Abstract
Meiotic recombination is a critical step in gametogenesis for many organisms, enabling the creation of genetically diverse haploid gametes. In each meiotic cell, recombination is initiated by numerous DNA double-strand breaks (DSBs) created by Spo11, the evolutionarily conserved topoisomerase-like protein1, but how these DSBs are distributed relatively uniformly across the four chromatids that make up each chromosome pair is poorly understood. Here we employ S. cerevisiae to demonstrate distance-dependent DSB interference in cis (in which the occurrence of a DSB suppresses adjacent DSB formation)—a process that is mediated by the conserved DNA damage response kinase, Tel1/ATM. The inhibitory function of Tel1 acts on a relatively local scale, while over large distances DSBs have a tendency to form independently of one another even in the presence of Tel1. Remarkably, over very short distances, loss of Tel1 activity causes DSBs to cluster within discrete zones of concerted DSB activity. Our observations support a hierarchical view of recombination initiation where Tel1/ATM prevents clusters of DSBs, and further suppresses DSBs within the surrounding chromosomal region. Such collective negative regulation is likely to help ensure that recombination events are dispersed evenly and arranged optimally for genetic exchange and efficient chromosome segregation.
We sought to elucidate the mechanisms that regulate the spatial patterning of meiotic DSBs. The conserved DNA damage response (DDR) kinase Tel1/ATM inhibits excessive DSB formation in a number of organisms2–5, suggesting that it might influence this process. While increased DSB formation in Tel1/ATM mutants could arise from a loss of cis-interference3,5(within chromatids), trans-interference3,4 (between chromatids), or from global derepression of Spo11 catalytic activity, some observations in S. cerevisiae 6,7 can be explained by the loss of cis-interference (see Supplementary Discussion for further details).
To test the idea that Tel1/ATM acts in cis to suppress additional DSBs within broken chromosomes3,5, we assessed the frequency that four test chromosomes are cleaved multiple times in the presence and absence of Tel1 using strains that accumulate meiotic DSBs due to deletion of DMC1, the meiosis-specific RecA/Rad51 paralogue8. Fragmented chromosomes were separated by pulsed-field gel electrophoresis (PFGE) and detected with a probe positioned in the centre of each chromosome. Fragments shorter than the distance between the probe and the closest telomere must arise from at least two DSBs on the same chromatid. In line with our predictions, such molecules increased 1.3-to-1.7-fold upon deletion of TEL1 (Fig. 1a,b and Extended Data Fig. 1a). Moreover, we noted a non-linear inverse correlation between the fold-increase and the fragment length (Fig 1c), which, because there were only minor increases in the apparent frequency of broken chromosomes as measured by indirect end-labeling (Fig. 1d and Extended Data Fig. 1b), cannot solely be explained by an increase in DSBs (Extended Data Fig. 2). These data instead suggest that the closer two DSBs are, the more likely that coincident cleavage is derepressed in the tel1Δ strain—as expected for loss of cis-interference.
Figure 1. Tel1-mediates distance-dependent suppression of DSB formation in cis .
a, Agarose-embedded genomic DNA isolated at the indicated timepoints was fractionated by PFGE, transferred to nylon and hybridised with a probe recognising a central position on chromosome V. Example lane profiles depict the relative signal density for the 8 h timepoints. b, Quantification of multi-cut DSB signals as depicted in (a), for chromosomes III, VIII, V and XI (see Extended Data Fig. 1). Fold enrichment and statistical differences in average signal are indicated (t-test). c, Ratio of dmc1Δ tel1Δ vs dmc1Δ lane signal corresponding to multi-cut DSBs measured at 8 h (a, and Extended Data Fig. 1-2) plotted as a function of size. d, Average chromosome breakage for all four chromosomes (see Extended Data Fig. 1). e-h, Tel1-mediated DSB suppression spans less than 150 kb (see Extended Data Fig. 3 for details). e, As in (a) but hybridised with a probe recognising a central position on chromosome III. Main DSB sites are indicated. “Double-cut zone A-B”: double-cuts formed from DSBs arising in both zones A and B on the same molecule. f, Physical map of chromosome III showing relative position of DSB zones and probes. g, Summary of observed and expected frequencies (based on independent events) of zone A-B double-cuts using data from 8 h timepoints. h, Calculated DSB interference between DSB zones A and B. a-h, Error bars, s.d. n=3. p-values: t-test.
DSB interference has not previously been demonstrated. To investigate further the idea that Tel1 mediates DSB interference, for each of the following analyses we compared the observed frequency of coincident DSB formation (“double-cuts”) to that expected if DSBs were arising independently within the tested regions. We also used the ratio of these two values to calculate the strength of DSB interference between any two given DSB loci (see Methods). Positive values indicate strong DSB interference, whereas values close to zero indicate no DSB interference (i.e. independence). Negative values indicate concerted DSB formation.
Long-range: The distribution of DSBs on chromosome III allowed us to assess whether DSBs separated by large distances (~150 kb) are subject to interference (Fig. 1e-h and Extended Data Fig. 3). In both dmc1Δ and dmc1Δ tel1Δ strains, the frequency of chromatids cut simultaneously within the major left-arm and right-arm DSB zones was very close to that expected for independent behaviour (Fig. 1g-h). We conclude that, at this large scale, DSB events arise independently of one another in the presence and in the absence of Tel1.
Medium range: We next probed the interval between two prominent Spo11-DSB hotspots separated by ~20 kb (the widely-characterised HIS4::LEU2 hotspot9 and a second site that maps within the leu2::hisG locus1; Fig. 2a,b). To improve signal detection we included a second recombination mutant (sae2Δ), which, due to an inability to remove Spo11, accumulates DSBs without ssDNA resection, causing DSB molecules to migrate as discrete dsDNA bands10–12. In both sae2Δ and dmc1Δ cells, signals ranging from 16-60 kb—indicative of DSBs arising in the vicinity of HIS4::LEU2 and the leu2::hisG region simultaneously (double-cuts)—though detectable, were present at frequencies significantly below those expected for independent cleavage within the two hotspot regions (i.e. positive interference; Fig. 2b-d and Extended Data Fig. 4). By contrast, double-cutting increased in both strains upon TEL1 deletion, arising at frequencies similar to those expected for independent cleavage (Fig. 2c), and indicating a loss of interference (Fig. 2d). By comparison, in both sae2Δ and dmc1Δ strains, upon downregulation of the Mec1/ATR branch of the DDR checkpoint pathway (achieved by deletion of the Rad24/RAD17 DDR clamp loader), double-cut frequencies remained lower than expected for independent DSB formation (Fig. 2c), suggesting that interference remained largely intact (Fig. 2d).
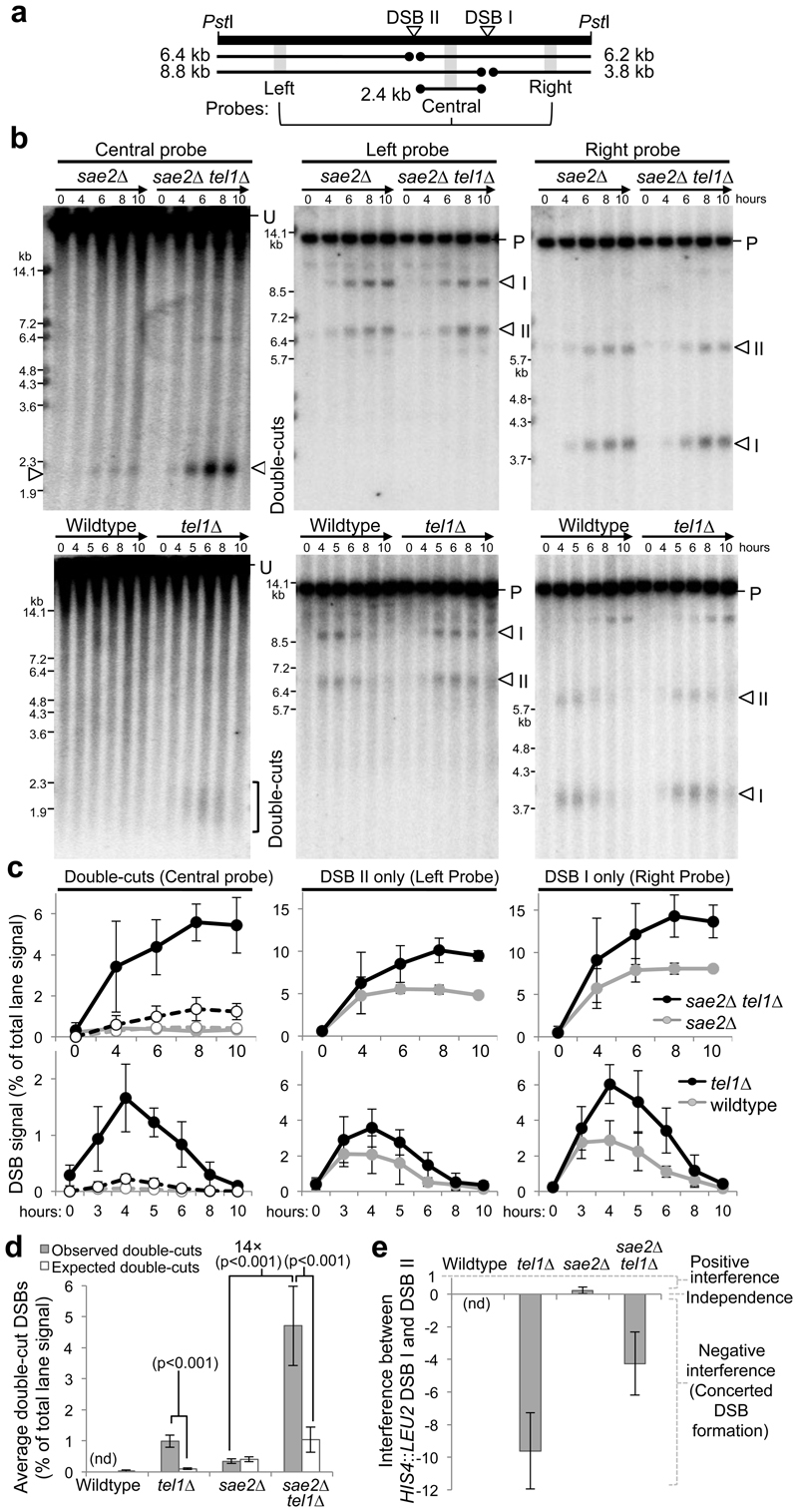
Figure 2. Tel1 suppresses adjacent meiotic DSB formation within a 70kb range.
