Abstract
Wnt/β-catenin signaling regulates progenitor cell fate decisions during lung development and in various adult tissues. Ectopic activation of Wnt/β-catenin signaling promotes tissue repair in emphysema, a devastating lung disease with progressive loss of parenchymal lung tissue. The identity of Wnt/β-catenin responsive progenitor cells and the potential impact of Wnt/β-catenin signaling on adult distal lung epithelial progenitor cell function in emphysema are poorly understood. Here, we used TCF/ Lef:H2B/GFP reporter mice to investigate the role of Wnt/β-catenin signaling in lung organoid formation. We identified an organoid-forming adult distal lung epithelial progenitor cell population characterized by a low Wnt/β-catenin activity, which was enriched in club and alveolar epithelial type (AT)II cells. Endogenous Wnt/β-catenin activity was required for the initiation of multiple subtypes of distal lung organoids derived from the Wntlow epithelial progenitors. Further ectopic Wnt/β-catenin activation specifically led to an increase in alveolar organoid number; however, the subsequent proliferation of alveolar epithelial cells in the organoids did not require constitutive Wnt/β-catenin signaling. Distal lung epithelial progenitor cells derived from the mouse model of elastase-induced emphysema exhibited reduced organoid forming capacity. This was rescued by Wnt/β-catenin signal activation, which largely increased the number of alveolar organoids. Together, our study reveals a novel mechanism of lung epithelial progenitor cell activation in homeostasis and emphysema.
Keywords: chronic lung disease, emphysema, lung epithelial progenitor, organoid, regeneration, Wnt/β-catenin
1. Introduction
The adult mammalian distal lung comprises functionally distinct regions including a branched network of conducting and respiratory airways and a dense lattice of alveolar sacs where gas exchange occurs. Maintenance and repair upon injury of this highly complex structure relies on distinct progenitor cell populations and their regulation by signaling pathways in a spatiotemporally controlled manner. Several progenitor cell populations have been identified, including club cells and alveolar type II (ATII) cells, which collectively are able to repopulate distal airway as well as alveolar epithelium.1–3 Regeneration upon acute and severe distal mouse lung injury (eg, following influenza infection) is proposed to involve activation of quiescent, multipotent progenitors capable of generating both airway and alveolar cell types.4–8 The function and potential impairment of lung epithelial progenitor cells upon chronic and progressive lung injury, which underlies many lung diseases including chronic obstructive pulmonary disease (COPD), however, remains largely unexplored.9
COPD is the third leading cause of death worldwide. One major pathological feature of COPD is emphysema, characterized by the progressive loss of functional parenchymal lung tissue and thus loss of alveolar gas exchange area. Currently, emphysema cannot be cured or reversed, underscoring a large unmet medical need for novel treatment options.10,11 Important risk factors for emphysema are age and genetic predisposition, cigarette smoking, or occupational exposures.10,12 It is known that ongoing inflammation, oxidative stress, and protease/antiprotease imbalance lead to matrix degradation and progressive tissue destruction in emphysema. Importantly, endogenous regenerative mechanisms of the lung are severely compromised in emphysema. Recent work by our laboratory and others has demonstrated that the activity of the Wnt/ β-catenin pathway, which is critical for lung development and lung tissue homeostasis, is reduced in the alveolar epithelium in human emphysema as well as in mouse models.9,13–17 Notably, ectopic activation of Wnt/β-catenin signaling induced intrinsic alveolar repair in mouse models of emphysema and 3D lung tissue culture derived from emphysema patients.9,18 These studies suggest that tissue regeneration can be initiated in adult human emphysematous lungs and that Wnt/β-catenin signaling serves as a potential therapeutic target to achieve tissue repair in emphysema. However, the identity of potential lung progenitor cells that respond to Wnt/β-catenin activation upon chronic injury to regenerate alveoli in emphysema, and the role of Wnt/β-catenin signaling during their transition from quiescence to activation in homeostasis and disease are poorly defined.
Here, we investigated the role of Wnt/β-catenin signaling in adult distal lung progenitor cells using a lung organoid assay. We aimed to identify and characterize the Wnt/β-catenin responsive epithelial progenitor cell populations in the adult lung, and furthermore, to investigate the potential for Wnt pathway modulation to rescue changes in regenerative potential in a mouse model of emphysema.
2. Materials and Methods
2.1. Mice
TCF/Lef:H2B/GFP mice (The Jackson Laboratory, 013752) of >8 weeks of age were used for all experiments. Mice were maintained in specific pathogen-free conditions. All animal experiments were performed according to the Ethics Committee guidelines of the Helm-holtz Zentrum München and Government of Bavaria and the institutional and regulatory guidelines of University of Colorado Institutional Animal Care and Use Committee.
2.2. Elastase treatment
Mice were injected with porcine pancreatic elastase (PPE, 40 U/kg body weight in 80 μL) oropharyngeally as described previously.19 The control mice received 80 μL of saline. Lung function measurement and lung epithelial isolation were performed at day 21 post-PPE injection. N = 6 animals per group and were repeated at least three times.
2.3. Lung epithelial cell isolation
Distal lung epithelial cells were isolated from adult mouse lung with antibody-conjugated magnetic beads as previously described.20–23 Detailed procedure is included in the Methods and Material section in Supporting Information.
2.4. Flow cytometric analysis and fluorescence-activated cell sorting
The distal lung epithelial cell suspension was stained with fluoro-chrome-conjugated antibodies, as described in the Methods and Material section in Supporting Information. Flow cytometric analysis was performed using a Fortessa cell analyzer (BD Bioscience). Fluorescence-activated cell sorting (FACS) was performed using a FACSAria Fusion cell sorter (BD Bioscience). A FACSDiva software was used for data analysis.
2.5. Microarray
A total of 18 RNA samples were prepared with RNeasy Micro kit (Qiagen 74004, Germantown, Maryland) from flow sorted Wntneg/low/high cells (n = 6/group, 3-4 mice pooled). The integrity of extracted RNA was analyzed using an Agilent Tapestation 2200 and the RNA integrity numbers are all well above 8. Transcriptional microarray assay was done at the University of Colorado Denver Genomics and Microarray Core facility. Following the manufacturer's protocol, 8 ng of starting total RNA was converted to cDNA with the Affymetrix GeneChip WT Pico Kit. Processed samples were then hybridized to a Clariom D arrays in the GeneChip Hybridization Oven 645 with rotation at 60 rpm for 16 hours at 45°C The arrays were washed and stained using FS450_0002 protocol, followed by examination with an Affymetrix GeneChip Scanner 3000 7G.
Upon data acquisition for TCF:GFP samples, subsequent data analysis was performed using R studio v1.1 and R v3.4.4. Probes and oligos were annotated using BrainArray CDF.24 The principle component analysis was performed using a singular-value decomposition method implemented in stats packages. Gene Set enrichment analysis was performed using GAGE pathway analysis method25 and gene set database MsigDB.26,27 This data set is publicly available in GEO under accession no. GSE150957. Emphysema human gene expression data set GSE47460 from GPL14550 platform was obtained and reanalyzed. Emphysema group is represented by samples with computerized tomography-emphysema score of greater than 30%.28 The pathway enrichment analysis is performed using Fisher's exact test and the gene set database MsigDB. To account for multiple testing, Hochberg P value correction method was used.
2.6. Organoid culture
The organoid assay is based on established and published protocols.3,29–31 Briefly, the freshly sorted distal lung epithelial cells were cocultured with the mitomycinC (10 μg/mL, Sigma #M4287) treated Mlg ([MLg2908, CCL206], ATCC) cell in 50% of Matrigel (Corning, 354263) in 24-well plate trans-well inserts with 0.4 μm of membrane pore size (Falcon 353095). Cultures were maintained in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) media (Gibco, 11330-032) containing 5% Fetal Bovine Serum (FBS), pen/ strep (100 U/mL), 1% GlutaMax (Life Technologies, 35050-061), 1X amphotericin B (Gibco, 15290018), 1X insulin-transferrin-selenium (Gibco #51300-044), recombinant mouse Epidermal Growth Factor (EGF) (0.025 μg/mL, Sigma, SRP3196), Cholera toxin (0.1 μg/mL, Sigma C8052), bovine pituitary extract (30 μg/mL, Sigma, P1476), and freshly added all-trans retinoic acid (0.01 μM, Sigma, R2625). Y-27632 (10 μM, Tocris, 1254) was added for the first 48 hours of culture to prevent anoikis. Media was refreshed every 2 to 3 days. Details of organoid culture and immunofluorescence (IF) stainings of organoids are described in the Methods and Material section in Supporting Information.
