Abstract
Emotions encompass cognitive and behavioural responses to reward and punishment. Using contests as a case-study, we propose that short-term emotions underpin animals' assessments, decision-making and behaviour. Equating contest assessments to emotional ‘appraisals', we describe how contestants appraise more than resource value and outcome probability. These appraisals elicit the cognition, drive and neurophysiology that governs aggressive behaviour. We discuss how recent contest outcomes induce long-term moods, which impact subsequent contest behaviour. Finally, we distinguish between integral (objectively relevant) and incidental (objectively irrelevant) emotions and moods (affective states). Unlike existing ecological models, our approach predicts that incidental events influence contest dynamics, and that contests become incidental influences themselves, potentially causing maladaptive decision-making. As affective states cross contexts, a more holistic ethology (incorporating emotions and moods) would illuminate animal cognition and behaviour.
Keywords: affective state, assessment, cognition, resource-holding potential, resource value, winner/loser effects
1. Introduction
Consider this: animal behaviour is underpinned by emotions and moods (‘affective states'; see [1–11]). We define emotions as short-term states elicited by stimuli (or their predictors) that animals will work to acquire (rewards; e.g. prey) or avoid (punishments; e.g. predators [5,12,13]). Moods are long-term states, which represent the cumulative average of emotions over time [14,15]. These functional definitions apply to any organism with a central nervous system [1]. Animal welfare scientists, neuroscientists and psychopharmacologists now recognize that affective states play a key role in decision-making [6,7]. However, behavioural ecologists and fundamental ethologists have not yet embraced emotions and moods [16].
Two main dimensions characterize affective states: valence and arousal [6,17–19] (figure 1). Valence, which ranges from positive to negative, encapsulates the fitness benefits and costs associated with a stimulus, either anticipated or actual [7]. Meanwhile, arousal (emotional intensity) indicates stimulus importance or urgency. High-arousal affective states divert attentional resources to the stimulus [20] and predispose vigorous action [21]. As well as emotions and moods, valence and arousal define sensations (e.g. pain) and interoception (which internal stimuli elicit; e.g. hunger [10]). Burgdorf & Panksepp [22] hypothesized that positive-valence, high-arousal states represent the activation of a reward acquisition system, whereas negative-valence, high-arousal states represent the activation of a punishment avoidance system. By conceptualizing affective states in terms of reward and punishment, this dimensional approach captures their evolutionary function and avoids categorical labels that can lead to anthropomorphism (e.g. [8]).
Figure 1.
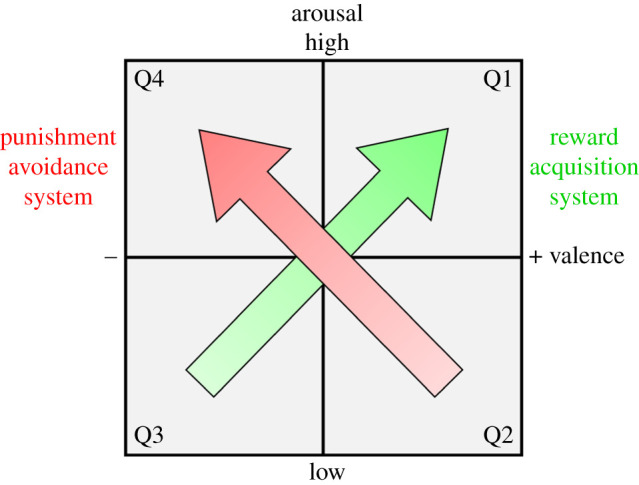
Valence and arousal define affective states (grey box), which encompasses emotions and moods [6]. Moving from Q3–Q1 is increasingly appetitive; Q2–Q4 is increasingly aversive. (Online version in colour.)
Anderson & Adolphs [1] identified two further characteristics of affective states. As well as having valence and arousal (scalability), emotions ‘generalize': various stimuli and situations can induce a particular affective state, and affective states can be associated with various behavioural responses. Affective states also ‘persist’ after stimulus removal. Thus, emotions do not mediate fixed responses to specific stimuli, because fixed responses neither generalize nor persist. Examples of non-affective behaviours therefore include withdrawal reflexes (which are genetically encoded from birth) and sexual imprinting (which is learnt during development and subsequently invariant). Emotions, on the other hand, facilitate flexible behaviour in complex, variable environments [23].
We propose that animal contests are an example of affective behaviour. Contests are direct inter-individual interactions that determine access to resources, such as food, mates or territory (i.e. rewards [24]). Resource value (RV) is the fitness benefit of the resource [25]. Contest costs include energy and time expenditure, injury and even death (i.e. punishments [26]). Greater potential benefits justify greater costs, so increasing RV increases investment [27,28]. However, contest costs and outcomes are not fixed. Resource-holding potential (RHP) is the ability to win contests, comprising traits like size, skill and weaponry [29–31]. Animals with a higher RHP are better at winning, so they are more likely to gain resources. Contests involve acquiring resources and avoiding punishments (valence), vary in intensity and escalation (arousal), are elicited by diverse stimuli and exhibited in various ways (generalization), and continue after the inciting event (persistence). These features imply an internal (i.e. affective) state mediating the link between reward, punishment and contest behaviour.
Previous researchers have not comprehensively applied affective state theory to animal contests. However, conceptualized as responses to rewards, punishments and their predictors, emotions cover contest information-gathering, decision-making and behaviour. This novel approach extends and refines contest motivation models. For example, Elwood & Arnott [32] explained contest dynamics in terms of two dimensions: RV and costs. A contestant engages if RV exceeds costs and withdraws if costs exceed RV. Whereas RV usually remains stable, costs accumulate throughout the contest. If costs increase enough to exceed RV, a contestant's strategy switches from engage to withdraw. This model approximates the valence dimension of affective states—RV representing positive valence and costs representing negative valence—except that valence is not specific to contests [6,7,14,15].
In this review, we use contests as a case study for applying emotion theory to behavioural ecology and fundamental ethology. We argue that contestants evaluate contest benefits and costs, and that these ‘appraisals' elicit emotional episodes encompassing contest decisions and behaviour. We describe how the affective outcome of contests might produce experience effects: prior winners' tendency to initiate and win (and prior losers' tendency to avoid and lose) subsequent contests. Unlike traditional ecological models, our perspective predicts that affective states previously induced in other behavioural contexts will impact contest dynamics. These objectively irrelevant influences could mediate contest decisions and cause maladaptive behaviour.
2. Structure of emotions
Emotions are elicited by appraisals: evaluations of stimuli, their context and their personal significance [33]. Scherer [34] proposed that humans sequentially appraise stimulus novelty, intrinsic valence, congruence with personal goals, outcome probability, discrepancy from expectations, situation controllability, other individuals' responsibility and whether potential responses are socially acceptable. Appraisal outcomes determine and differentiate emotions [35], with continuously updated re-appraisals regulating the response [36]. Other mammals, birds and fish also appear to appraise stimuli [23]. In lambs (Ovis aries), for example, stimulus novelty, discrepancy from expectations, controllability and social context impact physiology and behaviour [37]. These inferred appraisals elicit flexible emotional responses, which account for current conditions and personal circumstances, as well as intrinsic stimulus characteristics.