a, Example PFG with location of main DSBs (HIS4::LEU2 and leu2::hisG) in left arm of chromosome III, detected by the CHA1 probe, and diagram of the range of double-cuts detected by the FRM2 probe in panels (b) and (e). Major double-cut band is indicated with a star in (a, b, e). b-e, Agarose-embedded genomic DNA isolated from the indicated timepoints and strains was fractionated by PFGE, transferred to nylon and hybridised with FRM2. In dmc1Δ cells, the migration of the double-cut molecules was more variable and slightly retarded—at least in part due to extensive ssDNA resection25,26. c, Quantification of observed (b) and expected double-cut frequencies. Expected frequencies of double-cut molecules (as if forming independently) were calculated from measured single-cut frequencies (Extended Data Fig. 4). Statistically significant differences are indicated. d, Calculated DSB interference between HIS4::LEU2 and leu2::hisG (see Extended Data Fig. 4). e, Detection of double-cuts by PFGE as in (b) (left panel) and quantification of double-cuts (right panel) in the indicated strains. Error bars, s.d. n=3, unless indicated. p-values: t-test.
In dmc1Δ tel1Δ cells, although double-cut events plateaued after ~6 hours (Extended Data Fig. 5a), they were first detectable at the earliest point that single DSBs were also detectable (2.5 h), suggesting that double-cuts do not arise from the accumulation of unrepaired DSBs in dmc1Δ and sae2Δ strains. Indeed, double-cuts were also readily detectable in the otherwise recombination-proficient tel1Δ single mutant (~1.2% of total lane signal)—a situation that was not observed in wildtype cells (Fig. 2e). Thus, over medium distances, Tel1 suppresses the formation of adjacent DSBs on the same chromatid in both recombination-deficient and recombination-proficient cells. TEL1 deletion also caused coincident formation of DSBs separated by 10-70 kb at other genomic loci (Extended Data Fig. 5b). While the increase in double-cutting at these loci may partly be due to increased global DSB levels, our results collectively support the view that Tel1 mediates DSB interference in cis over domains of up to at least 70 kb, but that do not extend to 150 kb.
Short range: To investigate the role of Tel1 at closely-spaced DSBs we focused on the HIS4::LEU2 locus, which consists of two strong DSB hotspots separated by only ~2.4 kb (Fig. 3a). Despite such spatial proximity, molecules of ~2.4 kb were visible in the sae2Δ single mutant (~0.35 ±0.07% of lane signal)—indicative of simultaneous breakage at the two sites even in the presence of a functional TEL1 pathway (Fig. 3b-d). Deletion of TEL1 further increased the frequency of these molecules ~14-fold (4.71 ± 1.28% of lane signal). Such double-cut molecules were also detectable in the tel1Δ single mutant (~0.99 ± 0.19% of lane signal), albeit at appreciably lower signal intensity due to the transient nature of DSBs in repair-proficient cells, and to ssDNA resection causing the DSB signals to migrate heterogeneously during electrophoresis, hampering detection (Fig. 3b-d). Double-cut molecules in wildtype cells were below the detection limit of our assays.
Figure 3. Concerted DSB formation within the HIS4 :: LEU2 hotspot.
a, Diagram of HIS4::LEU2 locus showing location of DSBs, fragment sizes and probes used. b, Genomic DNA isolated from the indicated timepoints and strains was fractionated by electrophoresis, transferred to nylon and hybridised with probes as indicated. U, uncut parental DNA, P, PstI digested parental DNA. DSB signals are marked with open triangles or bracket. c, Quantification of DSB and double-cut signals in (b). Left panels: for comparison, expected double-cuts frequencies (dashed lines) are plotted alongside measured double-cut frequencies (plain lines). d-e, Summary chart of observed and expected (based on independent events) double-cuts (d) and interference values (e) calculated by averaging the 3-8 h (wildtype and tel1Δ) or 4-10 h (sae2Δ and sae2Δ tel1Δ) timepoints from each repeat. Wildtype double-cut data were omitted from panels (c-e) because the signal was below our detection limit. a-e, Error bars, s.d. n=4. p-values: t-test.
Surprisingly, both the tel1Δ and the tel1Δ sae2Δ strain displayed substantially greater levels of simultaneous Spo11-DSB formation than expected from the measured DSB frequencies at the two sites (Fig. 3d)—a phenomenon referred to as negative interference (Fig. 3e). Furthermore, the two DSBs within HIS4::LEU2 displayed no interference in the sae2Δ strain even though the suppressive Tel1 pathway is presumably active (Fig. 3d,e). Thus, in contrast to the more widely spaced DSB hotspots characterised above (medium-range), DSBs within HIS4::LEU2 do not interfere, and actually appear to form concertedly in the absence of TEL1.
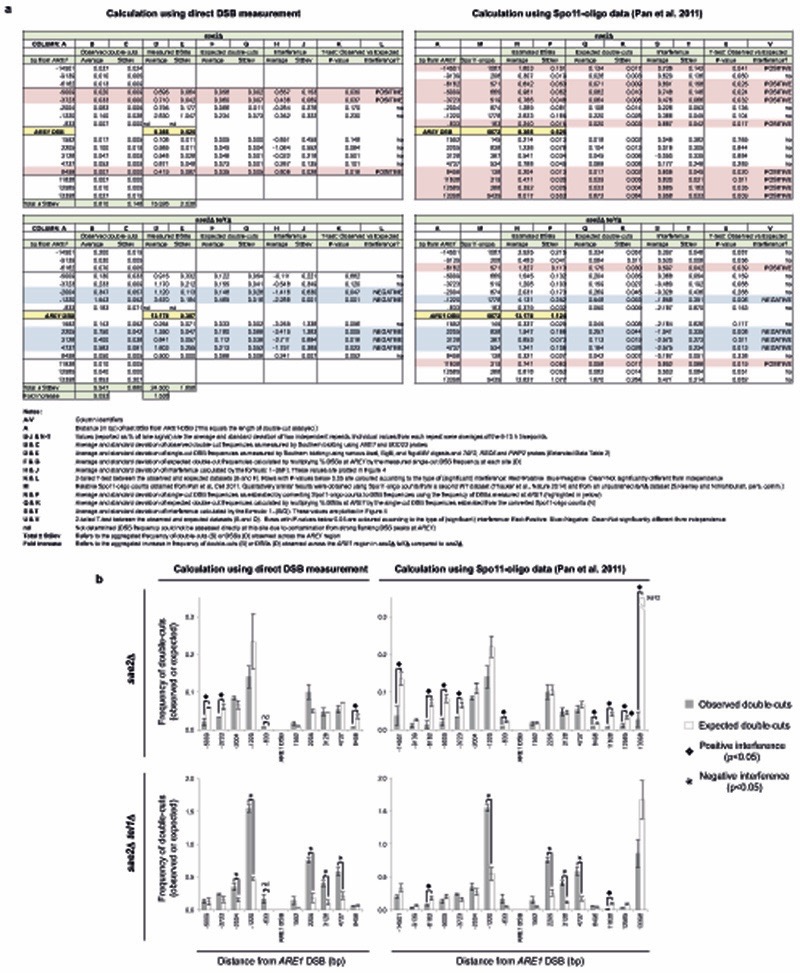
To investigate whether this phenomenon was unique to HIS4::LEU2, we measured the frequency of simultaneous cleavage arising between the natural ARE1 hotspot and each of the many minor DSB sites that flank this locus (Fig. 4a,b). In absolute terms, loss of TEL1 activity resulted in a 9.1-fold increase in the observed frequency of double-cutting across the ARE1 locus (Extended Data Fig. 6a). We then calculated the strength of interference between the ARE1 hotspot and each minor site using two methods to estimate the single-cut DSB frequencies: Direct measurement using Southern blotting, and that calculated using three independent Spo11-oligo datasets (two from wildtype cells1,13 and one from tel1Δ; S. Keeney and N. Mohibullah, pers. comm.; Fig. 4c,d, Extended Data Fig. 6, Methods, and data not shown). In the sae2Δ control, sites greater than 3-4kb from ARE1 displayed significant positive interference, whereas a zone of reduced DSB interference was observed for those DSBs in close proximity to ARE1 (Fig. 4c and Extended Data Fig. 6). In the absence of TEL1, this differential effect was dramatically increased: Cleavage of sites greater than 5 kb from ARE1 arose at similar to the expected frequency (i.e. no interference), whereas DSB sites much closer to ARE1 were disproportionately elevated, with observed double-cut frequencies being ~4-fold greater than expected if DSB formation was arising independently at each location (Fig. 4d and Extended Data Fig. 6). Collectively, we delimited a ~8 kb zone of strong negative DSB interference centred on the ARE1 hotspot (Fig. 4d).
