Abstract
Objectives
This study aims to characterize lateralization of sounds and localization of sounds in children with bilateral conductive hearing loss (BCHL) when listening with either one or two percutaneous bone conduction devices (BCDs).
Design
Sound lateralization was measured with the minimum audible angle test in which children were asked to indicate from which of the two visible speakers the sound originated. Sound localization was measured with a test in which stimuli were presented from speakers that were not visible to the children. In the sound localization test, 150 ms broadband noise bursts were presented, and sound level was roved over a 20-dB range. Because speakers were not visible the localization response was not affected by any visual cue. The sound localization test provides a clear distinction between lateralization and localization of sounds. Ten children with congenital BCHL and one child with acquired BCHL participated.
Results
Both lateralization and sound localization were better with bilateral BCDs compared with the unilaterally aided conditions. In the bilateral BCD condition, lateralization was close to normal in nearly all the children. The localization test demonstrated lateralization rather than sound localization behavior when listening with bilateral BCDs. Furthermore, in the unilateral aided condition, stimuli presented at different sound levels were mainly perceived at the same location.
Conclusions
This study demonstrates that, in contrast to listening with two BCDs, children demonstrated difficulties in lateralization of sounds and in sound localization when listening with just one BCD (i.e., one BCD turned off). Because both lateralization and sound localization behavior were tested, it could be demonstrated that these children are more able to lateralize than localize sounds when listening with bilateral BCDs. The present study provides insight in (sub-optimal) sound localization capabilities of children with congenital BCHL in the unilateral-aided and bilateral-aided condition. Despite the sub-optimal results on sound localization, this study underlines the merits of bilateral application of BCDs in such children.In patients with congenital bilateral conductive hearing loss (BCHL), bilateral application of bone conduction devices (BCDs) is not the standard treatment (Liu et al. 2013). Based on systematic reviews of the existing literature, several authors have concluded that more studies are needed to provide convincing evidence on the advantage of bilateral BCD application over listening with a unilateral BCD (Colquitt et al. 2011; Janssen et al. 2012; Johnson et al. 2017). Patients with BCHL cannot access binaural cues when listening with only one BCD. With two BCDs, these patients may gain increased access to binaural cues, allowing for improved localization abilities (Zeitooni et al. 2016). Binaural processing of our auditory world provides important benefits like, improved directional hearing, increased safety (Stelling-Kończak et al. 2016), feelings of comfort and understanding of speech in noisy listening conditions (Avan et al. 2015). Hence, it is important to provide patients with BCHL and their caretakers with evidence on the merits of hearing rehabilitation with two BCDs.
Keywords: aural atresia, bilateral conductive hearing loss, bone-anchored hearing aid, boneconduction device
Introduction
An operational tool to assess binaural processing (i.e., processing interaural differences in level and timing), is a sound localization test. Several studies have reported improvements in sound localization in children with BCHL (Priwin et al. 2007; Dun et al. 2013) and in adults with BCHL (Bosman et al. 2001), fitted bilaterally with percutaneous BCDs. However, concomitant stimulation of the contralateral cochlea, due to the limited transcranial attenuation of bone conduction vibrations, might limit the ability to process these interaural differences in level and timing (Stenfelt & Goode 2005).
Often sound sources are also visible and it is suggested that lateralization of sounds is already helpful, especially in dynamic situations, when combining audio and visual information.
In the studies demonstrating an improvement in sound localization in aided children with BCHL (Priwin et al. 2007; Dun et al. 2013), only a limited number of speakers were used. Therefore, these studies tested only the ability to lateralize sounds. By using both a lateralization test (the minimum audible angle [MAA] test) and a sound localization test, we aim to demonstrate whether bilaterally fitted children can indeed utilize binaural cues. With the MAA test lateralization of sounds is investigated. With the localization test, we are able to discriminate sound localization (i.e., processing of binaural cues) from lateralization.
Materials and Methods
Patients With BCHL
We identified 33 children with BCHL from our clinical database who were implanted bilaterally with percutaneous BCDs. All children had at least 6 months experience with bilateral BCDs. Six children with performal IQ ≤ 80 and/or poor cooperation during previous testing were excluded. Children who used only one of the BCDs, were not invited (n = 5). We were not successful in contacting three of the children, and therefore, in total, 19 children (i.e., parents of children) were invited to participate in the present study. Eleven children accepted the invitation and participated in the present study. These children indicated that they were (very) satisfied with the BCDs. Ten out of these 11 children were tested with the lateralization test, that is, MAA test, and all 11 children participated in the sound localization test. Patient characteristics are presented in Table 1. Ten children had congenital BCHL due to aural atresia (n = 9) or ossicular chain anomalies (n = 1), and one child suffered from acquired bilateral chronic otitis media (i.e., acquired BCHL). Four children participated in a previous study on directional hearing (Dun et al. 2013).
