Abstract
Leishmaniasis is a vector borne parasitic disease affecting millions of people worldwide and is spreading into further areas because of global warming. The development of new active substances against these single-cell eukaryotic parasites is of great importance. Leishmania tarentolae promastigotes (LtP) are non-pathogenic for mammals and serve as model organisms for pathogenic Leishmania in basic research. However, it is important to refine methods to study the process of the infection of mammalian macrophages by LtP and pathogenic Leishmania. Important stages of the infection are phagocytosis by macrophages and multiplication of Leishmania amastigotes in the phagolysosome of macrophages. In this study, advanced methods using electron spin resonance (ESR) spectroscopy and genetically manipulated LtP were used to monitor the infection of adherent J774 macrophages with LtP. An ESR method was established to detect the formation of superoxide radicals directly in adherent J774 cells and to investigate the effect of LtP on this activity. J774 cells responded with a burst of superoxide radicals in the presence of phorbol myristate acetate as positive control. In contrast, challenging J774 cells with LtP resulted in a much lower burst of superoxide radicals. To facilitate LtP detection in the phagolysosome of J774 macrophages, LtP expressing enhanced green fluorescent protein (EGFP-LtP) were constructed. After different infection times with EGFP-LtP, the J774 cells were visualized by phase contrast microscopy and the cell number was determined. The intramacrophage Leishmania tarentolae amastigotes (LtA) expressing EGFP were detected by fluorescence microscopy and then counted with ImageJ. These experiments showed that LtP are taken up by J774 cells and form intraphagolysosomal amastigotes. LtA under our conditions multiplied intracellularly and were able to persist about 48 h in J774 cells. These experiments showed that ESR spectroscopy of attached macrophages and the use of the EGFP-LtP are suitable methods to study the initial phase of Leishmania infection in vitro.
Keywords: Leishmania tarentolae, electron spin resonance, superoxide radicals, J774 macrophages
Introduction
Leishmania tarentolae is not pathogenic to humans and naturally infects lizards of the Old World, for example the gecko family Gekkonidae.
Nevertheless, ancient Leishmania tarentolae DNA sequences were found in bone marrow of a 300-years-old human Brazilian mummy (Novo et al., 2015). It is possible that these DNA sequences still circulate in the reptilian reservoir nowadays (Novo et al., 2015).
It was demonstrated that some virulence factors (i.e. GP63), which are well characterized in pathogenic Leishmania, are also expressed in Leishmania tarentolae (Raymond et al., 2012). This is not unexpected since there is a strong conservation of genes in Leishmania tarentolae in comparison to other pathogenic Leishmania and they share numerous genes with each other (Raymond et al., 2012).
Preclinical testing of potential antileishmanial compounds was done in several model systems. One option is to perform first drug screens in promastigotes and axenic amastigotes because they can be easily automatized and allow high-throughput screening (Taylor et al., 2010). A good correlation of IC50 values for drugs in Leishmania tarentolae with the corresponding models in pathogenic Leishmania was observed (Taylor et al., 2010). In parallel, selectivity of drugs is often tested in mammalian monocyte / macrophage cell lines, such as J774, U937, and THP-1 or alternatively in macrophages from mice and humans (Taylor et al., 2010; Geroldinger et al., 2017; Konoiordou et al., 2017). Mammalian drug toxicity is often assayed in HepG2 cells (Osorio et al., 2011) but also in many other mammalian cell types.
Leishmania tarentolae promastigotes and axenic amastigotes were used for screening antileishmanial drugs and compared with Leishmania species infective for humans including Leishmania braziliensis and Leishmania panamensis (Taylor et al., 2010). These studies demonstrated that IC50 values for major drugs, such as antimonials and amphotericin B, more depended on the parasite life stage than on the Leishmania species.
However, in vitro models of macrophages infected with Leishmania are more complex (Hsiao et al., 2011). The infection of macrophages by Leishmania proceeds in two different phases. In a first step the uptake of Leishmania by phagocytosis occurs, which is accompanied by NADPH oxidase activation and superoxide radical production by macrophages (van Assche et al., 2011). Despite the sensitivity of Leishmania to reactive oxygen and nitrogen species parasites can survive this oxidative burst by their antioxidative defense or by downregulation of macrophagal reactive oxygen species production. It was shown that Leishmania can interfere in the assembly of the NADPH oxidase complex in macrophages (Lodge et al., 2006). Whether Leishmania tarentolae are able to interfere in macrophagal phagocytosis is not known to our knowledge.
In the second phase pathogenic Leishmania persist in the phagolysosome of macrophages and start to multiply. For Leishmania tarentolae there have been only few reports that these are taken up by macrophages (Novo et al., 2015), but systematic quantitative characterization was rarely performed (Taylor et al., 2010). Furthermore, it was shown that Leishmania tarentolae were taken up by attached human macrophage U937 cells, where they persisted for 96 h. Bolhassani and coworkers (Bolhassani et al., 2011) demonstrated that the infectivity rate in J774 macrophages with Leishmania tarentolae was lower than those with Leishmania infantum and Leishmania major. However, little is known about the intraphagolysosomal transformation and persistence of Leishmania tarentolae in other macrophage cell lines (Hsiao et al., 2011). On the other hand it has been shown that even infection of J774 macrophages by Leishmania infantum is only successful under optimized conditions (Mendez et al., 1996). Nevertheless, the response of intraphagolysosomal Leishmania amastigotes in their macrophagal environment to drugs is believed to be the most predictive in vitro model. A methodical challenge in these tests is the staining and the time-consuming counting of intracellular amastigotes in infected macrophages. Although methods with GFP-tagged Leishmania have been used, in these studies infected macrophages usually were detached for flow cytometric analysis (Bolhassani et al., 2011), which is a harsh treatment for the cells.
Since we have investigated Leishmania tarentolae and J774 macrophages in previous mechanistic studies separately (Geroldinger et al., 2017), the aim of this work was to establish and apply methods to monitor the superoxide radical production during phagocytosis of Leishmania tarentolae by attached J774 macrophages via electron spin resonance spectroscopy and to verify the transformation and persistence of Leishmania tarentolae amastigotes in J774 cells using EGFP-tagged Leishmania tarentolae promastigotes.
