Abstract
In this study we present a first limnological characterization of Lake Billy Mitchell [1,013 m above sea level (a.s.l.), 88.3 m depth, 3 km2 surface area] in central Bougainville Island, Papua New Guinea. Physicochemical depth profiles indicated mixis of the entire water body with oxygen saturation reaching 55% in the deepest layers. A shallow thermocline was eroded at night, indicating atelomixis. and Na+, Ca2+, Mg2+ were the dominant anions and cations, respectively, leading to a conductivity of around 1,230 μS cm−1. The pH was close to neutral throughout the water column, and no accumulation of CO2 was observed at greater depths. With a total phosphorus concentration of around 25 μg liter−1 the lake can be considered as meso-to eutrophic. The phytoplankton community consisted of 18 taxa. The dinophyte Peridiniopsis cf. penardii and the filamentous green alga Planctonema lauterbornii dominated in the uppermost layer and reached a total biovolume around 16 mm3 liter−1. Six macrophyte taxa were found (three Spermatophyta/three Bryophyta), with the water chestnut Eleocharis dulcis covering the shoreline and Ceratophyllum demersum spreading to at least 3 m depth. Seven ciliate species were detected (<5 individuals ml−1) with bacterivorous scuticociliates and the prostomatid Coleps hirtus hirtus dominating the assemblage. The micrometazoan plankton community comprised the rotifer Anuraeopsis fissa, the copepod Mesocyclops cf. affinis, and a cladoceran species with-in the Ceriodaphnia cornuta group all concentrating in the upper water column. The only fish species found in the lake was the eel Anguilla megastoma, whereas in the effluent river this species occurred together with Anguilla marmorata.
This study provides a first limnological description of Lake Billy Mitchell on Bougainville Island, which fills the volcanic crater bearing the same name. The caldera formed after a major eruption only about 400 yr ago. Until now, the chemical composition and the species community of the lake were unknown. Among all surface waters, volcanic lakes show the widest pH ranges, from acid conditions to 12 (Pecoraino et al. 2015, Varekamp 2015). Their chemical composition ranges from concentrated hyperacidic fluids derived from the input of volcanic gases rich in SO2, HCl, HF, and CO2, to dilute, largely meteoric water, and to saline, alkaline fluids arising from evaporative concentration of Na+, , and rich waters. The compositional signatures largely depend on the fluctuations in hydrothermal input, variations in meteoric water fluxes, biological activity in the ecosystem, and chemical processes in the lake (Pecoraino et al. 2015, Varekamp 2015). Decreasing volcanic activity is accompanied by less extreme conditions (Taran et al. 2013).
Some crater lakes pose serious danger to human settlements through phreatic eruptions and gas release or volcanic mudflows (lahars) (see Manville 2015). In 1986 the explosive degassing of CO2 from the supersaturated monimolimnion of the meromictic Lake Nyos in Cameroon resulted in the eruption of ca. 1 km3 of gas that asphyxiated 1,700 people (Kling 1987, 1988). Since medieval times, the summit crater lake of the Kelut Volcano in eastern Java, Indonesia, has ejected water numerous times, and lahars killed more than 15,000 people (Thouret et al. 1998). On Bougainville Island, Billy Mitchell is considered a dangerous volcano due to the possibility of phreatomagmatic eruptions, lahars, and potential CO2 gas bursts. McKee et al. (1990) speculated that travertine (precipitated CaCO3) deposits at the ouflow of Lake Billy Mitchell may indicate an active hydrothermal system next to the lake generating CO2-rich fluids accumulating in the hypolimnion. Their hazard maps show that a substantial proportion of the human population could be affected by eruptive activity. Hence, understanding the mixis regime of this large crater lake is crucial to assess the hazard of potential degassing events to prevent the loss of human lives.
Due to its position on the Pacific Ring of Fire, Papua New Guinea has numerous lakes of volcanic origin, but limnological studies in this country are scarce (Chambers 1987, Osborne 1995). To our knowledge, the only detailed studies of crater lakes in Papua New Guinea have been conducted by Ball and Glucksman (1978, 1980), who investigated Lake Wisdom on Long Island and Lake Dakataua on New Britain Island. Lake Wisdom (pH 7.0–8.1) has a surface area of 95 km2 and oxygen is found down to maximum depth of 360 m, whereas Lake Dakataua (pH 6.9–7. 9) is only 45 km2 and exhibits anoxic conditions from approximately 60 m down to 120 m maximum depth. The ratio of the lake diameter to the height of the crater rim, basin morphology, wind stress, water budget, and thermal and chemical properties determine the extension of the mixed layer depth (Melack 1978). In addition, convection currents created by local geothermal heating may also transport oxygen down into the hypolimnion in lakes near active volcanism (Ball and Glucksman 1978).
Naturally, species richness and diversity often increase over time as the lakes age and volcanic activity decreases or ceases altogether (Schabetsberger, Drozdowski, et al. 2009). For example, Lake Wisdom fills a caldera that formed after one of the largest historical eruptions in Papua New Guinea, around A.D. 1660 (30 km3 of erupted magma), whereas the caldera of Lake Dakataua emerged around A.D. 800 [10 km3 (Global Volcanism Program)]. Consequently, the biological community of the older lake is much more diverse (Ball and Glucksman 1980).
Materials and Methods
Study Area
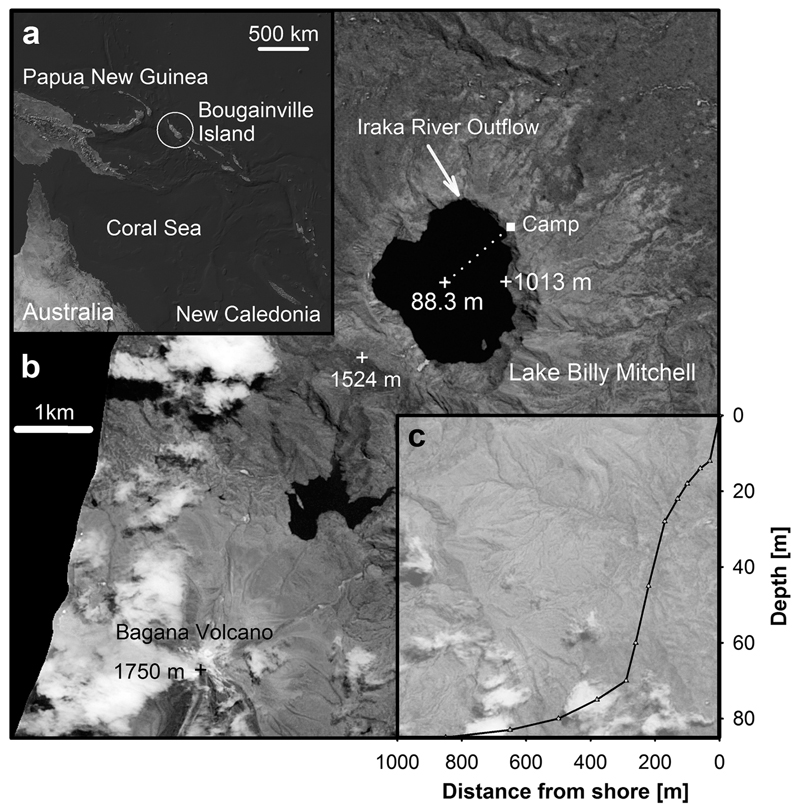
Lake Billy Mitchell [deepest point 88.3 m sounded at 06° 05.427′ S, 155° 13.472′ E, area of 3 km2, maximum length 2.26 km, 1,013 m above sea level (a.s.l.)] fills a caldera (maximum altitude 1,524 m) that is located 6 km northeast of the Bagana shield volcano (1,750 m) in central Bougainville Island (Figure 1). A lava dome 1 ha wide forms an island near its southern shore. The lake drains through a gorge into the Iraka River that flows down the eastern flank of the volcano and discharges into the sea after 23.5 km. The flanks of the Billy Mitchell caldera are still unstable with recurring landslides. The volcano has erupted twice during historical times. The most recent eruption, around A.D. 1580, likely formed the nearly vertical, steep-walled caldera and emitted about 14 km3 of tephra and ignimbrite. It was one of the largest Holocene eruptions on Papua New Guinea and may have been partly responsible for a global short-term atmosphere cooling event (Briffa et al. 1998). An earlier large explosive eruption, about half the size of the most recent one, occurred around A.D. 1030. Both events formed an ash layer of about 3,500 km2 (McKee et al. 1990, Global Volcanism Program 2016).
