Abstract
Background
The first cases of avian influenza A (H5N1) in humans in Vietnam were detected in early 2004, and Vietnam has reported the second highest number of cases globally.
Methods
We obtained retrospective clinical data through review of medical records for laboratory confirmed cases of influenza A (H5N1) infection diagnosed in Vietnam from January 2004 through December 2006. Standard data was abstracted regarding clinical and laboratory features, treatment, and outcome.
Results
Data were obtained for 67 (72%) of 93 cases diagnosed in Vietnam over the study period. Patients presented to the hospital after a median duration of illness of 6 days with fever (75%), cough (89%), and dyspnea (81%). Diarrhea and mucosal bleeding at presentation were more common in fatal than in nonfatal cases. Common findings were bilateral pulmonary infiltrates on chest radiograph (72%), lymphopenia (73%), and increased serum transaminase levels (aspartate aminotransferase, 69%; alanine aminotransferase, 61%). Twenty-six patients died (case fatality rate, 39%; 95% confidence interval, 27%–51%) and the most reliable predictor of a fatal outcome was the presence of both neutropenia and raised alanine aminotransferase level at admission, which correctly predicted 91% of deaths and 82% of survivals. The risk of death was higher among persons aged ≤16 years, compared with older persons (P< .001), and the risk of death was higher among patients who did not receive oseltamivir treatment (P = .048). The benefit of oseltamivir treatment remained after controlling for potential confounding by 1 measure of severity (odds ratio, 0.15; 95% confidence interval, 0.026–0.893; P = .034).
Conclusion
In cases of infection with Influenza A (H5N1), the presence of both neutropenia and raised serum transaminase levels predicts a poor outcome. Oseltamivir treatment shows benefit, but treatment with corticoste-roids is associated with an increased risk of death.
The re-emergence of cases of avian influenza A (H5N1) infection in humans in December 2003 was first detected when a series of children were admitted to the National Pediatric Hospital in Hanoi, Vietnam, with severe viral pneumonia of unknown etiology [1]. Since then, the clinical understanding of H5N1 infections in humans has increased but remains constrained by the relatively small number of cases, their widespread geographical distribution, and their occurrence in locations with limited resources. The World Health Organization has successfully pooled information from a range of sources to produce useful summaries of the clinical aspects of H5N1 infection, but these initiatives have been hampered by lack of access to disaggregated data, making detailed analysis impossible [2, 3].
To better describe the clinical features of human H5N1 infection, the progression of disease, the effectiveness of treatment, and the factors associated with death, we undertook a retrospective case note review of laboratory-confirmed cases of influenza A (H5N1) infection in Vietnam from 1 January 2004 through 31 December 2006.
Patients and Methods
The study population included all hospitalized patients with laboratory-confirmed influenza A (H5N1) infection diagnosed from 1 January 2004 through 31 December 2006. A case patient was defined as a patient with clinical illness who was admitted to hospital with a suspected diagnosis of H5N1 infection and for whom either influenza A (H5N1) RNA was detected in a clinical specimen with use of reverse-transcriptase polymerase chain reaction (RT-PCR) or influenza A (H5N1) virus was isolated. Clinical data for confirmed cases were abstracted from original medical records with use of a data abstraction sheet.
Clinical data for 10 of these cases have been previously published, but the data presented here are based on new abstraction and analysis [1, 4]. The study was approved by the Science and Ethics Committee of the Ministry of Science and Technology of Vietnam.
Virologic investigations
Influenza A (H5N1) subtype-specific RNA was detected in clinical samples by RT-PCR that used primers targeting regions of the hemagglutinin gene of the influenza H5N1 virus; the primers were developed by the World Health Organization, the US Centers for Disease Control and Prevention, and the Government Virus Unit of Hong Kong, as described elsewhere [1].
Clinical specimens were inoculated onto monolayers of Madin-Darby canine kidney cells for virus isolation. Virus isolates were identified by RT-PCR and hemagglutination inhibition assay with use of reference antiserum to influenza A/Duck/HK/820/80 (H5N3).
Statistical analysis
Vital signs and laboratory values were dichotomized into normal or abnormal on the basis of normal ranges for children and adults (table 1). Children were defined as those aged ≤16 years.
Table 1. Age-specific definitions used for clinical parameters.
| Age, years |
Tachypnea, respiratory rate, breaths/min |
Tachycardia, pulse rate, beats/min |
Hypotension, systolic blood pressure, mm Hg |
|---|---|---|---|
| 0–4 | >30 | >130 | ≤70 |
| 5–9 | >24 | >115 | ≤80 |
| 10–16 | >22 | >100 | ≤90 |
| >16 | >20 | >100 | ≤100 |
NOTE. Adapted from [5]. Definitions used to classify laboratory parameters: hypoxemia SaO2, ≤ 90% on room air; lymphopenia, <1500 lymphocytes/mm3; leukopenia, <4000 leukocytes/mm3; thrombocytopenia, <150,000 platelets/mm3; neutropenia, <2200 neutrophils/mm3; neutrophilia, >8250 neutro-phils/mm3; raised aspartate aminotransferase level, >40 U/L; raised alanine aminotransferase level, >37 U/L; raised serum creatinine level, >120 μmol/L; raised blood urea level, >7.5 mmol/L.