2. Methods
2.1. Chemicals
Diethylenetriaminepentaacetic acid (DTPA), dimethyl sulfoxide (DMSO), glucose, penicillin-streptomycin (PenStrep), phorbol-12-myristate-13-acetate (PMA) were obtained from Merck (Germany). Brain heart infusion (BHI, brain heart broth) medium, formaldehyde, hemin, superoxide dismutase (SOD) were purchased from Sigma (USA). Dihydroethidium (DHE) was from Fluka (Switzerland). Desferal (DFO) was from Novartis Pharma (Germany) and 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-HCl (CMH) was from Noxygen (Germany). KCl, K2HPO4, KH2PO4 and NaCl were from Roth (Germany). Dulbecco’s Modified Eagle Medium (DMEM) was obtained from Gibco and fetal calf serum (FCS, low LPS) from Bio&Sell (Germany). Yeast extract powder was supplied by Amresco (USA). Nourseothricin (NTC) was bought from Jena Bioscience (Germany).
2.2. LtP culture
LtP (LEXSY host strain P10, biosafety level 1, Jena Bioscience) were grown in sterile 50 mL tubes with gas-permeable caps (VWR, Austria) in an incubator (Memmert, Germany) at 26 °C in a tube shaker with agitation (0.05 s-1). They were cultivated in BHI medium (37 g/L) completed with hemin (5 mg/L) and 50,000 U/L penicillin 50 mg/L streptomycin to prevent bacterial contamination. LtP were passaged three times a week.
2.3. Macrophage culture
The macrophage cell line J774A.1 (TIB-67; ATCC, Manassas, VA, USA) was used for all experiments of the present study. J774 cells were grown in DMEM with high glucose (25 mM) and 1.5 g/L NaHCO3, 50,000 U/L penicillin 50 mg/L streptomycin and 10% FCS (heat-inactivated). Cells were grown in 50 mL TPP TubeSpin bioreactor tubes (VWR, Austria) in a roller culture apparatus of own design (5 rpm) in an incubator (Heraeus, Germany) at 37 °C and 5% CO2. The J774 cells were passaged twice a week.
2.4. Counting LtP and J774 cells
The number of LtP per mL was determined by measuring the optical density (OD) at 600 nm with a U1100 photometer (Hitachi, Japan). The cell-free BHI medium completed with hemin and PenStrep was used as a reference. The cell count was calculated from a calibration curve by the following formula: CCell (106/mL) = OD600nm * 0.969 * 124 (Fritsche, 2008). The number of J774 cells per mL was determined with the help of a Neubauer chamber (VWR, Austria) after fixation with 1% formaldehyde.
2.5. Electron spin resonance (ESR) spectroscopy
For cell incubations 24-well plates (polystyrene, flat bottom, non-tissue culture treated, VWR, Austria) were used. Round cover glasses from Thermo Scientific (∅12 mm #1.5, VWR, Austria) were autoclaved, subsequently washed with 70% ethanol and after drying under the laminar flow hood (Heraeus, Thermo Scientific, Austria) placed in each well of the 24-well plate. Aliquots with 0.25×106 J774 cells were added in each well and filled up to 1 mL with DMEM supplemented with PenStrep and FCS. These plates were incubated for 48 h at 37 °C and 5% CO2.
Detection of superoxide radicals by ESR spectroscopy was based on the reaction of these radicals with CMH and formation of the stable nitroxyl radical CM•.
After 48 h the supernatant of one well was removed and adherent J774 cells were washed with 1 mL PBS supplemented with 14 mM glucose. Afterwards the PBS/glucose solution was replaced by 0.5 mL phosphate buffered saline (PBS, 136 mM NaCl, 1.15 mM KH2PO4, 14 mM Na2HPO4, 2.7 mM KCl, pH 7.4)/glucose (14 mM) solution supplemented with 100 μM DFO and 24 μM DTPA. Then, 10 μL CMH stock solution was added to give a final concentration of 400 μM. Depending on the experimental group either 8 μL DMSO vehicle (J774 group), 8 μL PMA giving 5 μM final concentration (J774 + PMA group), 6.25×106 LtP (J774 + LtP group) or the respective volume of LtP-free medium were added to the well. Then, the plate was incubated for 20 min at room temperature. This procedure was repeated subsequently for all 24 wells. Six samples per group were prepared. For ESR measurements of superoxide radical formation in macrophages during phagocytosis the mixture in the well was eliminated, the round cover glass with the attached cells was lifted and the back side of the disc (without cells) was dried by filter paper. As a carrier for ESR measurements a microscope slide (cut from a standard slide to 12 mm width) was used.
To prevent contamination of the ESR resonator, rectangular spacers made of glass (12 × 4 mm) were mounted at both ends of the microscope slide. For manipulation at the upper end of the carrier a stripe of adhesive tape was attached as a handle.
On this carrier, 1.5 μL Milli-Q water was pipetted at 3 cm above the lower end and then the glass disc with the cells facing upwards was placed. After that the carrier with the glass disc and cells was transferred into the quartz dewar insert for TM flat cells (Wilmad-Labglass, SP Industries, USA) located in the resonator (TM 9403, Bruker, Germany) of the ESR spectrometer (EMX spectrometer, Bruker, Germany). The ESR spectra were measured with the following parameters: microwave frequency 9.67 GHz; modulation frequency 100 kHz, sweep width 100 G; power 20.02 mW; scan time 419 s; modulation amplitude 2 G; center field 3422.82 G; time constant 0.8192 s and gain 2*105. For each sample, five consecutive measurements were performed and the peak-to-peak intensity of the middle peak from ESR spectrum of CM• (Fig. 1) was measured by the software ESRView of own design.
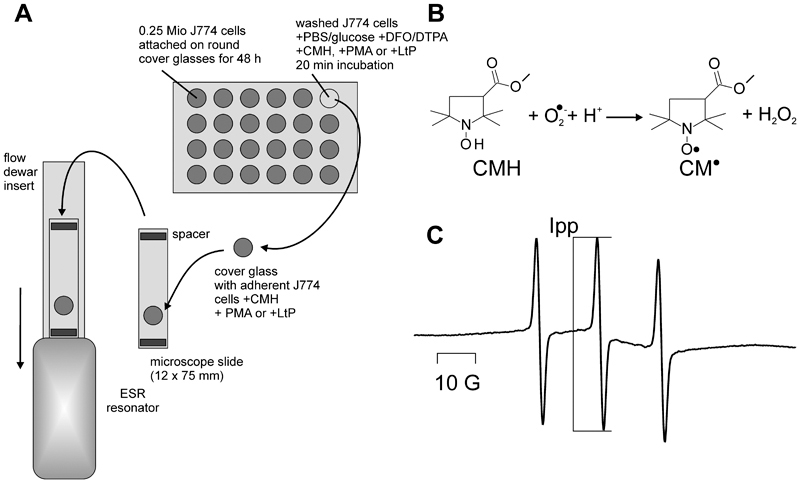
Fig. 1.
