts of the short transect from 5°N to 1°S at 155°E and five transects from 5°S to 25°S of latitude and from 160°E to 140°W of longitude in the western South Pacific were made at every other IKMT station (Fig. 2). As a result, casts were made at every one degree of latitude except for two transects where there were fewer stations due to the avoidance of the Solomon Islands in the 160°E transect and time constraints along 140°W (Fig. 1). This resulted in there being 21 CTD casts per transect, except in the westernmost (18 casts) and easternmost (16 casts) transects. The data from the CTD casts were used to plot hydrographic sections of temperature and salinity along each transect to a depth of 800 m using the Ocean Data View (ODV) program (https://odv.awi.de/). Current velocity vectors at 100 m were plotted using Acoustic Doppler Current Profiler (ADCP) current data from an ADCP device (38 kHz, TRDI) attached to the bottom of the ship except for the Phoenix Islands Protected Area (PIPA) in the Republic of Kiribati.
2.2. Morphology and genetic identification
The majority of the nearly 4000 anguilliform leptocephali that were collected in the survey were identified morphologically to be the larvae of many different families of marine eels. All anguillid leptocephali collected were examined morphologically on board using a stereo microscope while fresh following the identification methods of Miller and Tsukamoto (2004). Counts were made of the position of their last vertical blood vessel (LVBV), preanal myomeres (PAM), predorsal myomeres (PDM) and total myomeres (TM), and their number of anodorsal myomeres (distinguishes shortfin and longfin species) could then be determined from the PAM and PDM counts. The onboard myomere counts for the small larvae were only approximate. Some of the leptocephali from the southern region were photographed using a microscope camera (DXM1200F, Nikon) attached to a stereo microscope (SMZ-1500, Nikon). A portion of tissue of all specimens was preserved in 99% ethanol for further genetic analysis.
An onboard real-time PCR system was used to identify selected anguillid species (e.g., Tsukamoto et al., 2011), except in this case with more species potentially being present during the cruise. The final identifications were made by comparing about 500 base pairs of the mitochondrial DNA 16S ribosomal RNA gene sequences between the collected leptocephali and morphologically well-identified yellow eel specimens, as was done in a previous study following the procedure of Aoyama et al. (2007).
2.3. Otolith analysis
The ages of the southern region anguillid leptocephali collected in this study were analyzed using counts of their otolith daily growth rings and the ages were used to back-calculate their hatching dates. Each sagittal otolith was embedded in epoxy resin (Epofix, Struers), mounted on a glass slide, ground to near the core using a grinding machine (70 and 13 µm diamond cup-wheels, Discoplan-TS, Struers) and further polished to expose the core (3 µm Silicon Carbide grinding paper and OP-S suspension, Planopol-V, Struers). After cleaning the surface, each otolith was etched with 0.05 M HCl and vacuum coated with Pt-Pd in an ion-sputterer (E-1030, Hitachi) for scanning electron microscope observations (S-4800, Hitachi). The age of each specimen was determined by counting the number of successive daily rings from the hatch check to the otolith edge at magnifications of 600–1200×. Hatching date was back-calculated from age and collection date of each specimen.
3. Results
3.1. Hydrographic features and currents
The ADCP current vector data at 100 m depths showed some areas with strong currents, others with weak flow patterns and other areas that were possibly dominated by large mesoscale eddies. Strong eastward flows were observed within the North Equatorial Countercurrent (NECC), and the northern areas of the two eastern long transects had strong westward flows, which were likely part of the northern branch of the SEC. Strong westward flows also occurred at SEC latitudes along the 160°E transect and at two locations of the 175°E transect passing to the west of Fiji. At higher latitudes at 20–25°S, the current directions consistently alternated, suggesting large eddies were present (Fig. 1).
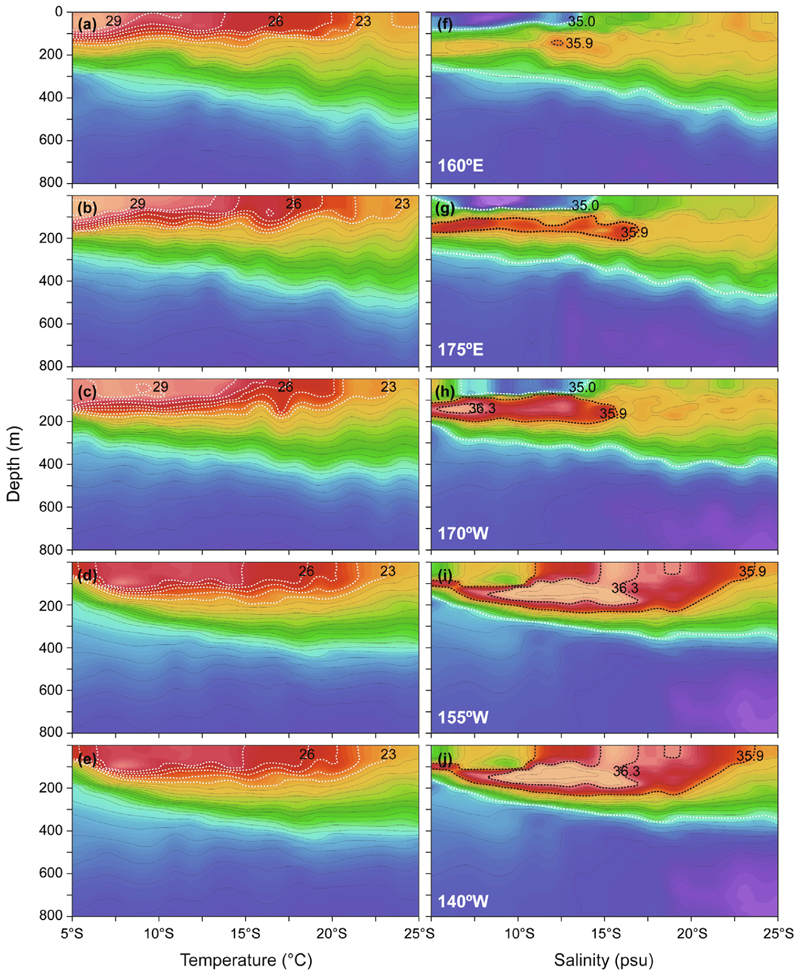
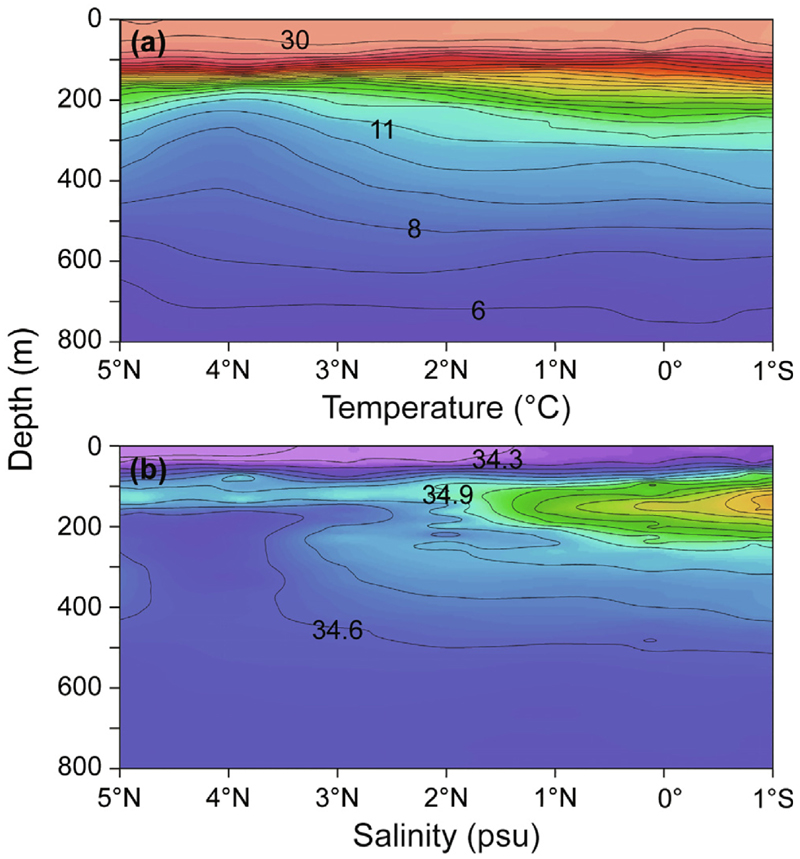
The hydrographic sections plotted from the five long transects of CTD casts across the latitudes of the SEC region from 155°E to 140°W (Fig. 2) showed there was a generally consistent pattern of a warmer layer of surface water (25–30 °C) being present in the upper 100 m extending down to at least 20°S across the region (Fig. 3a–e). The surface water cooled slightly in the east, with maximum temperatures being 28 °C (155°W) and 27 °C (140°W), compared to mostly 29 °C in the western transects. In contrast, the salinity sections showed considerable variations from west to east (Fig. 3f–j) that were caused by the low salinity surface-layer referred to as the Fresh Pool and the subsurface-layer of high salinity STUW. The Fresh Pool was distinctly present in the three western transects (minimum of 34.3 