Abstract
Overcoming drug-resistance remains a key challenge to cure patients with acute and chronic B cell malignancies. Here we describe a stroma cell autonomous signaling pathway, which contributes to drug resistance of malignant B cells. We show that protein kinase C (PKC)-β-dependent signals from bone marrow derived stroma cells markedly decrease the efficacy of cytotoxic therapies. Conversely, small molecule PKC-β inhibitors antagonize pro-survival signals from stroma cells and sensitize tumor cells to targeted and non-targeted chemotherapy, leading to enhanced cytotoxicity and prolonged survival in vivo. Mechanistically, stromal PKC-β controls the expression of adhesion- and matrix proteins, required for Phosphoinositide 3-kinases (PI3K) activation and the ERK-mediated stabilization of BCL-XL in tumor cells. Central to the stroma-mediated drug resistance is the PKC-β dependent activation of transcription factor EB (TFEB), regulating lysosome biogenesis and plasma membrane integrity. Stroma directed therapies, enabled by direct inhibition of PKC-β, enhance the effectiveness of many anti-leukemic therapies.
Introduction
Over the past decade next generation sequencing technologies provided opportunities to comprehensively describe the spectrum of genomic abnormalities found in various different B cell malignancies(1–4). Ultimately, this has improved our understanding of the underlying genetic mutations contributing to uncontrolled proliferation and extended cell survival while enabling the development of targeted therapies. Notably, drug resistance remains one of the most challenging clinical problems, reflected by the invariable disease recurrence of all low-grade lymphoma-, chronic lymphocytic leukemia (CLL) patients and a substantial fraction of patients suffering from acute lymphoblastic leukemia and high-grade lymphoma. Thus, although a majority of patients achieve disease remissions, relapses occur from cells surviving cancer-therapies. Identifying and targeting cells, which have acquired properties to survive these treatments may ultimately allow to fully eradicate these tumor cells and to achieve cure.
It has been recognized for some time that the tumor microenvironment plays a pivotal role for drug resistance by providing protective niches for tumor cells(5), allowing them to survive targeted and non-targeted therapies through the provision of anti-apoptotic signals. The bone marrow microenvironment appears to be of crucial importance for lymphoid diseases as minimal-residual disease detected in this compartment has a strong prognostic power to predict disease relapse in acute and chronic leukemia and lymphomas(6, 7). Several intermediates of the bone marrow microenvironment to tumor cell communications have been identified, but their targeting has been proven to be difficult and to result in minor effects at best (8),(9).
Here we describe a stroma cell autonomous signaling pathway, dependent on the expression and activation of protein kinase C-β (PKC-β) and subsequent activation of the transcription factor EB, both being of key importance to protect malignant B cells from cytotoxic therapies. Importantly, the dependency of malignant B cells on PKC-β-activity in the microenvironment can be therapeutically exploited with small molecule inhibitors, to treat patients with different B cell malignancies, including CLL, mantle cell lymphoma (MCL) and B cell acute lymphoblastic leukemia (B-ALL).
Results
Stroma PKC-β is essential for survival, but not homing or proliferation of TCL1-tg B cell tumors
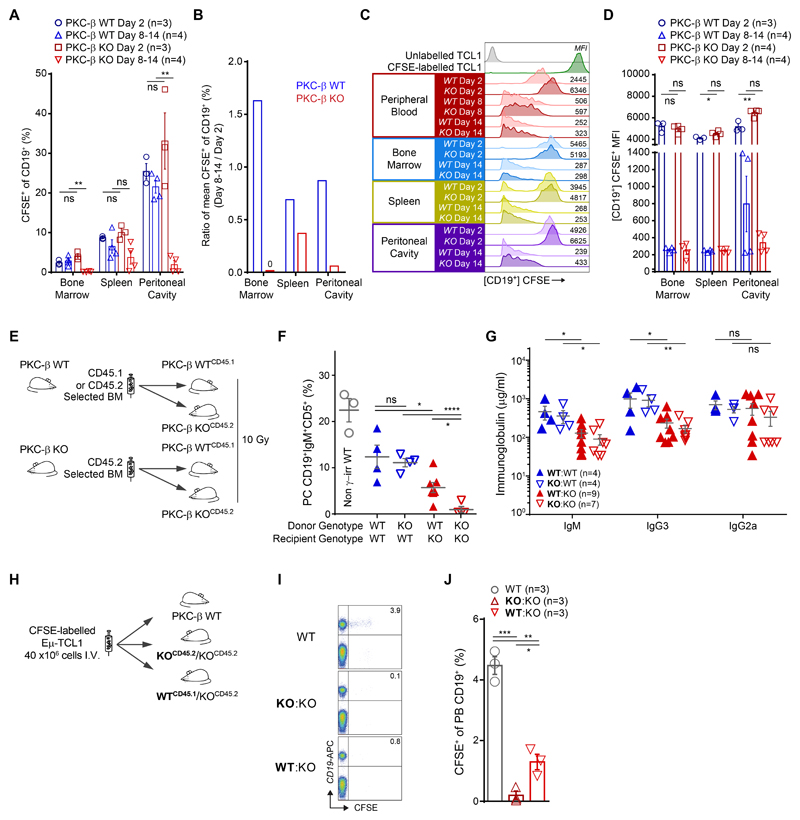
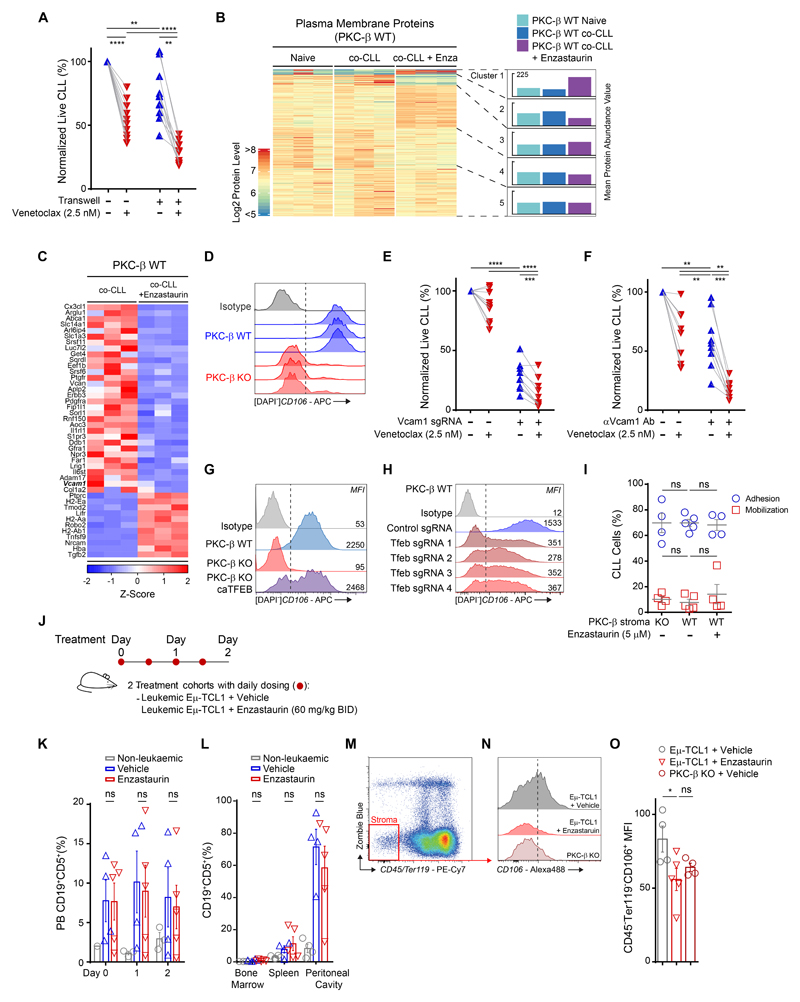
Our previous data demonstrated that PKC-β activity in mesenchymal stroma cells (MSC) is essential for the engraftment of malignant B cells derived from Eμ-TCL1-tg mice(10), a model resembling human CLL(11). To further investigate the mechanisms underlying this phenotype, our experiments set out to characterize the role of stromal PKC-β for tumor cell homing, proliferation and survival. Carboxyfluorescein-succinimidyl-ester(CFSE)- labeled CD5+CD19+ tumor cells from diseased TCL1-tg mice were transplanted intravenously (i.v.) and intraperitoneal (i.p.) into either PKC-β knock out (KO) or wild-type (WT) recipient mice. In addition, malignant cells were transplanted into WT mice, which were pre-treated for 48 hours with the PKC-β-inhibitor enzastaurin (60 mg/kg BID) or vehicle control and continued to receive treatment for 48 hours post-transplantation (fig. S1A). Tumor cells homed to the spleen and bone marrow of all recipient mice, irrespectively of PKC-β expression in the microenvironment or its pharmacological inhibition (fig. S1, B and C). A separate cohort of PKC-β KO and WT recipient mice was followed for 2 weeks and tumor engraftment was assessed in the peripheral blood, lymphatic tissues and the peritoneal cavity. A similar number of CFSE-labeled tumor cells were detectable in the peripheral blood of KO and WT mice on day 2. However, from day 8 onwards, we observed a marked increase of tumor cells in the peripheral blood of WT, but not in KO mice (fig. S1D). Two weeks after transplantation of tumor cells, malignant B cells were virtually absent in the bone marrow of KO mice and decreased in the spleen and peritoneal cavity of KO mice, in contrast to WT control mice where tumor cells were maintained (Fig. 1A and fig. S1E). During the first 2 weeks, disease progression was more pronounced in the bone marrow, which showed a stronger dependency on PKC-β compared to spleen or peritoneal cavity (Fig. 1B). The continuous decay of the CFSE-label with each cell division did not differ in KO and WT recipient mice (Fig. 1, C and D), indicating that PKC-β expression in the tumor microenvironment is dispensable for tumor cell homing or proliferation but required to provide pro-survival factors, essential for engraftment and disease progression.
Figure 1.
