Abstract
The Hedgehog (Hh) morphogen regulates growth and patterning. Since Hh signaling is also implicated in carcinogenesis, it is conceivable that de novo Hh-secreting organizers, if formed in association with oncogenic hit could be tumor-cooperative. Here we validate this hypothesis using the Drosophila model of cooperative epithelial carcinogenesis. We generate somatic clones with simultaneous loss of tumor suppressor, Lgl, and gain of the posterior compartment selector, Engrailed (En), known to induce synthesis of Hh. We show that lgl UAS-en clones in the anterior wing compartment trigger Hh signaling cascade via cross-talk with their Ci-expressing wild type cell neighbors. Hh-Dpp signaling from clone boundaries of such ectopically formed de novo organizers in turn drive lgl carcinogenesis. By contrast, Ci-expressing lgl clones transform by autocrine and/or juxtracine activation of Hh signaling in only the posterior compartment. We further show that sequestration of the Hh ligand or loss of Dpp receptor, Tkv, in these Hh-sending or –receiving lgl clones arrested their carcinogenesis. Our results therefore reveal a hitherto unrecognized mechanism of tumor cooperation by developmental organizers, which are induced fortuitously by oncogenic hits.
Keywords: Hh signaling, Developmental organizer, lgl, Tumor cooperation
1. Introduction
Cancer cells often display perturbation in key signaling pathways, many of which are required for early embryonic growth and development. One such highly conserved signaling pathway is the Sonic Hedgehog/Hedgehog (Shh/Hh) signaling pathway (Hartl and Scott, 2014; Lum and Beachy, 2004), which plays an essential role in early growth and patterning of the embryonic appendages (for a recent review, see Lopez-Rios, 2016). Transcriptional regulation of Hh is under Engrailed (En), a homeo-box containing transcription factor in Drosophila (Fig. 1A-C) (Basler and Struhl, 1994; Tabata et al., 1992), while in mammals, Shh transcription in the developing limb bud is regulated by the members of the Hox-D gene cluster (Zakany et al., 2004). In the Drosophila appendage primordia—for instance, the wing imaginal discs—secreted Hh from En-expressing cells of the posterior compartment serve as a short-range morphogen that triggers synthesis of its targets patched (ptc) and dpp that culminate in secretion of the long-range Dpp morphogen (TGF-β family protein in mammals) from the adjacent cells of the anterior compartment (Fig. 1D and E), see (Basler and Struhl, 1994; Dominguez et al., 1996; Tabata and Kornberg, 1994). Thus, juxtapositioning of Hh-sending (En-expressing) and Hh-receiving (Ci-expressing) cells at the anterior-posterior (A/P) compartment boundary create morphogen secreting organizers in Drosophila append-ages, which trigger the Hh-Dpp, signaling cascade (Dominguez et al., 1996). Sonic hedgehog (Shh)—the mammalian counterpart of Drosophila Hh—is secreted by the cells in the zone of polarizing activity (ZPA) of developing limb buds, where it is regulated by collinear expressions of members of the Hox-D cluster, namely Hoxd11, Hoxd12, and Hoxd13, thereby, triggering a Shh-TGF-β (Hh-Dpp, in Drosophila) signaling cascade (Riddle et al., 1993; Zakany et al., 2004).
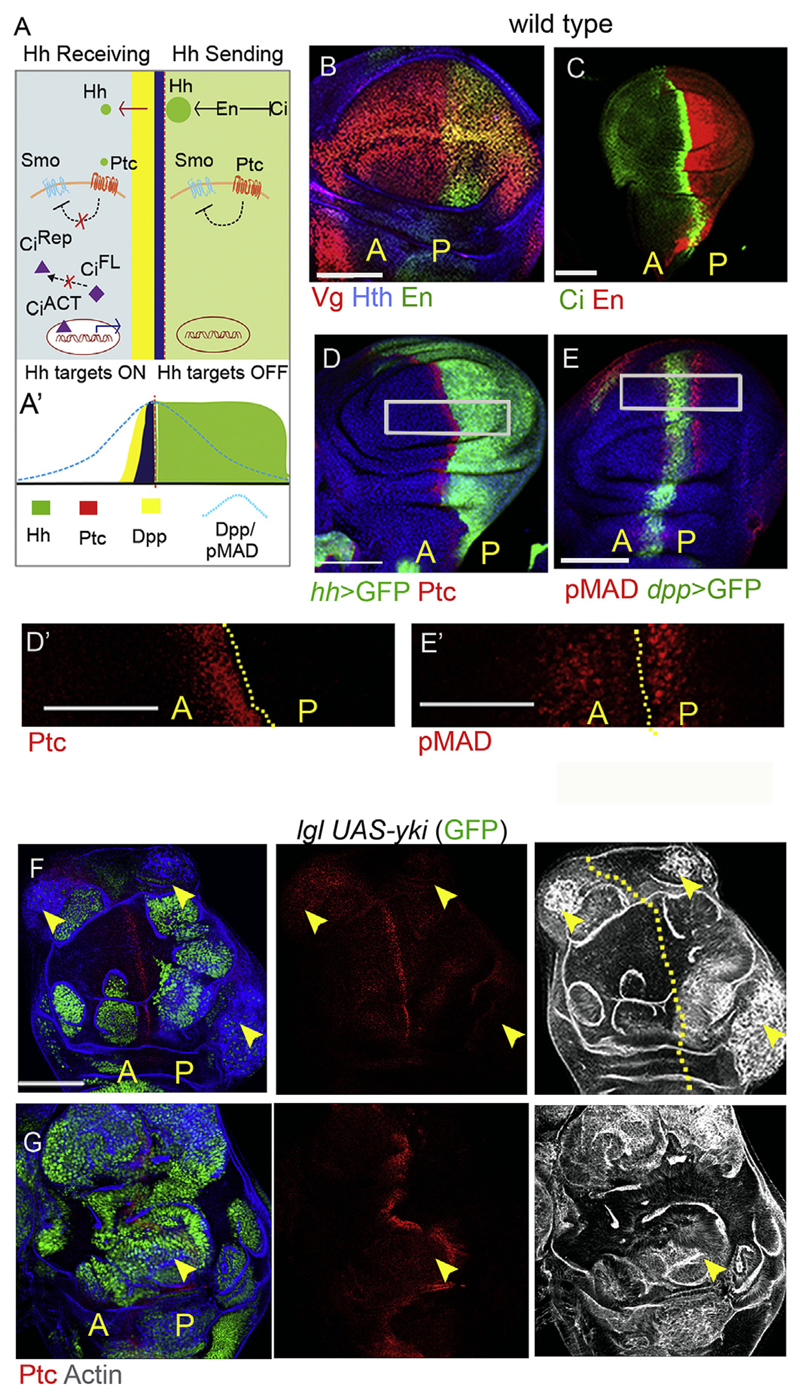
Fig. 1. lgl neoplasia does not entail recruitment of endogenous Hh signaling.
