Abstract
Purpose
Antimicrobial resistance (AMR) is of increasing global concern, threatening to undermine recent progress in reducing child and neonatal mortality. Repurposing older antimicrobials is a prominent strategy to combat multidrug- resistant sepsis. A potential agent is fosfomycin, however, there is scarce data regarding its in vitro activity and pharmacokinetics in the paediatric population.
Methodology
We analysed a contemporary, systematically collected archive of community-acquired (CA) and hospital-acquired (HA) paediatric Gram-negative bacteraemia isolates for their susceptibility to fosfomycin. MICs were determined using agar serial dilution methods and validated by disk diffusion testing where breakpoints are available. Disk diffusion antimicrobial susceptibility testing was also conducted for current empirical therapies (ampicillin, gentamicin, ceftriaxone) and amikacin (proposed in the literature as a new combination empirical therapeutic option).
Results
Fosfomycin was highly active against invasive Gram-negative isolates, including 90% (202/224) of Enterobacteriaceae and 96% (22/23) of Pseudomonas spp. Fosfomycin showed high sensitivity against both CA isolates (94%, 142/151) and HA isolates (81%, 78/96; P =0.0015). CA isolates were significantly more likely to be susceptible to fosfomycin than the current first-line empirical therapy (96% vs 59%, P <0.0001). Extended spectrum ß-lactamases (ESBL) production was detected in 34% (85/247) of isolates with no significant difference in fosfomycin susceptibility between ESBL-positive or -negative isolates (73/85 (86%) vs 147/162 (91%) respectively, P=0.245). All isolates were susceptible to a fosfomycinamikacin combination.
Conclusion
Gram-negative paediatric bacteraemia isolates are highly susceptible to fosfomycin, which could be combined with aminoglycosides as a new, carbapenem-sparing regimen to achieve excellent coverage to treat antimicrobial-resistant neonatal and paediatric sepsis.
Introduction
Antimicrobial resistance (AMR) is of increasing concern to global health, threatening to undermine the significant progress made in combating child mortality over the past two decades.1 Sepsis remains an important cause of mortality in children and neonates, responsible for almost one-quarter of all neonatal deaths, with the international literature now concluding that up to one-third of these deaths are directly attributable to AMR.2 For the treatment of sepsis in neonates, infants and children the World Health Organization (WHO) guidelines currently recommend first-line treatment with ampicillin (or penicillin) plus gentamicin, with third-generation cephalosporins recommended as second-line therapy.3
However, recent systematic reviews have reported high rates of AMR to the current regimen, documenting nonsusceptibility to penicillin/gentamicin and third generation cephalosporins of 44 and 43% (respectively) with overall rates of non-susceptibility to ampicillin (80%), gentamicin (22%) and ceftriaxone (74%) among Enterobacteriaceae,1,4,5 reflecting the global emergence and spread of extended spectrum β-lactamases (ESBLs). Simultaneously, carbapenem-resistant Enterobacteriaceae (CRE) are responsible for an increasing number of hospital-acquired outbreaks and substantial morbidity and mortality.6,7
With a global shortage of newly discovered or effective and affordable alternative antibiotics available, the repurposing of older antimicrobials (with improved susceptibility profiles) to update empirical therapeutic guidelines has recently received increasing international attention.8 One promising agent is fosfomycin, a bactericidal antibiotic that was first discovered in 1969. Its use was quickly overshadowed by the heavily promoted discovery of cephalosporins, resulting in infrequent international use and subsequently low global resistance rates.9
The WHO has classified fosfomycin in the category of a ‘critically important’ antimicrobial for investigation in light of its efficacy against multi-resistant Gram-negative organisms,10 and it has been identified as an antimicrobial which holds great promise worldwide for managing MDR Gram-negative infections due to its affordability and efficacy as a carbapenem-sparing regimen,10,11 Fosfomycin’s rapidly bactericidal activity is due to a unique mechanism of action of inhibition of the synthesis of peptidoglycan by blocking the formation of N-acetylmuramic acid at an earlier step than β-lactams, interfering with cell wall synthesis in both Gram-positive and Gram-negative bacteria.12 This affords the opportunity for synergistic mechanisms with other antimicrobials13,14 and helps circumvent the resistance caused by β-lactamase-producing organisms, resulting in efficacy against many problematic pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), glycopeptide-resistant Enterococci and multidrug-resistant (MDR) Enterobacteriaceae.15–17 Fosfomycin is also able to penetrate biofilms, including those produced by vancomycin-resistant enterococci (VRE).18 However, activity against Acinetobacter baumannii and Burkholderia spp. and the anaerobic species Bacteroides is limited.17,19