2.7. IF staining
Fresh frozen tissue embedded in optimal cutting temperature (O.C.T.) was sectioned and fixed with ice-cold acetone/methanol (1:1), followed by IF staining. Cytospin samples were generated with freshly sorted EpCAM+ Wntneg/low/high cells and stained with the same method for tissue sections. Full methods and antibodies used are described in the Methods and Material section in Supporting Information.
2.8. Statistical analysis
Data were analyzed with GraphPad Prism 8.0. Data are presented as mean ± SEM within the text. N refers to number of independent experiments starting from an independent EpCAM+ isolation, and n refers to number of organoids. The statistical tests used are stated in the figure legends. Differences at P < .05 were considered significant.
3. Results
3.1. Wnt/β-catenin activity defines distal lung epithelial populations with distinct transcriptome
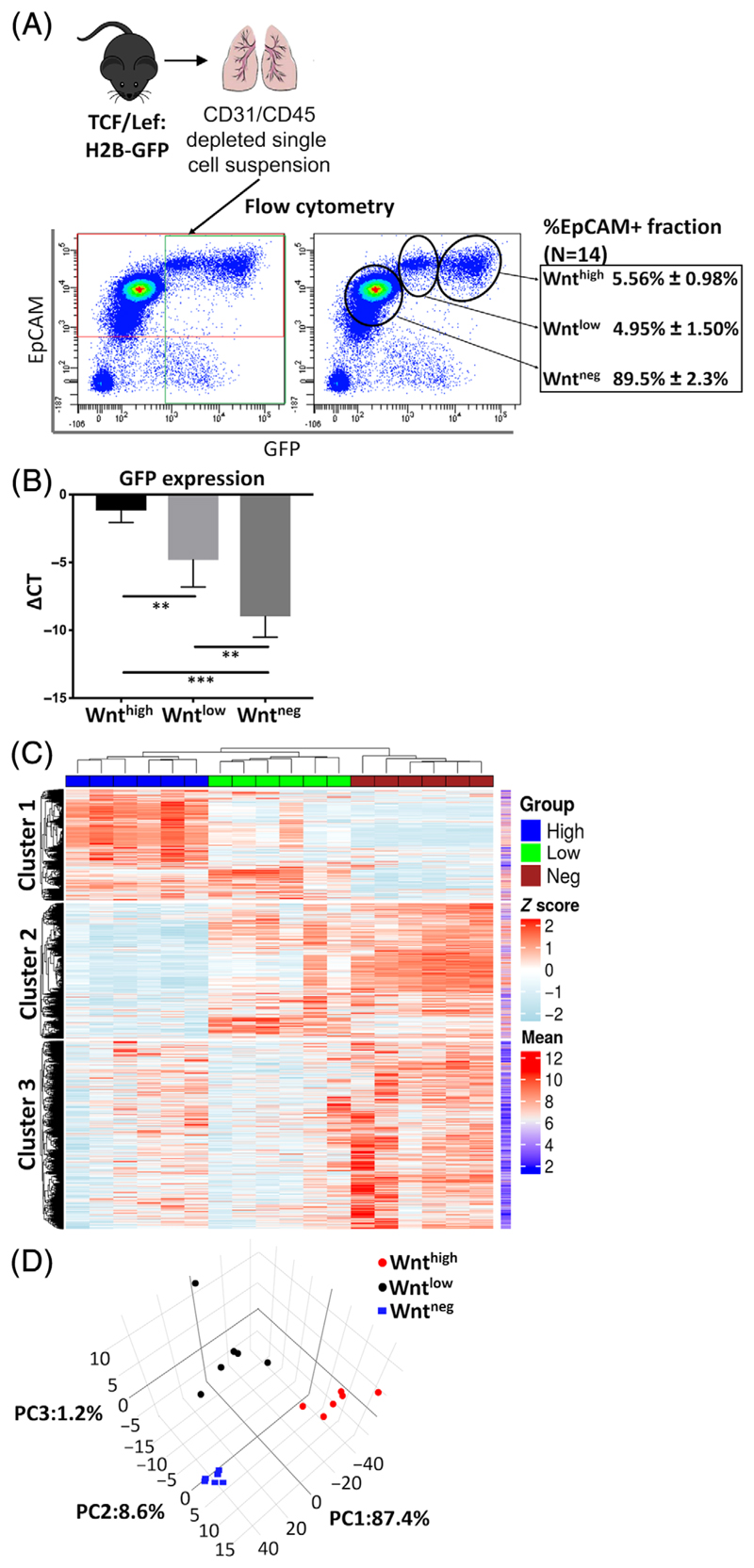
We investigated the contribution of Wnt/β-catenin signaling to adult distal lung epithelial progenitor cell function using a TCF/Lef:H2B-GFP (TCF:GFP) reporter mouse line, which harbors a histone 2B-tagged enhanced green fluorescent protein (EGFP) fusion gene driven by six copies of a TCF/Lef responsive element.32 Total EpCAM+ cells were isolated from the distal lung of 8 to 16 weeks old TCF:GFP mice by trachea removal and dispase digestion, followed by microbead (MACS)-based CD31+/CD45+ cell depletion and EpCAM+ cell selection (Figures 1A and S1A). Flow cytometry of cell suspensions from TCF:GFP mouse lungs stained for EpCAM and GFP revealed three distinct populations (EpCAM+ GFP neg, GFP low, and GFPhigh), comprising 89.5% ± 2.3%, 4.95% ± 1.5%, and 5.56% ± 0.98% of the EpCAM+ fraction, respectively (N = 14, Figure 1A). We further confirmed active β-catenin/TCF activity by quantitative reverse transcription polymer-ase chain reaction (qRT-PCR) for EGFP using sorted GFP neg/low/high
Figure 1.
The activity of Wnt/β-catenin signaling defines distal lung epithelial populations with distinct transcriptome. A, Experimental design and gating strategy for FACS sorting EpCAM+Wntneg/low/high fractions from TCF/Lef:H2B-GFP (TCF:GFP) mouse lungs. The proportion of the total EpCAM+ population represented by each fraction is shown. Mean ± SEM (N = 14). B, Expression of EGFP mRNA in the freshly sorted EpCAM+Wnthigh, Wntlow, and Wntneg cells from the TCF:GFP mouse lung. Mean ± SEM (n = 6). Statistics were performed on ΔCT values (ΔCT = CTHPRT - CTtarget). *P < .05, **P < .01, ***P < .001, Student's t test. C, Heatmap of the expression of 19 112 mouse genes showing distinct transcriptome of the sorted Wntneg/low/high cells. D, Principle component analysis using the top 500 differentially expressed genes in the Wntneg, low and high cells (N = 6). EGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting cells (Figure 1B) and thus refer to these cells as Wntneg/low/high cells from here on.
To further characterize the EpCAM+ Wntneg, Wntlow, and Wnthigh populations, we analyzed the transcriptome of freshly sorted cells by microarray. EpCAM+ Wntneg, Wntlow, and Wnthigh populations exhibited distinct gene expression patterns (19 112 genes were detected in total) (Figure 1C). Furthermore, principle component analysis of the top 500 genes by coefficient of variation demonstrated that the Wntneg population can be distinguished from the Wnt active populations by PC1 (87.4%), and that the Wntlow population possess a unique transcriptome that can be distinguished from the other two populations by PC2 (8.6%) (Figure 1D).