PKC-β expression in stroma cells is essential for tumor cell engraftment and normal B1 cell development
We were then interested in investigating whether the lack of tumor cell engraftment in PKC-β KO mice was entirely attributed to its absence in stroma cells or whether hematopoietic cells in the microenvironment also contributed. Germ-line deletion of PKC-β in mice causes immunodeficiency with a marked reduction of peritoneal B1 cells and a reduction in serum IgM and IgG3(12). Notably, no differences of white blood cells (WBC), hemoglobin or platelets were observed between WT and KO cells (fig. S2, A and B), also reflected by the presence of similar numbers of Lin-Sca1+C-Kit+ (LSK) and CD45+EPCR+CD150+CD48-(ESLAM) hematopoietic stem cells in the bone marrow (fig. S2C). In addition, the development of normal B cells was not affected by the germ-line deletion of PKC-β, with B-cell progenitor fractions (Hardy fractions A-D(13)) statistically similar between genotypes for both the frequency and absolute number of cells present per femur (fig. S2, D to F).
By generating mixed chimeras, differing only in the expression of PKC-β in the hematopoietic system, we could address whether the engraftment-dependence on microenvironment PKC-β signals is due to the malignant transformation or reflects properties of the cell-of-origin. The cell-of-origin is thought to be a CD5+ B cell(14), in mouse most likely a CD5+ B1 cell, an innate type of B cell responsible for the production of natural antibodies. We generated PKC-β chimeric mice by transplanting PKC-β WT CD45+ hematopoietic bone marrow cells into lethally irradiated (10 Gy) KO animals. To allow for the assessment of chimerism WT CD45.1+ bone marrow cells were transplanted into CD45.2+ KO recipient mice. As controls, KO CD45.2+ BM cells were transplanted into CD45.1+ WT recipient mice (Fig. 1E and fig. S3A). Strikingly, in BM-reconstituted WT recipient animals we found no difference in the number of peritoneal B1 cells derived from either PKC-β KO or WT donor cells. Conversely, the development of peritoneal B1 cells in KO recipient animals transplanted from WT bone marrow was significantly (p=0.02) reduced compared to WT recipient animals. Notably, the number of peritoneal B1 cells was still higher in these mice than in PKC-β KO control recipients reconstituted with KO bone marrow (Fig. 1F and fig. S3B). Analogous to the germ-line deletion of PKC-β, reconstitution of WT bone marrow in KO recipients was associated with reduced serum titers of the natural antibodies IgM and IgG3, whereas KO cells produced equivalent amounts of antibodies as WT cells in a WT background (Fig. 1G). These data demonstrate that PKC-β is an important cell-extrinsic factor for B cell development. To assess whether the differences in serum IgM and IgG3 titers were further associated with a PKC-β-dependent skewing of plasma cell differentiation, we compared chimerism of donor CD45+, CD138+ to donor CD45+, CD138- cells in the bone marrow and spleen. We found similar engraftment of either genotype in the bone marrow of PKC-β WT and KO recipient animals with no differences between CD138+ and CD138- cells (fig. S3, D and E). PKC-β WT donor cells also differentiated equally well in the spleens of either WT or KO recipient animals, whereas PKC-β KO donor cells gave rise to fewer splenic CD45+ CD138+ cells in a WT background (fig. S3, D and F).
To address whether PKC-β expressed in hematopoietic cells could rescue tumor cell survival in PKC-β KO recipient animals, we injected TCL1-tumor cells into KO animals, previously transplanted with KO or WT CD45+ bone marrow cells (Fig. 1H). All recipient chimeric mice were deficient for PKC-β in non-hematopoietic cells, but either did (WT→KO) or did not (KO→KO) contain PKC-β-expressing hematopoietic cells. Similar to our transplantation studies into non-chimeric mice, engraftment of tumor cells in the peripheral blood of WT→KO animals compared to WT control mice was significantly (p=0.001) impaired (Fig. 1I and J). On the other hand, WT→KO recipients contained only slightly more malignant cells than KO→KO recipients, indicating that PKC-β-mediated survival signals by non-hematopoietic cells play a predominant role in tumor maintenance.
Inhibition of stromal PKC-β mitigates environment-mediated drug resistance
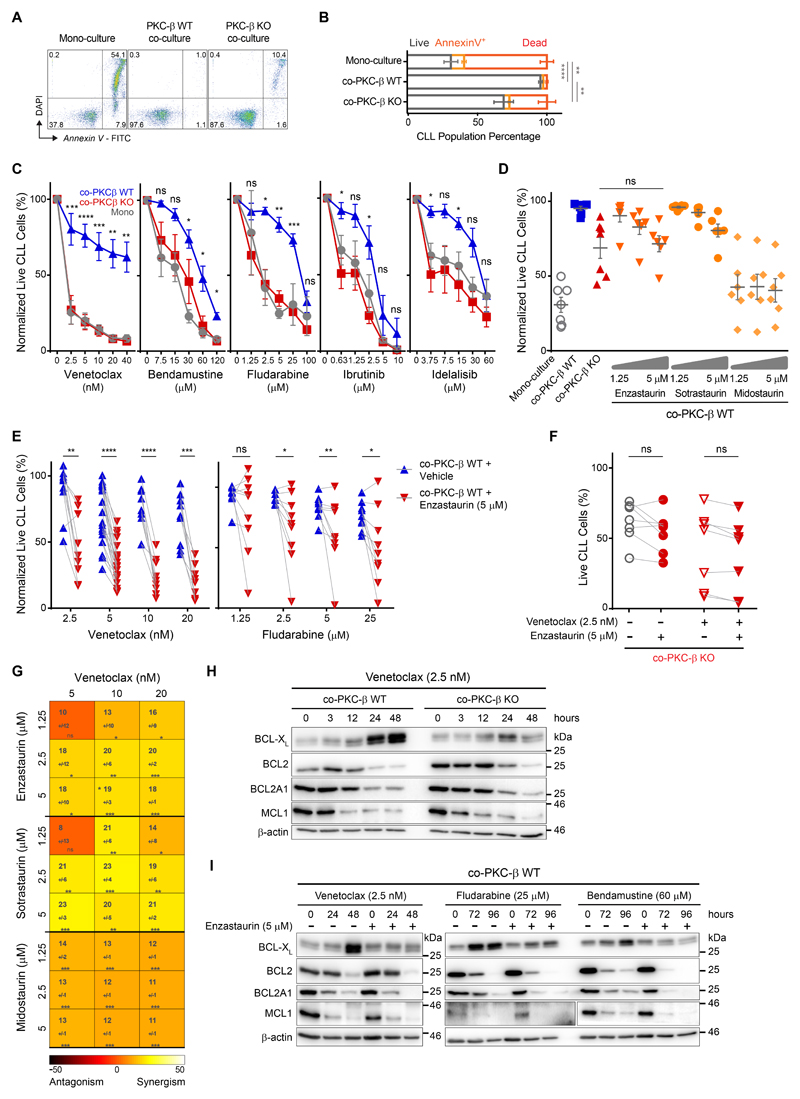
Signals derived from the microenvironment contribute not only to the development of normal hematopoietic cells, but also to drug resistance of malignant cells (termed environment-mediated drug resistance; EMDR)(5). The marked dependency of malignant B cell survival on PKC-β activity in the microenvironment raised the possibility that this interaction could be exploited therapeutically. To test this hypothesis, we cultured primary human CLL cells on bone marrow derived MSCs from either KO or WT mice. Notably, human MSCs were indistinguishable from mouse MSCs with regard to PKC-β activation and survival support(10). In line with our previous observations, PKC-β deficiency in bone marrow derived MSCs mitigated the anti-apoptotic effects of stromal cells on CLL cells (Fig. 2, A and B). To test the role of stromal PKC-β in EMDR, parallel co-cultures were exposed 24 hours post-CLL seeding to increasing doses of venetoclax (BCL2-inhibitor), bendamustine (alkylating agent), fludarabine (purine analogue) or ibrutinib and idelalisib, (inhibitors of B-cell receptor (BCR) - induced kinases), before assessing the viability of CLL cells 48 hours later. Expectedly, contact with WT MSCs enhanced the resistance of CLL cells to these cytotoxic drugs when compared to suspension cells in mono-culture (Fig. 2C). In particular, we observed strong protective effects of MCSs on CLL cells for venetoclax and fludarabine-treatments, while EMDR to BCR-inhibitors was less pronounced. The absence of PKC-β in MSCs completely abolished the protective effects seen with WT stromal cells under all treatments except for bendamustine, where PKC-β-deficiency nevertheless also strongly decreased stroma protection. Notably, PKC-β expression in monocytes, which are derived from the hematopoietic system and also support leukemogenesis(15), was dispensable for response to cytotoxic therapies (fig. S4, A to D). These data indicate that PKC-β expression in MSCs is crucial for EMDR of primary CLL cells.
Figure 2.
We previously demonstrated that PKC-β kinase activity in MSCs is essential for microenvironment-mediated survival support of leukemia cells(10). Therefore, we tested whether ablation of EMDR could be achieved with small molecule PKC-inhibitors. Enzastaurin and sotrastaurin are orally bioavailable, reversible ATP-competitive inhibitors of PKCs with enzastaurin being more specific for the β-isoform (16, 17). Midostaurin is a multi-kinase inhibitor with activity against PKC-isoforms and was recently approved for the treatment of FLT3-ITD+ AML(18). Co-cultures of WT MSCs and primary CLL cells were exposed to increasing low-doses of these inhibitors for 48 hours. Similar to co-culturing CLL cells on KO MSCs, each PKC-inhibitor reduced the viability of CLL cells (Fig. 2D). Importantly, the cytotoxic effects of enzastaurin and sotrastaurin did not exceed the survival-disadvantage of KO MSCs and were dose-dependent, whereas midostaurin induced CLL cell death beyond this effect similarly across all doses. This suggests that midostaurin also inhibits other kinases in MSCs and/or has direct cytotoxic effects on primary CLL cells. To test whether PKC-β inhibitors can sensitize malignant B cells to cytotoxic agents, we treated MSC/CLL-co-cultures for 48 hours with low dose (5 μM) enzastaurin and increasing doses of different cytotoxic agents. Assessment of apoptotic CLL cells after 48 hours in co-culture indicated that enzastaurin sensitized CLL cells to venetoclax, bendamustine and fludarabine, pheno-copying the experiments with KO stromal cells (Fig. 2E; fig. S4, E and F; patient characteristics are provided in table S1), but not consistently to ibrutinib and idelalisib (fig. S4F). Using the Combenefit platform(19) for the assessment of drug-synergy, the Bliss-independence and the Loewe-additivity models demonstrated that the cytotoxic effects of combinational treatments with venetoclax and PKC-β-inhibitors were indeed synergistic (Fig. 2G and fig. S4G). Similar to enzastaurin, the PKC-inhibitors sotrastaurin and midostaurin also chemo-sensitized malignant B cells to cytotoxic agents (Fig. 2G), indicating a class-rather than a drug-specific effect.