in 175°E), but it became less distinct in the two eastern transects (minimum of 35.2 in 140°W). The STUW forms in the eastern region of the South Pacific and extends westward, so it was least distinct in the westernmost transect, and the salinity values in each transect steadily increased in the eastern direction (maximum of 35.9 in the west and 36.5 in the east). The STUW extended farther north in the two eastern transects and it reached the surface in the easternmost transect because that transect was within the region of high evaporation where it forms. The shorter 155°E transect near the equator in the northwestern area showed some slightly different hydrographic characteristics (Fig. 4). There was a consistent and slightly deeper mixed layer of 29–30 °C water in the upper 75 m above the thermocline across those latitudes. The surface layer had slightly lower salinity (34.1) and the STUW also reached a lower value (35.6) compared to the transects. Comparing the ADCP data and the contour structure, the NECC was present in about the southern two-thirds of the short transect and there was no STUW north of the eastward NECC flow (Figs. 1 and 4).
Fig. 3.
Hydrographic sections of temperature (°C) and salinity (psu) constructed using data from a conductivity temperature depth profiler (CTD) along the five transects of 160°E (24–30 July), 175°E (6–12 August 2016), 170°W (14–24 August 2016), 155°W (26 August–2 September 2016) and 140°W (5–10 September 2016) routes that are labeled in Fig. 1.
Fig. 4.
Hydrographic sections of (a) temperature and (b) salinity constructed using data from a conductivity temperature depth profiler (CTD) along the 155°E transect (20–22 July 2016) northeast of New Guinea that is labeled in Fig. 1.
3.2. Size and age of anguillid leptocephali
The onboard morphometric observations and subsequent DNA sequencing found that 29 anguillid leptocephali of six species; A. bicolor (n = 12), A. marmorata (n = 5), A. australis (n = 7), A. reinhardtii (n = 2), A. megastoma (n = 2), and A. obscura (n = 1), were collected in the equatorial region and the South Pacific (2.5°N–16°S, 155°E–170°W) during the period of July–August 2016 (Table 1, Fig. 2). No anguillid larvae were collected in the two eastern transects of 155°W and 140°W or at any of the southern stations from 16.5°S to 25°S, although sampling was conducted in the same way in those areas.
Table 1.
Collection data, total length (mm), total myomeres, and age (days) of the anguillid leptocephali collected in the southern region during the R/V Hakuho Maru KH-16-4 cruise.
| No. | Collection date | St. | Latitude | Longitude | Species | Total length | Total myomeres | Age |
|---|---|---|---|---|---|---|---|---|
| 257 | 22 July 2016 | 24 | 2°30′N | 155°00′E | A. bicolor pacifica | 23.0 | 103 | 55 |
| 258 | 22 July 2016 | 24 | 2°30′N | 155°00′E | A. bicolor pacifica | 21.2 | 105 | 46 |
| 268 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 33.0 | 106 | 62 |
| 269 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 34.9 | 106 | 84 |
| 270 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 35.0 | 104 | 91 |
| 271 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 35.1 | 104 | 78 |
| 272 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 34.0 | 106 | 75 |
| 273 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 36.1 | 105 | 83 |
| 274 | 23 July 2016 | 29 | 0°00′ | 155°00′E | A. bicolor pacifica | 38.7 | 106 | 91 |
| 297 | 23 July 2016 | 30 | 0°30′S | 155°00′E | A. bicolor pacifica | 36.5 | 107 | 72 |
| 298 | 23 July 2016 | 30 | 0°30′S | 155°00′E | A. bicolor pacifica | 41.1 | 106 | 110 |
| 299 | 23 July 2016 | 30 | 0°30′S | 155°00′E | A. bicolor pacifica | 37.3 | 106 | 71 |
| 342 | 24 July 2016 | 34 | 6°00′S | 160°00′E | A. marmorata | 25.7 | 101 | 46 |
| 374 | 25 July 2016 | 35 | 6°30′S | 160°00′E | A. marmorata | 24.5 | 110 | 51 |
| 375 | 25 July 2016 | 35 | 6°30′S | 160°00′E | A. marmorata | 29.5 | 108 | 60 |
| 512 | 26 July 2016 | 42 | 13°00′S | 160°00′E | A. marmorata | 46.4 | 107 | 120 |
| 526 | 27 July 2016 | 44 | 14°00′S | 160°00′E | A. reinhardtii | 12.5 | ~113 | 26 |
| 527 | 27 July 2016 | 44 | 14°00′S | 160°00′E | A. australis | 9.0 | ~111 | 16 |
| 531 | 27 July 2016 | 44 | 14°00′S | 160°00′E | A. reinhardtii | 12.4 | 107 | 20 |
| 536 | 27 July 2016 | 45 | 14°30′S | 160°00′E | A. australis | 21.8 | 111 | 46 |
| 541 | 27 July 2016 | 47 | 15°30′S | 160°00′E | A. australis | 15.1 | 108 | 27 |
| 542 | 27 July 2016 | 47 | 15°30′S | 160°00′E | A. australis | 16.8 | 106 | 29 |
| 1260 | 9 August 2016 | 87 | 16°00′S | 175°00′E | A. australis | 12.0 | ~114 | 30 |
| 1261 | 9 August 2016 | 87 | 16°00′S | 175°00′E | A. australis | 10.8 | ~113 | 19 |
| 1267 | 9 August 2016 | 91 | 14°00′S | 175°00′E | A. obscura | 20.0 | 108 | 37 |
| 1280 | 9 August 2016 | 91 | 14°00′S | 175°00′E | A. australis | 12.0+a | 77+a | 25 |
| 1334 | 10 August 2016 | 97 | 11°00′S | 175°00′E | A. megastoma | 46.8 | 108 | 110 |
| 1345 | 10 August 2016 | 98 | 10°30′S | 175°00′E | A. megastoma | 37.7 | 112 | 71 |
| 1696 | 16 August 2016 | 130 | 12°30′S | 170°00′W | A. marmorata | 7.8 | ~110 | 19 |
Larva was not measured accurately due to damage.