Figure 1.
Map of Southwest Pacific region (a), aerial photograph of Lake Billy Mitchell and surroundings (b), and depth profile from the campsite to the deepest point of the lake (c). © by eoVision.
Sampling
Water depths were gauged with a 100 m portable sonar sensor (Lixada) along a transect line from the deepest point recorded to the northeastern shoreline. An anchor was set near the center of the lake at 06° 05.154′ S, 155° 13.690′ E (depth 76.3 m). Water samples for in situ measurements were collected with a 1.5-liter trap (Schindler-Patalas) on 4 April (1430–1800 hours) and 5 April (1000– 1130 hours) 2015 from 0, 5, 10, 15, 20, 25, 30, 40, 50, 60, and 75 m depths. Temperature (±0.1°C) was measured with a liquid-in-glass thermometer mounted inside the trap. Dissolved oxygen, oxygen saturation, pH, and conductivity were assessed with a 40d electrode (HACH HQ), which was placed inside the trap.
Physicochemistry
Water samples for chemical analyses were collected on 6 April 2015 between 0730 and 0900 hours at 0, 25, 50, and 75 m depths. For the determination of total phosphorus (Ptot), 100 ml of unfiltered water was stored without preservation. The rest of the water was filtered through precombusted GF/F filters (Whatman) using a 100 ml syringe tipped with a 50 mm filter holder (Sartorius). For each depth, a 100 ml and a 250 ml plastic bottle each were rinsed with filtered water from the trap and filled completely. The former sample was acidified with 0.75 ml of 2M HCl to approx. pH 2, the other one was stored without preservation. All samples were wrapped in aluminum foil and stored in a thermobag to prevent heating during transport from the lake. Samples were then stored at around 4°C in the dark until analyses in the Innsbruck University laboratory, Austria, were undertaken between 27 April and 8 May 2015. Ions (, Na+, K+, Mg2+, Ca2+) were quantified by ion chromatography using a Dionex ICS 1000. Dissolved organic carbon (DOC) and dissolved nitrogen (DN) concentrations were analyzed from the acidified samples using a Total Organic Carbon Analyzer (Shimadzu TOC-Vc series) equipped with a Total Nitrogen Module (TNM-1). Both parameters were detected simultaneously after combustion and catalytic oxidation of the injected sample. Ptot, dissolved phosphorus (Pdis), and dissolved reactive silica (DRSi) were analyzed using the Molybdate methods after Vogler (1966) and Smith and Milne (1981). Total alkalinity was measured by Gran titration.
Biological Sampling
Submerged plants were sampled through free diving at 06° 5.90′ S, 155° 13.85′ E from the shore to around 3 m depth, preserved in 50% ethyl alcohol (EtOH) or dried, and identified in the laboratory. Taxonomic literature used was as follows: Bryophyta, Buck et al. (2002), Higuchi and Nishimura (2002); Spermatophyta, Casper and Krausch (1981), Womersley (1995), Lunkai and Strong (2010), Ganie et al. (2015). All samples are deposited at R.S.’s laboratory at the University of Salzburg, Austria.
Plankton samples were collected at 0, 25, 50, and 75 m depths. Phytoplankton and ciliate samples were taken on 5 April between 1000 and 1130 hours; 100 ml of unfiltered water was preserved in Lugol’s solution for phytoplankton biovolume estimations and another 45 ml was preserved with a few drops of formaldehyde for additional taxonomy. For biovolume estimations, cells for each taxon were first enumerated using an inverted microscope (Nikon Diaphot) according to Utermöhl (1958). For large forms, 100× magnification and the whole chamber area was used; for small taxa, 400× and chamber diagonals. For abundant forms, around 500 and 2,000 individuals were counted to guarantee statistical quality. Individual algal biovolumes were calculated using geometric formulas of shapes similar to the respective phytoplankton cells (Hillebrand et al. 1999). At least 40 cells of each taxon were measured for estimating individual biovolumes, which were then used to calculate the mean biovolume of the respective taxon. The overall biovolume was calculated by multiplying cell number with mean biovolume of respective taxa. Photos were taken with a Zeiss Axio-Imager, equipped with a Zeiss Axiocam HRc camera. Taxonomic literature used was as follows: Chlorococales, Komarek and Fott (1983), Bock and Krienitz (2012); Ulotrichales, Skuja (1956), Remond and Hindák (1994); Chrysophyceae, Starmach (1985); Bacillariophyceae, Krammer and Lange-Bertalot (1988, 1991); Dinophyta, Popovsky and Pfister (1990).
Ciliates were identified from both living individuals and after quantitative protargol stain (QPS) (after Skibbe 1994 and Pfister et al. 1999). For live observations, around 0.5 liter from each sampling depth was filtered with a 10 μm mesh net and kept in 45 ml vials without preservation. The vials were opened at least every 2 days to ensure oxygen supply for the protists. Living specimens were observed 1 month after lake sampling. For QPS analyses, 20 ml of unfiltered water was preserved with EtOH (50% and increased to around 70% final concentration 1 month later). All ciliate samples were observed under magnifications up to 1,000× with an Olympus BX51 microscope equipped with brightfield and digital interference contrast. Images were taken with a Jenoptik camera and software connected to the microscope. Ciliate identification followed the keys of Foissner et al. (1991, 1994, 1999). The permanent QPS slides are deposited in the Biology Centre of the Museum of Upper Austria and are available upon request.
Micrometazoan zooplankton samples were collected on 5 April between 1400 and 1500 hours. For quantitative analyses, 1.5 liters of water was filtered through a net (mesh size 30 μm). An integrated sample was taken with a plankton net (30 μm mesh size, 45 cm diameter) on 6 April (0900 hours) from 25 m to the surface. All samples were immediately preserved with 2% formaldehyde, and around 3 hr later the final concentration was raised to 4%. In the laboratory, organisms were stained with Bengal Rose for at least 24 hr. Copepods, cladocerans, and rotifers were counted with a Leitz Labovert inverted microscope at 100× magnification using the sedimentation method after Utermöhl (1958). Taxonomic literature used was as follows: Rotifera, Koste (1978); Cladocera, Kořínek (2002); Copepoda, Holyńska and Stoch (2012).
Mollusks and insect larvae and imagos were collected at the same location as macrophytes for about 2 hr by dipnetting and preserved in 50% EtOH. Taxonomic literature used was as follows: Mollusca, Starmühlner (1976); Ephemeroptera, Theischinger and Hawking (2006); Heteroptera, Andersen and Weir (2004).
Freshwater eels (Genus Anguilla) were caught by local fishermen with hand nets and by hook and line in the lake and its outflow as well as in the lower stretches of the Iraka River. Species were identified according to dentition in the upper jaw following Ege (1939). Total length was measured to the nearest millimeter, and total weight was recorded to the nearest 10 g.