(A-A′) Schematic representation of the Hh signaling pathway displaying cross-talk between Hh-sending and Hh-receiving cells of the posterior [P] and Anterior [A] compartments respectively. (B–C) Different developmental domains of the wing disc: the distal wing pouch is marked by Vestigial, (Vg), the non-pouch domain is marked by Homothorax, (Hth); Engrailed marks the posterior (B) and Ci the anterior (C) compartments of the wing disc. (D) Hh signaling target Ptc is expressed in cells of the anterior compartment abutting the A/P boundary, while (E) Dpp target pMad (E) straddles the A/P boundary. D′ and E′ are higher magnifications of marked areas in D and E respectively. (F–G) lgl UAS-yki (GFP) clones do not display either ectopic (arrowheads, F) nor endogenous (arrowhead, G) gain of Hh target Ptc. lgl mutant clones display neoplastic transformation (disrupted F-actin filament, F and G) in both [A] and [P] wing compartments. Abbreviations: [A] = Anterior and [P] = Posterior in all figures. Scale bars 100 μm. D0 -E’: 50 μm.
Constitutive gain of Hh signaling in cancers of diverse genetic and tissue origins have been reported wherein the pathway is activated in a ligand-independent manner: for instance, due to a loss of its negative regulator, Patched (PTCH1), or activation of its transducer, Smoothened (SMO) (for review, see Barakat et al., 2010; Pak and Segal, 2016). On the other hand, secreted Hh ligand–dependent activation of the pathway are also frequently seen in various cancers, such as of the esophagus, stomach, pancreas (Berman et al., 2003) and in small cell lung carcinomas (Watkins et al., 2003). In most of these instances of Hh ligand-dependent cancers, however, mechanistic underpinnings of the trigger for Shh-TGF-β signaling remains poorly understood (Teglund and Toftgard, 2010; Theunissen and de Sauvage, 2009).
Here we show that Hh-Dpp signaling cascade from ectopic developmental organizers formed in association with an oncogenic hit drives tumor progression in Drosophila imaginal disc epithelia. Thus, when somatic clones in imaginal disc epithelia displaying loss of the tumor suppressor, Lgl, simultaneously gain the En selector, these trigger Hh signaling in cross-talk with their Ci-expressing cell neighbors. Following the well-known developmental paradigms, Hh ligand secreted by these lgl mutant clones induce synthesis of the long-range Dpp morphogen, but only in the Ci-expressing (thus Hh receiving) cell neighbors in the anterior compartment. The resultant Hh-Dpp signaling cascade then co-operates for lgl neoplasia. Conversely, when lgl mutant clones receive the Hh-ligand due to gain of Ci, these transform by receiving the Hh ligand from the En expressing (thus Hh-sending) cell neighbors of the posterior compartment. Our findings thus reveal that Hh ligand-mediated tumor cooperation are context-dependent and follow the essential ground rules of animal development, as seen at Hh signaling organizers.
2. Results and discussion
2.1. Endogenous Hh signaling is not recruited during lgl neoplasia in Drosophila wing
Mutant clones displaying loss of the tumor suppressor, Lgl, in imaginal epithelia are growth disadvantaged and are eliminated by cell competition (Agrawal et al., 1995; Froldi et al., 2010; Khan et al., 2013; Menendez et al., 2010). However, these lgl mutant clones survive and transform when provided with survival benefit, such as gain of Yki (Khan et al., 2013; Menendez et al., 2010), the transcription co-factor of the Hippo signaling pathway or when lgl mutant clones are generated in a cell competition-compromised Minute mutant genetic background (Froldi et al., 2010; Khan et al., 2013). Previously, we carried out a whole-genome transcriptomic analysis of wing imaginal epithelia that were mosaic for lgl mutant clones. Our analysis of the transcriptome revealed perturbations in a host of cellular signaling pathways (Khan et al., 2013); however, strikingly, we failed to observe significant enrichment of the Hh signaling pathway (Fig. S1A). Further, lgl mutant clones transformed by gain of Yki (lgl UAS-yki; Fig. 1F and G) or those induced in Minute genetic (M/+) background (Fig. S1B and C), too, did not display gain of Hh signaling pathway as revealed by the absence of Hh target, Ptc (Fig. 1F and Fig. S1B), or its downstream Dpp signaling target, pMAD (Fig. S1C). Since Hh is a short-range secreted ligand, we also examined the expression of Ptc, in lgl mutant clones abutting the endogenous A/P boundary; however, these did not display gain of Ptc (arrowhead, Fig. 1G) suggesting thereby that their transformation was not driven by gain of endogenous Hh signaling per se. We note that lgl mutant somatic clones transform in both anterior and posterior compartments (Fig. 1F and G), which is consistent with the fact that cells of both the compartments of wing appendage transform in lgl mutant larva (Agrawal et al., 1995). Collectively, these observations indicate that Hh signaling pathway per se is not perturbed or recruited during neoplastic transformation of lgl mutant clones.
