Abstract
Germinal center (GC) responses are controlled by T follicular helper (Tfh) and T follicular regulatory (Tfr) cells and are crucial for the generation of high affinity antibodies. Although the biology of human circulating and tissue Tfh cells has been established, the relationship between blood and tissue Tfr cells, defined as CXCR5+Foxp3+ T cells, remains elusive. Here, we found that blood Tfr cells are increased in Sjögren syndrome, especially in patients with high autoantibody titres, as well as in healthy subjects upon influenza vaccination. While, blood Tfr cells correlated with humoral responses they lack full B-cell suppressive capacity, despite being able to suppress T-cell proliferation. Blood Tfr cells have a naïve-like phenotype, although they are absent from human thymus or cord blood. Here, we found these cells were generated in peripheral lymphoid tissues prior to T-B interaction, as they are maintained in B-cell deficient patients. Therefore, blood CXCR5+Foxp3+ T cells in human pathology indicate ongoing humoral activity, but are not competent Tfr cells.
Introduction
Germinal center (GC) responses are crucial for the generation of high affinity antibodies during T-dependent immune responses. Within the GC resides a specialized subset of CD4+ T cells – the T follicular helper (Tfh) cells – which are essential for GC development and function (1, 2). It is now clear that Tfh cells play a central role in productive vaccine responses, while defects in their formation or function can contribute to immunodeficiency or autoimmunity (3, 4). More recently, the discovery of T follicular regulatory (Tfr) cells, a subset of suppressive regulatory T cells that participate in the GC, added an additional layer of complexity in the biology of GC responses (5–8).
Tfr cells, generally defined by Bcl-6+CXCR5+PD-1+ICOS+Foxp3+, are a distinct subset of thymic Foxp3+ regulatory T cells (Tregs) present in lymphoid tissues. Like the Tfh cell differentiation pathway, Tfr cell-commitment require both dendritic cell and B cell interactions, as well as CD28, SAP, ICOS, and PD-1 signaling (6, 9, 10). A tight balance between expression of transcription factors Bcl-6 and Blimp-1 regulates the differentiation of Tfr cells (6). Tfr cells have specialized functions in controlling the magnitude of GC responses and in limiting the outgrowth of non-antigen-specific B cell clones(5, 6). However, the precise mechanisms of Tfr cell suppression remain elusive, although CTLA-4 and regulation of metabolic pathways seem to play a key role (11–13).
Although, Tfh and Tfr cells are characterized by their location in lymphoid tissues an increasing number of studies have described putative circulating counterparts of these cells in peripheral blood. This is particularly relevant for studying the biology of these cells in humans, as access to secondary lymphoid tissues can be limiting. Human blood CXCR5+ T cells have been established as memory Tfh-like cells, based on the their ability to recapitulate bona fide Tfh cell functions: human blood CXCR5+ T cells can promote plasmablast differentiation, AID expression and class switch recombination by naïve B cells. However, they are phenotypically distinct from tissue Tfh cells and do not express the transcriptional repressor Bcl-6 (14–16). Furthermore, an immunization leading to GC and antibody responses correlates with an increase in the frequency of circulating ICOS+ Tfh cells, suggesting that they indicate ongoing Tfh cell responses in secondary lymphoid tissues (14, 16–19). Human circulating Tfh cells comprise a heterogeneous population concerning their phenotype and the quality of help they provide to B cells (14, 17).
In mice, CXCR5+Foxp3+ Tfr-like cells were found in peripheral blood after immunization, and shown to represent a circulating counterpart of tissue Tfr cells (9, 10).
Although, CXCR5-expressing Tregs and GC Foxp3-expressing T cells have been found in humans (7, 20, 21), so far, no study have addressed the biological significance of these putative circulating Tfr-like cells in humans. Human tonsil CD25+CD69- T cells have been shown to directly suppress B cell responses, but the relationship of these putative Tregs to Bcl-6+CXCR5+PD-1+ICOS+Foxp3+ Tfr cells is unclear (22, 23). Peripheral blood CXCR5+ Tregs are being studied as circulating Tfr cells in many different human diseases, despite the biological relevance of these cells being unclear (24–28). Additionally, Foxp3 upregulation by non-regulatory human T cells and transient CXCR5 expression by T cells undergoing activation challenge the assumption that peripheral blood CXCR5+ Tregs are indeed bona fide circulating Tfr cells (29, 30).
Here, we found that human blood Tfr cells, defined as CXCR5+Foxp3+ T cells, are generated in peripheral lymphoid tissues as humoral immune responses are established. However, in contrast to tissue Tfr cells and conventional CXCR5- Tregs, circulating Tfr cells have a naïve-like phenotype. Our data provide the first clear evidence that blood Tfr cells are generated following the initial steps that lead to germinal centre responses being distinct from tissue Tfr cells.
Results
Blood Tfr cells indicate ongoing germinal center responses
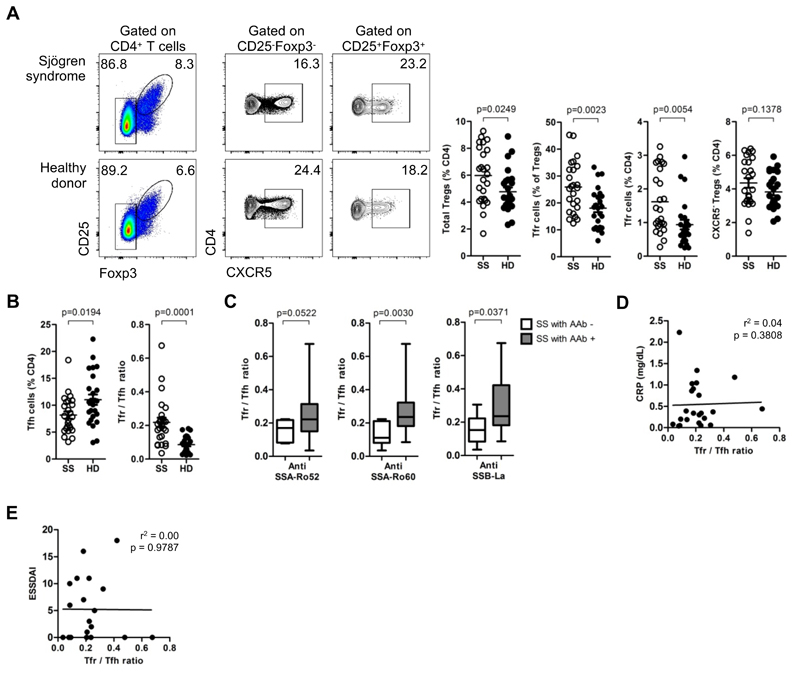
To address the impact of Tfr:Tfh ratio in human autoimmunity, we studied Sjögren syndrome (SS), a systemic autoimmune disease characterized by the lymphocytic infiltration of salivary and lachrymal glands with formation of ectopic lymphoid structures demonstrating the pathogenic involvement of B-T cell interactions (31, 32). We studied a cohort of 25 patients with recently diagnosed SS, accordingly to American European Consensus Group (AECG) criteria (33), under no immunosuppressive treatment other than prednisolone (less than 7.5 mg per day or equivalent) or hydroxycloroquine (Table S1). Unexpectedly, we found a striking increased frequency of circulating Tfr cells in SS as compared to age-matched healthy donors (Figure 1A, Figure S1A,B). Interestingly, this CXCR5+ Treg subset was specifically increased providing an explanation for the high Treg frequency observed in SS patients (Figure 1A). SS patients showed a significantly increase in the Tfr:Tfh ratio compared to healthy donors (Figure 1B) (8, 10). Furthermore, among SS patients, the increased Tfr:Tfh ratio is associated with patients with serum autoantibodies (Figure 1C). On the contrary we found no correlation between high Tfr:Tfh ratio with c-reactive protein or disease activity score (ESSDAI) (Figure 1D,E).
Figure 1. Blood Tfr cells are indicators of ongoing humoral activity.