Figure 4. Tel1 suppresses concerted DSB formation within chromatin loop domains.
a, Genomic DNA isolated from the indicated timepoints and strains was fractionated by electrophoresis, transferred to nylon and hybridised with either BUD23 or ARE1 probes. b, Diagram of ARE1 locus ±15 kb showing relative RMM binding profile14 with Spo11-oligo (DSB) peaks overlaid1. (ChIP/WCE=chromatin IP/input signal; hpM/bp=hits per million reads per basepair). Inferred chromosome axes and loop sites are highlighted. c-d, The frequency of each double-cut species in (a) was quantified, and interference between each pair of DSBs plotted after using the normalised frequency of Spo11-oligos1 at each site to estimate expected frequencies of double-cutting (solid line, see Supplementary Methods), or the measured frequency of DSB formation obtained for a subset of sites from Southern blotting experiments (dashed line, Extended Data Fig. 6). Plotted points show averages ± standard deviation for two independent repeats (individual values within each repeat are averages of the 6-10 h timepoints). Plotted lines are 3-period moving averages. Comparable results were obtained when Spo11-oligo counts obtained from a second wildtype13 or a tel1Δ strain were used (S. Keeney and N. Mohibullah, pers. comm.). Comparison of observed and expected frequencies, and statistical analyses are provided in Extended Data Fig. 6. e, Cartoon highlighting how Tel1/ATM suppresses DSB formation within (heavy dashed lines) and adjacent to (light dashed lines) active loop domains.
Our results suggest that the ARE1 region is acting as a domain in which concerted DSB formation readily occurs, but which is to a large extent repressed by Tel1 activity. Recently a model for Spo11-DSB catalysis has gained favour in which short chromosomal domains—equal in size to individual chromatin loops—become tethered to the chromosome structural axis in order to trigger Spo11-DSB catalysis (the Tethered Loop–Axis model14,15). We superimposed the binding position of chromosome axis components (RMM profile14) onto our map of simultaneous Spo11-DSB events and observed that the peak of negative DSB interference mapped to a trough in axis-protein binding signal—indicative of the centre of a chromatin loop (Fig. 4b,c). Such a correlation between regions of strong negative DSB interference and putative loop DNA was also observed at other tested loci, YCRO61W, SRB2, and CCT6 (Extended Data Fig. 7).
We propose that our observation of negative DSB interference at close range—apparently confined within a chromatin loop domain—is a previously unconsidered expectation of the tethered loop model. Specifically, loop-axis tethering of a specific region (to create a DSB-permissive subchromosomal domain) within only a subpopulation of cells will mean that population average measures of DSB frequency become underestimates of the DSB frequency within the tethered (active) fraction. Consequently, negative interference can be explained by loss of interference within a loop that is tethered in only a fraction of the population (Extended Data Fig. 8 and Supplementary Discussion for further details). While we favour this view, it is also possible that short-range concerted DSB formation is simply a consequence of DSBs being formed in regions of increased local DSB potential that are present in only a subpopulation of cells, and which map within chromosome loop domains (because that is primarily where DSBs occur) but are not caused by them.
In summary, our observations suggest that Tel1 mediates DSB interference in cis over domains that span ~100 kb, via a process that may be modulated by the unique organizational structure of the meiotic chromosome (Fig. 4e). Consequently, when Tel1 activity is lost, DSBs separated by medium-to-large distances (>10 kb) form independently of each other, whereas at close range (<10 kb), Tel1 suppresses DSB clustering within domains that may be defined by the boundary of local chromatin loops.
Whereas our work strongly indicates that Tel1 mediates DSB interference in cis, previous work concluded that Tel1 suppresses DSB formation in trans 4. We propose that these apparently distinct inhibitory roles are simply two consequences of a single process: distance-dependent inhibition of DSB formation, which—due to their close association—is transmitted along the pair of sister chromatids (i.e. in cis and in trans; see Supplementary Discussion for further details and analysis). In mouse and flies, ATM mutants appear to undergo increased rates of recombination initiation, failed DSB repair, and apoptosis2,3,16–18. Our work predicts that these phenotypes may arise from an excess of DSBs within highly-localised active domains spanning a pair of sister chromatids3.
Recent work suggested that in budding yeast, Rec114 (an evolutionarily conserved axis-associated accessory protein required for Spo11-DSB formation19) is negatively regulated by Tel1-dependent phosphorylation5. The meiosis-specific chromosomal checkpoint adapter protein, Hop1 (similar to mouse HORMAD1-2) and histone H2A are two other targets of Mec1/Tel1-mediated regulation20,21. However, double-cutting is not increased in strains harbouring nonphosphorylatable alleles of Rec114, Hop1, or H2A (V.G., R.A., M.J.N., unpublished observations), suggesting either that these factors act redundantly, or that DSB suppression is mediated via another target or function of Tel1.
Looking more broadly, our revelation that the strength of DSB interference varies nonuniformly with distance (Fig. 4) will have implications for the modeling of fine-scale recombination distributions in all sexually reproducing organisms and particularly in mutants or under conditions that modulate Tel1/ATM and Mec1/ATR signaling. Furthermore, we note that clustered DSBs might behave as double-strand gaps—the initiators of recombination in the original model of DSB repair22.
Our study also allows us to draw a parallel with the ATM-dependent repression of programmed DSB formation during VDJ recombination23. In both cases, potential cleavage sites are sequestered into active subdomains in which Tel1/ATM suppresses concerted DSB formation23. It is interesting to consider whether similar mechanisms regulate other types of programmed, yet ostensibly stochastic, biological events—such as firing of DNA replication origins, a process itself regulated by the ATR kinase24.
Methods
Yeast strains and culture methods
Meiotic cultures were prepared as described26. Strains were derived from SK1 using standard techniques. sae2Δ, exo1Δ, dmc1Δ and tel1Δ are full replacements of the ORF with kanMX4 or hphNT2. A full strain list is provided in Extended Data Table 1.
Molecular techniques
DSB signals were detected via hybridisation with specific DNA probes (detailed in Extended Data Table 2) after Southern blotting genomic DNA fractionated in agarose gels using standard techniques. For chromosome-scale analysis, genomic DNA was isolated in agarose plugs26,28. Radioactive signals were collected on phosphor screens, scanned with a Fuji FLA5100, and quantified using ImageGauge software.
DSB analysis by Southern blotting
Genomic DNA was isolated from aliquots of synchronously sporulating cultures as described previously27 but with minor modifications. Briefly, spheroplasts were prepared in 1 M sorbitol / 0.1 M EDTA / 0.1 M NaHPO4 pH 7.5 / 1% BME and 200 µg/ml zymolyase 100T for 1 hour at 37 °C, and lysed by adding SDS to 0.5% and proteinase K to 200 µg/ml with incubation for 4 hours to overnight at 60 °C. Protein was removed by mixing with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1), and nucleic acids precipitated by adding one-tenth volume of 3 M NaAc pH5.2 and an equal volume of 100% ethanol. Precipitates were washed in 70 % ethanol and dissolved in 1 x TE overnight at 4 °C. RNAse was added at 100 µg/ml, incubated for 60 minutes at 37 °C, genomic DNA was reprecipitated with ethanol / NaAc and DNA pellets left to dissolve in 1 x TE overnight at 4 °C. Signals were detected by Southern blotting of genomic DNA after fractionation on agarose gels as described previously27.
For measuring the DSBs frequencies (“single-cuts”) at various locations (for Fig. 3, 4, Extended Data Fig. 6, 7), genomic DNA was digested with the appropriate restriction enzyme, fractionated on agarose in 1× TAE for ~18 hours, transferred to nylon membrane under denaturing conditions, and hybridised with a probe allowing detection of DSBs to be quantified (as indicated in Extended Data Table 2).