3.2. Alveolar and airway epithelial progenitor cells are enriched in the Wntneg and Wntlow populations
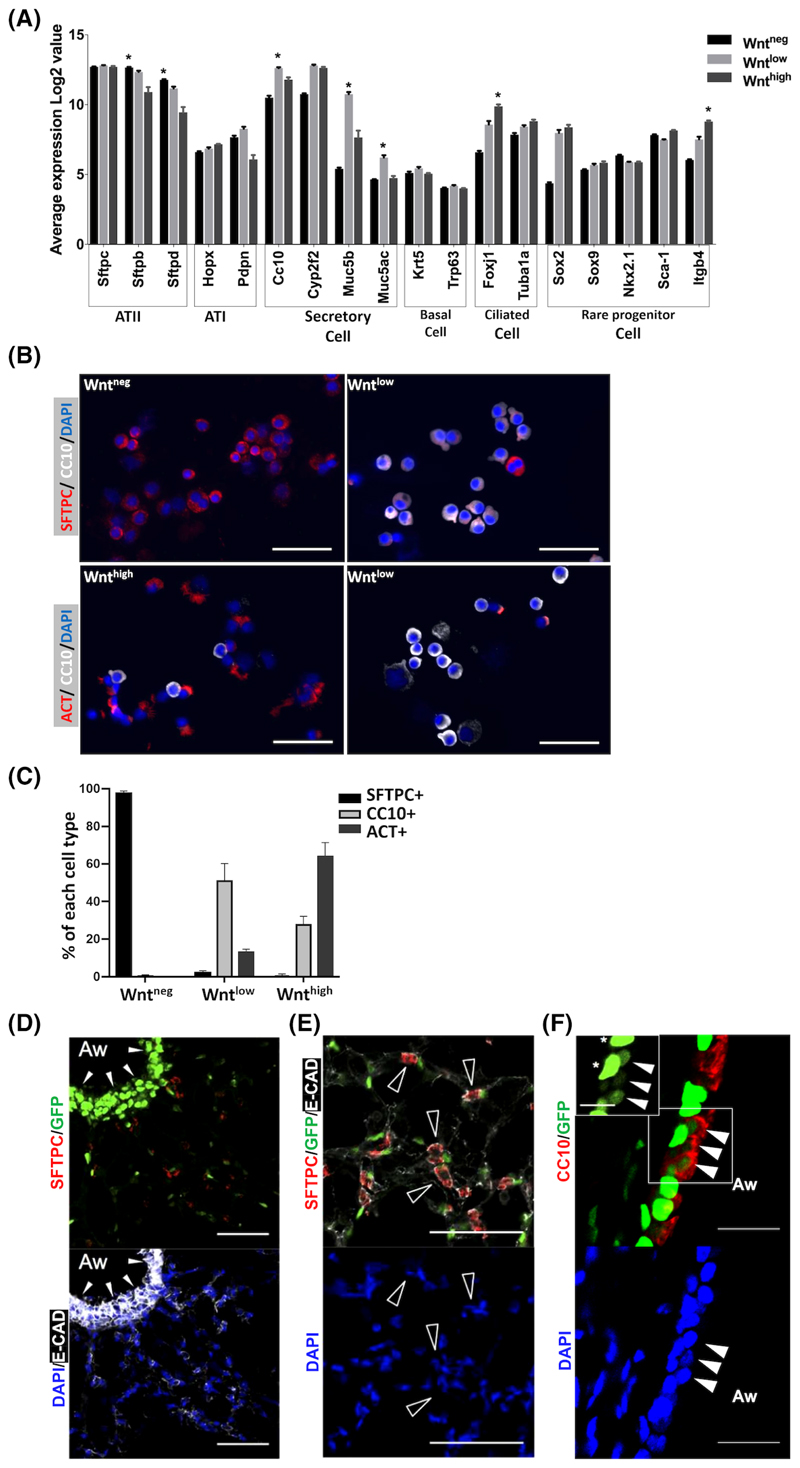
To identify the cell types labeled by the TCF:GFP reporter in the mouse distal lung, we applied the microarray data to analyze the expression of markers of known lung epithelial cells, including ATI, ATII, club cells, ciliated cells, basal cells, and rare populations of lung epithelial stem/progenitor cells reported previously.4,33 The expression of ATII cell markers (Sftpb and Sftpd) were higher in Wntneg cells compared to Wntlow and Wnthigh cells, while the mRNA of Sftpc was highly expressed in all three populations. The Wntlow population was found to be highly enriched in mRNA for the club cell marker CC10 and secretory cell markers Muc5b and Muc5ac, which are known to be expressed by club cells in mouse lungs, suggesting an enrichment of club cells in the Wntlow population (Figure 2A). Furthermore, the ciliated cell marker Foxj1 was enriched in Wnthigh cells. Itgβ4 and Sca1 label putative progenitor cells in adult mouse lung.4,33 We found that the Itgβ4 was enriched in Wnthigh cells, while no significant differences in the expression of Sca-1 were found when comparing all three populations. The mRNA expression levels of basal cell markers Krt5 and Trp63 were very low in all populations, indicating that basal cells are not a dominant cell type in the three populations of mouse distal lung. These results are further confirmed by qRT-PCR using sorted Wntneg/low/high cells (Figure S2A).
Figure 2.
Airway and alveolar epithelial progenitors are enriched in the Wntneg and Wntlow populations. A, Expression of lung epithelial cell markers in the Wntneg/low/high cells. Data presented is mean ± SEM of the expression Log2 value. N = 6. *P < .05 compared to other two groups. Student's t test. B, IF for CC10 (white), SFTPC (red, top panels), and ACT (red, bottom panels) on cytospins of FACS sorted Wntneg/low/high cells from adult TCF:GFP mouse lung. Scale bar = 50 μm. Nuclei stained with DAPI (blue). C, Quantification of CC10+, SFTPC+, and ACT+ cells in (B). Data shown is mean ± SEM. N = 4 individual animals. D,E, IF costaining of TCF:GFP lung sections for GFP (green) and lung cell markers (red). D, SFTPC (red, top) and ECAD (white, bottom). White arrowheads show GFP enriched in airway epithelium. E, An alveolar region, ECAD (white), SFTPC (red, top). Empty arrowheads show SFTPC+GFP-cells adjacent to GFP+ (white arrows), probably nonepithelial cells. F, CC10 (red, top). White arrowheads: CC10+GFPlow cells, stars: CC10-GFPhigh cells. Scale bar = 50 μm. ACT, acetylated α-tubulin; DAPI, 40,6-diamidino-2-phenylindole; ECAD, E-cadherin; FACS, fluorescence-activated cell sorting; IF, immunofluorescence
We next used a flow cytometry-based strategy to further characterize the cell types in EpCAM+Wntneg, Wntlow, and Wnthigh according to their CD24 and Sca-1 levels. CD24 and Sca-1 have been used previously to define subsets of mouse lung epithelial populations: ATII cells are CD24negSca-1neg, ciliated cells are CD24hi and Sca-1+, whereas the CD24low fraction is mostly comprised of CC10+ club cells.34 Gating of EpCAM+Wntneg, Wntlow, and Wnthigh cells to the bivariate CD24 vs Sca-1 dot plot, respectively, further confirmed that the majority of Wntneg cells were ATII cells (95% ± 0.3% CD24negSca-1neg), whereas Wnthigh cells were comprised primarily of ciliated cells (69.8% ± 2.1% CD24hi and Sca-1+). The Wntlow cells entailed a more heterogeneous cell population, including ATII cells, club cells, and ciliated cells (Figure S2A). In accordance with the microarray and flow cytometric data, IF staining and quantification of cytospins of FACS sorted EpCAM+ Wntneg/low/high cells from TCF:GFP lungs revealed that the CC10+ club cells are enriched in the Wntlow population (Figure 2B,C). The Wntneg cells are mainly SFTPC+ ATII cells, while the Wnthigh cells are mainly ciliated cells expressing acetylated α-Tubulin (ACT) (Figure 2B,C).
