Summary
Mutations in keratin genes underlie a variety of epidermal and non-epidermal cell-fragility disorders and are the genetic basis of many inherited palmoplantar keratodermas. Epidermolytic PPK (EPPK) is an autosomal dominant disorder that can be due to mutations in the keratin 1 gene (KRT1). Epidermolytic ichthyosis (EI), the major keratinopathic ichthyosis, is characterized by congenital erythroderma, blistering and erosions of the skin. Causative mutations in KRT1 and KRT10 have been described, with PPK being present primarily in association with the former. We report four unrelated cases - one with sporadic EI and three with autosomal dominant PPK, due to two novel and two recurrent KRT1 mutations. Mutations in KRT1 are not only scattered throughout keratin 1, as opposed to being clustered, but can result in a range of phenotypes as further confirmed by these mutations, giving a complex genotype/phenotype pattern.
Keywords: epidermolytic ichthyosis, palmoplantar keratoderma, keratin 1, autosomal dominant
Keratins constitute the intermediate filament proteins of keratinocytes, with the keratin cytoskeleton acting as an important structural stabilizer of epithelial cells.1 Mutations in keratin genes underlie a variety of epidermal and non-epidermal cell-fragility disorders and are the genetic basis of a number of the inherited palmoplantar keratodermas (PPKs).2 Epidermolytic PPK (EPPK; Vorner type; OMIM 144200) is an autosomal dominant disorder that can be caused by dominant-negative mutations in the keratin 1 gene (KRT1).3 Epidermolytic ichthyosis (EI; EHK; BCIE; OMIM 113800), the major keratinopathic ichthyosis, is characterized by congenital erythroderma, blistering and erosions of the skin.4 Causative mutations in KRT1 and KRT10 have been described, with PPK being present primarily in association with the former.
We report four unrelated cases with KRT1 mutations - one with sporadic EI and three with autosomal dominant PPK, due to two previously unreported and two recurrent KRT1 mutations.
Report
Genetic testing was performed with informed consent and ethical approval by an Institutional Review Board that complies with principles of the Helsinki Accord. Genomic DNA was extracted from peripheral blood leukocytes or from saliva collected in an Oragene DNA sample collection kit (DNA Genotek, Ontario, Canada). PCR and Sanger sequencing was performed to screen the coding regions and exon/intron boundaries of KRT1 (primer sequences available on request). Variants were confirmed as pathogenic by reference to the in silico prediction tool, Mutation Taster and by sequencing other family members when available. None of the variants were present on the database of Single Nucleotide Polymorphisms (dbSNP), 1000 Genome Project, NHLBI Exome Variant Server, Exome Aggregation Consortium (ExAC) or the Genome Aggregation Database (gnomAD).
Cases
Families 1 and 4 presented to their local dermatology services. Families 2 and 3 were identified through the International Pachyonychia Congenita Research Registry. All reported PPK since early childhood with a positive family history in cases 2, 3 and 4. No affected individuals had a history of collodion membrane, hair, teeth or cardiac abnormalities.