Twelve middle-sized A. bicolor leptocephali (21.2–41.1 mm, 46–110 days) were collected in the NECC area to the northeast of New Guinea, but none were found anywhere else. Four middle to large-sized leptocephali of A. marmorata (24.5–46.4 mm, 46–120 days) were collected to the north and south of the Solomon Islands and a small leptocephalus of that species (7.8 mm, 19 days, Fig. 5a) was caught near the northeast side of Samoa (Fig. 2). That leptocephalus is the easternmost and the smallest specimen of that species ever found in the South Pacific.
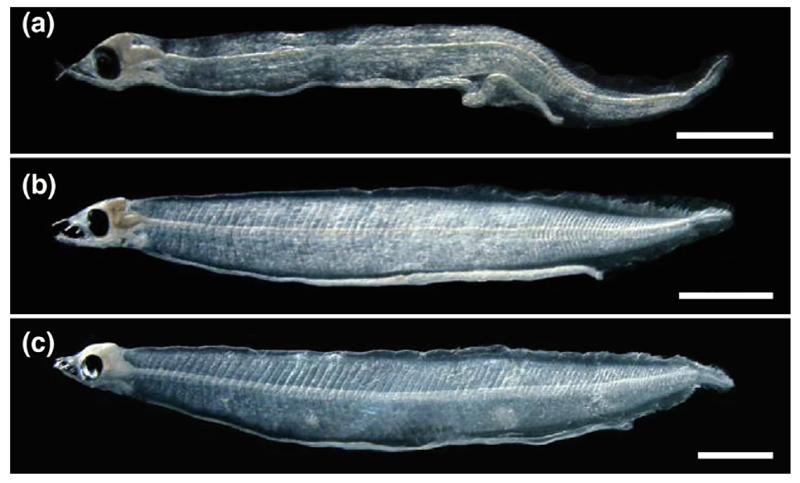
Fig. 5.
Photographs of leptocephali showing (a) Anguilla marmorata (No. 1696, 7.8 mm) collected at 12.5°S, 170°W, on 16 August 2016, (b) A. australis (No. 1261, 10.8 mm) collected at 16°S, 175°E, on 9 August 2016, and (c) A. reinhardtii (No. 526, 12.5 mm) collected at 14°S, 160°E, on 27 July 2016. Scale bars indicate 1 mm.
Four leptocephali of A. australis (9.0–21.8 mm, 16–46 days) were collected at three stations south of the Solomon Islands in the northeastern Coral Sea along the 160°E transect and three other larvae of this species (10.8–~12.0 mm, 19–30 days, Fig. 5b) were caught almost 1600 km to the east, not far to the northwest of Fiji along 175°E (Fig. 2). One ~12.0 mm A. australis specimen could not be accurately measured because of damage to the caudal fin.
Two small leptocephali of A. reinhardtii (12.4 and 12.5 mm, 20 and 26 days, Fig. 5c) were collected at one of the same stations (14°S, 160°E) with A. australis in the westernmost transect. Two A. megastoma (37.7 and 46.8 mm, 71 and 110 days) and one A. obscura leptocephali (20.0 mm, 37 days) were caught in the region to the northwest of Fiji (Fig. 2). The A. megastoma larvae were collected a little farther north than the A. obscura larva.
3.3. Hatching season
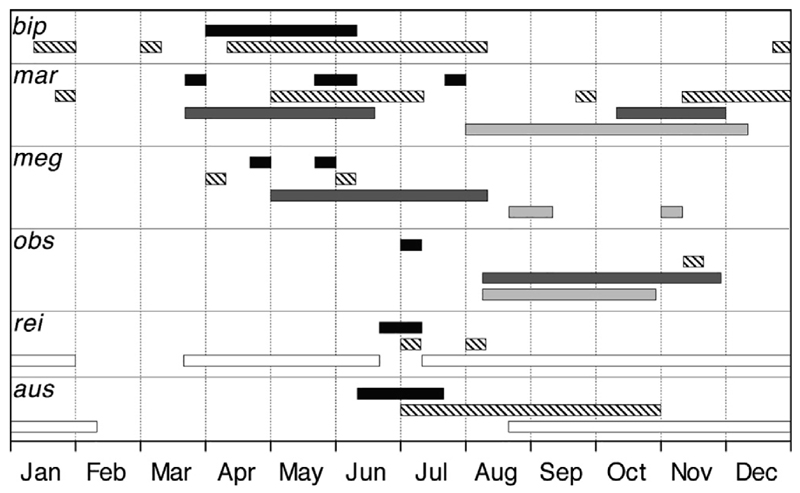
Hatching dates of each anguillid leptocephalus specimen in the southern area were calculated from the estimated age determined from their otolith daily rings. The hatching season for the six species ranged across the five months from late March to late July 2016 before the larvae were collected in July and August, with the range of months for each species depending on the size range of the collected larvae (Fig. 6). The wide range in sizes of A. marmorata larvae resulted in hatching dates from late March to late July. The other species were apparently hatched across ranges of within one to three months.
Fig. 6.
Hatching seasons of Anguilla bicolor pacifica (bip), A. marmorata (mar), A. megastoma (meg), A. obscura (obs), A. reinhardtii (rei), and A. australis (aus). Hatching seasons were estimated from leptocephali collected in this cruise (black bars) and previous cruises (striped bars) from Kuroki et al. (2006) for bip in both the North and South Pacific, and from Kuroki et al. (2008) for five other species collected in the South Pacific, and for glass eels recruited to Fiji (dark gray bars) from Hewavitharane et al. (2019), glass eels recruited to Tahiti (light gray bars) from Sasal et al. (unpublished data), and glass eels recruited to Australia and New Zealand (while bars) from Shiao et al. (2001, 2002).
4. Discussion
4.1. Possible spawning areas of three anguillid eel species
The extensive sampling grid and hydrographic data of the 2016 survey that included the short transect northeast of New Guinea and five long transects, provided several types of interesting new information and clues about the spawning areas of anguillid eels in the region. Leptocephali < 15 mm were collected of three species; A. marmorata (smallest 7.8 mm), A. australis (smallest 9.0 mm), and A. reinhardtii (smallest 12.4 mm). Each location where these small larvae were collected provides information about where the larvae could have been spawned when they are combined with the otolith aging estimates and the possible current flows in each area. The back calculated hatching data also provided useful information about when these eels may spawn. Combining the results of both present and previous studies estimated from leptocephali (Kuroki et al., 2008) and glass eels (Shiao et al., 2001, 2002; Helme et al., 2018; Hewavitharane et al., 2018, 2019), the spawning of each species appears to occur over a wide range of months. Hatching date estimates of A. marmorata from the various studies occurred in almost all months of the year, and almost to the same extent for A. australis and A. reinhardtii (Fig. 6).
As the survey moved south into the eastern Coral Sea the smallest A. australis larva ever collected (9.0 mm) was caught along with several 15–20 mm larvae and similar sized A. reinhardtii larvae. The morphology of these species of small larvae was quite similar to other small anguillid larvae (Fig. 5). This is the same location where 20–34 mm larvae of these species were also collected at about the same season of year in 1995 (Kuroki et al., 2008). The 9 mm A. australis larva collected about 700 km west of Vanuatu was estimated to be only 16 days old, so considering the general ADCP measurements and the likely presence of eddies (20 cm/s current would transport a larva approximately 276 km in 16 days), this larva was probably spawned on the west side of the Vanuatu Archipelago (Figs. 1 and 2). A few small 10–12 mm A. australis larvae were also collected to the northwest of Fiji in the 175°E transect. That location is almost 1600 km east of where the other small A. australis larvae were collected. Therefore, regardless of possible counter-current transport, it seems unlikely that the two groups of larvae were spawned in the same area. There were also small anguillid leptocephali (n = 25, 8.8–12.9 mm) collected in the same area northeast of Fiji (14–15°S, 175°E) in mid-August 1995 (Aoyama et al., 1999; Miller et al., 2006) that could not be genetically identified (formalin preserved), which could also have included A. australis larvae.