Continuous variables were tested for normal distribution with use of the Shapiro-Wilk test, and if they did not follow a normal distribution, they were summarized using medians and ranges. The Wilcoxon rank-sum test was used to compare continuous data. Categorical variables were summarized as proportions with exact binomial 95% confidence intervals (CIs). Pearson’s 2-sided x2 test or, if the expected count in any cell was <5, Fisher’s exact test were used to compare binary data. The Kaplan-Meier product-limit method was used to analyze patient survival. Patients who were discharged from the hospital because they were cured were assumed to survive after discharge. The log-rank test was used to compare survival distributions.
To identify patient characteristics that predicted death, multivariable logistic regression was performed. All variables with a P value of ≤.05 in the univariable analysis were considered for inclusion in the initial multivariable model. Colinearity of these variables was first assessed by generating a correlation coefficient matrix. A backward step-wise variable selection strategy was used to construct a final model, with a significance level of >.1 for removal and <.05 for re-entry.
Results
Demographic and epidemiological findings
Information was retrieved for 67 (72%) of 93 cases of human H5N1 infection diagnosed in Vietnam from January 2004 through December 2006. Demographic, epidemiological, clinical, and laboratory characteristics of the patients at admission are shown in table 2. The median age of patients was 25 years, which is similar (P = .19) to the median age (23 years) of patients involved in all 93 cases that were diagnosed in Vietnam during the study period. Exploratory comparisons between male and female patients did not show any statistically significant differences; therefore, data were aggregated by sex for all subsequent analyses.
Table 2. Demographic, epidemiological, clinical, and laboratory characteristics at hospital admission of patients with influenza A (H5N1) infection.
| Variable | All (n = 67) |
Nonfatal cases (n = 41) |
Fatal cases (n = 26) |
P |
|---|---|---|---|---|
| Age, median years (IQR) | 25 (16–42) | 30 (21–50) | 18 (10–30) | .002a |
| Male sex | 37/67 (55) [43–67] | 23/41 (56) [40–71] | 14/26 (54) [33–73] | .86b |
| Exposure to potential sources of H5N1 during 7 days before illness onset | ||||
| Direct contact with sick poultry | 24/67 (36) [24–18] | 14/41 (34) [20–51] | 10/26 (38) [20–59] | .72b |
| Direct contact with person with respiratory illness | 9/67 (13) [6–24] | 6/41 (15) [6–29] | 3/26 (11) [2–30] | >.99c |
| Visited poultry outbreak area | 13/67 (19) [11–31] | 6/41 (15) [6–29] | 7/26 (27) [12–48] | .21b |
| No known exposure | 21/67 (31) [20–14] | 15/41 (37) [22–53] | 6/26 (23) [9–44] | .24b |
| Time from onset of symptoms to hospitalization, median days (range) | 6 (1–2C) | 6 (1–20) | 6 (2–10) | .54a |
| Duration of hospitalization, median days (range) | 11 (1–81) | 16 (2–81) | 5 (1–15) | <.001a |
| Symptoms | ||||
| Cough | 6C/67 (89) [82–97] | 35/41 (85) [74–97] | 25/26 (96) [88–100] | .23c |
| Diarrhea | 7/67 (1C) [3–18] | 1/41 (2) [0–7] | 6/26 (23) [6–40] | .012c |
| Chest pain | 22/65 (34) [22–16] | 14/40 (35) [19–50] | 8/25 (32) [12–52] | .8b |
| Signs | ||||
| Body temperature 338≥C | 5C/67 (75) [64–85] | 27/41 (66) [55–81] | 23/26 (88) [75–100] | .038b |
| Tachypnea | 54/67 (81) [71–9C] | 30/41 (73) [59–87] | 24/26 (92) [81–100] | .054b |
| Tachycardia | 28/67 (42) [3C–54] | 11/41 (27) [13–41] | 17/26 (65) [46–85] | .002b |
| Sa02, mean % (range) on room air | 85 (3C–99) | 90 (50–99) | 78 (30–98) | <.004d |
| Hypoxemia | 27/59 (46) [33–59] | 10/33 (30) [14–47] | 17/26 (65) [46–85] | .007b |
| Hypotension | 19/66 (29) [17–4C] | 8/40 (20) [7–33] | 11/26 (42) [22–63] | .05b |
| Mucosal bleeding | 5/67 (7) [1–14] | 0/41 (0) | 5/26 (19) [3–35] | .007c |
| Hepatomegaly | 13/66 (2C) [1C–29] | 6/40 (15) [3–27] | 7/26 (27) [9–45] | .23b |
| Rales on auscultation | 55/67 (82) [73–91] | 33/41 (80) [68–93] | 22/26 (85) [70–99] | .75c |
| Pulmonary infiltrates on chest radiograph | 54/64 (84) [75–93] | 33/40 (82) [70–95] | 21/24 (87) [73–100] | .73c |
| Blood indices | ||||
| Hemoglobin level, median g/dL | 12.8 | 12.7 | 12.8 | .77a |
| Leukopenia | 29/65 (45) [32–57] | 10/41 (24) [11–38] | 19/24 (79) [62–97] | <.001b |
| Lymphopenia | 46/63 (73) [62–84] | 26/41 (63) [48–79] | 20/22 (91) [78–100] | .019b |
| Neutropenia | 19/63 (30) [18–42] | 5/41 (12) [2–23] | 14/22 (64) [42–85] | <.001b |
| Neutrophilia | 19/63 (3C) [18–42] | 16/41 (39) [23–55] | 3/22 (14) [0–29] | .036b |