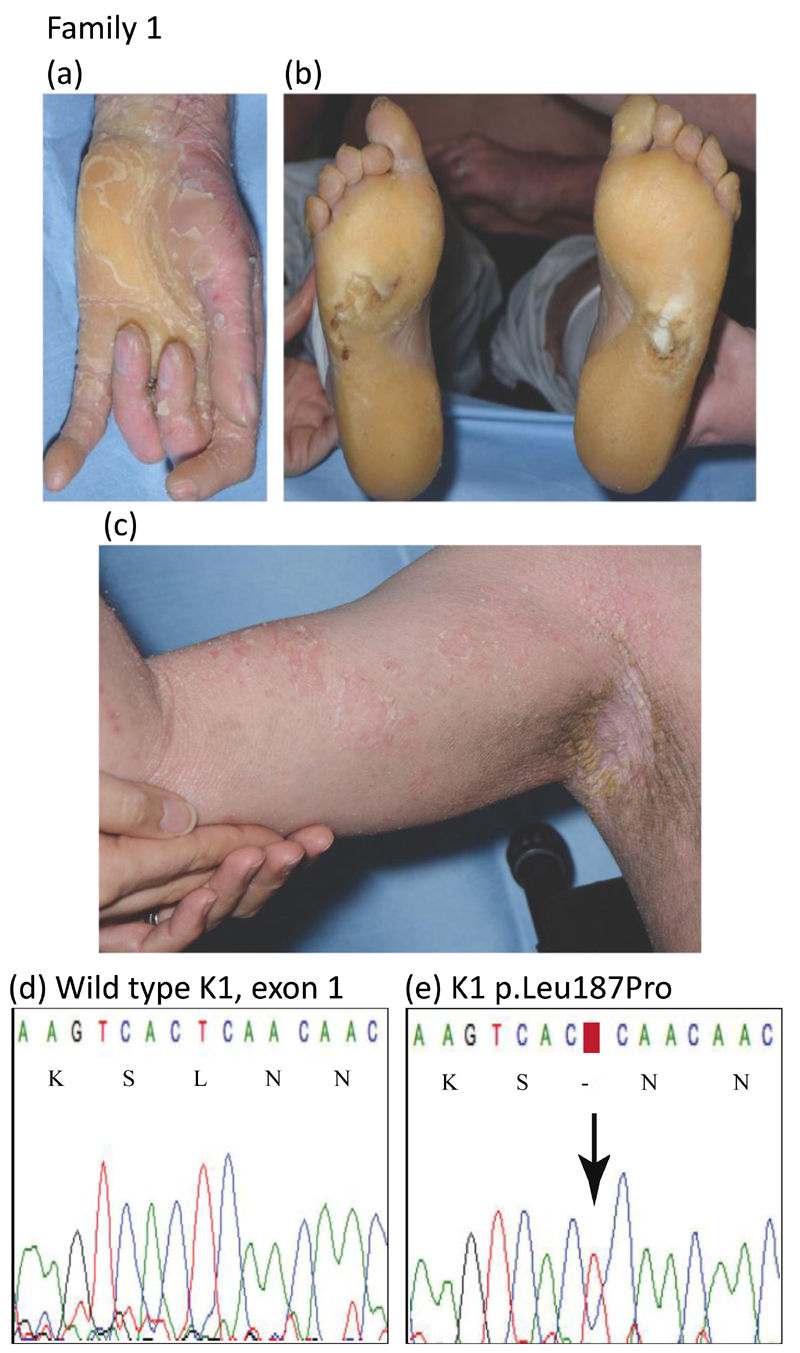
In family 1, the 23-year-old wheelchair-bound proband presented as an adult with diffuse hyperkeratosis on his trunk and limbs in addition to acral sites, having failed to seek medical attention for many years. Skin fragility and blistering were reported at sites of trauma, with a history of blistering, scaling and erythroderma being present from birth. Severe flexion contractures of the fingers and toes were evident, with surgical correction considered limited in terms of benefit (Fig.1a-c). Osteopenia, chronic pain, and the psychological impact of his debilitating skin disease resulting in social isolation and childhood bullying necessitated a multidisciplinary management approach. There was no known family history, although the proband was estranged from most close relatives so further clinical and genetic information was unobtainable. Mutation analysis of the proband in family 1 identified a previously unreported heterozygous missense mutation in exon 1 of the keratin 1, p.Leu187Pro; c.560T>C; (Figs. 1d,e and 4). DNA was not available from any other family members. This mutation is within the 1A domain of keratin 1 and another pathogenic variant has previously been reported at the same codon, p.Leu187Phe in two cases with typical EI (Table 1, www.interfil.org).5
Figure 1.
Clinical features and mutation analysis of family 1
(a) hyperkeratosis of the proband’s digits and palm
(b) diffuse plantar hyperkeratosis
(c) warty flexural hyperkeratosis
(d) wild-type KRT1 sequence of exon 1 (nucleotides 553-567)
e) the equivalent region of KRT1 from the proband of family 1. The arrow indicates a heterozygous mutation c.560T>C resulting in an amino acid substitution of leucine to proline, p.Leu187Pro, in the keratin 1 protein
Figure 4.