The 2016 specimens themselves though, are enough to suggest that there could be two different or overlapping spawning areas of A. australis in this western SEC region. Two anguillid species are known to spawn in overlapping areas in the Atlantic and North Pacific (Schmidt, 1922; Kuroki et al., 2009). But in the case of the two subspecies of A. australis, and considering the presence of the distinct geographic landmarks of large islands and the Vanuatu Archipelago, the two subspecies may have evolved separate spawning areas within the same region, which would help maintain their morphological and genetic differentiation that has been observed (Watanabe et al., 2006; Shen and Tzeng, 2007). Schmidt (1928) had speculated that A. australis australis from Australia might spawn on the west side of the New Caledonian submarine ridge and that A. australis schmidtii from New Zealand might spawn on the east side of the ridge. Various other studies have subsequently evaluated where the spawning locations of these eels might be within the same general region between New Caledonia and Tahiti (e.g., Jespersen, 1942; Castle, 1963; Jellyman, 1987). The possible routes/ distances of silver eel migration and durations of larval drifting before reaching recruitment areas might be two factors to consider when speculating about where it might be more adaptive for migrating silver eels of A. australis schmidtii from New Zealand or those of A. australis australis from Australia to spawn, if they do actually spawn in slightly different areas.
The larvae of A. reinhardtii were only collected to the west of Vanuatu during this and the previous surveys (Castle, 1963; Kuroki et al., 2008), suggesting their spawning area is only in one region of the SEC in the northeastern edge of the Coral Sea. That area seems to be the only area where A. reinhardtii leptocephali have been collected so far, and their glass eels recruit to areas ranging from northeastern Australia (farther north than A. australis australis) to eastern Tasmania, but not quite as far south as A. australis australis (Sloane, 1984; Shiao et al., 2002). Studies on the glass eels in Australia found that both A. reinhardtii and A. australis have a cline in age at recruitment from younger and smaller in size in the north to older and larger in the south (Shiao et al., 2002).
A few A. reinhardtii seem to recruit to northern New Zealand (McDowall et al., 1998), and A. dieffenbachii whose leptocephali have never been collected and identified, only recruits to New Zealand. The glass eels of A. australis schmidtii that recruit to the South Island of New Zealand were not much different in size or age than those of A. australis australis from southern Australia (Shiao et al., 2001), suggesting that the leptocephali take similar periods to reach both areas. The mean ages of A. dieffenbachii glass eels recruiting to New Zealand were found to be older than those of A. australis schmidtii (Marui et al., 2001). Considering the limited number of early life history studies on glass eels, and the mixture of sampling locations, times and years among the studies, it is difficult to reach any conclusions yet about the precise spawning areas and larval migrations of these Australian and New Zealand anguillid eel species and subspecies beyond what can be determined from the collection data of their small larvae in the SEC, and their absence at other latitudes.
It is similarly difficult to understand the spawning areas and life histories of other anguillid species in the South Pacific, because of their wide species distributions on most of the islands across the region and the possibility that they have more than one spawning population. Anguilla marmorata is the most widely distributed anguillid species because it is present across the entire Indo-Pacific from the western Indian Ocean to French Polynesia (Ege, 1939; Watanabe, 2003). Population genetic and morphology research indicates there are multiple populations of this species across its range, and there may be some population segregation between the eastern side (French Polynesia) and western side (Samoa, Fiji, New Caledonia, New Guinea) of the South Pacific (Ishikawa et al., 2004; Minegishi et al., 2008; Watanabe et al., 2008). Two tropical eel species of A. megastoma and A. obscura are similarly widely distributed and inhabit the same islands sympatrically with A. marmorata across the region (Jellyman, 2003; Watanabe, 2003), with A. obscura using the lower reaches of rivers, A. marmorata using the middle reaches, and A. megastoma using the upper reaches (Marquet and Galzin, 1991). Vertebral counts have also suggested there may be eastern and western populations of A. megastoma, but no differences were found for A. obscura (Watanabe et al., 2011). The present study provided the first clear evidence of a spawning location for these three species when the 7.8 mm A. marmorata leptocephalus (19 days old) was collected near Samoa. The region northeast of Vanuatu and northwest of Fiji is where biologging research using PSATs on silver eels from Vanuatu have suggested there is a sympatric spawning area of A. marmorata and A. megastoma (Schabetsberger et al., 2013, 2016). Larvae of both those species have been collected in that area before the 2016 survey, including a 19.0 mm A. marmorata (Kuroki et al., 2008). There were also relatively small A. marmorata larvae (n = 4, 16.5–18.3 mm), about a month after hatching, collected at 8°S, 170–180°E in February 2013 (Kuroki et al. unpublished data) farther north closer to the pop-up locations of the PSATs. The 7.8 mm A. marmorata larva from this study would not be from that area though, and it seems unlikely that the larvae from northwest of Fiji would have been transported that far to the northwest from near Samoa, so at least this species may spawn at two different locations in the more western region of the South Pacific. A more recent PSAT study released eels of A. marmorata from Samoa and did not find evidence of the eels attempting to make a long migration away from that area (Schabetsberger et al., 2019), which is consistent with the collection of the 7.8 mm larva there.
4.2. Larval distribution of other anguillid eel species
The western Pacific subspecies of A. bicolor pacifica is one of the species for which there is little evidence about the location of their spawning area, and the short transect northeast of New Guinea sampled during the first part of the 2016 survey collected relatively small leptocephali (21.2, 23.0 mm) as well as larger ones within the strong eastward flow of the NECC. The previous collection of A. bicolor pacifica (24.0 mm) near New Guinea in 1995 (Kuroki et al., 2006) and the newly collected larvae that would have been transported eastward, suggests that their spawning area might be somewhere north of New Guinea as previously suggested (Kuroki et al., 2006; Miller and Tsukamoto, 2017). The other subspecies of A. bicolor bicolor inhabits the Indian Ocean and has at least one spawning area there off West Sumatra (Jespersen, 1942; Aoyama et al., 2007; Kuroki et al., 2007). The apparent spawning area of A. bicolor pacifica is in an area with interconnecting currents and eddy systems (Miller and Tsukamoto, 2017) that could result in the larvae becoming widely distributed to areas where they recruit, such as the Indonesian Seas (Sugeha et al., 2001), the Philippines (Shirotori et al., 2016), and as far north as southern Japan (Yamamoto et al., 2001). However, no larvae of this species have been collected and identified at sizes < 20 mm so far.
The same lack of collections of small larvae of several of the anguillid species in the South Pacific make it difficult to estimate the locations of their spawning areas, but some life history information is beginning to obtained from studies on their glass eels. The 20.0 mm A. obscura caught northwest of Fiji was the smallest leptocephalus of that species ever to be collected and identified. This 37 days old larva could have been transported a considerable period by the SEC or eddies. Anguilla obscura is often the most abundant species that recruits to a small river area along the southern coast of the main island of Fiji, taking 141–204 days until recruitment there, which was somewhat longer than A. megastoma (126–163 days) and A. marmorata (124–183 days) that also recruited there (Hewavitharane et al., 2018, 2019). Two A. megastoma leptocephali (37.7 and 46.8 mm) caught at about 10°S to the northwest of Fiji were too large to provide any spawning location information. The glass eels of A. megastoma were much less abundant in Fiji (Hewavitharane et al., 2018). These tropical species having some glass eel recruitment during at least two periods of year or throughout most of the year (e.g., Sugeha et al., 2001; Hewavitharane et al., 2018) is different than the life-histories of temperate eel species that have single recruitment seasons.
