Abstract
DNA-encoded chemical libraries (DEL) are increasingly being used for the discovery and optimization of small organic ligands to proteins of biological or pharmaceutical interest. The DNA fragments, that serve as amplifiable identification barcodes for individual compounds in the library, are typically used in double-stranded DNA format. To the best of our knowledge, a direct comparison of DEL selections featuring DNA in either single- or double-stranded DNA format has not yet been reported. In this article, we describe a comparative evaluation of selections with two DEL libraries (named GB-DEL and NF-DEL), based on different chemical designs and produced in both single- and double-stranded DNA format. The libraries were selected in identical conditions against multiple protein targets, revealing comparable and reproducible fingerprints for both types of DNA formats. Surprisingly, selections performed with single-stranded DNA barcodes exhibited improved enrichment factors compared to double-stranded DNA. Using high-affinity ligands to carbonic anhydrase IX as benchmarks for selection performance, we observed an improved selectivity for the NF-DEL library (on average 2-fold higher enrichment factors) in favor of single-stranded DNA. The enrichment factors were even higher for the GB-DEL selections (approximately 5-fold), compared to the same library in double-stranded DNA format. Collectively, these results indicate that DEL libraries can conveniently be synthesized and screened in both single- and double-stranded DNA format, but single-stranded DNA barcodes typically yield enhanced enrichment factors.
Keywords: DNA-encoded chemical libraries, High throughput sequencing, protein selections
Introduction
The encoding of individual organic molecules with DNA fragments, serving as amplifiable identification barcodes, allows the construction and screening of combinatorial chemical libraries of unprecedented size[1–13]. Screening procedures are often performed by affinity capture on protein targets immobilized on solid supports, followed by PCR amplification of DNA barcodes and high-throughput sequencing. The frequency of sequence counts for individual library members can be graphically displayed as “fingerprints”, which facilitate the identification of preferentially enriched compounds[14–16].
The construction of DEL libraries typically relies on either “DNA-directed synthesis” (in which pre-formed single-stranded DNA template molecules drive the stepwise addition of building blocks onto a nascent chemical moiety by means of complementary oligonucleotide derivatives) or on “DNA-recorded synthesis” (in which the synthetic steps used for library construction are followed by the ligation of suitable DNA fragments which code for the identity of the building blocks)[2, 5, 11, 17]. Libraries constructed using DNA-directed synthesis methodologies typically feature DNA in single-stranded format[18], while libraries obtained using DNA-recorded procedures are commonly used in double-stranded DNA format[2, 5, 11, 12, 19]. Our group has previously described and implemented a DNA-recorded synthesis procedure that relies on “splint” ligation of single-stranded oligonucleotides with the help of a short complementary “bridge” adaptor, that can subsequently be removed[7, 11, 12]. The splint ligation technology allows the generation and screening of individual DEL libraries in either single-stranded or double-stranded DNA format. This property has been used in this article in order to assess the impact of DNA format on selection outcome.
We have recently constructed two DEL libraries (named GB-DEL and NF-DEL), containing 366’600 and 670’752 compounds, respectively. Thanks to the use of splint ligation procedures, the two libraries were encoded in both single-stranded and double-stranded DNA format and submitted to selections in identical experimental conditions. Analysis of DEL screening results against multiple targets revealed an extremely high level of similarity for fingerprints derived from the two alternative DNA formats. A close inspection of enrichment factors for the two sets of selections revealed, surprisingly, that selections performed in single-stranded DNA format would consistently give higher enrichment factors, compared to similar selections performed with the library in double-stranded DNA format. These findings provide comfort for the future use of the two alternative encoding methodologies and suggest that single-stranded DNA barcodes should be more frequently considered for DEL selections.