For measuring the frequency of double-cuts (for Fig. 3, 4, Extended Data Fig. 6, 7) undigested genomic DNA was fractionated and transferred using similar conditions. Membranes were hybridised with probes located between DSBs of interest (as indicated in figures and detailed in Extended Data Table 2).
Analysis of double-cutting at the HIS4::LEU2 locus (Fig. 3) necessitated taking into account of the fact that the strains used in this study contain three copies of the LEU2 gene: at the his4X::LEU2, leu2::hisG and nuc1::LEU2 loci. Specifically, because the probe designed to detect double-cuts between sites I and II within the his4X::LEU2 hotspot recognises these three loci, numerous cross-reacting bands arise when probing DNA digested with restriction enzymes. Therefore undigested DNA was fractionated and transferred as for other loci. However, double-cut values recorded by this method were multiplied by three to correct for the fact that only ~1/3rd of the uncut parental DNA signal originated from the HIS4::LEU2 locus. We note that double-cut frequencies measured using PstI-digested DNA were very similar to when using undigested DNA, but resulted in blots that were more complicated to analyse (data not shown). Radioactive signals were collected on phosphor screens, scanned with a Fuji FLA5100, and quantified (ImageGauge software, FujiFilm). Background subtraction was performed as described below.
DSB analysis by PFGE
DNA was prepared in agarose plugs as described26,28. For PFGE on Fig. 1, Extended Data Fig. 1 and 3, chromosomes were fractionated using a CHEF-DRIII PFGE system (BioRad) using the following conditions: 1.3% agarose in 0.5× TBE; 14· C; 6 V/cm; switch angle 120·; switch time of 20–60 seconds for 28 h. For PFGE on Fig. 2a, 2b and Extended Data Fig. 4, the following conditions were changed: switch time of 30 seconds for 3 hours and 3-6 seconds for 37 hours. For PFG on Fig. 2e and Extended Data Fig. 5: switch time of 30 seconds for 3 hours and 3-6 seconds for 22 hours. After transfer to nylon membrane under denaturing conditions26,28, membranes were hybridised with DNA probes specific to: central, left and right sub-telomeric regions of four chromosomes (Fig. 1, Extended Data Fig. 1 and 3); the FRM2 region between HIS4::LEU2 and leu2::hisG (Fig. 2 and Extended Data Fig. 4 and 5a); or to regions between specific DSB sites on chromosome V (POL5), chromosome IX (DOT5), or chromosome III (CTR86 and YCR061W) (Extended Data Fig. 5b). Probe details are listed in Extended Data Table 2. We note that due to small differences in the length of chromosome IX between otherwise isogenic isolates, DSB signals migrate at slightly different positions in the panel of strains in Extended Data Fig. 5. The positions of expected double-cuts are not affected by these differences, however, because the relative distance between each DSB is unaltered regardless of absolute chromosome length. Radioactive signals were collected on phosphor screens, scanned with a Fuji FLA5100, and quantified (ImageGauge software, FujiFilm). Background substraction was performed as described below.
DSB and double-cut quantification
Radioactive signals were collected on phosphor screens, scanned with a Fuji FLA5100, and quantified using ImageGauge software. Background signal caused by exposure fogging and non-specific membrane background (based on vacant areas of the blot) was removed using linear subtraction. The contribution of sheared parental DNA to DSB and double-cut signal was removed using a gradient drawn along the lane profile starting from the base of the parental band down to the lane end. Signal above this cut-off were quantified as specific signal (DSBs or double-cuts). For quantification of double-cut molecules on membranes obtained from undigested DNA, signal that was retained in the wells (10-30% of the total lane signal) was added to, and treated as if it were, parental signal. This latter correction assumes that only parental DNA, and no double-cut DNA, is selectively retained in the well. In reality, some double-cut species probably do also get retained in the well, suggesting that our double-cut measurements may be slight underestimates.
Measurement of distance-dependent increases in multi-cut signal ratio
For Figure 1c, for each chromosome, the signal intensity (expressed as a proportion of total lane signal) running through the multicut region (8 hour timepoints from Extended Data Fig. 1a) of the tel1Δ dmc1Δ samples were each divided by the same signal obtained from the dmc1Δ control sample. The resulting ratio was plotted on the Y-axis against apparent double-cut length (in kb) on the X-axis. The latter values were based on the approximate migration of the signal intensity on the PFG realtive to lambda concatemer molecular weight markers. Due to the very low signal intensities towards the very bottom of the gels (and subsequently very erratic ratio calculations), the presented data was trimmed at ~50 kb. Double-cut signal length is indicative of the relative distance between any two given DSBs. Loss of Tel1 activity disproportionately increases the frequency of the shorter double-cut products (with little effect on fragments >100kb), suggesting that Tel1 mediates distance-dependent DSB interference in cis. We note that due to the fact that molecules with ssDNA regions migrate more slowly during PFGE25 (i.e. DSBs and double-cuts with resected DNA ends), the actual X-axis (kb) values presented may be slight overestimates (perhaps +25%), and thus the distance that Tel1-dependent suppression is propagated may be somewhat shorter than the data make it appear.
To test whether the non-linear increase in double-cutting frequency for shorter molecules could alternatively be explained by increases in DSB formation unassociated with any change in DSB interference, we developed a computer program that simulates DSB formation on a linear model of chromosome V (576 kb). This program was initially written in Sinclair BASIC using a plugin for the TextMate editor (macromates.com/) and the FUSE emulator for MacOSX (fuse-emulator.sourceforge.net/), and subsequently rewritten in MATLAB (www.mathworks.co.uk/). The simulation iterates 1 million times for each of the mean values of 2.5, 3, 3.5, and 4 DSBs per chromatid using DSB frequencies (per round of simulation) described by the Poisson distribution for the specified mean. To simulate the frequency distributions of fragments detected by an interstitial probe, tallies were made of only those fragments that include the simulated probe position (FIR1 at position ~220kb). Subsequently, ratios were calculated for each position within each of these simulated distributions and equivalent simulated distributions generated with mean DSB frequencies 1.5–4-fold greater. See Extended Data Fig. 2 for further details of our analysis. MATLAB code for this simulation is available on request.
Calculations of DSB interference
For calculations of interference between the left (zone A) and right (zone B) arm of chromosome III (Fig. 1e-h and as described in Extended Data Fig. 3), total DSB frequencies in each zone were measured using CHA1 and GIT1 probes, respectively. Expected frequencies of coincident cutting were obtained by multiplying these values. Observed frequencies of coincident cutting were estimated by measuring the total signal falling in a ~120-200 kb window (the approximate distance between zones A and B) after probing using the central, SYP1 probe. Mean and standard deviation of the DSB frequencies for each timepoint are those of the three experimental repeats. A two-tailed t-test was used to compare the observed and expected samples. Although no timepoint showed statistically significant interference (neither positive nor negative), there were some notable trends: dmc1Δ cells display moderate negative interference at early timepoints, plateauing over time to display independence. Weak negative interference at early timepoints is actually expected, since at these timepoints, it is probable that only a subfraction of cells have initiated DSB formation, and thus expected double-cutting (calculated using the population average DSB frequency, which includes these inactive cells) will be an underestimate of that observed in the active fraction of cells. By contrast, dmc1Δ tel1Δ displays more positive interference than dmc1Δ TEL1+, also increasing with time. Although this might seem counterintuitive compared to the rest of the tel1Δ observations made in this study, we believe it is an artifact of the analysis, and is explained by the fact that in dmc1Δ tel1Δ cells the frequency of additional DSBs in the central zone (creating the smeared 50-100kb zone towards the bottom of the gel in Extended Data Fig. 3g) are moderately increased at all timepoints compared to dmc1Δ, thus potentially cutting the “zone A-B double-cuts” into smaller fragments. As a result, our calculation of interference moderately underestimates the frequency of double-cutting in zone A and zone B simultaneously—and more so over time as total DSB frequency increases. Although not perfect in numerical value, we believe that these analyses are sufficient to demonstrate that there is little or no measureable interference between zone A and zone B on chromosome III. For calculations of interference between HIS4::LEU2 and leu2::hisG (Fig. 2, and as described in Extended Data Fig. 4), total DSB frequency across the entire HIS4::LEU2 locus (both DSB sites plus two telomere-proximal DSBs) and across the leu2::hisG and two flanking minor hotspots (within ~10 kb) were measured using PFGE in the various strains and the resulting values were multiplied to obtain an expected frequency of double-cutting (see Extended Data Fig. 4 for all calculations and statistical analysis). DSB frequencies for each repeat were averages of the 6-10 h timepoints, and the mean and standard deviation are those of the three experimental repeats. A two-tailed t-test was used to compare the observed and expected samples.