(A) Frequency of total Tregs, Tfr cells and CXCR5- Tregs in peripheral blood of Sjögren syndrome patients (SS) and age-matched healthy donors (HD) (n = 25, unpaired Student T-test with Welch’s correction for variance). Representative plots (left) and pooled data (right). (B) Blood Tfr:Tfh ratio in SS patients and HD (n = 25, unpaired Student t-test with Welch’s correction for variance). (C) Blood Tfr:Tfh ratio in SS patients with and without serum autoantibodies (anti-SSA/Ro52, anti-SSB/Ro60, and anti-SSB/La) (n = 25, unpaired Student t-test). (D) Variation of blood Tfr:Tfh ratio accordingly to c-reactive protein (CRP). Analysis by linear regression. (E) Variation of blood Tfr:Tfh ratio accordingly to disease severity score (ESSDAI). Analysis by linear regression. Bars represent SEM (a,c) or minimum and maximum values (in box and whiskers graphs) (c).
Blood and tissue Tfr cells present different follicular and regulatory markers
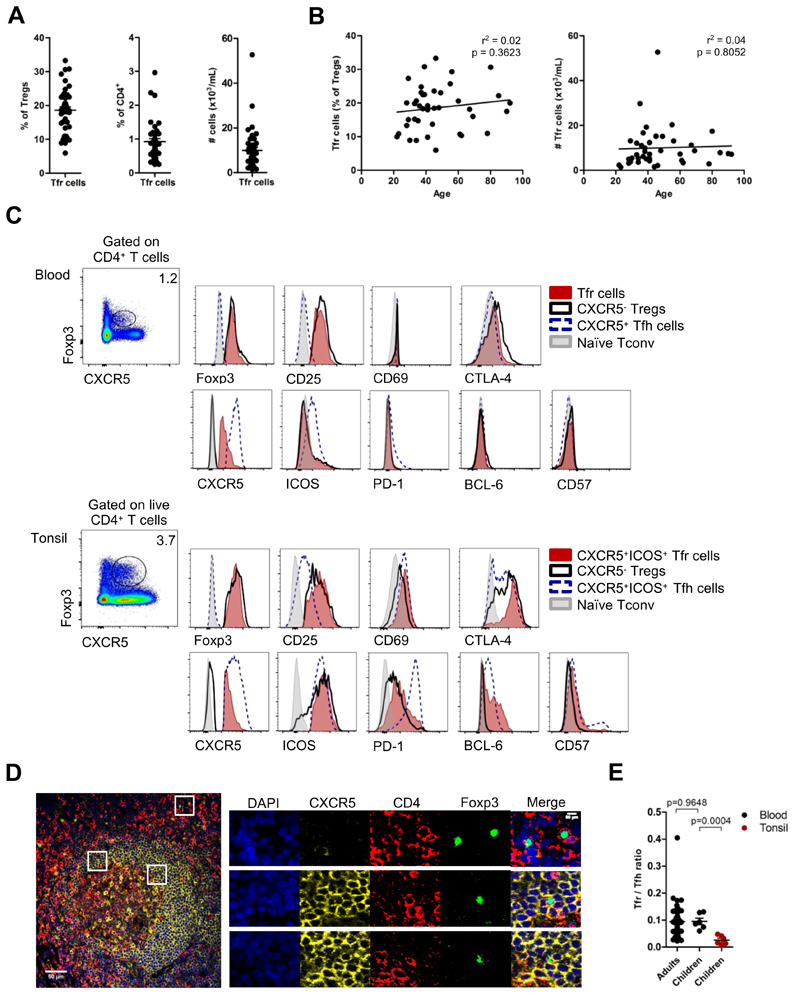
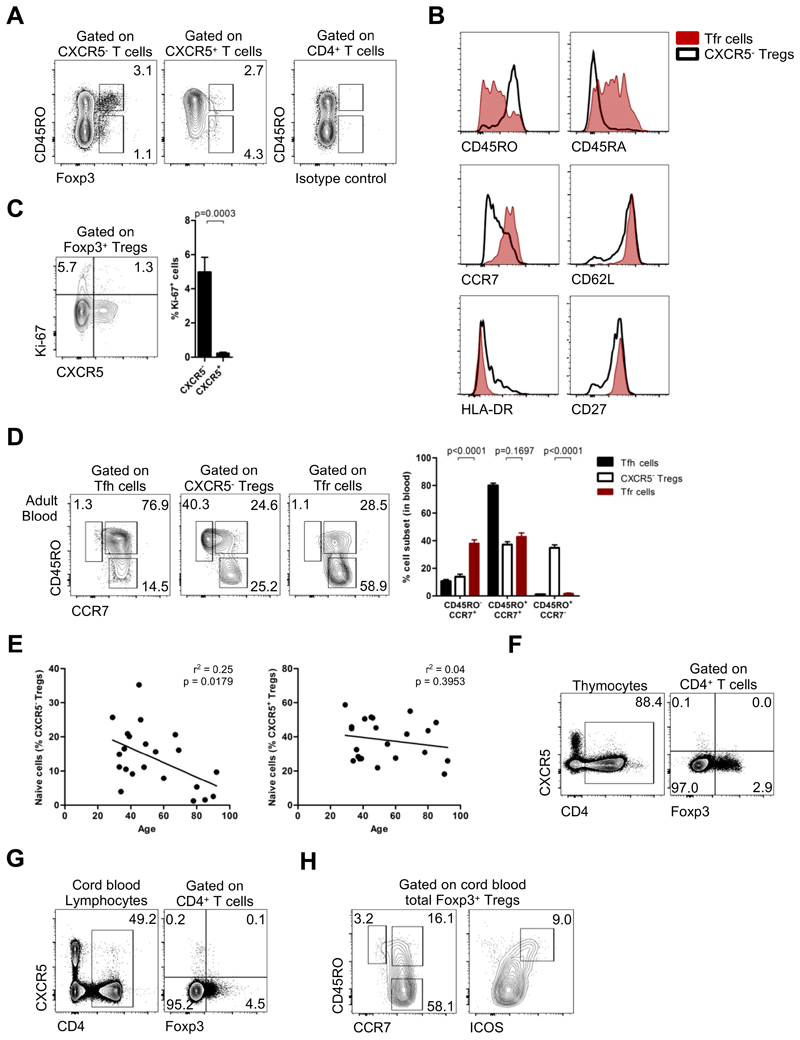
To test whether CXCR5+Foxp3+ Tfr cells in human peripheral blood are circulating counterparts of tissue Tfr cells, we studied peripheral blood from a cohort of 42 healthy volunteers between 22 and 92 years old (mean age 46.76 ± 18.14 years old, 30 females and 12 males). We found that CXCR5 was expressed by 18.57 ± 6.55% of total Tregs (defined as CD4+CD25+Foxp3+ T cells) (Figure 2A). The frequency and number of CXCR5+Foxp3+ T cells did not change with aging (Figure 2B, Figure S2A).
Figure 2. Blood Tfr cells show expression of follicular and regulatory markers.
(A) CXCR5+ Tfr cells constitute 18.57 ± 6.55% of Tregs (left) and 0.93 ± 0.56% of total CD4+ T cells (middle), representing 9985 ± 9043 cells per mL of blood (right) (n = 42, adult healthy donors). (B) Variation of blood Tfr cells frequency (left) and absolute number per ml of blood (right) accordingly to age (age range: 22 – 92 years old). (n = 42, linear regression). (C) Expression of Foxp3, CD25, CD69, CTLA-4, CXCR5, ICOS, PD-1 Bcl-6, and CD57 by Tfh cells (blue), CXCR5- Tregs (black) and Tfr cells (red), in children blood (top rows) and in tonsils (bottom rows). Naïve CD4+ T cells were used as control (gray). Representative plots from 6 healthy children. CXCR5+ subsets in tonsils were defined as CXCR5+ICOS+ cells (Fig. S2B). (D) Immunofluorescence microscopy of formalin-fixed paraffin-embedded human tonsils stained for DAPI (blue), CXCR5 (yellow), CD4 (red) and Foxp3 (green). Top, middle and bottom outlined areas indicate top, middle and bottom enlarged areas on the right, respectively. Data are representative of tonsil sections from 5 healthy children. (E) Blood Tfr:Tfh ratio in adult blood, children blood (tonsil donors) and in tissues (tonsils). Black and red dots represent blood and tonsil results, respectively (n = 42 for adults and n = 6 for children, Student t-test). Error bars represent SEM.