Notably, PKC-β expression is not restricted to MSCs, but also found at high amounts in malignant B cells(20). To prove that the synergistic effects are indeed mediated by the inhibition of stromal PKC-β and not attributed to off-target effects or inhibition of PKC-β expressed in tumor cells, we cultured primary CLL cells on PKC-β KO stromal cells and then exposed co-cultures to enzastaurin in the absence or presence of venetoclax. Analyses of apoptotic B cells after 48 hours demonstrated that enzastaurin did not affect the survival of CLL cells cultured on PKC-β KO stromal cells. Furthermore, under these conditions enzastaurin also did not enhance the cytotoxic effects of venetoclax (Fig. 2F). In conclusion, by genetically removing the target protein for the kinase inhibitor, these data prove that the PKC-β inhibitor sensitizes malignant B cells to cytotoxic drugs by ablating microenvironment-mediated, PKC-β-dependent survival signals and drug resistance.
The chemo-sensitizing effects of enzastaurin were most pronounced in combination with venetoclax. The efficacy of this BCL-2 inhibitor is largely dependent on the relative expression of other anti-apoptotic proteins. To understand how stromal PKC-β inhibits the cytotoxicity of venetoclax, we analyzed the expression of anti-apoptotic proteins in CLL cells cultured either on WT or KO stromal cells. In CLL cells cultured on WT stromal cells and treated with a low-dose of venetoclax, BCL-XL was significantly (p=0.009 at 48 hours post-treatment) up-regulated, whereas expression of BCL2, BCL2A1 and MCL-1 decreased after 48 hours. In contrast to WT stromal cells, the enhanced expression of BCL-XL was markedly mitigated in CLL cells cultured on KO stromal cells (Fig. 2H, for protein-quantification see fig. S5A). Similar to co-culture on KO stromal cells, treatment of WT stroma-CLL co-cultures with venetoclax or other chemotherapies in combination with enzastaurin also blocked the up-regulation of BCL-XL in CLL cells (Fig. 2I). Notably, the stroma-dependent stabilization of BCL-XL was not regulated by enhanced transcription as BCL-XL transcripts were readily detectable in co-cultured CLL cells and remained stable following venetoclax treatment (fig. S5, B and C). In conclusion, the activation of PKC-β in the microenvironment contributes to drug resistance by controlling the post-transcriptional regulation of BCL-XL in malignant B cells.
Stromal PKC-β is essential for ERK-activation and BCL-XL stabilization in CLL cells
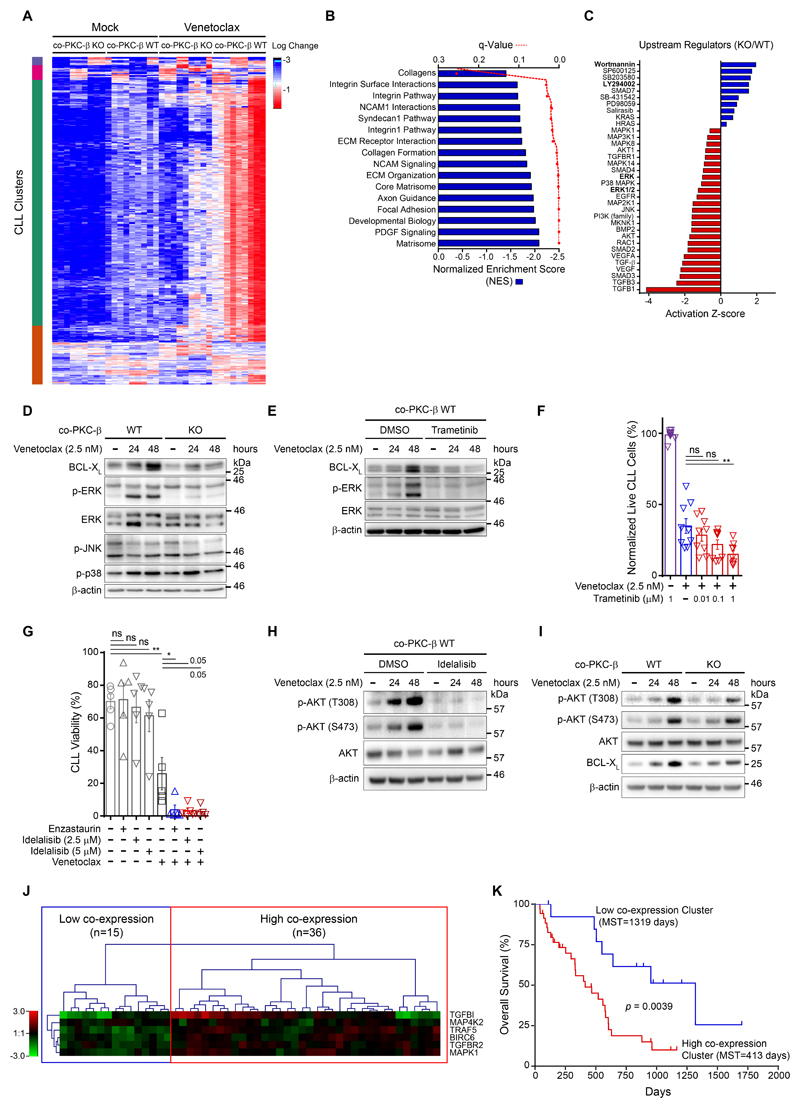
To further understand the role of stromal PKC-β in EMDR, RNA-seq expression profiling was performed on primary CLL cells cultured on either WT or KO stroma in the presence of venetoclax (used at a minimal concentration of 1.25 nM to avoid substantial cell death (see Fig. 2C)). Clustering of the global expression changes between conditions demonstrate a considerable difference in the CLL response to venetoclax when co-cultured with WT versus KO stroma (Fig. 3A). Pairwise comparison of gene expression in CLL cells cultured on either WT or KO stroma in the presence of venetoclax identified 810 differentially expressed genes (DEG); of these, 755 were significantly up-regulated in CLL cells cultured on WT stroma compared to cells co-cultured on KO stroma, while 55 genes were down-regulated (adjusted p-value <0.01; log2 fold-change >1). Gene set enrichment analysis indicates that co-culture on WT stromal cells enhanced the expression of genes required for ECM-remodeling and cell-cell interactions (Fig. 3B and data file S1). We then used Ingenuity Pathway Analysis (IPA) to identify canonical pathways and regulators operating up-stream of these transcriptional changes. These analyses indicated that PI3K and MAPK signaling was inhibited in CLL cells cultured on KO stroma (Fig. 3C) associated with effects on integrin- and TGF-β pathways (fig. S6A). Indeed, immunoblot analysis showed ERK-, but not p38- or JNK-pathway activation in CLL cells cultured on WT stroma under venetoclax exposure. This activation was severely mitigated in venetoclax-treated tumor cells cultured on KO stromal cells (Fig. 3D, fig. S6B) or cultured on WT stroma in the presence of enzastaurin (fig. S6C). Notably, ERK-activation was associated with enhanced BCL-XL expression, suggesting a functional link between the two. Indeed, the MEK1/2-inhibitor Trametinib antagonized venetoclax induced ERK-activation, and was associated with decreased BCL-XL protein expression (Fig. 3E) and enhanced cytotoxicity (Fig. 3F).
Figure 3.
Since activation of PI3K has been shown to limit the efficacy of venetoclax (21, 22), we investigated whether the PI3K inhibitor idelalisib also synergized with venetoclax in inducing apoptosis in CLL cells. Indeed, in combination with the PI3K inhibitor, venetoclax-induced apoptosis was enhanced (Fig. 3G) and AKT-phosphorylation, used as a surrogate to assess PI3K activity in malignant B cells, was abolished (Fig. 3H). To distinguish between idelalisib-effects in tumor or stroma cells, we performed immunoblotting from CLL cells cultured in the presence of venetoclax on either WT or KO stroma cells. Results from this experiment indicated that PI3K is activated in venetoclax treated tumor cells and that stroma PKC-β activity contributes to this activation, though this dependency was less pronounced than effects observed on MAPK-signaling (Fig. 3I and fig. S6D).
To provide further evidence that the up-regulation of PKC-β-mediated and EMDR-associated genes has clinical relevance, we retrospectively assessed the impact of stroma-mediated, de-regulated pathways on progression-free and overall survival in a cohort of fludarabine-refractory CLL patients(23). Co-expression of genes in CLL, regulated by stroma PKC-β, was found as a signature in a majority of the drug-resistant cohort (Fig. 3J). Moreover, patients with high co-expression signatures demonstrated a worse prognosis to salvage treatment, compared to low-expression signature patients (Fig. 3K and fig. S6E), emphasizing the relevance of these genes, identified in co-culture, to clinical observations. Conclusively, our in vitro data show that stroma-mediated drug resistance to venetoclax is mediated by ERK- and PI3K-signaling, dependent on PKC-β activity in stromal cells.