Next, we aimed to localize Wnt active/Wntpos lung cells in situ by IF of whole lung sections. In line with our findings above, Wntpos cells were abundant in airway epithelium (Figure 2D) and were also observed throughout alveolar tissue in situ (Figure 2D,E). However, only a few SFTPC+ ATII cells were Wntpos. Of note, Wntneg ATII cells were often situated adjacent to alveolar Wntpos cells, which may include nonepithelial Wntpos cell types observed in the flow cytometric data (Figures 1A and 2E). CC10+ cells in the airway epithelium frequently coexpressed GFP (Figure 2F). Notably, costaining with GFP indicates that these cells exhibit lower GFP expression compared to non-CC10+ cells with bright GFP expression (Figure 2F). Altogether, these data demonstrate that subpopulations of known lung epithelial progenitor cells, namely club cells and ATII cells, exhibit baseline Wnt/β-catenin activity in vivo and ex vivo. Thus, these cells might contribute to (impaired) repair in the emphysematous lung.
3.3. The organoid forming progenitors are enriched in the Wntlow epithelial population
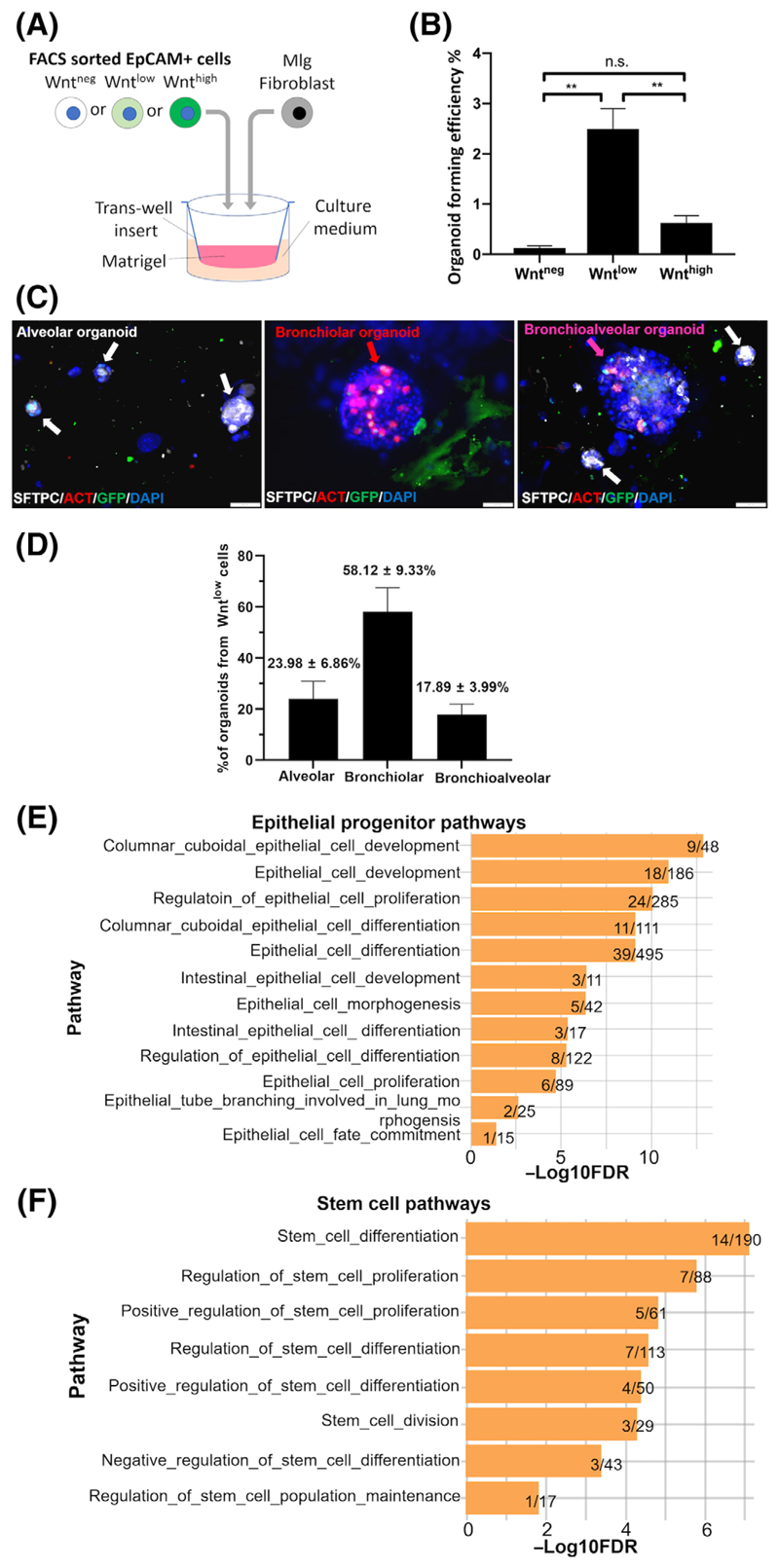
To investigate whether Wnt/β-catenin activity contributes to the capability of the lung epithelial cell progenitors to form organoids in vitro,35 EpCAM+ Wntneg/low/high cells were separately cocultured with Mlg fibroblasts in Matrigel (Figure 3A). Organoids developed over a time period of 14 days and formation of a lumen enclosed by E-Cadherin+ epithelial cells expressing apical ZO-1 was widely observed (Figure S3A), indicating apical-basal polarization. Notably, organoid-forming ability was only contained in Wntpos cells and specifically the Wntlow fraction (2.49% ± 0.74%), with a significantly lower level of organoid formation in the Wnthigh fraction (0.62% ± 0.27%). In contrast, organoids were virtually absent in the Wntneg fraction (0.13% ± 0.08%) (Figure 3B). These data support the notion that Wnt/TCF activity is required for adult distal lung epithelial progenitors to form organoids, and that the most potent organoid-forming cells are found in the Wntlow population. We next analyzed the subtypes of lung epithelial organoids that have been formed by the Wntlow cells: these are commonly separated into alveolar (SFTPC+), bronchiolar (ACT+), and bronchioalveolar (SFTPC+ACT+).31,36 Notably, the Wntlow cells gave rise to all three types of organoids: bronchiolar organoids (ACT+, 58.12% ± 9.33%), alveolar (SFTPC+, 23.98% ± 6.86%), as well as bro-nchioalveolar (SFTPC+ACT+, 17.89% ± 3.99%) organoids, as labeled by IF (Figure 3C,D). These findings further confirm enrichment of both alveolar and airway epithelial progenitor cells in the Wntlow cell population. In line with this, by performing gene ontology pathway enrichment analysis using 1024 genes that are significantly upregulated in the Wntlow cells (false discovery rate [FDR] < 0.05) compared to both Wnthigh and Wntneg cells, we found 33 pathways related to epithelial progenitor development (Figure 3E), stem cell function (Figure 3F), and cell cycle (Figure S3C) enriched in the Wntlow population. More than 10% of genes of these pathways were significantly upregulated in the Wntlow cells (FDR < 0.05, Figure S3D-F).
Figure 3.