Materials and Methods
Libraries design and synthesis
GB-DEL was synthetized starting from 4-azido-5-methoxy-5-oxopentanoic acid as central scaffold which was linked via amide bond forming reaction[20, 21] to 287 different oligonucleotides code A:(5’-C6-amino-GGAGCTTCTGAATTCTGTGTGCTG-XXXXXX-CGAGCGTC AGGCAGC-3’). After addition of the first set of building blocks(amines) by amide bond forming reaction[20, 21] followed by pool and split technique, The second set of building blocks (alkynes and carboxylic acids) has been coupled and encoded by enzymatic splint ligation with (Code B: 5’-CTGTGTGCTG-XXXXXX-CGAGTCCCATGGCGC-3’) using a chimeric RNA adaptor (GB_adapt: 3’-GCAG-rU-rC-rC-rGTCGGArC-rA-rC-rA-CGAC-5’) bridging code A and B.
The final single stranded (ss) GB-DEL was obtained after removal of adaptor by a denaturing HPLC performed at 60 °C.
NF-DEL was synthesized starting from different derivatives of Iodo-Phenylalanine as central scaffolds which were coupled to a universal amino-modified oligonucleotide (5’-C6-amino-GGAGCTTCTGAATT-3’) [20, 21]. A first set of building blocks (Carboxylic acids, alkynes) was coupled to the central scaffolds through CuAAC[22] or amide coupling[20, 21] and encoded by enzymatic ligation code A:(5’-CTGTGTGCTG-XXXXXX-CGAGTCCCATGGCGC-3’) using a DNA adaptor NF_adapt 1:(5’-CAGCACACAGAATTCAGAAGCTCC-3’). After pool and split, the second set of building blocks consisting in boronates and alkynes was linked via Suzuki and Sonogashira cross couplings[22] and encoded via enzymatic ligation of code B:(5’-CGGATCGACG-YYYYYYY-GCGTCAGGCAGC-3’) using a DNA adaptor:(NF_ adapt 2: 5’–CGTCGATCCGGCGCCATGG-3’). The final single stranded (ss) NF-DEL was purified by a denaturing HPLC performed at 60 °C.
Details for the construction of the GB-DEL and NF-DEL libraries will be published elsewhere.
Double stranded DEL formation
The two ss_DELs were dissolved in 100uL 1X NEBuffer™. A 12-mer oligonucleotide complementary to the ss_DELs at the 3’ extremity was annealed by heating at 75°C for 15min. The elongation of the preannealed DNA strands was completed at 25°C with the addition of 2uL of Klenow polymerase enzyme (M0210L NEB). The obtained double-stranded (ds) libraries were purified by a low-temperature (25 °C) HPLC for Klenow polymerase removal.
Selection experiments
Affinity selections were performed in triplicates both for single and double stranded DELs, using a KingFisher magnetic particle processor (Thermo Fisher Scientific) following a standard procedure optimized in our laboratory[23]. The biotinylated target protein coated onto Dynabeads™ MyOne™ Streptavidin T1 magnetic beads (Invitrogen) has been incubated at a 2uM concentration with both GB-DEL and NF-DEL (10^7 copies per library member for each selection).
After removal of unbound library members, the selection eluate has been amplified by PCR introducing at the same time additional selection-specific DNA barcodes and submitted to Illumina® high-throughput DNA sequencing (HiSeq 2500, Functional Genomics Center Zurich).
Comparative evaluation and quantitative analysis of selection results
Selection results were decoded by an inhouse-written C++ program and visualized using MatLab software version R2018. Enrichment factor were calculated and compared as described suppl. info).