2.2. Hh signaling from de novo A/P organizer cooperates for tumor progression in lgl mutant clones
We next sought to examine fate of lgl mutant clones in a wild type cell-competitive background, when Hh signaling was deliberately gained. We thus tested the consequences of a gain of the upstream regulator of Hh synthesis, namely, En, in the lgl mutant clones. Rationale of this test was to confer posterior cell fate identity to lgl mutant clones and thus create de novo A/P boundaries at the clone-cell neighbor interface in the anterior wing compartment (Basler and Struhl, 1994; Tabata et al., 1995). Indeed, gain of En resulted in suppression of Ci in lgl mutant clones in the anterior wing compartment (Fig. 2A) thereby recapitulating the attribute of an ectopic gain of En (Fig. S2), also see (Tabata et al., 1995; Zecca et al., 1995). We noted that lgl UAS-en clones in the anterior compartment were larger compared to their posterior counterparts (Fig. 2C and D), particularly when located in the proximal hinge domain of the wing imaginal discs. In the wing pouch domain, however, these were much smaller irrespective of their anterior or posterior origin (Fig. 2C and D). We note that these outcomes are in agreement with the previous observations on tumor resistance of the wing pouch domain (Froldi et al., 2010; Khan et al., 2013; Tamori et al., 2016). Further, anterior wild type cells abutting the overgrown proximal lgl UAS-en clones displayed cell death (Fig. 2B); we interpret these outcomes as a consequence of their elimination by cell competition-a feature often associated with neoplastic tumors of clonal origin (Froldi et al., 2010; Khan et al., 2013; Menendez et al., 2010).
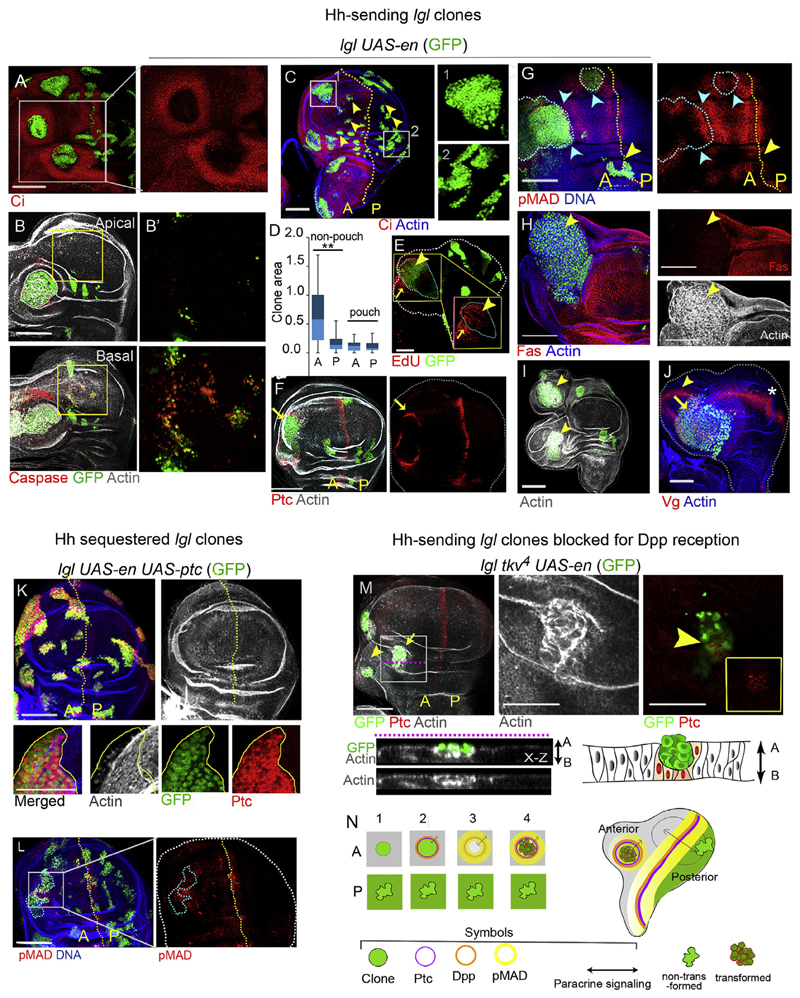
Fig. 2. Hh signaling from de novo A/P organizers transform Hh-sending lgl mutant clones in anterior wing compartment.
(A) Hh-sending lgl mutant clones, lgl UAS-en (GFP) display loss of Ci in [A] compartment. (B) Cell death (Caspase) in lgl UAS-en clones of the distal wing (boxed area) and in cells surrounding the clone in the wing hinge. Apical and basal (B0) sections of boxed area in distal wing are magnified on the right. (C) lgl UAS-en (GFP) clones are larger in the [A] compartment and display smooth boundary (box 1) compared to the clones in [P] compartment wherein the clones display wiggly clone boundary (box 2). (D) Comparison of lgl UAS-en clone sizes in the [A] and [P] compartments in the wing epithelium. Clones in the non-pouch region of the anterior compartment are significantly larger than their posterior counterparts (t-test, P = 2.84E-07, N = 70); whereas the difference in clone sizes is insignificant between the two compartments of the wing pouch (t-test, P = 0.422, N = 35). (E) Cell proliferation (as seen by EdU uptake) within (arrowhead) and in surrounding cells (arrow) of lgl UAS-en clone; magni ed view of the boxed area provided in the inset. (F) Gain of Ptc in wild type cells abutting the clone boundary (arrow). (G) Dpp target pMAD is gained on either side of the clone boundary (marked by blue dotted line and blue arrowheads). (H) Disruption of F-actin and loss of FasIII in lgl UAS-en clone (arrowhead). (I–J) Duplicated wing fields (arrows, I, J) associated with lgl UAS-en clone displaying wing fate marker Vg (yellow arrowhead, J; star marks endogenous wing domain). (K) Hh-sequestered lgl mutant clones (lgl UAS-en, UAS-ptc) display intact F-actin in both [A] and [P] compartments. Note absence of Ptc outside clone boundary (magnified field in box below). (L) lgl UAS-en, UAS-ptc display reduced pMad levels (compare with 2G). (M) lgl UAS-en clones blocked for Dpp reception (lgl tkv 4 ;UAS-en) grow poorly (arrow and arrowhead) and undergo epithelial extrusion (arrow); some cells display gain of Ptc (arrow head) in the magnified view of the boxed region displayed on the right. Lower panel displays x-z view and schematic depiction of lgl tkv 4 ;UAS-en clone. (N) Schematic summary of compartment-specific carcinogenesis of Hh-sending lgl mutant clones. Scale bars 100 μm.