For calculations of interference between the two DSBs within the HIS4::LEU2 locus (Fig. 3), double-cut signals derived from the central (LEU2) probe were multiplied by three to take into account that this probe hybridises to three parental genomic locations (HIS4::LEU2, leu2::hisG, nuc1::LEU2). Expected frequencies of double-cutting were calculated by multiplying the frequency of DSB I and DSB II as measured using probes on the right and left of the hotspot respectively (Fig. 3c). An independent method to estimate double-cutting frequency, based on the observed difference in the frequency of DSB site I or site II when using left versus right probes, yielded similar values (also yielding negative interference), but appeared more prone to quantification error. Details of this method are available on request.
For calculations of interference across the ARE1 locus (Fig. 4 and Extended Data Fig. 6), two methods were employed to calculate expected double-cut frequencies. Primarily, we converted Spo11 oligo counts1 (reads per million; RPM) at individual DSB sites to % DSBs by normalizing to the BUD23–ARE1 DSB signal measured by Southern blotting analysis, where BUD23–ARE1 (2721 RPM) = 8.37 ±0.53% DSBs (sae2Δ) or = 13.2 ±0.39% DSB (sae2Δ tel1Δ). Expected double-cut frequencies were then calculated by multiplying the frequencies of DSB formation at the test site and at the ARE1 hotspot. Comparable DSB interference values were obtained when Spo11-oligo counts obtained from a second wildtype dataset13 were used, and when unpublished Spo11-oligo counts from a tel1Δ strain were used (data not shown; S. Keeney and N. Mohibullah, pers. comm), indicating the degree of robustness of our analysis, and highlighting the fact that DSB frequencies vary relatively uniformly +/− TEL1. Nevertheless, to independently confirm these findings, we used Southern blotting to directly measure the individual DSB frequencies at nine of the DSB sites that flank ARE1 and calculated expected double-cut frequencies as above. This latter method, which in principle is more accurate because it directly assesses DSB frequency at each site using the same genomic DNA samples used to also measure observed double-cutting (and therefore precisely estimates expected double-cutting in sae2Δ and sae2Δ tel1Δ strains), produced results that agreed very well with the analysis using normalised Spo11-oligo datasets (see Fig. 4 and Extended Data Fig. 6 for a comparison). DSB and double-cut frequencies for each repeat were averages of the 6-10 h timepoints, and the mean and standard deviation are those of the three experimental repeats. A two-tailed t-test was used to compare the observed and expected samples.
In Extended Data Fig. 7, where possible we used Southern blotting to directly measure the individual DSB frequencies at each DSB site in sae2Δ and sae2Δ tel1Δ strains (using the average value across the 6-10 h timepoints), and used this value to calculate expected frequencies of double-cutting. However, there were a few DSB sites that were either below signal detection by Southern blotting, or that proved refractory to probes/digest combinations. For these sites we converted the reported Spo11-oligo counts1 to % DSB frequency as follows:
For the analysis of interval A in Extended Data Fig. 7d-f, the frequency of Spo11-oligos at the BRL1–PUT2 locus (138 RPM) was converted to an estimated DSB frequency of 0.38 ±0.03% in sae2Δ and 0.41 ±0.03% in sae2Δ tel1Δ based on normalising the frequency of Spo11-oligos at the main SRB2–NCP1 hotspot (3639 RPM) to 10.01 ±0.76% DSBs (sae2Δ) or 10.8 ±0.68% DSBs (sae2Δ tel1Δ) following direct measurement of DSB formation at the SRB2–NCP1 hotspot using Southern analysis.
For the analysis of intervals B, C, and D in Extended Data Fig. 7g-i, the frequency of Spo11-oligos at the respective loci (177 RPM, 169 RPM, and 400 RPM) were converted to estimated DSB frequencies of 0.63%, 0.60%, and 1.42% in sae2Δ and 0.83%, 0.79%, and 1.87% in sae2Δ tel1Δ based on normalising the frequency of Spo11-oligos at the main YDR186C–CCT6 hotspot (3997 RPM) to 14.15% DSBs (sae2Δ) or 18.72% DSBs (sae2Δ tel1Δ) following direct measurement of DSB formation at the YDR186C–CCT6 hotspot using Southern analysis.
Potential caveats with double-cut quantification using the methods described
Our high-resolution analysis of interference in Fig. 4, Extended Data Fig. 6, 7 assesses the frequency of coincident DSB formation at any pair of tested DSB sites (double-cuts) compared to the frequency of expected coincident cutting calculated by multiplying the measured single cut DSB frequencies at the pair of sites being tested. On any given side of a strong hotspot, double-cuts of increasing length are measured using the same probe (anchored close to a major DSB hotspot). A caveat of this method is that as the second site becomes more distant from the first (the anchor point), the ability to detect the assayed double-cut product will be impeded by the presence of any intervening additional DSB. However, for the genomic loci we have investigated, this small systematic error will have minimal impact on our data collection, as explained in detail below.
Given a molecule with DSBs arranged in linear order: A, B, C. If all DSBs form independently, the likelihood of an intervening DSB “B” cutting a molecule that has already been cut at site A and C, is directly proportional to the frequency of DSB formation at “B”. If DSB B = 5%, the observed frequency of A–C double-cuts will actually be only 95% of its actual value (5% of the time it is cut by B). Thus the corrected frequency of double-cut A–C is obtained by dividing the observed value by 0.95 (=multiplied by 1.053). Note that many interstitial DSBs are far weaker than 5%, and therefore will have an even lesser effect (see Extended Data Fig. 6 column D and N for examples of DSB frequencies across the ARE1 locus). This means that larger double-cuts are only very weakly underestimated (unless the sum of all the intervening cuts is very large, which in general is not true; see above comment). Even if we were to attempt to correct for this systematic error, it would result in only a subtle increase in the frequency of double-cuts in the larger range, resulting in a slightly wider and stronger spread of reduced/negative interference in tel1Δ. Note that this correction will not fundamentally change our observation that negative interference is centred within a loop.
Alternatively, if DSBs are forming concertedly (as we propose occurs in loop domains), then the frequency that A–C is cut by B is not proportional to the population average frequency of DSBs at B, but instead, A–C will be cut at whatever frequency B cuts in situations when A (and/or C) are activated. In this scenario, A–C might be more severely underestimated. As above, while this might result in a pattern of negative interference that spreads more broadly and more strongly than depicted in Fig. 4, it again will not change the observation of negative interference in the loop. Moreover, if negative interference is indeed restricted to loops (as we propose in this manuscript), the apparently disproportionate underestimate in double-cuts due to intervening DSBs will only be true of DSBs within the activated region. Outside of this concerted region, we would expect DSBs to behave independently (and have less impact, as above).
We note that using both correction methods (independent DSBs or concerted DSBs) will actually strengthen the phenomenon of negative interference that we observe in the absence of TEL1. Our small underestimate of double-cutting might also explain why weak interference is still retained over moderate ranges (>10kb) even in the absence of TEL1 (i.e. such weak interference that remains may be due to moderately underestimating long double-cuts, rather than actual retained interference; observed in both Fig. 2d and Fig. 4d).
Bioinformatics
Raw Spo11-oligo datasets1 containing signals at 1 bp step sizes were smoothed via Hann windows of varying size and zero values were filtered/removed to reduce file size via grep commands. Mei4-HA, Mer2-HA and Rec114-HA ChIP/WCE signals (sampled at t=4 hours and normalised by the authors14) were averaged with equal weighting and a continuous and smoothed dataset was constructed via spline interpolation on MATLAB R2013+. Resulting datasets were exported directly into .bedGraph files and additional .BigWig files were created via the precompiled UCSC bedGraphToBigWig tool.
Extended Data
Extended Data Figure 1. Tel1 suppresses the formation of multiple DSBs on the same chromatid.
a, Top: Agarose-embedded genomic DNA isolated at the indicated timepoints was fractionated by PFGE, transferred to nylon and hybridised with probes recognising a central position on chromosome III, VIII, V and XI. Example lane profiles depict the relative signal density for the 8 h timepoints. Representative blots are shown. Areas defined for quantification of multi-cut DSBs (bottom panel) are indicated. Asterisk: cross-hybridisation band. b, Quantification of total chromosome breakage measured in (a). c, As in (a) but using probes specific to the left (top panel), or right (bottom panel) telomere. In agreement with more DSBs per chromatid being formed in the absence of Tel1, close inspection of the PFGE lane profiles revealed that dmc1Δ tel1Δ cells had an increased frequency of shorter chromosome fragments, yet also fewer large chromosome fragments. Because a similar shift in DSB distribution towards shorter molecules is also observed when chromosomes are probed from their opposite end (compare top and bottom panels), this apparent shift can be explained by an increase in the frequency of multiple DSBs arising on the same chromatid in dmc1Δ tel1Δ relative to dmc1Δ. a-c, Error bars, s.d. n=3.