Results and Discussion
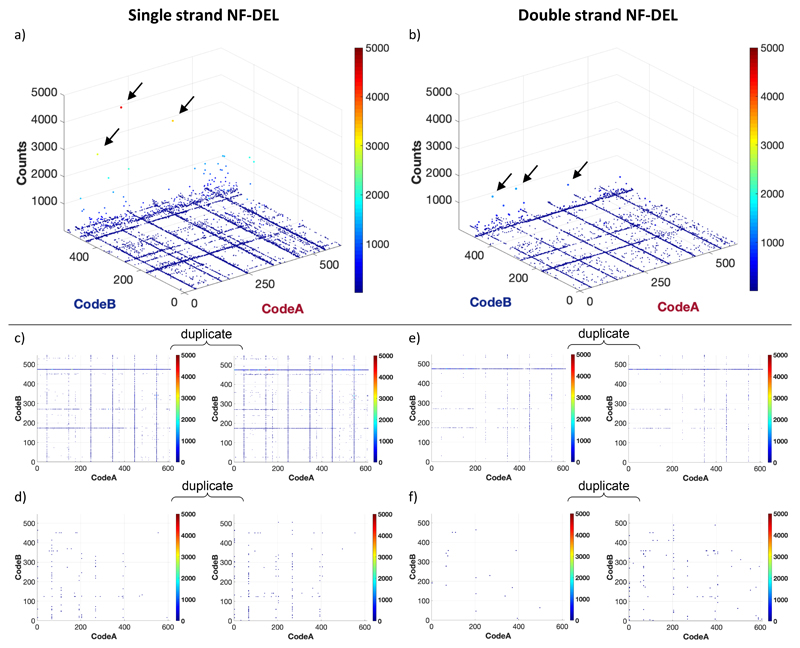
Figure 1 describes the general strategy used for the creation of DEL libraries in single-stranded and double-stranded DNA format. The stepwise assembly of two sets of building blocks (termed “A” and “B”) was followed by splint ligation encoding techniques as previously described [7, 11]. At the end of these procedures, the library was either used in single-stranded DNA format or converted into double-stranded DNA with the help of a complementary oligonucleotide primer and a Klenow polymerase fill-in reaction [Figure 1a]. A low-temperature HPLC purification was performed in order to remove residual Klenow polymerase prior to library selections in double-stranded DNA format. The general design of the GB-DEL and NF-DEL libraries is reported in Figure 1b. The GB-DEL library is based on the double functionalization of a glutamic acid scaffold, coupled to amino-tagged DNA via the carboxylic acid in gamma position. The NF-DEL library was constructed on the basis of iodo-phenyl alanine scaffolds, that were combinatorially modified using Suzuki coupling, Sonogashira coupling, Click chemistry and/or amide bond formation. Selections were performed using biotinylated proteins on streptavidin-coated magnetic beads for affinity capture procedures as previously described[11, 23]. Individual library members were present in approximately 107 copies prior to the affinity capture step, as we had previously shown that selection performance may drop when less than 105 copies of individual compounds are used[24, 25]. The DNA recovered at the end of the affinity capture procedure was PCR amplified and submitted to high-throughput sequencing according to previously published methodologies[23]. The number of DNA counts for individual library members can be graphically represented in a three-dimensional plot, giving rise to “fingerprints” which facilitate the assessment of DEL selections and the identification of preferentially binding compounds [Figure 1a].
Figure 1.
a) Schematic representation of DNA-encoded chemical libraries in single-stranded and double-stranded DNA format, which were used for affinity capture selections. b) Structural design of the GB-DEL and NF-DEL libraries.
Representative fingerprints for DEL selections performed with the GB-DEL and NF-DEL libraries against multiple targets can be found in Supplementary Figure 1. We focused on selections against carbonic anhydrase IX (“CAIX”, a target of pharmaceutical interest[26]) for a detailed comparison of the two libraries and of the screening methodologies with DNA in single-stranded or double-stranded format. Figure 2 shows representative fingerprints for selections of the NF-DEL library against CAIX. A striking similarity between selection results in single-stranded and in double-stranded format was clearly visible, but higher count numbers were observed when DNA was used in single-stranded format [Figure 2a,b]. The panels in Figure 2c-f show two-dimensional representations of selection results, in which individual library members are unambiguously identified by the Code A and Code B number, while the sequence counts were displayed as color codes. The use of different cut-off values and ranges for frequency counts reveals that, on average, enrichment factors for selections performed with single-stranded DNA were higher than with double-stranded DNA.