Organoid forming progenitor cells are enriched in the Wntlow epithelial population. A, Schematic of organoid assay experimental setup. B, Organoid forming efficiency (Organoid count/ Number of seeded cells × 100, %) of sorted Wntneg/low/high fractions in the organoid assay. Data are shown as mean ± SEM (N = 20). Statistics were performed using the mean of triplicate or quadruplicate wells. **P < .01, one-way ANOVA with Bonferroni post-test. C, Representative IF images of alveolar (white arrows), bronchiolar (red arrow), and bronchioalveolar (pink arrow) organoids derived from the Wntlow cells labeled by IF for SFTPC (white), ACT (red), GFP (green), and DAPI (blue). Scale bars = 50 μm. D, Quantification of each type of organoids formed by Wntlow cells. Data are shown as mean ± SEM (N = 3). E,F, Pathways enriched in the Wntlowcells related to, E, epithelial progenitor and, F, stem cell functions. Plotted values are the -Log10FDR values. Data shown on the right end of each bar: the numbers of significantly changed genes (both Wntlow vs Wntneg and Wntlow vs Wnthigh)/total number of genes in each pathway. ACT, acetylated α-tubulin; ANOVA, analysis of variance; DAPI, 40,6-diamidino-2-phenylindole; FDR, false discovery rate; GFP, green fluorescent protein; IF, immunofluorescence
3.4. Wnt/β-catenin signaling is required for initial formation of alveolar organoids by Wntlow cells but not for their subsequent proliferation
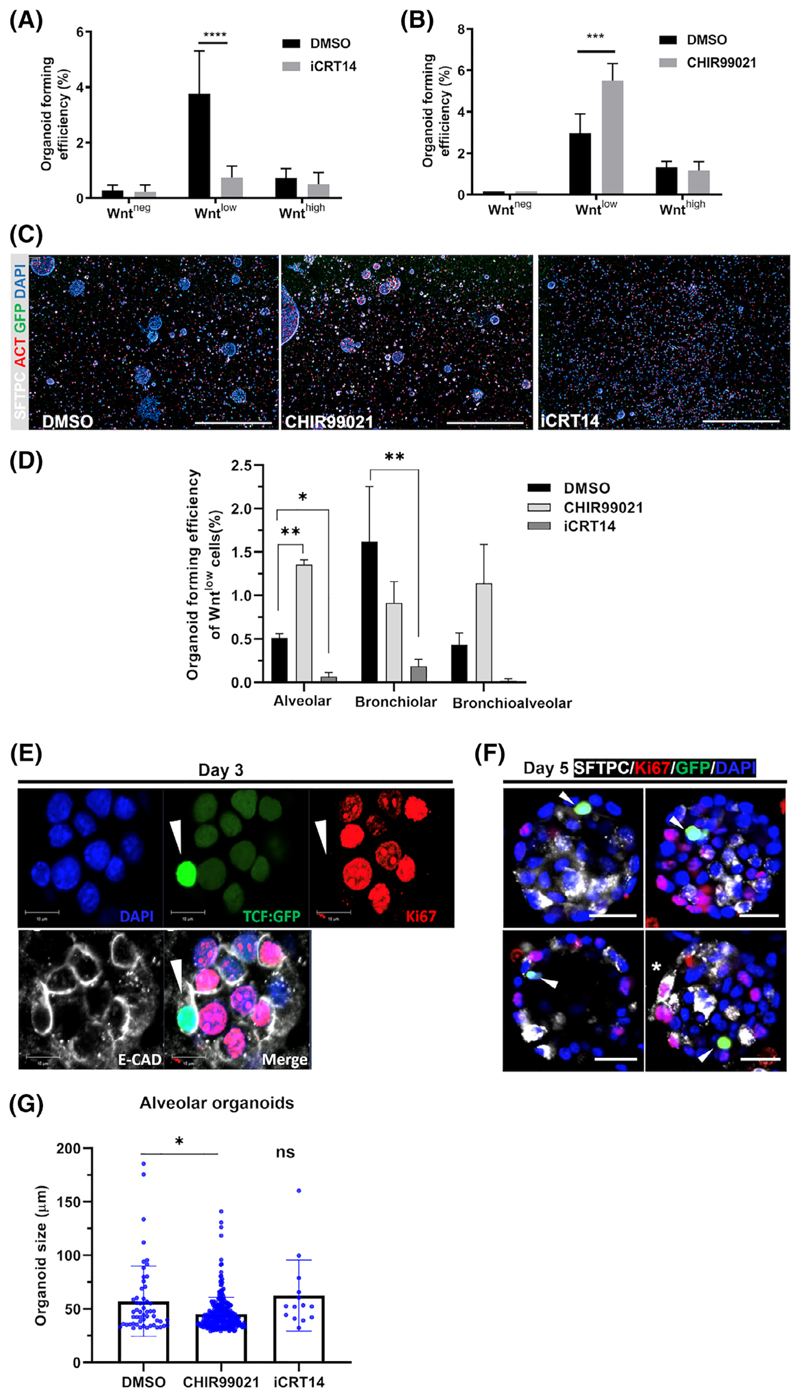
We next investigated the role of Wnt/β-catenin signal activity in progenitor function and organoid formation. Using organoids generated from sorted EpCAM+ Wntneg, Wntlow, and Wnthigh cells, we either inhibited endogenous Wnt/β-catenin signaling by adding the antagonist iCRT14, or augmented Wnt/β-catenin signaling by applying the agonist CHIR99021, to organoid cultures immediately after seeding. The Wnt/β-catenin antagonist iCRT14 significantly reduced organoid forming efficiency selectively in the Wntlow population [80% reduction compared to dimethyl sulfoxide (DMSO) control, P < .001]. In contrast, the Wnt/β-catenin agonist CHIR99201 increased organoid-forming efficiency of the Wntlow fraction (185% compared to DMSO control, P < .0001). Importantly, neither Wnt/β-catenin modulator significantly affected the organoid forming efficiency of Wntneg or Wnthigh cells (Figure 4A,B), demonstrating that mainly organoids formed by lung epithelial progenitor cells within the Wntlow population selectively responded to Wnt/β-catenin modulation.
Figure 4.
Wnt/β-catenin signaling is required for initial formation of alveolar organoids by Wntlow cells but not for their subsequent proliferation. A,B, The effects of treatments with DMSO control, A, Wnt/ β-catenin inhibitor iCRT14 (10 μM) (N = 4) and, B, GSK3 inhibitor CHIR99021 (2 μM) (N = 4) added from day 0 on the organoid forming efficiency of Wntlow cells. Data presented as mean ± SEM. ***P < .001, ****P < .0001, oneway ANOVA with Bonferroni post-test. C, Representative immunofluorescence images of day 14 organoids treated with DMSO, CHIR99021, and iCRT14, respectively. Scale bar = 1000 μm. D, Quantification of alveolar, bronchiolar, and bronchioalveolar organoids with DMSO/CHIR99021/iCRT14 treatments (N = 3). Mean ± SEM. *P < .05, **P < .01, Student's t test. E,F, Representative whole-mount immunofluorescence images showing GFP+ cells do not express proliferation marker Ki67 in organoid at, E, day 3 and alveolar organoids expressing SFTPC (white) at, F, day 5. Scale bars = 10 μm (E) and 20 μm (F). G, Effect of DMSO, iCRT14, or CHIR99021 on organoid diameter measured at day 14 (n > 120 organoids, N = 3). Mean ± SEM. *P < .05 compared to corresponding DMSO control, one-way ANOVA with Bonferroni post-test. ANOVA, analysis of variance; DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; ns, nonsignificant
We next determined whether Wnt/β-catenin modulation alters the formation of different subtypes of Wntlow cell derived organoids (Figure 4C,D). The inhibition of endogenous Wnt/β-catenin signaling led to reduced number of all organoid subtypes (Figure 4D), indicating that endogenous Wnt/β-catenin signaling is required for the formation of all types of organoids.Notably, we observed that ectopic Wnt/ β-catenin activation by CHIR99021 led to a significantly higher number of alveolar organoids, but did not change the number of bronchio-lar organoids (Figure 4D), suggesting that additional Wnt/β-catenin stimulation is critical for alveolar differentiation in organoids.
We next investigated the role of Wnt/β-catenin signaling in cell proliferation during organoid formation. IF staining for both GFP and the proliferation maker Ki67 in organoids at day 3 revealed that early in culture, cells with active Wnt/β-catenin signaling as labeled by GFP expression (Figure S4A) were not proliferating (Figure 4E). Moreover, proliferating cells did not show active Wnt/β-catenin signaling, as significantly fewer GFP+ cells were proliferating compared to GFP- cells in the organoids at day 3 (Figure S4B-D). The nonoverlapping pattern of Ki67 and GFP was maintained at day 5, the earliest stage to exhibit expression of ATII marker SFTPC in organoids (Figure 4F). These results support the notion that cell proliferation following initial onset of alveolar organoid formation does not require Wnt/β-catenin activation. In line with this, the sizes of remaining alveolar organoids were not affected by treatment with iCRT14 (Figure 4G). Interesting, CHIR99021 slightly reduced the size of alveolar organoids (Figure 4G).