Results
Physicochemistry
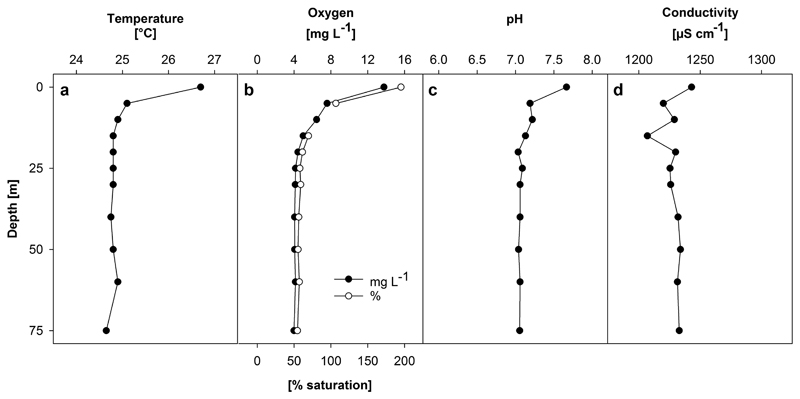
Water temperatures ranged from 26.7°C at the surface to 24.7°C at 75 m close to the lake bottom (Figure 2a ). Surface temperatures fluctuated between 25.2°C at 0800 hours to 27.4°C at around 1430 hours. Oxygen levels showed supersaturation near the surface (13.8 mg liter−1, 195%) and then declined quickly to 7.6 mg liter−1 (107%) at 5 m. With depth, oxygen concentration decreased gradually to 4.0 mg liter−1 (55%) at 75 m (Figure 2b ). pH decreased from 7.7 at the surface to 7.1 at 15 m and remained constant to the deepest point (Figure 2c ). Conductivity showed some fluctuation between 1,243 and 1,207 μS cm−1 in the upper 15 m and remained around 1,230 μS cm−1 towards the lake bottom (Figure 2d ).
Figure 2.
Depth profiles of (a) temperature, (b) oxygen, (c) pH, and (d) conductivity in Lake Billy Mitchell on 4 and 5 April 2015.
Ion contents are characteristic of wellbuffered carbonate waters exhibiting nearly constant concentrations throughout the water column (Table 1). A distinct vertical gradient could be observed only for nutrients, especially phosphorus, which reached maximum values near the sediment. Contrarily, DOC and DN concentrations were highest near the surface. Around 85% of Ptot was available as Pdis. NO3-N could only be detected at very low concentrations in 75 m, and NH4-N was generally below detection limit. Relatively high DRSi was observed at all depths.
Table 1.
Water Chemistry of Lake Billy Mitchell on 6 April 2015
| Depth | [meq L−1] | [meq L−1] | Cl− [meq L−1] | Na+ [meq L−1] | K+ [meq L−1] | Mg2+ [meq L−1] | Ca2+ [meq L−1] | [μg L-−1] | [μg L−1] | Ptot [μg L−1] | Pdis [μg L−1] | DOC [mg L−1] | DN [mg L−1] | DRSi [mg L−1] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0m | 6.46 | 3.36 | 3.47 | 5.42 | 0.37 | 2.20 | 5.77 | 0 | 0 | 21.2 | 17.6 | 6.91 | 0.45 | 35.46 |
| 25 m | 6.50 | 3.34 | 3.45 | 5.40 | 0.37 | 2.19 | 5.76 | 0 | 0 | 25.9 | 22.4 | 6.13 | 0.42 | 36.06 |
| 50 m | 6.44 | 3.26 | 3.41 | 5.31 | 0.37 | 2.16 | 5.67 | 0 | 0 | 25.9 | 22.1 | 6.90 | 0.49 | 36.11 |
| 75 m | 6.46 | 3.38 | 3.58 | 5.37 | 0.37 | 2.19 | 5.74 | 12 | 0 | 30.6 | 24.7 | 4.59 | 0.35 | 35.35 |
Biological Communities
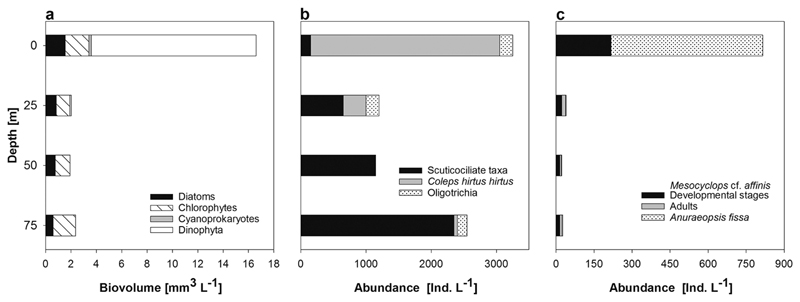
Eighteen phytoplankton taxa were recognized (Table 2, Figure 3), belonging to Cyanoprokaryota (1), Chlorophyta (9), Heterokontophyta (6), Chrysophyceae (1), and Dinophyta (1). The most prominent taxon was assigned by its thecal plates to Peridiniopsis cf. penardii (Table 2), followed by the filamentous green alga Planctonema lauterbornii. Algal biovolume reached 16.6 mm3 liter−1 at the surface, but in deeper layers values were generally <3 mm3 liter−1 (Figure 4a ).
Table 2.
List of Species Found in Lake Billy Mitchell
| Taxon | |
|---|---|
| Cyanoprokaryota | Amoebozoa |
| Anabaena sp. | Centrohelida |
| Chloro- and Streptophyta | Ciliophora |
| Coelastrum astroideum De Notaris, 1867 | Cinetochilum margaritaceum (Ehrenberg) Perty, 1849 |
| Cosmarium sp. | Coleps hirtus hirtus (Müller) Nitzsch, 1827 |
| Crucigenia cf. quadrata Morren, 1830 | Oligohymenophorea, Scuticociliatia (3 indet. species) |
| Franceia javanica (Bernard) Hortobagyi, 1962 | Pelagohalteria viridis (Fromentel), Foissner, Skogstad |
| Hindakia sp. | & Pratt, 1988 |
| Monorhaphidium sp. | Strobilidium caudatum (Fromentel) Foissner, 1987 |
| Mucidosphaerium pulchellum (Wood) Bock, Pröschold & Krienitz, 2011 | Rotifera |
| Anuraeopsis fissa Gosse, 1851 | |
| Planctonema lauterbornii Schmidle, 1903 | Cladocera |
| Tetrastrum triangulare (Chodat) Komarek, 1974 | Ceriodaphnia cornuta Group (Sars, 1885) |
| Heterokontophyta-Bacillariophyceae | Copepoda |
| Cocconeis sp. | Mesocyclops cf. affinis van de Velde, 1987 |
| Cyclotella meneghiniana Kützing, 1844 | Mollusca |
| Encyonema sp. | Amerianna sp. |
| Nitzschia cf. perminuta (Grunow) M. Peragallo, 1903 | Melanoides tuberculatus (Müller, 1774) |
| Orthoseira sp. | Ephemeroptera |
| Ulnaria ulna (Nitzsch) Compère, 2001 | Anax sp. |
| Heterokontophyta-Chrysophyceae | Aeshnidae indet. |
| Lagynion sp. | Coenagrionidae indet. |
| Dinophyta | Ischnura sp. |
| Peridiniopsis cf. penardii (Lemmermann) Bourrelly, 1968 | Libellulidae indet. |
| Tramea eurybia Selys, 1878 | |
| Bryophyta | Heteroptera |
| Hydrogonium sp. | Anisops spp. (3 species) |
| Racopilum sp. | Microvelia sp. |