Fosfomycin is well-distributed within tissues and achieves clinically beneficial concentrations throughout serum, abscess fluid, and renal and cardiorespiratory systems although it does not achieve sufficient concentrations in the CSF to be used as monotherapy for meningitis.20–22 Fosfomycin is not considered an appropriate empirical monotherapy option for sepsis due to the potential for the rapid emergence of resistance, and higher in vitro MICs observed in certain Gram-negative bacteria, such as Pseudomonas aeruginosa.12 For this reason, investigation of a broad-spectrum fosfomycin-amikacin combination therapy for the treatment of neonatal sepsis and meningitis is currently promoted in the international literature.8
Previous research has identified fosfomycin as a relatively safe drug with high tolerability in children.12 However, as fosfomycin’s discovery occurred prior to the current international standards of drug development and approval procedures, significant knowledge gaps regarding its in vitro capacity exist. The minimal in vitro data that is available appears promising, revealing susceptibility to (predominantly urinary) Gram-positive and Gram-negative isolates (including ESBL- producing E. coli and MRSA).23,24 However, there is a lack of published evidence for the in vitro efficacy of fosfomycin on isolates causing bacteraemia.
With the possibility that fosfomycin may be a promising candidate as an empirical therapeutic regimen in neonatal and paediatric sepsis, its potential efficacy in a paediatric population is particularly important. We therefore aimed to address this research gap by evaluating the susceptibility profiles of fosfomycin in contemporary invasive Gram-negative blood isolates of a neonatal and paediatric population, and to analyse its susceptibility profile in comparison to the currently recommended empirical therapy antibiotics: ampicillin, gentamicin and ceftriaxone. A second objective was to evaluate potential carbapenem-sparing fosfomycin-amikacin combination, currently under investigation as a new empirical therapeutic regimen for neonatal sepsis.8
Methods
Patients
The study examined isolates systematically collected from children aged 0–5 years admitted between 28 February 2012 to 25 May 2017 to Kilifi County Hospital (KCH). KCH is a first-level referral hospital situated in a rural coastal region of Kenya, with a 35-bed paediatric ward, five-bed research high dependency ward and a neonatal room, which serves approximately 4000 patients per year. As part of the routine admission investigations, blood cultures are systematically performed on admission for all children, unless admitted for elective surgery or minor trauma.25 Blood cultures are repeated in the case of clinical deterioration whilst in hospital and the date of collection recorded, allowing classification of cultures as either community- or hospital- acquired infections.
Bacterial isolates
Culture and antimicrobial susceptibility testing were undertaken at the KEMRI/Wellcome Trust Research Programme, which is co-located at KCH. All isolates were identified at the time of collection by conventional methods with confirmation provided by the API system (bioMérieux, Marcy l'Etoile, France), then stored at −80°C in tryptic soy broth with 15% glycerol. The KEMRI/Wellcome Trust laboratory is Good Clinical Laboratory Practice (GCLP) accredited and participates in external quality assessment (including the UK National External Quality Assessment Service, NEQAS).
For this study, consecutive non-duplicate blood culture isolates were retrieved for the following Gram-negative organisms: Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Pseudomonas luteola, Enterobacter cloacae complex, Pantoea spp., Salmonella spp., Shigella flexneri and Serratia marcescens. Upon retrieval, isolates were sub-cultured to blood agar and incubated for 18–24 h at 37°C in air. Isolates were classified as community-acquired (CA) if they were collected within 48h of admission, or hospital acquired (HA) if collected beyond 48h of admission.