Emotions have multiple components that can be measured empirically [10,38] (figure 2). These include changes in (i) cognition (information-gathering and processing), (ii) drive (manifested as the work animals will invest to access reward or avoid punishment) and (iii) neurophysiology (central and peripheral nervous system activity, and neuroendocrine function). Such changes facilitate the performance of (iv) behaviour, producing an organism-level response to reward and punishment [5,39,40]. Threatening stimuli, for instance, impact (i) cognition (increasing attention to the threat), (ii) drive (maximizing the work animals will invest in performing freeze, fight or flight responses) and (iii) neurophysiology (activating both the sympathetic nervous system and hypothalamic–pituitary–adrenal axis). These changes prepare the individual for (iv) behaviour (avoiding, attacking or escaping the threat).
Figure 2.
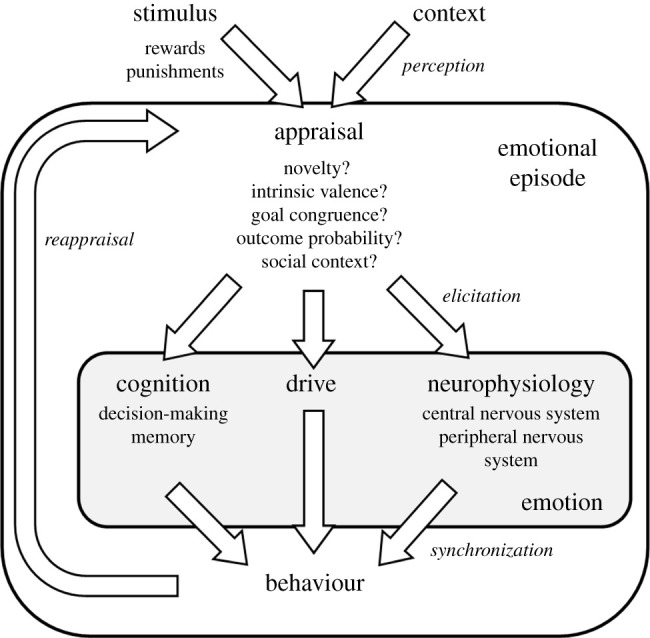
An emotional episode (white box). Appraisals of stimuli, their context and their personal significance elicit the emotion (grey box), whose components include cognition, drive and neurophysiology. These components govern the expression of behaviour. Conscious ‘feelings’ (not shown) are another potential component, but not essential.
Conscious feelings, another potential emotion component, cannot be directly measured. Humans describe feelings through language, which animals cannot do. As a result, animal researchers usually study other emotion components and remain agnostic about feelings [4,9,10]. Indeed, many human psychologists recognize unconscious emotion, where measurable components occur without corresponding feelings [41]. For example, Winkielman et al. [42] showed people positive or negative facial expressions. The images appeared too briefly for conscious awareness. When subsequently offered a novel drink, subjects shown the positive expression poured more, drank more and paid more than subjects shown the negative expression. Self-reported affective states did not differ between treatments. In animals, the relationship between feeling and non-feeling emotion components is an important area for future research [43]. However, for present purposes, we view emotions as functional states that may or may not be accompanied by feelings.
Animal welfare scientists and psychopharmacologists investigate affective states through both experiment and observation. In emotion induction experiments, rewards (e.g. enrichment [44]) induce positive emotions, and punishments (e.g. social defeat [45]) induce negative emotions [13]. Pharmacological manipulations may also induce positive- and negative-valence states [46]. Observationally, the measurable components of an emotional episode can indicate valence [4]. This includes changes in (i) cognition (attention [47], judgement [48,49] and memory biases [50]), (ii) drive (the work animals will invest to access reward or avoid punishment [51,52]), (iii) neurophysiology (brain and neuroendocrine circuits [5,8], and peripheral nervous system activity [2]) and (iv) behaviour (approach, exploration and play are often positively valenced, whereas avoidance and hiding are often negatively valenced [2]). A detailed discussion of empirical methods is beyond our scope, but we direct readers to previous reviews on measuring affective states in animals [2,4,6–8,10,11,47–49,51,52].
3. Initiating, escalating and quitting contests
Contest theorists emphasize two key assessments: animals assess RV (which determines fitness benefits and motivation) and RHP (which predicts fitness costs and outcome likelihood [25,29]). Contestants may assess only their own RHP (self-assessment [53,54]) or compare their RHP to their opponent's (mutual assessment [26,28,55]). In a meta-analysis of 36 species' assessment strategies, Pinto et al. [56] found that self-assessment is more common than mutual assessment.
Appraisal theory articulates and extends contest theory. The former predicts broader evaluations of the resource, opponent and context, all related back to the individual's own goals. Under Scherer's [34] sequential theory, contestants would first appraise novelty. Familiar resources are valued above novel resources (e.g. residency effects [57,58]), while dominance hierarchies reduce aggression towards familiar rivals [59]. Second, contestants would appraise the resource's intrinsic valence (objective RV; e.g. the calories in food). Third, contestants would appraise whether the resource contributes to their goals (subjective RV; e.g. starving animals value food most [60]). Fourth, contestants would appraise outcome probability (which covers RHP assessments). Animals avoid or de-escalate contests they will probably lose [29]. Fifth, contestants would appraise discrepancy from expectations. Compared to unconditioned controls, animals trained that a stimulus signals reward become more aggressive when the stimulus is unrewarded [61–63]. Sixth, contestants would appraise their response's compatibility with social context. Observer presence can modify animals' behaviour (audience effects [64–66]) and watching contests can modify the observers' subsequent behaviour (bystander effects [64,67]). During ongoing contests, animals also reappraise assessments, adjusting their behaviour as information and costs accumulate [31,55]. These appraisals have all been empirically documented, but several are not incorporated into current contest theory.
We further postulate that appraisals unify reward and punishment inputs into a decision-making common currency [68,69]. This facilitates cross-context comparisons between competing emotions, moods, sensations and interoception. For instance, food-deprived goldfish (Carassius auratus) endure more electric shocks to feed than well-fed goldfish [60]. Following shocks, fewer hermit crabs (Pagurus bernhardus) evacuate preferred Littorina shells than non-preferred Gibbula shells [70]. We conceptualize valence as the common currency in these reward/punishment trade-offs. Contestants likewise weigh RV against potential contest costs and outcome likelihood [32]. In self-assessment, contestants' affective states integrate RV and own RHP information. Animals persist until they reach a negative-valence threshold: the maximum cost they will pay for the resource. This threshold may be energetic [71,72] or include injury costs as well [73]. In mutual assessment, affective states integrate RV, own RHP and opponent RHP information. Animals withdraw when they establish that their opponent has a higher RHP [55], perhaps when they tip below neutral valence. Both self- and mutual assessment models require unidimensional (valence) comparisons of fitness-relevant information.
Affective states may also determine an assessment strategy. Researchers traditionally viewed assessment strategies as fixed (e.g. [29,32,74]), but now recognize individual- and population-level variation [75–77]. For example, green anoles (Anolis carolinensis) [78], mangrove killifish (Kryptolebias marmoratus) [79] and fiddler crabs (Uca mjoebergi) [80] use mutual assessment when deciding whether to escalate a contest, and self-assessment during the fight. Humans in positive affective states rely on heuristics (i.e. rules of thumb) more than humans in negative affective states [81]. When assessing the strength of an argument, for instance, people experiencing positive emotions use the author's expertise, whereas people in neutral states judge the content (i.e. deeper processing [82,83]). In animal contests, positive valence may also promote less cognitively demanding assessment strategies, such as self-assessment or rules of thumb (e.g. ‘resident wins'; see [84]). Future research could manipulate affective states to test this. We hypothesize that prior reward will lead to self-assessment, whereas prior punishment will lead to mutual assessment.