PKC-β dependent lysosome biogenesis is required for EMDR of B cells
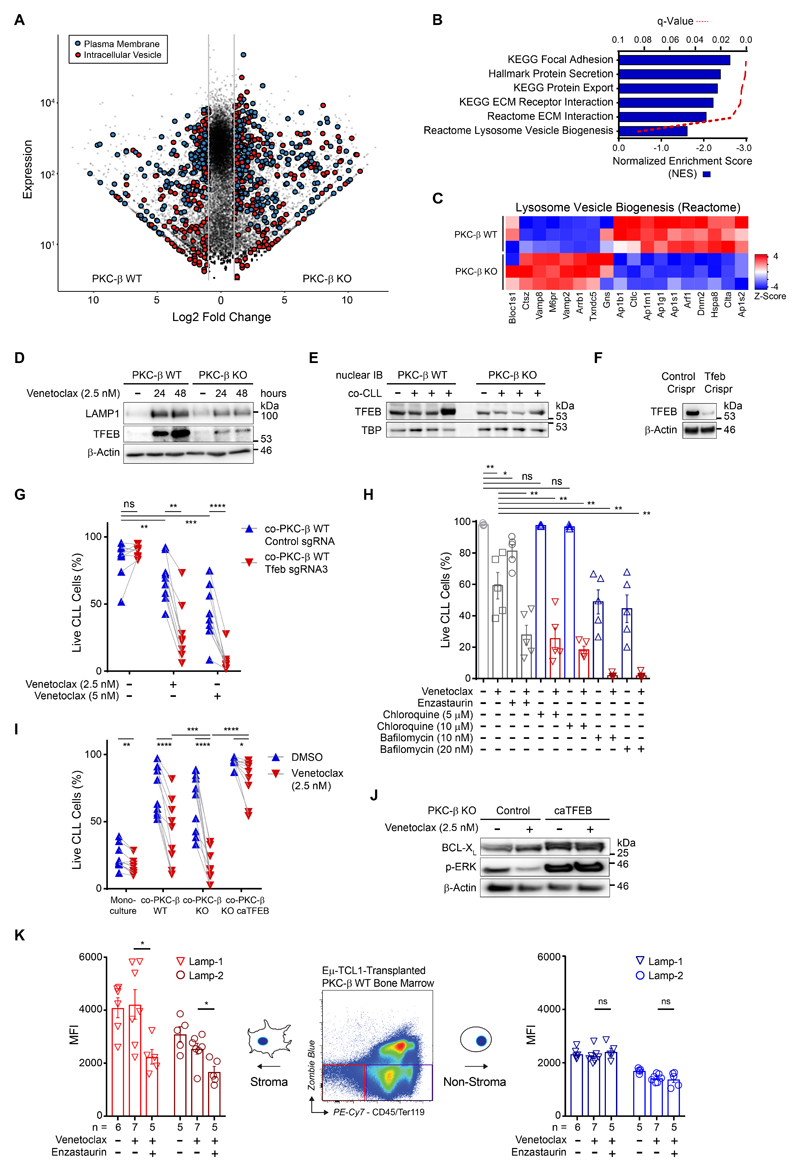
These results prompted us to also investigate the molecular events orchestrated by PKC-β in stromal cells. RNA sequencing analysis was performed using PKC-β WT and KO stromal cells co-cultured with primary CLL cells for 48 hours. A total of 3,352 genes with significantly changed expression (fold-change >2; adjusted p-value <0.05) were identified in PKC-β KO cells, compared with WT cells (Fig. 4A). This demonstrates a broad impact of PKC-β on stromal cell characteristics, including stark differences in the expression of genes encoding plasma membrane- and intracellular vesicular proteins. Nearly half of all annotated plasma membrane and intracellular vesicular protein genes were differentially regulated between PKC-β WT and KO stroma, with 438 and 287 genes exhibiting greater than a two-fold change in either gene set, respectively (adjusted p-value <0.05). Gene set enrichment analysis (GSEA) revealed PKC-β WT stromal enrichment of genes involved in ECM interactions and lysosome vesicle biogenesis (Fig. 4, B and C). Analysis of lysosome biogenesis by lysosome staining showed a substantially reduced lysosomal content in PKC-β KO cells compared to WT stroma (fig. S7A). Moreover, PKC-β KO stroma was also resistant to chloroquine-induced lysosome accumulation compared to WT stroma. In line with our observations, lysosome biogenesis in osteoclasts has been shown to be controlled by PKC-β mediated serine phosphorylation of the transcription factor EB (TFEB) C-terminal motif(24). Phosphorylation of TFEB increased its nuclear abundance and enhanced transcriptional activity (25).
Figure 4.
TFEB is a member of the MiT-TFE family of transcription factors, which are major factors regulating lysosome biogenesis and autophagy (26). To ascertain whether lysosome deregulation extended to stroma in co-culture with CLL under stress conditions, immunoblots for lysosome component protein, LAMP-1, and lysosome biogenesis transcription factor, TFEB, were conducted. Immunoblots showed markedly increased LAMP1 and TFEB protein expression in WT stroma at 24 and 48 hours, respectively, as compared to KO stroma in similarly treated CLL co-cultures (Fig. 4D). Further analysis found increased nuclear expression of TFEB in co-cultured PKC-β WT compared to KO stroma (Fig. 4E and fig. S7B). To assess the importance of TFEB for stroma-mediated drug resistance, we generated TFEB deficient cells using CRISRP/Cas9 deletion (Fig. 4F). While TFEB deficient stroma cells and control counterparts provided equal survival support for primary CLL cells in the absence of drugs, exposure of co-cultures to venetoclax demonstrated that TFEB is an essential factor for EMDR (Fig. 4G). We used chloroquine and bafilomycin to interrogate whether the inhibition of lysosome function equally inhibits EMDR. Chloroquine-treated co-cultures did not show impaired survival of malignant B cells in contrast to bafilomycin, which reduced the anti-apoptotic effects from stroma on CLL cells. However, both compounds markedly enhanced the cytotoxic effects of venetoclax (Fig. 4H). To disentangle direct inhibitory effects of chloroquine and bafilomycin on CLL and stroma cells, stroma cells were pre-treated with both compounds before their washout, preceding primary CLL co-culture and subsequent venetoclax exposure. Pre-treatment of stroma cells similarly mitigated EMDR, indicating that the synergistic effects were attributed to the inhibition of stroma- and not CLL-lysosomes (fig. S7C). Lastly, complementary to the deletion of TFEB from stroma cells, parental KO stroma cells were transduced with a constitutively-active TFEB variant (caTFEB)(27) or vector control. CLL co-cultured with PKC-β KO stroma expressing caTFEB demonstrated a significantly (p=3.71E-07) higher resistance to venetoclax compared to cells cultured on control stroma (Fig. 4I and fig. S7D). Additionally, venetoclax exposed CLL cells, co-cultured on PKC-β KO stroma expressing caTFEB, demonstrated increased amounts of BCL-XL and increased ERK-phosphorylation compared to CLL cells co-cultured on control KO stroma (Fig. 4J). To ascertain whether PKC-β inhibition impairs in vivo lysosomal biogenesis, TCL1-transplanted recipient mice were treated with enzastaurin. Stroma-restricted down-regulation of both lysosomal-associated membrane protein (LAMP)-1 and LAMP-2 was observed in the enzastaurin co-treated cohort, in comparison to venetoclax-treated and vehicle control cohorts, respectively (Fig. 4K). In contrast, these lysosome-associated proteins were unchanged across all cohorts in the non-stroma compartment, signifying a stromal-specific response to PKC-β inhibition in vivo.
These data demonstrate that PKC-β mediated activation of TFEB and lysosome biogenesis in stromal cells are central for EMDR and the reciprocal stabilization of BCL-XL in tumor cells.
Plasma membrane protein composition of stromal cells is regulated by PKC-β
Our gene expression profiling of stroma cells indicates a disparate cell surface phenotype between PKC-β WT and KO cells. To define the contribution of cell-cell interactions and response to venetoclax, we separated CLL cells from stromal cells using transwells. Disruption of cell-cell contact increased spontaneous apoptosis and venetoclax-induced cell death, indicating that cell-cell contact is of predominant importance for EMDR (Fig. 5A). To identify proteins on the surface of stromal cells relevant for contact-dependent survival of CLL cells, we performed plasma-membrane profiling (PMP) of MSCs, cultured in the absence or presence of CLL cells ± enzastaurin. Quantitative proteomic analysis identified changes in the composition of cell surface proteins of MSCs induced by contact with CLL cells (Fig. 5B and data file S2). Importantly, inhibition of PKC-β with enzastaurin altered the expression of cell surface proteins of CLL-activated MSCs, whereby the amounts of 79 and 123 proteins were up- and down-regulated by enzastaurin (q-value <0.05), respectively. A cluster of proteins with increased CLL-dependent cell surface expression was appreciably reduced by enzastaurin (cluster 2), whereas the surface expression of other proteins was increased after treatment with the PKC-β inhibitor (clusters 1,3). Enzastaurin altered the expression of proteins that have potential roles in adhesion, including collagen1A2 and several matrix glycoproteins (Fig. 5C). In addition, surface proteins with a potential role for activating MSCs themselves were detected. The PKC-β dependent expression of IL1Rl1, ADAM17 and IL6ST may contribute to the observed inflammatory gene signature of tumor-cell activated MSCs(10). Importantly, PMP of MSCs indicated that VCAM1 was also down-regulated by enzastaurin. Flow cytometry analysis on primary MSCs from WT and KO mice demonstrated a significantly higher expression of VCAM1 on WT cells (Fig. 5D), which was down-regulated on CLL-activated stroma cells upon PKC-β kinase inhibition (fig. S7E). VCAM1 can bind to integrins expressed on a variety of leukocytes, contributing to cell adhesion and survival (28). We therefore hypothesized that diminished EMDR observed in KO stromal cells was partly attributed to a reduced expression of VCAM1. To test this, we generated Vcam1-deficient bone marrow MSCs using CRISPR/Cas9. Assessment of viability of CLL cells, co-cultured on Vcam1 proficient or deficient stromal cells, indicated that VCAM1 contributes to the anti-apoptotic effects on malignant B cells. This dependency was further augmented by treatment with venetoclax (Fig. 5E). Similarly, a blocking antibody against VCAM1 enhanced spontaneous and venetoclax-induced apoptosis of CLL cells, indicating that VCAM1 is important for EMDR (Fig. 5F). Importantly, overexpression of constitutively active TFEB not only rescued EMDR (Fig. 4I), but also partially restored VCAM1 expression on KO cells (Fig. 5G). In support of this finding, deletion of TFEB caused a marked down-regulation of VCAM on stroma cells (Fig. 5H and fig. S7F), indicating that PKC-β-dependent activation of TFEB and lysosome biogenesis is important for plasma membrane integrity.