Antimicrobial susceptibility testing
Fosfomycin susceptibility was determined by the agar dilution method for all isolates, and additionally by disk diffusion methods for E. coli only: as described by the Clinical Laboratory Standards Institute (CLSI since disk diffusion breakpoints have not yet been validated for other Enterobacteriaceae regarding fosfomycin.26
Disk diffusion testing was performed using Mueller–Hinton agar (BBL Microbiology Systems, Cockeysville MD). For E. coli isolates, fosfomycin disks containing 200μg of fosfomycin and 50μg of glucose-6-phosphate (Sigma Aldrich, Dorset, UK) were used; for all other Enterobacteriaceae, disks containing amikacin (30μg), ampicillin (10μg), gentamicin (10μg) and ceftriaxone (30μg) (BBL Micro-biology Systems, Cockeysville MD). E. coli ATCC 25922, P. aeruginosa ATCC 27953 and S. aureus ATCC 25923 were utilised as control strains and compared to documented quality control reference ranges.27 Following overnight incubation in aerobic conditions at 35°C, zone diameters were measured and interpreted using CLSI guidelines.27
For susceptibility testing by agar dilution, Mueller–Hinton agar (BBL Microbiology Systems, Cockeysville MD) plates were prepared by serial dilution using fosfomycin disodium salt powder supplemented with 25μg ml−1 of glucose-6-phosphate (Sigma Aldrich, Dorset, UK).14,28 For each isolate, 10μl of a 0.5ml McFarland suspension was inoculated onto the test plate using a transfer loop. Control strains, including E. coli ATCC 25922 and S. aureus ATCC 25923 were included in each set of tests. The inoculated plates were incubated in aerobic conditions at 35°C for 18–24 h. The MIC of each antimicrobial agent was defined as the lowest concentration that inhibited visible growth of the organism.
Interpretation of susceptibility results
For the purpose of this study assessing Gram-negative bacteraemia, criteria for susceptibility categories by MIC were applied using the EUCAST interpretive criteria for all isolates of Enterobacteriaceae, as CLSI interpretive criteria are only available for urinary tract isolates of E. coli (Table 1).27,29 For P. aeruginosa, although EUCAST/CLSI breakpoint criteria have not yet been established, we applied a breakpoint of ≤64 μg/ml to designate susceptibility, which was inferred from previous evaluation of clinical isolates,30 and is within the EUCAST epidemiological cut-off value (ECOFF) of <128μg/ml.31 Breakpoints for commonly used clinical antibiotic therapy (ampicillin, gentamicin, ampicillin/ gentamicin combination therapy, ceftriaxone, amikacin and imipenem) were also analysed for isolates, which were found to be non-susceptible to fosfomycin (defined by EUCAST MIC criteria, ≥32μg /ml). Statistical analysis of the results was performed using STATA statistical software for Windows (Data Analysis and Statistical Software, version 15.0).
Table 1. Overview of species tested and ESBL status.
| Species | Total | Community Acquired | Hospital Acquired | ESBL positive | ESBL negative |
|---|---|---|---|---|---|
| Escherichia coli | 56 | 89% (50/56) | 11% (6/56) | 14% (8/56) | 86% (48/56) |
| Klebsiella spp. | 87 | 26% (23/87) | 74% (64/87) | 78% (68/87) | 22% (19/87) |
| Enterobacter cloacae | 17 | 53% (9/17) | 47% (8/17) | 24% (4/17) | 76% (13/17) |
| Pantoea | 10 | 70% (7/10) | 30% (3/10) | 10% (1/10) | 90% (9/10) |
| Pseudomonas spp. | 23 | 61% (14/23) | 39% (9/23) | 0% (0/23) | 100% (23/23) |
| Salmonella spp. | 35 | 91% (32/35) | 9% (3/35) | 5% (2/35) | 94% (33/35) |
| Serratia marcescens | 15 | 87% (13/15) | 13% (2/15) | 13% (2/15) | 87% (13/15) |
| Shigella flexneri | 3 | 67% (2/3) | 33% (1/3) | 0% (0/3) | 100% (3/3) |
| Salmonella Typhi | 1 | 100% (1/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) |
| Total | 247 | 61% (151 /247) | 39% (96/247) | 35% (85/247) | 66% (162/247) |
Results
A total of 247 consecutive non-duplicate archived Gram- negative aerobic blood culture were retrieved (Table 2): 132 isolates (53%) from neonates (aged <28 days); 88 (36%) from children aged 28 days to <2 years; and 27 (11%) from children aged 2 to 5 years. Fifty-eight (23%) were isolated from children outside the neonatal age with severe acute malnutrition.