As CXCR5 is used to identify human circulating Tfh cells, we compared the phenotype of circulating CXCR5+Foxp3+ Tfr cells with that of circulating Tfh cells and CXCR5-conventional Tregs. Peripheral blood CXCR5+Foxp3+ T cells share characteristics with both circulating Tfh cells and CXCR5- Tregs (Figure 2C). Taking advantage of routine tonsillectomies performed due to tonsil hypertrophy in otherwise healthy children, we compared the cell phenotype of paired blood and tissue samples from the same child (Figure 2C and Fig. S2B). We found that circulating Tfh cells were phenotypically distinct from their tissue counterparts, in line with previous reports, especially regarding their PD-1, ICOS and Bcl-6 expression (Figure 2C) (14, 15). In a similar way, circulating CXCR5+Foxp3+ T cells were also ICOS-PD-1-Bcl-6-CD57-, and consequently distinct from tonsil Tfr cells (Figure 2C). In addition, we confirmed that these cell populations displayed a similar phenotype in adults (Figure S2,D). Our results are consistent with murine studies showing that blood and tissue Tfr cells are phenotypically distinct (9). Notably, ICOS was not differentially expressed by Tregs and Tfr cells in tonsils (Figure 2C). Bcl-6 expression was not detected in any population by real-time PCR (Fig. S1F), consistently with previous reports showing that blood Tfh cells do not express Bcl-6 (15–18, 34). We also confirmed that tissue CXCR5+Foxp3+ T cells are localized within germinal centers, therefore, corresponding to Tfr cells (Figure 2D, Figure S2G). Curiously, we observed different Tfr:Tfh ratios in the blood and tonsils (Figure 2E).
CXCR5+Foxp3+ Tfr cells are a distinct subset of suppressive Foxp3+ T cells
It has been described that CXCR5 expression can transiently occur upon human T cell activation(30, 35, 36). Moreover, human T cells can also transiently express Foxp3 upon in vitro TCR stimulation in a TGF-β dependent manner (29, 37).
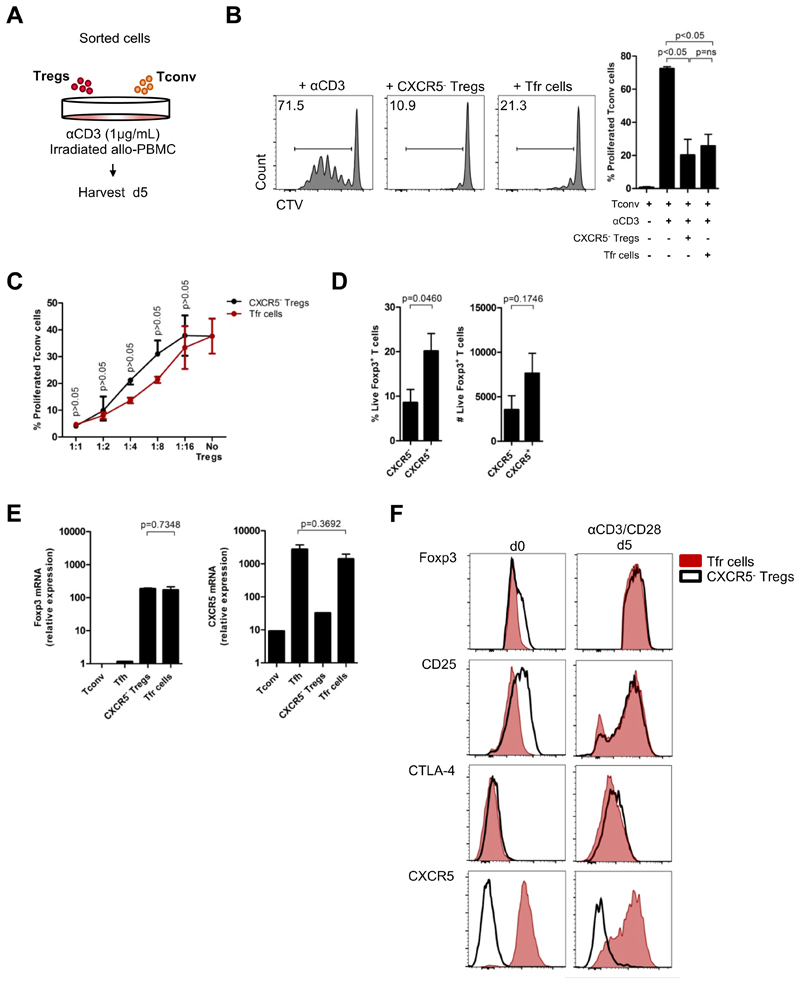
To address whether ex vivo CXCR5+Foxp3+ Tfr cells were bona fide regulatory cells, we sorted that cell population, as well as CXCR5- conventional Tregs (Fig. S3A,B), and cultured them with CTV-labeled conventional T cells. Proliferation of responder cells was analyzed after 5 days of soluble αCD3 stimulation (Figure 3A). Blood CXCR5+Foxp3+ Tfr cells significantly reduced conventional T cell proliferation (Figure 3B,C), definitely demonstrating their regulatory function.
Figure 3. Blood Tfr cells are a distinct subset of suppressive Tregs.
(A) Schematic representation of in vitro suppression assay. FACS-sorted 25 x 103 CXCR5-CD25-CD127+CD4+ Tconv cells were co-cultured with 25 x 103 CXCR5-CD25+CD127-CD4+ Tregs or CXCR5+CD25+CD127-CD4+ Tfr cells under stimulation by anti-CD3 (1 μg/mL), in presence of 105 irradiated (2500 rad) allo-PBMC. After 5 days cells responder cells were analyzed for CTV dilution by flow cytometry. Sorting strategy is described in Fig. S2a,b. (B) Proliferation of Tconv cells without Tregs or in the presence of either CXCR5- Tregs or Tfr cells. Representative plots (left) and pooled data (right) (n = 3, each with technical triplicates, One-way ANOVA with post-test Turkey’s Multiple Comparison). (C) Suppression curve of CXCR5- Tregs and Tfr cells in different ratios, using the same conditions described in Fig. 3A, B) (n = 1, with technical triplicates, Two-way ANOVA). (D) Stability of Foxp3 expression by sorted CXCR5- Tregs and CXCR5+ Tfr cells after 5 days of in vitro culture under αCD3/αCD28 (1 μL/well) stimulation. Percentage (left) and cell number (right) (n = 5, each with technical triplicates, Student t-test). (E) Relative expression of Foxp3 and CXCR5 by sorted Tconv, Tfh cells, CXCR5-Tregs and Tfr cells from blood, by real-time RT-PCR. Gene expression normalized to housekeeping genes (B2M, G6PD and ACTB) (n = 2, each with technical duplicates, Student t-test). (F) Expression of Foxp3, CD25, CTLA-4 and CXCR5 by sorted CXCR5-Tregs and Tfr cells at baseline (d0) and after 5 days of in vitro culture under αCD3/αCD28 (1 μL/well) stimulation. Representative histograms of 3 independent experiments, each one with technical triplicates. Error bars indicate SEM. (ns = not significant).
Stability of Foxp3 expression is required for the suppressive function of Tregs cells (38). In order to determine whether blood Tfr cells have stable Foxp3 expression, we stimulated sorted Tfr cells and CXCR5- Tregs with anti-CD3/CD28 microbeads for 5 days, in the absence of exogenous IL-2. In the absence of IL-2 Treg cells do not survive well in culture. Under these conditions, both CXCR5- Tregs and Tfr cells retain a similar frequency of Foxp3+ cells, albeit lower than in the beginning of the culture (Figure 3D). Interestingly, the frequency of recovered live Foxp3-expressing cells was slightly higher for sorted Tfr cells as compared to CXCR5- conventional Tregs. Next, we analyzed the relative expression of Foxp3 and CXCR5 in sorted conventional T cells, Tfh cells, Tfr cells and CXCR5- Tregs from human blood, by real-time PCR. Although Foxp3 protein expression was lower in Tfr cells than in CXCR5- Tregs (Figure 2C, Figure S2D), Foxp3 gene expression was similar between the two subsets (Figure 3E). In addition, circulating Tfh cell and Tfr cells also showed comparable CXCR5 gene expression (Figure 3E).
To investigate whether activation of blood Tfr cells triggers upregulation of Foxp3, CD25 and CTLA-4, a phenomena known to be associated with increased Treg suppressive function (39), we analyzed the phenotype of sorted CXCR5+ and CXCR5- Tregs after 5 days of culture in presence of αCD3/CD28 microbeads. We found an upregulation of Foxp3 and CD25 by Tfr cells, while CTLA-4 was increased in both populations (Figure 3F, Figure S3C). The levels of expression of these markers by blood Tfr cells after activation resembled those from tissue Tfr cells (compare with Figure 2C). Importantly, CXCR5 upregulation was not detected in sorted CXCR5- Tregs showing that CXCR5+ Tfr cells are a distinct subset of human blood Tregs.