Further, we observed that lgl UAS-en clones in the anterior compartment sorted out from their Ci-expressing neighbors (Fig. 2C, box 1), while these remained wiggly in the posterior compartment (Fig. 2C, box 2). Such behavior was consistent with the altered Hh signaling status of lgl UAS-en clones in the anterior compartment, see, (Dahmann and Basler, 2000), Further, consistent with recapitulation of signaling events at the endogenous A/P boundary lgl UAS-en clones displayed gain of the short-range Hh target, Ptc, in the Ci-expressing wild type anterior cell neighbors abutting the clone boundary (arrow, Fig. 2F) –a signature of formation of an A/P organizer de novo (Dominguez et al., 1996; Zecca et al., 1995). Importantly, as seen at the endogenous A/P boundary in the wing disc (Fig. 1E), Dpp, the long-range target of Hh (Zecca et al., 1995), activated its target pMad on either side of the lgl UAS-en clone boundaries (blue arrowheads in Fig. 2G). Further to examine cell proliferation induced by the Dpp morphogen we carried out an EdU uptake, which revealed cell proliferation, within the proximal lgl UAS-en clones of the anterior compartment as well as in their surrounding wild type neighbors (Fig. 2E). A non-cell autonomous proliferation, is not surprising given that the Dpp morphogen synthesized at the clone boundary display long-range signaling for growth and cell proliferation (Capdevila and Guerrero, 1994; Martin-Castellanos and Edgar, 2002).
Finally, by the day 5 of clone induction, nearly a third of the Hh-sending lgl UAS-en clones (n = 6/27) in the anterior hinge domain of the wing imaginal discs displayed neoplastic transformation marked by loss of septate junction marker, FasIII, besides disruption of F-actin (Fig. 2H), thus displaying hallmarks of neoplastically transformed epithelial cells in Drosophila (Brumby and Richardson, 2003; Khan et al., 2013; Pagliarini and Xu, 2003). In the posterior hinge domains, however, lgl UAS-en clones failed to display activation of the downstream target of Hh signaling, Ptc, in the wild type cells abutting the clone boundary (Fig. 2F). This is not surprising since En-expressing posterior cells do not express Ci (Fig. 1C), a prerequisite for transduction of Hh signal. Further, consistent with the organizing activity of these A/P clone boundaries formed de novo in the anterior compartment, about half of the transformed Hh-sending lgl UAS-en clones (n = 8/15) displayed appendage duplications, marked by non-cell autonomous induction of supernumerary wings (Fig. 2I and J) with characteristic gain of the wing cell fate selector, Vestigial (Vg) (Fig. 2J) (Williams et al., 1991). We next examined if lgl UAS-en clones also display compartment-specific bias in neoplastic transformation in other imaginal discs that display anterior-posterior compartmentalization similar to the wing, such as the haltere and the leg epithelium (Fig. S3A and B). Indeed, in the haltere (Fig. S3C) and in the leg (Fig. S3D) imaginal discs, too, we noted an anterior compartment-specific bias in neoplastic transformation of the lgl UAS-en clones.
2.3. Loss of Hh-Dpp signaling from ectopic A/P organizers at clone boundaries inhibit lgl neoplasia
Transformation of lgl UAS-en clones is likely to be driven by Hh-Dpp paracrine signaling at the A/P organizers formed de novo. To test this prediction, we gained the receptor, and also negative regulator of the Hh pathway, Ptc (Chen and Struhl, 1996), in lgl UAS-en clones. Indeed, lgl UAS-en, UAS-ptc clones failed to activate Hh target, Ptc, in Ci-expressing cells abutting the clone boundary (Fig. 2K, compare with Fig. 2F) and displayed marked reduction in the expression of Dpp target, pMAD (Fig. 2L, for comparison see Fig. 2G). Not surprisingly therefore these failed to display neoplastic transformation (Fig. 2K). Next, we knocked-down reception of the Dpp ligand in lgl UAS-en clones by simultaneous loss of Dpp receptor, Tkv, due its null mutation, tkv 4 (Shen and Dahmann, 2005) in lgl UAS-en clones; these, too, were under-grown (Fig. 2M, n = 8/8) and displayed epithelial extrusion (see x-z section in Fig. 2M), a characteristic feature of Dpp-compromised clones (Gibson and Perrimon, 2005; Shen and Dahmann, 2005).
These findings (Fig. 2) therefore support our initial assumption that A/P organizers formed de novo in association with an oncogenic lesion could provide co-operative drive for tumorigenesis (Fig. 2N). Organizer-mediated oncogenic cooperation revealed here may not be a feature unique to the Drosophila model of carcinogenesis. Hox genes are found misexpressed across many cancers (Shah and Sukumar, 2010) and it may not be surprising if some of these (those functionally akin to En in Drosophila) could activate ectopic Hh synthesis. Thus, Hh-ligand-dependent tumors that do not display genetic lesion(s) in the members of Hh-signaling per se (Briscoe and Therond, 2013; Teglund and Toftgard, 2010; Theunissen and de Sauvage, 2009) are ideal candidates to screen for up-stream, HOX mutations that may induce synthesis of the Hh ligand.
2.4. Hh-Dpp signaling from ectopic A/P organizers drive neoplasia in only select domains of the compound eye-antennal imaginal disc
2.4.1. Hh-sending lgl mutant clones undergo neoplasia in only the head domain
Next, we sought to examine if Hh-sending lgl mutant clones trigger neoplasia in domains where Hh synthesis is not linked to En expression. The eye-antennal imaginal disc is particularly interesting in this regard. Here, cells of the presumptive head domain—located anterior to the morphogenetic furrow (MF)—expresses Ci while Hh-sending cells are located posterior to the MF (Fig. 3A and B) (Dominguez and Hafen, 1997; Heberlein et al., 1995) Thus, Hh secreted from cells posterior to the MF activates dpp and ptc in the Ci-expressing cells anterior to the MF (Fig.3A and B) leaving in its wake differentiated retinal cells (Dominguez and Hafen, 1997; Heberlein et al., 1995), which are marked by expression of neuronal marker, Elav (Robinow and White, 1991).