Table 1.
Demographic and Audiological Characteristics of the Participating Children
| Sex | Age at Current Evalu-ation (y) | Age Fitting of Bilateral Percuta-neous BCDs (y) | Age Fitting First BCD on (soft)Band (Y>m) | Age Fitting Second BCD on (soft)Band (Y>m) | Type of Hearing Loss | Etiology | Syndrome | Current Sound Processors | PTA4 AD AC | PTA4 AD BC | PTA4 AS AC | PTA4 AS BC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Female | 7Y | 4Y | 3M | 1Y | Congenital | Atresia III | Goldenhar | Baha 4 | 59 | 1 | 74 | 5 |
| P2 | Female | 7Y | 4Y | 4M | 1Y 11M | Congenital | Ossicular chain anomalies | Goldenhar | Baha 4 | 59 | −1 | 58 | 8 |
| P3 | Male | 7Y | 4Y | 5M | 1Y5M | Congenital | AS Atresia III AD Atresia I | Goldenhar | Divino | 32 | –3 | 61 | 10 |
| P4 | Female | 7Y | 5Y | 1Y | 4Y | Congenital | Atresia I la | De Grouchy | BP100 | 60 | 4 | 60 | 3 |
| P5 | Female | 8Y | 4Y | 7M | 5Y | Congenital | Atresia III | Treacher Collins | Divino | 75 | 15 | 70 | 13 |
| P6 | Female | 8Y | 7Y | 1y 3M | 4Y | Congenital | Atresia ill | Treacher Collins | BP100 | 64 | 0 | 59 | – |
| P7 | Male | 9Y | 4Y | 1y 9M | 4Y | Congenital | Atresia III* | BP100 | 34 | –1 | 70 | 6 | |
| P8 | Female | 14Y | 6Y | 9M | 3Y | Congenital | Atresia III | BP100 | 61 | 4 | 64 | 8 | |
| P9 | Male | 15Y | 7Y | 2M | 7Y | Congenital | Atresia III | BP100 | 53 | 3 | 68 | 10 | |
| P10 | Male | 16Y | 6Y | 1y 7M | 6Y | Congenital | Atresia I la | Divino | 54 | 3 | 58 | 0 | |
| P11 | Female | 16Y | 11Y | 8Y | 8Y | Acquired | Chronic otitis media | Divino | 44 | 10 | 66 | 10 |
Patient characteristics.
After aural atresia surgery AC = air conduction, AD = right ear, AS = left ear, BC = bone conduction, M = months, PTA4 = pure tone average 0.5/1/2/4 kHz, Y = years.
Percutaneous BCDs and test conditions
Hearing tests were performed in one session with the child’s own BCDs (either Baha Divino, Baha BP100, or Baha 4; Cochlear BAS, Gothenburg, Sweden). All tests were performed with the child’s own habitual volume and microphone settings with the devices in auto- or omnidirectional mode. The three listening conditions were randomized: (1) unilateral BCD on the left side, (2) unilateral BCD on the right side, and (3) bilateral BCDs. This study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee.
Lateralization tested with the MAA test
With the MAA test the smallest perceivable difference in azimuth between two visible sound sources in the horizontal plane, was investigated (Hartmann & Raked 1989; Litovsky 1997; Dun et al. 2013). A broadband noise burst (bandwidth 0.5–20 kHz, 500 ms duration) was presented at a randomly selected sound levels of 55, 60 or 65 dB SPL. Sound levels were roved over only 10 dB for practical reasons (i.e., reduced experimental time) to limit the effects of head shadow as a monaural localization cue (e.g., Van Wanrooij & Van Opstal 2004). The MAA test, described also by Dun et al. (2013), was carried out in a sound attenuated booth; the children were positioned in the center of an arc with a 1-m radius. After a practice run, the loudspeakers were positioned at −90° (far left) and +90° (far right). Stimuli were presented at random by one of the two loudspeakers and the child was asked to identify the loudspeaker. After four subsequent correct responses out of four stimuli, the loudspeakers were repositioned to −60° and +60°. This procedure was continued for positions at 30°, 15°, 10°, and 5°. In case of an incorrect answer, another series of four stimuli was presented in the previous speaker position. The final score was defined as the smallest angle at which a series of four subsequent stimuli was correctly identified in two out of the three runs. No explicit feedback was given during the measurements.
