Abstract
A wide variety of radiolabeled peptide analogs for specific targeting of cholecystokinin-2 receptors (CCK2R) has been developed in the last decades. Peptide probes based on the natural ligands minigastrin (MG) and cholecystokinin (CCK) have high potential for molecular imaging and targeted radiotherapy of different human tumors, such as medullary thyroid carcinoma (MTC) and small cell lung cancer (SCLC). MG analogs with high persistent uptake in CCK2R expressing tumors have been preferably used for the development of radiolabeled peptide analogs. The clinical translation of CCK2R targeting has been prevented due to high kidney uptake or low metabolic stability of the different radiopeptides developed. Great efforts in the radiopharmaceutical development have been undertaken to overcome these limitations. Various modifications in the linear peptide sequence of MG have been introduced mainly with the aim to reduce kidney retention. Furthermore, improved tumor uptake could be obtained by in situ stabilization of the radiopeptide against enzymatic degradation through co-injection of peptidase inhibitors. Recent developments focusing on the stabilization of the C-terminal receptor binding sequence (Trp-Met-Asp-Phe-NH2) have led to new radiolabeled MG analogues with highly improved tumor uptake and tumor-to-kidney ratio. In this review, all the different aspects in the radiopharmaceutical development of CCK2R targeting peptide probes are covered, giving also an overview on the clinical investigations performed so far. The recent development of radiolabeled MG analogs, which are highly stabilized against enzymatic degradation in vivo, promises to have a high impact on the clinical management of patients with CCK2R expressing tumors in the near future.
Keywords: cholecystokinin-2 receptor, molecular imaging, targeted radiotherapy, gastrin, cholecystokinin, radiometals
1. Introduction
Cellular structures like antigens, membrane receptors and enzymes are intensively studied in terms of their suitability for early cancer detection and their potential for different treatment strategies [1–3]. Especially G protein-coupled receptors (GPCR), involved in the cell signal transduction, are outstanding targets for novel anti-cancer drugs because these proteins are often overexpressed on the cell membrane of different cancer cells [2,4]. Their natural ligands like regulatory peptides are promising lead structures to develop new targeting agents for molecular imaging and targeted radiotherapy [5,6]. Molecular-based investigations allowing to target structures overexpressed from such malignancies are highly interesting for nuclear medicine applications. Here ionizing radiation is used to visualize the tumor or damage the cancer cells through targeting with radiolabeled peptide analogs derived from the natural ligands [7,8].
More than three decades ago somatostatin based 123I-iodinated [Tyr3]octreotide was the first radiolabeled peptide analog clinically investigated in patients suffering from neuroendocrine tumors (NETs) known to overexpress somatostatin receptors [9]. The introduction of the radionuclide iodine-123 into the structure of this somatostatin analog was achieved by a direct radiolabeling approach in which the radionuclide is covalently bound to a functional group of the molecule. This relatively easy labeling strategy has the major disadvantage that the formed covalent bond shows a high tendency of in vivo dehalogenation limiting the intended targeting effect [10,11]. Nowadays regulatory peptides are mainly labeled via an indirect approach, in which a bifunctional chelator is conjugated to the peptide sequence which can form complexes with different radiometals [11]. Such bifunctional chelators are divided into acyclic and macrocyclic chelators. The acyclic chelator diethylenetriaminepentaacetic acid (DTPA) and especially the macrocyclic chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) are two examples of bifunctional chelators, which have found their way into routine clinical use. In the last decades, special interest has arisen on combining the ability of a diagnostic and a therapeutic application in one radiopharmaceutical after labeling with a corresponding radiometal [12]. Such theranostic investigations had a high impact especially on the clinical management of NET patients. The clinical use of different somatostatin analogs, such as DTPA-octreotide (DTPA-OCT), DOTA-Tyr3-octreotide (DOTA-TOC) and DOTA-Tyr3 -octreotate (DOTA-TATE), is well established in nuclear medicine. Radiolabeling of the peptide analogs with the β+ emitter gallium-68 allows for tumor staging using high sensitive positron emission tomography (PET) [13,14]. The same peptide analog complexed with β-emitting lutetium-177 or yttrium-90 [8,15] is used for peptide receptor radionuclide therapy (PRRT). This theranostic approach provides a significant advantage compared to other cancer treatments such as chemotherapy or radiation therapy as a preselection of patients who will benefit from PRRT is possible [12]. Great efforts have been undertaken to translate the successful clinical use of somatostatin analogs also to other regulatory peptides targeting alternative GPCRs. One of these intensively investigated GPCRs is the cholecystokinin-2 receptor (CCK2R). CCK2R are expressed at high incidence in various tumors like medullary thyroid carcinoma (MTC), small cell lung cancer (SCLC), astrocytoma, stromal ovarian and gastrointestinal stromal tumors (GIST). CCK2R are further expressed in about 20% of gastroenteropancreatic (GEP) tumors, in particular insulinomas, and less frequently in other tumors of neuroendocrine origin and with different histology [4,16–18].
The development of CCK2R targeting radiopeptides was driven mainly by the need to improve the medical care of MTC patients. The clinical management of thyroid cancer is of particularly high historical significance for nuclear medicine. Already more than 70 years ago nuclear medicine images were acquired in patients suffering from thyroid disorders [12]. Since then molecular imaging played an important role in diagnosing and managing thyroid cancer. Scintigraphic imaging and radiotherapy based on the uptake of 99mTc-pertechnetate or radioiodine (iodine-123 and iodine-131) via the sodium iodide symporter is only possible in thyroid cancer subtypes originating from the follicular cells of the thyroid [19]. MTC originates from the parafollicular C cells, not presenting this symporter on the cell surface and therefore unable to uptake iodine and pertechnetate. Beside histopathology, calcitonin and carcinoembryonic antigen produced by MTC cells are used as serum markers for diagnosis and follow-up [20,21]. Due to the high frequency of local lymph node metastases and the risk to overlook them in preoperative neck ultrasonography, total thyroidectomy coupled with central lymph node dissection is often recommended as primary treatment [21–23]. However, about 50% of the patients show persistent or recurrent disease and in more than 10% of the patients distant metastases are present already at the time of diagnosis [20,24]. Chemotherapy and external beam radiation showed limited response rates in patients with advanced disease [23]. Two systemic therapy options approved by the European Medicines Agency and the Food and Drug Administration are currently available. Due to the significant toxicity associated with vandetanib and cabozantinib, the use of these tyrosine kinase inhibitors is indicated only in patients with progressive and symptomatic MTC with unresectable locally advanced or metastatic disease [20,21]. A lack of sensitive imaging modalities and adjuvant therapies exits for patients with persistent or recurrent disease. The combination of ultrasonography, computed tomography (CT), magnetic resonance imaging and bone scintigraphy allows the detection of tumor lesions in less than 50% of the patients with elevated postoperative calcitonin levels. PET/CT with 6-[18F]fluorolevodopa (18F-DOPA) showing a detection rate of 70% is more useful to detect persistent or recurrent lesions and provides better results when compared to [18F]fluoro-2-deoxy-D-glucose (18F-FDG) and 68Ga-labeled somatostatin analogs [20,24]. Radiopeptides targeting CCK2R have a high potential to improve the diagnosis of patients suffering from MTC, but more importantly would enable targeted therapy in patients with advanced disease.
The CCK2R is a GPCR belonging to the cholecystokinin receptor (CCKR) family. CCKR are classified into two receptors subtypes, CCK1R and CCK2R. CCK1R are mainly expressed in the gallbladder and the pancreas and are involved in the stimulation of the pancreatic secretion and gallbladder contraction. CCK2R are found in the central nervous system and the stomach and mediate anxiety, gastric acid secretion and other physiological actions [25,26]. The endogenous ligands for CCKR are members of the regulatory peptide families of cholecystokinin (CCK) and gastrin. Both share the C-terminal sequence Trp-Met-Asp-Phe-NH2, proven essential for receptor binding. The endogenous ligands differ in the position of a tyrosyl residue, which can be sulfated or nonsulfated. CCK1R mainly recognizes sulfated CCK, whereas CCK2R binds both CCK and gastrin and distinguishes less between nonsulfated and sulfated forms [26–29]. After ligand binding, the peptide-receptor complex is rapidly internalized therefore allowing specific targeting of CCK2R expressing tumors [30].
Over the last decades an intense radiopharmaceutical development has been undertaken to provide radiolabeled CCK or gastrin analogs with optimal properties for diagnostic and therapeutic use in nuclear medicine [5,11, 30–32]. However, the clinical translation of this promising nuclear medicine application has been hampered by either high kidney uptake or low metabolic stability of the radiopeptides developed so far. Thus, the development of radiolabeled CCK2R targeting ligands and their preclinical and clinical evaluation is still ongoing. This review focuses on the previous success, major drawbacks and the recent progresses in the development of radiolabeled peptide probes suitable for diagnostic and therapeutic application in CCK2R expressing diseases.
2. Development of Radiolabeled Peptide Analogs Targeting the CCK2R
For in vitro evaluations, exploring the binding affinity of a new radioligand to its biological target, as well as in vivo biodistribution studies, investigating the tumor uptake in animal xenograft models, a suitable cell model is substantial. This cell model should express the corresponding receptor at a sufficient level (>106 binding sites/cell) and without high variations over time.
Additionally, rapid and endless growth of the cell line is of particular importance as well as the possibility to generate subcutaneous tumor xenografts in immunodeficient mice or rats [33]. In earlier studies investigating CCK2R targeting peptide analogs the human medullary thyroid carcinoma cell line TT, with relatively slow growth of tumor xenografts after subcutaneous injection in mice [34,35], or the rat pancreatic cell lines AR42J [36] and CA20948 [37], known to naturally express the rat CCK2R, were used. To avoid variable receptor expression, several groups started to work with transfected cell lines artificially overexpressing the CCK2R, such as human embryonic kidney 293 cells (HEK293) [38] or Chinese hamster ovary cells (CHO) [39] stably transfected with human CCK2R. Using the human epidermoid carcinoma cell line A431, yielding subcutaneous tumors with high efficiency, Aloj et al. developed a novel cell model for the evaluation of CCK2R targeting peptides. The cell line was transfected with the plasmid pCR3.1 containing the full coding sequence of human CCK2R (A431-CCK2R) as well as with the empty vector alone for negative control (A431-mock) [33,40].
The A431-CCK2R/A431-mock cell model seems to be a more adequate model for evaluating CCK2R binding peptide analogs, as divergent findings in AR42J versus A431-CCK2R cells with a cyclic minigastrin (MG) analog were related to possible differences in the binding domain of rat versus human CCK2R [41]. Recently, the human medullary carcinoma cell line MZ-CRC-1 has been used for the preclinical evaluation of radiolabeled CCK2R targeting peptides [42]. This human cell line was established from a metastatic site of a 43-year old female MTC patient. The experience with this cell line is very limited and comparative data performed by different research groups are still missing. For this reasons the preliminary results obtained so far are not included in this review.
Starting from the natural ligands displayed in table 1, a variety of CCK2R targeting radiopeptides was developed and preclinically evaluated. The amino acid sequences of the different peptide analogs developed are given in table 2, whereas in table 3 all clinical relevant radioligands are summarized. For successful application in molecular imaging and targeted radiotherapy radiolabeled peptides need to meet important prerequisites, such as high receptor affinity, sufficient metabolic stability, as well as high and persistent tumor uptake together with low uptake in non-target tissue and preferential renal excretion [32].