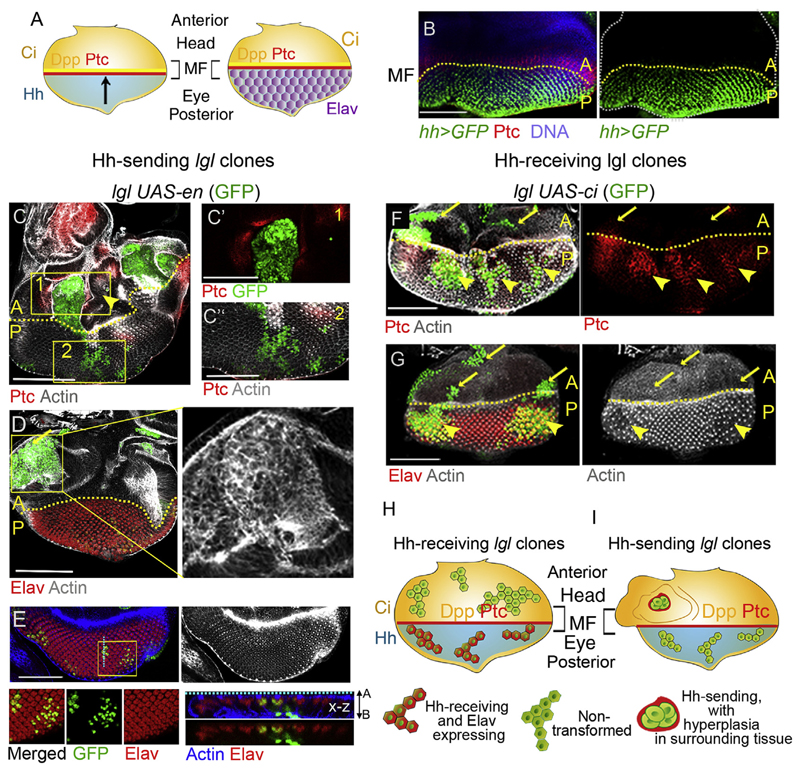
Fig. 3. Hh-induced cell differentiation in the eye primordium overrides its oncogenic drive.
(A) Schematic representation of Hh signaling domains in the eye epithelium. (B) Expression of Hh in cells posterior to the morphogenetic furrow [MF] and Ptc in cells anterior to the MF in the eye epithelium. (C) lgl UAS-en (GFP) clones in the head domain (anterior to the MF) display gain of Ptc (box 1) in abutting wild type cells and induce hyperplasia in surrounding tissue (arrowhead). Clones posterior to the MF do not gain Ptc (box 2). (C0 -C00) Boxed regions in (C) magnified on the right. (D) The lgl UAS-en (GFP) clones (arrow) undergo neoplastic transformation (altered F-actin) in the head domain. (E) These lgl clones in the eye domain fail to transform (intact F-actin) and continue to display retinal differentiation marker Elav (E). Boxed region magnified in the lower panels. x-z section along the boxed region on the far bottom right displays apico-basal localization of lgl UAS-en clone (GFP). (F–G) lgl UAS-ci clones (GFP) display activation of Hh signaling as seen by gain of Ptc (yellow arrowheads) in the eye domain, while clones anterior to the MF display absence of Ptc (arrow). (G) lgl UAS-ci clones in both head (arrows) and eye (arrowheads) domains fail to undergo neoplastic transformation (intact F-actin) and continue to express Elav (arrowheads, G). (H–I) Schematic representation of the outcomes of Hh-sending and Hh-receiving lgl mutant clones in the eye epithelium. Scale bars 100 μm.
We observed that similar to their counterparts in the anterior wing, lgl UAS-en clones anterior to the MF formed de novo A/P organizers with characteristic gain of Ptc outside clone boundary (Fig. 3C). Further, we observed hyperplastic growth in wild type cells outside the lgl UAS-en clone boundary marked by their excessively folded epithelium (yellow arrow, Fig. 3C and Fig S4), which is consistent with the induction of non-cell autonomous cell proliferation in lieu of induction of the long-range Dpp morphogen secretion from these clones boundaries (seeFig. 2G). These lgl UAS-en clones also displayed neoplastic transformation, as seen from their disrupted F-actin (Fig. 3D). By, contrast, in the eye primordium, these failed to either activate Hh signaling—as seen from the absence of Ptc (Fig. 3C, box 2)—nor displayed neoplasia (Fig. 3E) as seen by intact F-Actin (Fig. 3E) and continued to display the retinal differentiation marker Elav (Fig. 3E). We note that similar to their behavior in the posterior compartment of the wing imaginal discs the clones displayed poor growth compared to their anterior (Fig. 3C and D) counterparts. These outcomes, however, were not surprising given that both these clones and their cell neighbors are Hh-sending in nature and thus do not engage in signaling cross-talks.
2.4.2. Hh-induced cell differentiation overrides its tumor promoting role in the developing eye primordium
In the developing eye primordia, Ci is expressed in cells anterior to the MF (Dominguez and Hafen, 1997), while in the cells posterior to the MF, Ci is degraded by an Hh-induced, MATH and BTB domain containing protein, HIB (Zhang et al., 2006). Thus, Hh-sending cells, posterior to the MF, are refractory to their own secreted Hh ligand. We thus sought to examine the fate of lgl mutant cells in the eye primordium when these become Hh-receiving upon deliberate gain of Ci (lgl UAS-ci). Indeed, these clones displayed gain of Hh signaling target, Ptc (Fig. 3F). However, surprisingly, these failed to display neoplastic transformation and, instead, were marked by their persistent expression of retinal differentiation marker, Elav (Fig. 3G). Further, anterior to the MF, too, lgl UAS-ci mutant clones fail to undergo neoplastic transformation (arrows,Fig. 3F and G); this outcome is readily reconciled by the absence of signaling cross-talk with their cell neighbors, which also express Ci.
Thus, in the tissue context of the eye primordium (Fig. 3H and I), lgl UAS-en or lgl UAS-ci clones fail to induce cell fate reversal or neoplasia. This outcome is in agreement with our earlier observations that showed that in the absence of cell fate reversals lgl mutant clones in the eye primordium fail to display neoplastic transformation (Gupta et al., 2017; Khan et al., 2013). Such a developmental context-dependent outcome of Hh signaling offers possible explanation for the previous and apparently paradoxical reports of tumor-suppressive fallouts of stromal activation of Hh (Gerling et al., 2016), or absence of Hh target activation—despite secretion of the Hh ligand—as seen in a mouse models of colon cancer (Varnat et al., 2009).