Figure 5.
As a number of adhesion molecules were impacted by enzastaurin, we investigated whether blocking PKC-β resulted in a diminished capacity for adhesion of CLL cells in vitro. Patient CLL cells were flowed over PKC-β WT and KO stroma in defined channel slides (fig. S8A). Results indicated no difference existed between the adhesive capacity of both stromal genotypes in this assay (Fig. 5I, blue), with both stroma able to retain flowing CLL cells comparably equally and concordant with the in vivo homing data previously observed. Subsequently, treatment of adhered CLL on WT stroma with either vehicle or enzastaurin, demonstrated no difference in CLL cells mobilized in comparison between enzastaurin treated and untreated conditions. Additionally, KO stroma also showed comparable number of mobilized CLL cells upon channel flushing (Fig. 5I, red), indicating reduced Vcam1 expression does not impair stroma adhesion or retention of CLL under these conditions. To further validate this result, we treated diseased TCL1-tg mice for 2 consecutive days with enzastaurin before monitoring disease burden in the peripheral blood and lymphoid organs (Fig. 5J). In line with our in vitro data, we did not observe a mobilization of tumor cells into the peripheral blood. Likewise, disease burden in the peripheral blood, bone marrow, spleen and peritoneal cavity did not change over the course of the experiment (Fig. 5, K and L; fig. S8B and C). Notably, in vivo VCAM1 was markedly down-regulated on bone marrow derived CD45-Ter119- stroma cells from enzastaurin treated mice to similar amounts found on cells derived from KO mice (Fig. 5, M to O), indicating that the applied dose of enzastaurin was sufficient to inhibit stromal PKC-β in vivo. VCAM1 expression on bone marrow MSCs may therefore be a suitable and easily accessible biomarker for drug-efficacy in future studies. In conclusion, our data show that PKC-β kinase activity regulates the expression of numerous adhesion molecules on stromal cells. Their pharmacological down-regulation by kinase inhibitors does not affect positioning of malignant B cells within lymphoid niches but reduces EMDR.
Enzastaurin enhances chemo-sensitivity and prolongs survival in vivo
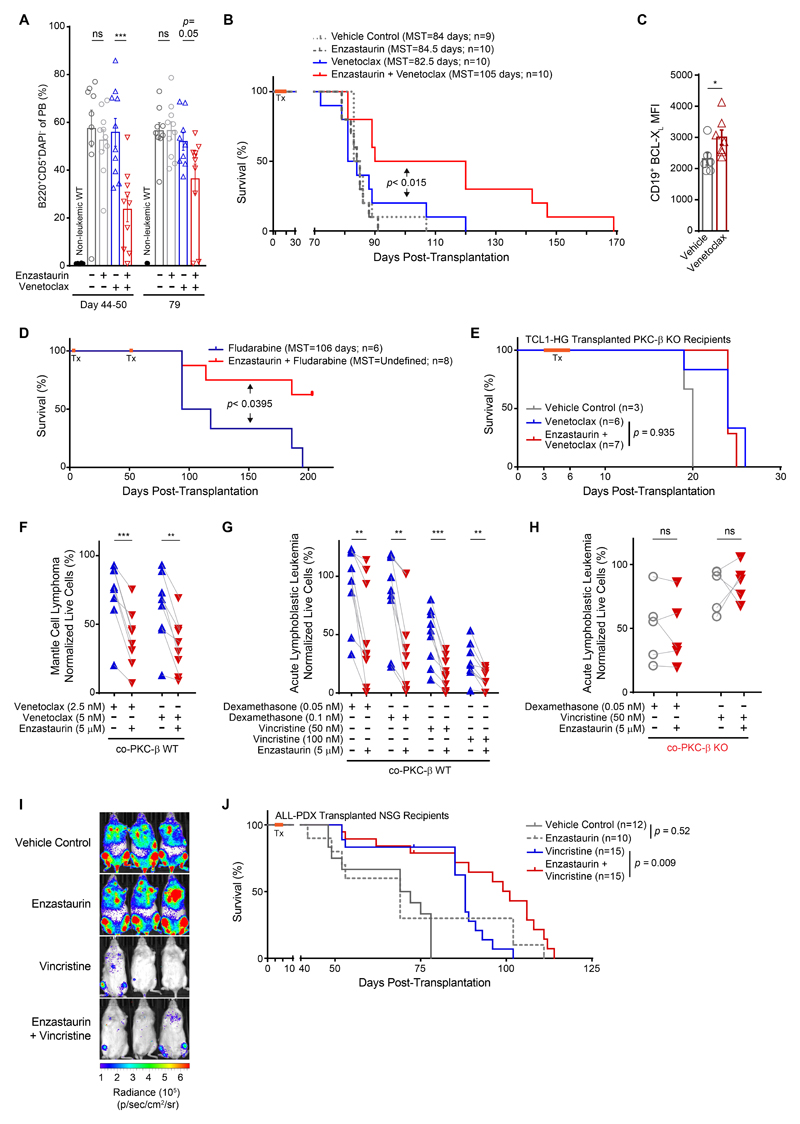
Encouraged by these results, we tested whether the chemo-sensitizing effects of PKC-β inhibitors observed in vitro could be translated into the treatment of murine B cell malignancies. To this end, we transplanted 3.5 to 5x106 splenocytes from several individual, diseased TCL1-tg mice i.p. into 4 cohorts of syngeneic C57B/6 mice. Three days after transplantation, mice were orally treated for 16 consecutive days with either enzastaurin, venetoclax or a combination of both (treatment scheme is shown in fig. S9A). Treatment was well tolerated without hematotoxicity (fig. S9B) or weight loss. However, venetoclax treated mice developed a loss of hair pigmentation, which was markedly enhanced with enzastaurin co-administration (fig. S9C). This is a known on-target side effect of venetoclax, explained by the exquisite dependency of melanoblasts on BCL-2(29). Monitoring of leukemogenesis in the peripheral blood revealed single agent treatment with enzastaurin or venetoclax, given over the course of 16 days, had no effect on the number of tumor cells in the peripheral blood or disease progression. In stark contrast, combination treatment reduced tumor burden and terminal spleen weights, whilst prolonging the life expectancy of mice (Fig. 6, A and B; fig. S9D). A separate assessment of BCL-XL in vivo in tumor cells of diseased animals showed that venetoclax induced its expression as anticipated from in vitro experiments (Fig. 6C).
Figure 6.
Similar to venetoclax, mice were also treated with fludarabine in combination with enzastaurin (fig. S9E). Disease progression, monitored in the peripheral blood 25-28 days after initial treatment, showed tumor cells in both cohorts with difference between treatment groups (32.4 ±3.4% of circulating tumor cells in the fludarabine group vs. 9.6% ±6.7% in the combination treatment group (fig. S9F)). Mice were then treated with a second cycle of therapy, 54 days after transplantation. The concurrent treatment of fludarabine and enzastaurin markedly prolonged survival of mice compared to fludarabine mono-therapy (Fig. 6D). No increased hematotoxicity was observed in mice concurrently treated with enzastaurin (fig. S9, G and H).
Our in vitro data demonstrated that the chemo-sensitizing effects of enzastaurin were ablated in co-cultures of tumor- and PKC-β KO stroma cells, indicating that no off-target effects contributed to the enhanced cytotoxicity of venetoclax (Fig. 2F). To prove that no off-target effects by co-administered enzastaurin contribute to the survival benefits observed in vivo, we generated TCL-1 driven B cell lymphoma which could overcome the strong dependency of Eμ-TCL1-tg cells on microenvironment PKC-β (Fig. 1). Eμ-TCL1-tg mice were crossed onto transposon harboring mice expressing dual transposases PiggyBac and Sleeping Beauty (Hyper-GRONC, HG)(30, 31). Transplanted TCL1-HG tumors overcame loss of microenvironment PKC-β, though displayed delayed leukemic progression compared to PKC-β wild-type recipients receiving the same TCL1-HG cells (fig. S9I). After engraftment of B cell tumors from this mouse model in PKC-β KO animals, mice were subsequently treated with venetoclax or a combination of enzastaurin and venetoclax. Venetoclax treatment prolonged life expectancy of diseased KO recipient mice. Importantly, the addition of enzastaurin did not result in extended survival (Fig. 6E), indicating that the chemo-sensitizing effects of the PKC-β inhibitor were not attributed to off-target effects or on-target inhibition of tumor-PKC-β.
Based on our previous observation that PKC-β was also activated in stromal cells following contact to tumor cells from ALL and MCL patients(10), we investigated whether PKC-β inhibition in WT stromal cells mitigated EMDR of co-cultured primary B cells from ALL and MCL patients. Analogous to CLL, enzastaurin enhanced the therapeutic effect of venetoclax on MCL cells (Fig. 6F and table S1). To demonstrate a chemo-sensitizing effect of PKC-β inhibition on primary cells from ALL patients, enzastaurin was used in combination with dexamethasone or vincristine, both established chemotherapies in the treatment of ALL patients. Inhibition of stromal PKC-β with enzastaurin increased the cytotoxicity of both drugs (Fig. 6G and table S1). Importantly, this effect was completely abolished in co-cultures with KO stromal cells (Fig. 6H), confirming that the PKC-β inhibitor sensitized B-ALL cells by targeting stromal PKC-β and excludes the contribution of off-target effects or inhibitory effects on PKC-β expressed in ALL cells. To assess whether PKC-β inhibition also sensitizes primary ALL cells to chemotherapy in vivo, NSG mice xenografted with luciferase-labelled ALL-PDX cells were treated for 3 days with vincristine ± enzastaurin. Bioluminescent imaging between 3 to 6 weeks post-transplantation revealed comparable leukemic burdens between vehicle and enzastaurin-treated mice (Fig. 6I and fig. S9J). As expected from our in vitro data, enzastaurin mono-therapy did not extend survival of ALL xenografted mice compared to vehicle controls, while the concomitant administration of enzastaurin and vincristine increased life-expectancy compared to single agent vincristine treatment (Fig. 6J).