Extended Data Figure 2. Non-linear increases in the frequency of closely spaced DSBs that arise upon TEL1 deletion cannot be explained by increases in absolute DSB frequency.
a-d, To test whether the non-linear increase in double-cutting frequency for shorter molecules (Fig. 1c) could alternatively be explained by increases in DSB formation unassociated with any change in DSB interference, DSB formation on chromosome V (576 kb) was simulated 1 million times for each of the mean values of 2.5, 3, 3.5, and 4 DSBs per chromatid using DSB frequencies (per round of simulation) described by the Poisson distribution for the specified mean. These frequencies are approximately equivalent to 217, 260, 304, and 347 DSBs per cell (~50 Mb). To simulate the frequency distributions of fragments detected by an interstitial probe, tallies were made of only those fragments that include the simulated probe position (FIR1 at position ~220kb). Subsequently, ratios were calculated for each position within each of these simulated distributions and equivalent simulated distributions generated with mean DSB frequencies 1.5x (red), 2x (green), 3x (purple), and 4x (blue) greater. Finally, these data simulations were overlaid with the experimental observations made from chromosome V using the FIR1 probe when comparing the ratio of the dmc1Δ tel1Δ : dmc1Δ (data from Fig. 1c; orange). In all cases, as in Fig. 1c, data has been trimmed for fragments shorter than 50 kb and greater than 300 kb. The asterisks indicate instances of similarity between simulated and observed patterns. We note that in no circumstances do the simulations match the steep non-linear curve, which is a hallmark of the experimental data caused by TEL1 deletion. The closest match is arguably simulating the ratio between a starting mean DSB frequency of 3.5 and that obtained from a 3–4-fold increase (c). While these simulations create a potential match, they both require the relatively high initial frequency of DSB formation in dmc1Δ cells of 304 DSBs per cell (note that the wildtype average frequency is estimated at ~160 DSBs per cell1), increasing to 900-1200 DSBs per cell upon TEL1 deletion. Moreover, in accord with the increased DSB frequency per cell, site-specific DSB frequencies would increase 3–4-fold in dmc1Δ tel1Δ cells relative to dmc1Δ cells to fit this simulation, something that we do not observe: average fold-changes in both sae2Δ and dmc1Δ strains are only ~1.5x upon TEL1 deletion (Fig. 3c, Extended Data Fig. 4c and Extended Data Fig. 6a), a fold-change that is modeled by each of the red plots—all of which show very poor correlations with the observed data. Thus we conclude that the non-linear inverse correlation between the fold-increase and the inter-DSB fragment length cannot solely arise from a global increase in DSB formation, but rather because the closer two DSBs are, the more likely that coincident cleavage is derepressed in the tel1Δ strain—as expected for a loss of cis-interference.
Extended Data Figure 3. Tel1-mediated DSB interference spans less than 150kb.
a, Physical map of chromosome III showing relative position of DSB zones and probes. b-d, Agarose-embedded genomic DNA isolated at the indicated timepoints was fractionated by PFGE, transferred to nylon and hybridised with probes recognising a left (b), right (c) or central position (d) on chromosome III. Probes, main DSB sites and areas selected for quantification of DSBs arising in individual zones are indicated. e-g, Quantification of DSB formation in zone A (e), zone B (f) and double-cuts arising from DSBs occurring in both A and B on the same molecule (g). h, Comparison of observed zone A-B double-cuts (g) to expected zone A-B double-cuts (calculated from single cut frequencies measured in e, f). We observe no statistical difference between observed and expected values at any time point (ttest: p-values all above 0.25 except dmc1Δ tel1Δ t=10 h sample, 0.061). i, Calculated DSB interference between DSB zones A and B. b-i, Error bars, s.d. n=3. See Supplementary Discussion for further details of this analysis.
Extended Data Figure 4. Analysis of DSB interference between HIS4::LEU2 and leu2::hisG .
a-b, Agarose-embedded genomic DNA isolated at the indicated timepoints was fractionated by PFGE, transferred to nylon and hybridised with probes recognising (a), the FRM2 locus located between the HIS4::LEU2 and leu2::hisG DSB hotspots, and (b), the CHA1 locus on the left telomere of chromosome III. Areas selected for quantification are indicated. c, Analysis of DSB interference between HIS4::LEU2 and leu2::hisG regions. The frequency of DSB formation within HIS4::LEU2 and leu2::hisG regions were measured in the various strains from PFGE using CHA1 (b) and FRM2 (a) probes, respectively, and the frequency of double-cuts were measured using the FRM2 probe (a). Total DSBs arising within the leu2::hisG region were calculated by summing double-cuts and leu2::hisG DSBs. Standard deviation indicates the variation between repeat analyses (n=3 for all samples except rad24Δ dmc1Δ: n=2). See notes below table for further details.
Extended Data Figure 5. Analysis of DSB double-cutting at various genomic loci.
a-b, Agarose-embedded genomic DNA isolated from the indicated timepoints and strains was fractionated by PFGE, transferred to nylon and hybridised with various probes: FRM2 (a); POL5, DOT5, CTR86, YCR061W (b). a, Detection of double-cut (left panel) and quantification (right panel). Major double-cut band corresponding to coincident DSBs at HIS4::LEU2 and leu2::hisG is indicated with a star. b, Detection of double-cuts on different chromosomes following PFGE in strains fully (dmc1Δ exo1Δ and sae2Δ) or partially (exo1Δ) defective for DSB repair (top panel). Asterisks: tel1Δ-specific double-cut signals. Diagram depicts possible double-cuts (bottom panels).
Extended Data Figure 6. Analysis of DSB interference across the ARE1 region.
a, DSB interference was calculated in sae2Δ (top) and sae2Δ tel1Δ (bottom) using the following formula: 1–f(observed double-cuts)/f(expected double-cuts), where the expected double-cut values were calculated using two methods: Left, single-cut frequencies were measured by Southern-blot using a TAF2 probe (for DSB sites on the left of ARE1) or a PWP2 and RSC6 probe (for the right-hand side of ARE1; Extended Data Table 2). Right, calculations were made after converting the measured Spo11-oligo frequency1 at each DSB site to a % DSB ± StDev value by using the measured DSB frequency at ARE1 in sae2Δ or sae2Δ tel1Δ for normalization (see Notes below table and Methods for further details). b, Chart of observed (column B) and expected (column F and Q) frequencies of double-cuts. Error bars, s.d. n=2. p-values: two-tailed t-test. Double-cut products that were present at a frequency that was statistically different from that for no interference (independence) were highlighted in (a) according to the type of interference present: red indicates positive DSB interference, blue indicates negative DSB interference (concerted DSB formation); in (b) the same statistical differences were indicated with open diamonds or asterisks, respectively.
Extended Data Figure 7. Tel1 suppresses concerted DSB formation within chromatin loop domains at numerous chromosomal loci.
a-i, DSB interference was calculated across three DSB hotspot regions located on three different chromosomes: chromosome III, BUD23–ARE1 to YCR061W–BUD31 (a-c); chromosome VIII, BRL1–PUT2 to SRB2–NCP1 (d-f); and chromosome IV, YDR186C–CCT6 to MSS116–REF2 (g-i). a, d, g, Upper panels: Genomic DNA isolated from sae2Δ or sae2Δ tel1Δ strains at the indicated timepoints was fractionated by agarose electrophoresis, transferred to nylon membrane and hybridised with the indicated probes: YCRO61W (a), SRB2 (d), CCT6 (g). Lower panels: Diagram of mean RMM binding profile14 overlaid with Spo11-DSB hotspot peaks1. Intervals between various detectable double-cut events are indicated below and specified with the letters A to D. Probes used for detecting double-cuts by southern blotting are indicated. b, e, h, Chart of observed and expected double-cuts for each of the indicated intervals, calculated as an average (per repeat) across the 4-10 h timepoints. Expected double-cut frequencies for each interval were calculated by multiplying the DSB frequencies (average across 4-10 h) at the two sites. Single-cut frequencies were measured by Southern-blot (see Extended Data Table 2 and Methods for details). For some intervals (superscript with a “+”), due to no Southern DSB data being available at the minor DSB site, calculations were made using the normalised Spo11-oligo frequency1 at the minor DSB site (as was performed in Fig. 4 and described in Methods). Asterisks and open diamonds indicate significant negative and positive interference, respectively. c, f, i, DSB interference was calculated by the following formula: 1–f(observed double-cuts)/f(expected double-cuts). Values above zero indicate positive DSB interference. Values below zero indicate negative DSB interference (concerted DSB formation). Conclusion: In addition to ARE1 (Fig. 4), at all three additional loci tested, concerted DSB formation is localised predominantly within a domain approximately demarcated by the RMM binding profile (see a, d and g lower panels). Notably, coincident formation of two DSBs, one within the BUD23-ARE1 domain and one within the YCR061W-BUD31 domain, arise independently in sae2Δ tel1Δ despite coincident DSB formation within each interval displaying negative interference. In (a), double-cuts in interval A were measured using the ARE1 probe (Fig 4a). Asterisk in (a) upper panel denotes a band that is a mixture of two tel1Δ-dependent double-cuts, which due to the relative location of the YCR061W probe and DSB sites cannot be unambiguously assigned and therefore were not analysed. Error bars, s.d. n=2, except (g-i) where only one experiment was performed. p-values: two-tailed t-test.