| Thrombocytopenia | 30/65 (46) [34–59] | 11/41 (27) [13–41] | 19/24 (79) [62–97] | <.001b |
| Aspartate aminotransferase level >40 U/L | 36/52 (69) [56–82] | 20/33 (61) [43–78] | 16/19 (84) [66–100] | .08b |
| Alanine aminotransferase level >37 U/L | 32/52 (61) [48–75] | 15/33 (45) [27–63] | 17/19 (89) [74–100] | .002b |
| Serum urea level >7.5 mmol/L | 13/59 (22) [12–35] | 7/35 (20) [8–37] | 6/24 (25) [10–47] | .65b |
| Serum creatinine level >120 mmol/L | 4/47 (8) [2–20] | 2/25 (8) [1–26] | 2/22 (9) [1–29] | >.99c |
| Therapy | ||||
| Mechanical ventilation | 30/66 (45) [33–58] | 4/40 (10) [3–24] | 26/26 (100) [87–100] | <.001b |
| Oseltamivir | 55/67 (82) [71–90] | 37/41 (90) [77–97] | 18/26 (69) [48–86] | .048c |
| Duration of oseltamivir treatment, median days | 5 | 5 | 2 | .002a |
| Vasopressors | 9/28 (32) [16–52] | 1/13 (8) [0–36] | 8/15 (53) [27–79] | .016c |
| Corticosteriods | 29/67 (43) [31–56] | 12/41 (29) [16–45] | 17/26 (65) [44–83] | .004c |
| Complications | ||||
| Development of increased neutrophil counte | 9/26 (35) [17–56] | 4/13 (31) [9–61] | 5/13 (38) [14–68] | >.99c |
| Pleural effusion | 13/67 (19) [11–31] | 5/41 (12) [4–26] | 8/26 (31) [14–52] | .06b |
| Pneumothorax | 6/67 (9) [3–18] | 2/41 (5) [1–16] | 4/26 (15) [4–35] | .20c |
NOTE. Data are proportion (%) [95% confidence interval] of patients, unless otherwise indicated. IQR, interquartile range. Hypoxemia, SaO2 ≤90% on room air; leukopenia, <4000 leukocytes/mm3; lymphopenia, <1500 lymphocytes/mm3; neutropenia, <2200 neutrophils/mm3; neutrophilia, >8250 neutrophils/mm3; thrombocytopenia, <150,000 platelets/mm3
Wilcoxon rank-sum test.
Pearson’s 2-sided x2 test.
Fisher’s exact test.
Independent samples t test.
Nonelevated neutrophil count at the time of admission but development of a count >8250 neutrophils/mm3 any time during the 6 days after hospital admission in subjects for whom serial blood count results were available. Excludes patients who received granulocyte macrophage colony-stimulating factor.
The most common exposure recorded in the clinical notes in the 7 days prior to illness onset was direct contact with sick poultry, but this exposure was recorded for only 24 (36%) of 67 cases, whereas for 21 (31%) of 67 cases, there was no record of an exposure to sick poultry or sick humans (table 2). Exposure to a person with a respiratory illness was recorded for 9 (13%) of 67 cases. In 4 of these 9 cases, the exposure was with another person with a confirmed case of H5N1 infection, and 1 of the 4 patients involved was a health care worker. However, insufficient data were available to draw conclusions about the possibility of person-to-person transmission in these cases. Data on the interval between exposure to potential sources of H5N1 viruses and onset of symptoms (the incubation period) were not available from the clinical records. Patients presented to the hospital after a median duration of illness of 6 days (range, 1–20 days).
Clinical findings on admission
At initial clinical assessment, the most common findings were fever (temperature, ≥38°C), tachypnea, and rales on auscultation of the lungs. The mean oxygen saturation with room air was 85%, with 27 (46%) of 59 patients having an oxygen saturation ≤90°/o. The median pulse rate was 100 beats/min, and the median systolic blood pressure was 110 mm Hg (range, 70–150 mm Hg). Applying age-specific normal ranges (table 1), 28 (42%) of 67 patients were tachycardic, and 19 (29%) of 66 patients were hypotensive. Chest radiographs taken on admission showed pulmonary infiltrates in 54 (84%) of 64 patients, of which 15 (28%) of 54 were unilateral focal, 14 (26%) of 54 were bilateral focal, and 25 (46%) of 54 were bilateral diffuse. On admission, 9 patients had a pleural effusion, and 1 nonventilated patient had bilateral pneumothoraces. Signs of mucosal bleeding were observed in 5 patients (1 adult with vaginal bleeding, 1 adult with bloody stool, and 3 children with gum bleeding and bleeding from venepuncture sites). One of these patients received a diagnosis of disseminated intravascular coagulation on the day of hospital admission, and another received the same diagnosis on day 3 after hospital admission; both died.
Median total leukocyte counts and lymphocyte counts were 5600 cells/mm3 (range, 300–19,900 cells/mm3) and 1173 cells/mm3 (range, 129–8,410 cells/mm3), respectively. The median platelet count was 174,000 platelets/mm3 (range, 14,000–520,000 platelets/mm3). Absolute lymphopenia (lymphocyte count <1500 cells/mm3) was the most common hematological abnormality, and was noted for 46 (73%) of 63 cases. Elevated serum transaminase levels were also a common finding.