In conclusion, enzastaurin increases the efficacy of different chemotherapy regimes in vivo by mitigating EMDR, demonstrated in pre-clinical CLL and ALL mouse models. In addition, ablation of EMDR by PKC-β inhibition was also observed in another B cells malignancy in vitro, indicating that the inhibition of PKC-β-dependent EMDR sensitizes a broad range of B cell malignancies to cytotoxic therapies.
Discussion
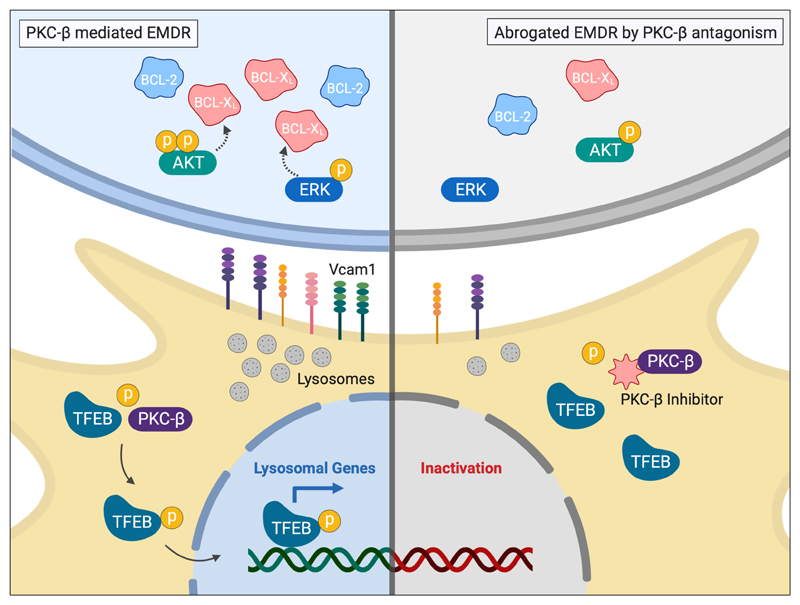
A growing amount of research has identified tumor-host interactions as essential processes regulating tumor cell immune evasion, metabolic adaptations, survival and proliferation (32). Here we describe a crucial dependency for survival of normal B1- and malignant B cells on PKC-β expressed in stromal cells. Under conditions of cytotoxic stress PKC-β regulates lysosome-biogenesis by stabilizing TFEB, which is essential for plasma membrane composition and EMDR, effectuated by BCL-XL expression in tumor cells. Genetic and pharmacological interference with this stress-response blunts this adaptation and increases the efficacy of chemotherapies (Fig. 7).
Figure 7.
Since neither homing nor proliferation of malignant B cells was affected by the removal of PKC-β from the microenvironment, we hypothesize that the predominant role of stromal PKC-β is the provision of survival signals to these cells that are intermittently required throughout their life-time. Under conditions of cytotoxic stress, PKC-β-dependent MAPK-mediated up-regulation of BCL-XL is essential for drug resistance and cell survival. However, it remains to be experimentally addressed whether similar mechanisms are in place under steady-state conditions and if so, to define their temporal and spatial requirements. It appears that the bone marrow is of particular importance for malignant B cells as the absence of PKC-β leads to a rapid loss of adoptively transferred cells in this compartment. This is in consonance with the clinical importance of this compartment for many B cells diseases, as reflected by the negative impact of bone marrow minimal-residual-disease (MRD)-positivity on progression-free-survival (7, 33). However, this conclusion is partly based on a lack of data, since MRD assessment in patients is restricted to easily accessible locations. Therefore, it remains unknown whether chemo-protective niches exist in other organs. While we have observed the strongest dependency on PKC-β in the bone marrow compartment during early disease stages, it is reasonable to assume that the spatial requirements and cellular composition of drug-protective niches are dynamic and change over time. In support of this hypothesis, in the Eμ-TCL-1 model the spleen appears to be the predominant site of disease at a terminal stage. The marked reduction of the spleen size in enzastaurin co-treated mice suggests that drug-protective niches also exist in this organ and are possibly more relevant at later disease stages. Indeed, we found that splenic follicular reticular cells (FRCs) can mediate PKC-β dependent drug resistance in vitro, similar to MSCs (fig S10A). Therefore, cell types other than bone marrow MSCs, present in different organs, are likely to also contribute to the observed effects in vivo. Their identification and molecular characterization is important and can be achieved through the employment of reporter mice.
In addition to tumor cells, we have observed that the physiological development of B1 cells also depends on PKC-β activity in the microenvironment. Although predominantly derived from fetal liver progenitors, B1 cells can also be derived from bone marrow HSCs(34, 35). Results from our bone marrow chimeras, showing impaired B1 cell development of WT bone marrow cells in a PKC-β KO background were unexpected and, in light of previously published data, indicate that PKC-β has a dual function in B cell development, maintenance and differentiation. Based on the observations made in germ-line deleted PKC-β KO mice(12), several groups have unambiguously demonstrated that intrinsically expressed PKC-β in B cells is essential for BCR-signaling through the recruitment of IKKs into lipid rafts and activation of IKKα/ NF-κB(36, 37). More recently, it was demonstrated that PKC-β deficient B cells fail to present antigens and to differentiate into plasma cells upon immunization (38). However, the capacity to form germinal centers ex vivo was per se not ablated in PKC-β KO B cells, indicating that the essential role of PKC-β for B cell differentiation is dependent on cell-cell interactions. A dual function of PKC-β in B cell physiology is also supported by our observation that WT bone marrow cells outperformed PKC-β KO cells in KO recipient mice with respect to the development of peritoneal B1 cells. In surprising contrast, the capacity of KO cells to produce B1 cells was indistinguishable from WT cells in WT recipient animals. In line with this observation, we found similar titers of the natural antibodies IgM and IgG3 in WT recipient animals reconstituted with KO CD45+ bone marrow cells. Although we cannot fully exclude the possibility that plasma cells survived lethal irradiation of recipient mice(39) and contribute to these antibody concentrations, reduced IgM and IgG3 titers in KO recipient animals reconstituted with WT CD45+ bone marrow cells strongly suggest that extrinsically expressed PKC-β is crucial for the production of natural antibodies. These findings reveal that the exquisite dependence of malignant B cells, especially under therapy, on PKC-β-dependent functions in their environment is already an integral part of normal B cell physiology.
Central to the environment-mediated drug resistance observed in our studies was a PKC-β-mediated increase in BCL-XL expression, in response to various chemotherapeutics with differing mechanisms of drug action. It was previously recognized that BCL-XL can confer a multi-drug resistant phenotype in hematopoietic- (40) and non-hematopoietic cells (41), making it a key target for therapy. Interestingly, CLL cells respond to the cellular stress of BCL-2 antagonism by a microenvironment-mediated post-transcriptional increase in BCL-XL expression. However, our data show that the increased BCL-XL protein expression in CLL co-cultured with PKC-β WT stroma was not attributed to enhanced transcription. This raises questions about the underlying mechanisms causing an extrinsically-mediated increase of BCL-XL protein. Different post-translational modifications of BCL-XL, such as ubiquitination(42) and deamidation(43), must also be considered as possible contributing factors.
BCL-XL was reported to be critical for the escape of self-reactive B-cells from negative selection: Self-reactive B-cells with enforced BCL-XL expression were shown to have some hallmarks of receptor editing and were uniformly anergic, after central deletion escape (44). The correlative ERK1/2 activation we experimentally observed in CLL cells may also be indicative of an anergic program, which has been reported to favor survival(45, 46). We may speculate though that the cellular stress of BCL-2 inhibition could re-invoke a microenvironment-mediated anergic survival program previously utilized by self-reactive B-cells to escape central deletion. In line with this speculation Eμ-TCL1 cells that failed to engraft in PKC-β-deficient mice might have failed to increase BCL-XL expression in vivo and succumbed to a retained autoimmunity checkpoint invoked by the absence of extrinsic PKC-β. Notwithstanding the exact physiological counterpart, we clearly demonstrate MEK1/2- or PKC-β inhibition represent very clinically attractive approaches to mitigate EMDR by preventing the up-regulation of BCL-XL in the malignant cells.
Our experiments demonstrate that VCAM1 expression on stromal cells is exquisitely dependent on PKC-β-mediated activation of TFEB. Previous studies have reported that the acute deletion of Vcam1 in adult mice led to a reduction of immature and mature B cells in the bone marrow (47, 48). In addition, homing of adoptively transferred mature B cells to the bone marrow was significantly impaired in these mice(47). In contrast, germ-line deletion of Vcam1, though is very often embryonic lethal, gave rise to a hypomorphic mouse model with no defects in B cell maturation (49), suggesting that low expression of VCAM1 is sufficient for B cell development or that compensatory mechanisms exist. It is reasonable to assume that the reduced expression of VCAM1 on bone marrow MSCs of PKC-β-deficient mice contributes to the PKC-β-deficient phenotypes. The low expression of constitutive VCAM1 on mesenchymal cells of PKC-β KO mice is possibly related to reduced activity of NF-κB, as Vcam1 is a direct transcriptional target(50). We have previously demonstrated the PKC-β dependent activation of NF-κB in stromal cells, essential for tumor cell survival, is dependent on Essential Modulator (NEMO)(10). Here we describe that the PKC-β dependent activation of TFEB and resultant lysosome biogenesis is of critical importance for plasma membrane composition and EMDR. Therefore, it is reasonable to speculate that the NF-κB deficiency observed in PKC-β KO stromal cells is, at least partially, attributed to a lack of lysosome-mediated degradation of IκB, as previously described(51, 52). However, lysosomes are multi-functional organelles, contributing to many cellular processes, including autophagy, exosome release, plasma membrane repair and adhesion. Therefore, other mechanisms may operate in parallel, including ECM-remodeling and enhanced integrin-signaling(53, 54), which could both further contribute to EMDR.