One limitation of the present survey for determining the origins of both the large and small leptocephali collected during the survey and exactly where their spawning areas are located was the wide spacing between transects. The long transects were effective to establish the latitudinal zones where larvae may have been present, but there were wide regions between the transects where many anguillid larvae could have been present. For example, it is possible that anguillid larvae may have been more abundant in the areas between the transects in the west, which may be one explanation for the low catches during the survey. This may also be one of the reasons why no anguillid larvae were collected in the two eastern transects. The 155°W transect did not pass near any major island groups where eels might live, and spawning far from any recruitment habitats at that longitude may not be advantageous. Similarly, the easternmost 140°W transect could have been on the upstream side of the majority spawning locations near the larger islands to the west where anguillid eels are known to live, such as Tahiti and the Marquesas Islands (Marquet and Galzin, 1991; Helme et al., 2018). The westward flow of the SEC in that region appears to be slow (5–10 cm/s), with frequent eastward countercurrents (Rougerie and Rancher, 1994) along with seasonal variability in the current flows (Martinez et al., 2009), so local spawning strategies may be effective. In that case, our two eastern transects may have been too far west and also too far east of the areas where most larvae were distributed. Our sampling could also have occurred during a time period when few larvae were present in those locations. It was recently reported that most recruitment of glass eels of the three species, A. marmorata, A. megastoma, and A. obscura, occurs from December to January in Tahiti and Moorea, with A. marmorata being by far the most abundant species at the single sampling locations on each island that have been studied (Helme et al., 2018).
4.3. Hydrographic features and spawning areas
An important aspect of the present survey were the five long hydrographic sections that could be plotted and compared to the catch locations of the larvae and the ADCP current velocity data. From a hydrographic perspective, these high-resolution transects of CTD casts at each station provided a geographic view of the hydrographic structure across a wide longitudinal zone of the South Pacific during one time period. For example, these sections provide clear documentation of the structure of the longitudinal changes in the STUW, which previously has been only evaluated through modelling or sources of data other than high-resolution salinity sections (e.g., Hasson et al., 2013; Qu et al., 2013). Four of the long hydrographic transects of the World Ocean Circulation Experiment (Koltermann et al., 2011) were made across this region in 1991–1993 (one was analyzed by Roden, 1998). But these hydrographic observations were made during various different months and years, which makes interpreting geographic differences more difficult.
Comparisons of the hydrographic structure to the catches of leptocephali during the 2016 survey showed that the small larvae of A. australis and A. reinhardtii were collected in a narrow area (~14–15°S) of the 160°E transect that was along the southern edge of the warm surface pool of water (Pacific Warm Pool; Delcroix, 1998; Delcroix et al., 2000) and also the low salinity Fresh Pool (Delcroix and Picaut, 1998; Hénin et al., 1998) (Fig. 3a,f). The small A. australis and A. obscura were collected along 175°E northwest of Fiji (~14–16°S) in a similar location in relation to these hydrographic features, and the small A. marmorata was caught slightly farther north along 170°W. The edge of these surface features may be likely locations for fronts to form, and both temperature (European and American eels) and salinity fronts (Japanese eel) appear to affect where spawning of the eels occurs (Kleckner and McCleave, 1988; Tsukamoto, 1992; Kimura and Tsukamoto, 2006; Aoyama et al., 2014). These locations in the western South Pacific were also above subsurface cores of the high salinity STUW, and both types of features have been hypothesized to potentially be able to provide hydrographic signposts that could be used by migrating anguillid eels as recently overviewed by Schabetsberger et al. (2016).
These larval catch locations were also likely associated with the westward flow of the SEC that occurs in the upper 300–400 m of the region and usually has a two-core structure with speeds that can reach > 20 cm/s (Delcroix et al., 1987; Kessler and Taft, 1987; Roden, 1998). It breaks up into several branches as it reaches the island groups of Vanuatu and New Caledonia, and then also bifurcates off north-eastern Australia, with the southern branch becoming the East Australian Current (EAC) (Ganachaud et al., 2014; Hu et al., 2015). This seems important for the species that recruit to eastern Australia after entering the southward flowing EAC, which is the western boundary current of the western South Pacific (Ridgway and Dunn, 2003; Hu et al., 2015), and those recruiting to New Zealand that must also enter the eastward flow across the Tasman Front (Tilburg et al., 2001).
There are various other complex seasonal currents or jets in the western region of the South Pacific however, which have likely influenced where spawning areas have been formed and how the larvae become distributed. Westward current jets can form such as north and south of New Caledonia and there are also eastward countercurrents that form at least during some seasons (Gourdeau et al., 2008; Ganachaud et al., 2014). Between the two branches of the SEC in the east, the South Equatorial Countercurrent (SECC) forms seasonally at about 5–10°S (Delcroix et al., 1987, 1992; Kessler and Taft, 1987; Roden, 1998), with peak flows in March (Chen and Qui, 2004). The western South Pacific can be viewed as a subtropical gyre with westward SEC flow in the north and eastward return flow to the south starting between New Zealand and Fiji (Morris et al., 1996; Hu et al., 2015). The current flows in the central part of the South Pacific such as in the French Polynesia region where three species of anguillid eels are present (Marquet and Galzin, 1991) and may spawn locally, seem less studied (Rougerie and Rancher, 1994; Martinez et al., 2009).
This complexity of current flows may be one reason why there is no evidence of a single regional spawning area for more than one species, because in the western South Pacific there is likely no single area that can result in the larvae reaching all the potential recruitment habitats. Therefore, it may be likely that some specific spawning areas have formed that enable recruitment to specific areas, such as the present study and previous information appears to indicate for A. marmorata (e.g., Robinet et al., 2008; Kuroki et al., 2014).
While the numbers of anguillid leptocephali collected in 2016 was relatively fewer than the previous survey in 1995 conducted in a similar season that included twice the number of IKMT tows per station (Aoyama et al., 1999), it is also important to note that the stations where anguillid larvae were not collected also provide useful information that can be compared to the hydrographic data. No anguillid leptocephali were collected to the south of the latitudes of the SEC. The hydrographic sections showed an interesting pattern of the highest levels of fluorescence from chlorophyll of phytoplankton being in the northern half of each transect (not shown). Those high-fluorescence areas corresponded to being within the thermocline below the warmer northern waters at latitudes where the westward flowing SEC would be. Those are the latitudes where the anguillid eels appear to spawn based on this study and previous collections of their larvae (Kuroki et al., 2008). This may suggest that those latitudes not only provide good westward flow for the leptocephali that need to go in that direction (also eastward just to the north in the SECC), but they also would provide a favorable environment for feeding (Miller et al., 2013; Feunteun et al., 2015; Liénart et al., 2016).
5. Summary and conclusions
The large-scale 2016 sampling survey to collect leptocephali in conjunction with extensive CTD casts in a short transect in the NECC northeast of New Guinea and along five long transects (5–25°S) crossing the SEC in the South Pacific provided new information about anguillid spawning areas and showed the hydrographic structure across the region. The sizes and ages of small leptocephali indicated spawning likely occurred in three different areas within the latitudes of the western SEC. Anguilla reinhardtii leptocephali were only collected on the west side of the Vanuatu Archipelago, whereas A. australis, which consists of two subspecies, was collected in widely separated areas on both sides of the archipelago. Combined with information from biologging or population structure studies, species such as A. marmorata appear to spawn in at least two areas within the New Caledonia to Samoa region, and also somewhere in French Polynesia, where no anguillid leptocephali have been collected yet. The warm Fresh Pool in the surface layer fades out in the eastern region where the high-salinity STUW is formed and extends subsurface within the thermocline into the western region. It is unclear if these hydrographic features help define any of the spawning areas that may be present. Future sampling surveys can now focus on a narrower range of latitudes to obtain further information about the spawning areas and larval ecology of the anguillid species in the South Pacific, and a more closely spaced series of transects sampled in the most likely spawning season will be useful for collecting the first anguillid leptocephali in the French Polynesia region. Other approaches using advanced techniques such as otolith microchemistry (e.g. isotope mapping), environmental DNA, numerical simulations, and high-precision PSAT tagging could also be informative about the early life histories and migration of the anguillid eels in the South Pacific.