Differences on admission between fatal and nonfatal cases
There were 26 fatalities among the 67 patients (39%; 95% CI, 27%–51%). The median duration between onset of illness and presentation to the hospital did not vary significantly between patients who died and those who survived. Patients with fatal cases were hospitalized for a median of 5 days (range, 1–15 days) before death, whereas those who survived were hospitalized for a median of 16 days (range, 2–81 days) before discharge.
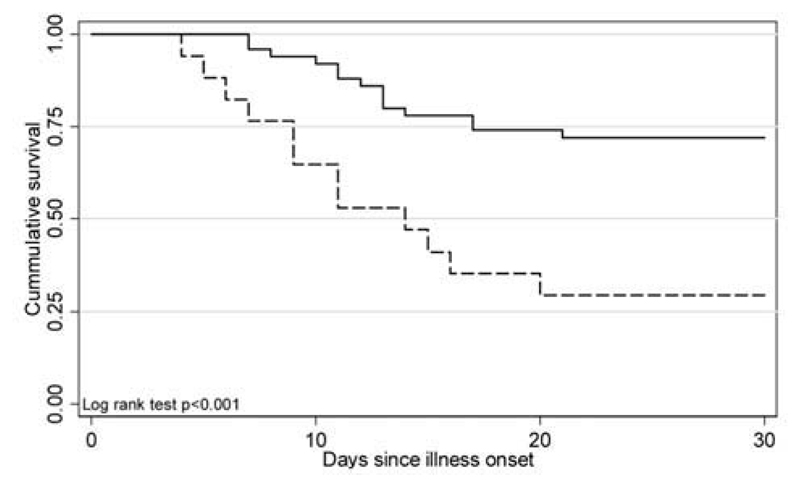
Patients with fatal cases were, on average, younger than those who survived (P= .002). The survival curve for persons aged ≤ 16 years, compared with older patients, demonstrates evidence for a survival difference between these 2 groups (P < .001) (figure 1).
Figure 1.
Survival plot for cases involving patients aged ≤16 years (dashed line), compared with cases involving patients aged >16 years (solid line), by duration since onset of illness.
Diarrhea was a more common presenting complaint in patients who died, compared with patients who survived, and 6 of 7 patients who had diarrhea died. On hospital admission, patients with fatal cases were more likely than patients with nonfatal cases to have a temperature ≥38°C, be tachycardic, be hypotensive, and have an oxygen saturation level on room air of ≤90%.
Blood tests on hospital admission showed that patients who subsequently died were more likely to be leukopenic, lymphopenic, neutropenic, and thrombocytopenic, compared with patients who survived. Thirteen (50%) of 26 patients who died were lymphopenic, neutropenic, and thrombocytopenic on hospital admission, compared with only 4 (10%) of 41 patients who survived. Elevated alanine aminotransferase (ALT) levels was a more common finding in fatal cases than in nonfatal cases.
Eleven variables with a P value ≤.05 were considered for inclusion in the logistic regression model. Leukopenia was excluded because of colinearity with neutropenia. The final model retained only neutropenia (odds ratio [OR], 8.97; P = .008) and elevated ALT (OR, 5.78; P= .057). Data on both neutrophil and ALT levels at hospital admission were available for 50 patients; in this subset, 10 of 11 patients with both neutropenia and increased ALT levels died (positive predictive value, 91%), and 32 of 39 patients who did not have both neutropenia and increased ALT levels survived (negative predictive value, 82%).
Treatment
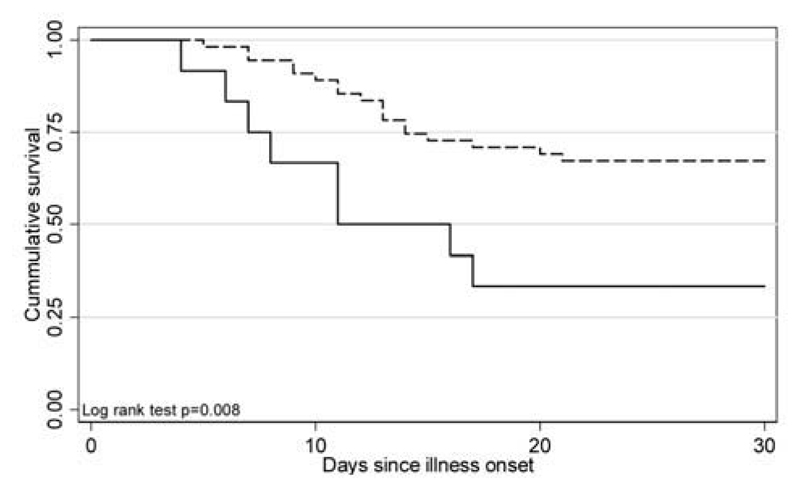
Oseltamivir was administered in 55 (82%) of 67 cases and was more commonly given to survivors than to those who died (P= .048). Figure 2 shows survival curves for those who did or did not receive treatment with oseltamivir (P= .008). Because both the likelihood of survival and the likelihood of receiving oseltamivir treatment decreased with age, a stratified analysis was performed to look for evidence of confounding or effect modification by age (table 3). The Mantel-Haenszel summary OR is still suggestive of a protective effect of oseltamivir treatment, but the difference is less pronounced than the difference in crude OR (0.39 vs. 0.24) and is not statistically significant. There was no evidence that age modified the effect of oseltamivir treatment on outcome (P = .62, by Breslow-Day test of homogeneity of OR). A stratified analysis of the effect of oseltamivir treatment on outcome after controlling for possible confounding by the presence or absence of neutropenia at hospital admission (as a marker of severity) still found evidence of a beneficial effect of oseltamivir treatment (Mantel-Haenszel summary OR, 0.15; 95% CI, 0.0260–893; P = .034). Two children received intravenous ribavirin for 3 days and 2 days, respectively, but not oseltamivir, because it was unavailable at that time; both patients died.