Measurement of superoxide radical formation in attached J774 macrophages by ESR spectroscopy. (A) Experimental procedure: Attachment of J774 macrophages to cover glasses in 24-well plates, incubation with different stimuli, transfer of cover glasses to a microscope slide which was then transferred into the resonator for ESR measurement. (B) Reaction of superoxide radicals with the cyclic hydroxylamine CMH under formation of a stable nitroxyl radical. (C) Typical ESR spectrum of the resulting nitroxyl radical CM• obtained in this measurement setup. Ipp indicates the peak-to-peak intensity which was used to quantify CMH conversion to CM• by superoxide radicals.
2.6. DHE assay
Aliquots containing 0.05x106 J774 cells were pipetted in wells of a 96-well microplate (black, untreated, polystyrene, flat bottom, Greiner Bio-One Fluotrac 200 VWR, Austria) and filled up to 200 μL with DMEM. This plate was incubated for 24 h at 37 °C and 5% CO2 in an incubator to allow attachment of J774 macrophages. After 24 h the supernatants of the J774 cells in the 96-well plate were removed and 200 μL PBS were added and distributed by aspiration three times. Then, PBS was replaced by 200 μL PBS/glucose in each well. After that DHE (25 μM), PMA (5 μM), SOD (20 μg/mL) and LtP (1.25×106) were added to the wells where required. Immediately after loading, the 96-well plate was given in the Plate Reader (EnSpire, PerkinElmer, Austria) and kinetics were measured with the following settings: temperature 30 °C, fluorescence mode, top reading, excitation 405 nm, emission 570 nm, delay phase 5 min and measurements 12.
2.7. Engineering of LEXSY expression strains
The LEXSY expression system (pLEXSY-sat2’ Cat.-No. EGE-234, Jena Bioscience, Germany) was designed for constitutive expression of target proteins following integration of the expression cassette into the chromosomal 18S rRNA locus (ssu). The target gene is transcribed constitutively (produced at a steady rate, independent of internal or external stimuli) by a constantly active host RNA polymerase I. The first step was to supply the open reading frame of the target gene with linker sequences, which contain restriction sites for insertion in the vector. For the cytosolic expression of the proteins, the target gene for enhanced green fluorescent protein (eGFP, 720 nt) was PCR-amplified (using a corresponding plasmid containing the gene of interest as a template) and cloned in the pLEXSY-sat2 expression vector (Jena Biosience) using NcoI and NheI restriction sites. EGFP is a fluorescent protein ideal for subcellular labelling and visualization to detect its expression. The preparation of expression plasmid for LEXSY host transfection was done by the digestion of the expression plasmid containing the gene of interest with SwaI restriction enzyme. This treatment generated a small fragment (2.9 kb) representing the E. coli part and a larger fragment (~ 6.7 kb) representing the linearized expression cassette with the target gene to be integrated into the chromosomal ssu locus of the LEXSY host by homologous recombination (double crossover). The linearized expression cassette was isolated using gel extraction Kit Omega (VWR, Austria). This purified expression cassette was used for LEXSY host P10 transfection. For efficient transfection the LtP culture was grown to approximately 274×106 cells/mL in BHI medium. Cells were put on ice for 30 min. Twenty microliters of the gel-purified or spin column-purified DNA fragment of EGFP were put in a sterile 1.5 mL Eppendorf tube and 30 μL Tris-buffer (20 mM, pH 7.4) was added. Then, 350 μL of the LtP suspension was added and the mixture was transferred into electroporation cuvettes (d = 2 mm; Bio-Rad). The electroporation was performed by following the low voltage protocol (exponential decay) with a Bio-Rad Gene Pulser (450 V, 450 μF, 1 pulse, pulse time of 5-6 ms). The transfected cells were then placed on ice for exactly 10 min and subsequently transferred to sterile 6 mL BHI medium supplemented with hemin and PenStrep. The parasites were incubated at 26 °C and after 3 h the LEXSY-NTC selection antibiotic (Jena Bioscience) was added. The EGFP-LtP were handled and counted like the wild type LtP except that NTC (0.1 mg/mL) was added to the culture.
After transfection, genomic DNA was extracted from recombinant and wild-type LtP and the presence and the integration of the expression cassette (of EGFP) into the ssu locus of the LEXSY host was confirmed by diagnostic PCR, using different primer combination (Sat-for F2999/ssu-rev F3002) and (ssu forward F3001/AP reverse primer A1715) as indicated in Jena Bioscience manual for LEXSYcon2.1 kit with pLEXSY-sat2.1. The presence of the EGFP was also confirmed by using EGFP-specific primer pair (plex-egfp_fwd and plex-egfp_rev-2). Used primer sequences were:
| Name | Sequence (5’to 3’) |
| sat forward primer F2999 | CCTAGTATGAAGATTTCGGTGATC |
| ssu reverse primer F3002 | CTGCAGGTTCACCTACAGCTAC |
| ssu forward primer F3001 | GATCTGGTTGATTCTGCCAGTAG |
| aprt reverse primer A1715 | TATTCGTTGTCAGATGGCGCAC |
| plex-gfp_fwd | TGCCACCAGATCTGCCATGGACCATGGTGAGCA |
| plex-gfp_rev2 | GTGGGTACCCTTAAGGCTAGCTTACTTGTACAGCTCGTCC |
2.8. Infection of J774 cells with EGFP-LtP
For these experiments a 24-well plate with a sterile, round cover glass in each well was used. Then, 0.25×106 J774 cells suspended in 500 μL DMEM were placed in each well and incubated for 1.5 h at 37 °C and 5% CO2. After that, unattached J774 cells were removed using 1 mL PBS. Afterwards, 500 μL DMEM was added in each well. The number of EGFP-LtP per mL of a stationary culture (four days after passage) was determined. Then, 6.25×106 EGFP-LtP were given in each well. Control plates with dead EGFP-LtP were made in the same way except that the EGFP-LtP were treated with 10 freeze-thaw cycles before infection of J774 cells.
At five different time points, cover glasses with cells from two wells were photographed. Therefore, the supernatants were aspirated from the wells and the cells were washed twice with 1 mL PBS. After removal of PBS, the round cover glasses with the infected J774 cells were mounted on a microscope slide with the size 75 × 25 mm (VWR, Austria). In a next step 1.5 μL PBS was pipetted on each of the round cover glasses and rectangular cover glasses with the size of 18 × 18 mm (VWR, Austria) were placed on them. Subsequently, the microscope slides were analyzed in the fluorescence microscope (Olympus 1X-71, Japan) and with the 40x objective (Olympus UPlanFLN 40x/0.75 Ph2, ∞/0.17/FN26.5) using a WHN 10x/22 ocular. Then, four positions were chosen and first photographed in the phase contrast mode and subsequently in the fluorescence mode (filter cube position: 2 (GFP, exciter: ET470/40x, beam splitter: 495LP, emitter: ET 525/50m)) at two different focus levels with the instrument software cellSens. Photos were taken with the following setting: exposure time 10-12 s, area full screen, sensitivity ISO 800, exposure compensation -2, live/film 1360x1024, single picture/process 1, live-picture-quality high.