Acknowledgements
We gratefully acknowledge the support of the captain and crew of the R/V Hakuho Maru. We also thank Dr. Don Jellyman, Dr. Christine Dupuy, Dr. Valerie Allain, Dr. Fabien Morat, Dr. Takashi Yamakawa, and Ms. Machiko Oya for their suggestions and cooperation about South Pacific research efforts, and we are grateful to all the other scientists, technicians, and students who helped to deploy the IKMT and CTD and to sort the many plankton samples to obtain anguillid larvae on board the KH-16-4 research cruise. Funding for various aspects of the survey was provided by JSPS KAKENHI Grant Number 26252030, 26450268, 17H03859, 17K19300. R.S. was supported by the Austrian Science Fund Number P28381.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aoyama J, Mochioka N, Otake T, Ishikawa S, Kawakami Y, Castle PHJ, Nishida M, Tsukamoto K. Distribution and dispersal of anguillid leptocephali in the western Pacific Ocean revealed by molecular analysis. Mar Ecol Prog Ser. 1999;188:193–200. [Google Scholar]
- Aoyama J, Wouthuyzen S, Miller MJ, Inagaki T, Tsukamoto K. Short-distance spawning migration of tropical freshwater eels. Biol Bull. 2003;204:104–108. doi: 10.2307/1543500. [DOI] [PubMed] [Google Scholar]
- Aoyama J, Wouthuyzen S, Miller MJ, Minegishi Y, Minagawa G, Kuroki M, Suharti SR, Kawakami T, Sumardiharga KO, Tsukamoto K. Distribution of leptocephali of the freshwater eels, genus Anguilla, in the waters off west Sumatra in the Indian Ocean. Environ Biol Fish. 2007;80:445–452. [Google Scholar]
- Aoyama J, Watanabe S, Miller MJ, Mochioka N, Otake T, Yoshinaga T, Tsukamoto K. Spawning sites of the Japanese eel in relation to oceanographic structure and the West Mariana Ridge. Plos One. 2014;9:e88759. doi: 10.1371/journal.pone.0088759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama J, Wouthuyzen S, Miller MJ, Sugeha HY, Kuroki M, Syahailatua A, Tantu FY, Hagihara S, Triyanto, Otake T, Tsukamoto K. Reproductive ecology and biodiversity of freshwater eels around Sulawesi Island Indonesia. Zool Stud. 2018;57:30. doi: 10.6620/ZS.2018.57-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle PHJ. Anguillid leptocephali in the southwest Pacific. Zool Publ Victoria Univ Wellington. 1963;33:1–14. [Google Scholar]
- Chen S, Qiu B. Seasonal variability of the south equatorial countercurrent. J Geophys Res. 2004;109 C08003. [Google Scholar]
- Delcroix T. Observed surface oceanic and atmospheric variability in the tropical Pacific at seasonal and ENSO timescales: a tentative overview. J Geophys Res. 1998;103:18611–18633. [Google Scholar]
- Delcroix T, Picaut J. Zonal displacement of western equatorial Pacific “fresh pool”. J Geophys Res. 1998;103:1087–1098. [Google Scholar]
- Delcroix T, Eldin G, Hénin C. Upper ocean water masses and transports in the western tropical Pacific (165°E) J Phys Oceanogr. 1987;17:2248–2262. [Google Scholar]
- Delcroix T, Eldin G, Radenac MH, Toole J, Firing E. Variation of the western Equatorial Pacific Ocean, 1986–1988. J Geophys Res. 1992;97:5423–5445. [Google Scholar]
- Delcroix T, Dewitte B, du Penhoat Y, Masia F, Picaut J. Equatorial waves and warm pool displacements during the 1992–1998 El Niño Southern Oscillation events: observation and modeling. J Geophys Res. 2000;105:26045–26062. [Google Scholar]
- Drouineau H, Durif C, Castonguay M, Mateo M, Rochard E, Verreault G, Yokouchi K, Lambert P. Endangered eels: a symbol of the effects of global change. Fish Fish. 2018;19:903–930. [Google Scholar]
- Ege V. A revision of the genus Anguilla Shaw: a systematic, phylogenetic and geographical study. Dana Report Carlsberg Foundation. 1939;16:8–256. [Google Scholar]
- Feunteun E, Miller MJ, Carpentier A, Aoyama J, Dupuy C, Kuroki M, Pagano M, Réveillac E, Sellos D, Watanabe S, Tsukamoto K, et al. Stable isotopic composition of anguilliform leptocephali and other food web components from west of the Mascarene Plateau. Prog Oceanogr. 2015;137:69–83. [Google Scholar]
- Ganachaud A, Cravatte S, Melet A, Schiller A, Holbrook NJ, Sloyan BM, Widlansky MJ, Bowen M, Verron J, Wiles P, Ridgway K, et al. The Southwest Pacific Ocean circulation and climate experiment (SPICE) J Geophys Res Oceans. 2014;119:7660–7686. [Google Scholar]
- Gordon AL, Giulivi CF, Busecke J, Bingham FM. Differences among subtropical surface salinity patterns. Oceanography. 2015;28:32–39. [Google Scholar]
- Gourdeau L, Kessler WS, Davis RE, Sherman J, Maes C, Kestenare E. Zonal jets entering the coral sea. J Phys Oceanogr. 2008;38:715–725. [Google Scholar]
- Gouriou Y, Toole J. Mean circulation of the upper layers of the western equatorial Pacific Ocean. J Geophys Res. 1993;98:22495–22520. [Google Scholar]
- Hasson A, Delcroix T, Boutin J. Formation and variability of the South Pacific Sea Surface Salinity maximum in recent decades. J Geophys Res Oceans. 2013;118:5109–5116. [Google Scholar]
- Helme H, Bertucci F, Moussa M, Wolff Y, Sasal P. Temporal dynamics of the recruitment of glass eels in two valleys of French Polynesia (Tahiti and Moorea Islands) Cybium. 2018;42:341–348. [Google Scholar]
- Hénin C, du Penhoat Y, Ioualalen M. Observations of seasurface salinity in the western Pacific fresh pool: large-scale changes in 1992–1995. J Geophys Res. 1998;103:7523–7536. [Google Scholar]
- Hewavitharane CA, Pickering TD, Ciro R, Mochioka N. Species composition, abundance and seasonal recruitment patterns of freshwater eels (Anguilla spp) to Viti Levu, Fiji Islands, in the western South Pacific. Mar Freshw Res. 2018;69:1704–1711. [Google Scholar]
- Hewavitharane CA, Pickering TD, Ciro R, Mochioka N. Early life history of tropical freshwater eels (Anguilla spp) recruiting to Viti Levu, Fiji Islands, in the western South. Pacific Mar Freshw Res. 2019 doi: 10.1071/MF19047. [DOI] [Google Scholar]
- Hu D, Wu L, Cai W, Gupta AS, Canachaud A, Qui B, Gordon AL, Lin X, Chen Z, Hu S, Wang G, et al. Pacific western boundary currents and their roles in climate. Nature. 2015;522:299–308. doi: 10.1038/nature14504. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Tsukamoto K, Nishida M. Genetic evidence for multiple geographic populations of the giant mottled eel Anguilla marmorata in the Pacific and Indian oceans. Ichthyol Res. 2004;51:343–353. [Google Scholar]
- Jacoby DMP, Casselman JM, Crook V, DeLucia M-B, Ahn H, Kaifu K, Kurwie T, Sasal P, Silfvergrip AMC, Smith KG, Uchida K, et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Global Ecol Conserv. 2015;4:321–333. [Google Scholar]
- Jellyman DJ. Review of the marine life history of Australasian temperate species of Anguilla. Am Fish Soc Symp. 1987;1:276–285. [Google Scholar]