| Vesicularia sp. | Pisces |
| Spermatophyta | Anguilla marmorata Quoy & Gaimard, 1824 |
| Ceratophyllum demersum L., 1753 | Anguilla megastoma Kaup, 1856 |
| Eleocharis dulcis (Burman) Trinius ex Henschel, 1833 | |
| Persicaria barbata (L.) H. Hara, 1966 |
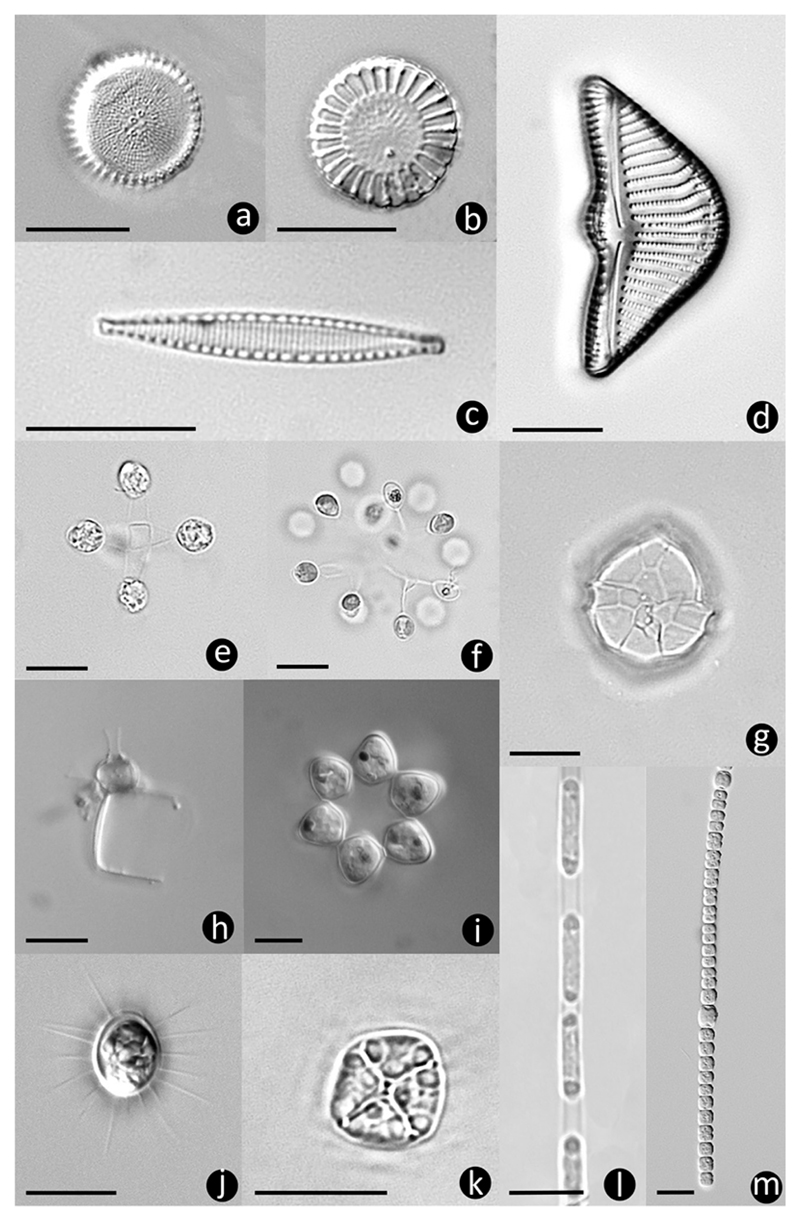
Figure 3.
Phytoplankton taxa: (a) Orthoseira sp. with central tube processes, (b) Cyclotella meneghiniana, (c) Nitzschia cf. perminuta, (d) Encyonema sp., (e) Mucidosphaerium pulchellum, (f) Hindakia sp., (g) Peridiniopsis cf. penardii, (h) Lagynion sp. attached to Orthoseira (cross-section through cell), (i) Coelastrum astroideum, (j) Franceia javanica, (k) Tetrastrum triangulare, (l) Planctonema lauterbornii with polar oil droplets in each cell, (m) Anabaena sp. Scale bars are 10 μm.
Figure 4.
Depth profiles of (a) phytoplankton biovolume, (b) ciliate abundance, and (c) crustacean and rotifer abundance on 4 and 5 April 2015.
More than 50% of the lakeshore was fringed by a belt of water chestnut, Eleocharis dulcis (approximately 5 m in width), and was interspersed with Persicaria barbata. Ceratophyllum demersum was the only submerged angiosperm detected. This species was frequent throughout the littoral zone, and single plants could also be observed at the maximum diving depth of approximately 3 m. Several mosses (Hydrogonium sp., Racopilum sp., and Vesicularia sp.) were found attached to rocks along the shoreline.
Four small hymenostomatid scuticociliate taxa (Cinetochilum margaritaceum and three indeterminate species), the prostomatid Coleps hirtus hirtus, the two oligotrichs Pelagohalteria viridis and Strobilidium caudatum, and a few specimens belonging to other taxa were found (Table 2, Figure 5a–g ). Total ciliate abundance in all depths was <5 individuals ml−1 (Figure 4b ). At the surface, C. hirtus hirtus dominated the ciliate assemblage, and with depth one scuticociliate taxon increased in number. Food vacuole analyses from preserved specimens revealed that C. hirtus hirtus fed on the co-occurring dominant dinoflagellate Peridiniopsis cf. penardii. Also in shallow water, the only mixotrophic (algal symbiontbearing) species, P. viridis, was found. Some other heterotrophic protists such as testate and naked Amoebozoa and Centrohelida were also observed.
Figure 5.
Ciliate (a–g) and micrometazoan (h–j) taxa from life (a–e), after a quantitative protargol stain (f–h), and after preservation with formaldehyde (i–j). (a–c) three different scuticociliate taxa, (d) Cinetochilum margaritaceum, (e) Coleps hirtus hirtus, (f) Pelagohalteria viridis, (g) Strobilidium caudatum, (h) Anuraeopsis fissa, (i) nauplius larva of Mesocyclops, (j) Mesocyclops adult. Scale bars in (a–h) are 20 μm, in (i) 50 μm, and in (j) 200 μm.
Only three micrometazoan zooplankton species were found (Table 2, Figure 5h–j ). Within rotifers, the only species detected was the cosmopolitan Anuraeopsis fissa, which reached an abundance of around 600 individuals liter−1 in the epilimnion (Figure 4c ). Deeper, the zooplankton community was dominated by the copepod Mesocyclops cf. affinis, occurring in all samples down to 75 m. Although nauplii and copepodids dominated at the surface (217 individuals liter−1), adults were found only below 25 m deep (2–14 individuals liter−1). Based on morphological analyses, this species showed intermediate features between M. affinis and M. dissimilis. A cladoceran species belonging to the Ceriodaphnia cornuta group was found in low numbers (0–1 individuals liter−1) down to 50 m. Only three individuals were detected in the quantitative samples; however, in the qualitative sample from 0 to 25 m depth this species was found more frequently.
Short sampling time and insufficient preservation resulted in a preliminary list of two mollusks, two heteropterans, and six ephemeropteran taxa (Table 2). In the lake, five eels (62–102 cm, 0.44–2.46 kg) were collected and assigned to Anguilla megastoma according to their upper jaw morphology. In the river, 22 Anguilla marmorata (37–102 cm, 0.12– 2.94 kg) and four A. megastoma (45–81 cm, 0.12–0.76 kg) were caught. One individual (51 cm, 0.28 kg) exhibited intermediate dentition. The stomach contents of one specimen of A. megastoma (102 cm, 2.46 kg) from the lake included eight Heteroptera, five Ephemeroptera larvae, one gastropod, and one Coleoptera.