Sound localization test
The localization experiment was conducted in a dark and sound-attenuated room. Children were seated in the center of the room. Stimuli were delivered from loudspeakers at a distance of 1.15 m from the child. Stimuli were presented at different azimuth positions (spatial resolution 2.5°, see Otte et al. 2013), ranging from −75° (left) to +75° (right). Broadband (bandwidth 0.5–8 kHz) noise bursts with a duration of 150 ms, were randomly presented from selected loudspeaker locations, at three random sound levels of 50, 60, or 70 dB SPL. A complete trial comprised 36 stimuli (12 stimuli per sound level). The response task was a head movement towards the noise source. Head movements were recorded with the magnetic search-coil induction technique, which has been shown to be adequate for testing normal-hearing children (Otte et al. 2013) and unilaterally hearing impaired children (Nelissen et al. 2016). We analyzed all responses separately for each condition and for each listener. Velocity and final head-position was used to determine the azimuth response (described previously: Otte et al. 2013; Nelissen et al. 2016; Agterberg et al. 2019). Head position had to be stable for at least 250 ms after velocity decreased to zero. Each child participated in a short practice session at the beginning of the experiment. During the measurements, children were only corrected when distracted or when they were in an incorrect seating position; no other feedback was provided.
Localization of visual stimuli
Some of the younger children (7–8 years old) seemed to have difficulties with performing and/or completing the sound localization task. To investigate whether this was related to their impaired hearing or related to understanding and execution of the task, a visual localization task was performed in three of the younger children (P1, P3, and P5) by replacing the auditory stimuli by visual stimuli (green LED) at the position of the loudspeakers. A series of 36 visual stimuli (duration 150 ms) was presented with light-emitting diodes.
Data analysis
Lateralization (MAA) and sound localization data were analyzed for each child and each condition separately. All data collected with the localization test are shown as target-response plots in Figure 1. Datasets are included after deleting the early (<100 ms after stimulus onset) and unstable head-saccades. In the acute unilateral BCD conditions, the bias (calculated with linear regression analysis of perceived versus presented sound-source azimuth as indicated in Otte et al. 2013 and Agterberg et al. 2019) is expected to be toward the site