- Jellyman DJ. The distribution and biology of the South Pacific species of Anguilla. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel Biology. Springer-Verlag; Tokyo: 2003. pp. 275–292. [Google Scholar]
- Jellyman DJ, Bowen M. Modelling larval migration routes and spawning areas of anguillid eels of New Zealand and Australia. Amer Fish Soc Symp. 2009;69:255–274. [Google Scholar]
- Jellyman DJ, Tsukamoto K. First use of archival transmitters to track migrating freshwater eels Anguilla dieffenbachii at sea. Mar Ecol Prog Ser. 2002;233:207–215. [Google Scholar]
- Jellyman DJ, Tsukamoto K. Vertical migrations may control maturation in migrating female Anguilla dieffenbachii. Mar Ecol Prog Ser. 2010;404:241–247. [Google Scholar]
- Jespersen P. Indo-Pacific leptocephalids of the genus Anguilla: systematic and biological studies. Dana Rep. 1942:22. [Google Scholar]
- Kessler WS, Taft BA. Dynamic heights and zonal geostrophic transports in the central tropical Pacific during 1979–1984. J Phys Oceanogr. 1987;17:97–122. [Google Scholar]
- Kimura S, Tsukamoto K. The salinity front in the North Equatorial Current: a landmark for the spawning migration of the Japanese eel (Anguilla japonica) related to the stock recruitment. Deep Sea Res II. 2006;53:315–325. [Google Scholar]
- Kleckner RC, McCleave JD. The northern limit of spawning by Atlantic eels (Anguilla spp) in the Sargasso Sea in relation to thermal fronts and surface water masses. J Mar Res. 1988;46:647–667. [Google Scholar]
- Koltermann KP, Gouretski VV, Jancke K. Hydrographic atlas of the World Ocean Circulation Experiment (WOCE) In: Sparrow M, Chapman P, Gould J, editors. Atlantic Ocean. Vol. 3 International WOCE Project Office; Southampton, UK: 2011. [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Wouthuyzen S, Arai T, Tsukamoto K. Contrasting patterns of growth and migration of tropical anguillid leptocephali in the western Pacific and Indonesian Seas. Mar Ecol Prog Ser. 2006;309:233–246. [Google Scholar]
- Kuroki M, Aoyama J, Wouthuyzen S, Sumardhiharga K, Miller MJ, Tsukamoto K. Age and growth of Anguilla bicolor bicolor leptocephali in the eastern Indian Ocean. J Fish Biol. 2007;70:538–550. [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Watanabe S, Shinoda A, Jellyman DJ, Feunteun E, Tsukamoto K. Distribution and early life history characteristics of anguillid leptocephali in the western South Pacific. Mar Freshw Res. 2008;59:1035–1047. [Google Scholar]
- Kuroki M, Aoyama J, Miller MJ, Yoshinaga T, Shinoda A, Hagihara S, Tsukamoto K. Sympatric spawning of Anguilla marmorata and Anguilla japonica in the western North Pacific Ocean. J Fish Biol. 2009;74:1853–1865. doi: 10.1111/j.1095-8649.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Miller MJ, Tsukamoto K. Diversity of early life history traits in freshwater eels and the evolution of their oceanic migrations. Can J Zool. 2014;92:749–770. [Google Scholar]
- Liénart C, Feunteun E, Miller MJ, Aoyama J, Mortillaro J-M, Hubas C, Kuroki M, Watanabe S, Dupuy C, Carpentier A, Otake T, et al. Geographic variation in stable isotopic and fatty acid composition of anguilliform leptocephali and particulate organic matter in the South Pacific. Mar Ecol Prog Ser. 2016;544:225–241. [Google Scholar]
- Marquet G, Galzin R. The eels of French Polynesia: taxonomy, distribution and biomass. La mer. 1991;29:8–17. [Google Scholar]
- Martinez E, Ganachaud A, Lefevre J, Maamaatuaiahutapu K. Central South Pacific thermocline water circulation from a high-resolution ocean model validated against satellite data: Seasonal variability and El Ninõ 1997–1998 influence. J Geophys Res. 2009;114 C05012. [Google Scholar]
- Marui M, Arai T, Miller MJ, Jellyman DJ, Tsukamoto K. Comparison of early life history between New Zealand temperate eels and Pacific tropical eels revealed by otolith microstructure and microchemistry. Mar Ecol Prog Ser. 2001;213:273–284. [Google Scholar]
- McCleave JD. Spawning areas of Atlantic eels. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel Biology. Springer-Verlag; Tokyo: 2003. pp. 141–156. [Google Scholar]
- McDowall RM, Jellyman DJ, Dijkstra LH. Arrival of an Australian anguillid eel in New Zealand: an example of transoceanic dispersal. Environ Biol Fish. 1998;51:1–6. [Google Scholar]
- Miller MJ, Tsukamoto K. Ocean Research Institute. The University of Tokyo; Tokyo: 2004. An Introduction to Leptocephali: Biology and Identification. [Google Scholar]
- Miller MJ, Tsukamoto K. The ecology of oceanic dispersal and survival of anguillid leptocephali. Can J Fish Aquat Sci. 2017;74:958–971. [Google Scholar]
- Miller MJ, Aoyama J, Mochioka N, Otake T, Castle PHJ, Minagawa G, Inagaki T, Tsukamoto K. Geographic variation in the assemblages of leptocephali in the western South Pacific. Deep-Sea Res I. 2006;53:776–794. [Google Scholar]
- Miller MJ, Chikaraishi Y, Ogawa NO, Yamada Y, Tsukamoto K, Ohkouchi N. A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol Lett. 2013;9 doi: 10.1098/rsbl.2012.0826. 20120826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Bonhommeau S, Munk P, Castonguay M, Hanel R, McCleave JD. A century of research on the larval distributions of the Atlantic eels: a reexamination of the data. Biol Rev. 2015;90:1035–1064. doi: 10.1111/brv.12144. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Westerberg H, Sparholt H, Wysujack K, Sørensen SR, Marohn L, Jacobsen MW, Freese M, Ayala DJ, Pohlmann JD, Svendsen JC, et al. Spawning by the European eel across 2000 km of the Sargasso Sea. Biol Lett. 2019;15 doi: 10.1098/rsbl.2018.0835. 20180835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Aoyama J, Tsukamoto K. Multiple population structure of the giant mottled eel Anguilla marmorata. Mol Ecol. 2008;17:3109–3122. doi: 10.1111/j.1365-294X.2008.03822.x. [DOI] [PubMed] [Google Scholar]
- Morris M, Roemmich D, Cornuelle B. Observations of variability in the South Pacific subtropical gyre. J Phys Oceanogr. 1996;26:2359–2360. [Google Scholar]
- Munk P, Hansen MM, Maes GE, Nielsen TG, Castonguay M, Riemann L, Sparholt H, Als TD, Aarestrup K, Andersen NG, Bachler M. Oceanic fronts in the Sargasso Sea control the early life and drift of Atlantic eels. Proc Roy Soc B. 2010;277:3593–3599. doi: 10.1098/rspb.2010.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pous S, Feunteun E, Ellien C. Investigation of tropical eel spawning area in the South-Western Indian Ocean: influence of the oceanic circulation. Prog Oceanogr. 2010;86:396–413. [Google Scholar]
- Qu T, Gao S, Fukumori I, Fine RA, Lindstrom EJ. Subduction of South Pacific waters. Geophys Res Lett. 2008;35 L02610. [Google Scholar]