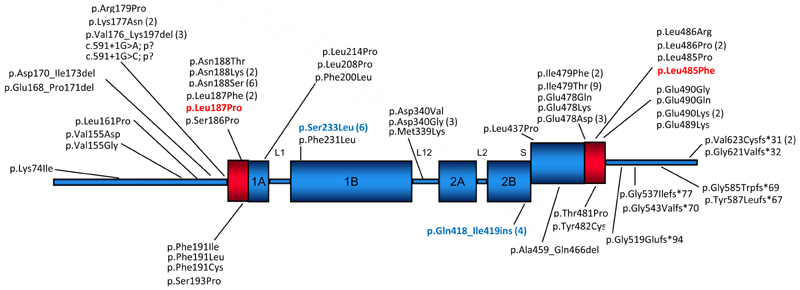
Schematic diagram showing the protein domain structure of keratin 1 and previously reported mutations. Those from this study are included; novel mutations are shown in red and recurrent ones in blue.
Table 1.
Molecular and clinical details of cases in this study (novel and recurrent, in bold) compared with previously reported cases.
| Family | Mutation - protein change | DNA change | Exon | Keratin domain | Clinical description | Reference |
|---|---|---|---|---|---|---|
| Family 1 | p.Leu187Pro | c.560T>C | 1 | 1A | EI, spontaneous case, with a history of blistering, scaling and erythema since birth. As an adult, diffuse hyperkeratosis on trunk, limbs and acral sites. | this study (novel mutation) |
| p.Leu187Phe | c.559C>T | 1 | 1A | EI, spontaneous case with erythroderma, erosions, and blisters on the entire body surface at birth and palmoplantar and flexural areas of hyperkeratosis in the later stage. | Ref 5 www.interfil.org | |
| p.Leu187Phe | c.559C>T | 1 | 1A | EI, spontaneous case, generalized hyperkeratosis involving palms and soles. | Ref 5 www.interfil.org | |
| Family 2 | p.Leu485Phe | c.1453C>T | 7 | 2B | PPK - familial case, diffuse PPK with sharp demarcation developed in early childhood. | this study (novel mutation) |
| p.Leu485Pro | c.1454T>C | 7 | 2B | Generalised severe EI including PPK (mother, EI naevus). | Ref 6 | |
| Family 3 | p.Ser233Leu | c.698C>T | 2 | 1B | PPK - familial case with diffuse well-demarcated plantar hyperkeratosis with slightly erythematous borders since infancy. Fissuring hyperkeratosis of the palms and fingertips. | this study (recurrent mutation) |
| p.Ser233Leu | c.698C>T | 2 | 1B | EPPK (Vörner type), familial case with diffuse PPK with a well-circumscribed erythematous margin. No hyperkeratosis affecting other body sites. | Ref 5 www.interfil.org | |
| p.Ser233Leu | c.698C>T | 2 | 1B | EPPK (Vörner type), familial case with diffuse PPK with a well-circumscribed erythematous margin. No hyperkeratosis affecting other body sites. | Ref 5 www.interfil.org | |
| p.Ser233Leu | c.698C>T | 2 | 1B | EPPK Vörner - familial case with diffuse yellowish hyperkeratosis on palms & soles developed shortly after birth, with a clear border & demarcated by an erythematous rim. | Ref 5 www.interfil.org | |