of the BCD, consequently negative when the left BCD is active, and positive when the right BCD is active. The MAE is the mean of all the (absolute) errors, in degrees, between the azimuth response and the position of the target loudspeaker. Unilateral MAE-score was compared with the bilateral MAE-score (paired-sample t-test). Data analysis was done using Matlab (the MathWorks) and Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Armonk NY; IBM Corp, Version 22).
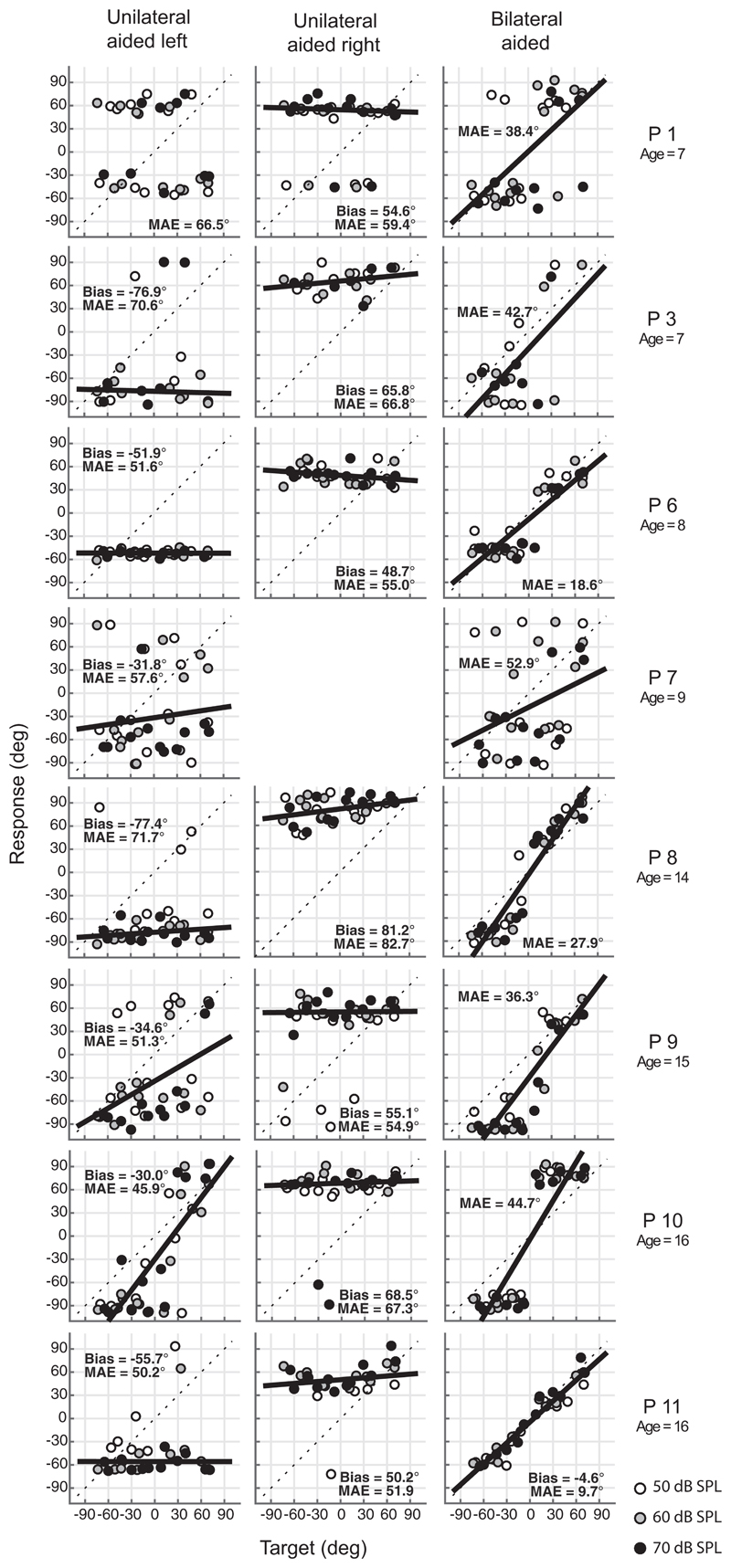
Fig. 1.
Sound-localization target-response plots of all children for broadband noise bursts. Responses of the three sound levels (white: 50, gray: 60, black: 70 dB SPL) are indicated in the unilateral left (left column), unilateral right (middle column), and bilateral (right column) BCD condition. Children demonstrate lateralization of stimuli in the bilateral BCD condition while P11 (the only child with an acquired BCHL) demonstrates good localization abilities. MAE = Mean absolute error, in degrees.
Results
Lateralization of Sounds With Bilateral BCDs
In Table 2, the outcomes of the lateralization test are presented. When listening with two BCDs, the MAA was significant smaller when compared with listening with one BCD (paired t-test MAA p<0.001). In 7 out of the 10 children, the MAA was 90° in one or both of the unilateral conditions, and ≤15° in the bilateral BCD condition. Only in child P5 the lateralization was not affected when listening with two BCDs; an MAA of 90° was found in all conditions.
Table 2.
The minimum audible angle in degrees (MAA) of all children (P1-P11) for the unilateral aided left, unilateral aided right and bilateral conditions with BB stimuli
| Unilateral Left MAA | Unilateral Right MAA | Bilateral MAA | |
|---|---|---|---|
| P1 | 90 | 90 | 15 |
| P2 | 15 | 60 | 5 |
| P3 | 90 | 90 | 15 |
| P4 | 90 | 90 | 10 |
| P5 | 90 | 90 | 90 |
| P6 | 90 | 90 | 5 |
| P7 | – | – | – |
| P8 | 90 | 30 | 5 |
| P9 | 90 | 90 | 30 |
| P10 | 15 | 90 | 5 |
| P11* | 60 | 90 | 10 |
Acquired conductive bilateral hearing loss.
Lateralization Instead of Localization When Listening With Two BCDs
Figure 1 shows the target-response plots of the localization test for eight children in both the acute unilateral aided left, unilateral aided right, and the bilateral BCD conditions. Data of P2, P4, and P5 were not included because the majority of their recorded head-saccades did not start with a stable head position. Responses to the three different sound levels are indicated in white (50 dB SPL), gray (60 dB SPL), and black dots (70 dB SPL). The overall bias and MAE are indicated in the panels.