The number of J774 cells in the phase contrast micrographs was counted manually with the help of the Multi Point Tool in ImageJ (Version 2.0.0). The J774 cells which were clipped at the border of the picture were only counted if more than a half of them was displayed. Then, the two fluorescence microscopy pictures were merged with a Python script of own design. Persistent EGFP-LtA (Leishmania tarentolae amastigotes expressing green fluorescent protein) were counted with two macros of own design in ImageJ, which were adjusted to count the EGFP-LtA with a certain size and intensity. Finally, the counts of all four positions from the two round cover glasses were calculated.
2.9. Data analysis and statistics
ESR raw data were analyzed with specific self-designed software ESRView. The infection of J774 cells with EGFP-LtP and the ESR signal intensities were calculated with Microsoft Excel. Graphs were made with Origin 6.1 (OriginLab). Results are shown as mean values ± standard deviation (SD) of data points. To find out whether the differences between experimental groups were significant, Student’s t-test (heteroscedastic, two-tailed) using Microsoft Excel was applied. Differences with p < 0.05 were considered significant.
3. Results
In a first set of experiments, the superoxide radical formation during phagocytic activity of attached J774 macrophages was studied (Fig. 1). After seeding and attachment of J774 cells on glass discs in 24-well plates, cells were washed with PBS/glucose and incubated for 20 min with CMH and a stimulus (PMA or LtP) if required. Subsequently, round cover glasses were transferred to a microscope slide as carrier and inserted into a resonator of an ESR spectrometer (Fig. 1A). Immediately, the data acquisition was started and the spectra of the reaction product of CMH with superoxide radicals were detected (Fig. 1B, C). For quantification of superoxide radical formation the increase of the middle peak-to-peak intensity (Ipp) during five consecutive scans was used.
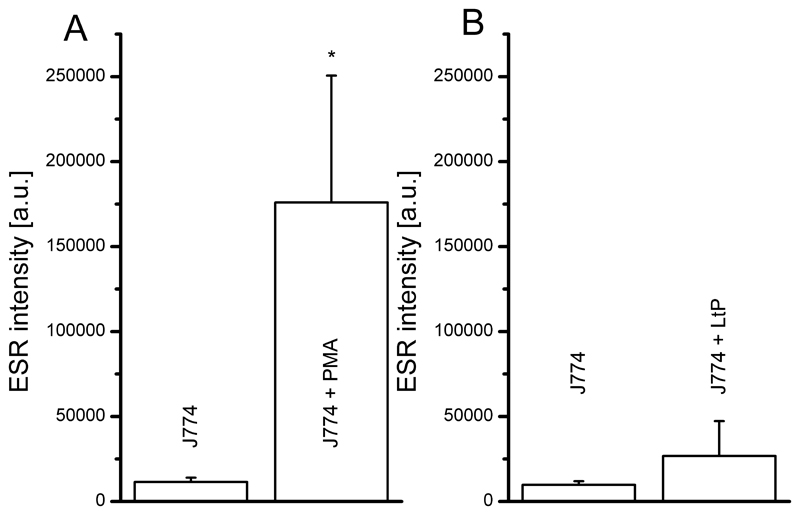
Using this method the superoxide radical formation in J774 cells was stimulated by PMA. As shown in Fig. 2A a more than 15-fold stimulation of radical formation vs. untreated J774 cells was observed. Stimulation of J774 cells by LtP also increased radical formation, but to a much lesser extent (about threefold, Fig. 2B).
Fig. 2.
Superoxide radical formation of attached J774 macrophages (0.25×106 cells seeded per well) upon stimulation with 5 μM PMA (A) or 6.25×106 LtP (B) compared to respective controls assessed by ESR spectroscopy. Data represent mean ± SD of experiments with each 6 wells per group (A, n = 7; B n = 3), * indicates significant differences vs. J774 on the level p < 0.05.
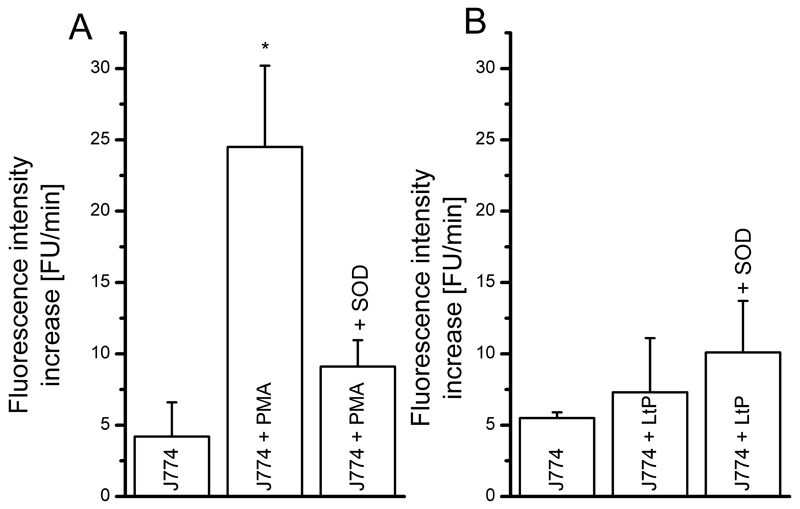
To verify superoxide radical formation under these conditions with an alternative method, we used the less specific fluorescence detection of DHE oxidation products (Fig. 3).
Fig. 3.
Oxidation of DHE (25 μM) as result of superoxide radical formation in J774 macrophages (0.05×106 per well seeded) upon stimulation with 5 μM PMA (A) or 1.25×106 LtP (B) assessed by a fluorescence plate reader. To verify the specificity of the detection method, 20 μg/mL (60 U/mL) SOD were added in the respective experiments. Data represent mean ± SD of 3 experiments (each 6 wells per type), * indicates significant differences vs. J774 on the level p < 0.05.