Having defined emotions as functional responses to reward and punishment, we can say that contest assessments (i.e. appraisals) elicit emotions. We propose that positive emotions about potential contests indicate that fitness benefits outweigh perceived costs, activating a reward acquisition system [6,7]. This system covers (i) cognition (information gathering and decisions to enter and escalate contests), (ii) drive (work invested to attack), (iii) neurophysiology (dopamine and opioid activity) and (iv) behaviour (threat displays and aggression). By contrast, negative emotions indicate that perceived contest costs outweigh fitness benefits, activating a punishment avoidance system. This system covers (i) cognition (information gathering and decisions to avoid and withdraw), (ii) drive (work invested to escape), (iii) neurophysiology (reduced serotonergic activity) and (iv) behaviour (submission and retreat).
From a human perspective, linking positive valence and aggressive behaviour may seem counterintuitive. Anger, for instance, feels negative [85], but causes aggression [86,87]. However, this perspective is based on our conscious experience of emotion (i.e. the feeling component). The non-feeling components indicate that anger is a reward acquisition emotion (i.e. positive valence), not a punishment avoidance emotion (i.e. negative valence [88]). Anger drives approach towards the inducing stimulus, whereas negative-valence emotions drive withdrawal [88]. As a result, our functional definition of emotion—which does not require conscious feeling—categorizes anger as positively valenced. Negative-valence emotions can lead to aggressive behaviour, but only when withdrawal is not an option (e.g. cornered animals lashing out). In the present manuscript, we only consider positive-valence aggression, where the aim is resource acquisition.
This review focuses on contest initiation, winning and losing, but affective states might also govern behavioural transitions within contests, such as levels of display or escalated aggression (e.g. [78–80]). From an emotion standpoint, the transitions at either end of contests are more empirically tractable. Applying an emotional event pre-contest indicates how emotions influence initiation, for example, whereas applying an emotional event between contests indicates how emotions disrupt experience effects. Tracking emotions during contests is more challenging, as contests are ongoing emotional events. To resolve this issue, we propose startling contestants at set points during a contest [29,89]. Motivation theorists interpret faster contest resumption (i.e. shorter startle latencies) as stronger motivation to fight [33]. However, affective state influences the startle reflex [47]. In humans [90], rhesus macaques (Macaca mulatta) [91] and rats (Rattus norvegicus) [90], negative-valence states increase startle duration and magnitude. Future researchers could use startle duration to understand how valence relates to within-contest behavioural transitions.
To summarize, emotion theory correctly predicts that contest assessments cover more than RV and RHP. Animals assess the resource, opponent and context in relation to individual circumstances. We hope researchers investigate whether additional human appraisals influence contest dynamics in other species. For example, perhaps agency appraisals (who was responsible? what did they intend?) influence contest decision-making. Under our definition of emotion, these appraisals elicit emotional responses that reflect personalcircumstances and prevailing conditions. Conceptualizing cognition, drive and neurophysiology as a unified affective state underpinning behaviour explains existing results and generates new hypotheses.
4. Contest outcome and experience effects
Contest outcomes indicate how an individual's RHP compares with the population's RHP [92,93]. Assuming self-assessment, wins signal relatively high personal RHP and losses signal relatively low personal RHP. Winners, therefore, initiate, escalate and win more subsequent contests (winner effects), whereas losers avoid and lose more subsequent contests (loser effects [94–96]). We conceptualize contests as emotional events, so winning induces positive-valence emotions that increase aggressive behaviour and losing induces negative-valence emotions that reduce aggressive behaviour (even if actual RHP does not change). By reflecting cumulative emotional outcomes, winner and loser effects represent long-term moods (figure 3).
Figure 3.
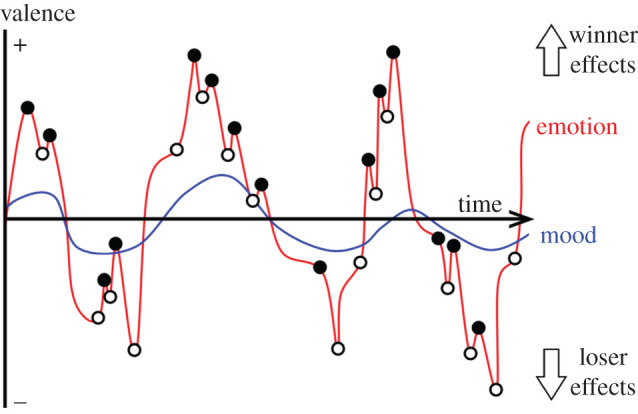
Cumulative emotional valence determines mood [11] (manifested in aggression). Considering only integral (objectively contest-relevant) influences, white dots are wins and black dots are losses. Considering both integral and incidental (objectively contest-irrelevant) influences, white dots are rewards and black dots are punishments. (Online version in colour.)
Both emotions and moods cause cognitive changes, such as judgement and decision-making biases [97]. People in positive affective states interpret ambiguous stimuli more optimistically than people in negative affective states [81], whereas pessimistic judgements characterize depression and anxiety [81,98–100]. Animals also exhibit judgement biases. Under ambiguity, mammals, birds, fish and insects in positive affective states have higher expectations of reward and lower expectations of punishment than animals in negative affective states [7,46,48,49,101]. Assuming reward and punishment experience predicts likely outcomes in the present, moods indicate whether ambiguous stimuli signal positive or negative outcomes, leading to judgement biases [14,49]. We therefore suggest that mood-induced judgement bias underlies contest experience effects. Winners gain fitness-enhancing resources, so winning is positively valenced. Thus, previous winners should be relatively optimistic about unknown rewards (RV) and outcome likelihood (RHP), and correspondingly more aggressive. Losing, meanwhile, is negatively valenced, so losers should be more pessimistic and less aggressive. Indeed, perceived RHP, rather than actual RHP, influences winner and loser effects [94,95] (cf. [102]).
Empirical evidence suggests that contests induce judgement biases. Researchers have trained both dominant and subordinate animals to associate one stimulus with a high-value reward (leading to shorter response latencies) and another stimulus with a low-value reward or no reward (leading to longer response latencies). When subsequently presented with untrained intermediate stimuli, dominant animals respond faster and more frequently than subordinates (rats [103]; pigs, Sus scrofa [104]; tufted capuchins, Sapajus apella [105]). We interpret the dominant animals' higher reward anticipation as optimism, which may reflect wins inducing positive valence. In similar tasks, rats [45] and Murray cod (Maccullochella peelii) [106] that repeatedly lose contests exhibit lower reward anticipation towards ambiguous stimuli, which we interpret as pessimism. Equivalent opponent-directed behaviour—the reduced likelihood of attacking an ambiguous rival—would constitute a loser effect. As judgement biases influence responses to ambiguity more than responses to predictable outcomes [7,49], we hypothesize that judgement biases impact behaviour in contests with unpredictable outcomes (where opponents have similar RHP) more than contests with predictable outcomes (where opponents’ RHP differs markedly).
Experience effects also suggest that contests can be intrinsically rewarding [107]. In addition to yielding external reward, aggressive behaviour itself (and particularly winning) seems to induce positive affective states, which may inform future decisions. For example, mice (Mus musculus) learn instrumental responses to access and attack submissive opponents [108]. Responses decline for non-submissive opponents, revealing that outcome matters. Moreover, winning induces conditioned place preference in mice [109], Syrian hamsters (Mesocricetus auratus) [110] and green anoles [111]. From an affective state perspective, positive emotions reward this conditioning. Affective reinforcement might also occur within a single contest. For instance, accurate strikes [30] or appropriate assessments [112] may be rewarding.