Table 2. EUCAST interpretive criteria of fosfomycin and amikacin for Enterobacteriaceae.
| MIC (μg/ml) | Zone Diameter Breakpoints (mm) | |||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| Fosfomycin | ≤32 | >32 | ≥24* | NA | <24* | |
| Amikacin | ≤16 | 32 | ≥64 | ≥17 | 15-16 | ≤14 |
| Gentamicin | ≤4 | 8 | ≥16 | ≥15 | 13-14 | ≤12 |
| Ampicillin | ≤8 | 16 | ≥32 | ≥17 | 14-16 | ≤13 |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≥23 | 20-22 | ≤19 |
| Imipenem | ≤1 | 2 | ≥4 | ≥23 | 20-22 | ≤19 |
For E. coli only. NA: Not Available
MIC distributions – Enterobacteriaceae and Pseudomonas spp
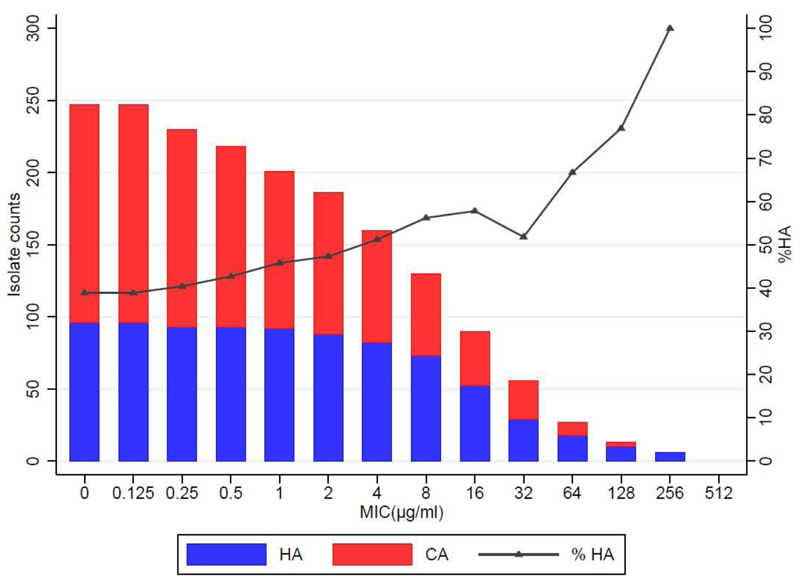
The MIC distributions of the 247 Gram-negative isolates are presented in Fig. 1, with analysis by species presented in Fig. 2. Fosfomycin was highly active against the Enterobacteriaceae isolates examined, with 90% (202/224) of all isolates classified as sensitive (MIC ≤32 μg/ml) as per EUCAST criteria. Among Pseudomonas spp., 96% (22/23) were sensitive as per the ECOFF criteria (≤64 μg/ml).31
Figure 1. Frequency histogram showing the number of isolates exhibiting growth at each fosfomycin agar dilution concentration level, identifying those classified as sensitive.
(MIC≤32μg/mL for Enterobacteriaceae, or ECOFF <128μg/mL for Pseudomonas spp.)
CA = Community-acquired
HA = Hospital-acquired
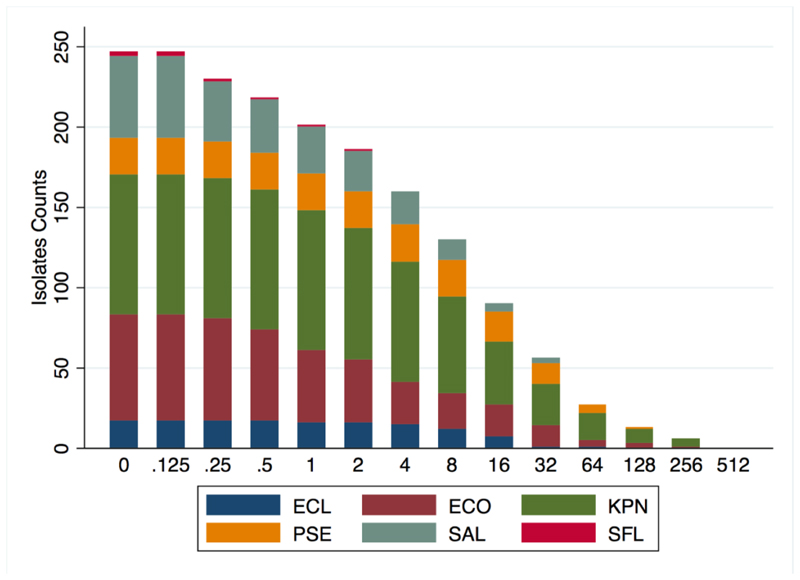
Figure 2. Frequency histogram of the number of isolates exhibiting growth at each fosfomycin dilution level, by species.