2.5. Ci-expressing lgl clones display neoplastic transformation when induced in the Hh-synthesizing posterior wing compartment
Ectopic gain of En down-regulates Ci in cells of the anterior wing compartment and, thereby, induces their anterior-to-posterior cell fate switch (Dominguez et al., 1996). By contrast, ectopic gain of Ci in the posterior wing compartment does not extinguish En expression; these clones, therefore, retain their posterior-cell fate (Hepker et al., 1997) also see Fig. S5A. As a result cells of the posterior compartment with ectopic gain of Ci are capable of transducing the Hh signal (Dominguez et al., 1996; Hepker et al., 1997, also see Fig. S5 and C). We sought to examine the fate of lgl UAS-ci clones in the posterior compartment of the wing primordium where these are likely to receive Hh ligand cell autonomously. As anticipated, these Hh-receiving lgl mutant clones in the posterior wing compartment displayed activation of the Hh target, Ptc (Fig. 4AA), and also its down-stream Dpp target, pMAD (Fig. 4B). Activation of the Hh-Dpp signaling cascade in these clones is likely to be autocrine since lgl UAS-ci clones continue to express En (Fig. 4C). Not surprisingly, lgl UAS-ci clones in the proximal wing of the posterior compartment were larger, compared to their anterior counterparts (Fig. 4D and E) and over half (n = 15/23) of these clones displayed neoplastic transformation (disrupted F actin, Fig. 4A, C). We observed that transformation of lgl UAS-ci clones could be arrested by sequestration of the Hh ligand by a gain of Ptc (lgl UAS-ci UAS-ptc; Fig. 4F), or by loss of the Dpp receptor, Tkv (lgl tkv 4 UAS-ci, Fig. 4G). Finally, we note that in the haltere or in the leg imaginal discs, too, lgl UAS-ci clones displayed selective neoplastic transformation in only their respective posterior compartments (Fig. S5D and E). Thus, unlike the eye primordium (Fig. 3), Hh-receiving clones in the wing, haltere or leg epithelial primordia transform when the Hh-ligand is endogenously synthesized.
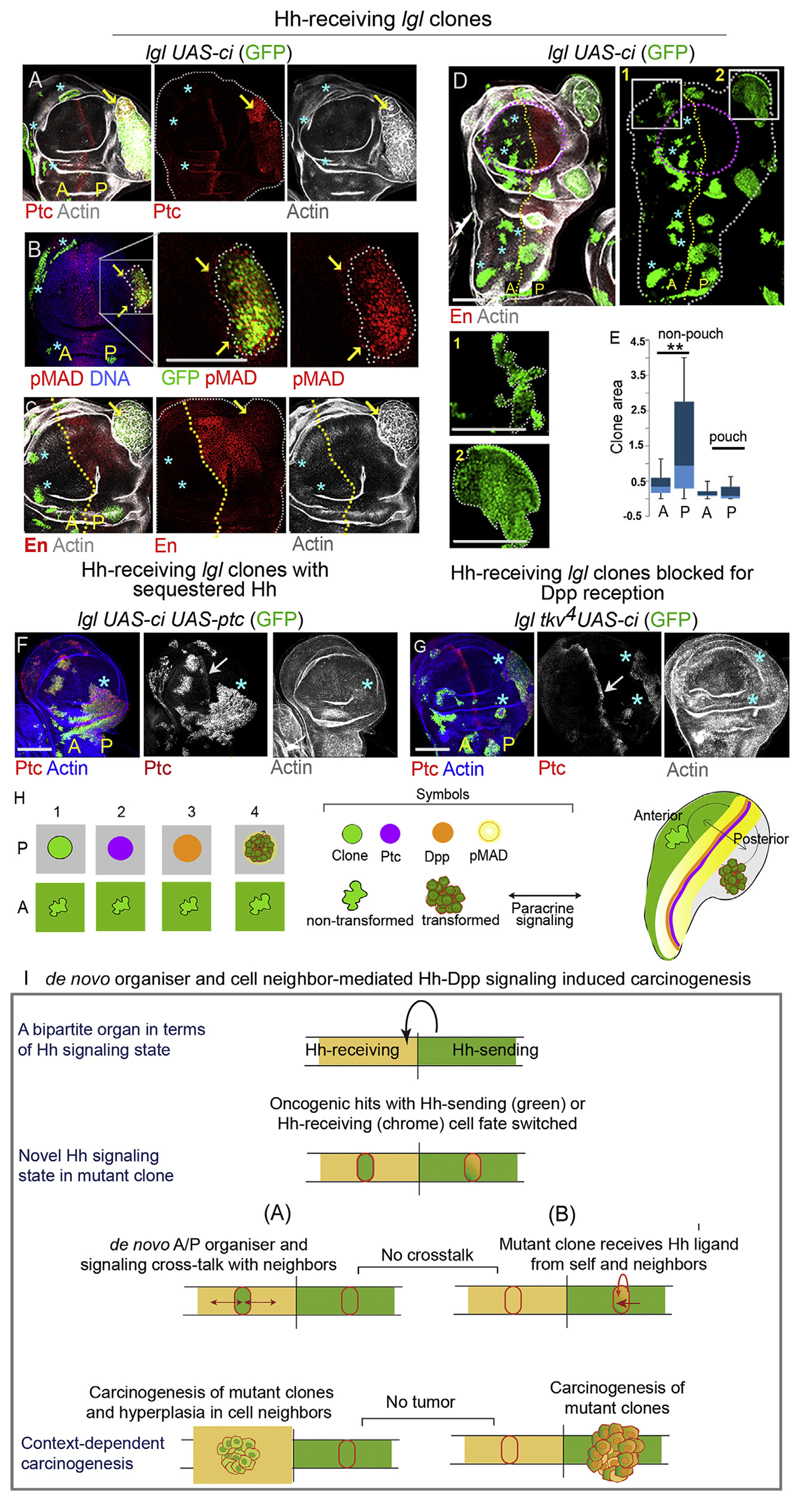
Fig. 4. Hh-receiving lgl mutant clones undergo neoplasia in posterior Hh sending compartment of wing imaginal discs by cell autonomous gain of Hh signaling.