To recap, we suggest that moods, which reflect contest outcome experience, mediate expectations about unknown RV and future outcomes. Mood-induced judgement bias and affective reinforcement may underpin these experience effects. To investigate judgement bias, contest researchers could measure optimism pre- and post-contest (see [48,49]). We predict that wins induce optimism and losses induce pessimism, with state optimism producing winner effects and state pessimism producing loser effects. Exploring the role of neurotransmitters linked to reward, such as opioids, could reveal whether contests are intrinsically rewarding.
5. Crossing behavioural contexts
So far, we have considered adaptive affective states. There are clear fitness benefits to cumulative experience informing reliable assessments, but existing optimality models already predict these effects. How do emotions and moods advance our understanding?
Integral affective states are objectively relevant to a cognitive process. In humans, for example, sunshine (stimulus) induces positive valence (emotion) that causes a decision (cognition) to go outside (behaviour). Incidental affective states, on the other hand, influence objectively unrelated cognitive processes [38,81,113–115]. For example, people rate their overall life satisfaction higher on sunny days than rainy days [116]. Sunshine (stimulus) induces positive valence (emotion) that causes an objectively unrelated assessment (cognition) to be reported positively (behaviour). Incidental affective states, thus, distinguish optimal and affective decision-making. Optimality models only use integral information, whereas affective states incorporate incidental influences as well.
Although understudied in behavioural ecology, incidental affective states influence animal cognition and behaviour. Starlings (Sturnus vulgaris) with enriched housing judge unrelated temporal stimuli more optimistically [44], while honeybees (Apis mellifera) shaken aversively judge unrelated olfactory stimuli more pessimistically [117]. Moreover, isolating rats improves recall of unrelated light and sound stimuli [50]. It follows that incidental information may influence contest behaviour, and that rewards and punishments in general—not wins and losses specifically—induce ‘winner' and ‘loser' effects (figure 3). For instance, positive valence female interactions increase aggressive behaviour in male speckled wood butterflies (Pararge aegeria) [118] and wolf spiders (Venonia coruscans) [119], whereas negative-valence predator exposure decreases aggressive behaviour in daffodil cichlids (Neolamprologus pulcher) [120]. However, a note of caution: apparently incidental influences may be functionally integral. The presence of a potential mate, for example, increases contest benefits, and predation risk increases contest costs [118–120]. We must understand a species's ecology to determine whether cross-context variables are objectively relevant, and hence whether they are integral or incidental. We welcome new research to fill this knowledge gap. Contest researchers could borrow affective state research methods from animal welfare science and psychopharmacology. Exposing fish to antidepressants and anxiolytics in wastewater has produced equivocal results: venlafaxine increases aggression [121], but fluoxetine reduces aggression [122]. To test whether incidental affective states influence contest behaviour, we need controlled interventions in more species.
Incidental affective states not only influence contests; contests might also induce incidental affective states and influence objectively unrelated cognitive processes (see [123]). For example, rats that repeatedly lose contests develop anhedonia (reduced reward sensitivity, expressed in non-contest situations and linked to depression in humans). Giving the rats unrelated but signalled food rewards reverses this effect [124]. Compared to tufted capuchins with subordinate bystanders, capuchins exposed to aggressive bystanders allocate more attention towards humans [125]. Dominant capuchins [105] and pigs [104] expect more positive outcomes from ambiguous spatial stimuli (i.e. optimism), while subordinate cod expect fewer positive outcomes from ambiguous spatial stimuli [106] (i.e. pessimism). Contest-induced incidental affective states may influence virtually any decision. Is brightly coloured prey toxic or a mimic? Are rustling leaves a predator or the wind? When moods bias decisions, the most encountered emotional stimuli with the longest duration and most polar valence might determine behaviour, regardless of objective relevance. It is possible that frequently winning contests, for example, may induce optimism that rare prey is edible, even if the prey is usually toxic. This example illustrates how decision-making using incidental information can negatively impact fitness. Incidental affective states may cause maladaptive behaviour [38].
Given their maladaptive potential, we suggest two reasons for incidental affective states. First, to be selected, cross-context affective states must increase fitness on average—not necessarily every time. Nettle & Bateson [14] noted that the recent environment and physical condition persist across behavioural contexts. Lame animals, for instance, cannot fight, forage or flee from predators, so information from each of these contexts is integral to the others. Cross-context affective states will be selected if most are integral, even if some are incidental. In humans, various measures increase the likelihood that cross-context affective states only influence relevant cognition [114,115]. For example, people associate their affective states with concurrent cognitive processes [126]. Incidental emotional influences are also less common than incidental moods, because emotions usually have an obvious cause [114]. Animal research may reveal similar mechanisms to limit incidental affect.
The second possible explanation is that incidental affective states dominate when animals lack reliable information, or when acquisition and storage costs outweigh the benefits [59]. This is why humans evaluating ambiguous stimuli (e.g. brand names without product details) rely on incidental affective states [127]. In animal contests, a fight indicates rival RHP most accurately, but entails substantial investment and potential injury [64,67]. Assessments in other contexts carry their own cost/accuracy trade-offs. Bystander effects avoid fight costs and reflect individual RHP, but they require individual discrimination and recall [32]. Winner and loser effects are less cognitively demanding, but based on previous opponents' RHP. This measure will predict future opponents' RHP less accurately than individual assessments. We hypothesize that mood does not even distinguish between behavioural contexts, further reducing both cognitive requirements and accuracy. Incidental affective states may therefore influence decisions when contestants have less reliable information or high information-gathering costs (e.g. intruders). From this perspective, incidental affective states are the ‘best of a bad job'.
In summary, integral affective states are objectively relevant and adaptive, whereas incidental affective states are objectively irrelevant and potentially maladaptive. Incidental influences may nonetheless seep in when integral information is unavailable or costly. Despite preliminary evidence, we do not yet know the extent of incidental affective states in animal decision-making. We hope that future researchers test whether objectively unrelated stimuli impact contest dynamics. Without integral influences, we predict that generic rewards increase aggression and generic punishments decrease aggression.
6. Conclusion
An affective-states approach generates novel predictions and opens new avenues for behavioural ecology (table 1). Both emotions and contest behaviour rely on assessments of stimuli and their personal significance; both enlist cognition, drive and neurophysiology; and both reflect reward and punishment experience. We equate contest assessments to emotional appraisals, which determine contest decision-making and behaviour. We explain experience effects as wins inducing positive moods and losses inducing negative moods. This hypothesis, and our conception of contests as emotional episodes, predicts that manipulating affective state will modify contest behaviour. As well as integral influences, incidental affective states may impact contests, and contest-induced affective states may impact objectively unrelated behaviours. We hypothesize that high-frequency, long-lasting, polar-valence events disproportionately influence animal decision-making and behaviour, even if incidental. Moreover, despite our focus on contests, emotion theory may underpin all non-reflexive behaviour—from signalling to mate choice to parental care. Behavioural ecologists and fundamental ethologists study these fields separately, but affective states transcend boundaries. We need a more holistic ethology to understand affective cognition and behaviour.
Table 1.