Table 1. Amino acid sequence of the most important members of the gastrin/CCK family.
| Natural ligand | Amino acid sequence* |
|---|---|
| Gastrin-17 (gastrin-I) | Glp-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| Gastrin-13 (minigastrin) | Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| Cholecystokinin-8 (CCK-8) | Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2 |
| Cholecystokinin-4 (CCK-4) | Trp-Met-Asp-Phe-NH2 |
bold letters = amino acids known to be involved in receptor binding
Table 2. Amino acid sequence of different CCK2R targeting peptide analogs suitable for labelling with radiometals.
| Peptide analog | Amino acid sequence |
|---|---|
| DTPA-minigastrin | DTPA-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-sCCK8 | DOTA-Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-CCK | DOTA-DAsp-Tyr-Nle-Gly-Trp-Nle-Asp-Phe-NH2 |
| DTPA-CCK | DTPA-DAsp-Tyr-Nle-Gly-Trp-Nle-Asp-Phe-NH2 |
| DTPA-MG0 | DTPA-DGlu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-MG0 | DOTA-DGlu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-MG11 | DOTA-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-G-CCK8 | DOTA-Gly-Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2 |
| SA106 | DOTA-DAsp-Phe(p-CH2SO3H)-HPG-Gly-Trp-HPG-Asp-Phe-NH2 * |
| Sargastrin | DOTA-Gln-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
| APH070 | DOTA-His-His-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-cyclo-MG1 | DOTA-cyclo[γ-D-Glu-Ala-Tyr-D-Lys]-Trp-Met-Asp-Phe-NH2 |
| DOTA-cyclo-MG2 | DOTA-cyclo[γ-D-Glu-Ala-Tyr-D-Lys]-Trp-Nle-Asp-Phe-NH2 |
| MGD5 | DOTA-Gly-Ser-Cys-(Glu-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2)2 |
| DOTA-PP-F6 | DOTA-DGln-DGln-DGln-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-PP-F10 | DOTA-DGln-DGln-DGln-DGln-DGln-DGln-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-PP-F16 | DOTA-DGln-DGlu-DGln-DGlu-DGln-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-PP-F11/CP04 | DOTA-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-PP-F11-L | DOTA-Glu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| DOTA-PP-F11N | DOTA-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
HPG = homopropargylglycine
Table 3. Clinical studies with radiolabeled CCK2R targeting peptide derivatives in patients with MTC or other CCK2R expressing tumors.
| Peptide derivative | Radionuclide | Radiopeptide | Injected activity (MBq) | Injected peptide amount (µg) | Tumor type | Number of patients | Reference |
|---|---|---|---|---|---|---|---|
| gastrin-I | iodine-131 | 131I-gastrin-I | 370 | 0.5/kg BW | metastatic MTC | 1 | [34,43] |
| [DTPA0]minigastrin | indium-111 | 111In-DTPA-MG | 130-170 | 0.5/kg BW | metastatic MTC occult MTC | 2 1 |
[43,75] |
| [DTPA0,DAsp1,Nle 3,6]CCK8 | indium-111 | 111In-DTPA-CCK | 158-211 | 7.2-8.5 1 x 18.7 |
metastatic MTC occult MTC | 2 5 |
[37,76] |
| [DTPA0DGlu1minigastrin | indium-111 | 111In-DTPA-MG0 | 185-259 | 5-10 | metastatic MTC occult MTC metastatic carinoid astrocytoma (grade III) | 23 22 1 1 |
[77,78] |
| 150-200 | 5.5-7.3 | metastatic MTC occult MTC | 22 5 |
[79] | |||
| 150-200 | 5.5-7.3 | neuroendocrine tumors | 61 | [80] | |||
| yttrium-90 | 90Y-DTPA-MG0 | 1110-1850* | Not stated | advanced metastatic MTC | 8 | [77,78,81] | |
| [DTPA0,DAsp1,Nle3,6]CCK8 [DOTA0,DGlu1,DesGlu2-6]minigastrin [N4-Gly0,DGlu1]minigastrin |
indium-111 indium-111 technetium-99m |
111In-DOTA-CCK 111In-DOTA-MG11 99mTc-Demogastrin-2 |
216±19 186±34 773±97 |
11.1±0.6 10.8±1.7 9.5±1.5 |
metastatic MTC | 6 | [82] |
| [HYNIC0,DGlu1,DesGlu2-6]minigastrin | technetium-99m | 99mTc-HYNIC-MG11 | 500-700 | 7-9 | presurgical MTC postsurgical MTC |
7 23 |
[83,84] |
| [DOTA0,DGln1-6]minigastrin | gallium-68 | 68Ga-DOTA-PP-F10 | 347 MBq | ~20 | metastatic MTC | 1 | [58] |
| [DOTA0,DGlu1-6]minigastrin | gallium-68 | 68Ga-DOTA-PP-F11 | Not stated | Not stated | presurgical MTC | 1 | [85] |
| [DOTA0,DGlu1-6]minigastrin | indium-111 | 111In-DOTA-PP-F11 | 200±20 | 10 or 50 | advanced MTC | 6 | [86] |
| [DOTA0,DGlu1-6,Nle11]minigastrin | lutetium-177 | 177Lu-DOTA-PP-F11N | 1000§ | <100 | histologically proven MTC | 16 | [87] |
Up to 4 injections in intervals of 4-6 weeks (MBq/m2);
two injections with/without Gelofusine within 4 weeks
The feasibility of CCK2R targeting was first shown using the 17 amino acid long peptide derivative gastrin-I labeled with iodine-131. In TT-xenografted female nude mice a tumor uptake of up to 9% injected activity per gram tissue (% IA/g) at 1 h post injection (p.i.) was reported for 131I-gastrin-I giving high promise for the diagnostic and therapeutic use in patients [34]. Soon thereafter, the 13 amino acid long sequence of human MG as well as a peptide analog derived from the 8 amino acid long sequence of CCK-8, conjugated to the acyclic chelator DTPA or to the macrocyclic chelator DOTA suitable for labeling with trivalent radiometals, were developed [37,43]. It was expected that the use of a “residualizing radiolabel” leading to “trapping” of the radiometal-chelate in the cell after internalization may allow to enhance the retention of radioactivity in the target tissue as well as the tumor-to-background ratio [37,43]. When comparing 111In-DTPA-MG and 111In-DTPA-sCCK8 in TT-xenografted nude mice a similar tumor uptake of 5% IA/g at 1 h p.i. was observed. Due to the higher background activity of 111In-DTPA-sCCK8 tumor visualization was superior for 111In-DTPA-MG [33]. To increase the kinetic stability of DTPA complexes, leucine (Leu) in position 1 of the peptide sequence of DTPA-MG was substituted with D-glutamic acid (Glu) leading to DTPA-MG0. For both, 90Y-DTPA-MG0 and 111In-DTPA-MG0, a higher resistance towards transchelation was found in DTPA solution and human serum, suggesting an involvement of Glu in the complexation of the radiometal. Substitution with DGlu was connected with lower blood levels as well as decreased bone and liver uptake, whereas no influence on the tumor uptake could be observed [44]. The biodistribution of 111In-DOTA-MG0 was further compared to sulfated 111In-DOTA-sCCK8, known to show a higher tumor uptake when compared to the nonsulfated peptide. In female BALB/c nude mice xenografted with HEK293 cells stably transfected with CCK2R as well as the same cell line transfected with a splice variant of the receptor (CCK2i4svR), the radiopeptides showed a comparable tumor uptake (~3% IA/g at 24 h p.i.). However, the kidney retention of 111In-DOTA-MG0 was remarkably higher (~30% versus ~3% IA/g at 24 h p.i.) [38].
The high kidney uptake of radiolabeled MG analogs was related to the pentaglutamate (penta-Glu) sequence in the linear peptide sequence. Preclinical studies in TT-xenografted female nu/nu mice showed that the kidney uptake of 111In-DTPA-MG0 was efficiently reduced by up to 90% through co-injection of penta-Glu [45]. Also the commercially available gelatin-based plasma expander Gelofusine showed to be useful in this respect and allowed to reduce renal accumulation of 111In-DTPA-MG0 by 45% in male Wistar rats [46]. Truncation of the penta-Glu sequence in 111In-DOTA-MG11, led to significantly lower kidney uptake in comparison with 111In-DOTA-MG0 (<0.4% versus 10% IA/g at 24 h p.i.), as studied in AR42J tumor-bearing Lewis rats. However, the depletion of the penta-Glu sequence was connected with a lower serum stability, indicating that the lower tumor uptake of 111In-DOTA-MG11 (0.3% versus 0.6% IA/g observed with 111In-DOTA-MG0 at 24 h p.i.) was related to lower metabolic stability [47]. Similar results were obtained with 99mTc-labeled peptide analogs based on MG or CCK [36,48–52]. The drawbacks related to high kidney retention or low metabolic stability hindered the development of a radiolabeled peptide analog suitable for therapeutic use. In the attempt to improve the pharmacokinetics and tumor targeting profile a variety of peptide analogs with different modifications, such as the introduction of linkers or unnatural amino acids, modification of peptide bonds, as well as multimerization or cyclization of the peptide sequence was developed by different research groups [31,32,53–58]. With the major goal of developing a radiolabeled CCK2R targeting ligand for clinical use in PRRT nine European research groups from Athens, Basel, Freiburg, Innsbruck, Ljubljana, London, Naples, Nijmegen and Rotterdam evaluated a series of different peptide analogs within a research network funded by the European Cooperation in Science and Technology (COST BM0607: Targeted Radionuclide Therapy). The study included twelve DOTA-conjugated CCK2R targeting peptide analogs, which were shared between the groups to perform different steps of preclinical testing in side by side comparative studies under standardized conditions [41,59,60]. Besides comprising DOTA-MG0 and DOTA-MG11 for comparison, different peptide derivatives with several modifications, such as addition, elimination or substitution of amino acids, cyclisation or dimerization of the peptide sequence, were included in the study. The different amino acid modifications were mainly applied in the N-terminal part of the peptide sequence. In the CCK analog G-CCK-8, a glycine residue was introduced as a spacer in the peptide sequence of CCK-8. The same peptide analog was previously evaluated using the linear chelator DTPAGlu coupled to N-terminal Gly spacer through the carboxyl function of the side chain of Glu [48]. For APH070 DGlu in MG11 was replaced by the L-form of the amino acid and two histidine residues were introduced in the aim to reduce the peptide reabsorption in the kidneys [61]. Another attempt to decrease kidney retention, focused on the replacement of the penta-Glu sequence by different combinations of D-glutamine or DGlu residues in DOTA-PP-F6, DOTA-PP-F10, DOTA-PP-F11 and DOTA-PP-F16. These peptide analogs were chosen from a previous study investigating the influence of different nonionic spacers, such as PEG or D-amino acids like D-Ser and D-Gln, on the metabolic stability and pharmacokinetic profile. Especially hydrophilic uncharged Gln spacers were found to be most promising to improve pharmacokinetics. The reduction of negative charges led to decreased kidney uptake, whereas the increased metabolic stability was related to the adoption of a more stable secondary structure [58]. To improve the pharmacokinetic profile in terms of stability against enzymatic degradation, cyclisation of the linear sequence of MG11 was achieved by incorporating DGlu in position 1 through the γ-carboxylic group and replacing glycine with D-lysine in position 4 allowing the introduction of an internal amide bond leading to DOTA-cyclo-MG1 [56,57]. A few C-terminal substitutions focused on the replacement of oxidation sensitive methionine (Met) by homopropargylglycine (HPG) in the CCK-8 analog SA106, a peptide analog which is additionally modified with a sulfonate isostere of tyrosine sulfate (Phe(p-CH2SO3H)). The influence of the introduction of Phe(p-CH2SO3H) in combination with the exchange of Met for Nle or HPG on the CCK2R affinity and tumor targeting properties was studied previously [62,63]. In sargastrin, derived from human gastrin-I, as well as in MGD5, a dimeric form of MG11, Met was replaced by norleucine (Nle). The possibility of replacing Met in the C-terminal receptor-specific region by Nle was reported also earlier [43,47,56]. HPG and methoxinine (O-methyl-L-homoserine) are other surrogates of Met allowing to maintain CCK2R affinity [64], whereas for replacement by isoleucine, methionine-sulfoxide and methionine sulfone loss of receptor affinity was reported [47,65]. Some of the N-terminal modifications introduced in the series of peptide analogs studied led to an increased enzymatic stability as well as increased tumor-to-kidney ratios in in vivo biodistribution studies performed in female BALB/c nude mice tumor xenografted with A431-CCK2R and A431-mock cells, as compared to 111In-DOTA-MG11 or 111In-DOTA-MG0. Three peptide derivatives, the dimeric peptide conjugate MGD5, the cyclic peptide DOTA-cyclo-MG1 and the linear peptide DOTA-PP-F11 with the penta-Glu moiety exchanged for the D-isomeric form, showed a favorable biodistribution profile with tumor values of ~10% IA/g at 1 h p.i. combined with improved tumor-to-kidney ratio [41,59,60]. For these three derivatives, however, different enzymatic cleavage sites have been reported, mainly in the receptor-specific C-terminal sequence. No intact radiopeptide was detectable in the blood and urine of female BALB/c mice intravenously injected with the 177Lu-labeled peptide derivatives already at 10 min after injection [59].
111In-DOTA-PP-F11 was selected for clinical translation and further preclinical evaluations of its pharmacology, pharmacokinetics and toxicology were carried out [66,67]. A kit formulation with two different doses of DOTA-PP-F11 (10 μg and 50 μg) for the straightforward preparation of 111In-DOTA-PP-F11 in the clinical setting was developed. By special attention on reducing metal traces, such as copper, zinc or ferric ions, as well as addition of ascorbic acid and Met to suppress Met oxidation a high shelf life of the kit as well as a high radiochemical purity and stability of the radiolabeled peptide analog was obtained [66]. Furthermore, biodistribution and dosimetry studies, as well as an extended single dose toxicity study in rats, based on the microdosing concept described in the ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals (CPMP/ICH/286/95), was performed. In these studies, a very low toxicity of DOTA-PP-F11 with a median lethal dose (LD50) ≥178.5 μg/kg body weight and a no-observed-adverse-effect-level (NOAEL) of 89 μg/kg body weight was confirmed. For calculating the human equivalent dose (HED) a safety factor of 10 was considered recommending a starting dose (MRSD) for a first-in-human clinical trial of 1.4 μg/kg. In the biodistribution studies performed with 111In-DOTA-PP-F11 in male Swiss albino mice up to 72 h p.i., a rapid blood clearance of the radiopeptide and predominant uptake in CCK2R-positive stomach and in the kidneys as main route of excretion was observed. Dosimetric calculations were based on extrapolating animal to human data and assuming a radioactivity dose of 220 MBq predicting a whole body radiation dose of 10 mSv and identified the kidneys as the organ with the highest absorbed dose (0.124 mGy/MBq) [67]. Kidney uptake was efficiently reduced by co-injection of the plasma expander Gelofusine (~2% versus ~6% IA/g at 4 h p.i.) [67].