Discussion
Physicochemical profiles measured during this single visit to Lake Billy Mitchell indicated (1) mixis of the entire water body and (2) low oxygen consumption at greater depths. Oxygen saturation (55%) was still found down to the deepest point at 88.3 m depth, although the lake is protected by a steep crater rim of about 100–300 m height. Obviously, the maximum lake diameter of more than 2 km permits enough wind friction to erode a permanent chemocline and allow transport of oxygen to the deepest layers from time to time. However, it cannot be excluded that convection currents resulting from geothermal heating carry oxygen into deeper layers. Solar heating and night cooling within the first few meters of the water column possibly indicated “atelomixis,” the formation and erosion of the thermocline on a daily basis (see Barbosa and Padisák 2002, Tavera and Martínez-Almeida 2005, Fetahi et al. 2014, Degefu and Schagerl 2015). A more pronounced stratification may develop during the dry season (May–September). Nevertheless, high supersaturation with CO2 in the hypolimnion is highly unlikely, even during more stagnant periods.
The chemical signature places Lake Billy Mitchell into the group of quiescent volcanic lakes with neutral pH (Varekamp et al. 2000, Marini et al. 2003). The ion content reflects surface runoff and erosion of volcanic bedrock, and the DRSi concentrations indicate silicate weathering. Additional ion input results from the inflow of volcanic springs, as observed on the eastern lakeshore. Previous studies on Lake Letas, a large caldera lake in Vanuatu, clearly showed high ion input and a fertilizing effect through hot inflows (Sichrowsky et al. 2015). Sulfides, chlorides, and bicarbonates as the main products of the acidifying agents derive from volcanic gases and are balanced by cations from water-rock interactions, in Lake Billy Mitchell mainly sodium and calcium (Marini et al. 2003). Atelomixis may partly explain the species composition of phytoplankton, which comprised a combination of buoyant and heavier forms. Two buoyant species prevailed in the lake: the filamentous chlorophyte Planctonema lauterbornii has loosely arranged cells, each equipped with two terminal oil droplets; the dinoflagellate Peridiniopsis cf. penardii has the ability to move vertically depending on nutrient and light availability and grazing pressure (Frempong 1984, Xu et al. 2010). On the other hand, heavy forms of the diatom genera Nitzschia, large Ulnaria, and Encyonema were also observed, which indicates daily turnover of large water masses. Atelomixis is probably one of the key factors in structuring plankton communities, especially in tropical highmountain lakes (Barbosa and Padisák 2002, Souza et al. 2008, Barbosa et al. 2013, Fetahi et al. 2014, Degefu and Schagerl 2015). In temperate lakes, nighttime convection is comparatively weak during calm periods, so phytoplankton species with less resistance to sinking fail to remain in the epilimnion (Padisák et al. 2003).
Phytoplankton species number was low (18 taxa), which can partly be explained by the method applied and by the single sampling event. Another study investigating the plankton assemblage of a crater lake located in Costa Rica (43 taxa) (Umaña and Jiménez 1995) used centrifugation to consider rare forms also. Moreover, those authors visited the system three times, which also contributed to higher species numbers. Cocquyt et al. (2010) in their study of the crater lake Kyaninga (Uganda) identified more than 150 taxa in their 1 yr study of planktonic and benthic forms. Phytoplankton abundance was also low with the exception of the surface sample, where buoyant taxa were concentrated, although Ptot concentrations of ~25 μg liter−1 indicated meso- to eutrophic conditions. Nitrogen limitation could be responsible for low phytoplankton biomass, because was below the detection limit except in the deepest water sample, but this assumption needs to be proved in further investigations. Nitrogen limitation of phytoplankton seems to be common in tropical lakes and probably results from denitrification during anoxia over prolonged times of stagnation (Talling and Lemoalle 1998, Lewis 2002), which may indicate a more stable stratification and anoxic conditions during the dry season. On the other hand, a substantial proportion of dissolved nitrogen may also be available as DON, which can act as an important N source for primary production (Berman and Bronk 2003, Bronk et al. 2007). In addition, deeper water layers were likely shaded, slowing down primary productivity. Direct observations from free diving showed that transparency of the surface water was low. The low light penetration was likely caused by phytoplankton and increased concentrations of DOC, which may result from long retention times and accumulation of poorly degradable humic substances.
The macrophyte vegetation consisted of three spermatophyte and three moss species. The wild form of the water chestnut, Eleocharis dulcis, is a perennial freshwater wetland species (Ash and Ash 1984) widely distributed in the tropics. In Oceania its range extends from Melanesia and Palau in the western Pacific to Tonga and Samoa in Polynesia (Smith 1979). The cultivated form is used as building material, and the corms are eaten as a vegetable (Ghazanfar 2001). The second species occurring along the lakeshore, Persicaria barbata, is distributed from Saudi Arabia to eastern Asia, Himalayas to western and southern China, Bhutan, India, Indonesia, Malaysia, Myanmar, Nepal, New Guinea, Philippines, Sri Lanka, Thailand, and Vietnam (Gupta 2013) and is typically found along streams and lakes at an altitude of 600 to 1,800 m a.s.l. The only completely submerged macrophyte found was the cosmopolitan (Cook 1996) and shade-adapted (Wells et al. 1997) Ceratophyllum demersum. Under appropriate conditions it forms dense monospecific stands and thus can dominate entire bodies of water. It competes against other macrophytes and also against phytoplankton for inorganic nitrogen and light (Mjelde and Faafeng 1997). Furthermore it generally suppresses the growth of other autotrophic organisms by allelopathy (Gross et al. 2003). The three bryophyte genera Hydrogonium, Racopilum, and Vesicularia all have a tropical or subtropical distribution (Frey et al. 2009, eFlores 2016, Moss Flora of China 2016).
Scuticociliates are common in eutrophic waters or in the hypolimnia of temperate deep lakes (Beaver and Crisman 1989, Müller et al. 1991, Sonntag et al. 2006, Zingel et al. 2006). Such filter-feeding ciliates are efficient bacterial grazers and usually highly abundant (Posch et al. 2001, Ong’ondo et al. 2013). Cinetochilum margaritaceum is a cosmopolitan eurysaprobic species found in almost every habitat all over the world including remote lakes in the Pacific as well as high-mountain lakes (see Foissner et al. 1994, Sichrowsky et al. 2015; B. Kammerlander, pers. comm.).
Planktonic ciliates such as the omnivorous Coleps hirtus hirtus and Strobilidium caudatum as well as the mixotrophic Pelagohalteria viridis are widespread. Mixotrophic species are often predominant in the euphotic zone of temperate lakes and may dominate ciliate assemblages during the warm season (Sonntag et al. 2006, 2011).
The rotifer and crustacean plankton community was depauperate with only three species, probably due to the fairly recent formation of the lake basin, limited range of food, and potential geothermal activity affecting the water body from time to time. The cosmopolitan and warm stenothermic rotiferan species Anuraeopsis fissa is widespread throughout the South Pacific region (Schabetsberger, Drozdowski, et al. 2009) and can be considered a colonizer species. It has previously been found in higher concentrations near the thermocline in other tropical crater lakes (Schabetsberger et al. 2004; Schabetsberger, Rott, et al. 2009; Sichrowsky et al. 2014). The taxonomy and distribution of the genus Mesocyclops in Oceania is not yet fully resolved (Holyńska and Stoch 2012). The copepod Mesocyclops cf. affinis could not clearly be assigned, because it showed transitions between M. affinis and M. dissimilis. The distributions of these two taxa were reported to extend from New Guinea to Indochina and from Japan to Vietnam, respectively. They may be one entity or incipient species (Fabio Stoch, pers. comm.). The developmental stages of Mesocyclops populated the layers near the surface, whereas adult copepods dominated deeper water. The exact taxonomic status of the cladoceran species belonging to the Ceriodaphnia cornuta group remains unclear. The taxon was originally described from Australia, where three cryptic sibling species may exist (Sharma and Kotov 2013). In addition, C. cornuta–like morphotypes are widely distributed across the globe in the tropics and subtropics, consisting of several cryptic species with no morphological diagnostic traits identified to date (Sharma and Kotov 2013). Too few specimens were caught in the quantitative samples to assess its vertical distribution.