Since P11 is the only child with acquired BCHL, data were not included for further analysis, however, presented to indicate the child’s good sound localization abilities when listening with bilateral BCDs (Fig. 1). The data points lie along the diagonal and yielded a small MAE of 10°, close to that of normal-hearing children.
In all tested children with congenital BCHL, the data of the bilaterally aided condition were not uniformly distributed; see Figures 1 and 2B. Most children showed a bimodal response pattern, reflecting sound lateralization and not sound localization. Children could not identify the correct sound locations and perceived the stimuli mainly from approximately one location at the left and one location at the right side. Still, the data indicate that the children were able to distinguish whether stimuli were presented at either the left or the right side.
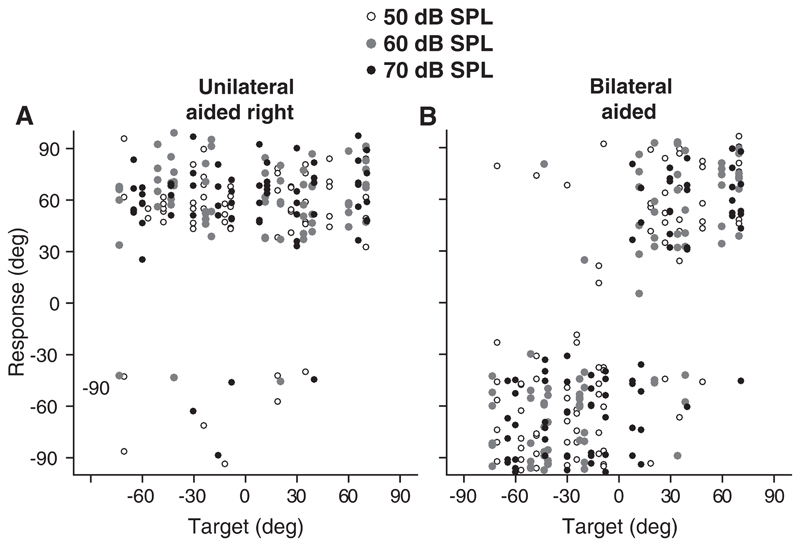
Fig. 2.
Azimuth target-response plots pooled for unilateral right (A) and bilateral BCD (B) conditions for the congenital BCHL patients. Levels are indicated, open, gray, and black (50, 60, and 70 dB SPL).
Sound level independent bias when localizing with one BCD
Figure 2 demonstrates the target-response data for all the children with congenital BCHL in the unilateral right (Fig. 2A) and bilateral (Fig. 2B) condition (P11 excluded). Sound levels are indicated by different symbols (open = 50 dB SPL, gray = 60 dB SPL, black = 70 dB SPL; see Figure 2 in Supplemental Digital Content http://links.lww.com/EANDH/A635 with color code). In the unilaterally aided situation, 92% of the stimuli were perceived toward the aided side while the response was not affected by the level of the stimuli (level independent bias, paired t-test, p = 0.4). For example, for child P8, the bias in the unilateral left condition is −77.4° and the bias in the unilateral right condition is 81.2° (Fig. 1). In the unilateral conditions noise bursts presented at the lowest sound level (50 dB SPL) were not always perceived. For the three different sound levels 50 dB SPL, 60 dB SPL, and 70 dB SPL, respectively, 87%, 95%, and 93% of the stimuli were perceived toward the aided side (Fig. 2A). The overall MAE pooled for levels was larger in both the unilateral aided left (mean MAE 58°) condition (paired-sample t-test; p < 0.005), and the unilateral aided right (mean MAE 62°) condition (paired-sample t-test; p < 0.001), compared with the MAE in the bilateral aided (mean MAE 34°) condition.
Localization of visual stimuli, tested in three of the children, was accurate (MAE values were close to MAE values of normal-hearing children), demonstrating that the inaccurate localization of auditory stimuli is related to impaired hearing and not to limitations in understanding and/or performing the behavioral localization response.