| p.Ser233Leu | c.698C>T | 2 | 1B | EPPK Vörner - familial case with diffuse yellowish hyperkeratosis on palms & soles developed shortly after birth, with a clear border & demarcated by an erythematous rim. | Ref 5 www.interfil.org | |
| p.Ser233Leu | c.698C>T | 2 | 1B | EPPK (Vörner type), developed shortly after birth. | Ref 8 | |
| Family 4 | p.Gln418_Ile419ins18 | c.1254+1G>A | 6 | 2B | PPK - familial case with diffuse palmoplantar hyperkeratosis that developed shortly after birth. | this study (recurrent mutation) |
| p.Gln418 Ile419ins18 | c.1254+1G>A | 6 | 2B | EPPK - familial case, hyperkeratosis restricted to the palms and soles developed in childhood. Varying severity between family members from patchy plantar keratoderma to confluent plantar keratoderma. A violaceous erythematous border was observed at the edge of the keratoderma in severe cases. | Ref 3 | |
| p.Gln418 Ile419ins18 | c.1254+1G>A | 6 | 2B | EPPK - familial case, hyperkeratosis restricted to the palms and soles developed in childhood. Varying severity between family members from patchy plantar keratoderma to confluent plantar keratoderma. A violaceous erythematous border was observed at the edge of the keratoderma in severe cases. | Ref 3 | |
| p.Gln418 Ile419ins18 | c.1254+1G>A | 6 | 2B | EPPK - familial case, hyperkeratosis restricted to the palms and soles developed in childhood. Varying severity between family members from patchy plantar keratoderma to confluent plantar keratoderma. A violaceous erythematous border was observed at the edge of the keratoderma in severe cases. | Ref 3 |
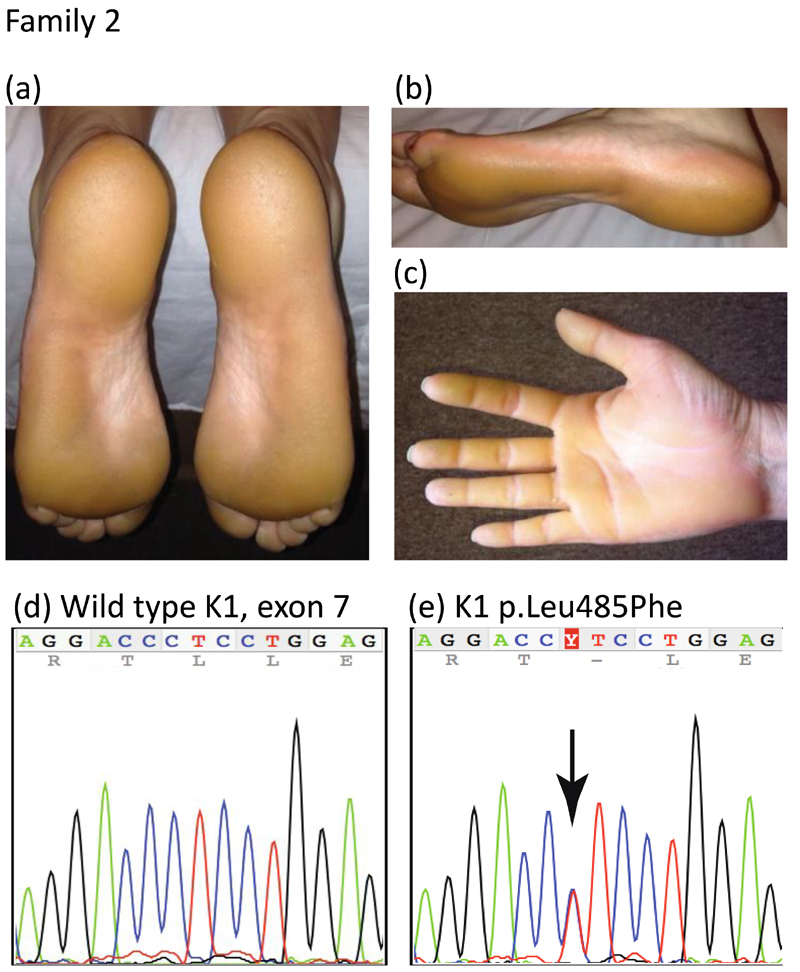
The 39-year-old female proband of family 2 presented with a history of diffuse keratoderma of bilateral palms and plantar feet with sharp demarcation and yellow hue since early childhood, with milder callosities and fissuring affecting the palms and fingertips (Fig. 2a-c). Her older brother, mother and maternal grandmother all had similar findings. A previously unreported mutation, p.Leu485Phe; c.1453C>T (Figs. 2d,e and 4) was identified in exon 7 of KRT1 in the proband of family 2. The mutation was present in two other affected family members, her brother and mother. Another pathogenic missense mutation has previously been reported at this position in the 2B domain of keratin 1, p.Leu485Pro; c.1454T>C in a case with PPK (Table 1).6
Figure 2.