Further studies were carried out to investigate the influence of the stereochemistry of the N-terminal penta-Glu sequence of DOTA-PP-F11 on the enzymatic stability and pharmacokinetics. In circular dichroism spectroscopy experiments a type II reverse turn conformation was reported for DOTA-PP-F11, whereas a preferential α-helical conformation was adopted when replacing the six DGlu residues by LGlu residues (DOTA-PP-F11-L). In the biodistribution studies performed in AR42J xenografted male Lewis rats, in line with the results obtained in binding affinity and internalization studies, an up to 2-fold lower tumor uptake was reported for 111In-labeled DOTA-PP-F11 compared to DOTA-PP-F11-L. However also the kidney retention of 111In-DOTA-PP-F11 was 20-fold lower leading to the assumption that not only the charge but also the secondary structure of the peptide plays an important role for the kidney uptake and retention [68].
Considering a possible therapeutic use, the influence on the tumor uptake of the injected peptide amount was evaluated for DOTA-PP-F11. Biodistribution studies in A431-CCK2R xenografted female BALB/c nude mice with 111In-DOTA-PP-F11 at an injected peptide amount in the range of 0.05 to 15 nmol revealed saturation effects leading to decreased uptake in tumor xenografts and stomach with a peptide amount >0.15 nmol, whereas kidney uptake was not affected. The dosimetry calculations, considering a similar biodistribution profile and the molar activities achievable for radiolabeling of DOTA-PP-F11 with different therapeutic radiometals, pointed out that for larger xenografts multiple injections of 90Y-DOTA-PP-F11 are preferable, whereas for smaller xenografts alpha emitting bismut-213 seems preferable over beta minus emitting lutetium-177 and yttrium-90 [69]. No therapeutic preclinical study was performed with DOTA-PP-F11 radiolabeled with a therapeutic radionuclide. Such a study was performed only for two 177Lu-labled cyclic MG analogs in A431-CCK2R/A431-mock xenografted BALB/c nude mice of both sexes. The study included three groups: a control group receiving no treatment (injection of physiological saline), a low activity group receiving 15 MBq of the 177Lu-labeled peptide analogs corresponding to 0.6 nmol peptide and a high activity group receiving 30 MBq corresponding to 1.2 nmol peptide. For both groups treated with the radiolabeled peptide analogs, a significantly reduced tumor growth was reported for A431-CCK2R xenografts, whereas in CCK2R-negative A431-mock xenografts tumor growth was reduced to a lesser extent in comparison with the control group receiving no treatment. The treatment was connected with a transient medullary toxicity as well as increased serum creatinine and urea levels [70]. In another study, the risk of nephrotoxicity was preclinically evaluated for different 111In-labeled peptide analogs, including 111In-DOTA-MG0, after injection of a rather high radioactivity of 40 MBq. An absorbed kidney dose of more than 40 Gy was connected with long-term nephrotoxicity [71]. No further preclinical studies analyzing the long-term toxicity of radiolabeled CCK2R targeting peptides were carried out.
For potential imaging studies with the diagnostic radiometals gallium-68, indium-111 and copper-64, PP-F11 was conjugated to the alternative macrocyclic chelators, 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), and 1-(1,3-carboxypropyl)-4,7-carboxymethyl-1,4,7-triazacyclononane (NODAGA). The biodistribution and imaging profile of all chelator/radionuclide combinations was evaluated in female SCID mice bearing A431-CCK2R tumor xenografts. With the 64Cu-labeled peptide analogs, highest tumor uptake was observed. However, instability of the 64Cu-labeled chelator complexes also led to high uptake in non-target tissue. Interestingly, the different chelators did not show a high influence on the biodistribution profile of 111In- and 68Ga-labeled PP-F11. From the radiopeptides studied 68Ga-DOTA-PP-F11 seemed most promising for PET/CT imaging [72]. LC-MS studies on the complexes of DOTA-PP-F11 with the natural isotopes of gallium, yttrium and lutetium revealed that for the complexation of yttrium and lutetium three carboxylic groups of DOTA are involved, whereas two carboxylic groups are involved in the coordination of gallium [73].
The peptide sequence of DOTA-PP-F11 contains a Met residue in the C-terminal receptor binding sequence which poses the risk of oxidation during the radiolabeling process connected with loss of receptor affinity [46,74]. Substitution of Met by Nle, a commonly known concept to overcome this disadvantage [43,47,56], was applied in DOTA-PP-F11N in the aim to develop a chemically stabilized therapeutic agent. A lower susceptibility against enzymatic degradation was found in vitro for 177Lu-DOTA-PP-F11N, when compared to 177Lu-DOTA-MG11 and 177Lu-DOTA-PP-F11. However, the biodistribution studies in female nude mice revealed a very similar tumor uptake of ~7% IA/g at 4 h p.i. for 177Lu-DOTA-PP-F11 and 177Lu-DOTA-PP-F11N in A431-CCK2R xenografts [42].
3. Clinical use of Radiolabeled CCK2R Targeting Peptide Analogs
The very first imaging study with a radiolabeled CCK2R targeting peptide analog was reported in 1998. This study was performed with 131I-gastrin-I. Scintigraphic whole body imaging in a 71-year-old male patient with metastatic MTC, beside confirming the physiological uptake in CCK2R expressing tissues, such as stomach, pancreas and gallbladder, visualized mediastinal lymph node and lung metastases [34,43].
Soon thereafter, a chelator-based labeling approach with radiometals was developed allowing the use of indium-111 with superior physical imaging characteristics when compared to iodine-131. In analogy to 111In-labeled DTPA-OCT (111In-DTPA-OCT), finding increasing clinical use for the diagnosis of neuroendocrine tumors at the time, the linear chelator DTPA was introduced into the amino acid sequence of MG as well as a peptide analog derived from CCK-8. Scintigraphic imaging with 111In-DTPA-MG visualized known metastatic lesions in two patients (lymph node, lung, liver and bone metastases) as well as an unknown liver lesion in a patient with occult disease, which could be confirmed by conventional CT imaging only four weeks later [43,75]. 111In-DTPA-CCK was studied in seven MTC patients, from which only two had radiological evidence of metastases [37,76]. Only for one of these two patients some of the known liver lesions could be visualized, whereas the known lesions in liver and bone were missed in the second patient. Scintigraphy of the five patients with occult disease suggested a possible pathology in one additional patient. With both radiotracers physiological uptake in the CCK2R expressing stomach together with predominant renal excretion and minor intestinal activity was observed. The uptake of 111In-DTPA-CCK in liver and kidneys was lower when compared to 111In-DTPA-OCT [37,76]. However, a relatively high soft tissue accumulation was observed at 4 h p.i., leading to visualization of the breast and nipples. Thus, a better visualization of the lesions was possible at 24 and 48 h p.i. [76]. 111In-DTPA-MG showed a lower liver uptake in comparison with 111In-DTPA-OCT favoring the possible detection of liver metastases [43].
111In-DTPA-MG0 with Leu in position 1 replaced by DGlu was studied in healthy volunteers in comparison with 111In-DTPA-MG. Scintigraphic imaging with both radiotracers revealed main physiological uptake in stomach, together with minor uptake in gallbladder and breast tissue. Additional uptake occurred in the kidneys as main route of excretion together with transient bowel activity indicating additional biliary excretion [35,75,77]. In line with improved complex stability, scintigraphic imaging with 111In-DTPA-MG0 confirmed a significantly lower liver and bone marrow uptake when compared to the Leu or DLeu containing derivative [77]. The development of a chelator-based radiolabeling approach, allowing stable radiolabeling with different trivalent radiometals was an important step also towards the further development of CCK2R targeted PRRT.
In subsequent studies with 111In-DTPA-MG0 a total of 45 MTC patients was examined [77,78]. In 23 patients with known metastatic MTC, already at 1 h p.i. all lesions known from conventional imaging modalities could be visualized. The tumor-to-background activity ratios increased over time. Thus, at 24 h p.i. the best contrast was obtained. In 20 of 22 patients with occult metastatic MTC at least one lesion was visualized, therefore reaching a very promising sensitivity of 91%. Furthermore, almost no physiological uptake occurred in liver and spleen, suggesting that the detection of metastatic lesions can be highly improved when compared to 111In-DTPA-CCK, as well as somatostatin receptor scintigraphy with 111In-DTPA-OCT.
Similar results were reported also in a consecutive study including 27 MTC patients [79]. In a subgroup of 19 patients undergoing both gastrin receptor scintigraphy with 111In-DTPA-MG0 and somatostatin receptor scintigraphy with 111In-DTPA-OCT, 94.2% of the lesions known from other imaging methods were detected with 111In-DTPA-MG0 and 40.7% of the lesions were detected with 111In-DTPA-OCT. Another subgroup of 26 patients was examined with 111In-DTPA-MG0 and 18F-FDG. In this subgroup, 87.3% of the lesions confirmed by two imaging methods were detected with 111In-DTPA-MG0, 76.1% by CT and 67.2% by 18F-FDG PET. Using the combination of 111In-DTPA-MG0 and CT 96.7% of the lesions could be localized. In the subgroup of 6 patients with occult MTC only one unknown lesion could be detected with 111In-DTPA-MG0. Using 111In-DTPA-MG0 also patients with other tumor entities were examined, reporting CCK2R-specific uptake in the liver lesions of a patient with metastatic carcinoid as well as in a patient with grade III astrocytoma [77]. A broader study was carried out comparing gastrin and somatostatin receptor scintigraphy in patients with neuroendocrine tumors [80]. Sixty-one patients with histologically verified neuroendocrine tumors were included in the study, comprising 51 carcinoid tumors, 3 gastrinomas, 2 glucagonomas, 1 insulinoma, 3 paragangliomas, as well as 1 presumed pulmonary carcinoid which proved to be small cell lung cancer and was therefore excluded from the study. From the 60 patients evaluated, 54 had known metastatic disease, 5 recurrent disease and one no known metastases. In this study, an overall tumor-detection rate of 73.7% and 82.1% was reported for 111In-DTPA-MG0 and 111In-DTPA-OCT, respectively. Interestingly, in 6 of 11 patients showing no uptake of 111In-DTPA-OCT, positive uptake was seen with 111In-DTPA-MG0. In additional 6 of 49 patients with positive 111In-DTPA-OCT uptake, a total of 18 additional lesions was visualized with 111In-DTPA-MG0. Conventional imaging methods were used to confirm lesions that were detected only by 111In-DTPA-MG0. When considering the number of tumor sites and degree of uptake, 111In-DTPA-MG0 was superior to 111In-DTPA-OCT in 21.7% of the patients. The authors, therefore, concluded that gastrin receptor scintigraphy should be performed in selected patients with neuroendocrine tumors showing equivocal or absent 111In-DTPA-OCT uptake.
Based on the promising imaging results with 111In-DTPA-MG0, an initial therapeutic study was carried out with 90Y-DTPA-MG0 in eight patients with advanced metastatic MTC. The study was designed as dose escalation study with increasing activity dose levels. The first group of patients received up to four injections of 1110 MBq/m2 in intervals of 4−6 weeks. Subsequently, the activity injected was increased to 1480 MBq/m2 and 1850 MBq/m2 in the second and third group, respectively [77]. Treatment of the first three patients with 1110 MBq/m2 was well tolerated with no signs of toxicity. Dose escalation in the subsequent three patients to 1480 MBq/m2 was connected with transient hematologic toxicity (≤ grade 3) in all three patients. One patient without signs of renal toxicity during treatment developed chronic renal failure requiring hemodialysis one year after treatment. In the group treated with 1850 MBq/m2 only two patients were included. In one of these two patients transient hematologic toxicity (grade 3) was overserved, whereas the second patient developed a combined hematologic (grade 4) and reversible renal toxicity (grade 1) followed by myelodysplastic syndrome and chronic myelomonocyctic leukemia two years after treatment. In terms of therapeutic effect, in one patient a partial remission and in another patient a minor response was observed, four additional patients showed a stabilization of the disease lasting for 6 to 36 months [81]. Due to the observed severe nephrotoxic side effects, no further treatments were carried out with 90Y-DTPA-MG0.