ECL = E. cloacae; ECO = E. coli; KPN = K. pneumonia; PSE = Pseudomonas spp. SAL = Salmonella Spp; SFL = S. flexneri
Among the Enterobacteriaceae, K. pneumoniae exhibited the highest frequency of non-susceptibility (20%, 17/83), followed by (in order of non-susceptibility) E. coli (5%, 3/55) and E. cloacae (6%, 1/17), while all Salmonella spp. and S. flexneri samples were fully sensitive.
Community-acquired versus hospital-acquired isolates
In total, 151 (61%) isolates were CA samples while 96 were HA (collected >48 h after admission), of which 64/96 (67%) were isolated from neonates. E. coli was the most frequently isolated CA sample (51/151, 34%), followed by Klebsiella spp. (22/151, 15%). Klebsiella spp. (n=64) were responsible for 67% (64/96) of the HA isolates, followed by Pseudomonas spp. (n=9). HA isolates were more likely to exhibit fosfomycin non-susceptibility (19%, 18/96) than CA isolates (6%, 9/151, P=0.0015).
ESBL-producing isolates
Overall, 85 (34%) of the 247 isolates tested were ESBL-producing organisms, of which the majority (n=67, 79%) were Klebsiella spp. Fosfomycin was highly active against both ESBL-positive and -negative isolates, revealing susceptibility in 91% (147/162) of ESBL-negative isolates and 86% (73/85) of ESBL-positive isolates (P=0.2).
Zone diameter interpretive criteria
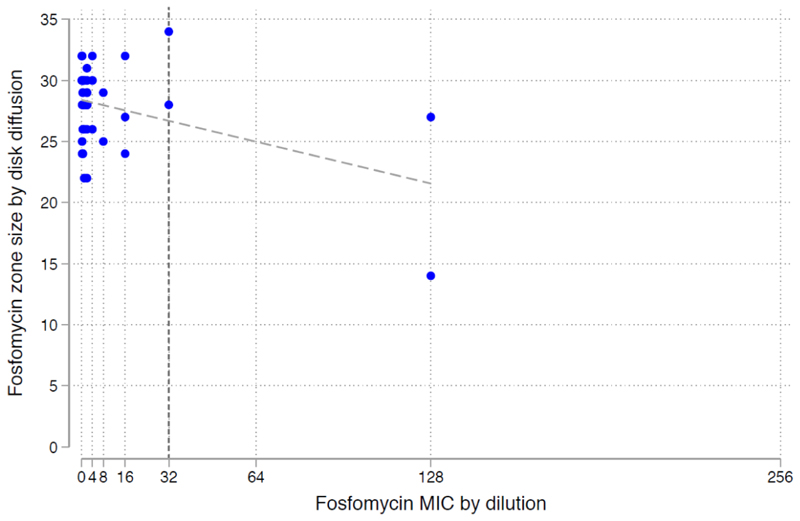
Fig. 3 documents the MIC versus zone diameters for E. coli, illustrating high concordance between agar dilution sensitivity MIC and zone diameter breakpoints by disk diffusion.
Figure 3. Fosfomycin sensitivity of E. coli isolates, by MIC agar dilution and disk diffusion.
The vertical line indicates the EUCAST breakpoint for Enterobacteriaceae (≤32μg/mL) The diagonal line is the fitted linear regression.
Comparison of fosfomycin susceptibility with currently used first-line agents
The analysis of fosfomycin susceptibility to Gram-negative infections for commonly used antimicrobials is presented in Table 3. Of 224 Enterobacteriaceae, 92 (41%) were non-susceptible to the current WHO first-line therapy (ampicillin and gentamicin), including 16% of CA isolates (24/151) and 71% (68/96) of HA isolates. Altogether, 80 of these 92 isolates (87%) were susceptible to fosfomycin (alone), and all (100%) were susceptible to either fosfomycin or amikacin (Table 3). Similarly, of the 93 isolates (26 CA, 67 HA) not susceptible to ceftriaxone, 79 (85%) were susceptible to fosfomycin and all (100%) were susceptible to the combination of fosfomycin and amikacin.