Figure 2.
Survival plot for cases in which patients received oseltamivir (dashed line), compared with cases in which patients did not receive oseltamivir (solid line), by duration since onset of illness (censored at day 30, because all deaths had occurred by day 21 after illness onset).
Table 3. Analysis of relationship between oseltamivir treatment and outcome, stratified by age group.
| Variable | Survived | Died | OR (9S% CI) | P a |
|---|---|---|---|---|
| All ages | ||||
| Oseltamivir not given | 4 | 8 | 0.24 (0.065–0.916) | .048 |
| Oseltamivir given | 37 | 18 | … | |
| Aged <16 years | ||||
| Oseltamivir not given | 1 | 6 | 0.25 (0.021–2.94) | .34 |
| Oseltamivir given | 4 | 6 | … | |
| Aged >16 years | ||||
| Oseltamivir not given | 3 | 2 | 0.54 (0.081–3.67) | .61 |
| Oseltamivir given | 33 | 12 | … | |
| Overallb | … | … | 0.39 (0.09–1.71) | .21 |
NOTE. P = .62, by the Breslow-Day test of homogeneity of odds ratio (OR). CI, confidence interval.
Fisher’s exact test.
Mantel-Haenszel summary OR.
All but 4 patients were treated with intravenous antibiotics, most commonly a third-generation cephalosporin plus either levofloxacin, gatifloxacin, or amikacin. Treatment with gluco-corticoids (methylprednisolone, 1–3 mg/kg/day for up to 7 days) was administered to 29 (43%) of 67 patients and was more commonly given to patients who died than survivors (65% vs. 29%; P = .004). A stratified analysis of the effect of steroid treatment on outcome, after controlling for possible confounding by the presence or absence of neutropenia at admission (as a marker of severity), still found evidence of an increased risk of death (Mantel-Haenszel summary OR, 4.11; 95% CI, 1.14–14.83; P = .027).
Six pediatric patients received treatment with granulocyte macrophage colony-stimulating factor (GMCSF). An increase in the white blood cell and platelet counts after GMCSF treatment was observed for all 5 patients for which serial results were available, but 5 of the 6 patients died (table 4).
Table 4. Patients with influenza A (H5N1) infection who received treatment with granulocyte macrophage colony-stimulating factor (GMCSF).
| Day after hospital admission | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient, variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1: male, aged 1 year | ||||||||||
| Received GMCSF 0.5 MIU/kg | … | Yes | Yes | Yes | … | … | … | … | … | … |
| Neutrophil count, cells/mm3 | 406 | 1705 | 2520 | 6720 | … | … | … | … | … | … |
| Platelet count, platelets/mm3 | 31,000 | 95,000 | 38,000 | 58,000 | … | … | … | … | … | … |
| Death | … | … | … | Died | … | … | … | … | … | … |
| 2: female, aged 8 years | ||||||||||
| ReceivedGMCSF0.5MIU/kg | … | Yes | … | … | … | … | … | … | … | … |
| Neutrophil count, cells/mm3 | 290 | 798 | 15,040 | … | … | … | … | … | … | … |
| Platelet count, platelets/mm3 | 81,000 | 162,000 | 269,000 | … | … | … | … | … | … | … |
| Death | … | … | Died | … | … | … | … | … | … | … |
| 3: female, aged 10 years | ||||||||||
| Received GMCSF 0.5 MIU/kg | … | … | … | … | Yes | … | … | … | … | … |
| Neutrophil count, cells/mm3 | 1904 | 1421 | 2701 | 2448 | 5395 | 21,760 | … | … | … | … |
| Platelet count, platelets/mm3 | 13,500 | 143,000 | 144,000 | 130,000 | 151,000 | 245,000 | … | … | … | … |
| Death | … | … | … | … | … | … | Died | … | … | … |
| 4: male, aged 4 years | ||||||||||
| Received GMCSF 0.5 MIU/kg | … | … | … | … | Yes | Yes | Yes | … | … | … |
| Neutrophil count, cells/mm3 | 1081 | 476 | 1976 | … | … | … | 7344 | 9040 | … | … |
| Platelet count, platelets/mm3 | 15,000 | 200,000 | 79,000 | … | … | … | 121,000 | 344,000 | … | … |
| Death | … | … | … | … | … | … | … | … | Died | … |
| 5: female, aged 1 year | ||||||||||
| ReceivedGMCSF1MIU/kg | Yes | … | … | … | … | … | … | … | … | … |
| Neutrophil count, cells/mm3 | 300 | … | … | … | … | … | … | … | … | … |
| Platelet count, platelets/mm3 | 31,000 | … | … | … | … | … | … | … | … | … |
| Death | … | Died | … | … | … | … | … | … | … | … |
| 6: male, aged 4 years | ||||||||||
| Received GMCSF 0.5 MIU/kg | … | … | Yes | … | … | … | … | … | … | … |
| Neutrophil count, cells/mm3 | … | 1748 | 576 | … | 6060 | … | … | … | … | 4600 |
| Platelet count, platelets/mm3 | … | 73,000 | 59,000 | … | 242,000 | … | 353,000 | … | … | 376,000 |