Low sampling success and insufficient preservation resulted in an incomplete list of macroinvertebrates. We identified the euryoecious gastropod Melanoides tuberculatus, which is found throughout the Indo-Pacific region and has been introduced to Central and South America. The species is common in lakes and rivers and is viviparous and parthenogenetic (Berry and bin Haji Kadri 1974). Amerianna spp. are usually found in lentic bodies of water, lay eggs, and are distributed in warm regions of Australasia; they are also tolerant to differences in water quality (Starmühlner 1976). The dragonfly Tramea eurybia has been reported from the Andaman Islands, Indonesia, New Guinea, and Fiji (Theischinger and Hawking 2006).
The single fish species found in Lake Billy Mitchell was Anguilla megastoma. It usually inhabits the upper stretches of rivers and creeks (Ege 1939, Marquet and Galzin 1991). For example, the indigenous name in Tahiti is puhi-mauá (“eel of the mountains”) (Schmidt 1927). It was also caught close to the sea, but the dominant species in the lower stretches of the Iraqa River was Anguilla marmorata, the only freshwater eel previously documented from Bougainville Island (Powell and Powell 1999). We did not find this species in the lake. On Gaua Island, Vanuatu, elvers of A. marmorata and A. megastoma both surmount Siri Falls (120 m high) to reach Lake Letas 7 km inland. Hybridization between both eel species has been observed (Schabetsberger et al. 2015), which may explain the occurrence of individuals with intermediate dentition caught during this study.
Difficult access to the lake prevented a more detailed survey of Lake Billy Mitchell, and future studies are required to better understand its mixis regime and to provide a more complete species inventory. There likely is some weak geothermal activity at the currently dormant volcano, and hence the hazard of gas release could only be assessed by a long-term monitoring program. In addition, Lake Loloru fills another caldera of the volcano with the same name in southern Bougainville. McKee et al. (1990) considered it even more hazardous than the larger Lake Billy Mitchell, because more densely populated areas could be affected by airfall tephra and lahars. Hence both lakes should be studied in more detail.
Acknowledgments
Thanks to Johannes Enroth, Regine Jahn, Alexey Kotov, Milen Marinov, Higuchi Masanobu, Veronika Mayerhofer, Fabio Stoch, Thomas von Rintelen, and Herbert Zettel for species identification. We thank Josef Franzoi, Salvador Morales Gomez, and Gry Larsen for help in the laboratory and Natalja Ring for preparing the ciliate permanent preparations (QPS). Two anonymous reviewers provided numerous constructive comments.
Literature Cited
- Andersen NM, Weir TA. Entomomonograph. Vol. 14 Apollo Books, Stenstrup, Denmark; CSIRO Publishing; Collingwood, Australia: 2004. Australian water bugs: Their biology and identification (Hemiptera-Heteroptera, Gerromorpha and Nepomorpha) [Google Scholar]
- Ash J, Ash W. Freshwater wetland vegetation of Viti Levu. Fiji N Z J Bot. 1984;22:377–391. [Google Scholar]
- Ball E, Glucksman J. Limnological studies of Lake Wisdom, a large New Guinea caldera lake with a simple fauna. Freshwater Biol. 1978;8:455–468. [Google Scholar]
- Ball E, Glucksman J. A limnological survey of Lake Dakataua, a large caldera lake in West New Britain, Papua New Guinea, with comparison to Lake Wisdom, a younger nearby caldera lake. Freshwater Biol. 1980;10:73–84. [Google Scholar]
- Barbosa FAR, Padisák J. The forgotten lake stratification pattern: Atelomixis. Verh Int Ver Limnol. 2002;28:1385–1395. [Google Scholar]
- Barbosa LG, Barbosa FAR, Araujo JMG, de Bicudo CEM. The dominance of desmids in tropical monomictic lakes (SE Brazil) Limnetica. 2013;32:71–86. [Google Scholar]
- Beaver JR, Crisman TL. The role of ciliated protozoa in pelagic fresh-water systems. Microb Ecol. 1989;17:111–136. doi: 10.1007/BF02011847. [DOI] [PubMed] [Google Scholar]
- Berman T, Bronk DA. Dissolved organic nitrogen: A dynamic participant in aquatic ecosystems. Aquat Microb Ecol. 2003;31:279–305. [Google Scholar]
- Berry AJ, bin Haji Kadri A. Reproduction in the Malayan freshwater cerithiacean gastropod Melanoides tuberculate . J Zool (Lond) 1974;172:369–381. [Google Scholar]
- Bock C, Krienitz L. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia. 2012;698:295–326. [Google Scholar]
- Briffa KR, Jones PD, Schweingruber FH, Osborn TJ. Influence of volcanic eruptions on the Northern Hemisphere summer temperature over the past 600 years. Nature (Lond) 1998;393:450–455. [Google Scholar]
- Bronk DA, See JH, Bradley P, Killberg L. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences. 2007;4:283–296. [Google Scholar]
- Buck WR, Vitt DH, Malcolm WM. Flora of Australia. Australian Biological Resources Study; Canberra: 2002. Supplementary Series No. 14. [Google Scholar]
- Casper SJ, Krausch H-D. Pteridophyta und Anthophyta, Part 2: Saururaceae to Asteraceae. In: Ettl H, Gerloff J, Heyming H, editors. Vol. 24 Süßwasserflora von Mitteleuropa; Gustav Fischer, Stuttgart: 1981. [Google Scholar]
- Chambers MR. The freshwater lakes of Papua New Guinea: An inventory and limnological review. J Trop Ecol. 1987;3:1–23. [Google Scholar]
- Cocquyt C, Plisnier P-D, Gelorini V, Rumes B, Verschuren D. Observations on the limnology and phytoplankton community of crater Lake Kyaninga (Uganda), with special attention to its diatom flora. Plant Ecol Evol. 2010;143:365–377. [Google Scholar]
- Cook CDK. Aquatic plant book. SPB Academic Publishing; Amsterdam, New York: 1996. [Google Scholar]
- Degefu F, Schagerl M. The phytoplankton community of tropical highmountain crater Lake Wonchi, Ethiopia. Hydrobiologia. 2015;755:197–208. [Google Scholar]
- eFlores. Bryophyte flora of North America. [Accessed 8 April 2016];2016 http://www.efloras.org/
- Ege V. A revision of the Genus Anguilla Shaw. Dana Rep. 1939;16:8–256. [Google Scholar]
- Fetahi T, Schagerl M, Mengistu S. Key drivers for phytoplankton composition and biomass in an Ethiopian highland lake. Limnologica. 2014;46:77–83. [Google Scholar]
- Foissner W, Blatterer H, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems, Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1991;1/91:1–478. [Google Scholar]
- Foissner W, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems, Band III: Hymenostomata, Prostomatida, Nassulida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1994;1/94:1–548. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Frempong E. A seasonal sequence of diel distribution patterns for the planktonic dinoflagellate Ceratium hirundinella in a eutrophic lake. Freshwater Biol. 1984;14:401–421. [Google Scholar]