Major predictions and outstanding questions that arise from applying emotion theory to animal contests.
| major predictions | outstanding questions |
|---|---|
| Contest appraisals cover more variables than traditionally recognized (i.e. RV and RHP) | Are contest appraisals sequential? Do untested human appraisals (e.g. perceived agency) modify contest dynamics in animals? |
| Positive affective states induce self-assessment; negative states induce mutual assessment | Do assessment strategies vary with affective state? How might this influence the outcome? |
| Winner effects are associated with optimistic responses to judgement bias tasks; loser effects are associated with pessimistic responses | What neurocognitive mechanisms underpin judgement bias? Are they equivalent to the mechanisms underpinning winner/loser effects? |
| Incidental affective influences modify contest behaviour | Do incidental affective states commonly impact contests in nature? Why evolve a generalized (rather than domain-specific) affective system? |
| Humans and animals share rules that increase the likelihood of incidental influences (e.g. concurrence, ambiguity, and link to moods) | What mechanisms minimize incidental influences? How do these affect fitness? |
| The above predictions apply only to animals with a central nervous system | Do all animals with a central nervous system have affective states? Are contest dynamics fundamentally different in organisms without a central nervous system? |
Supplementary Material
Acknowledgements
We thank Robert Elwood, an anonymous reviewer and Innes Cuthill for their useful comments and insights on this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
A.C. and G.A. conceived the review; A.C. drafted the manuscript; all authors revised and commented on the manuscript and accept responsibility for its contents.
Competing interests
We declare we have no competing interests.
Funding
A.C. received a postgraduate studentship from Northern Ireland's Department for the Economy. This paper is part of a project that has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme, grant no. 851145.
References
- 1.Anderson DJ, Adolphs R. 2014. A framework for studying emotions across species. Cell 157, 187–200. ( 10.1016/j.cell.2014.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissy A, et al. 2007. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397. ( 10.1016/j.physbeh.2007.02.003) [DOI] [PubMed] [Google Scholar]
- 3.Gygax L. 2017. Wanting, liking and welfare: the role of affective states in proximate control of behaviour in vertebrates. Ethology 123, 689–704. ( 10.1111/eth.12655) [DOI] [Google Scholar]
- 4.Kremer L, Klein Holkenborg SEJ, Reimert I, Bolhuis JE, Webb LE. 2020. The nuts and bolts of animal emotion. Neurosci. Biobehav. Rev. 113, 273–286. ( 10.1016/j.neubiorev.2020.01.028) [DOI] [PubMed] [Google Scholar]
- 5.LeDoux J. 2012. Rethinking the emotional brain. Neuron 73, 653–676. ( 10.1016/j.neuron.2012.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendl M, Burman OHP, Paul ES. 2010. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B 277, 2895–2904. ( 10.1098/rspb.2010.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendl M, Paul ES. 2020. Animal affect and decision-making. Neurosci. Biobehav. Rev. 112, 144–163. ( 10.1016/j.neubiorev.2020.01.025) [DOI] [PubMed] [Google Scholar]
- 8.Panksepp J. 2011. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci. Biobehav. Rev. 35, 1791–1804. ( 10.1016/j.neubiorev.2011.08.003) [DOI] [PubMed] [Google Scholar]
- 9.Paul ES, Mendl MT. 2018. Animal emotion: descriptive and prescriptive definitions and their implications for a comparative perspective. Appl. Anim. Behav. Sci. 205, 202–209. ( 10.1016/j.applanim.2018.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul ES, Sher S, Tamietto M, Winkielman P, Mendl MT. 2020. Towards a comparative science of emotion: affect and consciousness in humans and animals. Neurosci. Biobehav. Rev. 108, 749–770. ( 10.1016/j.neubiorev.2019.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb LE, Veenhoven R, Harfeld JL, Jensen MB. 2018. What is animal happiness? Ann. N Y Acad. Sci. 1438, 1–15. ( 10.1111/nyas.13983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carver CS. 2001. Affect and the functional bases of behavior: on the dimensional structure of affective experience. Pers. Soc. Psychol. Rev. 5, 345–356. ( 10.1207/S15327957PSPR0504_4) [DOI] [Google Scholar]
- 13.Rolls ET. 2005. Emotion explained. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Nettle D, Bateson M. 2012. The evolutionary origins of mood and its disorders. Curr. Biol. 22, 712–721. ( 10.1016/j.cub.2012.06.020) [DOI] [PubMed] [Google Scholar]
- 15.Trimmer PC, Paul ES, Mendl MT, McNamara JM, Houston AI. 2013. On the evolution and optimality of mood states. Behav. Sci. 3, 501–521. ( 10.3390/bs3030501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser D. 2009. Animal behaviour, animal welfare and the scientific study of affect. Appl. Anim. Behav. Sci. 118, 108–117. ( 10.1016/j.applanim.2009.02.020) [DOI] [Google Scholar]
- 17.Posner J, Russell JA, Peterson BS. 2005. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol 17, 715–734. ( 10.1017/S0954579405050340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JA. 1980. A circumplex model of affect. J. Pers. Soc. Psychol. 39, 1161–1178. ( 10.1037/h0077714) [DOI] [Google Scholar]
- 19.Russell JA. 2003. Core affect and the psychological construction of emotion. Psychol. Rev. 110, 145–172. ( 10.1037/0033-295X.110.1.145) [DOI] [PubMed] [Google Scholar]
- 20.Storbeck J, Clore GL. 2008. Affective arousal as information: how affective arousal influences judgments, learning, and memory. Soc. Pers. Psychol. Compass 2, 1824–1843. ( 10.1111/j.1751-9004.2008.00138.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach DR, Dayan P. 2017. Algorithms for survival: a comparative perspective on emotions. Nat. Rev. Neurosci. 18, 311–319. ( 10.1038/nrn.2017.35) [DOI] [PubMed] [Google Scholar]
- 22.Burgdorf J, Panksepp J. 2006. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 30, 173–187. ( 10.1016/j.neubiorev.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 23.Faustino AI, Oliveira GA, Oliveira RF. 2015. Linking appraisal to behavioral flexibility in animals: implications for stress research. Front. Behav. Neurosci. 9, 104 ( 10.3389/fnbeh.2015.00104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briffa M, Hardy ICW. 2013. Introduction to animal contests. In Animal contests (eds Hardy ICW, Briffa M), pp. 1–4. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.Arnott G, Elwood RW. 2008. Information gathering and decision making about resource value in animal contests. Anim. Behav. 76, 529–542. ( 10.1016/j.anbehav.2008.04.019) [DOI] [Google Scholar]