Figure 2.
Comparison of NF-DEL fingerprints obtained using carbonic anhydrase IX as target protein. Selection fingerprints were obtained using the NF-DEL library in single-stranded (a) or in double-stranded (b) format. The resulting fingerprints for selection on target proteins (c-d) or in negative controls (e-f) in duplicate were displayed in pseudo-two-dimensional format, in which the color code indicates the number of sequence counts.
Figure 3 shows the results of similar selections, performed with the GB-DEL library. An excellent reproducibility was observed for replicate selections and in the pairwise comparison between the use of single-stranded and double-stranded DNA. As for the NF-DEL library, fingerprints of CAIX selections revealed higher enrichment factors for selections performed using DNA in single-stranded format.
Figure 3.
Comparison of GB-DEL fingerprints obtained using carbonic anhydrase IX as target protein. Selection fingerprints were obtained using the NF-DEL library in single-stranded (a) or in double-stranded (b) format. The resulting fingerprints for selection on target proteins (c-d) or in negative controls (e-f) in duplicate were displayed in pseudo-two-dimensional format, in which the color code indicates the number of sequence counts.
In order to provide a more quantitative assessment of the enrichment factors for aromatic sulfonamides that were recovered with high sequence counts from the NF-DEL and GB-DEL libraries, we displayed the structures of the compounds together with the experimental enrichment factors from CAIX selections and standard deviations [Figure 4]. Enrichment factors were calculated by dividing the sequence counts of individual compounds by the average number of counts for all compounds in a given selection [11, 27, 28]. The analysis revealed that enrichment factors were approximately two-fold higher for the NF-DEL library in single-stranded DNA format, compared to the use of double-stranded DNA. Similar findings were observed for the GB-DEL library, but in this case enrichment factors were approximately 5-fold higher.
Figure 4.
Examples of enrichment factors ratios (EF) of selected CAIX binding compounds in single-stranded and double-stranded format for the NF-DEL library (top panel) and GB-DEL library (bottom panel).
There may be a number of possible factors influencing the good screening performance of libraries in single-stranded DNA format. It is possible that the DNA moiety may interact with the solid support used for affinity capture, thus leading to a more efficient recovery. The percentage of binders recovered at the end of selections has recently been quantified by our groups and by others, using quantitative PCR methodologies. High-affinity binders can be selected with high yields, which however are rarely quantitative [24, 25, 29]. Alternatively, the Klenow polymerization step may lead to some undesired damage to the library, which had to undergo an HPLC purification step at low temperature, to remove residual enzyme and nucleotide triphosphates. It should be mentioned, however, that both library formats led to the confident identification of lead compounds [Figure 2,3 and Supplementary Figure 1].
The vast majority of DEL selections reported so far in the literature corresponds to libraries constructed in double-stranded DNA format. However, the group of David Liu at Harvard University has previously described DEL selections and successful identification of binders using single-stranded DEL libraries. In some case the authors used affinity capture procedures, similar to the ones described in this article[2, 19, 23, 30]. In other cases, ingenious selection methodologies [e.g., based on interaction-dependent PCR procedures] were performed[9, 31–33]. To the best of our knowledge, the analysis described in this article represents the first direct comparison of the same libraries in the two alternative DNA formats. The use of single-stranded DNA barcodes may not only perform better compared to double-stranded DNA, but may be also more versatile. For example, individual molecules on a single DNA filament can hybridize to complementary DNA strands, carrying different chemical moieties. This property has been used for the hybridization of libraries with a complementary strand carrying a photo-crosslinker, in order to enable light-controlled formation of covalent bonds with the cognate target of interest[29, 34, 35]. Alternatively, sub-libraries of compounds based on single-stranded DNA barcodes can be hybridized to each other, leading to encoded self-assembling chemical (ESAC) libraries[7, 36–38].