In this assay PMA triggered an about 6-fold increase of DHE oxidation vs. control cells which was strongly sensitive to SOD. In contrast, LtP triggered only a minor increase in radical formation in J774 cells, which was also not sensitive to SOD. These findings suggest that LtP do not overly trigger J774 superoxide radical formation. This raised the question whether LtP were phagocytized by J774 macrophages at all.
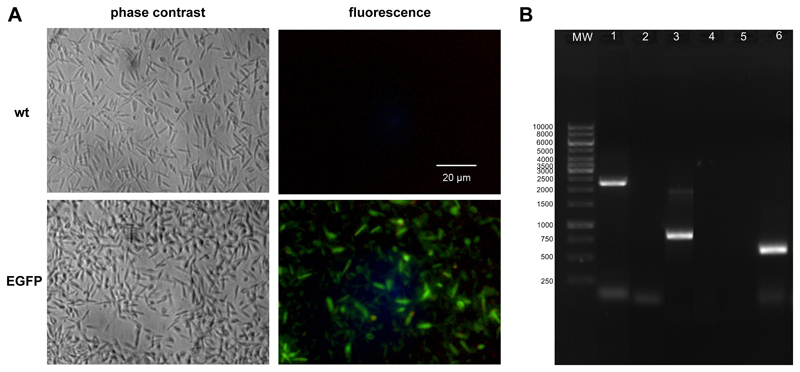
To study this question LtP were transfected with a plasmid containing EGFP obtaining a nourseothricin-in-sensitive strain which constitutively expresses EGFP (Fig. 4). While both strains of LtP are visible in phase contrast micrographs, in fluorescence microscopy only the EGFP strain is detected.
Fig. 4.
Characterization of EGFP-expressing LtP. (A) Micrographs of LtP (P10) in their wild type (wt) and EGFP-transfected form in the phase contrast and fluorescence mode (FITC B-2A filter, Nikon). (B) Agarose gel electrophoresis of the PCR products from transgenic and wt-LtP using different primer combinations: lane 1, EGFP-LtP using F3002/F2999 primer pair; lane 2, wt-LtP negative control using F3002/F2999 primer pair; lane 3, EGFP-LtP using 12 F3001/A1715 primer pair; lane 4, wt-LtP negative control using F3001/A1715 primer pair; lane 5, wt-LtP negative control using EGFP-specific primer; lane 6, EGFP-LtP using EGFP specific primer.
Gel electrophoresis of PCR products using primer pair F2999/ F3002 gives an amplified fragment with about 2.2 kb (Fig. 4B, lanes 1, 2), whereas with primer pair F3001/A1715 the amplified fragment was about 1 kb (Fig. 4B, lanes 3, 4). The expected bands of 2.2 kb and 1 kb, respectively, were only observed from transformed cells indicating the correct integration of the expression constructs into ssu locus. Using an EGFP-specific primer pair, a product of ~ 720 bp was observed in EGFP-LtP, but not in wt-LtP (Fig. 4B, lanes 5, 6).
Green fluorescence in EGFP-LtP is evenly distributed across the whole parasite and, therefore, seems to be suitable to track the fate of LtP in macrophages.
In order to verify whether LtP are phagocytosed / taken up by macrophages at all, we incubated attached J774 cells with either freeze-thawed LtP (inactivated) (Fig. 5) or viable LtP (Fig. 6).
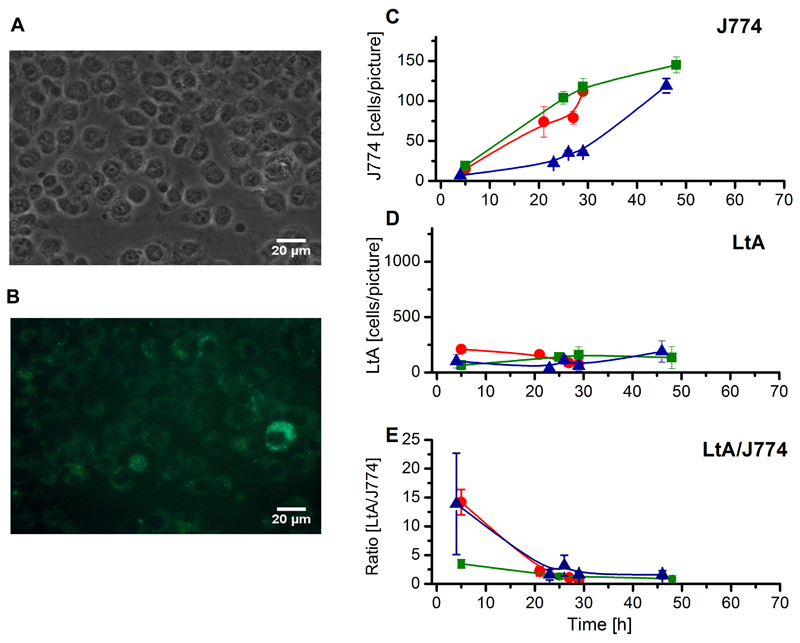
Fig. 5.
Phase contrast and fluorescence images of J774 cells infected with freeze-thawed EGFP-LtP and their quantitative evaluation. (A) Phase contrast and (B) fluorescence images taken 20 h post infection at identical positions. (C) J774 cell number obtained from manual counting. (D) LtA count obtained from ImageJ counting. (E) LtA/J774 cell count ratio. (C-E) Each colored curve represents an independent cell batch and for each data point in the respective colored curves micrographs from four positions of two cover glasses were evaluated. Data represent mean ± SD.
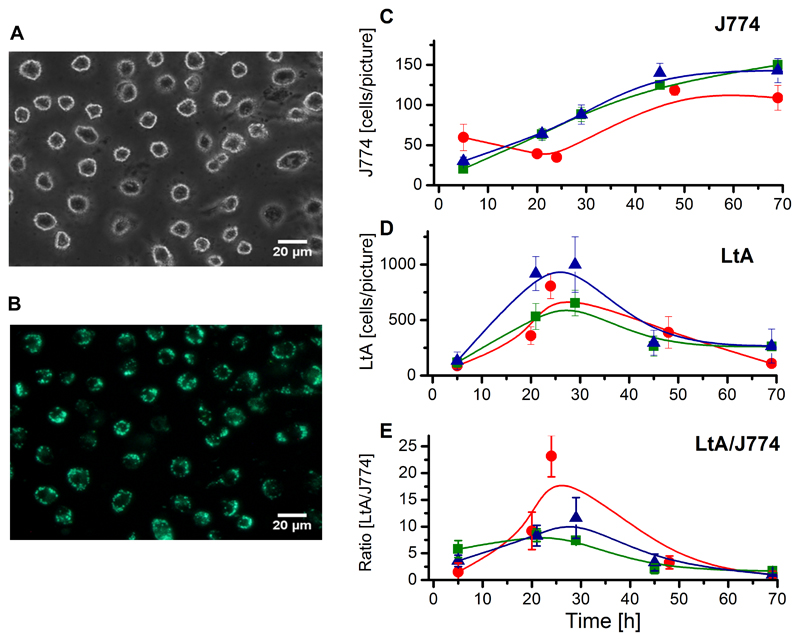
Fig. 6.