| Death | … | … | … | … | … | … | … | … | … | … |
Progression and complications
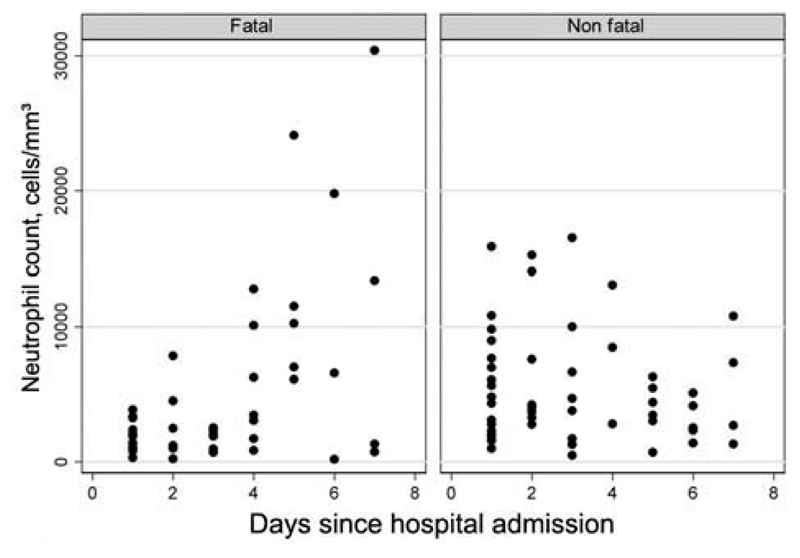
Serial full blood count data could be retrieved for 36 patients; 18 who died and 18 who survived. Excluding 5 patients who were treated with GMCSF, mean lymphocyte and platelet counts remained below normal levels for patients who died during the first 7 days after hospital admission. Although the risk of developing absolute neutrophilia (neutrophil count, >8250 cells/mm3) was not significantly different between patients who died and patients who survived (table 2), a relative increase in neutrophil count was more common in fatal cases (9 [64%] of 14) than nonfatal cases (8 [47%] of 17); the magnitude of the increase was also greater among fatal cases (mean increase in neutrophil count, 10,955 cells/mm3 vs. 3974 cells/mm3) (figure 3). After hospital admission, 5 patients developed pneumothoraces (4 while undergoing ventilation), and 1 patient developed a pleural effusion while undergoing ventilation.
Figure 3.
Neutrophil count for fatal and nonfatal cases (n = 31), by duration since hospital admission, excluding 5 patients who were treated with granulocyte macrophage colony-stimulating factor.
Virology and serology
Virus culture was successful for 20 (30%) of the 67 patients. Twelve of the 20 patients with culture-confirmed infection died (case fatality rate, 60%; 95% CI, 36%–81%), whereas 14 of 47 cases which were confirmed to be positive by RT-PCR but not culture resulted in death (case fatality rate, 30%; 95% CI, 17%–45%).
All viral isolates were clade 1, and all contained mutations that conferred resistance to amantadine. Oseltamivir resistance was detected in 2 isolates from cases that were reported elsewhere [4, 6]. During 2004, only clade 1 viruses were detected in poultry in Vietnam. During 2005, >95% of viruses isolated from poultry were clade 1. In 2006, no viruses were isolated from poultry.
Cause of death
Patients in all fatal cases experienced progressive respiratory failure and required mechanical ventilation, with 8 (31%) of 26 also requiring vasopressor treatment to maintain blood pressure. A limited postmortem analysis was conducted for 3 pediatric patients, and the results have been reported elsewhere [7]. In all 3 patients, the lung tissue revealed diffuse alveolar damage with hyaline membrane formation consistent with acute respiratory distress syndrome, as well as infiltration with macrophages and lymphocytes. Influenza A (H5N1) was successfully cultured from postmortem transthoracic lung tissue aspiration samples from 2 adult patients.
Discussion
We describe, to our knowledge, the second largest series of human cases of H5N1 infection to date; comprising 67 (63%) of 106 cases reported in Vietnam and 67 (17%) of 387 cases globally. Compared with all cases reported to World Health Organization as of January 2008, the series of cases we report involves a higher median age (25 vs. 18 years) and a lower case fatality rate (39% vs. 61%) [2]. The reasons that Vietnam has experienced a greater proportion of cases that were less severe and involved older patients than the global average are not clear but could include chance, differences in case ascertainment, differences in age-specific susceptibility or exposure, or differences in the infecting viruses. Our findings, however, are not outside the limits of other case series. The median age of patients in 26 cases detected inmainland China (29 years) was higher than that in Vietnam, and mortality rates of 33% (6 of 18 patients) and 40% (22 of 50 patients) have been observed in Hong Kong and Egypt, respectively [2, 8–11]. Although confirmation of virus clade was only available for 30% of cases, it is likely that, based on the pattern of poultry isolates during the period, all of the cases reported here were caused by clade 1 viruses. In animal studies, different clades are associated with different severity of disease, and there is no reason that the same should not be true in humans [12]. Cases of H5N1 infection occurring in Vietnam since the end of 2006 have been caused by clade 2.3.4 viruses, and the fatality rate has been 77% (10 of 13 patients) [13]. Therefore, spatial and temporal differences in mortality may be attributable to differences in the infecting viruses.