- 26.Enquist M, Leimar O. 1990. The evolution of fatal fighting. Anim. Behav. 39, 1–9. ( 10.1016/S0003-3472(05)80721-3) [DOI] [Google Scholar]
- 27.Enquist M, Leimar O. 1987. Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 127, 187–205. ( 10.1016/S0022-5193(87)80130-3) [DOI] [Google Scholar]
- 28.Hammerstein P, Parker GA. 1982. The asymmetric war of attrition. J. Theor. Biol. 96, 647–682. ( 10.1016/0022-5193(82)90235-1) [DOI] [Google Scholar]
- 29.Arnott G, Elwood RW. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004. ( 10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 30.Briffa M, Lane SM. 2017. The role of skill in animal contests: a neglected component of fighting ability. Proc. R. Soc. B 284, 20171596 ( 10.1098/rspb.2017.1596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 32.Elwood RW, Arnott G. 2012. Understanding how animals fight with Lloyd Morgan's canon. Anim. Behav. 84, 1095–1102. ( 10.1016/j.anbehav.2012.08.035) [DOI] [Google Scholar]
- 33.Moors A, Ellsworth PC, Scherer KR, Frijda NH. 2013. Appraisal theories of emotion: state of the art and future development. Emot. Rev. 5, 119–124. ( 10.1177/1754073912468165) [DOI] [Google Scholar]
- 34.Scherer KR. 2001. Appraisal considered as a process of multilevel sequential checking. In Appraisal processes in emotion: theory, methods, research (eds Scherer KR, Schorr A, Johnstone T), pp. 92–120. New York, NY: Oxford University Press. [Google Scholar]
- 35.Moors A. 2013. On the causal role of appraisal in emotion. Emot. Rev. 5, 132–140. ( 10.1177/1754073912463601) [DOI] [Google Scholar]
- 36.Uusberg A, Taxer JL, Yih J, Uusberg H, Gross JJ. 2019. Reappraising reappraisal. Emot. Rev. 11, 267–282. ( 10.1177/1754073919862617) [DOI] [Google Scholar]
- 37.Veissier I, Boissy A, Désiré L, Greiveldinger L. 2009. Animals' emotions: studies in sheep using appraisal theories. Anim. Welfare 18, 347–354. [Google Scholar]
- 38.Lerner JS, Li Y, Valdesolo P, Kassam KS. 2015. Emotion and decision making. Annu. Rev. Psychol. 66, 799–823. ( 10.1146/annurev-psych-010213-115043) [DOI] [PubMed] [Google Scholar]
- 39.Damasio A, Carvalho GB. 2013. The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 14, 143–152. ( 10.1038/nrn3403) [DOI] [PubMed] [Google Scholar]
- 40.Nesse RM, Ellsworth PC. 2009. Evolution, emotions, and emotional disorders. Am. Psychol. 64, 129–139. ( 10.1037/a0013503) [DOI] [PubMed] [Google Scholar]
- 41.Winkielman P, Berridge KC. 2004. Unconscious emotion. Curr. Dir. Psychol. Sci. 13, 120–123. ( 10.1111/j.0963-7214.2004.00288.x) [DOI] [Google Scholar]
- 42.Winkielman P, Berridge KC, Wilbarger JL. 2005. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers. Soc. Psychol. Bull. 31, 121–135. ( 10.1177/0146167204271309) [DOI] [PubMed] [Google Scholar]
- 43.Birch J, Schnell AK, Clayton NS. 2020. Dimensions of animal consciousness. Trends Cogn. Sci. 24, 789–801. ( 10.1016/j.tics.2020.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matheson SM, Asher L, Bateson M. 2008. Larger, enriched cages are associated with ‘optimistic’ response biases in captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 109, 374–383. ( 10.1016/j.applanim.2007.03.007) [DOI] [Google Scholar]
- 45.Papciak J, Popik P, Fuchs E, Rygula R. 2013. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 256, 305–310. ( 10.1016/j.bbr.2013.08.036) [DOI] [PubMed] [Google Scholar]
- 46.Neville V, Nakagawa S, Zidar J, Paul ES, Lagisz M, Bateson M, Løvlie H, Mendl M. 2020. Pharmacological manipulations of judgement bias: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 108, 269–286. ( 10.1016/j.neubiorev.2019.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crump A, Arnott G, Bethell EJ. 2018. Affect-driven attention biases as animal welfare indicators: Review and methods. Animals 8, 136 ( 10.3390/ani8080136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bethell EJ. 2015. A ‘how-to’ guide for designing judgment bias studies to assess captive animal welfare. J. Appl. Anim. Welf. Sci. 18, S18–S42. ( 10.1080/10888705.2015.1075833) [DOI] [PubMed] [Google Scholar]
- 49.Mendl M, Burman OHP, Parker RMA, Paul ES. 2009. Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Appl. Anim.Behaviour Science 118, 161–181. ( 10.1016/j.applanim.2009.02.023) [DOI] [Google Scholar]
- 50.Takatsu-Coleman AL, et al. 2013. Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: mood-congruent memory in male mice? J. Psychiatry Neurosci. 38, 259 ( 10.1503/jpn.120050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen MB, Pedersen LJ. 2008. Using motivation tests to assess ethological needs and preferences. Appl. Anim. Behav. Scie. 113, 340–356. ( 10.1016/j.applanim.2008.02.001) [DOI] [Google Scholar]
- 52.Kirkden RD, Pajor EA. 2006. Using preference, motivation and aversion tests to ask scientific questions about animals' feelings. Appl. Anim. Behav. Sci. 100, 29–47. ( 10.1016/j.applanim.2006.04.009) [DOI] [Google Scholar]
- 53.Maynard SJ. 1974. The theory of games and the evolution of animal conflicts. J. Theor. Biol. 47, 209–221. ( 10.1016/0022-5193(74)90110-6) [DOI] [PubMed] [Google Scholar]
- 54.Mesterton-Gibbons M, Marden JH, Dugatkin LA. 1996. On wars of attrition without assessment. J. Theor. Biol. 181, 65–83. ( 10.1006/jtbi.1996.0115) [DOI] [Google Scholar]
- 55.Enquist M, Leimar O. 1983. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 102, 387–410. ( 10.1016/0022-5193(83)90376-4) [DOI] [Google Scholar]
- 56.Pinto NS, Palaoro AV, Peixoto PEC. 2019. All by myself? Meta-analysis of animal contests shows stronger support for self than for mutual assessment models. Biol. Rev. 94, 12509 ( 10.1111/brv.12509) [DOI] [PubMed] [Google Scholar]
- 57.Fuxjager MJ, Mast G, Becker EA, Marler CA. 2009. The ‘home advantage’ is necessary for a full winner effect and changes in post-encounter testosterone. Horm. Behav. 56, 214–219. ( 10.1016/j.yhbeh.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 58.Kemp DJ, Wiklund C. 2004. Residency effects in animal contests. Proc. R. Soc. Lond. B 271, 1707–1711. ( 10.1098/rspb.2004.2775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobson EA. 2020. Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr. Opin. Psychol. 33, 209–215. ( 10.1016/j.copsyc.2019.09.010) [DOI] [PubMed] [Google Scholar]
- 60.Millsopp S, Laming P. 2008. Trade-offs between feeding and shock avoidance in goldfish (Carassius auratus). Appl. Anim. Behav. Sci. 113, 247–254. ( 10.1016/j.applanim.2007.11.004) [DOI] [Google Scholar]
- 61.Duncan IJH, Wood-Gush DGM. 1971. Frustration and aggression in the domestic fowl. Anim. Behav. 19, 500–504. ( 10.1016/S0003-3472(71)80104-5) [DOI] [PubMed] [Google Scholar]