Clinical features and mutation analysis of family 2
(a, b) diffuse plantar (c) palmar keratoderma of the proband
(d) mutation analysis showing DNA sequence (nucleotides 1447-1461) of exon 7 of KRT1 in an unaffected control compared to (e) the same region of KRT1 in the proband of family 2. The arrow indicates a heterozygous mutation c.1453C>T leading to missense mutation p.Leu485Phe.
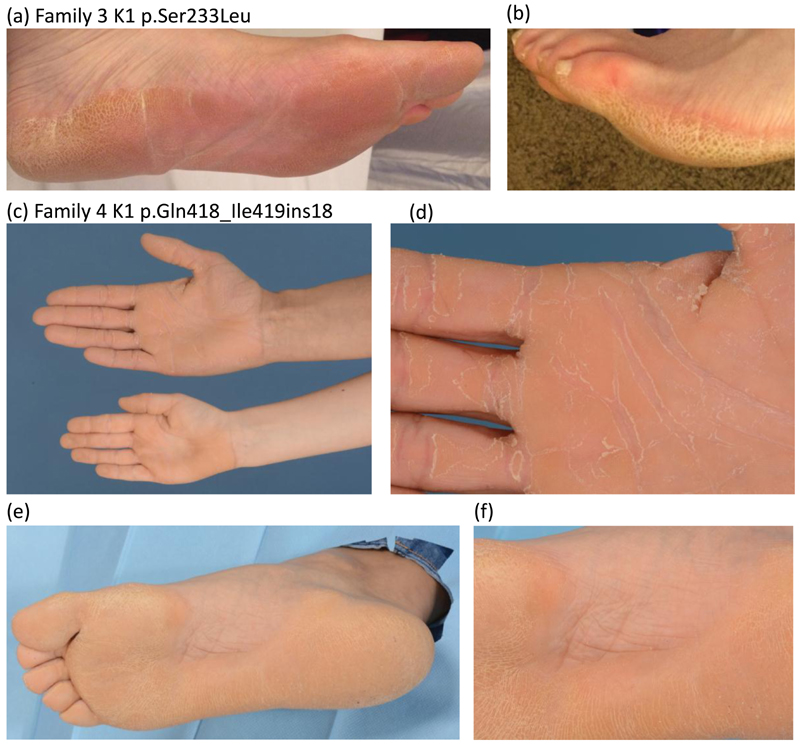
In Family 3, the 43-year-old female proband had diffuse well-demarcated plantar hyperkeratosis with slightly erythematous borders since infancy (Fig. 3 a,b). Fissuring hyperkeratosis of the palms and fingertips was present but no nail dystrophy, transgrediens or blistering. Her father, paternal grandmother, paternal great-grandmother, and paternal great-aunt all had similar symptoms. The proband of family 3 was found to have a mutation in the 1B domain of KRT1, p.Ser233Leu; c.698C>T. This has been previously reported in several cases with EPPK (Table 1, Fig 4, www.interfil.org).5
Figure 3.
Clinical features of families 3 and 4
(a and b) diffuse well-demarcated plantar hyperkeratosis with slightly erythematous border of the proband of family 3, with mutation K1 p.Ser233Leu.
(c) diffuse hyperkeratosis of palms of proband and daughter from family 4, with mutation K1 p.Gln418_Ile419ins18.
(d) peeling of skin on the palm associated with oral acitretin therapy;
(e, f) diffuse hyperkeratosis of the soles with some sparing of the arch of proband of family 4.