Discussion
This study demonstrates the advantage of bilateral application of BCDs in children with congenital BCHL. When one BCD was turned off, children demonstrated difficulty in discriminating horizontal sound positions (Table 2 and Fig. 2). In this condition, the majority of children had an MAA above 60°. Moreover, in the sound localization test, the unilateral aided children perceived the stimuli mainly at one position on the aided side. When bilateral BCDs were used, both lateralization (Table 2), as well as sound localization (Figs. 1 and 2) was better compared with listening in the acute unilateral BCD condition. These results support subjective observations (Ho et al. 2009; Dutt et al. 2002) and objective outcomes (Bosman et al. 2001; Priwin et al. 2007; Dun et al. 2013) in patients with BCHL. Nevertheless, the directional hearing abilities when listening with bilateral BCDs can be mainly characterized as “lateralization” instead of “localization.” This indicates that these children are able to distinguish whether sounds are coming from left or right side, but incapable to indicate the exact sound source location.
Interestingly, when comparing the localization data of child P10 with child P11 (Fig. 1), the target response plots for the bilateral aided condition are clearly different. However, the pure lateralization of child P10 is reflected by a better score of 5° (MAA test; see table 2), compared with child P11 who had a score of 10°. These results demonstrate that the reported benefit depends on the test that is selected. This is supported by the observation that, in contrast to the data in the present study demonstrating lateralization, Bosman et al. (2001) reported that adults with congenital BCHL demonstrated adequate sound localization instead of lateralization, when listening with two BCDs. In their setup, sound localization was tested with just 7 visible speakers positioned 30° apart, and instead of good sound localization the reported good performance might reflect appropriate lateralization.
The sound localization test was validated with a visual localization test in three of the youngest children. This test demonstrated that visual stimuli were localized correctly, implicating that the children had no difficulties with the test procedure and that poor scores on the sound localization test were related to poor localization abilities and not to limited cognitive abilities.
Children with BCHL due to microtia and/or aural atresia, which is a relatively rare condition with an incidence of 1:50.000 newborns, have no access to pinna cues. The absence of at least one normal-hearing ear, and the possibility that intensity differences are less well perceived at the cochlea because of “gain characteristics” of the device, stresses the need of listening with a second BCD.
Limitations of the present study are the potential selection bias, the variability in pre- and post-surgical (bilateral) bone conduction hearing experience, and the relatively large age range of the tested children. Ideally, children are able to localize sounds instead of just being able to indicate whether a sound is coming from the left or from the right (i.e., sound lateralization). The studied population is rather unique and the present study does not contain enough data to conclude whether any of the above mentioned factors are crucial for localization.
Regarding lateralization and sound localization in the unilateral BCD condition, this condition was acute and probably rather new to all children (i.e., as they were accustomed to listening mostly with bilateral inputs). Therefore, we cannot exclude that unilaterally implanted children with long-term experiences with one BCD, would perform better on both the lateralization test and the sound localization test. Furthermore, it would be of interest to investigate if localization abilities might improve after more localization training with bilateral BCDs. In the group of 33 children, a substantial portion (15% or 5 children), indicated they frequently only used one BCD. Further research is needed to investigate why some children with BCHL indicated to use only one BCD.
Conclusion
The present study provides an overview of lateralization and localization abilities in children with congenital BCHL. The results support the potential benefit on directional hearing after bilateral implantation, and are in agreement with previous studies investigating these abilities comparing listening with one or two BCDs (Bosman et al. 2001; Priwin et al. 2007; Dun et al. 2013).
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com)
Acknowledgments
The research was supported by the William Demants og Hustra Ida Emilies Fond (M.J.H.A.), the FP7-PEOPLE-2013-ITN Marie Curie Initial Training Network iCare (K.V. and M.J.H.A.), and by European Union Horizon-2020 ERC Advanced Grant 2016 (ORIENT, Grant No. 693400) (A.F.M.S.). All authors contributed significantly to the study, with C.A.d.B., A.J.B., and M.J.H.A. mainly collecting the data; C.A.d.B. and K.V. analyzing the data; C.A.d.B., A.J.B., A.F.M.S., M.K.S.H., and M.J.H.A. writing the article.
C.A.d.B., M.K.S.H. report financial support to the authors institution for conducting 2 clinical studies from Oticon Medical AB (Askim, Sweden) and from Cochlear Bone Anchored Solutions AB (Mölnlycke, Sweden), outside the submitted work.