- 62.Papini MR, Dudley RT. 1997. Consequences of surprising reward omissions. Rev. Gen. Psychol. 1, 175–197. ( 10.1037/1089-2680.1.2.175) [DOI] [Google Scholar]
- 63.Vindas MA, Folkedal O, Kristiansen TS, Stien LH, Braastad BO, Mayer I, Øverli Ø. 2012. Omission of expected reward agitates Atlantic salmon (Salmo salar). Anim. Cogn. 15, 903–911. ( 10.1007/s10071-012-0517-7) [DOI] [PubMed] [Google Scholar]
- 64.Darden SK, May MK, Boyland NK, Dabelsteen T. 2019. Territorial defense in a network: audiences only matter to male fiddler crabs primed for confrontation. Behav. Ecol. 30, 336–340. ( 10.1093/beheco/ary169) [DOI] [Google Scholar]
- 65.Miles MC, Fuxjager MJ. 2019. Social context modulates how the winner effect restructures territorial behaviour in free-living woodpeckers. Anim. Behav. 150, 209–218. ( 10.1016/j.anbehav.2019.02.011) [DOI] [Google Scholar]
- 66.Montroy K, Loranger MJ, Bertram SM. 2016. Male crickets adjust their aggressive behavior when a female is present. Behav. Processes 124, 108–114. ( 10.1016/j.beproc.2015.11.003) [DOI] [PubMed] [Google Scholar]
- 67.Oliveira RF, Lopes M, Carneiro LA, Canário AVM. 2001. Watching fights raises fish hormone levels. Nature 409, 475 ( 10.1038/35054128) [DOI] [PubMed] [Google Scholar]
- 68.Cabanac M. 1992. Pleasure: the common currency. J. Theor. Biol. 155, 173–200. ( 10.1016/S0022-5193(05)80594-6) [DOI] [PubMed] [Google Scholar]
- 69.Levy DJ, Glimcher PW. 2012. The root of all value: a neural common currency for choice. Curr. Opin Neurobiol. 22, 1027–1038. ( 10.1016/j.conb.2012.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elwood RW, Appel M. 2009. Pain experience in hermit crabs? Anim. Behav. 77, 1243–1246. ( 10.1016/j.anbehav.2009.01.028) [DOI] [Google Scholar]
- 71.Payne RJH, Pagel M. 1996. Escalation and time costs in displays of endurance. J. Theor. Biol. 183, 185–193. ( 10.1006/jtbi.1996.0212) [DOI] [Google Scholar]
- 72.Payne RJH, Pagel M. 1997. Why do animals repeat displays? Anim. Behav. 54, 109–119. ( 10.1006/anbe.1996.0391) [DOI] [PubMed] [Google Scholar]
- 73.Payne RJH. 1998. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 56, 651–662. ( 10.1006/anbe.1998.0835) [DOI] [PubMed] [Google Scholar]
- 74.Taylor PW, Elwood RW. 2003. The mismeasure of animal contests. Anim. Behav. 65, 1195–1202. ( 10.1006/anbe.2003.2169) [DOI] [Google Scholar]
- 75.Camerlink I, Turner SP, Farish M, Arnott G. 2017. The influence of experience on contest assessment strategies. Sci. Rep. 7, 1–10. ( 10.1038/s41598-017-15144-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chapin KJ, Enrique P, Peixoto C, Briffa M. 2019. Further mismeasures of animal contests: a new framework for assessment strategies. Behav. Ecol. 30, 1177–1185 ( 10.1093/beheco/arz081) [DOI] [Google Scholar]
- 77.Mesterton-Gibbons M, Heap SM. 2014. Variation between self- and mutual assessment in animal contests. Am. Nat. 183, 199–213. ( 10.1086/674443) [DOI] [PubMed] [Google Scholar]
- 78.Garcia MJ, Paiva L, Lennox M, Sivaraman B, Wong SC, Earley RL. 2012. Assessment strategies and the effects of fighting experience on future contest performance in the green anole (Anolis carolinensis). Ethology 118, 821–834. ( 10.1111/j.1439-0310.2012.02072.x) [DOI] [Google Scholar]
- 79.Hsu Y, Lee SP, Chen MH, Yang SY, Cheng KC. 2008. Switching assessment strategy during a contest: fighting in killifish Kryptolebias marmoratus. Anim. Behav. 75, 1641–1649. ( 10.1016/j.anbehav.2007.10.017) [DOI] [Google Scholar]
- 80.Morrell LJ, Backwell PRY, Metcalfe NB. 2005. Fighting in fiddler crabs Uca mjoebergi: what determines duration? Anim. Behav. 70, 653–662. ( 10.1016/j.anbehav.2004.11.014) [DOI] [Google Scholar]
- 81.Blanchette I, Richards A. 2010. The influence of affect on higher level cognition: a review of research on interpretation, judgement, decision making and reasoning. Cogn. Emot. 24, 561–595. ( 10.1080/02699930903132496) [DOI] [Google Scholar]
- 82.Mackie DM, Worth LT. 1989. Processing deficits and the mediation of positive affect in persuasion. J. Pers. Soc. Psychol. 57, 27–40. ( 10.1037/0022-3514.57.1.27) [DOI] [PubMed] [Google Scholar]
- 83.Worth LT, Mackie DM. 1987. Cognitive mediation of positive affect in persuasion. Soc. Cogn. 5, 76–94. ( 10.1521/soco.1987.5.1.76) [DOI] [PubMed] [Google Scholar]
- 84.Hutchinson JMC, Gigerenzer G. 2005. Simple heuristics and rules of thumb: where psychologists and behavioural biologists might meet. Behav. Processes 69, 97–124. ( 10.1016/j.beproc.2005.02.019) [DOI] [PubMed] [Google Scholar]
- 85.Harmon-Jones E, Harmon-Jones C, Amodio DM, Gable PA. 2011. Attitudes toward emotions. J. Pers. Soc. Psychol. 101, 1332 ( 10.1037/a0024951) [DOI] [PubMed] [Google Scholar]
- 86.Cabral JCC, de Almeida RMM. 2019. Effects of anger on dominance-seeking and aggressive behaviors. Evol. Hum. Behav. 40, 23–33. ( 10.1016/j.evolhumbehav.2018.07.006) [DOI] [Google Scholar]
- 87.Veenstra L, Bushman BJ, Koole SL. 2018. The facts on the furious: a brief review of the psychology of trait anger. Curr. Opin. Psychol. 19, 98–103. ( 10.1016/j.copsyc.2017.03.014) [DOI] [PubMed] [Google Scholar]
- 88.Carver CS, Harmon-Jones E. 2009. Anger is an approach-related affect: evidence and implications. Psychol. Bull. 135, 183 ( 10.1037/a0013965) [DOI] [PubMed] [Google Scholar]
- 89.Elwood RW, Wood KE, Gallagher MB, Dick JTA. 1998. Probing motivational state during agonistic encounters in animals. Nature 393, 66–68. ( 10.1038/29980) [DOI] [Google Scholar]
- 90.Koch M. 1999. The neurobiology of startle. Prog. Neurobiol. 59, 107–128. ( 10.1016/S0301-0082(98)00098-7) [DOI] [PubMed] [Google Scholar]
- 91.Winslow JT, Parr LA, Davis M. 2002. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol. Psychiatry 51, 859–866. ( 10.1016/S0006-3223(02)01345-8) [DOI] [PubMed] [Google Scholar]
- 92.Fawcett TW, Johnstone RA. 2010. Learning your own strength: winner and loser effects should change with age and experience. Proc. R. Soc. B 277, 1427–1434. ( 10.1098/rspb.2009.2088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mesterton-Gibbons M. 1999. On the evolution of pure winner and loser effects: a game-theoretic model. Bull. Math. Biol. 61, 1151–1186. ( 10.1006/bulm.1999.0137) [DOI] [PubMed] [Google Scholar]
- 94.Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. Camb. Philos. Soc. 81, 33–74. ( 10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 95.Hsu Y, Lee IH, Lu CK. 2009. Prior contest information: mechanisms underlying winner and loser effects. Behav. Ecol. Sociobiol. 63, 1247–1257. ( 10.1007/s00265-009-0791-9) [DOI] [Google Scholar]