Blood Tfr cells do not preferentially suppress humoral responses
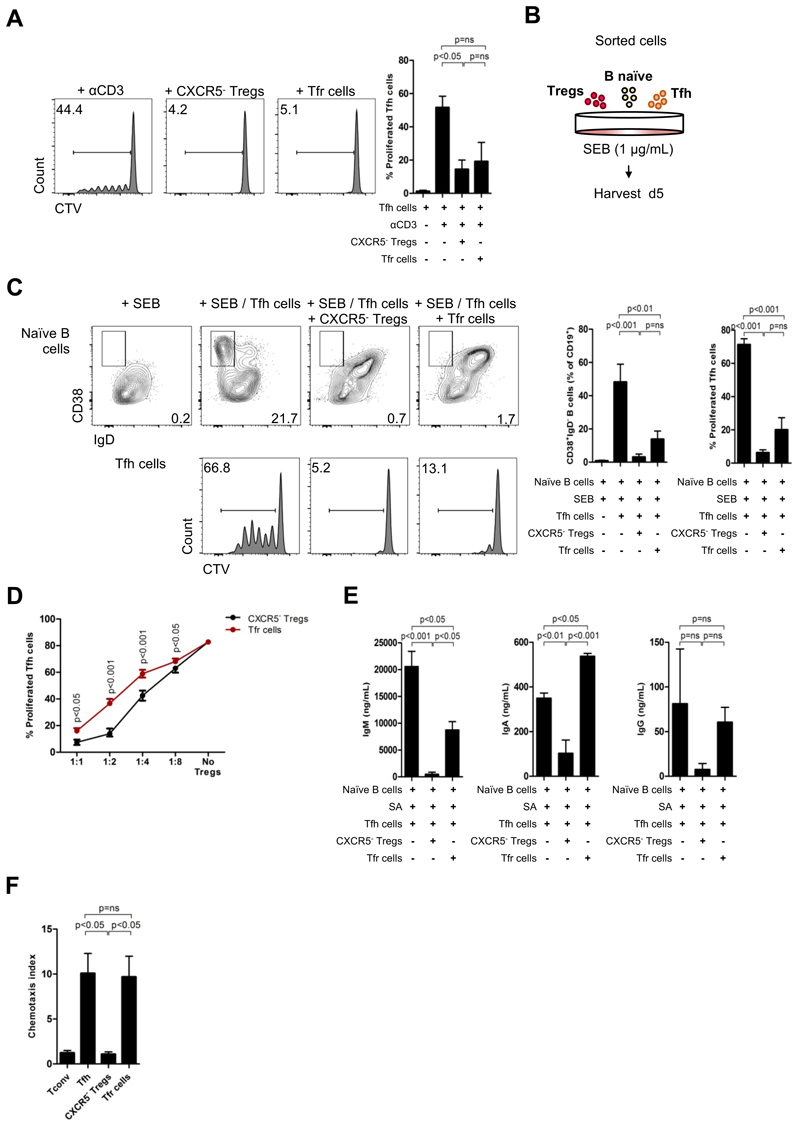
In order to address the function of blood Tfr cells we first investigated if this population could directly suppress Tfh cells. Using a similar in vitro assay used to prove the regulatory capacity of blood Tfr cells, but with sorted Tfh cells as responders, we found that blood Tfr cells strongly suppressed Tfh cell proliferation, however without a specific advantage when compared with CXCR5- Tregs (Figure 4A).
Figure 4. Blood Tfr cells do not show specialized humoral regulatory capacity.
(A) Proliferation of CXCR5+CD25-CD127+CD4+ Tfh cells without regulatory T cells or in the presence of either CXCR5- Tregs or Tfr cells after 5 days of in vitro culture as described in figure 3a. Representative plots (left) and pooled data (right) (n = 3, each with technical triplicates, One-way ANOVA with post-test Turkey’s Multiple Comparison). (B) Schematic representation of suppression co-culture assay. FACS-sorted 25 x 103 CXCR5+CD25-CD127+CD4+ Tfh cells (or CXCR5-CD25-CD127+CD4+ Tconv cells) were co-cultured for 5 days with 25 x 103 CXCR5+CD25+CD127-CD4+ Tregs (or CXCR5-CD25+CD127-CD4+ Tregs) under stimulation by SEB (1 μg/mL) and in the presence of 30 x 103 CD27-IgD+CD19+ naïve B cells. (C) Upregulation of CD38 and downregulation of IgD by naïve B cells (top) and proliferation of Tfh cells by CTV dilution (bottom) without Tregs or in the presence of either CXCR5- Tregs or Tfr cells. Representative plots (left) and pooled data (right) (n = 5, each with technical triplicates, One-way ANOVA with post-test Turkey’s Multiple Comparison). (D) Suppression curve of CXCR5- Tregs and Tfr cells in different ratios, using the same conditions described in Fig. 4B, C) (n = 1, with technical triplicates, Two-way ANOVA). (E) ELISA determination of for IgA, IgM and total IgG in supernatants after 10 days of in vitro co-culture performed as described in (d), but using SEB (1μg/mL) + SEA (10g/mL) + SEE (10ng/mL) + TSST-1 (10ng/mL) as superantigen stimulation. (n = 3, each with technical triplicates, One-way ANOVA with post-test Turkey’s Multiple Comparison). (E) In vitro migration of 75 x 103 sorted Tconv, Tfh, CXCR5- Tregs, and Tfr cells towards a CXCL13 gradient (2 μ/mL), expressed by chemotaxis index (n = 3, each with technical triplicates, One-way ANOVA with post-test Turkey’s Multiple Comparison). Error bars indicate SEM. (ns = not significant).
Next, to directly assess the impact of blood Tfr cells on B cell activation, we used in vitro T-B co-cultures in presence of SEB superantigen (Figure 4C). After 5 days of culture, B cells upregulated CD38 and downregulated IgD only in presence of Tfh cells (Figure 4C). Both CXCR5- and CXCR5+ Tregs impaired the generation of CD38+IgD- GC-like B cells. Consistent with our results from suppression assays with Tfh cells (Figure 4A), Tfh cell proliferation was similarly inhibited by CXCR5- and CXCR5+ Tregs (Figure 4C,D, Figure S4A). As expected Tfh cells showed better proliferation responses in co-culture with B cells.
To further address the function of blood Tfr cells on humoral responses we analyzed class switch recombination by naïve B cells 10 days after superantigen stimulation. We found that blood Tfr cells, although able to reduce activation of naïve B cells and proliferation of Tfh cells as shown before, did not significantly limit class switch recombination by B cells, as no impact on IgA nor IgG production was observed (Figure 4E). On the contrary, CXCR5- Tregs efficiently suppressed humoral responses (Figure 4E).
CXCR5/CXCL13-dependent migration to GC is critical for suppression of humoral responses by Tfr cells (5, 7) and plasma CXCL13 levels have been correlated to ongoing GC responses in humans (40). To prove that blood Tfr cells were capable to migrate towards a CXCL13 gradient we conducted in vitro chemotaxis assays with sorted populations from human peripheral blood. We found that, although the CXCR5 MFI of peripheral Tfh and Tfr cells was slightly different (Figure 2C, Figure S2D), both populations shared their ability to migrate towards a CXCL13 gradient, showing functional capacity of blood Tfr cells to enter CXCL13 enriched tissues (Figure 4F).
Blood Tfr cells have a distinctive naïve-like phenotype
To explain the surprising observation that blood Tfr cells do not suppress antibody production we hypothesized that this population could represent thymus-derived precursors of Tfr cells not yet fully committed to regulate humoral responses. Indeed, we found that blood Tfr cells were predominantly CD45RO-Foxp3lo resting Tregs (Figure 5A), expressing high levels of CD45RA, CCR7, CD62L, CD27 and low levels of HLA-DR, reminiscent of a naïve phenotype (Figure 5B). Virtually, all blood Tfr cells were quiescent Ki-67- non-proliferating cells when analyzed ex vivo (Figure 5C). Moreover, circulating Tfr cells were virtually devoid of CD45RO+CCR7- effector-memory cells in striking contrast to CXCR5- Tregs, a phenotype more similar to circulating Tfh cells (Figure 5D). While the vast majority of blood Tfh cells were CD45RO+CCR7+ central-memory cells, consistently with previous reports (14–17), a significant proportion of Tfr cells were CD45RO-CCR7+ naïve cells (Figure 5D). Furthermore, the few CD45RO- Tfh cells did not express high levels of CD45RA indicating that those cells were not really naïve, in contrast to Tfr cells. (Fig. S5A). Therefore, blood Tfr cells constitute a pool of naïve resting cells.