Extended Data Figure 8. Stochastic loop tethering (activation) predicts apparent short-range negative interference.
a, In this model, DSBs A and B reside within a single loop domain (subject to tethering-dependent DSB formation), which is active in only a subpopulation of cells. The expected frequency of coincident DSB formation (double-cutting), assuming no DSB interference, is calculated for different frequencies of loop activation/tethering per chromatid assuming a model where DSB formation is wholly dependent on loop activation/tethering. In summary, loop activation/tethering at a frequency of X, will result in apparent negative interference of 1–1/X. See text for further details. b and c, Cartoons (left) and worked examples (right) for situations in which 50% (b), or 20% (c) of the chromatids within the assayed population are active/tethered at the test locus. The cartoons depict the tethering state of an average sample of 10 chromatids from the population. It is also possible that loop tethering and loop activation are not synonymous processes. In principle, activation of a loop might precede and enable tethering, but not be caused by it.
Extended Data Table 1. Table of strains used in this study.
All strains are of the SK1 background. Genetic modifications were generated by transformation or intercrossing using standard methods. Laboratory origin of strains is indicated.
| Strains | Reference | Genotype |
|---|---|---|
| SG147 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, dmc1#x0394;::LEU2/” | Gray et al, 2013 |
| SG343 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, dmc1Δ::HphMX/”, tel1Δ::HphMX/”, | This study |
| MJ781 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, dmc1Δ::LEU2/”, rad24Δ::Hyg/” | Gray et al, 2013 |
| MJ315 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, sae2Δ::KanMX6/” | Gray et al, 2013 |
| SG346 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp or bgl?/”, leu2::hisG or leu2Δ?/”, his4X::LEU2/”, nuc1::LEU2/”, tel1Δ::HphMX4/”, sae2Δ::KanMX6 | This study |
| SG103 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, rad24Δ::Hyg/”, sae2Δ::KanMX/”, | Gray et al, 2013 |
| VG402 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG,/” his4X::LEU2/”, nuc1::LEU2/”, sae2Δ::KanMX4/”, tel1Δ::HphMX4/” | This study |
| MJ6 | MATa/alpha, ho::LYS2/”’, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/” | Neale et al, 2005 |
| SG344 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, tel1Δ::HphMX4 | This study |
| VG392 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, exo1Δ::KanMX4/” | This study |
| VG393 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG,/” his4X::LEU2/”, nuc1::LEU2/”, exo1Δ::KanMX4/”, tel1Δ::HphMX4/” | This study |
| VG376 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, dmc1Δ::HphMX4/”, tel1Δ::HphMX4/”, exo1Δ::KanMX4/” | This study |
| VG377 | MATa/alpha, ho::LYS2/”, lys2/”, ura3/”, arg4-nsp/”, leu2::hisG/”, his4X::LEU2/”, nuc1::LEU2/”, dmc1Δ::HphMX4/”, exo1Δ::KanMX4/” | This study |
Extended Data Table 2. Table of primers/probes used in this study for DSB and double-cut detection.
Indicated columns: Location of probes (gene locus) for Southern blots, primer sequences used to generate these probes (by PCR), and reference to figure(s) within this manuscript in which probes were used for data collection. For quantification of PFGE and double-cuts (DC), no DNA digestion was performed prior to electrophoresis. For quantification of single-cut DSB frequencies at specific loci, DNA was first digested to completion with the indicated restriction enzyme prior to electrophoresis.
| Probe | ORF chromosome coordinates | primers | Digestion | Comments |
|---|---|---|---|---|
| Figure 1 and Extended Data Figure 1 | ||||
| RMD6 | LChrV: 13720 to 14415 | RMD6_F@+13 CTTGCAACATCGTTATACTCCCAG RMD6_R@+592 GAACTTTGAACCTTTGCACCTCTAC |
NA | |
| YER186C | R ChrV: 562625 to 561705 | YER186C_F@+4 TGTGGCATCCTGATGGTTACGAGC YER186C_R@+674 TCCTCTATGCTATCACCCACCTCTG |
NA | |
| FIR1 | RChrV: 215063 to 217693. | FIR1_F@+1 ATGAGCCTCCCTGTTACACCTGTCAA FIR1_R@+944 ATTCCAAGAAGCTTATCAGCATCTGC |
NA | |
| CHA1 | LChrlll: 16880 to 15798 | CHA1_F@-9 ACCAGCGAGATGTCGATAGTCTAC CHA1_R@+1052 TCTGGAAATATGAAATTGTCAGCG |
NA | Also Extended Data Figure 3 & 4 |
| GITI | R Chrlll: 298605 to 297049 | GIT1_F@+35 GGAAGTGAACGAGAACACTAATCC GIT1_R@+891 AACGGAACTGATAATTGTTGAACTG |
NA | Also Extended Data Figure 3 |
| SYP1 | R Chrlll: 176438 to 173826 | SYP1_F@+1016 ACACCCTAAGATCTAAAGTGGGCTC SYP1_R@+1764 GGATTTAGTTCTCTTAGCTCGCCAG |
NA | Also Extended Data Figure 3 |
| CBP2 | L ChrVIII: 25509 to 23617 | CBP2_F@+712 CGCCACTTTGCACCTTGAATGAA CBP2_R@+1356 TTTCGATTTTGTCAGCACGGTTTG |
NA | |
| CRG1 | R ChrVIII: 519437 to 520312 | CRG1_F@+177 TTTAAGGAAGTGATTGGGATTGAT CRG1_R@+749 RGATTATCTCTAGCCCAAGAAGTG |
NA | |
| CIC1 | ChrVIII: 210848 to 211978 | CIC1_F@+476 CTTAAAGACCGTTTACAAGGCATATGAG CIC1_R@+1116 CTTGACAGCTTCTGACTCGCTAGATTC |
NA | |
| JEN1 | L ChrXI:22234 to 24084 | JEN1_F@-2 ATATGTCGTCGTCAATTACAGATGAG JEN1_R@+620 GGCCACTTTCTGGAAGACTTATC |
||
| SIRI | R ChrXI: 640540 to 642504 | SIR1_F@+14 CTCCAGGCTTGCAGTTATTGATG SIR1_R@+581 CATTTTGTTAAGCCAACCTGACTC |
||
| NUP100 | ChrXI: 310199 to 313078 | NUP100_F@+128 ATTCCACCAGTAACAATGCCCAATCAG NUP100_R@+895 GAGTGCTGCTGTTCATCGAGTTTTGTC |
||
| Figure 2 and Extended Data Figure 4 & 5 | ||||
| FRM2 | LChrlll: 75285 to 74704 | FRM2_F@+27 GCTATTACAAACCGTCGTACCATC FRM2_R@+645 CATCGCTfiAGGTATCATTACTTCAT |
NA | Figure 2a, b, and e, Extended Data Figure 4a & 5a |
| POLV | LChrV: 51539 to 48471 | POL5_F@+1 ATGACAGGGAAAGTCAACAGAGACCT POL5_R@+900 ACCAAACAACGGTAGCAGAACACTC |
NA | Extended Data Figure 5b |
| DOT5 | R ChrIX: 334882 to 335529 | DOT5_F@+1 ATGGGTGAAGCACTACGTAGATCAAC DOT5_R@+882 AATAGTGCCCGTTCTCAATGTAAACC |
NA | Extended Data Figure 5b |
| YCR061W (I) | R Chrlll: 225563 to 227458 | YCR061W_F@+58 CCCATGATGACATGGACATGGAC YCR061W_R@+884 GGTATGTCTTGAGGAAGCAGAGG |
NA | Extended Data Figure 5b Also Extended Data Figure 7a |
| Figure 3 | ||||
| Leu2 | LEU2_F ATATACCATTCTAATGTCTGC LEU2_R ACCATTTTCTTAACTTCTTCGGCG |
NA | Central probe | |
| LEU2LH> | LEU2LH_F GTACGTACAGACCGTCCTGACGG LEU2LH_R CTTTGTCGGAAGCCTTCACCACGTCC |
Pstl | Left probe | |
| MRX2 | Chrlll: 63282 to 62776 | HIS4_F@+5170 CGTGAAGTGGAACGATGCCC HIS4_R@+5493 GCAACTGTTTCCAGCCTTCACC |
Pstl | Right probe |
| Figure 4 | ||||
| BUD23 | R Chrlll: 211546 to 210718 | BUD23_F@1 ATGTCACGTCCTGAGGAGTTGG BUD23_R@+800 GTGAACTTGGAGTCCTTCGCAAC |
NA | Quantification of DC between ARE1 and hotpots on the left of ARE1 |
| ARE1 | R Chrlll: 211929 to 213761 | ARE1_F@+54 ACTCAATTCCGCAGAAGCCA ARE1_R@+715 TTGCCAAGTCCAACATTGCG |
NA | Quantification of DC between ARE1 and hotpots on right of ARE1 |
| TAF2 | R Chrlll: 205397 to 201174 | TAF2_F@+23 CCACTCCTAGAGCCATTGTTAG TAF2_R@+693 TCATCAAGCAAATCGACACATGG |
Asel
NgoMIV |
Quantification of DSB% at ARE1 and hotspots on the right of ARE1 |
| PWP2 | R Chrlll: 223228 to 220457 | PWP2_F@+35 GTACGGTCTACAGGCAAGGTAAC PWP2_R@+815 TTGCTGGATGGAAGGTGACACAC |
NgoMIV | Quantification of DSB% at ARE1 and hotspots on the right of ARE1 |
| RSC6 | R Chrlll: 214994 to 216445 | Bg/II | Quantification of DSB% at ARE1 and hotspots on the right of ARE1 | |
| CTR86 | R Chrlll: 220067 to 218376 | CTR86_F@+50 TACCATGATGAAGAACGACCCATGTTG CTR86_R@+898 ATTGCAATATCTGCAACAAAGTGGTG |
Bg/II | Quantification of DSB% at YCR054W |
| YCR061W (I) | R Chrlll: 225563 to 227458 | YCR061W_F@+58 CCCATGATGACATGGACATGGAC YCR061W_R@+884 GGTATGTCTTGAGGAAGCAGAGG |
Bg/II | Quantification of DSB% at YCR061W |
| YCR061W (II) | R Chrlll: 225563 to 227458 | YCR061W_F@+1283 GGTCCACCAACATCTTCTTGGAG YCR061W_R@+2176 TCAGAGAGAACCTCCAGTAGAGTC |
Bg/II
Pstl EcoRI |
Quantification of DSB% at YCR061W |