In seek of alternative radioligands, truncated DOTA-MG11 missing the penta-Glu sequence and displaying improved tumor-to-kidney ratios in preclinical animal studies [47], was therefore selected for further clinical studies. Furthermore, also a 99mTc-labeled MG0 derivative conjugated to an open chain tetraamine chelator (99mTc-Demogastrin-2) with most favorable tumor-to-nontarget ratios seemed promising for clinical translation [52]. In a comparative study investigating the diagnostic performance of 111In-DOTA-MG11, 111In-DOTA-CCK and 99mTc-Demogastrin-2 in the same six patients, 99mTc-Demogastrin-2 was clearly superior to the 111In-labeled peptide analogs. Only with 99mTc-Demogastrin-2 all known tumor lesions as well as additional unknown lesions in neck (2 patients), brain (1 patient), bone (1 patient), and liver (1 patient) were visualized. From the lesions detected with 99mTc-Demogastrin-2, some of the neck and liver lesions were not visualized with 111In-DOTA-MG11 (2 patients) and 111In-DOTA-CCK (4 patients). The lower diagnostic performance of the 111In-labeled peptides was attributed to suboptimal imaging characteristics of indium-111, lower injected radioactivity as well as lower stability against metabolic degradation [74,82]. The uptake of the DOTA-conjugated peptides in the tumor lesions was, however, too low to consider a potential application in PRRT. Similar disappointing results were observed with a 99mTc-labeled MG11 derivative conjugated to the monodentate ligand hydrazinonicotinic acid (99mTc-HYNIC-MG11). In a comparative study, the diagnostic performance of gastrin receptor scintigraphy with 99mTc-HYNIC-MG11 and somatostatin receptor scintigraphy with 99mTc-labeled HYNIC-TOC (99mTc-HYNIC-TOC) was evaluated in 30 MTC patients. Somatostatin receptor scintigraphy was positive in 20 patients (66.7%), whereas gastrin receptor scintigraphy was positive only in 11 patients (36.7%). The lower diagnostic performance of 99mTc-HYNIC-MG11 was attributed to possible oxidation of Met in the C-terminal receptor specific region during the heating step of the radiolabeling process leading to loss of receptor affinity. Physiological CCK2R related uptake in stomach was observed only in 19 of the 30 patients studied. However, a high intestinal tracer accumulation occurred in all patients potentially influencing the interpretation of the scans. Uptake in spleen, liver and kidneys was lower when compared to 99mTc-HYNIC-TOC [84].
The use of 68Ga-labeled somatostatin analogs for PET/CT staging of neuroendocrine tumors greatly improved the staging and follow-up of neuroendocrine tumors. In a prospective study, including 84 patients with known or suspected neuroendocrine tumors, the diagnostic performance of 68Ga-labeled DOTA-TOC (68Ga-DOTA-TOC) PET was compared to conventional somatostatin receptor scintigraphy and CT. In this study 68Ga-DOTA-TOC PET clearly showed a higher diagnostic efficacy compared to SPECT and CT [14]. In the attempt to use the improved detection rate of PET imaging also for CCK2R based receptor targeting, a first PET/CT imaging study with 68Ga-DOTA-PP-F10 was performed in a 59-year-old female patient with metastatic MTC. The revealed receptor positive liver metastasis was in accordance with a previous 68Ga-DOTA-TOC PET/CT. Standardized uptake values (SUVs) in the range of 0.5-1.0 were found for liver, lung and gluteus muscle, whereas the SUV for stomach mucosa was 3.1 [58]. Furthermore, a PET/CT imaging study with 68Ga-DOTA-PP-F11 was performed in a 75-year-old male patient diagnosed with MTC by fine-needle aspiration biopsy [85]. Preoperative staging was additionally performed with the 68Ga-labeled DOTA-TATE (68Ga-DOTA-TATE) for comparison. The PET/CT images obtained with both PET tracers confirmed increased uptake in the right thyroid mass without signs of lymph node metastases. 68Ga-DOTA-PP-F11 showed physiological uptake in the stomach, together with highly reduced uptake in liver, spleen and kidneys compared to 68Ga-DOTA-TATE [85].
The intense preclinical research undertaken over the last years focused on the further optimization of the peptide sequence and led to new developments. Based on the promising preclinical results obtained with DOTA-PP-F11 and DOTA-PP-F11N, recently new clinical trials have been initiated. In a phase I multi-center study 111In-DOTA-PP-F11 is currently investigated in patients with advanced MTC (ClinicalTrials.gov Identifier: NCT03246659) at two different peptide dose levels, 10 μg (as a safety step in first administrations) and 50 μg (suitable also for PRRT). The study goals include the evaluation of the safety of the intravenous administration and of the diagnostic potential to detect tumor lesions, as well as of the dosimetry to identify critical organs and of the co-administration of Gelofusine to reduce kidney retention [86,88]. Overall 16 patients were enrolled in the study, confirming the safe administration of 200 MBq 111In-DOTA-PP-F11 at both peptide dose levels as well as a high sensitivity and specificity for the detection of unknown MTC lesions. Co-administration of the gelatin-based plasma expander Gelofusine resulted in a reduction of kidney retention and could therefore also be important for the future therapeutic assessment of 177Lu-DOTA-PP-F11. In a phase 0 single-center proof of principle study (ClinicalTrials.gov Identifier: NCT02088645) 6 patients with advanced MTC received two administrations of 1 GBq 177Lu-DOTA-PP-F11N with and without co-administration of Gelofusine, in a random cross-over order within 4 weeks [87]. The short infusion over 5 min of a peptide dose of ~100 μg was well tolerated with minor signs of toxicity, such as hypotension, flushing, hypokalemia. In four patients 177Lu-DOTA-PP-F11N SPECT/CT could be compared to 18F-DOPA PET/CT, a PET tracer with higher sensitivity and specificity in the characterization of MTC lesions when compared to 18F-FDG PET [89]. With 177Lu-DOTA-PP-F11N 41 of the 49 lesions identified with 18F-DOPA could be visualized, confirming a good correlation of the two imaging methods. The dosimetry calculation revealed that with 177Lu-DOTA-PP-F11N a lower tumor radiation dose (0.88 Gy/GBq) is reached in comparison with 177Lu-labeled DOTA-TATE (177Lu-DOTA-TATE) in patients with neuroendocrine tumors [90]. The tumor accumulation seems, however, sufficiently high for a potential therapeutic application, as the median absorbed doses to kidneys (0.11 Gy/GBq) and bone marrow (0.028 Gy/GBq) were at least three times lower in comparison with 177Lu-DOTA-TATE. The stomach was identified as the main dose-limiting organ (0.42 Gy/GBq) whereas co-administration of Gelofusine did not affect the tumor-to-kidney ratio. When combined with a renal-protective agent, treatment with 177Lu-DOTA-TATE resulted to be associated with transient hematologic events, whereas no evidence of renal toxic effects occurred within the median duration of follow-up of 14 months [15]. A similarly low toxicity profile can be expected also for the treatment with 177Lu-labeled CCK2R targeting peptides.
4. Ongoing Preclinical Investigations and Future Directions
When developing new radiopharmaceuticals based on regulatory peptides, different requirements have to be taken under consideration. The most important step at the beginning of any new development is to prove that the chosen target (e.g. a receptor or an enzyme) for the new radioligand is predominantly and highly overexpressed on the tumor tissue. A high incidence of CCK2R was reported for neuroendocrine tumors, in particular MTC (>90%), whereas only about 20% of gastroenteropancreatic (GEP) tumors express CCK2R. When investigating stromal tumors, all tissue samples of stromal ovarian cancers and ~50% of GIST expressed CCK2R. An incidence of about 50% was also found for SCLC and astrocytoma. A receptor density of >5000 dpm/mg tissue was found for MTC and GIST and also SCLC shows a high receptor density [16,18]. Therefore, these tumors seem most promising for CCK2R targeting. High receptor incidence and density, together with a rapid clearance of the radioligand from the blood and non-targeted tissues lays the basis for achieving sufficient target-to-non-target ratios [30]. These requirements are often fulfilled by regulatory peptide based radiopharmaceuticals acting as GPCR-agonists, which are internalized into the cell. Binding of a receptor agonist is followed by internalization of the peptide-receptor complex. After internalization, the receptor either undergoes degradation in the lysosome or is recycled to the cell surface. Radiometal-chelator conjugated amino acids formed by intralysosomal degradation of radiolabeled peptide analogs are not recognized by transport proteins and therefore cause accumulation and long-term retention of radioactivity in the target cells [75,77]. Internalization of the radiopeptide was assumed to be a basic requirement for successful diagnostic and therapeutic application [91]. Most of the radiolabeled CCK2R targeting peptide analogs developed are based on the natural ligands CCK and MG and are therefore agonists.
In the last years, several studies, especially focusing on somatostatin analogs, revealed that also non-internalizing receptor antagonists, due to their higher number of binding sites show a high potential to be used for nuclear medicine applications [92–94]. On the basis of benzodiazepines non-peptidic radioiodinated CCK2R antagonists haven been developed [95]. Via introduction of different spacers, the CCK2R antagonist Z-360 was conjugated to a chelating agent and radiolabeled with technetium-99m. Biodistribution studies in nu/nu mice xenografted with HEK293 cells stably transfected with CCK2R and CCK2i4svR revealed a high tumor uptake of 12% IA/g 4 h p.i., however, also the uptake in liver and kidneys was comparably high [96]. The use of an antagonist would have the clear advantage of less side effects in comparison to the possible biological effects of an agonist. The administration of radiolabeled CCK2R targeting peptides acting as agonists can be associated with various side effects, including nausea, flushes, cough, sensations of pressure in the chest and abdomen, sensations of paresthesia in the extremities, dizziness, as well as temporary change in blood pressure and heart rate with no change of the ECG pattern. The effects reported were generally mild, lasted only a few minutes and resolved spontaneously without intervention. Slow injection of the radiopeptide is recommended to avoid such biological effects.
Not only the chelator, but the radionuclide too plays a crucial role in the pharmacological behavior of the radiolabeled peptide analog. Furthermore, the same chelator labeled with different radiometals can form metal chelates with varying charge and hydrophilicity influencing the biodistribution profile of the radioligand. Chelator and radiometal were shown to greatly affect the targeting properties of radiolabeled somatostatin antagonists [6]. For CCK2R targeting peptide analogs only a limited influence on the tumor uptake and tumor-to-kidney ratio was described, as exemplified by PP-F11 conjugated to different chelators and labeled with different radiometals [72].
The main limitation of the CCK2R targeting peptide analogs currently investigated in clinical trials remains their high sensitivity toward enzymatic degradation in vivo explaining the rather lower tumor radiation doses achievable with 177Lu-DOTA-PP-F11 and 177Lu-DOTA-PP-F11N. It seems therefore of particular importance to avoid fast degradation during blood circulation to allow that the radiopeptide can reach its target in an intact state. In preclinical investigations it was demonstrated for different peptide analogs, such as somatostatin, bombesin and gastrin analogs [32,97], that by increasing the in vivo stability and bioavailability of the radiopeptide through co-administration of enzyme inhibitors a highly improved targeting profile can be achieved. These findings give high promise that stabilization against enzymatic degradation may lead to higher diagnostic sensitivity as well as improved therapeutic efficacy. It has been shown in previous studies that angiotensin-converting enzyme (ACE), neutral endopeptidase (NEP or neprilysin) and aminopeptidase A (APA) are major enzymes involved in the degradation of CCK and gastrin analogs [98,99]. Both, CCK and gastrin analogs, are potential substrates of ACE and NEP. However, MG analogs containing two or more Glu residues seem to be ACE resistant [32]. Radiolabeled peptide analogs with N-terminal conjugation of a chelator are protected against degradation by APA. Therefore, only the effect of ACE inhibition using lisiniprol (Lis) as well as NEP inhibition with phosphoramidon (PA) or thiorphan (TO) was evaluated for radiolabeled gastrin analogs [42,51,100,101]. With both NEP inhibitors a highly improved tumor targeting was achieved for 111In-DOTA-MG11, which was higher for co-injection of PA versus TO, whereas no effect was observed for ACE inhibition with Lis [101]. Comparative studies comparing the effect of PA for three 111In-labeled peptide analogs revealed that NEP inhibition was effective only for 111In-DOTA-MG11 and resulted in 70% intact radiopeptide in the blood of male Swiss albino mice 5 min p.i. (versus <4% without PA). This stabilization also led to a significantly increased tumor uptake in xenografted male SCID mice with values of ~15% IA/g 4 h p.i. in AR42J xenografts and 10-16% IA/g in A431-CCK2R xenografts versus 2-3% IA/g without PA [101]. For 111In-DOTA-MG0 and 111In-DOTA-PP-F11 containing the penta-Glu sequence only a minor effect on the in vivo stability (>85% intact radiopeptide versus 70% without PA) was observed. Still, the tumor uptake in A431-CCK2R xenografts increased from 8% to 16% IA/g at 4 h p.i. for 111In-DOTA-PP-F11 and from 12% to 17% IA/g at 4 h p.i. for 111In-DOTA-MG0, underlining that minor improvements in stability lead to highly improved targeting properties [100]. Similar results have been reported also for 99mTc-labeled gastrin analogs of different length [51]. When analyzing the susceptibility of 177Lu-DOTA-PP-F11N, 177Lu-DOTA-PP-F11 and 177Lu-DOTA-MG11 against different extracellular and intracellular proteases in vitro, cleavage by NEP was confirmed for 177Lu-DOTA-MG11 and 177Lu-DOTA-PP-F11, whereas 177Lu-DOTA-PP-F11N with replacement of Met by Nle was not susceptible to NEP. NEP inhibition with PA and TO in vivo was, however, effective only for 177Lu-DOTA-MG11 leading to a highly improved targeting profile with more than 3-fold increased uptake in A431-CCK2R xenografts in female nude mice. For 177Lu-DOTA-PP-F11 and 177Lu-DOTA-PP-F11N only a slightly enhanced uptake was observed in A431-CCK2R xenografts, confirming the limited utility of enzyme inhibitors for the radiolabeled MG analogs currently investigated in clinical studies [42]. For PA no extensive toxicity studies are available and the clinically certified NEP inhibitors seem to show a lower effect on the targeting properties. Different issues such as solubility, safety, efficacy and potential route of administration still need to be addressed, hindering the clinical translation of this concept [100,101].