Figure 5. Blood Tfr cells are immature but are not committed as such in the thymus.
(A) Backgate of CXCR5- and CXCR5+ Tregs accordingly to CD45RO and Foxp3 expression. (B) Expression of CD45RO, CD45RA, CCR7, CD62L, HLA-DR and CD27 by Tfr cells (red) and CXCR5- Tregs (black) in blood. (C) Expression of Ki-67 by CXCR5+ Tregs and CXCR5- Tregs in blood (n = 22, Student t-test). (D) CD45RO+CCR7-effector-memory (EM), CD45RO+CCR7+ central-memory (CM) and CD45RO-CCR7+ naïve subsets of Tfr cells and CXCR5- Tregs in adult blood. Representative plots (left) and pooled data (right) (n = 22, Student t-test). Tfh cells are represented in blue, CXCR5-Tregs in black and Tfr cells in red. (E) Variation of CD45RO-CCR7+ naïve Tfr cells and CXCR5- Tregs frequency in blood accordingly to age (n = 22, linear regression). (F) Expression of CXCR5 by Foxp3+CD4+ thymocytes (n = 4). (G) Expression of CXCR5 by cord blood Foxp3+CD4+ T cells (n = 3). (H) Expression of CD45RO, CCR7 and ICOS by cord blood Tregs (n = 3). Bars represent SEM. (ns = not significant).
To test whether blood Tfr cells were indeed thymus-derived precursors of tissue Tfr cells we analyzed the frequency of these cells accordingly to age. Contrary to thymic derived naïve Tregs, CD45RO-CCR7+ naïve Tfr cells did not significantly decrease with increasing age (Figure 5E). In addition, the expression of CD31, a marker used to identify recent thymic emigrants in human blood (41–43), was not specifically enriched in the population (Fig. S5B,C). Although these observations suggest blood Tfr cells are not a thymic population, this was not conclusive. Therefore, we directly examined CXCR5-expressing T cells in the human thymus and neonatal cord blood. There was not a population of CXCR5+ Tregs detected in any of those tissues (Figure 5F,G and Fig. S5D). Although, CXCR5-expressing Tregs were not found in cord blood, some Foxp3+ cells expressed CD45RO, suggesting that additional activation signals not present before birth are required to shape a CXCR5 phenotype in circulating Tregs. Consistent with our previous data, ICOS+ Tregs were detected in cord blood, indicating that ICOS cannot be used as a specific follicular marker in circulating human Treg cells (Figure 5H).
Blood Tfr cells emerge from lymphoid organs before B-cell interaction
Having demonstrated that circulating Tfr cells did not egress from the thymus, we investigated whether Tfr cells recirculate from secondary lymphoid tissues before being fully committed to tissue Tfr cells.
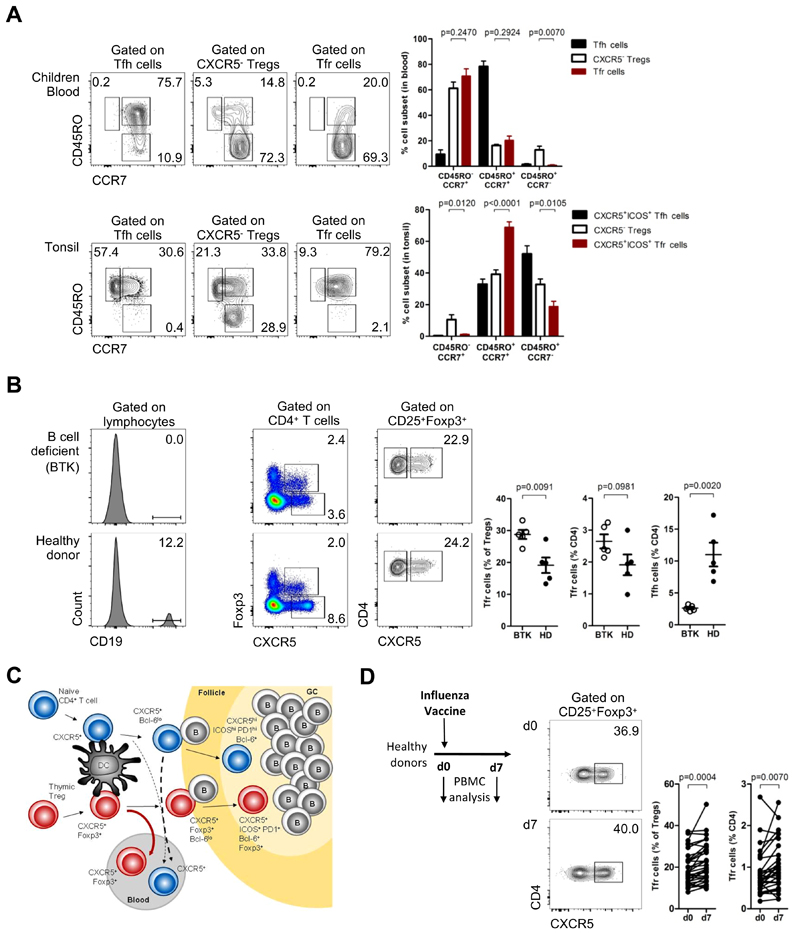
We compared both CXCR5+ and CXCR5- Treg subsets from children paired blood and tissue (tonsils) concerning their effector-memory, central-memory and naïve composition. We found that CD45RO+CCR7- effector Tfr cells were present in lymphoid tissues but not in blood, suggesting that effector Tfr cells are selectively retained in tissues, similarly to effector Tfh cells (Figure 6A). Therefore, it is unlikely that blood CD45RO-Tfr cells derive from the fully mature tissue Tfr cells that express CD45RO, as the few CD45RA-re-expressing end-stage memory CD4+ T cells do not become CD45RO- (Figure S6A) (44, 45).
Figure 6. Blood Tfr cells are lymphoid tissue derived Tfr precursors.
(A) CD45RO+CCR7- EM, CD45RO+CCR7+ CM and CD45RO-CCR7+ naïve subsets of Tfr cells and CXCR5- Tregs in children blood (top) and in tissues (bottom). Representative plots (left) and pooled data (right) (n = 6, Student t-test). Tfh cells are represented in blue, CXCR5- Tregs in black and Tfr cells in red. CXCR5+ subsets in tonsils were defined as CXCR5+ICOS+ cells (Fig. S2B). (C) Blood Tfh and Tfr cells from X-linked Agammaglobulinemia (BTK-deficient) patients, compared to sex and age-matched healthy donors. Representative plots (left) and pooled data (right) (n = 5, Student t-test). (C) Model of CXCR5+ follicular helper and regulatory cells T cells generation and recirculation in humans, upon antigen stimulation. Tfh cells in red and Tfr cells in blue. (D) Frequency of peripheral blood Tfr cells on the day of influenza vaccination (d0) and 7 days later in healthy volunteers. Schematic representation and representative plots (left) and pooled data (right) (n = 32, Student t-test). Bars represent SEM. significant).
Our data suggest that blood Tfr cells are generated in secondary lymphoid tissue prior to full differentiation towards mature Tfr cells. It has been know that full differentiation of follicular T cells requires a two-step process with an initial activation mediated by dentritic cells and a subsequent B cell interaction in the B-T border. We investigated whether blood Tfr cells, given their immature phenotype could be generated before the B cell interactions required for acquisition of terminal differentiation. To investigate this issue we analyzed peripheral blood from X-linked Agammaglobulinemia (BTK-deficient) patients, with a complete absence of CD19+ cells. We observed a striking decrease in blood Tfh cells in those patients, in line with previous reports (Figure 6B) (46). However, frequency of blood Tfr cells were not decreased in B-cell deficiency patients (Figure 6B). These observations are conclusive in establishing that blood Tfr cells enter the circulation before B-cell contact, while the majority of blood Tfh cells require B cell interactions. To investigate whether CD45RO+ and CD45RO- blood Tfr cells could discriminate between Tfr cells recirculating before and after B-cell interaction, we analyzed these two populations in peripheral blood of patients with B-cell deficiency, as well as in SS patients. We found no significant differences in CD45RO+ or CD45RO-Tfr cells in these two diseases (Figure S5E), suggesting that although CD45RO is an activation marker on Tfr cells, its upregulation occurs before B-cell interaction.