- Qu T, Gao S, Fine RA. Subduction of South Pacific tropical water and its equatorward pathways as shown by simulated passive tracer. J Phys Oceanogr. 2013;43:1551–1565. [Google Scholar]
- Réveillac E, Robinet T, Rabenevanana M-W, Valade P, Feunteun E. Clues to the location of the spawning area and larval migration characteristics of Anguilla mossambica as inferred from otolith microstructural analyses. J Fish Biol. 2009;74:1866–1877. doi: 10.1111/j.1095-8649.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Ridgway KR, Dunn JR. Mesoscale structure of the mean East Australian Current System and its relationship with topography. Prog Oceanogr. 2003;56:189–222. [Google Scholar]
- Righton D, Aarestrup K, Jellyman D, Sébert P, van den Thillart G, Tsukamoto K. The Anguilla spp migration problem: 40 million years of evolution and two millennia of speculation. J Fish Biol. 2012;81:365–386. doi: 10.1111/j.1095-8649.2012.03373.x. [DOI] [PubMed] [Google Scholar]
- Robinet T, Lecomte-Finiger R, Escoubeyrou K, Feunteun E. Tropical eels Anguilla spp recruiting to Réunion Island in the Indian Ocean: taxonomy, patterns of recruitment and early life histories. Mar Ecol Prog Ser. 2003;259:263–272. [Google Scholar]
- Robinet T, Réveillac E, Kuroki M, Aoyama J, Tsukamoto K, Rabenevanana MW, Valade P, Gagnaire PA, Feunteun E. New clues for freshwater eels (Anguilla spp) migration routes to eastern Madagascar and surrounding islands. Mar Biol. 2008;154:453–463. [Google Scholar]
- Roden G. Upper ocean thermohaline, oxygen, nutrient, and flow structure near the date line in the summer of 1993. J Geophys Res. 1998;103:12919–12939. [Google Scholar]
- Rougerie F, Rancher J. The Polynesian south ocean: features and circulation. Mar Poll Bull. 1994;29:14–25. [Google Scholar]
- Schabetsberger R, Økland F, Aarestrup K, Sichrowsky U, Kalfatak D, Tambets M, Dall’Olmo G, Kaiser R, Miller P. Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 2013;475:177–190. [Google Scholar]
- Schabetsberger R, Miller MJ, Dall’Olmo G, Kaiser R, Økland F, Watanabe S, Aarestrup K, Tsukamoto K. Hydrographic features of anguillid spawning areas: potential signposts for migrating eels. Mar Ecol Prog Ser. 2016;554:141–155. doi: 10.3354/meps11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabetsberger R, Scheck A, Kaiser R, Leaana R, Gubili C, Økland F. Oceanic migration behaviour of Pacific eels from Samoa. Fish Manag Ecol. 2019;26:53–56. doi: 10.1111/fme.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. The breeding places of the eel. Phil Trans R Soc Lond Ser B. 1922;211:179–208. [Google Scholar]
- Schmidt J. The fresh-water eels of Australia, with some remarks on the short-finned species of Anguilla. Rec Aust Mus. 1928;16:179–210. [Google Scholar]
- Schmidt J. Carlsberg Foundation. Copenhagen: 1935. Danish eel investigations during 25 years, 1905–1930; p. 16. [Google Scholar]
- Shen KN, Tzeng WN. Genetic differentiation among populations of the short-finned eel Anguilla australis from East Australia and New Zealand. J Fish Biol. 2007;70:177–190. [Google Scholar]
- Shiao JC, Tzeng WN, Collins A, Jellyman DJ. Dispersal pattern of the glass eel stage of Anguilla australis revealed by otolith growth increments. Mar Ecol Prog Ser. 2001;219:241–250. [Google Scholar]
- Shiao JC, Tzeng WN, Collins A, Iizuka Y. Role of marine larval duration and growth rate of glass eels in determining the distribution of Anguilla reinhardtii and A australis on Australian eastern coasts. Mar Freshw Res. 2002;53:687–695. [Google Scholar]
- Shinoda A, Aoyama J, Miller MJ, Otake T, Mochioka N, Watanabe S, Minegishi Y, Kuroki M, Yoshinaga T, Yokouchi K, Fukuda N, et al. Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev Fish Biol Fisher. 2011;21:591–611. [Google Scholar]
- Shirotori F, Ishikawa T, Tanaka C, Aoyama J, Shinoda A, Yambot AV, Yoshinaga T. Species composition of anguillid glass eels recruited at southern Mindanao Island, the Philippines. Fish Sci. 2016;82:915–922. [Google Scholar]
- Sloane RD. Invasion and upstream migration by glass-eels of Anguilla australis australis Richardson and A reinhardtii Steindachner in Tasmanian freshwater streams. Aus J Mar Freshw Res. 1984;35:47–59. [Google Scholar]
- Sugeha HY, Arai T, Miller MJ, Limbong D, Tsukamoto K. Inshore migration of the tropical eels, Anguilla spp, recruiting to the Poigar River estuary on Sulawesi Island. Mar Ecol Prog Ser. 2001;221:233–243. [Google Scholar]
- Tilburg CE, Hurlburt HE, O’Brien JJ, Shriver JF. The dynamics of the East Australian Current System: The Tasman front, the East Auckland Current, and the East Cape Current. J Phys Oceanogr. 2001;31:2917–2943. [Google Scholar]
- Tsukamoto K. Discovery of the spawning area for Japanese eel. Nature. 1992;356:789–791. [Google Scholar]
- Tsukamoto K, Lee T-W, Fricke H. Spawning areas of the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel Biology. Springer-Verlag; Tokyo: 2003. pp. 121–140. [Google Scholar]
- Tsukamoto K, Aoyama J, Miller MJ. The present status of the Japanese eel: resources and recent research. In: Casselman J, Cairns D, editors. Eels at the edge Am Fish Soc Symp. Vol. 58. Bethesda, Maryland: 2009. pp. 21–35. [Google Scholar]
- Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun. 2011;2 doi: 10.1038/ncomms1174. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. Taxonomy of the freshwater eels, Genus Anguilla Schrank, 1798. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel Biology. Springer-Verlag; Tokyo: 2003. pp. 3–18. [Google Scholar]
- Watanabe S, Aoyama J, Tsukamoto K. Reconfirmation of morphological differences between A australis australis Richardson and A australis schmidtii Phillipps. NZ J Mar Freshw Res. 2006;40:325–331. [Google Scholar]
- Watanabe S, Aoyama J, Miller MJ, Ishikawa S, Feunteun E, Tsukamoto K. Evidence of population structure in the giant mottled eel, Anguilla marmorata, using total number of vertebrae. Copeia. 2008;2008:680–688. [Google Scholar]
- Watanabe S, Aoyama J, Tsukamoto K. A new species of freshwater eel Anguilla luzonensis (Teleostei: Anguillidae) from Luzon Island of the Philippines. Fish Sci. 2009;75:387–392. [Google Scholar]
- Watanabe S, Miller MJ, Aoyama J, Tsukamoto K. Analysis of vertebral counts of the tropical anguillids, Anguilla megastoma, A obscura, and A reinhardtii, in the western South Pacific in relation to their possible population structure. Environ Biol Fish. 2011;91:353–360. [Google Scholar]
- Yamamoto T, Mochioka N, Nakazono A. Seasonal occurrence of anguillid glass eels at Yakushima Island, Japan. Fish Sci. 2001;67:530–532. [Google Scholar]
- Zhang L, Qu T. Low-frequency variability of South Pacific Tropical Water from Argo. Geophys Res Lett. 2014;41:2441–2446. [Google Scholar]