Phase contrast and fluorescence images of J774 cells infected with viable EGFP-LtP and their quantitative evaluation. (A) Phase contrast and (B) fluorescence images taken 20 h post infection at identical positions. (C) J774 cell number obtained from manual counting. (D) LtA count obtained from ImageJ counting. (E) LtA/J774 cell count ratio. (C-E) Each colored curve represents an independent cell batch and for each data point in the respective colored curves micrographs from four positions of two cover glasses were evaluated. Data represent mean ± SD. The percentages of infected macrophages were: (red curve) 5 h: 52 ± 12%; 20 h: 80 ± 13%; 24 h: 86 ± 13%; 48 h: 45 ± 18% and 69 h: 20 ± 13%; (green curve) 5 h: 45 ± 9%; 21 h: 77 ± 14%; 29 h: 79 ± 10%; 45 h: 52 ± 16% and 69 h: 53 ± 9%; (blue curve) 5 h: 53 ± 25%; 21 h: 85 ± 5%; 29 h: 49 ± 22%; 45 h: 23 ± 12% and 69 h: 10 ± 5%.
In phase contrast pictures (Fig. 5A, 6A) the number of macrophages was counted manually. As can be seen from quantitative evaluation (Fig. 5C, 6C), J774 cells continuously grow, showing that under both conditions J774 cell growth was comparable. In the fluorescence micrographs (Fig. 5B, 6B), green fluorescence can only arise from LtP or their partially degraded cell components. Internalized LtP and transformed LtA can be recognized by green high intensity spots. Based on this feature quantitative evaluation was done by ImageJ using a cell-counting macro of own design. While the fluorescence pictures of J774 cells incubated with inactivated LtP showed only a mostly diffuse green fluorescence (Fig. 5B), in incubations with living EGFP-LtP (Fig. 6B) numerous high intensity spots are visible inside J774 cells. The absence of flagella and the round shape of these spots suggested the intramacrophagal conversion into LtA. A quantitative comparison shows a maximum of LtA count after about 24 h upon incubation with viable LtP (Fig. 6D), while this is not observed with inactivated LtP (Fig. 5D). Accordingly, the calculated LtA/J774 ratio showed a maximum of around 10 LtA/J774 after about 24 h (Fig. 6E), which is not observed upon incubation with dead LtP (Fig. 5E).
4. Discussion
Leishmania tarentolae is a Leishmania species which is not pathogenic to humans and other mammals. It has a similar genome as pathogenic Leishmania (Leishmania donovani, Leishmania infantum, Leishmania amazonensis and others), shares most proteins and metabolic pathways as well as bioenergetic functions (Azizi et al., 2009; Raymond et al., 2012). All Leishmania have a digenic life cycle as promastigotes in the insect vector and as intracellular amastigote form in host macrophages (Raymond et al., 2012).
In preclinical drug-testing for identification of antileishmanial drugs cellular models include promastigotes, axenic amastigotes and intracellular amastigotes in their macrophage host cells, the latter one being the technically most difficult but most predictive model.
LtP and corresponding axenic amastigotes are currently used as in vitro models (Taylor et al., 2010). That Leishmania tarentolae is also taken up by mammalian macrophages has been shown by Bolhassani et al. (Bolhassani et al., 2011). But phagocytosis and persistence of Leishmania tarentolae in J774 macrophages has not been extensively characterized yet. Phagocytosis by human macrophages was suggested to trigger a burst of oxygen and nitrogen radicals (van Assche et al., 2011). Oxygen radicals are involved in physiological processes such as oxidative phosphorylation in mitochondria and phagocytosis. To which extent Leishmania can trigger a strong burst of oxygen radicals during phagocytosis is still uncertain. The enzyme responsible for this oxidative burst is the NADPH oxidase (NOX2) which is located at the macrophagal cell membrane and the phagolysosomal membrane. In addition, inducible nitric oxide synthase contributes nitrogen radicals to this defense mechanism. However, Leishmania can obviously counteract this flood of oxygen and nitrogen radicals by specific antioxidant systems, such as glutathione and trypanothione (van Assche et al., 2011), or by preventing NADPH oxidase assembly by lipophosphoglycan (LPG) occurring in promastigote membranes (Lodge et al., 2006). Besides these problems the detection of superoxide radicals by fluorescence dyes was criticized in the past to be unspecific and quantitatively unreliable (Zielonka and Kalyanaraman, 2010). In our study the phagocytosis phase was examined for oxygen radical formation. Therefore, ESR spectroscopy (Fig. 2) and a DHE assay (Fig. 3) were applied in order to verify the stimulation of superoxide radical formation during phagocytosis in J774 cells by Leishmania.
An artificial trigger of oxygen radical formation in macrophages is PMA. It is similar to the natural activator diacylglycerol (DAG) and is used to activate the protein kinase C (PKC) (Niedel et al., 1983). P47phox, which is a cytosolic component of NADPH oxidase, enables the production of superoxide radicals via NADPH oxidase after being phosphorylated by activated PKC (Fontayne et al., 2002).
ESR spin trapping is an appropriate method to identify and quantify free radicals.
Abbas and coworkers (Abbas et al., 2015) demonstrated an interesting methodology for measurement of radical production in attached cells by ESR spectroscopy using nitrone spin traps. A disadvantage of this method is that spin trapping with common spin traps, such as 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and related nitrones, has a low efficiency and requires high intracellular spin trap concentrations in the millimolar range (Shi et al., 2005; Dikalov et al., 2011). An alternative approach is the reaction of the cyclic hydroxylamine CMH with superoxide radicals under formation of the ESR-detectable stable nitroxyl radical CM• (Fig. 1). CMH is a cell-permeable hydroxylamine, which is used in biological systems at concentration between 200 and 400 μM.