In summary, we have shown that DEL libraries can be conveniently used in both single- and double-stranded DNA format. The use of single-stranded DNA barcodes yields slightly superior selection results and may represent a more versatile methodology, enabling novel affinity capture procedures and the implementation of ESAC technology.
Supplementary Material
Acknowledgements
Financial support by the ETH Zürich, the Swiss National Science Foundation (grant number 310030_182003/1), the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 670603), and the Federal Commission for Technology and Innovation (KTI, grant number 12803.1 VOUCH-LS) is gratefully acknowledged.
Footnotes
Conflict of Interest
The authors note the following disclosure: Dario Neri is co-founder and shareholder of Philochem (www.philochem.com), a company that operates (inter alia) in the field of DNA-encoded chemistry.
References
- [1].Brenner S, Lerner RA. Encoded combinatorial chemistry. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5381–5383. doi: 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leimbacher M, Zhang Y, Mannocci L, Stravs M, Geppert T, Scheuermann J, Schneider G, Neri D. Discovery of small-molecule interleukin-2 inhibitors from a DNA-encoded chemical library. Chemistry. 2012;18:7729–7737. doi: 10.1002/chem.201200952. [DOI] [PubMed] [Google Scholar]
- [3].Winssinger N. Nucleic acid-programmed assemblies: translating instruction into function in chemical biology. Chimia (Aarau) 2013;67:340–348. doi: 10.2533/chimia.2013.340. [DOI] [PubMed] [Google Scholar]
- [4].Arico-Muendel C, Zhu Z, Dickson H, Parks D, Keicher J, Deng J, Aquilani L, Coppo F, Graybill T, Lind K, Peat A, et al. Encoded library technology screening of hepatitis C virus NS4B yields a small-molecule compound series with in vitro replicon activity. Antimicrob Agents Chemother. 2015;59:3450–3459. doi: 10.1128/AAC.00070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Franzini RM, Ekblad T, Zhong N, Wichert M, Decurtins W, Nauer A, Zimmermann M, Samain F, Scheuermann J, Brown PJ, Hall J, et al. Identification of structure-activity relationships from screening a structurally compact DNA-encoded chemical library. Angew Chem Int Ed Engl. 2015;54:3927–3931. doi: 10.1002/anie.201410736. [DOI] [PubMed] [Google Scholar]
- [6].Daguer JP, Zambaldo C, Ciobanu M, Morieux P, Barluenga S, Winssinger N. DNA display of fragment pairs as a tool for the discovery of novel biologically active small molecules. Chemical Science. 2015;6:739–744. doi: 10.1039/c4sc01654h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wichert M, Krall N, Decurtins W, Franzini RM, Pretto F, Schneider P, Neri D, Scheuermann J. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat Chem. 2015;7:241–249. doi: 10.1038/nchem.2158. [DOI] [PubMed] [Google Scholar]
- [8].Samain F, Ekblad T, Mikutis G, Zhong N, Zimmermann M, Nauer A, Bajic D, Decurtins W, Scheuermann J, Brown PJ, Hall J, et al. Tankyrase 1 Inhibitors with Drug-like Properties Identified by Screening a DNA-Encoded Chemical Library. J Med Chem. 2015;58:5143–5149. doi: 10.1021/acs.jmedchem.5b00432. [DOI] [PubMed] [Google Scholar]
- [9].Blakskjaer P, Heitner T, Hansen NJ. Fidelity by design: Yoctoreactor and binder trap enrichment for small-molecule DNA-encoded libraries and drug discovery. Curr Opin Chem Biol. 2015;26:62–71. doi: 10.1016/j.cbpa.2015.02.003. [DOI] [PubMed] [Google Scholar]