3.5. Wnt/β-catenin signaling attenuates the impaired organoid forming capacity of distal lung epithelial progenitors in emphysema
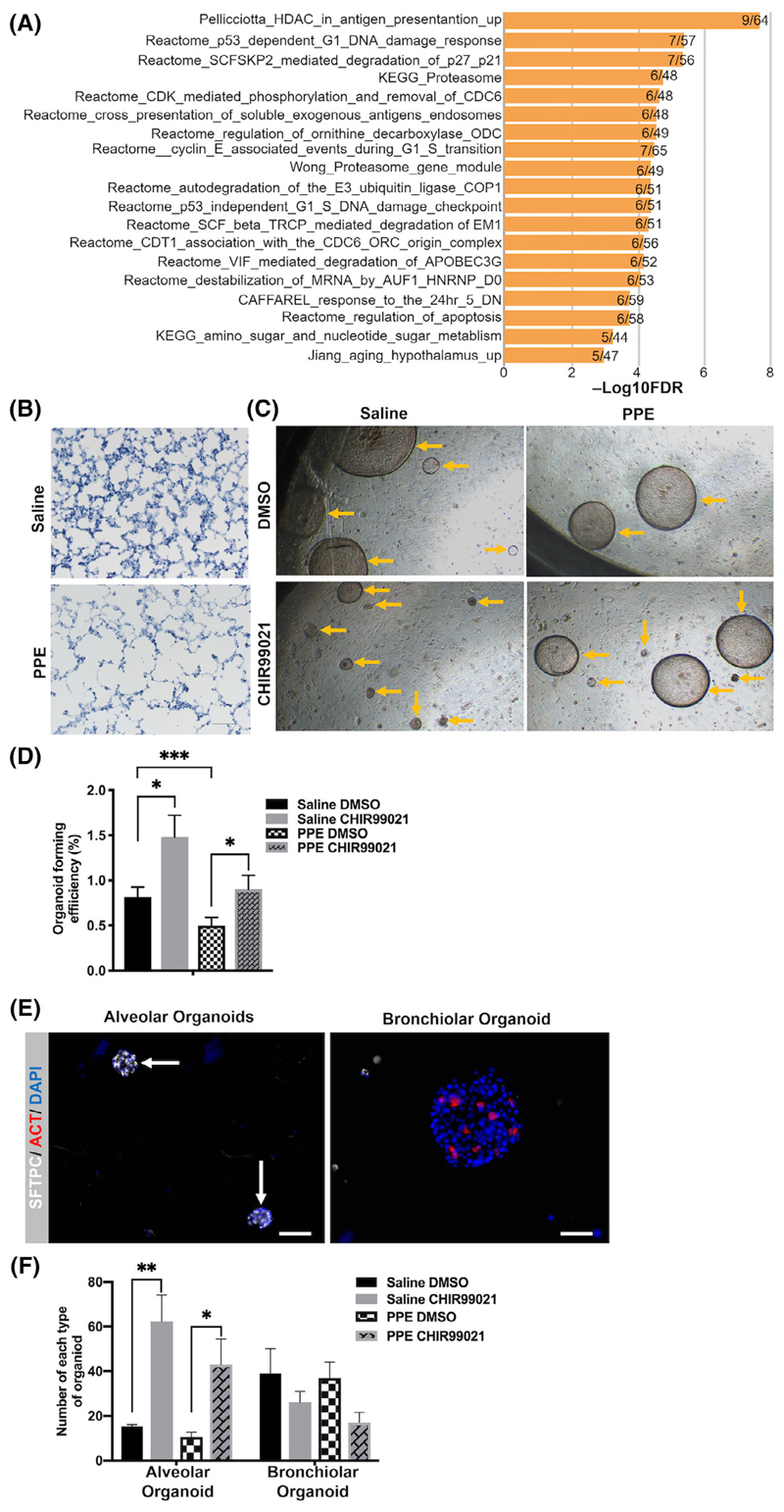
Wnt/β-catenin activity has been shown to be reduced in emphy-sema9,13-17 and our data indicate that Wnt/β-catenin activity is required for distal lung epithelial progenitors to form organoids. Thus, we aim to further investigate the function of distal lung epithelial progenitors in emphysema. We compared the gene expression profiles of Wntlow cells from the TCF:GFP mice with microarray data obtained from lung samples of emphysema patients (published microarray data set GSE4746037) and found that a significant number of transcripts enriched in normal Wntlow cells compared with Wntneg and Wnthigh (q ≤ 0.05) were downregulated in the whole lung tissue from human emphysematous lungs (Table S1). Among these genes, CLDN10, CHAD, and HP are expressed by club cells,38,39 which represent the major population in the Wntlow cells (Figure 2). Pathway analysis on these genes further identified pathways involving proteasome activity (Figure 5A), which has previously been shown to be impaired in COPD.40,41 Moreover, we identified genes encoding receptors in the Wnt/β-catenin pathway, and Wnt/β-catenin target genes which were upregulated in Wntlow cells, but downregulated in human emphysema (Figure S5A), further confirming previous findings of Wnt/β-catenin signal reduction in emphysema.9 Altogether, these results indicate a potential impairment of Wntlow progenitor cells in emphysema.
Figure 5.
Wnt/β-catenin signaling attenuates the impaired organoid forming capacity of distal lung epithelial progenitors in emphysema. A, Pathway enrichment analysis of genes enriched in the Wntlow cells but downregulated in COPD. Plotted values are -Log10FDR. Data shown on the right ends of bars are numbers of genes that are enriched in Wntlow cells but downregulated in COPD/ total number of genes in each pathway. B, H&E staining of lung tissue sections shows enlargement of airspace in the PPE treated lungs compared to those of saline treated mice after 21 days. Scale bar = 100 μm. C, Representative image of organoids at day 14 from PPE and saline treated lungs treated with DMSO and CHIR99021. Yellow arrows show organoids. Scale bar = 400 μm. D, Organoid forming efficiency of whole epithelial population from PPE and saline treated TCF:GFP mice at day 14 and effects of DMSO and CHIR99021 treatments added to the organoid culture at day 0. N = 6 individual animals per treatment. Data presented as mean ± SEM. ***P < .001, *P < .05, one-way ANOVA with Holm-Sidak post-test. E, Representative fluorescence images of small alveolar organoids (SFTPC+/ ACT-, left, arrows) and large bronchiolar organoids (ACT+/SFTCP-, right). Scale bar = 100 μm. Nuclei stained with DAPI (blue). F, Number of alveolar (SFTPC+/ACT-) and bronchiolar (SFTPC-/ACT+) organoids formed from 10 000 cells of each from PPE and saline treated TCF:GFP mice at day 14 and the effects of DMSO and CHIR99021 treatments added to the organoid culture from day 0. N = 4. Data presented as mean ± SEM. **P < .01, *P < .05, one-way ANOVA with Holm-Sidak post-test. ANOVA, analysis of variance; COPD, chronic obstructive pulmonary disease; DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; FDR, false discovery rate; PPE, porcine pancreatic elastase
To further test this hypothesis, we used TCF:GFP mice subjected to a single dose (40 U/kg) of PPE or saline through oropharyngeal injection. After 21 days, the lungs developed emphysema as assessed by lung function parameters (Figure S5B), and airspace enlargement as shown by histology (Figure 5B). We dissociated cells from the lungs of emphysematous and control TCF:GFP mice and analyzed them by flow cytometry (Figure S5E). Interestingly, while the percentage of the Wntneg/low/high cells isolated from emphysematous lungs were similar to those isolated from saline treated lungs (Figure S5F), IF analysis of the cytospins of the Wntneg/low/high cells revealed changes in the cellular composition of the subpopulations isolated from the emphysematous lung (Figure S5G).
We next compared the lung epithelial progenitor cell potential in the organoid assay. The unsorted distal lung epithelial cells from emphysematous lungs exhibited a significant reduced organoid forming efficiency with ~50% less than those from control lungs (Figure 5C,D). In cells isolated from control (non-emphasemouts) lungs, Wnt/β-catenin activation by CHIR99021 immediately after seeding increased organoid forming efficiency compared to DMSO control (Figure 5C). Critically, in cells isolated from emphysematous lungs, Wnt/β-catenin activation in culture also significantly increased organoid forming efficiency compared to DMSO control (Figure 5C,D).