The goal to optimize CCK2R targeting seems more likely achievable through chemical stabilization of the radiolabeled peptide itself. The stabilization strategies of CCK2R targeting peptide analogs reported so far mainly focused on the introduction of stabilizing substitutions close to the N-terminus of the peptide, although enzymatic cleavage sites are also known within the C-terminal receptor binding sequence Trp-Met-Asp-Phe-NH2 [42,59]. The different peptide derivatives developed still present enzymatic cleavage sites, thus a sufficient stabilization against enzymatic degradation seems to be achievable only by adequate stabilization of the receptor specific C-terminus. The development of a stabilization strategy for this particular region of the peptide sequence is challenging due to the risk of losing CCK2R affinity. An interesting approach directed to the stabilization of the peptide backbone is the amide-to-triazole substitution [102].
However, when comparing NEP-inhibition versus triazole substitution of different radiolabeled bombesin analogs, a higher in vivo stability of the radiopeptides was found for NEP-inhibition of the unsubstituted bombesin analog when compared to mono and bis-triazole-substituted analogs (88% intact radiopeptide versus 30-45% at 5 min p.i. in male Swiss albino mice). Biodistribution studies in SCID mice bearing human prostate adenocarcinoma PC-3 xenografts expressing the gastrin-releasing peptide receptor (GRPR) revealed a threefold higher tumor uptake for co-injection of PA in comparison to triazole-substitution (~25 versus ~8 %IA/g for h p.i.) [102]. Triazole substitution is currently investigated also for MG analogues with varying effects on receptor affinity and tumor uptake [104].
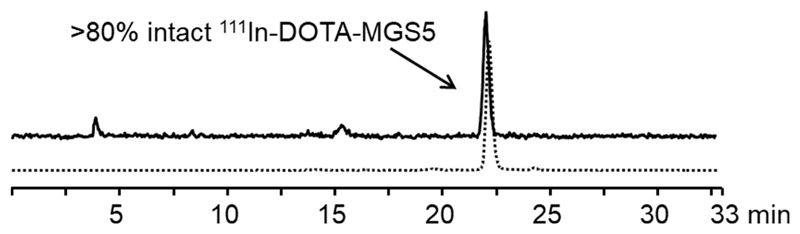
Based on evidence in the literature, that CCK analogs substituted within the C-terminal peptide sequence with unnatural amino acids containing bulky aromatic side chains or N-methylated peptide bonds are highly resistant to enzymatic degradation [105], a new series of radiolabeled MG analogs has been developed recently. By applying specific amino acid substitutions, such as aromatic and N-methylated amino acids, within the receptor specific C-terminus of MG11 new radiopeptides with promising preclinical characteristics were developed [106–108] which are summarized in Table 4. Single substitution of phenylalanine (Phe) with 1-naphthylalanine (1-Nal) or N-methyl-phenylalanine ((N-Me)Phe) as well as additional substitution of Met with phenylglycine or N-methyl-norleucine ((N-Me)Nle) was investigated. Based on the preclinical investigations performed, including receptor affinity and cell uptake assays, as well as stability and biodistribution studies, specific amino acid substitutions were identified, that allow to retain receptor affinity. Furthermore it was found that two substitutions in the C-terminal region seemed to be required for efficient stabilization and improved tumor targeting [106]. For 111In-DOTA-MGS4 showing replacement of Met by (N-Me)Nle and of Phe by (N-Me)Phe a promising in vivo stability (~80% intact radiopeptide 10 min p.i. in the blood of female BALB/c mice) as well as a high tumor uptake (~10% IA/g 4 h p.i. in A431-CCK2R xenografted female BALB/c nude mice) was observed. 111In-DOTA-MGS4 therefore combines the high tumor uptake of 111In-DOTA-MG0 with the reduced kidney uptake of 111In-DOTA-MG11 leading to an improved tumor-to-kidney ratio of ~3. With 111In-DOTA-MGS5 showing replacement of Met by (N-Me)Nle and of Phe by 1-Nal these promising results could be further improved. For 111In-DOTA-MGS5 an unexpectedly high cell uptake was observed in vitro (~50% in A431-CCK2R cells after 2 h incubation). In addition, as illustrated in fig. 1, an improved in vivo stability was confirmed in the blood of female BALB/C mice injected with 111In-DOTA-MGS5 (83% intact radiopeptide at 10 min p.i.; n=2). Similar results were obtained also for 177Lu-DOTA-MGS5 (86% and 70% intact radiopeptide at 10 min and 1 h p.i., respectively) [107]. The combination of increased cell uptake and improved resistance against enzymatic degradation led to a further increase in tumor uptake independently of using indium-111, gallium-68 or lutetium-177 for labeling (19-25% IA/g in A431-CCK2R tumor xenografts, 1 h or 4 h p.i.).
Table 4. Amino acid sequence of C-terminally stabilized CCK2R targeting peptide analogs suitable for labeling with radiometals.
| Peptide derivative | Radionuclide | Radiopeptide |
|---|---|---|
| [DOTA0,DGlu1,DesGlu2-6,1-Nal13]minigastrin | indium-111 | 111In-DOTA-MGS1 |
| [DOTA0,DGlu1,DesGlu2-6,(N-Me)Nle11,(N-Me)Phe13]minigastrin | indium-111 | 111In-DOTA-MGS4 |
| [DOTA0,DGlu1,DesGlu2-6,(N-Me)Nle11,1-Nal13]minigastrin | gallium-68 indium-111 lutetium-177 |
69Ga-DOTA-MGS5 111In-DOTA-MGS5 177Lu-DOTA-MGS5 |
| [HYNIC0,DGlu1,DesGlu2-6,(N-Me)Nle11,1-Nal13]minigastrin | technetium-99m | 99mTc-HYNIC-MGS5 |
| [HYNIC0,DGlu1,DesGlu2-6,(N-Me)Nle11,(N-Me)1-Nal13]minigastrin | technetium-99m | 99mTc-HYNIC-MGS11 |
Fig. 1.
Radio-HPLC analysis of the blood obtained from a BALB/c mouse 10 min after intravenous injection of 111In-DOTA-MGS5 (~10 MBq, ~2 nmol); dashed line showing the radiochromatogram of the radioligand after preparation.
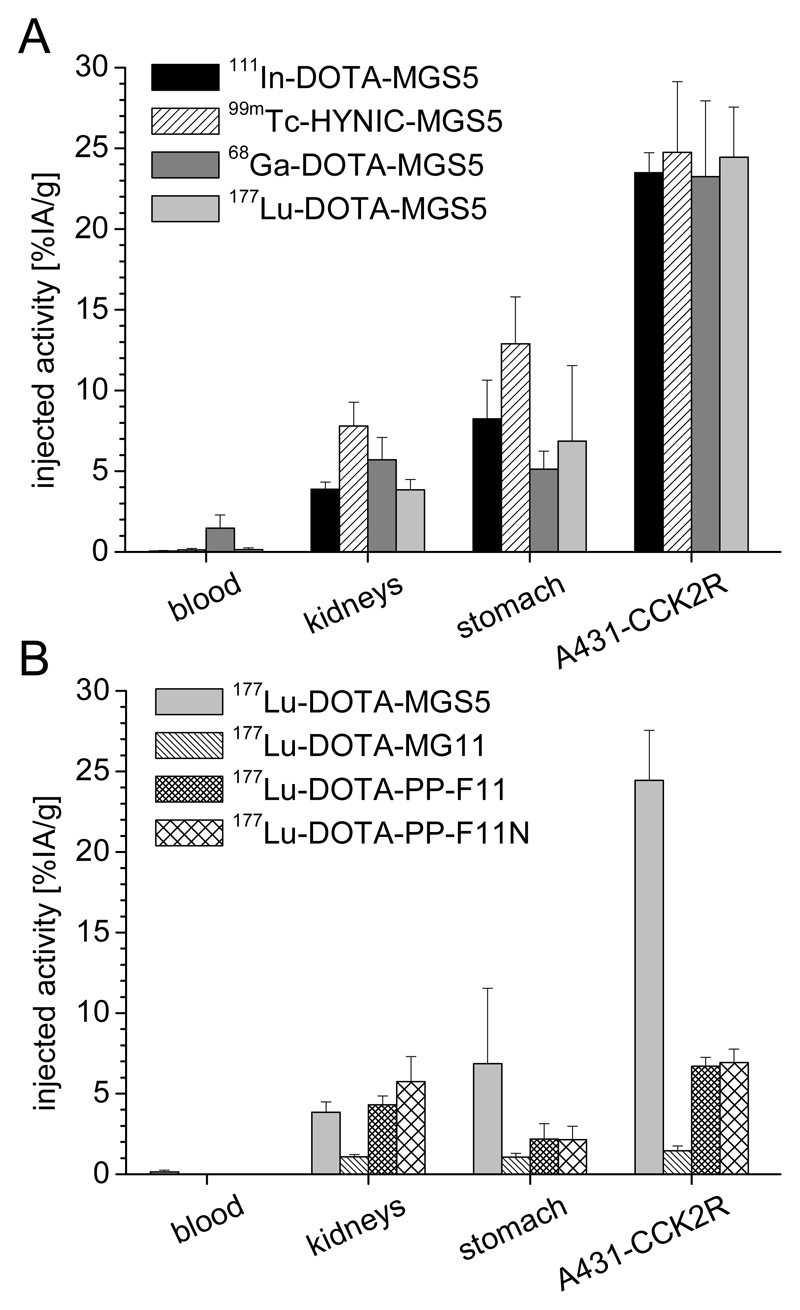
A somewhat increased uptake was additionally observed in CCK2R expressing stomach and pancreas, underlining a receptor-specific effect [107]. Based on these promising results also two HYNIC-conjugated peptide conjugates labeled with technetium-99m were developed. Besides investigating HYNIC-conjugated MGS5, a second N-methylated peptide bond between aspartic acid and 1-Nal was introduced in HYNIC-MGS11 in the attempt to further stabilize the C-terminal region. 99mTc-HYNIC-MGS5 showed an in vivo stability (~70% intact radiopeptide in the blood of female BALB/c mice 10 min p.i.) and tumor uptake (~25% IA/g in A431-CCK2R/mock xenografted female BALB/c nude mice 1 h and 4 h p.i.) comparable to 111In-DOTA-MGS5. The introduction of an additional N-methylated peptide bond in 99mTc-HYNIC-MGS11 led to a further increased resistance against enzymatic degradation (~95% intact radiopeptide) together with an almost doubled tumor uptake (~40% IA/g, 1 h and 4 h p.i.) when compared to 99mTc-HYNIC-MGS5. This increased tumor uptake was, however, also connected with increased kidney retention in comparison with 99mTc-HYNIC-MGS5 (~17% IA/g versus 8% IA/g at 4 h p.i.) resulting in comparable tumor-to-kidney ratios of 2-3 [108]. The new MG analogs clearly outperform the radiopeptides currently used in clinical trials. Of particular importance are the results achieved with DOTA-MGS5 and HYNIC-MGS5 (see fig. 2). The two peptide analogs radiolabeled with different radiometals, indium-111 or technetium-99m for SPECT, gallium-68 for PET and lutetium-177 for PRRT, show a three-fold higher tumor uptake in comparison with 177Lu-DOTA-PP-F11 and 177Lu-DOTA-PP-F11N (20-24% IA/g versus ~7 in A431-CCK2R xenografted nude mice at 1-4 h p.i.) [42,107]. Furthermore, the tumor-to-kidney ratio with values of 6.5 for 177Lu-DOTA-MGS5 at 4 h p.i. versus 1.2-1.6 reported for 177Lu-DOTA-PP-F11 and 177Lu-DOTA-PP-F11N is highly improved. DOTA-MGS5 therefore fulfils all important criteria for diagnostic and therapeutic use in nuclear medicine and might therefore be a powerful new peptide probe for the diagnosis and treatment of patients with CCK2R expressing tumors [109].
Fig. 2.
Biodistribution profile in A431-CCK2R xenografted BALB/c nude mice of (A) DOTA-MGS5 radiolabeled with different radiometals (111In/177Lu/99mTc: 4 h p.i.; 68Ga: 1 h p.i.) and (B) 177Lu-DOTA-MGS5 in comparison with other radioligands at 4 h p.i. [42,107]; data are expressed as mean ± SD.
Conclusion
Over the past decades, a variety of CCK2R targeting peptide analogs has been developed for both diagnostic and therapeutic use in nuclear medicine applications. Based on first preclinical studies comparing radiopeptides derived from the natural ligands, MG and CCK, MG analogs were selected as most promising for further development. Kidney uptake was efficiently reduced by truncation of the penta-Glu sequence. In order to counteract the thereby caused high susceptibility towards enzymatic degradation in vivo, various stabilization strategies, such as the introduction of different linkers or unnatural amino acids, modification of peptide bonds, as well as multimerization or cyclization of the peptide sequence, were investigated. In situ stabilization with enzyme inhibitors as well as modification of the peptide backbone has shown to increase the bioavailability of MG analogs in vivo. Recent developments achieving radiolabeled MG analogs with highly improved stability against enzymatic degradation in vivo through site-specific modifications of the C-terminal receptor specific sequence Trp-Met-Asp-Phe-NH2 promise a further improvement in tumor uptake while limiting the radiation dose delivered to the kidneys. Clinical studies investigating the diagnostic and therapeutic potential of DOTA-MGS5 radiolabeled with different radiometals, such as the use of 68Ga-DOTA-MGS5 for high sensitivity PET/CT as well as PRRT with 177Lu-DOTA-MGS5 in patients with progressive or metastatic MTC are warranted, to elucidate the potential of this new peptide analog in the clinical management of patients with CCK2R expressing tumors.