- 96.Rutte C, Taborsky M, Brinkhof MWG. 2006. What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21. ( 10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- 97.Paul ES, Harding EJ, Mendl M. 2005. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehav. Rev. 29, 469–491. ( 10.1016/j.neubiorev.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 98.Everaert J, Podina IR, Koster EHW. 2017. A comprehensive meta-analysis of interpretation biases in depression. Clin. Psychol. Rev. 58, 33–48. ( 10.1016/j.cpr.2017.09.005) [DOI] [PubMed] [Google Scholar]
- 99.Hirsch CR, Meeten F, Krahé C, Reeder C. 2016. Resolving ambiguity in emotional disorders: the nature and role of interpretation biases. Annu. Rev. Clin. Psychol. 12, 281–305. ( 10.1146/annurev-clinpsy-021815-093436) [DOI] [PubMed] [Google Scholar]
- 100.Stuijfzand S, Creswell C, Field AP, Pearcey S, Dodd H. 2018. Research Review: is anxiety associated with negative interpretations of ambiguity in children and adolescents? A systematic review and meta-analysis. J. Child Psychol. Psychiatry 59, 1127–1142. ( 10.1111/jcpp.12822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harding EJ, Paul ES, Mendl M. 2004. Cognitive bias and affective state. Nature 427, 312 ( 10.1038/427312a) [DOI] [PubMed] [Google Scholar]
- 102.Kasumovic MM, Elias DO, Sivalinghem S, Mason AC, Andrade MCB. 2010. Examination of prior contest experience and the retention of winner and loser effects. Behav. Ecol. 21, 404–409. ( 10.1093/beheco/arp204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barker TH, George RP, Howarth GS, Whittaker AL. 2017. Assessment of housing density, space allocation and social hierarchy of laboratory rats on behavioural measures of welfare. PLoS ONE 12, e185135 ( 10.1371/journal.pone.0185135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horback KM, Parsons TD. 2019. Judgement bias testing in group-housed gestating sows. Behav. Processes 159, 86–92. ( 10.1016/j.beproc.2018.12.021) [DOI] [PubMed] [Google Scholar]
- 105.Schino G, Massimei R, Pinzaglia M, Addessi E. 2016. Grooming, social rank and ‘optimism’ in tufted capuchin monkeys: a study of judgement bias. Anim. Behav. 119, 11–16. ( 10.1016/j.anbehav.2016.06.017) [DOI] [Google Scholar]
- 106.Rogers L, Sales E, Shamsi S, Kopf RK, Freire R. 2020. Aggressive encounters lead to negative affective state in fish. PLoS ONE 15, e0231330 ( 10.1371/journal.pone.0231330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.May ME. 2011. Aggression as positive reinforcement in people with intellectual disabilities. Res. Dev. Disabil. 32, 2214–2224. ( 10.1016/j.ridd.2011.05.029) [DOI] [PubMed] [Google Scholar]
- 108.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. 2016. Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci. 19, 596–604. ( 10.1038/nn.4264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martínez M, Guillién-Salazar F, Salvador A, Simon VM. 1995. Successful intermale aggression and conditioned place preference in mice. Physiol. Behav. 58, 323–328 ( 10.1016/0031-9384(95)00061-M) [DOI] [PubMed] [Google Scholar]
- 110.Meisel RL, Joppa MA. 1994. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol. Behav. 56, 1115–1118. ( 10.1016/0031-9384(94)90352-2) [DOI] [PubMed] [Google Scholar]
- 111.Farrell WJ, Wilczynski W. 2006. Aggressive experience alters place preference in green anole lizards, Anolis carolinensis. Anim. Behav. 71, 1155–1164. ( 10.1016/j.anbehav.2005.10.006) [DOI] [Google Scholar]
- 112.Reichert MS, Quinn JL. 2017. Cognition in contests: mechanisms, ecology, and evolution. Trends Ecol. Evol. 32, 773–785. ( 10.1016/j.tree.2017.07.003) [DOI] [PubMed] [Google Scholar]
- 113.George JM, Dane E. 2016. Affect, emotion, and decision making. Org. Behav. Hum. Dec. Process. 136, 47–55. ( 10.1016/j.obhdp.2016.06.004) [DOI] [Google Scholar]
- 114.Västfjäll D, Slovic P, Burns WJ, Erlandsson A, Koppel L, Asutay E, Tinghög G. 2016. The arithmetic of emotion: Integration of incidental and integral affect in judgments and decisions. Front. Psychol. 7, 325 ( 10.3389/fpsyg.2016.00325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wyer RS Jr, Dong P, Huang X, Huang Z, Wan LC. 2019. The effect of incidental emotions on judgments and behavior in unrelated situations: a review. J. Assoc. Cons. Res. 4, 198–207. ( 10.1086/701889) [DOI] [Google Scholar]
- 116.Schwarz N, Clore GL. 1983. Mood, misattribution, and judgements of well-being informative and directive functions of affective states. J. Pers. Soc. Psychol. 45, 513–523. ( 10.1037/0022-3514.45.3.513) [DOI] [Google Scholar]
- 117.Bateson M, Desire S, Gartside SE, Wright GA. 2011. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070–1073. ( 10.1016/j.cub.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bergman M, Olofsson M, Wiklund C. 2010. Contest outcome in a territorial butterfly: the role of motivation. Proc. R. Soc. B 277, 3027–3033. ( 10.1098/rspb.2010.0646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang S, Ai H, Li D. 2019. Mating changes a male contestant from a loser to a winner in male–male competition in a wolf spider. Biol. J. Linnean Soc. 128, 83–92. ( 10.1093/biolinnean/blz091) [DOI] [Google Scholar]
- 120.Reddon AR, Dey CJ, Balshine S. 2019. Submissive behaviour is mediated by sex, social status, relative body size and shelter availability in a social fish. Anim. Behav. 155, 131–139. ( 10.1016/j.anbehav.2019.06.026) [DOI] [Google Scholar]
- 121.Parrott JL, Metcalfe CD. 2018. Nest-defense behaviors in fathead minnows after lifecycle exposure to the antidepressant venlafaxine. Environ. Pollut 234, 223–230. ( 10.1016/j.envpol.2017.11.049) [DOI] [PubMed] [Google Scholar]
- 122.Perreault HA, Semsar K, Godwin J. 2003. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol. Behav. 79, 719–724. ( 10.1016/S0031-9384(03)00211-7) [DOI] [PubMed] [Google Scholar]
- 123.Niemelä PT, Santostefano F. 2015. Social carry-over effects on non-social behavioral variation: mechanisms and consequences. Front. Ecol. Evol. 3, 49 ( 10.3389/fevo.2015.00049) [DOI] [Google Scholar]
- 124.Van der Harst JE, Baars AM, Spruijt BM. 2005. Announced rewards counteract the impairment of anticipatory behaviour in socially stressed rats. Behav. Brain Res. 161, 183–189. ( 10.1016/j.bbr.2005.02.029) [DOI] [PubMed] [Google Scholar]
- 125.Boggiani L, Addessi E, Schino G. 2018. Receiving aggression triggers attention bias in tufted capuchin monkeys. Anim. Behav. 146, 173–180. ( 10.1016/j.anbehav.2018.10.021) [DOI] [Google Scholar]
- 126.Clore GL, Wyer RS, Dienes BPA, Gasper K, Gohm C, Isbell L. 2001. Affective feelings as feedback: Some cognitive consequences. In Theories of mood and cognition: a user's guidebook (eds Martin LL, Clore GL), pp. 27–62. Mahwah, NJ: Erlbaum. [Google Scholar]
- 127.Bakamitsos GA. 2006. A cue alone or a probe to think? The dual role of affect in product evaluations. J. Cons. Res. 33, 403–412. ( 10.1086/508525) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.