(A-D) lgl UAS-ci clones (GFP) display cell-autonomous gain of Ptc (A, arrow) and pMAD (B, arrow), Boxed region in B displayed at higher magnification on the right. lgl UAS-ci clones display neoplastic transformation (disrupted F-actin, arrows in A and C) in the [P] compartment. In the anterior compartment (stars) they remain untransformed. The lgl UAS-ci clones do not display loss of En (C, arrow). (D) These clones grow better in the [P] compartment and display smooth clone boundary compared to clones in the [A] compartment. Also see magnified view of clones in box 1 and box 2 displayed below. (E) Comparison of lgl UAS-ci clone sizes in the [A] and [P] compartments of wing pouch and non-pouch domains of the wing imaginal discs. Clones in the non-pouch region of the posterior compartment are significantly larger than their anterior counterparts (t-test, P = 0.0002, N = 78); whereas the difference in clone sizes is insigni cant between the two compartments of the wing pouch (t-test, P = 0.156, N = 25). (F) lgl UAS-ci, UAS-ptc clone in the [P] compartment (star) fail to undergo neoplastic transformation (intact F-actin, F). (G) lgl tkv 4 UAS-ci clones blocked for Dpp reception display gain of Ptc (stars) in [P] compartment, but fail to transform (intact F-actin). Arrows in F and G point the endogenous Ptc at the A/P boundary. (H) Schematic representation of developmental context-dependent transformation of lgl UAS-ci clones in the posterior compartment. (I) Schematic summary of context-dependent carcinogenesis via Hh ligand-mediated crosstalks between oncogenically mutated cells and their immediate cell-neighbors. Note that in this model, chance juxtaposition of cell neighbors displaying a complementary Hh signaling state represents the crucial trigger for signaling crosstalks with the lgl mutant clones that drive their tumor progression. Scale bars 100 μm.
3. Conclusion
Cancer is intrinsically linked to perturbations in developmental ho-meostasis (Potter, 1978; Rubin, 2009; Soto et al., 2008). Unraveling these links between developmental homeostasis and onset of cancer thus hold the keys to our understanding of some of the essential principles of carcinogenesis. Our study reveals one such hitherto unrecognized developmental mechanism, which when perturbed can provide trigger for carcinogenesis in the wake of an existing oncogenic lesion. Thus, we reveal how a switch in developmentally conferred compartmental fate results in the formation of de novo organizer thereby triggering runaway neoplasia in oncogenically mutant cells. In this principle of carcinogenesis, tumor cooperation is mediated by the Hh-Dpp cascade via formation of an ectopic A/P organizer (Fig. 4I(A)). On the other hand, switch of oncogenically targeted cells to an Hh-receiving cell state triggers clonal neoplasia in only Hh-sending cell neighborhood (Fig. 4I(B)).Thus, unlike previous instances of direct activation of signaling pathways (Brumby and Richardson, 2003; Chen et al., 2012; Khan et al., 2013; Menendez et al., 2010; Pagliarini and Xu, 2003) or cooperative trigger between two mutations (Wu et al., 2010), our current study reveals a cell fate-induced trigger for tumor cooperation between a tumor and its wild type cell neighbors. Our results therefore uncover a novel crossroad of cancer and developmental mechanisms. Conservation of both essential developmental and cancer mechanisms between fly and mammals also suggest that the developmental organizer-dependent carcinogenesis revealed here is also likely to be conserved. Our findings also address a vexing question as to why all oncogenic hits do not give rise to tumors (Bissell and Hines, 2011; Visvader, 2011). It can be noted that—while cells of both anterior and posterior lineages in the wing primordium are amenable to Hh-driven tumor progression—the nature of the mutational hits and the compartmental fates of the cell neighbors determines their cancerous transformation. Developmental underpinnings of cancer mechanisms revealed here further illustrate these complexities of cancer cells-of-origin.
4. Material and methods
Generation of genetic mosaics
Somatic clones were generated by flp/FRT-flip-out (Xu and Rubin, 1993) or MARCM (Lee and Luo, 2001) technique. Heat shock was given 48 h after egg laying (AEL) at 37°C for 30 min to induce hs-flp–mediated somatic recombination. Imaginal discs were dissected from third instar larvae on day 4 or 5 after heat-shock. For control clones larvae were dissected on day 3 after heat shock.
Immuno-fluorescence staining and microscopy
Briefly, larvae were dissected in 1X PBS, and imaginal discs were fixed in 4% paraformaldehyde and incubated in primary antibody overnight, followed by incubation with fluorescently tagged secondary antibodies, counter-stained with TO-PRO-3 (Invitrogen) and mounted using vectashield anti-fade mounting medium (Vector laboratories). Primary antibodies used were: Mouse anti-Engrailed (1:50; DSHB (Developmental Studies Hybridoma Bank)), Rat anti-Ci (1:50; DSHB), mouse anti-Ptc (1:50, DSHB), Rabbit anti-pSmad (1:200; Cell Signaling Technology), Mouse anti-Wingless (Wg) (1:250; DSHB), Rabbit anti-Vg (1:100; gift from S Carroll); Mouse anti–β-gal (1:500; Sigma Aldrich), Rabbit anti-Caspase 3 (1:1000, Sigma Aldrich); Alexa Fluor 555, 633 secondary antibodies (1:500), Phalloidin-633 (1:100). TO-PRO-3 (Invitrogen, 1:300) was used for nuclear marking, Images were acquired with Leica-SP5 confocal microscope and processed using Leica confocal software-LAS AF and Adobe Photoshop.
Clone Area Analysis
Clone areas were determined using ImageJ software, on projected optical sections that displayed maximum clone territory. Box-plots depicting clone areas were generated using MS Excel. t-test, assuming unequal variance was performed to calculate statistical significance of clones size differences in the [A] and [P] compartments, using MS Excel.
Cell proliferation assay
Cell proliferation was detected by EdU uptake using Click-iT Alexa-Fluor-555 kit by Invitrogen. Briefly, unfixed wing imaginal discs were incubated with100μM of EdU in Schneider’s insect medium, for 1 h at room temperature. Tissue was then fixed in 4% paraformaldehyde and incubated in secondary buffer containing fluorescent-tagged dye (following manufacturer’s instruction), for 1 h at room temperature and subsequently washed in phosphate buffer saline, counter-stained with TO-PRO-3 (Invitrogen) and mounted using anti-fade mountant.