We therefore hypothesized that blood Tfr cells are generated in secondary lymphoid tissue and enter the circulation before full differentiation towards tissue Tfr cells (Figure 6C). To test our model in vivo, we analyzed samples from healthy adults undergoing influenza vaccination. Previous studies have shown a positive correlation between circulating Tfh cell subsets and antibody responses following influenza vaccination in healthy adults (15, 18, 19). We, therefore, analyzed the impact of vaccination in circulating Tfr cells. Consistent with our hypothesis we found that circulating Tfr cells increased on day 7 following influenza vaccination (Figure 6D). This observation is in line with our prediction that during ongoing GC responses Tfr cells are generated and some exit from the tissue to the peripheral blood.
Discussion
Our comprehensive evaluation of human Tfr support a model in which blood Tfr cells are generated following the initial steps that lead to germinal centre responses in secondary lymphoid tissues, exiting the tissue prior to interactions with B cells that are required for complete differentiation towards tissue resident Tfr cells.
Although some studies have quantified blood CXCR5+ Tregs as circulating Tfr cells in different diseases, the human biology of CXCR5+ Tregs remains elusive (24, 25, 27, 47, 48). Moreover, most of the literature describe studies where what is defined as blood Tfh cells contain both Tfh and CXCR5+ Tfr cells, while many others studies identify as Tregs a mixture of bona fide conventional Tregs together with CXCR5+ Tfr cells. As such, results may be confounded by combining effector and regulatory cell populations. As an example, our cohort of SS patients show an increase in the frequency of Foxp3+ Tregs, compared with the control population. However, only CXCR5+ Tfr cells, and not conventional CXCR5- Tregs, are increased in those patients. As a consequence, the apparent increase of Tregs in the blood of SS patients is in fact an increase of CXCR5+ Tfr cells that reflect the ongoing humoral activity. It was the search for an explanation for this apparent counterintuitive observation that led us to establish the ontogeny and function of human circulating Tfr cells.
We found that Tfr cells in tonsils have follicular and regulatory markers and were found within germinal centers, whereas blood Tfr cells do not express ICOS, PD-1, or Bcl-6, apparently diverging these cells from follicular imprinting. Previous studies have described low ICOS and PD-1 expression, and no Bcl-6 expression in human blood Tfh cells (14). In mice, blood Tfr cells have also lower expression of ICOS(9). It was also reported that murine circulating Tfr cells can bypass the B cell zone and do not gain full activation as part of a memory programmed state (9). In line with these studies, the absence of ICOS, PD-1 and Bcl-6 from human blood CXCR5+ Tfr cells does not exclude their follicular ontogeny.
Our results show key differences between mice and humans regarding the function of blood CXCR5+ Tfr cells: while murine blood Tfr cells appear to be specialized in suppressing antibody production (despite their lower suppressive capacity when compared to tissue Tfr cells) (9, 10, 12, 49), human blood Tfr cells do not have the ability to fully suppress humoral responses.
Nevertheless, we found that blood Tfr cells specifically migrated towards CXCL13 gradient, suggesting these cells have the capacity to reach the follicles. Importantly, CXCR5- conventional Tregs did not upregulate CXCR5 upon in vitro activation, further confirming CXCR5-expressing Tfr cells as a distinctive subset.
We also found that blood Tfr cells have a prominent naïve phenotype. However, they are absent from the thymus and cord blood (where activated Tregs can already be found). These observations provide compelling evidence that activation signals generated in peripheral lymphoid organs are required to shape a CXCR5+ phenotype on human Foxp3+ T cells. Conversely, tissue Tfr cells are almost all CD45RO+ antigen experienced effector cells. Taken together, these observations led us to hypothesize that blood Tfr cells leave lymphoid tissues as immature cells, prior to B cell interaction in T-B border, and full differentiation into Tfr cells. This view was supported by the presence of blood Tfr cells in peripheral blood of patients lacking B-cells due to genetic defects. This finding provides an explanation for the incomplete suppressive function of blood Tfr cells.
An important limitation of our study is the difficulty in the isolation of tissue Tfr cells for functional assays, as the frequency of tonsil Foxp3-expressing cells among follicular CD4+CD25+CD127low cells is very low. However, the phenotype between blood and tissue Tfr cells is remarkably different in particular with respect to maturation markers.
Importantly, our results from vaccination and SS patient cohorts show that blood Tfr cells are indicative of ongoing humoral activity. In SS patients, where ongoing germinal center reactions promote the production of autoantibodies (31, 32), blood Tfr cells were significantly increased (directly contributing to an increased Tfr:Tfh ratio). Although, we expected to find a decrease in this putative humoral suppressive cell population in autoimmune conditions, our results suggest that blood Tfr cells indicate ongoing humoral activity and are not a measurement of suppressive potential. This is in line with recently published reports showing an increase in blood CXCR5+ Tregs in other autoimmune conditions and infectious diseases (27, 28, 48). Therefore, studies regarding blood CXCR5+ Tregs in different human settings should be carefully interpreted.
Given that circulating Tfr cells have an immature phenotype, it is not surprising that blood Tfr cells are not fully endowed with suppressive function, because the suppressive capacity of conventional Treg cells have been ascribed predominantly to those cells with a more mature phenotype. The TCR repertoire of Tfr cells is different from Tfh and probably skewed towards auto-antigens (50), it is possible that circulating Tfr cells represent a pool of cells ready to be recruited into subsequent GC responses as they retain the ability to migrate towards CXCL13.
In conclusion, our data support a model in which CXCR5+Bcl6- T cells egress from secondary lymphoid tissues during antigen-driven immune responses. While, the frequency of blood Tfh cells is reduced in the absence of B cells, Tfr cells do not require interactions with B cells. Thus, the acquisition of a CXCR5+Foxp3+ phenotype in the tissues precedes access to the follicle, where the cells acquire a fully mature phenotype. As a consequence, circulating Tfr cells represent lymphoid tissue derived Tfr precursors not yet endowed with full B-cell and humoral regulatory function.
Materials and Methods
Study Design
Samples sizes were estimated based on previous studies and accordingly to each cohort (see human samples). No outliers were excluded. Number of biological and technical replicates is stated in figure legends. Humans samples from different conditions were used (see human samples) with appropriate age-matched controls. This experimental study was performed unblinded.
Human samples
Fresh peripheral blood samples were collected from patients referred to Rheumatology Department of Hospital de Santa Maria, Centro Hospitalar Lisboa Norte for salivary gland biopsy due to clinical suspicion of SS. Blood samples were collected on the day of salivary gland biopsy. All patients with exclusion criteria for SS (33) or treated with biologic drugs, Disease Modifying Anti-Rheumatic Drugs (DMARDs) or more that 7.5mg per day of prednisolone were excluded. Patients diagnosed with an infectious disease in the previous month were also excluded, as well as those who received any vaccine in the same period of time. Patients were diagnosed as having SS if they met AECG diagnosis criteria (n = 25) (33). Routine c-reactive protein plasma levels (mg/dL) closest to blood collection were used. Age-matched healthy volunteers (from the cohort described bellow) were used for statistical comparison. Fresh peripheral blood samples were collected from adult healthy volunteers (n = 42). Fresh buffy-coats (blood collection in less than 24 hours) were used for in vitro suppression and co-culture assays. Tonsils and peripheral blood samples were collected from healthy children submitted to tonsillectomy due to tonsil hypertrophy (n = 6). Children with any clinical condition, under any drug treatment or submitted to tonsillectomy due to chronic tonsillitis were excluded. Umbilical cord blood samples were collected from healthy pregnancies during delivery (n = 3). Thymus tissue was collected from children submitted to cardiac surgery due to congenital heart disease who were otherwise healthy (n = 4). Blood samples were also collected from X-linked Agammaglobulinemia (BTK-deficient) patients during routine blood tests (n = 5). All blood samples were collected in in EDTA coated tubes. These studies were approved by the Lisbon Academic Medical Center Ethics Committee (Ref. n.° 505/14). Informed consent was obtained from all adult volunteers, parents or legal guardians.