Table 3. Proportion of Enterobacteriaceae resistant to individual antibiotics, with comparison to the likelihood of these isolates also being resistant to fosfomycin.
| Enterobacteriaceae n=224 | Number of resistant isolates as per disk diffusion testing36 | Expressed as a % of isolates | Number of isolates also resistant to fosfomycin (as per MIC)36 | % Resistant to both antibiotics | Difference in proportions (P-value) |
|---|---|---|---|---|---|
| Fosfomycin | 22 | 9.8% | - | - | - |
| Gentamicin | 92 | 41% | 12 | 13% | 28% (p<0.001) |
| Amikacin | 1 | 0.4% | 0 | 0% | 0.4% (p>0.05) |
| Ampicillin | 158 | 71% | 18 | 11% | 60% (p<0.001) |
| Ceftriaxone | 93 | 42% | 14 | 15% | 27% (p<0.001) |
| Imipenem | 1 | 0.4% | 0 | 0% | 0.4% (p>0.05) |
| Ampicillin/Gentamicin Combination | 92 | 41% | 12 | 13% | 28% (p<0.001) |
Fosfomycin-amikacin combination therapy
Fig. 4 illustrates the fosfomycin MIC against amikacin zone diameter breakpoints. All 247 Gram-negative isolates were sensitive to either fosfomycin or amikacin, the potential empirical combination therapy for neonatal sepsis.33
Fosfomycin sensitivity and mortality
Of the 247 isolates tested 87 (35%) were from children who died during that hospital admission; 47 (54%) from neonates aged <28 days, with the remaining mortality cases from children aged <48 months. Of the 87 isolates associated with mortality, 42 (48%) had community-acquired infections; 31% of these (13/42) were resistant to ampicillin and gentamicin, yet all of these isolates exhibited fosfomycin susceptibility.
Of the 87 isolates from children who died, 45 were associated with hospital-acquired infections, of which 49% (31/45) were non-susceptible to second-line therapy (ceftriaxone), yet 22 (71%) of these resistant HA isolates were susceptible to fosfomycin. Overall, there was a significantly greater proportion of fosfomycin non-susceptible isolates among the children who died (17%; 15/87) than among those who survived (7.5%, 12/160, P=0.02).
Discussion
Fosfomycin has potential as both a new empirical therapeutic regimen for clinical sepsis in neonates and children8 and as a carbapenem-sparing treatment strategy in MDR sepsis.34,35 In light of renewed interest in fosfomycin’s therapeutic potential, a range of studies have recently been published which acknowledge its promising in vitro activity16,32,36–39 and our results, unique due to their presentation within clinical data, add strength to this literature.
Our analysis of 247 Gram-negative bacteremia isolates from children reveals high susceptibility among both Enterobacteriaceae and Pseudomonas spp., including MDR and ESBL-producing organisms, in both community- and hospital-acquired infections and across both neonates and older children. We have revealed that several Gram-negative invasive isolates, which were not susceptible to current empirical therapeutic regimens (ampicillin/gentamicin or ceftriaxone), were significantly more likely to be susceptible to fosfomycin. Our findings also revealed 100% in vitro susceptibility to a combination of fosfomycin and amikacin, supporting the potential fosfomycin-amikacin empirical regimen for neonatal sepsis currently under discussion.8 These two agents have the potential to provide broad spectrum cover with adequate penetration of the meninges, with a promising safety profile, whilst minimising the likelihood of resistance emerging.12 Indeed, by circumventing the increasing global resistance challenges caused by ESBLs, this combination regimen would provide broad spectrum carbapenem-sparing cover, with a high likelihood of improved clinical efficacy.