Information regarding the reagents and their sources has been provided in the Key Resource Table.
4.1. Key Resources Table
Information regarding the reagents and their sources has been provided in the Key Resource Table.
| Reagent Or Resource | Source | Identifier |
|---|---|---|
| Anti-Engrailed/Invected | DSHB* | 4D9 |
| Anti-Ci | DSHB | 2A1 |
| Anti-Ptc (extracellular region) | DSHB | Apa 1 |
| Anti-Wg | DSHB | 4D4 |
| Anti-Elav | DSHB | 9F8A9 |
| Anti-pSmad | Cell Signaling Technology | #8828 |
| Anti–β-gal | Sigma-Aldrich | SAB4200805 |
| Anti-Lgl | Gift from Fumio Matsuzaki | |
| Anti-Vg | Gift from Sean Carroll | |
| Alexa Fluor Phalloidin-633 | Invitrogen | A22284 |
| TO-PRO-3 | Invitrogen | S33025 |
| Critical Commercial Assays | ||
| EdU labeling Kit | Invitrogen | Click-iT #C10337 |
| Experimental Models: Transgenic Fly lines | ||
| lgl4FRT40A | BDSCxref * | BDSC#36289 |
| UAS-ptc | BDSC | BDSC#5817 |
| UAS-ykiS168A | BDSC | BDSC#28818 |
| tkv4 | BDSC | BDSC#58786 |
| UAS-ci | Gift from Christian Dahmann, Institute of Genetics, Technische Universität Dresden | |
| UAS-en | Gift from Florence Maschat, Université de Montpellier | |
| Software | ||
| Leica Application Suite, AF, 2.6.0 build | Leica | |
| ImageJ | https://imagej.net/Welcome | |
Developmental Studies Hybridoma Bank, University of Iowa
Bloomington Drosophila Stock Center, Indiana University
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2019.09.011.
Acknowledgements
We thank Christian Dahmann, Florence Maschat and Bloomington Drosophila Stock Centre, for fly stocks; Jonaki Sen and Developmental Studies Hybridoma Bank for antibodies. AB is an Early Career Fellow of the Wellcome Trust/ DBT India Alliance. We acknowledge The Wellcome Trust-DBT India Alliance- Early Career Fellowship (IA/E/13/1/501271 to AB). Research in the laboratory of PS was supported by Department of Biotechnology (DBT/PR14716/BRB/10/876/2011), New Delhi and IIT Kanpur, India.
Footnotes
Authors contribution
AB and PS conceptualized the study, AB conducted the experiments and both AB and PS analysed the data and wrote the manuscript.
References
- Agrawal N, Kango M, Mishra A, Sinha P. Neoplastic transformation and aberrant cell-cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev Biol. 1995;172:218–229. doi: 10.1006/dbio.1995.0017. [DOI] [PubMed] [Google Scholar]
- Barakat MT, Humke EW, Scott MP. Learning from Jekyll to control Hyde: hedgehog signaling in development and cancer. Trends Mol Med. 2010;16:337–348. doi: 10.1016/j.molmed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci U S A. 2012;109:484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K. Opposing transcriptional outputs of Hedgehog signaling and engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell. 2000;100:411–422. doi: 10.1016/s0092-8674(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Brunner M, Hafen E, Basler K. Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science. 1996;272:1621–1625. doi: 10.1126/science.272.5268.1621. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Hafen E. Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye. Genes Dev. 1997;11:3254–3264. doi: 10.1101/gad.11.23.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froldi F, Ziosi M, Garoia F, Pession A, Grzeschik NA, Bellosta P, Strand D, Richardson HE, Grifoni D. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling M, Buller NV, Kirn LM, Joost S, Frings O, Englert B, Bergstrom A, Kuiper RV, Blaas L, Wielenga MC, Almer S, Kuhl AA, Fredlund E, van den Brink GR, Toftgard R. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat Commun. 2016;7 doi: 10.1038/ncomms12321. 12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Gupta RP, Bajpai A, Sinha P. Selector genes display tumor cooperation and inhibition in Drosophila epithelium in a developmental context-dependent manner. Biol Open. 2017;6:1581–1591. doi: 10.1242/bio.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl TA, Scott MP. Wing tips: the wing disc as a platform for studying Hedgehog signaling. Methods. 2014;68:199–206. doi: 10.1016/j.ymeth.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- Hepker J, Wang QT, Motzny CK, Holmgren R, Orenic TV. Drosophila cubitus interruptus forms a negative feedback loop with patched and regulates expression of Hedgehog target genes. Development. 1997;124:549–558. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- Khan SJ, Bajpai A, Alam MA, Gupta RP, Harsh S, Pandey RK, Goel-Bhattacharya S, Nigam A, Mishra A, Sinha P. Epithelial neoplasia in Drosophila entails switch to primitive cell states. Proc Natl Acad Sci U S A. 2013;110:E2163–E2172. doi: 10.1073/pnas.1212513110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J. The many lives of SHH in limb development and evolution. Semin Cell Dev Biol. 2016;49:116–124. doi: 10.1016/j.semcdb.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos C, Edgar BA. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development. 2002;129:1003–1013. doi: 10.1242/dev.129.4.1003. [DOI] [PubMed] [Google Scholar]
- Menendez J, Perez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A. 2010;107:14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Pak E, Segal RA. Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev Cell. 2016;38:333–344. doi: 10.1016/j.devcel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter VR. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br J Canc. 1978;38:1–23. doi: 10.1038/bjc.1978.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rubin H. Rethinking “cancer as a dynamic developmental disorder” a quarter century later. Cancer Res. 2009;69:2171–2175. doi: 10.1158/0008-5472.CAN-08-4213. [DOI] [PubMed] [Google Scholar]
- Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Soto AM, Maffni MV, Sonnenschein C. Neoplasia as development gone awry: the role of endocrine disruptors. Int J Androl. 2008;31:288–293. doi: 10.1111/j.1365-2605.2007.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–2645. doi: 10.1101/gad.6.12b.2635. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- Tamori Y, Suzuki E, Deng WM. Epithelial tumors originate in tumor hotspots, a tissue-intrinsic microenvironment. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002537. e1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–6010. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruizi Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell. 2006;10:719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.