Whereas no age differential among surviving patients was observed among H5N1 cases in Indonesia and China, and in Hong Kong the case fatality rate was higher among adults, we have observed a pattern that has been seen elsewhere, a decrease in the risk of death with an increase in age [2, 8, 11, 14–17]. One possible explanation for this pattern is the presence of cross-protective immunity conferred by lifelong exposure to a repertoire of influenza antigens. Although seroepidemiological studies have thus far not revealed any significant level of neutralizing antibodies to H5N1 in exposed populations [2, 18], cross-reactive humoral responses against H5N1 may be induced by other influenza subtypes [19–22]. There is also evidence that cytotoxic T lymphocyte responses play a role in heterosubtype immunity [23, 24], as well as evidence of cross-reactive cell mediated responses to H5N1 in H5N1-naive populations [19, 25–27]. Therefore, it is possible that previous exposure to seasonal influenza antigens or other avian influenza viruses may provide a degree of protection against H5N1.
Although epidemiological data derived retrospectively from medical records are not robust, our findings are similar to those of other groups, with ∼30% of subjects unable to report any significant exposures in the 7 days prior to onset of symptoms [28–30]. The case patients in our series presented primarily with a fever, cough, dyspnea, absolute lymphopenia, and pulmonary infiltrates on chest radiograph, with impaired gas exchange. This clinical picture is consistent with other published reports [1–3, 8, 11, 15, 17, 28, 30–40]. The diagnostic sensitivity or specificity of any of these factors cannot, however, be assessed with our data, and efforts should be made to compare H5N1 cases with non-H5N1 respiratory infections. The median duration of illness prior to hospitalization (6 days) was similar to that reported in Indonesia (median duration, 6 days), Turkey (mean duration, 6.6 days), and China (median duration, 6 days) [2, 8, 15, 28, 30]. Severe cases were more likely to involve symptoms and signs of multiorgan involvement at hospital admission, including diarrhea, mucosal bleeding, raised serum transaminase levels, and depressed neutrophil and platelet counts. Diarrhea and gum bleeding have been reported elsewhere, as has disseminated intravascular coagulation [8, 11, 1517, 38–42].
The most accurate predictor of a fatal outcome in our case series was the presence at hospital admission of both a low neutrophil count and raised ALT levels. The neutropenia observed in fatal cases could result from a range of processes, including apoptosis, reactive hemophagocytosis, or neutrophil sequestration. Infiltration of the lungs with neutrophils has been reported in animals that were experimentally infected with the 1918 influenza strain and H5N1 [43], and moderate, but not abundant, pulmonary neutrophils have been observed in the limited number of H5N1 postmortems analyses that have been reported thus far [7, 44, 45]. Although there is some evidence that neutrophils play a beneficial role in controlling influenza infection and neutrophil counts at hospital admission were, on average, higher among survivors [46, 47], 9 (64%) of 14 patients who died experienced an increase in neutrophil count during hospital admission, and treatment with GMCSF was not associated with improved survival, despite increases in neutrophil counts.
We observed improved survival among patients treated with oseltamivir, compared with untreated patients, which is consistent with other reports [8, 28]. This effect remained after controlling for potential confounding by illness severity, with use of neutropenia as a marker of severity. Although it is possible that this result is confounded by age, the tendency for a beneficial effect of oseltamivir remained after stratification by age, although it was no longer statistically significant. Unfortunately, data on the day of illness that oseltamivir treatment was started were not collected in our study, but improved survival with earlier treatment initiation has been reported elsewhere [28]. Treatment with increased doses, loading doses, or longer schedules may further enhance any beneficial effect of oseltamivir or other antiviral drugs. Because of variability in the sensitivity of H5N1 viruses to amantadine and oseltamivir and the recent emergence of oseltamivir resistance among H1N1 viruses, it is imperative that the safety, tolerability, and efficacy of combination therapies be studied. We found an association between the use of corticosteroids and death, which persisted after controlling for 1 measure of severity.
Through the compilation of data on a relatively large series of H5N1 cases, we have undertaken a more thorough analysis of the characteristics of human H5N1 infection than has previously been possible. However, the sample size is still too small for robust investigation of patterns in subgroups of patients. Moreover, the data presented here correspond to only 1 H5N1 virus clade, and comparison of different clades and resistance profiles would be very valuable. Therefore, cooperation between groups, to aggregate individual patient clinical data, is essential to permit more powerful analyses and to increase understanding and improve clinical management.
Acknowledgments
We thank the patients for kindly participating in this study, and we thank the doctors and nurses at the National Institute for Infectious and Tropical Diseases, the National Pediatric Hospital, and the Hospital for Tropical Diseases, Vietnam, for their assistance.