Analysis of organoid size revealed that epithelial cells isolated from emphysematous lung primarily formed larger organoids (diameter of 100-150 μm) and fewer small organoids (diameter of 50-100 μm) compared to cells isolated from control lungs (Figure S5C,D). Based on size and morphology, these organoids likely represent airway and alveolar organoids, respectively.31,36 Importantly, in cells isolated from both control and emphysematous lungs, CHIR99021 treatment increased the percentage of small, alveolar-like organoids compared to DMSO treatment (Figure S5C,D). In line with this, IF for SFTPC and ACT (Figure 5E) further confirmed that the numbers of alveolar organoids (SFTPC+/ACT~) formed by the cells from both healthy and emphysematous mouse lungs mice were significantly increased by the CHIR99021 treatment (Figure 5F).
4. Discussion
Loss of functional distal lung tissue is a hallmark of numerous chronic lung diseases including emphysema; however, the regenerative capabilities of adult distal lung epithelial progenitor cells in chronic lung diseases are poorly understood. Using a well-established organoid assay,3,29–31,42 we identified a murine distal lung epithelial progenitor population that requires Wnt/β-catenin signaling to form organoids. These data expand our knowledge on previous observations, reporting reduced Wnt/β-catenin signaling in the alveolar epithelium in murine and human emphysema.9,13 17 While it has been shown that Wnt/β-catenin activation in emphysema led to reversal of airspace enlargement and improved lung function,9,18 the underlying mechanisms and cells involved have not been studied. Here, we demonstrate for the first time an impaired organoid forming capacity of the distal lung epithelial progenitor cells isolated from a murine mouse model of emphysema.
We used a well-known Wnt/β-catenin reporter (TCF:GFP) mouse to identify three distinct lung epithelial populations. Our qRT-PCR analysis of freshly sorted Wntneg/low/high cells revealed expression of the EGFP gene consistent with GFP fluorescence as determined by flow cytometry, demonstrating different levels of TCF/Lef1-dependent gene transcription, and thus confirming Wnt/β-catenin activity.
Surprisingly, the highest organoid forming efficiency was found in Wntlow cells. Further analyses revealed that this Wnt/β-catenin active population was heterogenous with an enrichment of club cells. Wntlow cells formed three types of lung organoids: alveolar, bronchiolar, and bronchoalveolar. Importantly, ectopic activation of Wnt/β-catenin signaling promoted the formation of alveolar but not bronchiolar organoids. One explanation is that Wnt/β-catenin signaling directs the differentiation of uncommitted progenitor cells toward an alveolar fate. This is in line with a previous study reporting both airway and alveolar differentiation of club cells driven by niche-derived cues in vitro, and that high Wnt levels favor the alveolar differentiation of club cells.36 Thus, a subpopulation of Wnt/β-catenin-active club cells might account for alveolar organoids in our study.
Another possibility is that ectopic Wnt/β-catenin activation selectively activates alveolar-committed progenitors cells in the organoid assay. Accordingly, we identified a small population of SFTPC+ ATII cells in the Wntlow cell population. This idea is consistent with previous reports of a rare subpopulation of ATII cells labeled by the Wnt/ β-catenin target gene Axin2 that are Wnt-responsive, form alveolar organoids in vitro and can regenerative alveoli in vivo.43,44 Notably, we observed that the majority of ATII cells belonged to the Wntneg population which did not exhibit organoid forming capacity. Consistent with this, previous studies using Axin2 reporter mice showed that the majority of ATII cells are not labeled by the reporter and do not exhibit progenitor function at homeostasis.43–45 Moreover, we found that Wntneg cells did not respond to ectopic Wnt/β-catenin activation to form organoids in our culture setting in vitro. Whether these cells could be activated to form organoids in alternative culture conditions in vitro or regenerate alveolar tissue in vivo requires further investigation.
With regards to other niche cells, it is important to consider the possibility that within the organoid assay, both epithelial cells and fibroblasts might respond to Wnt/β-catenin activation. However, in a recent study, we demonstrated that pretreatment of fibroblasts with the Wnt/β-catenin activator CHIR99021 prior to organoid coculture with lung epithelial cells did not affect subsequent organoid formation.42 Thus, the increase in alveolar organoids is likely due to direct Wnt/β-catenin activation of epithelial progenitors.
We observed that after organoid formation had initiated, GFPpos cells did not proliferate during early stages of organoid formation. Moreover, addition of Wnt/β-catenin inhibitor to the culture did not affect the size of remaining alveolar organoids. These results provide evidence that proliferation after alveolar organoid onset is Wnt/ β-catenin-independent. In a previous study using the same reporter mouse to investigate proximal lung progenitor cells in the trachea, Lynch et al reported that Wnt/β-catenin signaling was activated in proliferating submucosal gland progenitor cells and surface airway epithelial stem cells in response to naphthalene-induced injury.46 These differences may reflect diverse functions of Wnt/β-catenin signaling in different stem cell populations in the proximal vs distal lung and/or in response to different types of injuries.
Interestingly, we did not find enrichment of classical Wnt/ β-catenin target genes, such as Axin2 and Lgr5 in the Wntpos populations in our microarray (data not shown), which could be explained by distinct target genes of Wnt/β-catenin signaling dependent on different cell types and functions. Moreover, transient target gene expression in response to early Wnt activation in vivo may explain their absence as well. While active Wnt/β-catenin-driven EGFP transcript expression corresponds with GFP level as determined by flow cytometric analysis, we cannot fully exclude potential GFP label retention in some GFP+ cells due to H2B-GFP stability.47 This needs to be further considered in particular for the Wnthigh population mainly consists of ciliated cells, which are terminally differentiated and do not exhibit progenitor potential in vivo.48 As EGFP mRNA expression was enriched in the Wnthigh population, it is possible that ciliated cells exhibit high active Wnt/β-catenin signaling at baseline, although its role might be different from that in other cell populations. Another possibility is that cell types other than ciliated cells that are also present within the Wnthigh population account for the high levels of EGFP expression in this population. In general, future investigations using single cell analysis will be critical to further dissect the cellular heterogeneity of the different Wntneg/low/high cell populations and thus allow further functional studies. Moreover, additional mouse models will be required to perform lineage tracing studies of the progenitor cells to confirm their role in vitro and in vivo.
Importantly, we report for the first the time impaired organoid formation of distal lung epithelial progenitor cells isolated from a murine model of elastase-induced emphysema. Upon pharmacological activation of Wnt/β-catenin signaling, organoid forming capacity of distal lung epithelial cells was largely restored. Notably, Wnt/ β-catenin activation increased the number of alveolar organoids of cells isolated from both control and emphysematous lungs. Given the distal alveolar injury in this model, it is likely that more “alveolar-primed” instead of ‘airway-primed” progenitor cells are activated by the Wnt/β-catenin activation signaling upon emphysematous lung post injury.
Finally, our data demonstrate a potential beneficial role of Wnt/ β-catenin activation for progenitor cell function and thus further adds to a growing amount of literature highlighting Wnt/β-catenin activation as a potential regenerative approach in chronic lung dis-eases.9,18,43,44 In this context, it is important to note that the temporal and spatial regulation and level of Wnt/β-catenin signaling is critical.14,49 Along these lines, we have recently reported that chronic prolonged Wnt/β-catenin signaling can lead to an increase in cellular senescence and fibrotic activation in cultured primary ATII cells,50 representing a novel mechanism that might be involved in aberrant repair in pulmonary fibrosis.51 Thus it will be important to further study and define the cell- and environment-specific effects of Wnt/β-catenin signaling to develop fine-tuned approaches for proper tissue repair.
In summary, we identified a Wnt/β-catenin-active and responsive distal lung epithelial progenitor population. In these cells, Wnt/β-catenin signal activation increased alveolar organoid formation, but was not required for subsequent proliferation of their progeny. Importantly, the organoid forming potential of the distal lung epithelial progenitors was impaired in a mouse model of emphysema, which could be partly rescued by Wnt/ β-catenin activation. Understanding how Wnt/β-catenin signaling co-ordinates with other instructive cues to initiate lung progenitor cell activation will help elucidate how failure of lung repair contributes to lung diseases and how endogenous progenitor cells in the lung could be utilized for the development of novel treatment options for chronic lung disease.