We believe that we have generated sufficient data to justify investigating the chemo-sensitizing properties of PKC-β inhibitors in a clinical setting. We have observed substantial survival benefits by co-administered enzastaurin in all our in vivo treatment experiments: Of note, our treatment with venetoclax was limited to 16 days. It can be expected that a continuous treatment with PKC-β inhibitors and venetoclax, as it is clinical practice in patients, will provide much greater survival benefits and deeper remissions. Similarly, the extended survival observed with combination treatment in our ALL-PDX model is a proof-of-principle that PKC-β inhibitors enhance the cytotoxic effects of vincristine. In contrast to the treatment of ALL patients, which receive cytotoxic therapies for 1-2 years, diseased animals received only a very short course of vincristine, which already lead to a substantial survival benefit. Enhanced effects of PKC-β inhibitors are therefore likely to be observed in clinical practice with repeated treatment cycles given to patients.
Conceptually similar to our proposed drug combinations, the concomitant treatment with BCL-2- and BTK-inhibitors is currently being investigated in lymphoma patients(55), aiming at blocking microenvironment interactions to enhance the cytotoxicity of venetoclax. Contrary to this concept, our data indicate that PKC-β inhibitors cause an “in-situ chemo-sensitization” of B cells as we have not observed an adherence-deficiency or a redistribution of cells into the peripheral blood upon kinase inhibition. The reduced expression of adhesion molecules on PKC-β deficient stromal cells likely reduces survival signals to leukemic cells, but without affecting niche residency. The dependency of different types of B cells, including normal B1 cells and immature malignant B cells, on PKC-β functions in the microenvironment, further indicates that PKC-β inhibitors may be clinically used beyond the spectrum of BCR-inhibitor sensitive disease entities. The treatment of auto-immune diseases with a pathogenic contribution from B cells, such as systemic lupus erythematous, may also benefit from the chemo-sensitizing effects of PKC-β inhibitors.
At present, the PKC-β inhibitor enzastaurin, an oral ATP-competitive small molecule inhibitor with a relative specificity for PKC-β(16), is in clinical development at the stage of phase III trials (e.g. NCT03263026). In addition, midostaurin, though less specific for the β-isoform, has recently been approved for the treatment of AML patients (18). Based on the observation that PKC-β is intrinsically expressed in malignant B cells, favorable phase II data and an excellent safety profile, the PRELUDE phase III trial was designed to test the efficacy of enzastaurin as maintenance mono-therapy for DLBCL patients in at least partial remission following treatment with R-CHOP. The trial failed its primary end point (disease free survival)(56), which led to a temporary halt in the clinical development of the drug. Notably, contrary to low-grade lymphomas or ALL, MRD is clinically not a problem for the vast majority of DLBCL patients, suggesting that EMDR may not play a substantial role for the majority of patients with high-grade NHL. This is reflected by the observation that 70% of patients in the PRELUDE trial were already cured at the end of immuno-chemotherapy, before commencing maintenance therapy.
Our data provide evidence that the chemo-sensitizing effects of enzastaurin depend on the lysosome-mediated remodeling of cell-surface and matrix-proteins. Since enzastaurin undergoes liver-mediated degradation and has a short half-life(57), contributing to a substantial inter-subject variability in the steady-state plasma concentration(58), we predict that the timing between administering a PKC-β inhibitor to mitigate EMDR and cytotoxic agents are important for their synergism. Biomarkers such as VCAM1 or LAMP1 expression in MSCs should therefore be used in future clinical trials to determine the optimal time between co-administering chemo-sensitizing- and cytotoxic therapy.
The incorporation of PKC-β inhibitors in treatment-regimens used for various B cells malignancies may have profound clinical and socioeconomic implications: improved clinical responses may ultimately allow for the reduction of the number of treatment cycles, lowering cumulative drug doses and minimizing side effects, costs and need for salvage therapies. Notably our data demonstrate that the application of PKC-β inhibitors can be limited to treatment days, minimizing their compound-specific side effects and costs. Clinical trials are now needed to address whether our data can be translated into improved patient care.
Material and Methods
Study Design
Our primary objective was to test whether the dependency of malignant B cells from patients with CLL, MCL and ALL patients on PKC-β expressed and activated in the tumor microenvironment could be harnessed therapeutically. By transferring normal bone marrow or labeled tumor cells into WT and KO recipient mice we demonstrate that stroma PKC-β is required for normal B1 cell development and the survival of malignant B cells. This dependency was further investigated through ex vivo co-culture experiments, in which we demonstrate that small molecule inhibitors targeting PKC-β mitigate environment mediated drug resistance by blocking the up-regulation of BCL-XL in tumor cells in an ERK and PI3K dependent manner. We employed RNAseq and mass spectrometry to delineate the molecular mechanisms in stromal cells, demonstrating that stromal PKC-β is essential for the activation of TFEB. Activated TFEB in stromal cells regulates the expression of adhesion molecules required for stroma-mediated protection from cytotoxic agents. Our data were validated through in vivo experiments, in which diseased C57B/6 or NSG- mice were treated with cytotoxic agents in combination with PKC-β inhibitors.
We did not use statistics to predetermine sample sizes. Animals were randomly assigned to genotypes or the 4 treatment cohorts (vehicle control, venetoclax or vincristine, enzastaurin, enzastaurin + venetoclax or vincristine). Sample sizes in the mouse experiments were based on own and published data (10, 59). Animal wellbeing was monitored daily and all experiments were conducted under the UK Home Office regulations. Investigators were not blinded to treatment allocations, but animal technicians who delivered care and decided independently upon which animals needed to be sacrificed (applying strict criteria for end points) were blinded. In vitro studies were conducted with multiple technical and biological replicates to ensure reproducibility of data.
Statistical Analyses
All in vitro experiments were repeated at least three times, and the means ± SEM were calculated. The exact sample size for each experiment is provided in the figure legends. Statistical analyses of results were performed using one-way ANOVA followed by two-tail Student t-tests, with respective unpaired and paired analyses experimentally dependent. Statistical annotations as previously noted were denoted with asterisks according to the following, ****p < 0.0001, *** p <0.001, **p<0.01, *p<0.05, and ns p >0.05. In vivo studies were carried out using multiple animals (3 to 15 per group, specified in figures), and Kaplan-Meier curves were generated from survival data.
Supplementary Material
Summary.
Inhibition of stroma cell PKC-β mitigates environment-mediated drug resistance in B cell malignancies.
Acknowledgements
We would like to express our deepest gratitude to patients who donated blood for research purposes. In particular, we thank Joanna Baxter and her team for enrolling patients in these studies. We would like to thank Anthony Green, Brian Huntly, George Vassiliou and Dan Hodson for scientific discussion. We are thankful for the generous provision of NestinGFP mice by Simon Mendez-Ferrer and the help of Claudia Korn for staining bone marrow sections. Eμ-TCL1-tg mice were kindly provided by Carlo Croce under an MTA.
Funding
This work was funded by Cancer Research UK (CRUK; C49940/A17480). I.R. is a senior CRUK fellow. M.S.S is supported by the DFG through SCHM2440/7-1 and CRC1243 (A12). L.G. & O.W. received funding from CWCUK (grant 14-169) and GOSHCC (grant V2617). A.E. receives research grants from the Austrian Science Fund (FWF; Transcan I2795-B28 to A.E. (FIRE-CLL), DACH grants I3282-B26 and I1299-B21 (FOR2036) and a grant from the Paracelsus Medical University (PMU Grant E-13/18/091-EGF). S.S. receives funding from the DFG (SFB1074, project B1), relevant to this work.
Footnotes
Author Contribution
E.P., J.C. and A.M. performed and analyzed experiments. S.F. analyzed all RNAseq and mass spectrometry data with support from J.R.B.. A.S. performed and analyzed HSC and progenitor populations in KO and WT mice (fig.S2). M.M., in collaboration with J.C.W. and P.J.L., performed the PMP experiment. H.S., A.E. and M.S.S. contributed conceptually to the project. V.E. and M.B. performed ALL-PDX experiments. Additionally, L.G. and O.W made a substantial contribution to carrying out the ALL-PDX studies and helped critique the output for important intellectual content. J.B. and S.S. provided data depicted in Fig. 3J and K; fig. S6E. Prkcb-KO mice were provided by M.L.; The manuscript was written by E.P., M.S.S. and I.R.
Competing Interests
The authors declare to have no conflicts of interest.
Data and Material Availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information.
The gene expression profile data have been deposited in the GEO database under accession numbers GSE119808 (CLL RNA-Seq, Fig. 3) and GSE119813 (Stromal RNA-seq, Fig. 4).