- Frey W, Stech M, Fischer E. Bryophytes and seedless vascular plants. Vol. 3 Gebrüder Bornträger; Berlin, Stuttgart: 2009. Syllabus of plant families. [Google Scholar]
- Ganie AH, Tali BA, Khuroo AA, Reshi ZA, Les DH. Ceratophyllum platyacanthum subsp. oryzetorum (Kom.) Les (Ceratophyllaceae): An addition to the flora of India from Kashmir Himalaya. 2015 Check List 11: Article 1661. [Google Scholar]
- Ghazanfar SA. Eleocharis dulcis (kuta), a plant of economic and cultural importance in the South West Pacific: Habitat restoration efforts in the vanua of Buca, Vanua Levu. Fiji S Pac J Nat Appl Sci. 2001;19:51–53. [Google Scholar]
- Global Volcanism Program. National Museum of Natural History, Smithsonian Institution. [Accessed 5 February 2016]; http://www.volcano.si.edu/
- Gross EM, Erhard D, Jvanyi E. Allelopathic activities of Ceratophyllum L. and Najas marina ssp. intermedia (Wolfgang) Casper. Hydrobiologia. 2003;506:583–589. [Google Scholar]
- Gupta AK. Persicaria barbata. [Accessed 8 April 2016];The IUCN Red List of Threatened Species 2013. 2013 http://www.iucnredlist.org/ [Google Scholar]
- Higuchi M, Nishimura N. Studies of the bryophyte flora of Vanuatu. 4. Hypnaceae (Musci) Ann Tsukuba Bot Gard. 2002;21:91–94. [Google Scholar]
- Hillebrand H, Durselen DC, Kirschtel D, Pollingher U, Zohary T. Biovolume calculation for pelagic and benthic microalgae. J Appl Phycol. 1999;35:403–424. [Google Scholar]
- Holyńska O, Stoch F. Mesocyclops (Crustacea, Copepoda, Cyclopidae) in the South Pacific islands. Zool Anz. 2012;251:237–252. [Google Scholar]
- Kling GW. Seasonal mixing and catastrophic degassing in tropical lakes, Cameroon, West Africa. Vol. 237. Science; Washington, D.C: 1987. pp. 1022–1024. [DOI] [PubMed] [Google Scholar]
- Kling GW. Comparative transparency, depth of mixing, and stability of stratification in lakes of Cameroon. West Africa Limnol Oceanogr. 1988;33:27–40. [Google Scholar]
- Komarek J, Fott B. Chlorophyceae, Ordnung: Chlorococcales. In: Huber-Pestalozzi G, editor. Das Phytoplankton des Süßwassers. Vol. 16 Schweizerbart’sche Verlagsbuchhandlung; Stuttgart: 1983. [Google Scholar]
- Kořínek V. Cladocera. In: Fernando CH, editor. A guide to tropical freshwater zooplankton. Backhuys Publishers; Leiden: 2002. pp. 69–122. [Google Scholar]
- Koste W. Die Rädertiere Mitteleuropas. Gebrüder Borntraeger; Berlin: 1978. Rotatoria. [Google Scholar]
- Krammer K, Lange-Bertalot H. Bacillariophyceae, Part 2: Bacillariophyceae, Ephithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D, editors. Süßwasserflora von Mitteleuropa. Vol. 1 Gustav Fischer; Stuttgart: 1988. [Google Scholar]
- Krammer K, Lange-Bertalot H. Bacillariophyceae, Part 3: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D, editors. Süßwasserflora von Mitteleuropa. Vol. 1 Gustav Fischer; Stuttgart: 1991. [Google Scholar]
- Lewis WM., Jr Causes for the high frequency of nitrogen limitation in tropical lakes. Verh Int Ver Limnol. 2002;28:210–213. [Google Scholar]
- Lunkai D, Strong MT. Eleocharis. Flora of China. In: Wu ZY, Raven PH, Hong DY, editors. Acoraceae through Cyperaceae. Vol. 23. Science Press; Beijing, and Missouri Botanical Garden Press, St. Louis: 2010. pp. 188–200. [Google Scholar]
- Manville V. Volcano-hydrological hazards from volcanic lakes. In: Rouwet D, Christenson B, Tassi F, Vandemeulebrouck J, editors. Volcanic lakes. Springer; Berlin Heidelberg: 2015. pp. 93–123. [Google Scholar]
- Marini L, Vetuschi Zuccolini M, Saldi G. The bimodal pH distribution of volcanic lake waters. J Volcanol Geotherm Res. 2003;121:83–98. [Google Scholar]
- Marquet G, Galzin R. The eels of French Polynesia: Taxonomy, distribution and biomass. La Mer. 1991;29:8–17. [Google Scholar]
- McKee CO, Johnson RW, Rogerson R. Explosive volcanism on Bougainville Island: Ingnimbrites, calderas, and volcanic hazards. Proc Pac Rim Congr. 1990;2:237–245. [Google Scholar]
- Melack J. Morphometric, physical and chemical features of the volcanic crater lakes of western Uganda. Arch Hydrobiol. 1978;84:430–453. [Google Scholar]
- Mjelde M, Faafeng B. Ceratophyllum demersum hampers phytoplankton development in some small Norwegian lakes over a wide range of phosphorus level and geographic latitude. Freshwater Biol. 1997;37:355–365. [Google Scholar]
- Moss Flora of China. [Accessed 8 April 2016];2016 http://www.efloras.org/
- Müller H, Geller W, Schöne A. Pelagic ciliates in Lake Constance: Comparison of epilimnion and hypolimnion. Verh Int Ver Limnol. 1991;24:846–849. [Google Scholar]
- Ong’ondo GO, Yasindi AW, Oduor SO, Jost S, Schagerl M, Sonntag B, Boenigk J. Ecology and community structure of ciliated protists in two alkaline-saline Rift Valley lakes in Kenya with special emphasis on Frontonia . J Plankton Res. 2013;35:759–771. [Google Scholar]
- Osborne PL. Limnology in the wet tropics: Papua New Guinea. In: Gopal B, Wetzel RG, editors. Limnology in developing countries. International Association of Theoretical and Applied Limnology; New Dehli: 1995. pp. 121–160. [Google Scholar]
- Padisák J, Soróczki-Pintér É, Rezner Z. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton: An experimental study. Hydrobiologia. 2003;500:243–257. [Google Scholar]
- Pecoraino G, D’Alessandro W, Inguaggiato S. The other side of the coin: Geochemistry of alkaline lakes in volcanic areas. In: Rouwet D, Christenson B, Tassi F, Vandemeulebrouck J, editors. Volcanic lakes. Springer; Berlin Heidelberg: 2015. pp. 219–237. [Google Scholar]
- Pfister G, Sonntag B, Posch T. Comparison of a direct live count and an improved quantitative protargol stain (QPS) in determining abundance and cell volumes of pelagic freshwater protozoa. Aquat Microb Ecol. 1999;18:95–103. [Google Scholar]
- Popovsky J, Pfister LA. Dinophyceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D, editors. Süßwasserflora von Mitteleuropa. Vol. 6 Gustav Fischer; Stuttgart: 1990. [Google Scholar]
- Posch T, Jezbera J, Vrba J, Šimek K, Pernthaler J, Andreatta S, Sonntag B. Size selective feeding in Cyclidium glaucoma (Ciliophora, Scuticociliatida) and its effects on bacterial community structure: A study from a continuous cultivation system. Microb Ecol. 2001;42:217–227. doi: 10.1007/s002480000114. [DOI] [PubMed] [Google Scholar]
- Powell JH, Powell RE. The freshwater ichthyofauna of Bougainville Island, Papua New Guinea. Pac Sci. 1999;53:346–356. [Google Scholar]