Supplementary Material
Significance statement.
The field of regenerative lung biology lacks understanding of mechanisms regulating lung progenitor behavior in homeo-stasis and in chronic diseases. This study demonstrates a distinct, broad, Wnt-responsive cellular landscape in the lung ex vivo and in vivo. This study reveals nuances in Wnt signaling dynamics during organoid formation that have not previously been described. Importantly, this study utilizes an elastase-induced mouse emphysema model to show for the first time that distal lung epithelial progenitor cell function is impaired in a chronic lung disease. The authors strongly believe that their study will advance the field, contributing to the understanding of how lung repair and regeneration upon chronic lung injury is controlled in distinct cell populations and thus will potentially aid the rational design of cell-specific therapeutics aimed at inducing lung regeneration in lung diseases, which represent the second leading cause of death worldwide.
Acknowledgments
We are grateful to all members of the transatlantic #PinkLab in Munich and Denver for fruitful discussions. We thank Kristi Hatakka and Anastasia van den Berg for their technical support. We are grateful to Dr Amy Firth for providing suggestions on experimental design. This project is funded by National Institutes of Health Grant R01HL141380 (M. K.) and F32HL149290-01(Y. H.), the Lung Foundation Netherlands (Longfonds) Grant 6.1.14.009 (R. G., M. K., J. S., P. S. H.), Grant 5.1.17.166 (R. G., M. K.), a Pulmonary Division Junior Investigator Pilot Award by Division of Pulmonary Science and Critical Care and Genomic and Microarray Core of University of Colorado Anschutz Medical Campus, Denver, CO, USA (Y. H.).
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: F32HL149290-01, R01HL141380; The Lung Foundation Netherlands, Grant/Award Numbers: 5.1.17.166, 6.1.14.009
Footnotes
Conflict of interest
R.G. declared research grants from Boehringer Ingelheim, Aquilo, Chiesi, and Novartis. The other authors declared no potential conflicts of interest.
Author contributions
Y.H., J.-P.N.-B.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; CO., C.C: collection and assembly of data, data analysis and interpretation; W.R.: data analysis and interpretation; P.S.H., J.S., R.G, M.K.: conception and design, data analysis and interpretation; All authors contributed to manuscript writing and final approval of manuscript.
Data Availability Statement
The data that support the findings of this study are openly available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150957, reference number GSE150957.
References
- 1.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman HA, Li X, Alexander JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray S, Chiba N, Yao C, et al. Rare SOX2(+) airway progenitor cells generate KRT5(+) cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Reports. 2016;7:817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock J, Konigshoff M. Endogenous lung regeneration: potential and limitations. Am J Respir Crit Care Med. 2012;186:1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan AE, Brumwell AN, Xi Y, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo W, Zhang T, Wu DZ, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kneidinger N, Yildirim AO, Callegari J, et al. Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med. 2011;183:723–733. doi: 10.1164/rccm.200910-1560OC. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ, Burney PG, Silverman EK, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.76. 15076. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff PG, Agusti A, Roche N, et al. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet. 2015;385:1789–1798. doi: 10.1016/S0140-6736(15)60693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skronska-Wasek W, Mutze K, Baarsma HA, et al. Reduced frizzled receptor 4 expression prevents WNT/β-catenin-driven alveolar lung repair in COPD. Am J Respir Crit Care Med. 2017;196(2):172–185. doi: 10.1164/rccm.201605-0904OC. [DOI] [PubMed] [Google Scholar]
- 14.Baarsma HA, Konigshoff M. ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax. 2017;72(8):746–759. doi: 10.1136/thoraxjnl-2016-209753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabler CT, Morrisey EE. Developmental pathways in lung regeneration. Cell Tissue Res. 2017;367:677–685. doi: 10.1007/s00441-016-2537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhl FE, Vierkotten S, Wagner DE, et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J. 2015;46:1150–1166. doi: 10.1183/09031936.00183214. [DOI] [PubMed] [Google Scholar]
- 19.John-Schuster G, Hager K, Conlon TM, et al. Cigarette smoke-induced iBALT mediates macrophage activation in a B cell-dependent manner in COPD. Am J Physiol Lung Cell Mol Physiol. 2014;307:L692–L706. doi: 10.1152/ajplung.00092.2014. [DOI] [PubMed] [Google Scholar]
- 20.Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res. 2012;38:363–373. doi: 10.3109/01902148.2012.713077. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Kubo H, Ota C, et al. The increase of microRNA-21 during lung fibrosis and its contribution to epithelial-mesenchymal transition in pulmonary epithelial cells. Respir Res. 2013;14:95. doi: 10.1186/1465-9921-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treutlein B, Brownfield DG, Wu AR, et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll B, Kikuchi A, Lau AN, et al. Isolation and characterization of distal lung progenitor cells. Methods Mol Biol. 2012;879:109–122. doi: 10.1007/978-1-61779-815-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai M, Wang P, Boyd AD, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo W, Friedman MS, Shedden K, et al. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics. 2009;10:161. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberzon A, Subramanian A, Pinchback R, et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YS, Kim EY, Ahn HK, et al. Prognostic significance of CT-emphy-sema score in patients with advanced squamous cell lung cancer. J Thorac Dis. 2016;8:1966–1973. doi: 10.21037/jtd.2016.06.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQualter JL, Yuen K, Williams B, et al. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teisanu RM, Chen H, Matsumoto K, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng-Blichfeldt JP, Schrik A, Kortekaas RK, et al. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine. 2018;36:461–474. doi: 10.1016/j.ebiom.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrer-Vaquer A, Piliszek A, Tian G, et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bro-nchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Matsumoto K, Brockway BL, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell. 2016;38:590–600. doi: 10.1016/j.devcel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Tammela T, Hofree M, et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer Y, Tedrow J, de Bernard S, et al. A novel genomic signature with translational significance for human idiopathic pulmonary fibro-sis. Am J Respir Cell Mol Biol. 2015;52:217–231. doi: 10.1165/rcmb.2013-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemke AC, Snyder JC, Brockway BL, et al. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol. 2009;40:340–348. doi: 10.1165/rcmb.2007-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guha A, Vasconcelos M, Zhao R, et al. Analysis of notch signaling-dependent gene expression in developing airways reveals diversity of Clara cells. PLoS One. 2014;9:e88848. doi: 10.1371/journal.pone.0088848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meiners S, Eickelberg O. What shall we do with the damaged proteins in lung disease? Ask the proteasome! Eur Respir J. 2012;40:1260–1268. doi: 10.1183/09031936.00208511. [DOI] [PubMed] [Google Scholar]
- 41.van Rijt SH, Keller IE, John G, et al. Acute cigarette smoke exposure impairs proteasome function in the lung. Am J Physiol Lung Cell Mol Physiol. 2012;303:L814–L823. doi: 10.1152/ajplung.00128.2012. [DOI] [PubMed] [Google Scholar]
- 42.Ng-Blichfeldt JP, de Jong T, Kortekaas RK, et al. TGF-beta activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am J Physiol Lung Cell Mol Physiol. 2019;317:L14–L28. doi: 10.1152/ajplung.00400.2018. [DOI] [PubMed] [Google Scholar]
- 43.Nabhan Ahmad N, Brownfield Douglas G, Harbury Pehr B, Krasnow Mark A, Desai Tushar J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flozak AS, Lam AP, Russell S, et al. β-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch TJ, Anderson PJ, Xie W, et al. Wnt signaling regulates airway epithelial stem cells in adult murine submucosal glands. Stem Cells. 2016;34:2758–2771. doi: 10.1002/stem.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buczacki SJ, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 48.Rawlins EL, Ostrowski LE, Randell SH, et al. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA; 2007. pp. 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basil MC, Katzen J, Engler AE, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehmann M, Hu Q, Hu Y, et al. Chronic WNT/beta-catenin signaling induces cellular senescence in lung epithelial cells. Cell Signal. 2020;70 doi: 10.1016/j.cellsig.2020.109588. 109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konigshoff M, Balsara N, Pfaff EM, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150957, reference number GSE150957.