Acknowledgements
The Austrian Science Fund (FWF) is acknowledged for financial support (project number P 27844).
Footnotes
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- [1].van der Meel R, Gallagher WM, Oliveira S, O'Connor AE, Schiffelers RM, Byrne AT. Recent Advances in Molecular Imaging Biomarkers in Cancer: Application of Bench to Bedside Technologies. Drug Discov Today. 2010;15(3–4):102–114. doi: 10.1016/j.drudis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- [2].Moody TW, Ramos-Alvarez I, Jensen RT. Neuropeptide G Protein-Coupled Receptors as Oncotargets. Front Endocrinol (Lausanne) 2018;9(June):345. doi: 10.3389/fendo.2018.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karpuz M, Silindir-Gunay M, Ozer AY. Current and Future Approaches for Effective Cancer Imaging and Treatment. Cancer Biother Radiopharm. 2018;33(2):39–51. doi: 10.1089/cbr.2017.2378. [DOI] [PubMed] [Google Scholar]
- [4].Reubi JC, Waser B. Concomitant Expression of Several Peptide Receptors in Neuroendocrine Tumours: Molecular Basis for in Vivo Multireceptor Tumour Targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- [5].Fani M, Peitl PK, Velikyan I. Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms. Pharmaceuticals (Basel) 2017;10(1):1–22. doi: 10.3390/ph10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fani M, Braun F, Waser B, Beetschen K, Cescato R, Erchegyi J, Rivier JE, Weber WA, Maecke HR, Reubi JC. Unexpected Sensitivity of Sst2 Antagonists to N-Terminal Radiometal Modifications. J Nucl Med. 2012;53(9):1481–1489. doi: 10.2967/jnumed.112.102764. [DOI] [PubMed] [Google Scholar]
- [7].Dash A, Chakraborty S, Pillai MRA, Knapp FF. Peptide Receptor Radionuclide Therapy: An Overview. Cancer Biother Radiopharm. 2015;30(2):47–71. doi: 10.1089/cbr.2014.1741. [DOI] [PubMed] [Google Scholar]
- [8].Nicolas GP, Morgenstern A, Schottelius M, Fani M. New Developments in Peptide Receptor Radionuclide Therapy. J Nucl Med. 2018;60(2):167–171. doi: 10.2967/jnumed.118.213496. [DOI] [PubMed] [Google Scholar]
- [9].Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, Lameris JS, Reubi JC, Lamberts SW. Localisation of Endocrine-Related Tumours with Radioiodinated Analogue of Somatostatin. Lancet (London, England) 1989;1(8632):242–244. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- [10].Lamberts SWJ, Reubi JC, Krenning EP. Validation of Somatostatin Receptor Scintigraphy in the Localization of Neuroendocrine Tumors. Acta Oncol (Madr) 1993;32(2):167–170. doi: 10.3109/02841869309083907. [DOI] [PubMed] [Google Scholar]
- [11].Fani M, Maecke HR. Radiopharmaceutical Development of Radiolabelled Peptides. Eur J Nucl Med Mol Imaging. 2012;39(S1):11–30. doi: 10.1007/s00259-011-2001-z. [DOI] [PubMed] [Google Scholar]
- [12].Turner JH. An Introduction to the Clinical Practice of Theranostics in Oncology. Br J Radiol. 2018;91(1091) doi: 10.1259/bjr.20180440. 20180440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kjaer A, Knigge U. Use of Radioactive Substances in Diagnosis and Treatment of Neuroendocrine Tumors. Scand J Gastroenterol. 2015;50(6):740–747. doi: 10.3109/00365521.2015.1033454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-Octreotide PET in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and CT. J Nucl Med. 2007;48(4):508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- [15].Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, et al. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reubi JC. Targeting CCK Receptors in Human Cancers. Curr Top Med Chem. 2007;7(12):1239–1242. doi: 10.2174/156802607780960546. [DOI] [PubMed] [Google Scholar]
- [17].Sanchez C, Escrieut C, Clerc P, Gigoux V, Waser B, Reubi JC, Fourmy D. Characterization of a Novel Five-Transmembrane Domain Cholecystokinin-2 Receptor Splice Variant Identified in Human Tumors. Mol Cell Endocrinol. 2012;349(2):170–179. doi: 10.1016/j.mce.2011.10.010. [DOI] [PubMed] [Google Scholar]
- [18].Reubi JC, Schaer JC, Waser B. Cholecystokinin(CCK)-A and CCK-B/Gastrin Receptors in Human Tumors. Cancer Res. 1997;57(7):1377–1386. [PubMed] [Google Scholar]
- [19].Liu H, Wang X, Yang R, Zeng W, Peng D, Li J, Wang H. Recent Development of Nuclear Molecular Imaging in Thyroid Cancer. Biomed Res Int. 2018;2018 doi: 10.1155/2018/2149532. 2149532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ganeshan D, Paulson E, Duran C, Cabanillas ME, Busaidy NL, Charnsangavej C. Current Update on Medullary Thyroid Carcinoma. Am J Roentgenol. 2013;201(6):867–876. doi: 10.2214/AJR.12.10370. [DOI] [PubMed] [Google Scholar]
- [21].Viola D, Elisei R. Management of Medullary Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48(1):285–301. doi: 10.1016/j.ecl.2018.11.006. [DOI] [PubMed] [Google Scholar]
- [22].Machens A, Dralle H. Biomarker-Based Risk Stratification for Previously Untreated Medullary Thyroid Cancer. J Clin Endocrinol Metab. 2010;95(6):2655–2663. doi: 10.1210/jc.2009-2368. [DOI] [PubMed] [Google Scholar]
- [23].Maia AL, Wajner SM, Ferreira Vargas CV. Advances and Controversies in the Management of Medullary Thyroid Carcinoma. Curr Opin Oncol. 2017;29(1):25–32. doi: 10.1097/CCO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- [24].Treglia G, Castaldi P, Villani MF, Perotti G, de Waure C, Filice A, Ambrosini V, Cremonini N, Santimaria M, Versari A, Fanti S, et al. Comparison of 18F-DOPA, 18F-FDG and 68Ga-Somatostatin Analogue PET/CT in Patients with Recurrent Medullary Thyroid Carcinoma. Eur J Nucl Med Mol Imaging. 2012;39(4):569–580. doi: 10.1007/s00259-011-2031-6. [DOI] [PubMed] [Google Scholar]
- [25].Magnan R, Masri B, Escrieut C, Foucaud M, Cordelier P, Fourmy D. Regulation of Membrane Cholecystokinin-2 Receptor by Agonists Enables Classification of Partial Agonists as Biased Agonists. J Biol Chem. 2011;286(8):6707–6719. doi: 10.1074/jbc.M110.196048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TVO. The Biology of Cholecystokinin and Gastrin Peptides. Curr Top Med Chem. 2007;7(12):1154–1165. doi: 10.2174/156802607780960483. [DOI] [PubMed] [Google Scholar]
- [27].Smeets RL, Fouraux MA, van Emst-de Vries SE, De Pont JJ, Willems PH. Protein Kinase C-Mediated Inhibition of Transmembrane Signalling through CCK(A) and CCK(B) Receptors. Br J Pharmacol. 1998;123(6):1189–1197. doi: 10.1038/sj.bjp.0701713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dufresne M, Seva C, Fourmy D. Cholecystokinin and Gastrin Receptors. Physiol Rev. 2006;86(3):805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- [29].Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. Structure, Distribution, and Functions of Cholecystokinin Receptors. Pharmacol Rev. 1999;51(4):745–781. [PubMed] [Google Scholar]
- [30].Fani M, Maecke HR, Okarvi SM. Radiolabeled Peptides: Valuable Tools for the Detection and Treatment of Cancer. Theranostics. 2012;2(5):481–501. doi: 10.7150/thno.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roosenburg S, Laverman P, van Delft FL, Boerman OC. Radiolabeled CCK/Gastrin Peptides for Imaging and Therapy of CCK2 Receptor-Expressing Tumors. Amino Acids. 2011;41(5):1049–1058. doi: 10.1007/s00726-010-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaloudi A, Nock BA, Krenning EP, Maina T, De Jong M. Radiolabeled Gastrin/CCK Analogs in Tumor Diagnosis: Towards Higher Stability and Improved Tumor Targeting. Q J Nucl Med Mol Imaging. 2015;59(3):287–302. [PubMed] [Google Scholar]
- [33].Aloj L, Panico MR, Caracó C, Zannetti A, Del Vecchio S, Di Nuzzo C, Arra C, Morelli G, Tesauro D, De Luca S, Pedone C, et al. Radiolabeling Approaches for Cholecystokinin B Receptor Imaging. Biopolymers. 2002;66(6):370–380. doi: 10.1002/bip.10347. [DOI] [PubMed] [Google Scholar]
- [34].Behr TM, Jenner N, Radetzky S, Béhe M, Gratz S, Yücekent S, Raue F, Becker W. Targeting of Cholecystokinin-B/Gastrin Receptors in Vivo: Preclinical and Initial Clinical Evaluation of the Diagnostic and Therapeutic Potential of Radiolabelled Gastrin. Eur J Nucl Med. 1998;25(4):424–430. doi: 10.1007/s002590050241. [DOI] [PubMed] [Google Scholar]
- [35].Behr TM, Béhé M, Angerstein C, Gratz S, Mach R, Hagemann L, Jenner N, Stiehler M, Frank-Raue K, Raue F, Becker W. Cholecystokinin-B/Gastrin Receptor Binding Peptides: Preclinical Development and Evaluation of Their Diagnostic and Therapeutic Potential. Clin Cancer Res. 1999;5(10 Suppl):3124s–3138s. [PubMed] [Google Scholar]
- [36].von Guggenberg E, Behe M, Behr TM, Saurer M, Seppi T, Decristoforo C. 99mTc-Labeling and in Vitro and in Vivo Evaluation of HYNIC- and (Nalpha-His)Acetic Acid-Modified [D-Glu1]-Minigastrin. Bioconjug Chem. 2004;15(4):864–871. doi: 10.1021/bc0300807. [DOI] [PubMed] [Google Scholar]
- [37].de Jong M, Bakker WH, Bernard BF, Valkema R, Kwekkeboom DJ, Reubi JC, Srinivasan A, Schmidt M, Krenning EP. Preclinical and Initial Clinical Evaluation of 111In-Labeled Nonsulfated CCK8 Analog: A Peptide for CCK-B Receptor-Targeted Scintigraphy and Radionuclide Therapy. J Nucl Med. 1999;40(12):2081–2087. [PubMed] [Google Scholar]
- [38].Laverman P, Roosenburg S, Gotthardt M, Park J, Oyen WJG, De Jong M, Hellmich MR, Rutjes FPJT, Van Delft FL, Boerman OC. Targeting of a CCK2 Receptor Splice Variant with 111In-Labelled Cholecystokinin-8 (CCK8) and 111In-Labelled Minigastrin. Eur J Nucl Med Mol Imaging. 2008;35(2):386–392. doi: 10.1007/s00259-007-0604-1. [DOI] [PubMed] [Google Scholar]
- [39].Laverman P, Béhé M, Oyen WJG, Willems PHGM, Corstens FHM, Behr TM, Boerman OC. Two Technetium-99m-Labeled Cholecystokinin-8 (CCK8) Peptides for Scintigraphic Imaging of CCK Receptors. Bioconjug Chem. 2004;15(3):561–568. doi: 10.1021/bc034208w. [DOI] [PubMed] [Google Scholar]
- [40].Aloj L, Caracò C, Panico M, Zannetti A, Del Vecchio S, Tesauro D, De Luca S, Arra C, Pedone C, Morelli G, Salvatore M. In Vitro and in Vivo Evaluation of 111In-DTPAGlu-G-CCK8 for Cholecystokinin-B Receptor Imaging. J Nucl Med. 2004;45(3):485–494. [PubMed] [Google Scholar]
- [41].Aloj L, Aurilio M, Rinaldi V, D'ambrosio L, Tesauro D, Peitl PK, Maina T, Mansi R, Von Guggenberg E, Joosten L, Sosabowski JK, et al. Comparison of the Binding and Internalization Properties of 12 DOTA-Coupled and 111In-Labelled CCK2/Gastrin Receptor Binding Peptides: A Collaborative Project under COST Action BM0607. Eur J Nucl Med Mol Imaging. 2011;38(8):1417–1425. doi: 10.1007/s00259-011-1816-y. [DOI] [PubMed] [Google Scholar]