- Remond O, Hindák F. Morphology and sheath architecture in the filamentous green alga Planctonema lauterbornii (Chlorophyceae, Ulotrichales) Bull Soc Neuchâtel Sci Nat. 1994;117:99–109. [Google Scholar]
- Schabetsberger R, Drozdowski G, Drozdowski I, Jersabek CD, Rott E. Limnological aspects of two tropical crater lakes (Lago Biao and Lago Loreto) on the island of Bioko (Equatorial Guinea) Hydrobiologia. 2004;524:79–90. [Google Scholar]
- Schabetsberger R, Drozdowski G, Rott E, Lenzenweger R, Jersabek C, Fiers F, Traunspurger W, Reiff N, Stoch F, Kotov A, Martens K, Schatz H, Kaiser R. Losing the bounty? Investigating species richness in isolated freshwater ecosystems of Oceania. Pac Sci. 2009;63:153–179. [Google Scholar]
- Schabetsberger R, Økland F, Kalfatak D, Sichrowsky U, Tambets M, Aarestrup K, Gubili C, Sarginson J, Boufana B, Jehle R, Dall’Olmo G, Miller MJ, Scheck A, Kaiser R, Quartly G. Genetic and migratory evidence for sympatric spawning of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 2015;521:171–187. [Google Scholar]
- Schabetsberger R, Rott E, Friedl G, Drozdowski G, Razafinandraivo E, Holmes C. First limnological characterization of the tropical crater lake Amparihibe in the Makira Protected Area, Madagascar. Eco-Mont. 2009;1:43–52. [Google Scholar]
- Schmidt J. Les Anguilles de Tahiti. 15. La Nature; Paris: 1927. Jul, pp. 57–65. [Google Scholar]
- Sharma P, Kotov AA. Molecular approach to identify sibling species of the Ceriodaphnia cornuta complex (Cladocera: Daphniidae) from Australia with notes on the continental endemism of this group. Zootaxa. 2013;3702:79–89. doi: 10.11646/zootaxa.3702.1.5. [DOI] [PubMed] [Google Scholar]
- Sichrowsky U, Schabetsberger R, Sonntag B, Stoyneva M, Maloney AE, Nelson DB, Richey JN, Sachs JP. Limnological characterization of volcanic crater lakes on Uvea Island (Wallis and Futuna, South Pacific) Pac Sci. 2014;68:333–343. [Google Scholar]
- Sichrowsky U, Schabetsberger R, Pall K, Sonntag B, Stoyneva M, Kalfatak D, Økland F, Scheck A, Tambets M. Limnological characterization of the largest freshwater lake in remote Oceania. Pac Sci. 2015;69:165–180. [Google Scholar]
- Skibbe O. An improved quantitative protargol stain for ciliates and other planktonic protists. Arch Hydrobiol. 1994;130:339–347. [Google Scholar]
- Skuja H. Taxonomische und biologische Studien über das Phytoplankton Schwedischer Binnengewässer. Nov Act Reg Soc Sci Upsala. 1956;16:1–404. [Google Scholar]
- Smith AC. Flora Vitiensis nova: A new flora of Fiji. Vol. 1 Pacific Tropical Botanical Garden; Läwa‘i, Hawai‘i: 1979. [Google Scholar]
- Smith JD, Milne PJ. Spectrophotometric determination of silicate in natural waters by formation of small alpha, Greek-molybdosilicic acid and reduction with a tin(IV)–ascorbic acid–oxalic acid mixture. Anal Chim Acta. 1981;123:263–270. [Google Scholar]
- Sonntag B, Posch T, Klammer S, Teubner K, Psenner R. Phagotrophic ciliates and flagellates in an oligotrophic deep alpine lake: Contrasting variability with seasons and depths. Aquat Microb Ecol. 2006;43:193–207. [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Are freshwater mixotrophic ciliates less sensitive to solar UV radiation than heterotrophic ones? J Eukaryot Microbiol. 2011;58:196–202. doi: 10.1111/j.1550-7408.2011.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MBG, Barros CFA, Barbosa F, Hajnal E, Pádisak J. Role of atelomixis in replacement of phytoplankton assemblages in Dom Helvécio Lake, South-East Brazil. Hydrobiologia. 2008;607:211–224. [Google Scholar]
- Starmach K. Chrysophyceae und Haptophyceae. In: Ettl H, Gerolff J, Heynig H, Mollenhauer D, editors. Süßwasserflora von Mitteleuropa. Vol. 1 Gustav Fischer; Stuttgart: 1985. [Google Scholar]
- Starmühlner F. Beiträge zur Kenntnis der Süßwassergastropoden pazifischer Inseln. Ergebnisse der österreichischen Indopazifik-Expedition des 1. Zoologischen Institutes der Universität Wien Ann Naturhist Mus Wien, Ser B Bot Zool. 1976;80:473–656. [Google Scholar]
- Talling JF, Lemoalle J. Ecological dynamics of tropical inland waters. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Taran Y, Inguaggiato S, Cardellini C, Karpov G. Posteruption chemical evolution of a volcanic caldera lake: Karymsky Lake, Kamtchatka. Geophys Res Lett. 2013;40:5142–5146. [Google Scholar]
- Tavera R, Martínez-Almeida V. Atelomixis as a possible driving force in the phytoplankton composition of Zirahuén, a warm-monomictic tropical lake. Hydrobiologia. 2005;533:199–208. [Google Scholar]
- Theischinger G, Hawking J. The complete field guide to dragonflies of Australia. CSIRO Publishing; Collingwood: 2006. [Google Scholar]
- Thouret J-C, Abdurachman KE, Bourdier J-L, Bronto S. Origin, characteristics, and behaviour of lahars following the 1990 eruption of Kelud volcano, eastern Java (Indonesia) Bull Vulcanol. 1998;59:460–480. [Google Scholar]
- Umaña GV, Jiménez C. The basic limnology of a low altitude tropical crater lake: Cerro Chato, Costa Rica. Rev Biol Trop. 1995;43:131–138. [Google Scholar]
- Utermöhl H. Zur Vervollkommnung der quantitativen PhytoplanktonMethodik. Mitt Int Ver Theor Angew Limnol. 1958;9:1–38. [Google Scholar]
- Varekamp JC. The chemical composition and evolution of volcanic lakes. In: Rouwet D, Christenson B, Tassi F, Vandemeulebrouck J, editors. Volcanic lakes. Springer; Berlin Heidelberg: 2015. pp. 93–123. [Google Scholar]
- Varekamp JC, Pasternack GB, Rowe GL., Jr Volcanic lake systematics II. Chemical constraints. J Volcanol Geotherm Res. 2000;97:161–179. [Google Scholar]
- Vogler P. Zur Analytik der Phosphorverbindungen in Gewässer. Limnologica. 1966;4:437–444. [Google Scholar]
- Wells RD, de Winton MD, Clayton JS. Successive macrophyte invasion within the submerged flora of Lake Tarawera, central North Island, New Zealand. N Z J Mar Freshwater Res. 1997;31:449–459. [Google Scholar]
- Womersley JS. Handbooks of the flora of Papua New Guinea. Vol. 1 Melbourne University Press; Victoria: 1995. [Google Scholar]
- Xu Y, Cai Q, Wang L, Kong L, Li D. Diel vertical migration of Peridiniopsis niei Liu et al., a new species of dinoflagellates in an eutrophic bay of Three-Gorge Reservoir, China. Aquat Ecol. 2010;44:387–395. [Google Scholar]
- Zingel P, Agasild H, Nõges T, Kisand V. Ciliates are the dominant grazers on picoand nanoplankton in a shallow, naturally highly eutrophic lake. Microb Ecol. 2006;53:134–142. doi: 10.1007/s00248-006-9155-4. [DOI] [PubMed] [Google Scholar]