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119808
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119813
The mass spectrometry proteomics data (Fig. 5) have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD011062 and 10.6019/PXD011062.
https://www.ebi.ac.uk/pride/archive/projects/PXD011062
All other remaining data are available within the Article and Supplementary Files, or available from the authors upon request
References
- 1.Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, Escaramis G, Jares P, Beà S, González-Díaz M, Bassaganyas L, et al. Campo, Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada V, Conde L, Villamor N, Ordóñez GR, Jares P, Bassaganyas L, Ramsay AJ, Beà S, Pinyol M, Martínez-Trillos A, López-Guerra M, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 3.Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, Lawrence MS, Roemer MGM, Li AJ, Ziepert M, Staiger AM, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679–690. doi: 10.1038/s41591-018-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, Hodson DJ, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 6.Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, Wood BL, Kelloff GJ, Jessup JM, Radich JP. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.0580. e170580–e170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, Fink AM, Bühler A, Zenz T, Wenger MK, Mendila M, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 8.Löwenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, Fey M, Schouten H, de Greef G, Ferrant A, Kovacsovics T, et al. Dutch-Belgian Hemato-Oncology Cooperative Group, Swiss Group for Clinical Cancer Research, Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349:743–752. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 9.Ossenkoppele GJ, Stussi G, Maertens J, van Montfort K, Biemond BJ, Breems D, Ferrant A, Graux C, de Greef GE, Halkes CJM, Hoogendoorn M, et al. Addition of bevacizumab to chemotherapy in acute myeloid leukemia at older age: a randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK) Blood. 2012;120:4706–4711. doi: 10.1182/blood-2012-04-420596. [DOI] [PubMed] [Google Scholar]
- 10.Lutzny G, Kocher T, Schmidt-Supprian M, Rudelius M, Klein-Hitpass L, Finch AJ, Dürig J, Wagner M, Haferlach C, Kohlmann A, Schnittger S, et al. Protein kinase c-β-dependent activation of NF-κB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell. 2013;23:77–92. doi: 10.1016/j.ccr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, Russo G, Hardy RR, Croce CM. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 13.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 14.Seifert M, Sellmann L, Bloehdorn J, Wein F, Stilgenbauer S, Dürig J, Küppers R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J Exp Med. 2012;209:2183–2198. doi: 10.1084/jem.20120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiffert M, Schulz A, Ohl S, Döhner H, Stilgenbauer S, Lichter P. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116:4223–4230. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]
- 16.Faul MM, Gillig JR, Jirousek MR, Ballas LM, Schotten T, Kahl A, Mohr M. Acyclic N-(azacycloalkyl)bisindolylmaleimides: isozyme selective inhibitors of PKCbeta. Bioorg Med Chem Lett. 2003;13:1857–1859. doi: 10.1016/s0960-894x(03)00286-5. [DOI] [PubMed] [Google Scholar]
- 17.Wagner J, von Matt P, Sedrani R, Albert R, Cooke N, Ehrhardt C, Geiser M, Rummel G, Stark W, Strauss A, Cowan-Jacob SW, et al. Discovery of 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]pyrrole-2,5-dione (AEB071), a potent and selective inhibitor of protein kinase C isotypes. J Med Chem. 2009;52:6193–6196. doi: 10.1021/jm901108b. [DOI] [PubMed] [Google Scholar]
- 18.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Veroli GY, Fornari C, Wang D, Mollard S, Bramhall JL, Richards FM, Jodrell DI. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics. 2016;32:2866–2868. doi: 10.1093/bioinformatics/btw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Gamal D, Williams K, LaFollette TD, Cannon M, Blachly JS, Zhong Y, Woyach JA, Williams E, Awan FT, Jones J, Andritsos L, et al. PKC-β as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood. 2014;124:1481–1491. doi: 10.1182/blood-2014-05-574830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojarczuk K, Wienand K, Ryan JA, Chen L, Villalobos-Ortiz M, Mandato E, Stachura J, Letai A, Lawton LN, Chapuy B, Shipp MA. Targeted inhibition of PI3Kα/δ is synergistic with BCL-2 blockade in genetically defined subtypes of DLBCL. Blood. 2019;133:70–80. doi: 10.1182/blood-2018-08-872465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, Almasan A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6:e1593–e1593. doi: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stilgenbauer S, Zenz T, Winkler D, Bühler A, Schlenk RF, Groner S, Busch R, Hensel M, Dührsen U, Finke J, Dreger P, et al. German Chronic Lymphocytic Leukemia Study Group, Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- 24.Ferron M, Settembre C, Shimazu J, Lacombe J, Kato S, Rawlings DJ, Ballabio A, Karsenty G. A RANKL-PKCβ-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 2013;27:955–969. doi: 10.1101/gad.213827.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Xu M, Ding X, Yan C, Song Z, Chen L, Huang X, Wang X, Jian Y, Tang G, Tang C, et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol. 2016;18:1065–1077. doi: 10.1038/ncb3407. [DOI] [PubMed] [Google Scholar]
- 26.Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young NP, Kamireddy A, Van Nostrand JL, Eichner LJ, Shokhirev MN, Dayn Y, Shaw RJ. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev. 2016;30:535–552. doi: 10.1101/gad.274142.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 29.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 30.Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, Rad L, Ellis P, Andrews R, Banerjee R, Grove C, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43:470–475. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedrich MJ, Rad L, Bronner IF, Strong A, Wang W, Weber J, Mayho M, Ponstingl H, Engleitner T, Grove C, Pfaus A, et al. Genome-wide transposon screening and quantitative insertion site sequencing for cancer gene discovery in mice. Nat Protoc. 2017;12:289–309. doi: 10.1038/nprot.2016.164. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram C-R, Arnold R, Fietkau R, Freund M, Ganser A, Ludwig W-D, Maschmeyer G, et al. German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia, Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- 34.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Düber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, Weiss S. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 36.Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, Kato RM, Kang S, Patrone L, Wall R, Teitell M, et al. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 37.Saijo K, Mecklenbräuker I, Santana A, Leitger M, Schmedt C, Tarakhovsky A. Protein kinase C beta controls nuclear factor kappaB activation in B cells through selective regulation of the IkappaB kinase alpha. J Exp Med. 2002;195:1647–1652. doi: 10.1084/jem.20020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsui C, Martinez-Martin N, Gaya M, Maldonado P, Llorian M, Legrave NM, Rossi M, MacRae JI, Cameron AJ, Parker PJ, Leitges M, et al. Protein Kinase C-β Dictates B Cell Fate by Regulating Mitochondrial Remodeling, Metabolic Reprogramming, and Heme Biosynthesis. Immunity. 2018 doi: 10.1016/j.immuni.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JJ, Cole LJ. The radiation resistance of long-lived lymphocytes and plasma cells in mouse and rat lymph nodes. J Immunol. 1967;98:982–990. [PubMed] [Google Scholar]
- 40.Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903–1910. [PubMed] [Google Scholar]
- 41.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 42.Kajihara R, Sakamoto H, Tanabe K, Takemoto K, Tasaki M, Ando Y, Inui S. Protein phosphatase 6 controls BCR-induced apoptosis of WEHI-231 cells by regulating ubiquitination of Bcl-xL. J Immunol. 2014;192:5720–5729. doi: 10.4049/jimmunol.1302643. [DOI] [PubMed] [Google Scholar]
- 43.Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosová I, Kulans LA, Fu X, Weinberg JS, Heinecke JW, Roth KA, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111:51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 44.Fang W, Weintraub BC, Dunlap B, Garside P, Pape KA, Jenkins MK, Goodnow CC, Mueller DL, Behrens TW. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- 45.Apollonio B, Scielzo C, Bertilaccio MTS, Ten Hacken E, Scarfò L, Ranghetti P, Stevenson F, Packham G, Ghia P, Muzio M, Caligaris-Cappio F. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood. 2013;121 doi: 10.1182/blood-2012-12-474718. 3879–88– S1–8. [DOI] [PubMed] [Google Scholar]
- 46.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, Caligaris-Cappio F, Ghia P. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112:188–195. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 47.Leuker CE, Labow M, Müller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedrich C, Cybulsky MI, Gutierrez-Ramos JC. Vascular cell adhesion molecule-1 expression by hematopoiesis-supporting stromal cells is not essential for lymphoid or myeloid differentiation in vivo or in vitro. Eur J Immunol. 1996;26:2773–2780. doi: 10.1002/eji.1830261133. [DOI] [PubMed] [Google Scholar]
- 50.Shu HB, Agranoff AB, Nabel EG, Leung K, Duckett CS, Neish AS, Collins T, Nabel GJ. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993;13:6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuervo AM, Hu W, Lim B, Dice JF. IkappaB is a substrate for a selective pathway of lysosomal proteolysis. Mol Biol Cell. 1998;9:1995–2010. doi: 10.1091/mbc.9.8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu L-Y, Hsueh Y-C, Cheng H-L, Wu KK. Cytokine-induced autophagy promotes long-term VCAM-1 but not ICAM-1 expression by degrading late-phase IκBα. Sci Rep. 2017;7 doi: 10.1038/s41598-017-12641-8. 12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akkari L, Gocheva V, Kester JC, Hunter KE, Quick ML, Sevenich L, Wang H-W, Peters C, Tang LH, Klimstra DS, Reinheckel T, et al. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev. 2014;28:2134–2150. doi: 10.1101/gad.249599.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, Burbury K, Turner G, Di Iulio J, Bressel M, Westerman D, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 56.Crump M, Leppa S, Fayad L, Lee JJ, Di Rocco A, Ogura M, Hagberg H, Schnell F, Rifkin R, Mackensen A, Offner F, et al. Randomized, Double-Blind, Phase III Trial of Enzastaurin Versus Placebo in Patients Achieving Remission After First-Line Therapy for High-Risk Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2016;34:2484–2492. doi: 10.1200/JCO.2015.65.7171. [DOI] [PubMed] [Google Scholar]
- 57.Welch PA, Sinha VP, Cleverly AL, Darstein C, Flanagan SD, Musib LC. Safety, tolerability, QTc evaluation, and pharmacokinetics of single and multiple doses of enzastaurin HCl (LY317615), a protein kinase C-beta inhibitor, in healthy subjects. J Clin Pharmacol. 2007;47:1138–1151. doi: 10.1177/0091270007304775. [DOI] [PubMed] [Google Scholar]
- 58.Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, Cole P, Sullivan R, Riddle J, Schmidt J, Enas N, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–4099. doi: 10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 59.Liem NLM, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD, MacKenzie KL, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 61.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 65.Weekes MP, Tan SYL, Poole E, Talbot S, Antrobus R, Smith DL, Montag C, Gygi SP, Sinclair JH, Lehner PJ. Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection. Science. 2013;340:199–202. doi: 10.1126/science.1235047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenwood EJ, Matheson NJ, Wals K, van den Boomen DJ, Antrobus R, Williamson JC, Lehner PJ. Temporal proteomic analysis of HIV infection reveals remodelling of the host phosphoproteome by lentiviral Vif variants. Elife. 2016;5:12112. doi: 10.7554/eLife.18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, Döhner H, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liem NLM, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD, MacKenzie KL, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.