- [10].Cuozzo JW, Centrella PA, Gikunju D, Habeshian S, Hupp CD, Keefe AD, Sigel EA, Soutter HH, Thomson HA, Zhang Y, Clark MA. Discovery of a Potent BTK Inhibitor with a Novel Binding Mode by Using Parallel Selections with a DNA-Encoded Chemical Library. Chembiochem. 2017;18:864–871. doi: 10.1002/cbic.201600573. [DOI] [PubMed] [Google Scholar]
- [11].Li Y, De Luca R, Cazzamalli S, Pretto F, Bajic D, Scheuermann J, Neri D. Versatile protein recognition by the encoded display of multiple chemical elements on a constant macrocyclic scaffold. Nature Chemistry. 2018;10:441–448. doi: 10.1038/s41557-018-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Favalli N, Biendl S, Hartmann M, Piazzi J, Sladojevich F, Gräslund S, Brown PJ, Näreoja K, Schüler H, Scheuermann J, Franzini R, et al. A DNA-encoded library of chemical compounds based on common scaffolding structures reveals the impact of ligand geometry on protein recognition. ChemMedChem. 2018;13:5. doi: 10.1002/cmdc.201800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scheuermann J, Neri D. DNA-encoded chemical libraries: a tool for drug discovery and for chemical biology. Chembiochem. 2010;11:931–937. doi: 10.1002/cbic.201000066. [DOI] [PubMed] [Google Scholar]
- [14].Goodnow RA, Jr, Dumelin CE, Keefe AD. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat Rev Drug Discov. 2017;16:131–147. doi: 10.1038/nrd.2016.213. [DOI] [PubMed] [Google Scholar]
- [15].Neri D, Lerner R. DNA-Encoded Chemical Libraries: A Selection System Based On Endowing Organic Compounds with Amplifiable Information. Annual Review of Biochemistry. 2018l;87:24. doi: 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Favalli N, Bassi G, Scheuermann J, Neri D. DNA-encoded chemical libraries - achievements and remaining challenges. FEBS Lett. 2018;592:2168–2180. doi: 10.1002/1873-3468.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Y, Zhao P, Zhang M, Zhao X, Li X. Multistep DNA-templated synthesis using a universal template. J Am Chem Soc. 2013;135:17727–17730. doi: 10.1021/ja409936r. [DOI] [PubMed] [Google Scholar]
- [18].Li X, Liu DR. DNA-templated organic synthesis: nature's strategy for controlling chemical reactivity applied to synthetic molecules. Angew Chem Int Ed Engl. 2004;43:4848–4870. doi: 10.1002/anie.200400656. [DOI] [PubMed] [Google Scholar]
- [19].Mannocci L, Zhang Y, Scheuermann J, Leimbacher M, De Bellis G, Rizzi E, Dumelin C, Melkko S, Neri D. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proceedings of the National Academy of Sciences. 2008;105:17670–17675. doi: 10.1073/pnas.0805130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li Y, Gabriele E, Samain F, Favalli N, Sladojevich F, Scheuermann J, Neri D. Optimized Reaction Conditions for Amide Bond Formation in DNA-Encoded Combinatorial Libraries. ACS Comb Sci. 2016;18:438–443. doi: 10.1021/acscombsci.6b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Franzini RM, Samain F, Abd Elrahman M, Mikutis G, Nauer A, Zimmermann M, Scheuermann J, Hall J, Neri D. Systematic evaluation and optimization of modification reactions of oligonucleotides with amines and carboxylic acids for the synthesis of DNA-encoded chemical libraries. Bioconjug Chem. 2014;25:1453–1461. doi: 10.1021/bc500212n. [DOI] [PubMed] [Google Scholar]