| Extended Data Figure 7 | ||||
| YCR061WW (I) | R Chrlll: 225563 to 227458 | YCR061W_F@+58 CCCATGATGACATGGACATGGAC YCR061W_R@+884 GGTATGTCTTGAGGAAGCAGAGG |
NA | Quantification of DC between YCR061W and YCR065W |
| YCR061W (II) | R Chrlll: 225563 to 227458 | YCR061W_F@+1283 GGTCCACCAACATCTTCTTGGAG YCR061W_R@+2176 TCAGAGAGAACCTCCAGTAGAGTC |
Pstl
EcoRI |
Quantification of DC between YCR06fWand YCR065W DSB% at YCR061W and weak hotspot within YCR061W DSB% at YCR063W and YCR065W |
| PUT2 | R ChrVIII: 181977 to 183704 | PUT2_F@+1 ATGCTATCAGCAAGGTGCCTC PUT2_R@+989 CCACTTGGGTGAACTAGATGG |
Stul | Quantification of DSB% at YHR039W, YHR040W and YHR042W |
| SRB2 | R ChrVIII: 189131 to 189864 | NCP1_F@-512 TTCCTTCGCTCAATTGCACTTTCCC NCP1_R@-56 CCACTACAGGAACGCAAACTTAAGC |
NA | Quantification of DC between SRB2INCP1 and YHRQ39W, YHR040W and YHR042W. |
| CCT6 | R ChrlV: 836421 | CCT6_F@+20 TCCGAAGGCTGAATCGTTGAG CCT6_R@+1025 CTTCCACAGAGTTCTGAGCTTC |
Pstl | Quantification of DC between CCT6and SLY1 DSB% at CCT6 |
| SLY1 | ChrlV: 838392 to 840392 | SLY1_F@+1 ATGGCTGTGGAGGAAATTGCGTCC SLY1_R@+1025 TTCTCTGCAGCTTCTGGGAATGGC |
EcoRI | DSB% at SLY1 |
Supplementary Material
Acknowledgements
V.G. was supported by an MRC New Investigator Grant to M.J.N. M.J.N. is supported by a University Research Fellowship from the Royal Society, a Career Development Award from the Human Frontiers Science Program Organisation, and a Consolidator Grant from the European Research Council. We thank S. Keeney and N. Mohibullah for sharing unpublished observations.
Footnotes
Author contributions. V.G. and M.J.N. designed the experiments and wrote the paper. V.G., R.A., S.G. and M.J.N. performed the experiments. T.J.C. provided data analysis and bioinformatics support.
Author information. Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
References
- 1.Pan J, Sasaki M, Kniewel R, Murakami H, et al. A Hierarchical Combination of Factors Shapes the Genome-wide Topography of Yeast Meiotic Recombination Initiation. Cell. 2011;144:719–731. doi: 10.1016/j.cell.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyce EF, Pedersen M, Tiong S, White-Brown SK, et al. Drosophila ATM and ATR have distinct activities in the regulation of meiotic DNA damage and repair. J Cell Biol. 2011;195:359–367. doi: 10.1083/jcb.201104121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange J, Pan J, Cole F, Thelen MP, et al. ATM controls meiotic double-strandbreak formation. Nature. 2011;479:237–240. doi: 10.1038/nature10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Kleckner NE, Storlazzi A, Kim KP. Meiotic double-strand breaks occur once per pair of (sister) chromatids and via Mec1/ATR and Tel1/ATM, once per quartet of chromatids. Proc Natl Acad Sci U S A. 2011;108:20036–20041. doi: 10.1073/pnas.1117937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carballo JA, Panizza S, Serrentino ME, Johnson AL, et al. Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003545. e1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitzblau HG, Hochwagen A. ATR/Mec1 prevents lethal meiotic recombination initiation on partially replicated chromosomes in budding yeast. Elife. 2013;2 doi: 10.7554/eLife.00844. e00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argunhan B, Farmer S, Leung WK, Terentyev Y, et al. Direct and indirect control of the initiation of meiotic recombination by DNA damage checkpoint mechanisms in budding yeast. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065875. e65875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop DK, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 9.Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- 10.Keeney S, Kleckner N. Covalent protein-DNA complexes at the 5' strand termini of meiosis-specific double-strand breaks in yeast. Proc Natl Acad Sci U S A. 1995;92:11274–11278. doi: 10.1073/pnas.92.24.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prinz S, Amon A, Klein F. Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics. 1997;146:781–795. doi: 10.1093/genetics/146.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee AH, Kleckner N. A general method for identifying recessive diploid-specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics. 1997;146:797–816. doi: 10.1093/genetics/146.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thacker D, Mohibullah N, Zhu X, Keeney S. Homologue engagement controls meiotic DNA break number and distribution. Nature. 2014;510:241–246. doi: 10.1038/nature13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panizza S, Mendoza MA, Berlinger M, Huang L, et al. Spo11-accessory proteins link double-strand break sites to the chromosome axis in early meiotic recombination. Cell. 2011;146:372–383. doi: 10.1016/j.cell.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Blat Y, Protacio RU, Hunter N, Kleckner N. Physical and functional interactions among basic chromosome organizational features govern early steps of meiotic chiasma formation. Cell. 2002;111:791–802. doi: 10.1016/s0092-8674(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 16.Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, et al. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol. 2005;25:7203–7215. doi: 10.1128/MCB.25.16.7203-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Giacomo M, Barchi M, Baudat F, Edelmann W, et al. Distinct DNA-damagedependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barchi M, Roig I, Di Giacomo M, de Rooij DG, et al. ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000076. e1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Bourbon HM, de Massy B. Functional conservation of Mei4 for meiotic DNA double-strand break formation from yeasts to mice. Genes Dev. 2010;24:1266–1280. doi: 10.1101/gad.571710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature. 2000;408:1001–1004. doi: 10.1038/35050000. [DOI] [PubMed] [Google Scholar]
- 21.Carballo JA, Johnson AL, Sedgwick SG, Cha RS. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell. 2008;132:758–770. doi: 10.1016/j.cell.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. The double-strandbreak repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 23.Steinel NC, Lee BS, Tubbs AT, Bednarski JJ, et al. The ataxia telangiectasia mutated kinase controls Igκ allelic exclusion by inhibiting secondary Vκ-to-Jκ rearrangements. J Exp Med. 2013;210:233–239. doi: 10.1084/jem.20121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toledo LI, Altmeyer M, Rask MB, Lukas C, et al. ATR Prohibits Replication Catastrophe by Preventing Global Exhaustion of RPA. Cell. 2013;155:1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Westmoreland J, Ma W, Yan Y, Van Hulle K, et al. RAD50 is required for efficient initiation of resection and recombinational repair at random, gamma-induced double-strand break ends. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000656. e1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray S, Allison RM, Garcia V, Goldman AS, Neale MJ. Positive regulation of meiotic DNA double-strand break formation by activation of the DNA damage checkpoint kinase Mec1(ATR) Open Biol. 2013;3 doi: 10.1098/rsob.130019. 130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia V, Phelps SE, Gray S, Neale MJ. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature. 2011;479:241–244. doi: 10.1038/nature10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami H, Borde V, Nicolas A, Keeney S. Gel electrophoresis assays for analyzing DNA double-strand breaks in Saccharomyces cerevisiae at various spatial resolutions. Methods Mol Biol. 2009;557:117–142. doi: 10.1007/978-1-59745-527-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.