The 32-year-old proband in family 4 had diffuse plantar and palmar hyperkeratosis which peeled significantly once acitretin 20mg od was introduced (Fig. 3 c-f). Her grandmother, mother, son and daughter were similarly affected. Analysis of the proband in family 4 identified recurrent pathogenic mutation c.1254+1G>A causing a splice site mutation in exon 6, leading to utilization of a novel in-frame splice site 54 bases downstream with subsequent insertion of 18 amino acids into the 2B rod domain, p.Gln418_Ile419ins18, previously reported in association with EPPK (Table 1, Fig 4).3
Keratin intermediate filaments are characterized by tissue-specific expression patterns, with the cell-type and tissue-specific expression profile of the mutated keratin dictating the cell type and body site that is affected in the disorder.2 KRT1 and KRT10 confer structural integrity to suprabasal keratinocytes and mutations impair the assembly of the keratin cytoskeleton. In some keratin disorders such as pachyonychia congenita, keratin mutations are predominantly clustered to the helix boundary domains at either end of the rod domain. However, the 84 cases previously reported with mutations in KRT1 (www.interfil.org) show that mutations in KRT1 are not only scattered throughout keratin 1 as shown in Fig. 4 but can result in a range of phenotypes6 as further confirmed by these mutations, giving a complex genotype/phenotype pattern. A wide range in phenotypic severity is also reported in association with KRT1 mutations particularly in EI7,8. Mutations in the keratin 9 gene (KRT9) also cause epidermolytic PPK (EPPK). With the development of new gene and mutation-specific therapies for inherited disorders of keratinization, it is becoming increasingly important to accurately document both genotype and phenotype. Our cases add to the mutational and clinical spectrum and demonstrate wide phenotypic variation in KRT1 mutations, despite their unifying molecular basis.
Learning Points.
Mutations in keratin genes underlie a variety of epidermal and non-epidermal cell-fragility disorders and are the genetic basis of a number of the inherited palmoplantar keratodermas.
Epidermolytic PPK (OMIM 144200) is an autosomal dominant disorder usually caused by mutations in the keratin 9 gene (KRT9) but mutations in the keratin 1 gene (KRT1) have been reported in association with this phenotype.
Epidermolytic ichthyosis (OMIM 113800), the major keratinopathic ichthyosis, is characterized by congenital erythroderma, blistering and erosions of the skin.
Causative mutations in KRT1 and KRT10 have been described in epidermolytic ichthyosis, with PPK being present primarily in association with the former.
Mutations in KRT1 are not only scattered throughout keratin 1 but can result in a range of phenotypes as further confirmed by these mutations, giving a complex genotype/phenotype pattern.
This report adds to the mutational and clinical spectrum, demonstrating wide phenotypic variation in KRT1 mutations, despite their unifying molecular basis.
Acknowledgements
We would like to thank the patients and their family members for their cooperation in this study. FJDS, NJW and MZ were supported by The Centre for Dermatology and Genetic Medicine at the University of Dundee, which is funded by a Wellcome Trust Strategic Award (098439/Z/12/Z to WHIMcL), and FJDS and NJW were supported by a grant from the Pachyonychia Congenita Project (to F.J.D.S, www.pachyonychia.org).
Footnotes
Conflict of interest:
None
References
- 1.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumours and cultures cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 2.Corden LD, McLean WHI. Human keratin diseases: Hereditary fragility of specific epithelial tissues. Exp Dermatol. 1996;5:297–307. doi: 10.1111/j.1600-0625.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatsell SJ, Eady RA, Wennerstrand L, et al. Novel splice site mutation in keratin 1 underlies mild epidermolytic palmoplantar keratoderma in three kindreds. J Invest Dermatol. 2001;116:606–609. doi: 10.1046/j.1523-1747.2001.13041234.x. [DOI] [PubMed] [Google Scholar]
- 4.Oji V, Tadini G, Akiyama M, et al. Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Sorèze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Szeverenyi I, Cassidy AJ, Chung CW, et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–60. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 6.Arin MJ, Oji V, Emmert S, et al. Expanding the keratin mutation database: novel and recurrent mutations and genotype-phenotype correlations in 28 patients with epidermolytic ichthyosis. Br J Dermatol. 2011;164:442–7. doi: 10.1111/j.1365-2133.2010.10096.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolling MC, Bladergroen, van Steensel MAM, et al. A novel mutation in the L12 domain of keratin 1 is associated with mild epidermolytic ichthyosis. Br J Dermatol. 2010;162:875–879. doi: 10.1111/j.1365-2133.2009.09617.x. [DOI] [PubMed] [Google Scholar]
- 8.Holtz A, Oji V, Bourrat E, et al. Expanding the clinical and genetic spectrum of KRT1, KRT2 and KRT10 mutations in keratinopathic ichthyosis. Acta Derm Venereol. 2016;96:473–478. doi: 10.2340/00015555-2299. [DOI] [PubMed] [Google Scholar]