Our results have some limitations. Firstly, the isolates evaluated in this study were collected from a single hospital in Kenya; expanding the geographic base to analyse further in vitro efficacy of fosfomycin would allow for improved generalisability. Secondly, isolates that were non-susceptible to fosfomycin were not sequenced to establish the mechanism of resistance, which would be informative for establishing regional fosfomycin susceptibility profiles. Enzymes conferring fosfomycin resistance have been categorized into four groups – FosA, FosB, FosC and FosX. The most important from an epidemiological point of view is FosA3 seen in E. coli, which is typically located on a conjugative plasmid that also carries the CTX-M-type ESBL – transmission of this plasmid-mediated resistance therefore causes both cephalosporin and fosfomycin resistance simultaneously.40 While FosA3 was first reported in Japan in 2006, it has now spread across Asia in both E. coli and K. pneumoniae; although there are no published reports regarding its presence on other continents.41
Our results have revealed very high susceptibility to fosfomycin in a range of common neonatal pathogens, systematic collection of cultures on admitted children to our unit (removing any potential bias caused by cultures only being collected on the most unwell children or those failing initial inpatient treatment), and the susceptibility testing was performed in an accredited laboratory, which participates in international external quality assurance.
This research therefore adds valuable data regarding the in vitro response of Gram-negative paediatric bacteraemia to fosfomycin, with interesting clinical outcome associations, including revelations on the likelihood of improved efficacy compared to the current levels of non-susceptibility to first- and second-line antimicrobial therapy in CA and HA infections. While our data reveal promising susceptibility profiles for fosfomycin, more clinical research is required before this therapy can be recommended as empiric therapy in a neonatal or paediatric population. An improved understanding of fosfomycin’s pharmacology, pharmacodynamics and pharmacokinetics in neonatal and paediatric populations is required, and randomised trials comparing the efficacy of the currently recommended empirical regimens to fosfomycin (potentially in combination with amikacin) are also necessary, to determine clinical efficacy and toxicity outcomes.
Funding
We are supported by grants from the MRC/DfID/Wellcome Trust Joint Global Health Trials Scheme, the Drugs for Neglected Diseases Initiative (DNDi) and the Bill and Melinda Gates Foundation.
References
- 1.Williams PCM, Isaacs D, Berkley JA. Antimicrobial resistance among children in sub-Saharan Africa. Lancet Infect Dis 2018 Feb. 18(2):e33–e44. doi: 10.1016/S1473-3099(17)30467-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. 2013 [PubMed] [Google Scholar]
- 4.Downie L, Armiento R, Subhi R, Kelly J, Clifford V, et al. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO’s currently recommended antibiotics -systematic review and meta-analysis. Arch Dis Child. 2013;98:146–154. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 5.Le Doare K, Bielicki J, Heath PT, Sharland M. Systematic review of antibiotic resistance rates among Gram-negative bacteria in children with sepsis in resource-limited countries. J Ped Inf Dis. 2015;4:11–20. doi: 10.1093/jpids/piu014. [DOI] [PubMed] [Google Scholar]
- 6.Saidel-Odes L, Borer A. Limiting and controlling carbapenem resistant Klebsiella pneumoniae. Infect Drug Resist. 2014;7:9–14. doi: 10.2147/IDR.S44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu D, Dong N, Zheng Z, Lin D, Huang M, et al. A fatal outbreak of ST11 carbapenem resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18:37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 8.Folgori L, Ellis SJ, Bielicki JA, Heath PT, Sharland M, et al. Tackling antimicrobial resistance in neonatal sepsis. Lancet Glob Health. 2017;5:e1066–1068. doi: 10.1016/S2214-109X(17)30362-5. [DOI] [PubMed] [Google Scholar]
- 9.Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, et al. Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 10.The World Health Organization. Critically important antimicrobials for human medicine, 3rd Revision. 2011 [Google Scholar]
- 11.Pulcini C, Bush K, Craig WA, et al. Forgotten antibiotics: an inventory in Europe, the United States, Canada, and Australia. Clin Inf Dis. 2012;54:268–274. doi: 10.1093/cid/cir838. [DOI] [PubMed] [Google Scholar]