- [42].Sauter AW, Mansi R, Hassiepen U, Muller L, Panigada T, Wiehr S, Wild AM, Geistlich S, Béhé M, Rottenburger C, Wild D, et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J Nucl Med. 2019;60(3):393–399. doi: 10.2967/jnumed.118.207845. [DOI] [PubMed] [Google Scholar]
- [43].Behr TM, Jenner N, Béhé M, Angerstein C, Gratz S, Raue F, Becker W. Radiolabeled Peptides for Targeting Cholecystokinin-B/Gastrin Receptor-Expressing Tumors. J Nucl Med. 1999;40(6):1029–1044. [PubMed] [Google Scholar]
- [44].Béhé M, Becker W, Gotthardt M, Angerstein C, Behr TM. Improved Kinetic Stability of DTPA-DGlu as Compared with Conventional Monofunctional DTPA in Chelating Indium and Yttrium: Preclinical and Initial Clinical Evaluation of Radiometal Labelled Minigastrin Derivatives. Eur J Nucl Med Mol Imaging. 2003;30(8):1140–1146. doi: 10.1007/s00259-003-1178-1. [DOI] [PubMed] [Google Scholar]
- [45].Béhé M, Kluge G, Becker W, Gotthardt M, Behr TM. Use of Polyglutamic Acids to Reduce Uptake of Radiometal-Labeled Minigastrin in the Kidneys. J Nucl Med. 2005;46(6):1012–1015. [PubMed] [Google Scholar]
- [46].Gotthardt M, van Eerd-Vismale J, Oyen W, de Jong M, Zhang H, Rolleman E, Maecke HR, Behe M, Boerman O. Indication for different mechanisms of kidney uptake of radiolabeled peptides. Eur J Nucl Med. 2007;48(4):596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- [47].Good S, Walter MA, Waser B, Wang X, Müller-Brand J, Béhé MP, Reubi JC, Maecke HR. Macrocyclic Chelator-Coupled Gastrin-Based Radiopharmaceuticals for Targeting of Gastrin Receptor-Expressing Tumours. Eur J Nucl Med Mol Imaging. 2008;35(10):1868–1877. doi: 10.1007/s00259-008-0803-4. [DOI] [PubMed] [Google Scholar]
- [48].Aloj L, Panico M, Caraco C, Del Vecchio S, Arra C, Affuso A, Accardo A, Mansi R, Tesauro D, De Luca S, Pedone C, et al. In Vitro and in Vivo Characterization of Indium-111 and Technetium-99m Labeled CCK-8 Derivatives for CCK-B Receptor Imaging. Cancer Biother Radiopharm. 2004;19(1):93–98. doi: 10.1089/108497804773391739. [DOI] [PubMed] [Google Scholar]
- [49].von Guggenberg E, Dietrich H, Skvortsova I, Gabriel M, Virgolini IJ, Decristoforo C. 99mTc-Labelled HYNIC-Minigastrin with Reduced Kidney Uptake for Targeting of CCK-2 Receptor-Positive Tumours. Eur J Nucl Med Mol Imaging. 2007;34(8):1209–1218. doi: 10.1007/s00259-006-0348-3. [DOI] [PubMed] [Google Scholar]
- [50].D'Andrea LD, Testa I, Panico M, Di Stasi R, Caracò C, Tarallo L, Arra C, Barbieri A, Romanelli A, Aloj L. In Vivo and in Vitro Characterization of CCK8 Bearing a Histidine-Based Chelator Labeled with 99mTc-Tricarbonyl. Biopolymers. 2008;90(5):707–712. doi: 10.1002/bip.21041. [DOI] [PubMed] [Google Scholar]
- [51].Kaloudi A, Nock BA, Lymperis E, Krenning EP, de Jong M, Maina T. 99mTc-Labeled Gastrins of Varying Peptide Chain Length: Distinct Impact of NEP/ACE-Inhibition on Stability and Tumor Uptake in Mice. Nucl Med Biol. 2016;43(6):347–354. doi: 10.1016/j.nucmedbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- [52].Nock BA, Maina T, Béhé M, Nikolopoulou A, Gotthardt M, Schmitt JS, Behr TM, Mäcke HR. CCK-2/Gastrin Receptor-Targeted Tumor Imaging with (99m)Tc-Labeled Minigastrin Analogs. J Nucl Med. 2005;46(10):1727–1736. [PubMed] [Google Scholar]
- [53].Sosabowski JK, Matzow T, Foster JM, Finucane C, Ellison D, Watson SA, Mather SJ. Targeting of CCK-2 Receptor-Expressing Tumors Using a Radiolabeled Divalent Gastrin Peptide. J Nucl Med. 2009;50(12):2082–2089. doi: 10.2967/jnumed.109.064808. [DOI] [PubMed] [Google Scholar]
- [54].Summer D, Kroess A, Woerndle R, Rangger C, Klingler M, Haas H, Kremser L, Lindner HH, von Guggenberg E, Decristoforo C. Multimerization Results in Formation of Re-Bindable Metabolites: A Proof of Concept Study with FSC-Based Minigastrin Imaging Probes Targeting CCK2R Expression. PLoS One. 2018;13(7):e0201224. doi: 10.1371/journal.pone.0201224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Summer D, Rangger C, Klingler M, Laverman P, Franssen GM, Lechner BE, Orasch T, Haas H, von Guggenberg E, Decristoforo C. Exploiting the Concept of Multivalency with 68Ga- and 89Zr-Labelled Fusarinine C-Minigastrin Bioconjugates for Targeting CCK2R Expression. Contrast Media Mol Imaging. 2018;2018 doi: 10.1155/2018/3171794. 3171794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].von Guggenberg E, Sallegger W, Helbok A, Ocak M, King R, Mather SJ, Decristoforo C. Cyclic Minigastrin Analogues for Gastrin Receptor Scintigraphy with Technetium-99m: Preclinical Evaluation. J Med Chem. 2009;52(15):4786–4793. doi: 10.1021/jm900400w. [DOI] [PubMed] [Google Scholar]
- [57].Von Guggenberg E, Rangger C, Sosabowski J, Laverman P, Reubi JC, Virgolini IJ, Decristoforo C. Preclinical Evaluation of Radiolabeled DOTA-Derivatized Cyclic Minigastrin Analogs for Targeting Cholecystokinin Receptor Expressing Malignancies. Mol Imaging Biol. 2012;14(3):366–375. doi: 10.1007/s11307-011-0506-2. [DOI] [PubMed] [Google Scholar]
- [58].Kolenc-Peitl P, Mansi R, Tamma M, Gmeiner-Stopar T, Sollner-Dolenc M, Waser B, Baum RP, Reubi JC, Maecke HR. Highly Improved Metabolic Stability and Pharmacokinetics of Indium-111-DOTA-Gastrin Conjugates for Targeting of the Gastrin Receptor. J Med Chem. 2011;54(8):2602–2609. doi: 10.1021/jm101279a. [DOI] [PubMed] [Google Scholar]
- [59].Ocak M, Helbok A, Rangger C, Peitl PK, Nock BA, Morelli G, Eek A, Sosabowski JK, Breeman WAP, Reubi JC, Decristoforo C. Comparison of Biological Stability and Metabolism of CCK2 Receptor Targeting Peptides, a Collaborative Project under COST BM0607. Eur J Nucl Med Mol Imaging. 2011;38(8):1426–1435. doi: 10.1007/s00259-011-1818-9. [DOI] [PubMed] [Google Scholar]
- [60].Laverman P, Joosten L, Eek A, Roosenburg S, Peitl PK, Maina T, Mäcke H, Aloj L, Von Guggenber E, Sosabowski JK, De Jong M, et al. Comparative Biodistribution of 12 111In-Labelled Gastrin/CCK2 Receptor-Targeting Peptides. Eur J Nucl Med Mol Imaging. 2011;38(8):1410–1416. doi: 10.1007/s00259-011-1806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mather SJ, Mckenzie AJ, Sosabowski JK, Morris TM, Watson SA. Selection of Radiolabeled Gastrin Analogs for Peptide Receptor – Targeted Radionuclide Therapy. J Nucl Med. 2007;48(4):615–622. doi: 10.2967/jnumed.106.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Roosenburg S, Laverman P, Joosten L, Eek A, Oyen WJG, de Jong M, Rutjes FPJT, van Delft FL, Boerman OC. Stabilized 111In-Labeled SCCK8 Analogues for Targeting CCK2-Receptor Positive Tumors: Synthesis and Evaluation. Bioconjug Chem. 2010;21(4):663–670. doi: 10.1021/bc900465y. [DOI] [PubMed] [Google Scholar]
- [63].Roosenburg S, Laverman P, Joosten L, Eek A, Rutjes FPJT, van Delft FL, Boerman OC. In Vitro and in Vivo Characterization of Three 68Ga- and 111In-Labeled Peptides for Cholecystokinin Receptor Imaging. Mol Imaging. 2012;11(5):401–407. [PubMed] [Google Scholar]
- [64].Grob NM, Behe M, von Guggenberg E, Schibli R, Mindt TL. Methoxinine - an Alternative Stable Amino Acid Substitute for Oxidation-Sensitive Methionine in Radiolabelled Peptide Conjugates. J Pept Sci. 2017;23(1):38–44. doi: 10.1002/psc.2948. [DOI] [PubMed] [Google Scholar]
- [65].Helbok A, Decristoforo C, Behe M, Rangger C, Guggenberg E. Preclinical Evaluation of In-111 and Ga-68 Labelled Minigastrin Analogues for CCK-2 Receptor Imaging. Curr Radiopharm. 2009;2(4):304–310. [Google Scholar]
- [66].Pawlak D, Rangger C, Kolenc Peitl P, Garnuszek P, Maurin M, Ihli L, Kroselj M, Maina T, Maecke H, Erba P, et al. From Preclinical Development to Clinical Application: Kit Formulation for Radiolabelling the Minigastrin Analogue CP04 with In-111 for a First-in-Human Clinical Trial. Eur J Pharm Sci. 2016;85:1–9. doi: 10.1016/j.ejps.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maina T, Konijnenberg MW, KolencPeitl P, Garnuszek P, Nock BA, Kaloudi A, Kroselj M, Zaletel K, Maecke H, Mansi R, Erba P, et al. Preclinical Pharmacokinetics, Biodistribution, Radiation Dosimetry and Toxicity Studies Required for Regulatory Approval of a Phase I Clinical Trial with 111In-CP04 in Medullary Thyroid Carcinoma Patients. Eur J Pharm Sci. 2016;91:236–242. doi: 10.1016/j.ejps.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kolenc Peitl P, Tamma M, Kroselj M, Braun F, Waser B, Reubi JC, Sollner Dolenc M, Maecke HR, Mansi R. Stereochemistry of Amino Acid Spacers Determines the Pharmacokinetics of 111In-DOTA-Minigastrin Analogues for Targeting the CCK2/Gastrin Receptor. Bioconjug Chem. 2015;26(6):1113–1119. doi: 10.1021/acs.bioconjchem.5b00187. [DOI] [PubMed] [Google Scholar]
- [69].Konijnenberg MW, Breeman WAP, de Blois E, Chan HS, Boerman OC, Laverman P, Kolenc-Peitl P, Melis M, de Jong M. Therapeutic Application of CCK2R-Targeting PP-F11: Influence of Particle Range, Activity and Peptide Amount. EJNMMI Res. 2014;4(1):47. doi: 10.1186/s13550-014-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rangger C, Klingler M, Balogh L, Pöstenyi Z, Polyak A, Pawlak D, Mikolajczak R, von Guggenberg E. 177 Lu Labeled Cyclic Minigastrin Analogues with Therapeutic Activity in CCK2R Expressing Tumors: Preclinical Evaluation of a Kit Formulation. Mol Pharm. 2017;14(9):3045–3058. doi: 10.1021/acs.molpharmaceut.7b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Melis M, Vegt E, Konijnenberg MW, de Visser M, Bijster M, Vermeij M, Krenning EP, Boerman OC, de Jong M. Nephrotoxicity in Mice after Repeated Imaging Using 111In-Labeled Peptides. J Nucl Med. 2010;51(6):973–977. doi: 10.2967/jnumed.109.074310. [DOI] [PubMed] [Google Scholar]
- [72].Roosenburg S, Laverman P, Joosten L, Cooper MS, Kolenc-Peitl PK, Foster JM, Hudson C, Leyton J, Burnet J, Oyen WJG, Blower PJ, Mather SJ, et al. PET and SPECT Imaging of a Radiolabeled Minigastrin Analogue Conjugated with DOTA, NOTA, and NODAGA and Labeled with 64Cu, 68Ga, and 111In. Mol Pharm. 2014;11(11):3930–3937. doi: 10.1021/mp500283k. [DOI] [PubMed] [Google Scholar]
- [73].Maurin M, Garnuszek P, Baran P, Pawlak D, Mikolajczak R. The Radiometal Makes a Difference. Synthesis and Preliminary Characterisation of DOTA-Minigastrin Analogue Complexes with Ga, Lu and Y. Nucl Med Rev Cent East Eur. 2015;18(2):51–55. doi: 10.5603/NMR.2015.0014. [DOI] [PubMed] [Google Scholar]
- [74].Breeman WAP, Fröberg AC, de Blois E, van Gameren A, Melis M, de Jong M, Maina T, Nock BA, Erion JL, Mäcke HR, Krenning EP. Optimised Labeling, Preclinical and Initial Clinical Aspects of CCK-2 Receptor-Targeting with 3 Radiolabeled Peptides. Nucl Med Biol. 2008;35(8):839–849. doi: 10.1016/j.nucmedbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [75].Behr TM, Béhé M, Becker W. Diagnostic Applications of Radiolabeled Peptides in Nuclear Endocrinology. Q J Nucl Med. 1999;43(3):268–280. [PubMed] [Google Scholar]