Financial support
Ministry of Science and Technology of the Socialist Republic of Vietnam, Wellcome Trust UK (grant WT081613MA), and National Institutes of Health National Institute of Allergy and Infectious Diseases, through the South East Asia Infectious Disease Clinical Research Network.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Tran TH, Nguyen TL, Nguyen TD, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–73. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 3.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Clinical Center. Age-appropriate vital signs. [Accessed 3 July];2008 Available at: http://www.cc.nih.gov/ccc/pedweb/pedsstaff/
- 6.Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 7.Liem NT, Nakajima N, Phat le P, et al. H5N1-infected cells in lung with diffuse alveolar damage in exudative phase from a fatal case in Vietnam. Jpn J Infect Dis. 2008;61:157–60. [PubMed] [Google Scholar]
- 8.Yu H, Gao Z, Feng Z, et al. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE. 2008:3–e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris JS, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–9. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KF, Chan PK, Chan KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–6. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim JK, Seiler P, Forrest HL, et al. Pathogenicity and vaccine efficacy of different clades of Asian H5N1 avian influenza A viruses in domestic ducks. J Virol. 2008;82:11374–82. doi: 10.1128/JVI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le MT, Wertheim HF, Nguyen HD, et al. Influenza A H5N1 clade 2.3.4 virus with a different antiviral susceptibility profile replaced clade 1 virus in humans in northern Vietnam. PLoS ONE. 2008:3–e3339. doi: 10.1371/journal.pone.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smallman-Raynor M, Cliff AD. Avian influenza A (H5N1) age distribution in humans. Emerg Infect Dis. 2007;13:510–2. doi: 10.3201/eid1303.060849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oner AF, Bay A, Arslan S, et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006;355:2179–85. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- 16.Areechokchai D, Jiraphongsa C, Laosiritaworn Y, Hanshaoworakul W, O’Reilly M. Investigation of avian influenza (H5N1) outbreak in humans—Thailand, 2004. MMWR Morb Mortal Wkly Rep. 2006;55(Suppl 1):3–6. [PubMed] [Google Scholar]
- 17.Kandun IN, Wibisono H, Sedyaningsih ER, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–94. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 18.Vong S, Coghlan B, Mardy S, et al. Low frequency ofpoultry-to-human H5NI virus transmission, southern Cambodia, 2005. Emerg Infect Dis. 2006;12:1542–7. doi: 10.3201/eid1210.060424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gioia C, Castilletti C, Tempestilli M, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis. 2008;4:121–8. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007:4–e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillim-Ross L, Subbarao K. Can immunity induced by the human influenza virus N1 neuraminidase provide some protection from avian influenza H5N1 viruses? PLoS Med. 2007:4–e91. doi: 10.1371/journal.pmed.0040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinohe T, Tamura S, Kawaguchi A, et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis. 2007;196:1313–20. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambhara S, Kurichh A, Miranda R, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–53. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- 24.Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr Opin Biotechnol. 2007;18:529–36. doi: 10.1016/j.copbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162:7578–83. [PubMed] [Google Scholar]
- 26.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol. 2008;180:1758–68. doi: 10.4049/jimmunol.180.3.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82:5161–6. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandun IN, Tresnaningsih E, Purba WH, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372:744–9. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Feng Z, Zhang X, et al. Human influenza A (H5N1) cases, urban areas of People’s Republic of China, 2005–2006. Emerg Infect Dis. 2007;13:1061–4. doi: 10.3201/eid1307.061557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sedyaningsih ER, Isfandari S, Setiawaty V, et al. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005-June 2006. J Infect Dis. 2007;196:522–7. doi: 10.1086/519692. [DOI] [PubMed] [Google Scholar]
- 31.Buchy P, Mardy S, Vong S, et al. Influenza A/H5N1 virus infection in humans in Cambodia. J Clin Virol. 2007;39:164–8. doi: 10.1016/j.jcv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11:201–9. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–75. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Feng Z, Shu Y, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371:1427–34. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Shu Y, Hu S, et al. The first confirmed human case of avian influenza A (H5N1) in mainland China. Lancet. 2006:367–84. doi: 10.1016/S0140-6736(05)67894-4. [DOI] [PubMed] [Google Scholar]
- 36.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Lan Y, Xu CL, et al. Study on a fatal pregnant woman died from by avian influenza (H5N1) [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:288–92. [PubMed] [Google Scholar]
- 38.Zhang W, Wen LY, Lu M, et al. Clinical characteristic analysis of the first human case infected by influenza A (H5N1) in Jiangxi Province [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:300–6. [PubMed] [Google Scholar]
- 39.Witayathawornwong P. Avian influenza A (H5N1) infection in a child. Southeast Asian J Trop Med Public Health. 2006;37:684–9. [PubMed] [Google Scholar]
- 40.Bay A, Etlik O, Oner AF, et al. Radiological and clinical course of pneumonia in patients with avian influenza H5N1. Eur J Radiol. 2007;61:245–50. doi: 10.1016/j.ejrad.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Apisarnthanarak A, Kitphati R, Thongphubeth K, et al. Atypical avian influenza (H5N1) Emerg Infect Dis. 2004;10:1321–4. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C, Fang P, Liu YN, et al. A retrospective study of one case of human infection by the highly pathogenic avian influenza A (H5N1) [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:9–13. [PubMed] [Google Scholar]
- 43.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008:4–e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobasa D, Takada A, Shinya K, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–7. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 45.Gu J, Xie Z, Gao Z, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370:1137–45. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82:2772–83. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]