Although CHM reacts with superoxide radicals by electron transfer and not by covalent binding as for DMPO-like spin traps, it is considered more specific for superoxide radicals (in addition to peroxynitrite) than most methods based on fluorescence dyes, such as dichlorofluorescein diacetate (Kohno, 2010; Kalyanaraman et al., 2012). Likewise, in biological systems a superoxide-dependent and a superoxide-independent oxidation product of DHE are formed side by side. Therefore, fluorescence assays with DHE (without HPLC separation of products) at commonly used wavelength can hardly distinguish both products. Recently, Nazarewicz et al. (Nazarewicz et al., 2013) developed a DHE assay with wavelength pairs, which should be more selective for the superoxide-related DHE oxidation product. However, this new method recommended bottom reading at lower excitation wavelength (405 nm) rather than the DHE standard method (480 nm), which did not work properly in standard clear microplate consumables probably due to autofluorescence. Therefore, we employed top reading of fluorescence in black microplates with the disadvantage that excitation and emission light had to pass the supernatant before detecting DHE changes in macrophagal cell layers. In our study the ESR/CMH method in combination with a microplate-based DHE method was used to ensure validity of superoxide measurement results.
The results of the ESR measurements (Fig. 1) proved that PMA is an effective trigger for oxygen radical formation in J774 macrophages (Fig. 2A). In the DHE assay, PMA also triggered a significant increase of superoxide radical formation, which was abolished by addition of SOD (Fig. 3A). ESR results showed that Leishmania tarentolae was also able to trigger oxygen radical formation in J774 cells (Fig. 2B) but much less than PMA. In contrast, the results of the DHE assay showed only a minor stimulating effect on superoxide radical formation with J774 cells phagocytizing LtP (Fig. 3B). Our data demonstrated that in J774 macrophages, both PMA and LtP trigger an oxidative burst in macrophages, but LtP to a much lesser extent. Likewise for Leishmania donovani it was shown that they are phagocytosed in the absence of strong NADPH oxidase activation (Lodge and Descoteaux, 2006). Mechanistically this could be based on the effect that Leishmania interfere in the assembly of the host macrophagal NADPH oxidase complex by lipophosphoglycanes (Lodge et al., 2006). In addition, it was discussed that Leishmania stimulate their own phagocytosis by macrophages (Hsiao et al., 2011), however, in a rather silent mode. The used model of attached J774 cells during incubation and analysis prevents artifacts of other methods which require detachment of cells prior to analysis, such as flow cytometry.
A major characteristic for pathogenic Leishmania is that they survive and multiply in the host macrophages, thereby hiding from the host immune system. This is partially achieved by manipulation of the host cell signaling and metabolism by Leishmania amastigotes (Kaye and Scott, 2011; Luz et al., 2012). Several but not all virulence factors of pathogenic Leishmania were identified (Felipe et al., 2011).
The usual experimental approach involves the infection of macrophage cell lines by Leishmania and the detection of intracellular amastigotes by stains or fluorescence methods. Commonly used stains to detect intracellular amastigotes are Giemsa stain, Wright Giemsa stain and Diff-Quick stain and require well-trained personal (Sathpathi et al., 2014). These stains are difficult to use for automatic detection in attached macrophages containing Leishmania amastigotes. In the last years the alternative detection of GFP-tagged Leishmania in macrophages by flow cytometry was proposed (Bolhassani et al., 2011). This, however, has the disadvantage that infected macrophages need to be detached prior to flow cytometric analysis.
In our study we created an EGFP-expressing strain of LtP. These EGFP-expressing LtP were used in order to automatize counting of the intracellular GFP-LtA in attached macrophages from phase contrast and fluorescence micrographs. The data show that the system of LtA/J774 macrophages is a suitable model for the phagocytosis of pathogenic Leishmania, but cannot mimic the long-term persistence of these pathogenic Leishmania species. However, this may depend on the experimental conditions as other publications have shown persistence and multiplication of LtA in U937 cells for at least four days (Taylor et al., 2010). According to these results the data support the hypothesis that Leishmania delay phagolysosome maturation (Hsiao et al., 2011), because the highest LtA/J774 ratio with the living Leishmania tarentolae was observed after 24 h (Fig. 6) and the highest ratio of the dead Leishmania tarentolae was after 5 h (Fig. 5). This study has also shown that Leishmania tarentolae can persist 48 h in J774 macrophages, but did not longer survive intracellularly under our conditions. In addition, it has been shown that the selection of the macrophage cell type may influence the amastigotes to macrophages ratio (Seifert et al., 2010). Since cell lines like J774 and RAW 246.7, in contrast to primary macrophages, are still multiplying under in vitro infection conditions (as ours) the outcome of the assay by decreasing the infection index may be influenced (Van den Kerkhof et al., 2018), These experiments demonstrate that the LtP/J774 system is a suitable model for the initial phase of Leishmania infection and can be used to investigate how drugs modulate the phagocytic activity during Leishmania uptake. However, the data also demonstrate that the system Leishmania tarentolae / J774 system is no replacement for in vitro drug screens with pathogenic Leishmania.
In summary, we demonstrated a new method to monitor the superoxide radical detection in attached macrophages during Leishmania infection with an electron spin resonance technique which is more specific for superoxide radicals than many fluorescence dyes. Furthermore, we suggested a new variant how EGFP-tagged Leishmania can be used to monitor infection directly in attached macrophages. By these proof-of-principle experiments we demonstrated that during infection of J774 macrophages by Leishmania tarentolae NADPH oxidases are not fully activated compared to a treatment with phorbol myristate. Furthermore, Leishmania tarentolae was transformed into amastigotes in J774 cells but they did not persist more than 48 in attached J774 macrophages under our conditions. This experimental approach might be useful to study other combinations of Leishmania with macrophages from different cell lines and animals.
Highlights.
Direct analysis of superoxide radical production in attached J774 macrophages during infection with Leishmania tarentolae by electron spin resonance spectroscopy.
Leishmania tarentolae do not provoke a high superoxide radical production during infection of J774 cells.
Construction of an EGFP-expressing Leishmania tarentolae strain and quantitative analysis of J774 infection by EGFP-expressing Leishmania tarentolae using light / fluorescence microscopy and image recognition algorithms.
Acknowledgements
The award of an Ernst Mach scholarship to Lianet Monzote by the Austrian Exchange Office (OEAD) is gratefully acknowledged. Special thanks to the Austrian Science Fund (FWF) for supporting the present study under grant P 27814-B22. In addition we thank Prof. Veronika Sexl for her support.
Abbreviations
- BHI
brain heart infusion
- CHM
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-HCl
- DFO
desferal
- DHE
dihydroethidium
- DMEM
Dubelco’s modified eagle medium
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- DMSO
dimethyl sulfoxide
- DTPA
diethylenetriaminepentaacetic acid
- EGFP
enhanced green fluorescent protein
- ESR
electron spin resonance
- FCS
fetal calf serum
- GSH
glutathione
- LtA
Leishmania tarentolae amastigotes
- LtP
Leishmania tarentolae promastigotes
- NTC
nourseothricin
- OD
optical density
- PBS
phosphate-buffered saline
- PenStrep-penicillin
streptomycin
- PMA
phorbol-12-myristate-13-acetate
- SD
standard deviation
- SOD
superoxide dismutase
- wt
wild type
Footnotes
Conflict of interest
All authors have no conflict of interest to declare.