For vaccination studies we used adult healthy volunteers (n = 32) recruited from the Cambridge BioResource as part of the vaccination study during the 2014 – 2015 winter season. Participants were excluded if they have had a previous adverse reaction to any vaccination, a known allergy to any components of the vaccine, were taking immune modulating medication, and women who are pregnant or breastfeeding. Participants were administered the inactivated influenza vaccine (split virion) BP vaccine (Sanofi Pasteur) by intramuscular injection in the right deltoid. Blood samples were collected in EDTA coated tubes on the day of vaccination (prior to administration of the vaccine) and 7 days following vaccination. The influenza vaccination study protocol was approved by the Health Research Authority, National Research Ethics Service committee South Central, Hampshire A, UK (REC reference:14/SC/1077).
Cell isolation and flow cytometry
PBMCs were isolated from blood samples by Ficoll-gradient medium (Histopaque-1077, Sigma-Aldrich) using SepMate tubes (StemCell Technologies). Lymphocytes from tonsils and thymocytes were also isolated by Ficoll-gradient medium after mechanical disruption. Before cell sorting, PBMCs from Buffy-coats were enriched for CD4+ T cells using MojoSort Human CD4 T Cell Isolation Kit (BioLegend). The CD4+ fraction was used for cell sorting of CD4+ T cell subsets. The CD4- fraction was used for cell sorting of naïve B cells (supplementary Figure 1a for sorting strategy). For flow cytometry cells were stained with anti-Bcl-6 (K112-91, BD Biosciences), anti-CCR7 (#150503, R&D Systems), anti-CD127 (eBioRDr5, eBioscience), anti-CD19 (HIB19, BioLegend), anti-CD25 (BC96, eBioscience), anti-CD27 (LG.7F9, eBioscience), anti-CD3 (OKT3, eBioscience), anti-CD31 (WM-59, eBioscience), anti-CD38 (HB-7, BioLegend), anti-CD4 (OKT4, BioLegend), anti-CD45RA (HI100, eBioscience), anti-CD45RO (UCHL1, BioLegend), anti-CD57 (HNK-1, BioLegend), anti-CD62L (DREG-56, BioLegend), anti-CD69 (FN30, BioLegend), anti-CD8 (RPA-T8, eBioscience), anti-CTLA-A (L3D10, BioLegend), anti-CXCR5 (J252D4, BioLegend), anti-Foxp3 (PCH101, eBioscience), anti-HLA-DR (G46-6, BD Biosciences), anti-ICOS (C398.4A, BioLegend), anti-IgD (IA6-2, BioLegend), anti-Ki67 (Ki-67, BioLegend), anti-PD-1 (EH12.2H7, BioLegend). For Bcl-6, CTLA-4, Foxp3, and Ki67 intracellular staining, Foxp3 Fix/Perm Kit (eBioscience) was used accordingly to manufacturer instructions. For cell viability staining, Live/Dead Fixable Aqua Dead Cell Stain Kit (Life Technologies) was used. Cell Trace Violet Cell Proliferation Kit (Life Technologies) was used for cell proliferation assessment. Cell sorting was performed in Aria IIu and Aria III instruments (BD Biosciences). Flow cytometry analysis was performed in a LSR Fortessa instrument (BD Biosciences) and further analyzed with FlowJo v10 software (TreeStar).
Cell culture and functional assays
For in vitro suppression assays 25 x 103 CXCR5-CD25-CD127+CD4+ conventional T cells or 25 x 103 CXCR5+CD25-CD127+CD4+ Tfh cells were plated with CXCR5-CD25+CD127-CD4+ Tregs cells or CXCR5+CD25+CD127-CD4+ Tregs cells in 1:1 ratio. Cells were cultured with 1μg/mL anti-CD3 (OKT3, eBioscience) in the presence of 105 irradiated (2500rad) allo-PBMCs. After 5 days cells were harvested and responder cells were analyzed for CTV dilution by flow cytometry. For TCR stimulation assays 25 x 103 CXCR5-CD25+CD127-CD4+ Tregs cells and CXCR5+CD25+CD127-CD4+ Tregs were plated with 1μL/well of anti-CD3/anti-CD28 MACSiBead particles (T Cell Activation Kit, Miltenyi Biotec). For co-culture in vitro suppression assays 25 x 103 CXCR5+CD25-CD127+CD4+ Tfh cells were plated with CXCR5-CD25+CD127-CD4+ Tregs cells or CXCR5+CD25+CD127-CD4+ Tregs cells in 1:1 ratio, in the presence of 30 x 103 CD27-IgD+CD19+ naïve B cells. Cells were cultured with 1μg/mL SEB (Sigma-Aldrich). After 5 days responder Tfh cells were analyzed for CTV dilution, B cells for CD38 upregulation and Tregs for follicular and activation markers. For immunoglobulin measurement 25 x 103 CXCR5+CD25-CD127+CD4+ Tfh cells were plated with CXCR5-CD25+CD127-CD4+ Tregs cells or CXCR5+CD25+CD127-CD4+ Tregs cells in 1:1 ratio, in the presence of 30 x 103 CD27-IgD+CD19+ naïve B cells. Cells were cultured with 1μg/mL SEB (Sigma-Aldrich) + 10ng/mL SEA (Toxin Technology) + 10ng/mL SEE (Toxin Technology) + 10ng/mL TSST-1 (Toxin Technology). After 10 days supernatants were collected and immunoglobulin concentration determined by ELISA. Cultures were performed in U-shape 96 wells plates in RPMI medium (RPMI 1640, Life Technologies) supplemented with 10% heat-inactivated Fetal Bovine Serum (Life Technologies), 1% HEPES (Sigma-Aldrich), 1% Sodium Pyruvate (Life Technologies), 1% PenStrep (Life Technologies), and 0.05% Gentamicin (Life Technologies) and in 37.° C, 5% CO2 incubator conditions.
ELISA
IgA, IgM and total IgG concentration were determined in supernatants from T-B co-culture (as described above) by ELISA using Human ELISA Ready Set Go Kit, according to manufacturer instructions (eBioscience).
Migration Assays
For in vitro chemotaxis assays 75 x 103 CXCR5-CD25-CD127+CD4+ conventional T cells, CXCR5+CD25-CD127+CD4+ Tfh cells, CXCR5-CD25+CD127-CD4+ Tregs cells and CXCR5+CD25+CD127-CD4+ Tregs cells were loaded on top wells of HTS Transwell 96-well permeable supports (5μm pore size) (Corning). Plain RPMI medium (RPMI 1640, Life Technologies) or that supplemented with 0.2μg/mL CXCL13 (Peprotech) was added to the bottom wells of the plate. After 4 hours of incubation (37.°C, 5% CO2), filters were removed and cells that migrated to the lower chamber were counted in a LSR Fortessa instrument (BD Biosciences) and further analyzed with FlowJo v10 software (TreeStar). Chemotaxis index was calculated as the ratio of cells migrating toward CXCL13 and cells randomly migrating.
Real-time RT-PCR
Total RNA was extracted and reverse transcribed from FACS-sorted CXCR5-CD25-CD127+CD4+ conventional T cells, CXCR5+CD25-CD127+CD4+ Tfh cells, CXCR5-CD25+CD127-CD4+ Tregs cells and CXCR5+CD25+CD127-CD4+ Tregs using RNeasy Micro Kit (Qiagen) according to manufacturer instructions. cDNA was generated using SuperScript III reverse transcriptase (Life Technologies), according to manufacturer instructions. Real-time PCR was set up with Power SYBR Green PCR Master Mix (Applied Biosystems) and performed on ViiA 7 Real-Time PCR System (Applied Biosystems), according to manufacturer instructions. The expression of each gene was normalized to housekeeping genes (B2M, ACTB or G6PD) and calculated by change-in-threshold method (ΔCt), using QuantStudio Real-Time PCR software v1.1 (Applied Biosystems).