- [76].Kwekkeboom DJ, Bakker WH, Kooij PPM, Erion J, Srinivasan A, de Jong M, Reubi J, Krenning EP. Cholecystokinin Receptor Imaging Using an Octapeptide DTPA-CCK Analogue in Patients with Medullary Thyroid Carcinoma. Eur J Nucl Med. 2000;27(9):1312–1317. doi: 10.1007/s002590000296. [DOI] [PubMed] [Google Scholar]
- [77].Béhé M, Behr TM. Cholecystokinin-B (CCK-B)/Gastrin Receptor Targeting Peptides for Staging and Therapy of Medullary Thyroid Cancer and Other CCK-B Receptor Expressing Malignancies. Biopolymers. 2002;66(6):399–418. doi: 10.1002/bip.10356. [DOI] [PubMed] [Google Scholar]
- [78].Behr TM, Béhé MP. Cholecystokinin-B/Gastrin Receptor-Targeting Peptides for Staging and Therapy of Medullary Thyroid Cancer and Other Cholecystokinin-B Receptor-Expressing Malignancies. Semin Nucl Med. 2002;32(2):97–109. doi: 10.1053/snuc.2002.31028. [DOI] [PubMed] [Google Scholar]
- [79].Gotthardt M, Béhé MP, Beuter D, Battmann A, Bauhofer A, Schurrat T, Schipper M, Pollum H, Oyen WJG, Behr TM. Improved Tumour Detection by Gastrin Receptor Scintigraphy in Patients with Metastasised Medullary Thyroid Carcinoma. Eur J Nucl Med Mol Imaging. 2006;33(11):1273–1279. doi: 10.1007/s00259-006-0157-8. [DOI] [PubMed] [Google Scholar]
- [80].Gotthardt M, Béhé MP, Grass J, Bauhofer A, Rinke A, Schipper ML, Kalinowski M, Arnold R, Oyen WJG, Behr TM. Added Value of Gastrin Receptor Scintigraphy in Comparison to Somatostatin Receptor Scintigraphy in Patients with Carcinoids and Other Neuroendocrine Tumours. Endocr Relat Cancer. 2006;13(4):1203–1211. doi: 10.1677/erc.1.01245. [DOI] [PubMed] [Google Scholar]
- [81].Behr T, Behe M, Gotthardt M, Angerstein C, Heufelder C, Becker W. Cholecystokinin(CCK(-B/ Gastrin-Receptor bining peptides for diagnosis and therapy of metastatic medullary thyroid cancer. Eur J Nucl Med. 2001;28(8):1023. [Google Scholar]
- [82].Fröberg AC, de Jong M, Nock BA, Breeman WAP, Erion JL, Maina T, Verdijsseldonck M, de Herder WW, van der Lugt A, Kooij PPM, Krenning EP. Comparison of Three Radiolabelled Peptide Analogues for CCK-2 Receptor Scintigraphy in Medullary Thyroid Carcinoma. Eur J Nucl Med Mol Imaging. 2009;36(8):1265–1272. doi: 10.1007/s00259-009-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kosowicz J, Czepczyński R, Ziemnicka K, Gryczyńska M, Sowiński J. [The Use of a New Analogue DGlu-Octagastrin in Scintigraphy of Medullary Thyroid Carcinoma] Endokrynol Pol. 2006;57(4):427–430. [PubMed] [Google Scholar]
- [84].Kosowicz J, Mikolajczak R, Czepczyński R, Ziemnicka K, Gryczyńska M, Sowiński J. Two Peptide Receptor Ligands (99m)Tc-EDDA/HYNIC-Tyr(3)-Octreotide and (99m)Tc-EDDA/HYNIC-(D)Glu-Octagastrin for Scintigraphy of Medullary Thyroid Carcinoma. Cancer Biother Radiopharm. 2007;22(5):613–628. doi: 10.1089/cbr.2006.368. [DOI] [PubMed] [Google Scholar]
- [85].Kunikowska J, Ziemnicka K, Pawlak D, Ruchala M, Kolasa A, Janicka-Jedynska M, Woźniak A, Mikolajczak R, Królicki L. Medullary Thyroid Carcinoma - PET/CT Imaging with 68Ga-Labelled Gastrin and Somatostatin Analogues. Endokrynol Pol. 2016;67(1):68–71. doi: 10.5603/EP.2016.0010. [DOI] [PubMed] [Google Scholar]
- [86].Hubalewska-Dydejczyk A, Mikolajczak R, Decristoforo C, Kolenc-Peitl P, Erba PA, Zaletel K, Maecke H, Maina T, Konijnenberg M, Garnuszek P, Trofimiuk-Muldner M, et al. Phase I Clinical Trial Using a Novel CCK2 Receptor-Localizing Radiolabelled Peptide Probe for Personalized Diagnosis and Therapy of Patients with Progressive or Metastatic Medullary Thyroid Carcinoma - Final Results. Eur J Nucl Med Mol Imaging. 2019;46(Suppl 1):S339. [Google Scholar]
- [87].Rottenburger C, Nicolas GP, McDougall L, Kaul F, Cachovan M, Vija AH, Schibli R, Geistlich S, Schumann A, Rau T, Glatz K, et al. Cholecystokinin-2 Receptor Agonist 177Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma - Results of the Lumed Phase 0a Study. J Nucl Med. 2019;41(0) doi: 10.2967/jnumed.119.233031. jnumed. 119.233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Erba PA, Maecke H, Mikolajczak R, Decristoforo C, Zaletel K, Maina-Nock T, Peitl PK, Garnuszek P, Froberg A, Goebel G, de Jong M, et al. A Novel CCK2/Gastrin Receptor-Localizing Radiolabeled Peptide Probe for Personalized Diagnosis and Therapy of Patients with Progressive or Metastatic Medullary Thyroid Carcinoma: A Multicenter Phase I GRAN-T-MTC Study. Polish Arch Intern Med. 2018;128(12):791–795. doi: 10.20452/pamw.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, Ambrosini V, Kjaer A, Delgado-Bolton R, Kunikowska J, et al. Guideline for PET/CT Imaging of Neuroendocrine Neoplasms with 68Ga-DOTA-Conjugated Somatostatin Receptor Targeting Peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44(9):1588–1601. doi: 10.1007/s00259-017-3728-y. [DOI] [PubMed] [Google Scholar]
- [90].Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, Fischer R, Rivier JEF, Reubi JC, Maecke HR, Weber WA. Comparison of Somatostatin Receptor Agonist and Antagonist for Peptide Receptor Radionuclide Therapy: A Pilot Study. J Nucl Med. 2014;55(8):1248–1252. doi: 10.2967/jnumed.114.138834. [DOI] [PubMed] [Google Scholar]
- [91].Reubi JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr Rev. 2003;24(4):389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- [92].Pauwels E, Cleeren F, Bormans G, Deroose CM. Somatostatin Receptor PET Ligands - the next Generation for Clinical Practice. Am J Nucl Med Mol Imaging. 2018;8(5):311–331. [PMC free article] [PubMed] [Google Scholar]
- [93].Bison SM, Konijnenberg MW, Melis M, Pool SE, Bernsen MR, Teunissen JJM, Kwekkeboom DJ, de Jong M. Peptide Receptor Radionuclide Therapy Using Radiolabeled Somatostatin Analogs: Focus on Future Developments. Clin Transl Imaging. 2014;2(1):55–66. doi: 10.1007/s40336-014-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Fani M, Nicolas GP, Wild D. Somatostatin Receptor Antagonists for Imaging and Therapy. J Nucl Med. 2017;58(Supplement 2):61S–66S. doi: 10.2967/jnumed.116.186783. [DOI] [PubMed] [Google Scholar]
- [95].Akgün E, Körner M, Gao F, Harikumar KG, Waser B, Reubi JC, Portoghese PS, Miller LJ. Synthesis and in Vitro Characterization of Radioiodinatable Benzodiazepines Selective for Type 1 and Type 2 Cholecystokinin Receptors. J Med Chem. 2009;52(7):2138–2147. doi: 10.1021/jm801439x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wayua C, Low PS. Evaluation of a Nonpeptidic Ligand for Imaging of Cholecystokinin 2 Receptor-Expressing Cancers. J Nucl Med. 2015;56(1):113–119. doi: 10.2967/jnumed.114.144998. [DOI] [PubMed] [Google Scholar]
- [97].Nock BA, Maina T, Krenning EP, de Jong M. “To Serve and Protect”: Enzyme Inhibitors as Radiopeptide Escorts Promote Tumor Targeting. J Nucl Med. 2014;55(1):121–127. doi: 10.2967/jnumed.113.129411. [DOI] [PubMed] [Google Scholar]
- [98].Dubreuil P, Fulcrand P, Rodriguez M, Fulcrand H, Laur J, Martinez J. Novel Activity of Angiotensin-Converting Enzyme. Hydrolysis of Cholecystokinin and Gastrin Analogues with Release of the Amidated C-Terminal Dipeptide. Biochem J. 1989;262(1):125–130. doi: 10.1042/bj2620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Migaud M, Durieux C, Viereck J, Soroca-Lucas E, Fournié-Zaluski MC, Roques BP. The in Vivo Metabolism of Cholecystokinin (CCK-8) Is Essentially Ensured by Aminopeptidase A. Peptides. 1996;17(4):601–607. doi: 10.1016/0196-9781(96)00036-8. [DOI] [PubMed] [Google Scholar]
- [100].Kaloudi A, Nock BA, Lymperis E, Krenning EP, de Jong M, Maina T. Improving the In Vivo Profile of Minigastrin Radiotracers: A Comparative Study Involving the Neutral Endopeptidase Inhibitor Phosphoramidon. Cancer Biother Radiopharm. 2016;31(1):20–28. doi: 10.1089/cbr.2015.1935. [DOI] [PubMed] [Google Scholar]
- [101].Kaloudi A, Nock BA, Lymperis E, Valkema R, Krenning EP, de Jong M, Maina T. Impact of Clinically Tested NEP/ACE Inhibitors on Tumor Uptake of [(111)In-DOTA]MG11-First Estimates for Clinical Translation. EJNMMI Res. 2016;6(1):15. doi: 10.1186/s13550-015-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Valverde IE, Bauman A, Kluba CA, Vomstein S, Walter MA, Mindt TL. 1,2,3-Triazoles as Amide Bond Mimics: Triazole Scan Yields Protease-Resistant Peptidomimetics for Tumor Targeting. Angew Chem Int Ed Engl. 2013;52(34):8957–8960. doi: 10.1002/anie.201303108. [DOI] [PubMed] [Google Scholar]
- [103].Maina T, Kaloudi A, Valverde IE, Mindt TL, Nock BA. Amide-to-Triazole Switch vs. in Vivo NEP-Inhibition Approaches to Promote Radiopeptide Targeting of GRPR-Positive Tumors. Nucl Med Biol. 2017;52:57–62. doi: 10.1016/j.nucmedbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [104].Grob N, Béhé M, Schibli R, Mindt T. 177Lu-Labelled Peptidomimetics: Novel Minigastrin Analogues for Improved Tumour Targeting. Nucl Med Biol. 2019;72–73(2019):S20. [Google Scholar]
- [105].Corringer PJ, Weng JH, Ducos B, Durieux C, Boudeau P, Bohme A, Roques BP. CCK-B Agonist or Antagonist Activities of Structurally Hindered and Peptidase-Resistant Boc-CCK4 Derivatives. J Med Chem. 1993;36(1):166–172. doi: 10.1021/jm00053a022. [DOI] [PubMed] [Google Scholar]
- [106].Klingler M, Decristoforo C, Rangger C, Summer D, Foster J, Sosabowski JK, von Guggenberg E. Site-Specific Stabilization of Minigastrin Analogs against Enzymatic Degradation for Enhanced Cholecystokinin-2 Receptor Targeting. Theranostics. 2018;8(11):2896–2908. doi: 10.7150/thno.24378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Klingler M, Summer D, Rangger C, Haubner R, Foster J, Sosabowski J, Decristoforo C, Virgolini I, von Guggenberg E. DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use. J Nucl Med. 2019;60(7):1010–1016. doi: 10.2967/jnumed.118.221283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Klingler M, Rangger C, Summer D, Kaeopookum P, Decristoforo C, von Guggenberg E. Cholecystokinin-2 Receptor Targeting with Novel C-Terminally Stabilized HYNIC-Minigastrin Analogs Radiolabeled with Technetium-99m. Pharmaceuticals (Basel) 2019;12(1) doi: 10.3390/ph12010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].von Guggenberg E, Klingler M, Garnuszek P, Mikolajczak R, Janota B, Hubalewska-Dydejczyk A, Kiec-Klimczak M, Przybylik-Mazurek E, Virgolini I. Gallium-68 Labelled Minigastrin Analogue for High Sensitivity PET Imaging of Cholecystokinin-2 Receptor Expressing Tumours. Eur J Nucl Med Mol Imaging. 2019;46(Suppl1):S268. [Google Scholar]