References
- Abbas K, Hardy M, Poulhes F, Karoui H, Tordo P, Ouari O, Peyrot F. Medium-throughput ESR detection of superoxide production in undetached adherent cells using cyclic nitrone spin traps. Free Radical Research. 2015;49:1122–1128. doi: 10.3109/10715762.2015.1045504. [DOI] [PubMed] [Google Scholar]
- Azizi H, Hassani K, Taslimi Y, Najafabadi HS, Papadopoulou B, Rafati S. Searching for virulence factors in the non-pathogenic parasite to humans Leishmania tarentolae. Parasitology. 2009;136:723–735. doi: 10.1017/S0031182009005873. [DOI] [PubMed] [Google Scholar]
- Bolhassani A, Taheri T, Taslimi Y, Zamanilui S, Zahedifard F, Seyed N, Torkashvand F, Vaziri B, Rafati S. Fluorescent Leishmania species: development of stable GFP expression and its application for in vitro and in vivo studies. Experimental Parasitology. 2011;127:637–645. doi: 10.1016/j.exppara.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Dikalov SI, Kirilyuk IA, Voinov M, Grigorev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radical Research. 2011;45:417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe IM, Aquino DM, Kuppinger O, Santos MD, Rangel ME, Barbosa DS, Barral A, Werneck GL, Caldas AJ. Leishmania infection in humans, dogs and sandflies in a visceral leishmaniasis endemic area in Maranhao, Brazil. Memorias Do Instituto Oswaldo Cruz. 2011;106:207–211. doi: 10.1590/s0074-02762011000200015. [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Fritsche CLE. Untersuchungen zur optimalen Kultivierung von Leishmania tarentolae. Halle-Wittenberg (Germany): Martin-Luther-Universität, (Dissertation); 2008. [Google Scholar]
- Geroldinger G, Tonner M, Hettegger H, Bacher M, Monzote L, Walter M, Staniek K, Rosenau T, Gille L. Mechanism of ascaridole activation in Leishmania. Biochemical Pharmacology. 2017;132:48–62. doi: 10.1016/j.bcp.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Hsiao CHC, Ueno N, Shao JQ, Schroeder KR, Moore KC, Donelson JE, Wilson ME. The effects of macrophage source on the mechanism of phagocytosis and intracellular survival of Leishmania. Microbes and Infection. 2011;13:1033–1044. doi: 10.1016/j.micinf.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biology and Medicine. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nature Reviews Microbiology. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- Kohno M. Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. Journal of Clinical Biochemistry and Nutrition. 2010;47:1–11. doi: 10.3164/jcbn.10-13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoiordou M, Patterson S, Wyllie S, Seifert K. Snapshot profiling of the antileishmanial potency of lead compounds and drug candidates against intracellular Leishmania donovani amastigotes, with a focus on human-derived host cells. Antimicrobial Agents and Chemotherapy. 2017;61:1–7. doi: 10.1128/AAC.01228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge R, Descoteaux A. Phagocytosis of Leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of NADPH oxidase activation. European Journal of Immunology. 2006;36:2735–2744. doi: 10.1002/eji.200636089. [DOI] [PubMed] [Google Scholar]
- Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cellular Microbiology. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Luz NF, Andrade BB, Feijo DF, Araujo-Santos T, Carvalho GQ, Andrade D, Abanades DR, Melo EV, Silva AM, Brodskyn CI, Barral-Netto M, Barral A, Soares RP, Almeida RP, Bozza MT, Borges VM. Heme oxygenase-1 promotes the persistence of Leishmania chagasi infection. Journal of Immunology. 2012;188:4460–4467. doi: 10.4049/jimmunol.1103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez S, Nell M, Alunda JM. Leishmania infantum: infection of macrophages in vitro with promastigotes. International Journal for Parasitology. 1996;26:619–622. doi: 10.1016/0020-7519(96)00037-9. [DOI] [PubMed] [Google Scholar]
- Nazarewicz RR, Bikineyeva A, Dikalov SI. Rapid and specific measurements of superoxide using fluorescence spectroscopy. Journal of Biomolecular Screening. 2013;18:498–503. doi: 10.1177/1087057112468765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol diester receptor copurifies with protein kinase C. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo SP, Leles D, Bianucci R, Araujo A. Leishmania tarentolae molecular signatures in a 300 hundred-years-old human Brazilian mummy. Parasites & Vectors. 2015;8:72. doi: 10.1186/s13071-015-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y, Travi BL, Renslo AR, Peniche AG, Melby PC. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. Plos Neglected Tropical Diseases. 2011;5:e962. doi: 10.1371/journal.pntd.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond F, Boisvert S, Roy G, Ritt JF, Legare D, Isnard A, Stanke M, Olivier M, Tremblay MJ, Papadopoulou B, Ouellette M, Corbeil J. Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Research. 2012;40:1131–1147. doi: 10.1093/nar/gkr834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathpathi S, Mohanty AK, Satpathi P, Mishra SK, Behera PK, Patel G, Dondorp AM. Comparing Leishman and Giemsa staining for the assessment of peripheral blood smear preparations in a malaria-endemic region in India. Malaria Journal. 2014;13:512. doi: 10.1186/1475-2875-13-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K, Escobar P, Croft SL. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. Journal of Antimicrobial Chemotherapy. 2010;65:508–511. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- Shi HL, Timmins G, Monske M, Burdick A, Kalyanaraman B, Liu Y, Clement JL, Burchiel S, Liu KJ. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Archives of Biochemistry and Biophysics. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Taylor VM, Munoz DL, Cedeno DL, Velez ID, Jones MA, Robledo SM. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Experimental Parasitology. 2010;126:471–475. doi: 10.1016/j.exppara.2010.05.016. [DOI] [PubMed] [Google Scholar]
- van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radical Biology and Medicine. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Van den Kerkhof M, Van Bockstal L, Gielis JF, Delputte P, Cos P, Maes L, Caljon G, Hendrickx S. Impact of primary mouse macrophage cell types on Leishmania infection and in vitro drug susceptibility. Parasitology Research. 2018;117:3601–3612. doi: 10.1007/s00436-018-6059-4. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radical Biology and Medicine. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]