Primers (Invitrogen): Foxp3_F 5’-GCAAATGGTGTCTGCAAGTG-3’; Foxp3_R 5’-GCCCTTCTCATCCAGAAGAT-3’; CXCR5_F 5’-CTGGAAATGGACCTCGAGAA-3’; CXCR5_R 5’-GCAGGGCAGAGATGATTTTC-3’; Bcl-6_F 5’-TTCCGCTACAAGGGCAAC-3’; Bcl-6_R 5’-CGAGTGTGGGTTTTCAGGTT-3’; B2M_F 5’-TATGCCTGCCGTGTGAACCAT-3’; B2M_R 5’-CGGCATCTTCAAACCTCCATG-3’; ACTB_F 5’-CTCTTCCAGCCTTCCTTCCT-3’; ACTB_R 5’-AGCACTGTGTTGGCGTACAG-3’; G6PD_F 5’-CCAAGCCCATCCCCTATATT-3’; G6PD_R 5’-CCACTTGTAGGTGCCCTCAT-3’.
Immunofluorescence microscopy
After paraffin removal and antigen retrieval by heat (HIER pH 9, Leica Biosystems) 3μm sections of formalin-fixed paraffin-embedded human tonsil were stained with anti-human CXCR5-Alexa-Fluor 488 (J252D4, BioLegend), anti-human CD4 (SP35, CellMarque) and anti-human Foxp3 (PCH101, eBioscience) primary antibodies. Alexa-Fluor 488 (anti-mouse), Alexa-Fluor 546 (anti-rabbit) and Alexa-Fluor (anti-Rat) were used as secondary antibodies, respectively. DAPI was used as nuclei counterstaining. Images were acquired with ZEN 2012 software on a Zeiss LSM 710 confocal point-scanning microscope (Carl Zeiss, Oberkochen, Germany) using a dry plan-apochromat 20x objective (200x magnification) and with a numerical aperture of 0.80. Images were further analyzed using Image-J FiJi software.
Statistical analysis
Unpaired, paired Student T-test and One-way ANOVA with post-test Turkey’s Multiple Comparison were used as described. Linear regression analysis was also conducted for some data. Results are presented as mean ± SD. P values of less than 0.05 were considered statistically significant. GraphPad Prism v5 software was used for statistical analysis.
Supplementary Material
One Sentence Summary.
Human blood Tfr cells are generated in the initial steps that lead to a germinal centre response, however, the Tfr in the circulation exit the tissue prior to completing differentiation into germinal centre Tfr cells.
Acknowledgments
We thank to Ana Catarina H Pinto for the recruitment of adult healthy volunteers, and for making the diagram. We thank the Instituto Português do Sangue e da Transplantação, the Centro Hospitalar Lisboa Ocidental – Hospital de Santa Cruz, and the Centro Hospitalar Lisboa Norte – Hospital de Santa Maria (Rheumatology, Otorhinolaryngology and Obstetrics departments) for human samples and collaborations. We thank the Cambridge BioResource staff for their help with volunteer recruitment. We thank members of the Cambridge BioResource SAB and Management Committee for their support of our study and the National Institute for Health Research Cambridge Biomedical Research Centre for funding. We gratefully acknowledge the participation of all volunteers. We also thank to José Faro for his opinions and statistical review.
Funding
This study was funded by HMSP-ICT/0034/2013, FAPESP/19906/2014, and PTDC/IMI-IMU/7038/2014 research grants. The vaccination study was funded by the European Research Council Start Grant TWILIGHT to MAL (637801). WP was funded by a Newton International Fellowship from the Royal Society. MAL is funded by the Bioscience and Biotechnology Research Council.
Footnotes
Author contributions: VRF designed research, performed experiments, analyzed data, and wrote the paper. AAD designed research, performed experiments and statistical analysis, and reviewed the paper. ARM and ARP performed experiments. WP performed the vaccination studies. SLS selected BTK-deficient patients. JEF, AES and ML designed research and reviewed the paper. LG designed research, and wrote the paper.
Competing interests: The authors declare no relevant patent applications or competing financial interests.
References and Notes
- 1.Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinuesa CG, Linterman MA, Yu D, MacLennan ICM. Follicular Helper T Cells. Annu Rev Immunol. 2016;34 doi: 10.1146/annurev-immunol-041015-055605. annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 3.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly — TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16 doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–60. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 6.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang Y-H, Lim H, Reynolds JM, Zhou X-H, Fan H-M, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sage PT, Sharpe AH. T Follicular Regulatory Cells in the Regulation of B cell Responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sage PT, Alvarez D, Godec J, Von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124:5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sage PT, Francisco LM, Carman CV, Sharpe aH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, Kuchroo VK, Haining WN, Chevrier N, Haigis M, Sharpe AH. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17:1436–1446. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Tsai LM, Leong Y, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, Zhu L, et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentebibel S, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, et al. Induction of ICOS+CXCR3+CXCR5+ Th cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005191. 176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentebibel S, Khurana S, Schmitt N, Kurup P, Mueller C, Obermoser G, Palucka AK, Albrecht RA, Garcia-Sastre A, Golding H, Ueno H. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6:26494. doi: 10.1038/srep26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 21.Wallin EF, Jolly EC, Suchánek O, Bradley JA, Espéli M, Jayne DRW, a Linterman M, Smith KGC. Human T follicular helper and T follicular regulatory cell maintenance is independent of germinal centers. Blood. 2014;124:2666–2675. doi: 10.1182/blood-2014-07-585976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 23.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Y, Yang B, Lu J, Zhang J, Yang H, Li J. Imbalance of circulating CD4+CXCR5+FOXP3+ Tfr-like cells and CD4+CXCR5+FOXP3- Tfh-like cells in myasthenia gravis. Neurosci Lett. 2016;630:176–182. doi: 10.1016/j.neulet.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 25.Dhaeze T, Peelen E, Hombrouck A, Peeters L, Van Wijmeersch B, Lemkens N, Lemkens P, Somers V, Lucas S, Broux B, Stinissen P, et al. Circulating Follicular Regulatory T Cells Are Defective in Multiple Sclerosis. J Immunol. 2015;195:832–40. doi: 10.4049/jimmunol.1500759. [DOI] [PubMed] [Google Scholar]
- 26.Pandya JM, Lundell A, Hallström M, Andersson K, Nordström I, Rudin A. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol. 2016;100:823–833. doi: 10.1189/jlb.5A0116-025R. [DOI] [PubMed] [Google Scholar]
- 27.Shan Y, Qi C, Zhao J, Liu Y, Gao H, Zhao D, Ding F, Wang J, Jiang Y. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clin Exp Pharmacol Physiol. 2015;42:154–61. doi: 10.1111/1440-1681.12330. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med. 2014;12:251. doi: 10.1186/s12967-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Schaerli P, Loetscher P, Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol. 2001;167:6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- 31.Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat Rev Rheumatol. 2013;9:544–56. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 32.Mavragani CP, Moutsopoulos HM. Sjögren’s Syndrome. Annu Rev Pathol Mech Dis. 2014;9:273–85. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 33.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, et al. European Study Group on Classification Criteria for Sjögren’s Syndrome, Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–68. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Förster R, Lipp M, Lanzavecchia A. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4 ϩ FOXP3 Ϫ T cells by T-cell receptor stimulation is transforming growth factor- nl – dependent but does not confer a regulatory phenotype. October. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 40.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Bélanger S, Kasturi SP, Landais E, Akondy RS, McGuire HM, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 42.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4+ Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–26. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 44.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012;24:476–481. doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Arlettaz L, Barbey C, Dumont-Girard F, Helg C, Chapuis B, Roux E, Roosnek E. CD45 isoform phenotypes of human T cells: CD4+CD45RA-RO+ memory T cells re-acquire CD45RA without losing CD45RO. Eur J Immunol. 1999;29:3987–3994. doi: 10.1002/(SICI)1521-4141(199912)29:12<3987::AID-IMMU3987>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 46.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, Bustamante J, Okada S, Stoddard JL, Deenick EK, Pelham SJ, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136:993–1006e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med. 2014;12:251. doi: 10.1186/s12967-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Li W, Zhang Y, Song X, Xu L, Xu Z, Zhou S, Zhu J, Jin X, Liu F, Chen G, et al. Distribution of peripheral memory t follicular helper cells in patients with schistosomiasis japonica. PLoS Negl Trop Dis. 2015;9:1–13. doi: 10.1371/journal.pntd.0004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, Kuchroo VK, Haining WN, Chevrier N, Haigis M, Sharpe AH. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17 doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maceiras AR, Almeida SCP, Mariotti-Ferrandiz E, Chaara W, Jebbawi F, Six A, Hori S, Klatzmann D, Faro J, Graca L. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun. 2017;8:15067. doi: 10.1038/ncomms15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.