References
- Agterberg MJH, Snik AFM, Van de Goor RMG, et al. Sound-localization performance of patients with single-sided deafness is not improved when listening with a bone-conduction device. Hear Res. 2019;372:62–68. doi: 10.1016/j.heares.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avan P, Giraudet F, Buki B. Importance of binaural hearing. Audiol Neurootol. 2015;20(1):3–6. doi: 10.1159/000380741. [DOI] [PubMed] [Google Scholar]
- Bosman AJ, Snik AF, van der Pouw CT, et al. Audiometric evaluation of bilaterally fitted bone-anchored hearing aids. Audiology. 2001;40:158–167. [PubMed] [Google Scholar]
- Colquitt JL, Loveman E, Baguley DM, et al. Bone-anchored hearing aids for people with bilateral hearing impairment: a systematic review. Clin Otolaryngol. 2011;36:419–441. doi: 10.1111/j.1749-4486.2011.02376.x. [DOI] [PubMed] [Google Scholar]
- Dun CA, Agterberg MJ, Cremers CW, et al. Bilateral bone conduction devices: improved hearing ability in children with bilateral conductive hearing loss. Ear Hear. 2013;34:806–808. doi: 10.1097/AUD.0b013e318291784e. [DOI] [PubMed] [Google Scholar]
- Dutt SN, McDermott A-L, Burrell SP, et al. Patient satisfaction with bilateral bone-anchored hearing aids: the Birmingham experience. J Laryngol Otol Suppl. 2002:37–46. [PubMed] [Google Scholar]
- Hartmann WM, Raked B. On the minimum audible angle-a decision theory approach. J Acoust Soc Am. 1989;85:2031–2041. doi: 10.1121/1.397855. [DOI] [PubMed] [Google Scholar]
- Ho EC, Monksfield P, Egan E, et al. Bilateral Bone-anchored Hearing Aid: impact on quality of life measured with the Glasgow Benefit Inventory. Otol Neurotol. 2009;30:891–896. doi: 10.1097/MAO.0b013e3181b4ec6f. [DOI] [PubMed] [Google Scholar]
- Janssen RM, Hong P, Chadha NK. Bilateral bone-anchored hearing aids for bilateral permanent conductive hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2012;147:412–422. doi: 10.1177/0194599812451569. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Xu J, Cox RM. Impact of Hearing Aid Technology on Outcomes in Daily Life III: Localization. Ear Hear. 2017;38:746–759. doi: 10.1097/AUD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY. Developmental changes in the precedence effect: estimates of minimum audible angle. J Acoust Soc Am. 1997;102:1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- Liu CC, Chadha NK, Bance M, et al. The current practice trends in pediatric bone-anchored hearing aids in Canada: a national clinical and surgical practice survey. J Otolaryngol Head Neck Surg. 2013;42:43. doi: 10.1186/1916-0216-42-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen RC, Agterberg MJ, Hol MK, et al. Three-year experience with the Sophono in children with congenital conductive unilateral hearing loss: tolerability, audiometry, and sound localization compared to a bone-anchored hearing aid. EurArch Otorhinolaryngol. 2016;273:3149–3156. doi: 10.1007/s00405-016-3908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte RJ, Agterberg MJ, Van Wanrooij MM, et al. Age-related hearing loss and ear morphology affect vertical but not horizontal sound-localization performance. J Assoc Res Otolaryngol. 2013;14:261–273. doi: 10.1007/s10162-012-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priwin C, Jonsson R, Hultcrantz M, et al. BAHA in children and adolescents with unilateral or bilateral conductive hearing loss: a study of outcome. Int JPediatr Otorhinolaryngol. 2007;71:135–145. doi: 10.1016/j.ijporl.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Stelling-Kończak A, Hagenzieker M, Commandeur JJF, et al. Auditory localisation of conventional and electric cars: Laboratory results and implications for cycling safety. Transportation Research Part F: Traffic Psychology and Behaviour. 2016;41:227–242. [Google Scholar]
- Stenfelt S, Goode RL. Bone-conducted sound: physiological and clinical aspects. Otol Neurotol. 2005;26:1245–1261. doi: 10.1097/01.mao.0000187236.10842.d5. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24:4163–171. doi: 10.1523/JNEUROSCI.0048-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitooni M, Mäki-Torkko E, Stenfelt S. Binaural Hearing Ability With Bilateral Bone Conduction Stimulation in Subjects With Normal Hearing: Implications for Bone Conduction Hearing Aids. Ear Hear. 2016;37:690–702. doi: 10.1097/AUD.0000000000000336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.