- [22].Favalli N, Bassi G, Zanetti T, Scheuermann J, Neri D. Screening of Three Transition Metal-Mediated Reactions Compatible with DNA-Encoded Chemical Libraries. Helvetica Chimica Acta. 2019;102 doi: 10.1002/hlca.201900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Decurtins W, Wichert M, Franzini RM, Buller F, Stravs MA, Zhang Y, Neri D, Scheuermann J. Automated screening for small organic ligands using DNA-encoded chemical libraries. Nat Protoc. 2016;11:764–780. doi: 10.1038/nprot.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Zimmermann G, Scheuermann J, Neri D. Quantitative PCR is a Valuable Tool to Monitor the Performance of DNA-Encoded Chemical Library Selections. Chembiochem. 2017;18:848–852. doi: 10.1002/cbic.201600626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sannino A, Gabriele E, Bigatti M, Mulatto S, Piazzi J, Scheuermann J, Neri D, Donckele EJ, Samain F. Quantitative Assessment of Affinity Selection Performance by Using DNA-Encoded Chemical Libraries. ChemBioChem. 2019;20:7. doi: 10.1002/cbic.201800766. [DOI] [PubMed] [Google Scholar]
- [26].Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- [27].Faver JC, Riehle K, Lancia DR, Jr, Milbank JBJ, Kollmann CS, Simmons N, Yu Z, Matzuk MM. Quantitative Comparison of Enrichment from DNA-Encoded Chemical Library Selections. ACS Comb Sci. 2019;21:75–82. doi: 10.1021/acscombsci.8b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buller F, Steiner M, Scheuermann J, Mannocci L, Nissen I, Kohler M, Beisel C, Neri D. High-throughput sequencing for the identification of binding molecules from DNA-encoded chemical libraries. Bioorg Med Chem Lett. 2010;20:4188–4192. doi: 10.1016/j.bmcl.2010.05.053. [DOI] [PubMed] [Google Scholar]
- [29].Denton KE, Krusemark CJ. Crosslinking of DNA-linked ligands to target proteins for enrichment from DNA-encoded libraries. Medchemcomm. 2016;7:2020–2027. doi: 10.1039/C6MD00288A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clark MA, Acharya RA, Arico-Muendel CC, Belyanskaya SL, Benjamin DR, Carlson NR, Centrella PA, Chiu CH, Creaser SP, Cuozzo JW, Davie CP, et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat Chem Biol. 2009;5:647–654. doi: 10.1038/nchembio.211. [DOI] [PubMed] [Google Scholar]
- [31].McGregor LM, Gorin DJ, Dumelin CE, Liu DR. Interaction-Dependent PCR: Identification of Ligand−Target Pairs from Libraries of Ligands and Libraries of Targets in a Single Solution-Phase Experiment. J Am Chem Soc. 2010;132:3. doi: 10.1021/ja107677q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McGregor LM, Jain T, Liu DR. Identification of ligand-target pairs from combined libraries of small molecules and unpurified protein targets in cell lysates. J Am Chem Soc. 2014;136:3264–3270. doi: 10.1021/ja412934t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- [34].Li G, Liu Y, Liu Y, Chen L, Wu S, Liu Y, Li X. Photoaffinity labeling of small-molecule-binding proteins by DNA-templated chemistry. Angew Chem Int Ed Engl. 2013;52:9544–9549. doi: 10.1002/anie.201302161. [DOI] [PubMed] [Google Scholar]
- [35].Zhao P, Chen Z, Li Y, Sun D, Gao Y, Huang Y, Li X. Selection of DNA-encoded small molecule libraries against unmodified and non-immobilized protein targets. Angew Chem Int Ed Engl. 2014;53:10056–10059. doi: 10.1002/anie.201404830. [DOI] [PubMed] [Google Scholar]
- [36].Melkko S, Scheuermann J, Dumelin CE, Neri D. Encoded self-assembling chemical libraries. Nat Biotechnol. 2004;22:568–574. doi: 10.1038/nbt961. [DOI] [PubMed] [Google Scholar]
- [37].Zimmermann G, Neri D. DNA-encoded chemical libraries: foundations and applications in lead discovery. Drug Discov Today. 2016;21:1828–1834. doi: 10.1016/j.drudis.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dal Corso A, Catalano M, Schmid A, Scheuermann J, Neri D. Affinity Enhancement of Protein Ligands by Reversible Covalent Modification of Neighboring Lysine Residues. Angew Chem Int Ed Engl. 2018;57:17178–17182. doi: 10.1002/anie.201811650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.