- 12.Grabein B, Graninger W, Rodríguez Baño J, Dinh A, Liesenfeld DB. Intravenous fosfomycin—back to the future. Systematic review and meta-analysis of the clinical literature. Clin Micro Inf. 2017;(23):363–372. doi: 10.1016/j.cmi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 13.MacLeod DL, Barker LM, Sutherland JL, Moss SC, Gurgel JL, et al. Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis. J Antimicrob Chemother. 2009;(64):829–836. doi: 10.1093/jac/dkp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz R. Fosfomycin: an old—new antibiotic. Clin Microbiol Infect. 2012;(18):4–7. doi: 10.1111/j.1469-0691.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 15.Allerberger F, Klare I. In-vitro activity of fosfomycin against vancomycin-resistant enterococci. J Antimicrob Chemother. 1999;(43):211–217. doi: 10.1093/jac/43.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, et al. Antimicrobial susceptibility of multi-drug resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;(35):240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. Fosfomycin for the treatment of infections caused by multidrug resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents. 2009;34:111–120. doi: 10.1016/j.ijantimicag.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Descourouez JL, Jorgenson MR, Wergin JE, Rose WE. Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob Agents Chemother. 2013;(57):1518–1520. doi: 10.1128/AAC.02099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyanova L. Susceptibility of anaerobes to fusidic acid and fosfomycin. Int J Antimicrob Agents. 2017;(45):560–561. doi: 10.1016/j.ijantimicag.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Frossard M, Joukhadar C, Erovic BM, Dittrich P, Mrass PE, et al. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother. 2000;(44):2728–2732. doi: 10.1128/aac.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhnen E, Pfeifer G, Frenkel C. Penetration of fosfomycin into cerebrospinal fluid across non-inflamed and inflamed meninges. Infection. 1987;(15):422–424. doi: 10.1007/BF01647220. [DOI] [PubMed] [Google Scholar]
- 22.Sauermann R, Schwameis R, Fille M, Ligios M, Zeitlinger M. Cerebrospinal fluid impairs antimicrobial activity of fosfomycin in vitro. J Antimicrob Chemother. 2009;(64):821–823. doi: 10.1093/jac/dkp261. [DOI] [PubMed] [Google Scholar]
- 23.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents. 2016;(47):269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Garau J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin Microbiol Infect. 2008;(14):198–202. doi: 10.1111/j.1469-0691.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 25.Berkley JA, Lowe BS, Mwangi I, et al. Bacteraemia among children admitted to a rural hospital in Kenya. New Eng J Med. 2005;(352):39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 26.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. CLSI supplement M100. [Google Scholar]
- 27.CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard. 12th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. CLSI document M02-A12. [Google Scholar]
- 28.Greenwood D, Jones A, Eley A. Factors influencing the activity of the trometamol salt of fosfomycin. Eur J Clin Microbiol. 1986;(5):29–34. doi: 10.1007/BF02013457. [DOI] [PubMed] [Google Scholar]
- 29.European Committee on Antimicrobial Susceptibility Testing. Fosfomycin: rationale for the EUCAST clinical breakpoints, version 1.0. 2013. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Fosfomycin_rationale_1.0_20130203.pdf.
- 30.Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother. 2015;(70):3042–3050. doi: 10.1093/jac/dkv221. [DOI] [PubMed] [Google Scholar]
- 31.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 2.0. 2012. www.eucast.org.
- 32.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother. 2010;(54):3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folgori L, Livadiotti S, Carletti M, Bielicki J, Pontrelli G, et al. Epidemiology and clinical outcomes of multidrug-resistant, gram-negative bloodstream infections in a European tertiary pediatric hospital during a 12-month period. Ped Inf Dis J. 2014;(33):929–932. doi: 10.1097/INF.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 34.Trecarichi EM, Tumbarello M. Therapeutic options for carbapenem resistant Enterobacteriaceae infections. Virulence. 2017;(8):470–484. doi: 10.1080/21505594.2017.1292196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2(2) doi: 10.1093/ofid/ofv050. ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those non-susceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;(54):526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Kapaskelis A, Samonis G. Antimicrobial susceptibility of Gram-positive non-urinary isolates to fosfomycin. Int J Antimicrob Agents. 2010;(35):497–499. doi: 10.1016/j.ijantimicag.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Falagas ME, Giannopoulou K, Kokolakis G, Rafailidis P. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;(46):1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 39.Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in gram-negative pathogens. J Antimicrob Chem-other. 2012;(67):255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Shen P, Wei Z, Liu L, He F, et al. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents. 2015;(45):66–70. doi: 10.1016/j.ijantimicag.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 41.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf.