Abstract
Within cell membranes numerous protein assemblies reside. Among their many functions, these assemblies regulate the movement of molecules between membranes, facilitate signaling into and out of cells, allow movement of cells by cell-matrix attachment, and regulate the electric potential of the membrane. With such critical roles, membrane protein complexes are of considerable interest for human health, yet they pose an enduring challenge for structural biologists since it is difficult to study these protein structures at atomic resolution in in-situ environments. In order to advance structural and functional insights for these protein assemblies, membrane mimetics are typically employed to recapitulate some of the physical and chemical properties of the lipid bilayer membrane. Extraction from native membranes can however sometimes change the structure and lipid binding properties of these complexes leading to conflicting results and fueling a drive to study complexes directly from native membranes. Here we consider the co-development of membrane mimetics with technological breakthroughs in both cryo-electron microscopy (cryo-EM) and native mass spectrometry (nMS). Together, these developments are leading to a plethora of high-resolution protein structures, as well as new knowledge of their lipid interactions, from different membrane-like environments.
Introduction
Membrane protein structure determination has undergone a well-documented transformation over the last decade1. Once poorly represented in databases, now more than 1000 unique membrane protein structures have been deposited (https://blanco.biomol.uci.edu/mpstruc/). This transformation in the ability to obtain structural information for membrane proteins, is due primarily to the development of high-resolution cryo-EM but can also be attributed to the introduction of many new detergent-free membrane mimetics. These mimetics are designed to help stabilise membrane protein complexes after removal from their native environment. For example, co-development of mimetics and EM has had a profound influence on our understanding of the structure and function of rotary ATP synthases. The first low-resolution structures of mitochondrial ATP synthase oligomers in situ, observed using cryo-electron tomography (cryo-ET), revealed complexes aligned along the cristae of mitochondria2. Early attempts to image the intact complex in detergent using cryo-EM3 struggled to distinguish the boundaries of the micelles from those of the protein subunits but paved the way for widespread structural studies (Figure 1). Fast forward another eight years and images of resolution good enough to build atomic models, at confidence, of dimeric and tetrameric mitochondrial ATP synthases were reported4,5. Just this year, the first structure to contain all subunits including 6.8PL of the ovine mitochondrial monomer ATP synthase, as well as the first full structure of the dimeric bovine mitochondrial ATP synthase, both indicating a different position for the 6.8PL subunit than previously observed, and with lipids within the c-ring, both structure were determined in detergent micelles6,7. This progress in less than a decade is phenomenal. New challenges are emerging however. Specifically, how do we ensure that the membrane mimetic selected does not alter the subunit composition, lipid binding properties or conformation of the protein assembly under investigation?
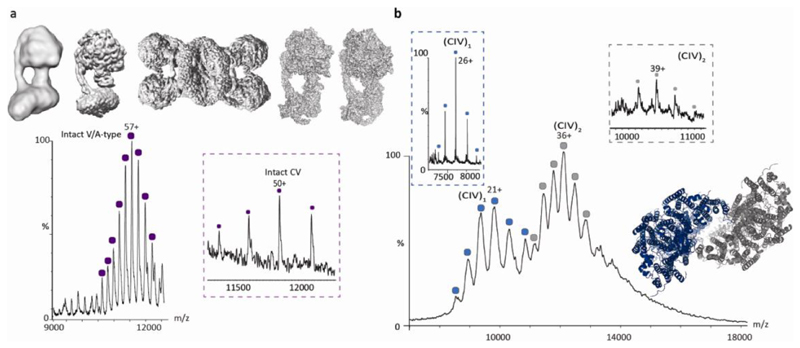
Fig. 1. The resolution revolution has transformed EM and MS.
a Cryo-EM structures of bovine F-type ATP synthase from 20123 (modelled from EMD-2091), 201588 (modelled from EMD-3164) and 2019 (reproduced with permission from 5), from left to right respectively. Mass spectrum of the V/A-type atpase from Thermus thermophilus recorded in 2011 in DDM micelles with a mass consistent with lipid binding in the centre of the Vo membrane ring14. Inset (purple box) mass spectrum of bovine F-type ATP synthase ejected from native membranes in 2018 with mass consistent with the presence of subunits e and 6.8 PL (orange)60 shown schematically in the structure of porcine ATP synthase5 b Mass spectrum of complex IV (Cco) recorded in 2015 in C8E4 detergent micelles reveals an equilibrium between monomeric (blue) and dimeric forms (grey)13. The broadness of the peaks is attributed to multiple lipids binding to the complex, forming a lipid plug between the two monomers. By contrast monomeric and dimeric forms ejected directly from membrane vesicles in 2018 yield well-resolved charge states in the case of the monomeric form (blue box, full width at half maximum = 3.8 m/z), with peak broadening for the dimer (grey box) (full width at half maximum=32.8 m/z)60. The structure is modelled from PDB:2OCC. % is relative intensity, CV is complex V. (CIV)1 is complex IV monomer, (CIV)2 is complex IV dimer. Each collored peak series represents an annotated protein complex.
Here we consider these questions, looking at the development and various attributes of different membrane mimetics and at the technological developments that have majorly contributed to the drive for their development.
Detergents as tools for extraction and as early membrane mimetics
Classically, proteins are extracted from their native membrane environment through the choice of synthetic detergents that are designed to mimic and replace lipids that surround the protein. The use of synthetic detergents to form micelles of varying sizes and physio-chemical properties8 has been the subject of intense research for decades. At an early stage methods were proposed to show that the size of the micelle can be factored into biophysical measurements when determining the oligomeric states of protein subunits9. Moreover, a number of activity assays also demonstrated that functional proteins can be maintained within micelles, often with lipids added exogenously10.
For mass spectrometry (MS) detergent micelles were the first means of protecting membrane protein complexes during their transition from solution to gas phase11. Removal of the micelle is achieved by high-energy collisions releasing the membrane protein complex often with lipid binding intact. The effects of different detergents on this MS process have been studied12 and the formation of lipid ‘plugs’ within these detergent-based environments has been demonstrated, Figure 1. For bovine mitochondrial complex IV13, this lipid plug was assigned to the binding of seven or eight phospholipids or three or four cardiolipins, whereas for the rotary ATPase from Enterococcus hirae, 10 cardiolipins were attached to the K10 membrane rotor14.
Many fascinating structures of membrane protein assemblies have been obtained using detergent micelles, for example G-protein coupled receptors (GPCRs). Explicitly functional states of the A2A adenosine receptor in solution were observed using 19F-NMR15. Using x-ray crystallography, the first structure was that of the prototypic bovine Rhodopsin16, followed by landmark structures of the β2 adrenergic receptor (β2AR) fused to T4 lysozyme in complex with a diffusible ligand and crystallised in lipidic cubic phase in 200717,18. This later structure, and the various stabilisation strategies employed, paved the way for a plethora of GPCRs and eventually led to the first structures of coupled G-protein receptors in 2011. For the coupled complex, the β2 AR interacting with a stimulatory G-protein was stabilized by nanobody binding to the Gαs protein, and crystallogenesis was again enhanced by a T4 lysozyme fusion at the N terminus of the receptor19. A new detergent Maltose neopentyl glycol-320 was employed to stabilize the purified complex in micelles prior to crystallisation in lipid cubic phase21. Phoscholine detergent micelles allowed the first mass spectra of a GPCR and highlighted the role of phosphatidylinositol 4,5-bisphosphate (PIP2) in enhancing coupling to its cognate G-protein22, a result in accord with the latest cryo-EM structure of a GPCR Arrestin complex in a nanodisc wherein PIP2 binding was also observe23. By 2020 some 400 GPCR structures have been deposited; the vast majority being solved by x-ray crystallography in detergent micelles. with a recent increase for EM structures of coupled receptors and larger assemblies (https://zhanglab.ccmb.med.umich.edu/GPCR-EXP).
The widespread and continued use of detergents in x-ray crystallography, cryo-EM, and other biophysical studies continues to motivate research to improve their properties. For example, the Maltose neopentyl glycol-3, employed for crystallisation of the β2 AR G-protein complex, was the first of a new generation of detergents with very low critical micelle concentrations (CMC) 20. As such this detergent is considered milder and easier to work with than established detergents, especially for cryo-EM where it induces low background and promotes high protein stability. The new amphiphile “glyco - diosgenin” (GDN), the synthetic substitute for digitonin, has also been found to confer enhanced stability to a variety of membrane proteins and has been successfully employed in a number of recent cryo-EM structure determinations, particularly for challenging membrane protein complexes24. Furthermore, oligoglycerol detergents were recently introduced to enable both the purification and MS of a range of membrane proteins including GPCRs25.
Despite the introduction of these new detergents many cautionary tales exist surrounding their use for protein structure determination. For example, the varying oligomeric state of the mechansosensitive channel of large conductance (MscL) in which the reorganization of a pentamer into a tetrameric complex was found to be a detergent-dependent process26. MS demonstrated an equilibrium between pentameric and tetrameric species of MscL that could be altered by detergent, disrupted by binding of specific lipids, and perturbed by increasing temperatures (37 °C)27. Specifically, the detergent LDAO, used in the crystallization process, appears to induce this oligomeric shift, whereas Triton X-100 and C8E5 do not. Likewise, a growing number of solution NMR studies have pointed to destabilization, or denaturation, of α-helical membrane proteins, particularly mitochondrial carriers, within phosphocholine detergents28.
Addition of lipids to proteins in detergent micelles is a common practice for improving the stability, structure and function of membrane proteins. Specifically the delicate balance between the detergent used and the amount of phospholipids copurifying is known to be critical to the formation of three-dimensional crystals. Examples include the 5 to 13 lipid molecules per protein that were required for crystal formation of the human erythrocyte anion exchanger29 and the sarco/endoplasmatic reticulum Ca2+-ATPase which requires a specific lipid binding site to be occupied for crystals to form30. Another instance in which lipids have been shown to have an effect includes the side chain conformations of the ammonia channel, which were stabilised by phosphatidyl glycerol in C8E4 detergent micelles31. Addition of cardiolipin and phosphatidyl glycerol to LeuT in octylglucoside micelles was also shown to stabilise dimeric interactions32. Moreover thermodynamic comparisons within phasphatidylcholine (PC)/phosphatidylethanolamine (PE)/phosphatidylglycerol (PG) bilayers show that charge and lateral pressure are important and can increase the thermodynamic stability of LeuT in bilayers over that in micelles33.
In addition to thermodynamic stability, there are other properties that cannot be recapitulated in micelles. These properties include the voltage or charge gradients across membranes, the lateral pressure and curvature within the bilayer environment, as well as the changes in polarity that occur during lipid dynamics34. Moreover, detergent micelles have been shown to encourage and support lipid interactions by both removing and promoting lipid associations25,35, leading to questions about the relevance of lipid binding within micelles with respect to those interactions that occur in native membranes.
These studies, while highlighting the power of detergent micelles for structure determination, also call for a greater understanding the impact of detergents and the role of exogenously added lipids on the precise composition and structure of membrane proteins. The use of detergents for protein extraction is often difficult to avoid since many of the detergent-free approaches used for analysis require detergent-based extraction in their initial preparation steps e.g. nanodiscs. Alternatives such as lipodsics (see below) are under development, but their extraction properties are not yet fully defined. There is, however, a general trend away from detergent micelles for structure determination and a move towards more lipidic environments.
Development of detergent-free membrane mimetics
Motivated by the many established and unknown differences between detergent micelles and the native membrane environment, a number of membrane mimetics have been developed that avoid the use of detergents during the final stages (Figure 2). Considering the properties of each membrane mimetic in turn, as outlined above, detergent micelles have been the mainstay of membrane protein research for decades. Amphipols, amphipathic chemical polymers that wrap around a detergent purified membrane protein, have shown utility for NMR and MS of individual membrane proteins36,37,38. One of the most notable NMR studies is of bacteriorhodopsin39 while the TRPM8 ion channel, the primary detector of cold, which was studied in amphipols following detergent solubilisation provides an excellent example of a cryo-EM study40. Structures of this TRPM8 channel in ligand-free, antagonist-bound, or calcium-bound forms, define conformational changes that would have been challenging to capture in detergent micelles.
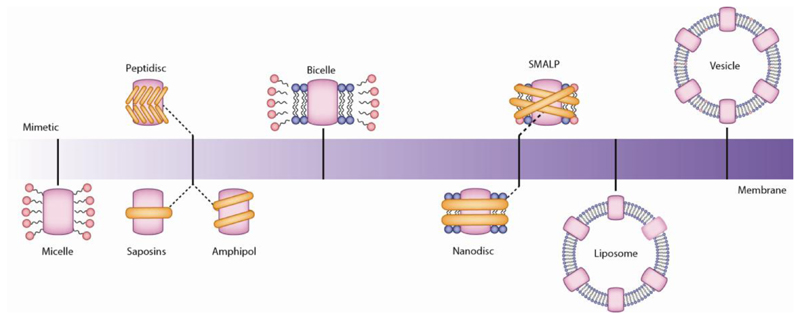
Fig. 2. From micelles to membranes.
The transition from detergent micelles through the various mimetics that have been developed over the last few decades, each with the overall goal of extracting the protein and reconstituting as closely as possible to its original membrane environment. The plethora of membrane protein structures coming to the fore can also be attributed to the new ways of stabilizing these protein complexes in membrane mimetics introduced in recent years. There now exists a wide range of such mimetics including nanodiscs, synthetic polymers, amphipols, peptidiscs and saposins complimenting more established approaches of bicelles, liposomes and vesicles. Each has their merits and compatibilities with various methods but rarely are they universal. For example bicelles, to which membrane protein extracted in detergents, that is followed by encapsulation in lipids flanked by detergent to mimic the membrane, are compatible with NMR89. Nanodiscs are often selected for structure determination via EM 42 and peptidiscs are employed with affinity preparations55. Moreover, while all are capable of capturing aspects of the membrane environment, inherent differences in their properties lead to more ready application for certain types of protein complexes or applications.
Nanodiscs encompass a variety of selected lipids within protein structural belts. Membrane proteins extracted following detergent purification can be inserted into these nanodiscs, and this has led to many recent structures of previously intractable complexes41 by means of both EM42 and solution phase NMR43. One of the first structures to maintain lipid interactions, determined in nanodiscs, was that of the rat TRPV1 channel, implicated in the sensation of heat44. This structure represented a substantial achievement since it was possible to locate both annular and regulatory lipids and to show how specific phospholipid interactions enhance binding of a spider toxin. The first step however requires detergent solublisation which may be problematic for fragile assemblies. Moreover, the length of the protein belt is a critical consideration for the size of the protein assembly under investigation. A membrane protein structural belt that is too short has been reported to generate more homogeneous nanodisc-protein particles, but has the potential to create discs wherein lipids are bound too tightly thereby restricting conformational change42.
Styrene maleic acid (SMA) copolymers are made of hydrophobic styrene and hydrophilic maleic acid units, giving rise to amphiphilic polymers. These can then interact with both the lipid bilayer and the solution to directly extract the protein complex of interest from the membrane45,46, therefore allowing the removal of the detergent extraction step present in other membrane mimetics. This process yields SMA lipid particles (SMALPs, also known as lipodiscs) with membrane protein complexes surrounded by the lipids from their native membrane making this an extremely attractive technology in principle. Recently, a respiratory supercomplex has been successfully isolated and visualized with associated native lipids in a SMALP following extraction using SMA47. Similarly the largest system reported to date, the Photosystem I - SMALP complex, was functional despite the selective loss of one transmembrane subunit, PsaF48. Labile interaction of PsaF with the PS-1 core was attributed to its selective displacement during copolymer insertion. Despite this observation, formation of the Photosystem I –SMALP complex represents an exciting breakthrough in the analysis of large assemblies using this membrane mimetic. Some perturbation of the lipids might be anticipated however when adding acid directly to membranes. Moreover, because SMALPs are only soluble under basic conditions, and precipitate in the presence of divalent cations, required for many downstream applications, this has motivated the introduction of additional polymers for excision49. Recently it was shown that positively charged poly(styrene-co-maleimide) (SMI) forms similar nanoparticles with comparable efficiency to SMA, while remaining functional at acidic pH and compatible with high concentrations of divalent cations. It should be noted, however, that despite these remarkable advancements, and the great hope that these completely detergent-free mimetics carry, this extraction technique remains challenging for use with EM, and for MS it has not yet been possible to define directly interactions with endogenous lipids using this approach.
Liposomes by contrast are constituted from pre-selected lipids to form large spherical-shaped vesicles, composed of one or more phospholipid bilayers. The membrane protein under study is then inserted into the liposome following detergent extraction. Liposomes are amenable to determination of pore structures, enabling determination of both their assembly and structure by cryo-EM50,51,52. Prior to the development liposomes enabled spherical reconstruction to determine the cryo-EM structure of the human large-conductance calcium- and voltage-activated potassium channel (BK) in a lipid environment 52,53. More recently, and with the direct detector in place, fascinating insights into the structure of Gasdermin D were enabled by liposomes revealing a 27-fold symmetric pore. The formation of the double ring structure of Gasdermin D was attributed to the absence of the natural lipid asymmetry which is difficult to recapitulate in such a preparation50,54. For E. coli hemolysin ClyA, oligomers (12-mer, 13-mer and 14-mers) were observed in a range of detergents, and on the surface of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)-1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS)-cholesterol liposomes, where cholesterol was identified as a critical factor in the assembly reaction51. With the exception of cryo-EM for pore forming complexes, the use of liposomes appears less widespread. This phenomenon is due in part to the introduction of the direct electron detector, but also to the numerous membrane mimetics that are less complicated to work with than liposomes but still allow high resolution structure determination.
Additional protein and peptide-based detergent-free encapsulation methods include Peptidiscs, which are formed from short amphipathic bi-helical peptides without additional lipids. Multiple copies of the peptide shield the membrane-exposed part of the target protein55. By contrast the Saposin family of protein-based discs allow for the reconstitution of membrane proteins in a lipid environment that is stabilized by a scaffold of saposin proteins56. Both show great promise for structural biology with peptidiscs recapitulating many associations between complexes, as evidenced via proteomics57 and saposins supporting cryo-EM of challenging membrane proteins, for example PeptTSo256. Moreover saposins have also enabled NMR studies of membrane proteins including GPCRs38. Given that both approaches have only recently been introduced the numbers of applications for both remain relatively low but are likely to increase markedly as methodologies and technologies advance.
Vesicles have also been used for studying membrane proteins in close to native membrane environments via cryo-EM. In an early example of this approach, extracellular vesicles enriched with a specific membrane protein, were formed from adherent mammalian cells transfected with the gene corresponding to the full-length protein of interest. Overexpression of the protein resulted in the accumulation of membrane protein containing extracellular vesicles in the growth medium, which were then separated by differential centrifugation of the supernatant58,59. The structures of these membrane proteins in this vesicle environment were first evidenced by cryo-EM and cryo-ET, and the development of this technology for use in conjunction with MS60. The main challenges associated with studying vesicles stem from the unexpected associations that have not been observed previously from experiments performed in detergent micelles and the immense variety of proteins embedded within the membrane. For both native MS and cryo-EM these complexities are overcome, at least in part, by the increase in resolution that is now possible for both approaches.
The resolution revolution
At the beginning of this decade, EM structures were limited heavily by resolution, lacking the molecular details that would be necessary to build atomic models and to properly identify subunits, let alone small molecules and lipids. With the invention and introduction of the direct electron detector, and additional algorithms to assess and reconstruct structures, the increase in resolution achieved by EM is leading to structural details that approach the level of detail available from x-ray crystallography. These developments have led to spectacular advances over recent years and have highlighted many technical challenges that remain61. Some high-resolution EM structures also now call into question earlier reports and add detailed lipid binding to previous observations. For example a human synaptic Type A γ-aminobutyric acid receptor (GABAAR) complex reconstituted into lipid nanodiscs62 reveals a different conformation to previous studies of this complex formed from engineered proteins and reconstituted into detergent micelles63,64. Moreover, in the structure obtained from nanodiscs each GABAAR pentamer harbours two phosphatidylinositol 4,5-bisphosphate (PIP2) molecules not seen in the structures obtained in detergent micelles.
For MS resolution has been considerably enhanced by the development of Orbitrap instruments, first for soluble protein complexes, and shortly after for membrane proteins, with the introduction of a Q-Exactive Extended Mass Range (EMR) instrument65,66. Subsequent development led to the introduction of a further high mass range and higher energy instrument the UHMR bringing sufficient resolution to observe post-translational modifications (PTMs) and small molecule binding at the level of soluble proteins in some cases67. Importantly, for membrane proteins this new instrumentation has enabled resolution of small molecule ligands such that the length of lipid side-chains (separated by 14 or 28 mass units) can be resolved while binding directly to membrane proteins68. The ability to define isoforms, PTMs and the detailed assignment of small molecules is important for direct identification of the often low resolution images of lipids, dynamic adducts and isoforms that define the origin of complexes from various tissues and organelles69 ‘ 70.
Enhanced resolution in conjunction with new membrane mimetics
While the use of detergent micelles has served MS well over the last decade11, new mimetics for use in conjunction with MS have also been explored. Specifically SMALPS were used to probe complexes within native lipid environments using laser induced liquid bead ion desorption (LILBID) MS71. For Nanodiscs the average number of lipids within the nanodisc complex could be defined by maintaining the intact assembly. Alternatively membrane proteins can be ejected from the nanodisc complex with associated lipids, using a charge manipulation strategy in conjunction with electrospray72.
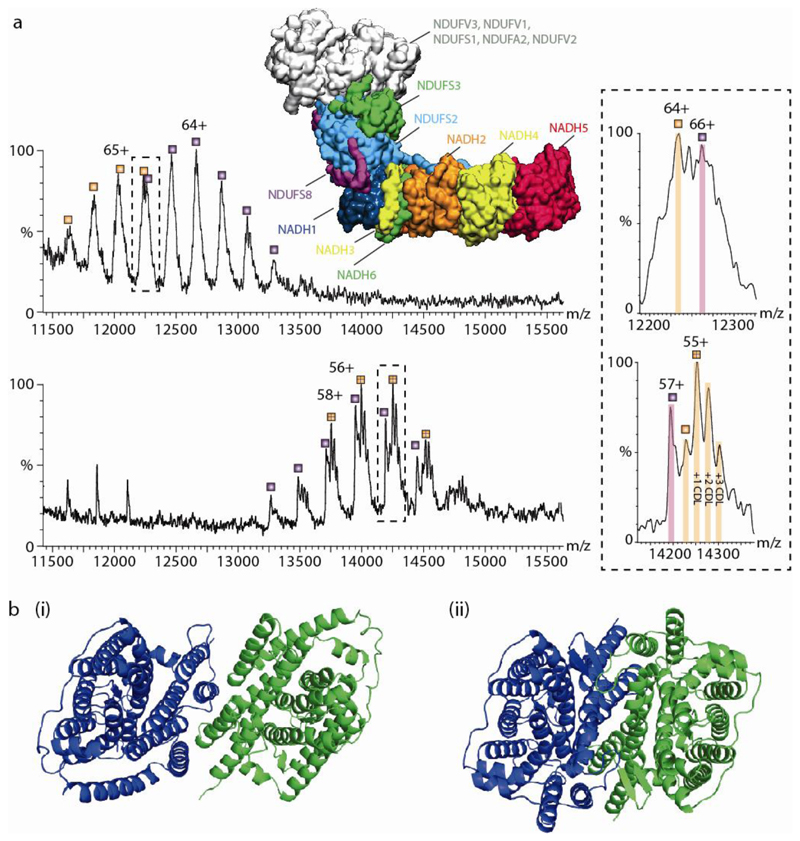
Avoiding detergents or polymers altogether has been a long-term goal and was recently demonstrated using Sonnicated Lipid Vesicle MS (SoLVe MS)60. The native lipid vesicles are generated by physical means, such as a cell press or application of osmotic pressure, or are naturally excreted with membrane proteins intact either at endogenous levels or via over-expression. In this approach the membrane protein complexes are charged at high field strengths and ejected directly from their native environments into a mass spectrometer. The key advantage of this approach is that the absence of chemicals preserves novel interactions that were not directly observed before by other membrane mimetic methods, including those between the bacterial F-type ATPase and the SecYEG translocon, which were subsequently validated using peptidiscs55. Using SoLVe-MS, many additional examples were reported, including those between the 39 membrane subunits of Complex I, in an assembly state observed previously in EM and linked to regeneration73. Interestingly different lipid binding properties were observed for the complex I membrane arm ejected directly from the membrane as opposed to the complex extracted in detergent micelles (Figure 3a). Molecular dynamics simulations of the hydrophobic complex I arm74 highlight a number of specific cardiolipin binding sites in close agreement with lipid binding pattern identified when the complex is ejected from a native membrane environment.
Fig. 3. The effect of detergents on lipid binding and structural integrity.
(a) Comparison of mass spectra of Complex I (CI) in detergent micelles (upper) and directly from vesicles (lower). Upper panel is mass spectrum recorded in a n-Dodecyl-β-D-Maltopyranoside (DDM) detergent micelle, which reveals two series of peaks (labeled orange and purple) differing in mass by 26,612 Da assigned to NDUFS3 (in green, within the protein model). The structure shown is from PDB:5LDW. Both series are assigned to the complete membrane region. The charge states differ between both conditions- the top panel is measured in 2x critical micellar concentration (CMC) of DDM, the bottom panel is CI lacking the N module ejected directly from native membranes with no recourse to detergents or other membrane mimetics. The lipid binding properties change in terms of both the extent and identity of bound lipids. However, the measured mass remains essentially the same for both the detergent extracted and SoLVe measurements (782,772 ± 35 Da compared to 782,478 ± 27 Da) for the membrane arm without NDUFS3 (orange series) and 809,269 ± 47 compared to 809,101 ± 57 Da for the membrane arm with NDUFS3 (purple series) respectively. (b) Comparison of UraA:Proton symporter in β-NG75, PDB: 3QE7 (i) whereby the dimer interface is deformed by the protrusion of the acyl chain of the detergent molecules between key helices in each monomer, and in fos-choline 9 and 1176 from PDB:5XLS (ii) showing a functional dimer.
For mitochondrial Complex IV, ejected from vesicles, both monomeric and a low population of a dimeric form were observed60 (Figure 1). Comparing this data with that reported previously from detergent micelles shows that the extent of lipid binding in the dimeric form ejected from vesicles is much less than for the detergent encapsulated form13. Similarly for bovine mitochondrial ATP synthase the protein 6.8PL was assigned by mass, in the absence of lipids normally observed in the mass spectra of detergent extracted rotary ATPases, in line with recent results from Cryo-EM5. This example, together with the recent V-type ATPase from synaptic vesicles visualizing additional subunits within the cavity of the c-ring, shows how increased resolution allows for the detection of previously unseen subunits and isoforms both from EM and MS70.
As resolution is enhanced, and membrane mimetics developed, new challenges arise. For instance, the plethora of high-resolution structures recently reported have indicated some variance in subunit stoichiometry. Selected examples include the bacterial UraA uracil:proton symporter, which was first thought to be active as a monomer as it crystalizes as a dimer whereby the dimer interface is distorted by perturbation of the protein structure with alkyl chain insertion of n-nonyl-β-D-glucopyranoside (β-NG)75. Later however, it was shown that crystallization in the presence of fos-choline gives rise to an intact dimer structure now believed to be the true active form of the enzyme76 (Figure 3b). Amotherexample is the membrane receptor Patched 1 (Ptch1) involved in the Hedgehog (Hh) signalling pathway, which is important in embryogenesis and associated with cancer (Figure 4a). The structure of the monomeric form of human Ptch1 was first reported in 2018 studied in DDM micelles with cholesterol hemisuccinate present77. Later that year a further EM structure of mouse Ptch1 was reported as a dimer within amphipols (comprising Poly (Maleic Anhydride-alt-1-Decene) substituted with 3-(Dimethylamino) Propylamine). These amphipols were visualized as transparent shells surrounding the transmembrane domains78. Early in 2019 an EM structure with four Ptch1 protomers, organized as a loose dimer of dimers, in GDN detergent micelles, was reported79. These three reports highlight the difficulties of comparing oligomeric state of membrane proteins from different preparations in different membrane mimetics.
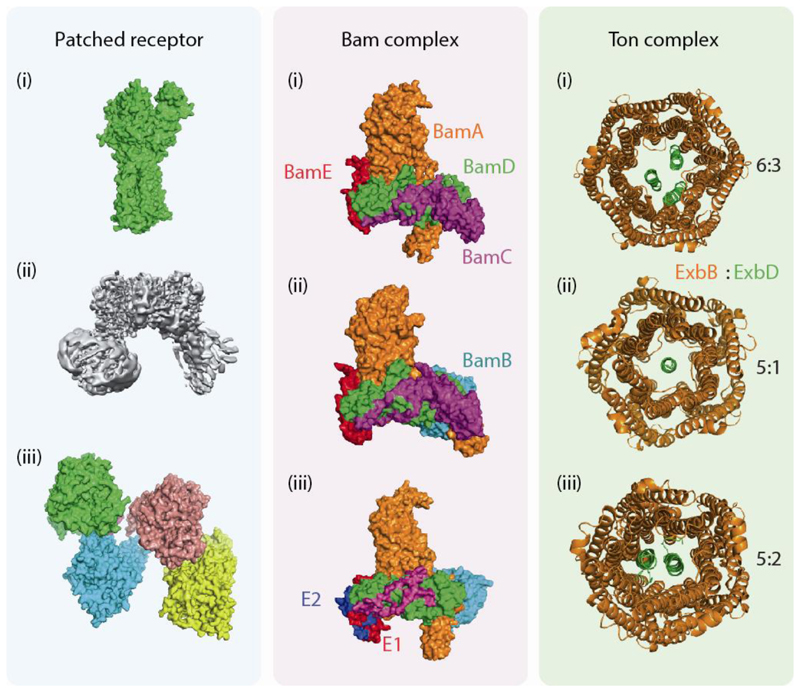
Fig. 4. Variation in the subunit stoichiometry of the the Patched receptor, Bam and Ton complexes.
For the patched receptor (green panel) three different EM structures have been reported. First a monomeric structure from DDM micelles in August 201877 (i) followed by a dimeric structure from amphipols in November 2018 (reproduced with permission from78) (ii) and a tetrameric structure in May 2019 from GDN micelles79 (iii). The Bam complex (blue panel) was first reported in 2016, using x-ray crystallography and C8E4 micelles shown to contain 4 subunits - BamACDE80 (i) later reported as a pentamer (BamABCDE) by means of cryo-EM82 (ii) and using native MS a hexamer of Bam containing two E subunits could also be assigned, both from native membranes and recombinant sources (iii). The Ton complex (orange panel) was shown from x-ray structures to exist in two forms 6ExbB: 3ExbD and 5ExbB:1ExbD84 (i-ii) and a stoichiometry of 5ExbB:2ExbD85 was deduced from EM (iii). When ejected from native membranes into a mass spectrometer it was assigned as 5ExbB:1ExbD60 (ii).
Interestingly, it is not always the detergent that influences the outcome of the experiment, because protein expression levels may also affect subunit stoichiometry, as seen in the BAM complex (Figure 4b). First expressed and crystallised in C8E4 micelles from one plasmid containing a single copy of all five genes (BamA - E) the structure of the complex was solved to 3.4 Å resolution via x-ray crystallography80. BamB was not present in this structure possibly due to proteolytic degradation during incubation. One month later all five encoding genes on a single plasmid lead to an x-ray structure at similar resolution (3.6 Å), also from detergent micelles, but this time BamB was present suggesting routes for the biogenesis of outer membrane proteins81. Later using cryo-EM, and the same plasmid, the pentameric form of the complex was resolved (4.9 Å) revealing conformational changes of the proteins and movement of the lateral gate82. Interestingly in this study, an alternative form of the complex was also observed containing an additional copy of BamE using electrospray MS from DDM micelles82. This alternative stoichiometry was attributed to the overexpression protocol. When ejected directly from E. coli inner membrane vesicles using SoLVe MS however the additional copy of BamE was also present, despite the complex resulting from endogenous expression. When this second copy of BamE was not present however up to three cardiolipin molecules were observed to bind to the complex60 suggesting a membrane targeting mechanism in the absence of the second copy of BamE.
The structure of the Ton complex, present in the inner membrane of Gram-negative bacteria, was first reported from a combination of biophysical approaches including x-ray crystallography, negative stain EM and double electron-electron resonance (DEER) spectroscopy with a stoichiometry consisting of a pentamer of ExbB, a dimer of ExbD, and at least one copy of TonB in lipid-protein-detergent solutions (Figure 4c)83. Two years later, a 6:3 ExbB and ExbD stoichiometry was determined from C8E4/C8E5 detergent micelles and 2D crystallography, supporting a model whereby the proton motive force may lead to changes in subunit stoichiometry of the complex84. Very recently a structure of pentameric ExbB and dimeric ExbD was reported from cryo-EM of the complex in lipid nanodiscs with crosslinking of ExbD supporting its dimerization85. The Ton complex was observed in a 5:1 ExbB to ExbD stoichiometry using crystallography84 or when ejected from E. coli outer membrane vesicles60.
This variance in subunit stoichiometry exemplified by the three examples described above could be a response to the method of extraction or reconstitution. Alternatively, multiple subunit stoichiometries may exist in vivo and one or more maybe destabilised by a particular extraction or reconstitution process.
Future perspectives
With the insights made possible by increased resolution and recent technological breakthroughs, comparison of wild-type structures in mimetics to structures recorded in detergent micelles, using heavily modified proteins, should be undertaken with caution and with the understanding that differences may arise. Looking forward, we are now able to begin answering many of the vexing questions that have perplexed the field for many years. Specifically, how do the structures of complexes change and respond within their native lipid environments? To this end, recent developments in MS, as well as cryo-EM, are particularly exciting. For cryo-EM advances with laser plate technology86,87 greatly enhance the contrast of biological specimens allowing for more robust structure determination, either as separated single molecules, or within cellular contexts. Though not able to provide detailed structural information, MS from native membranes captures complimentary information in the form of direct associations with other proteins, for example molecular chaperones, stoichiometries, native lipid binding interactions and incorporation of different isoforms. As such MS holds the promise of uncovering novel interacting partners for the multitude of complexes that reside within native bilayers. Bringing together insights from structure determination and MS in lipidic environments, will in turn stimulate research into the impact of membranes and provide a more complete picture of both healthy and diseased cells.
Acknowledgments
We thank all members of the Robinson group for helpful discussions and the many collaborators who have contributed to this work. We are also grateful to Sarah L. Rouse, Lindsay A. Baker, Nieng Yan and Christoph Gerle for critical review of the manuscript. We would also like to thank Firdaus Samsudin and Syma Khalid for the MD based model of the Bam Complex. We acknowledge with thanks funding from an ERC Advanced Grant ENABLE (695511) and a Wellcome Trust Investigator Award (104633/Z/14/Z).
References
- 1.Callaway E. The revolution will not be crystallized: a new method sweeps through structural biology. Nature. 2015;525:172–4. doi: 10.1038/525172a. [DOI] [PubMed] [Google Scholar]
- 2.Davies KM, Anselmi C, Wittig I, Faraldo-Gomez JD, Kuhlbrandt W. Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc Natl Acad Sci U S A. 2012;109:13602–7. doi: 10.1073/pnas.1204593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker LA, Watt IN, Runswick MJ, Walker JE, Rubinstein JL. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proc Natl Acad Sci U S A. 2012;109:11675–80. doi: 10.1073/pnas.1204935109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy BJ, et al. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo coupling. Science. 2019;364 doi: 10.1126/science.aaw9128. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, et al. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science. 2019;364:1068–1075. doi: 10.1126/science.aaw4852. [DOI] [PubMed] [Google Scholar]
- 6.Pinke G, Zhou L, Sazanov LA. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat Struct Mol Biol. 2020;27:1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 7.Spikes TE, Montgomery MG, Walker JE. Structure of the dimeric ATP synthase from bovine mitochondria. Proc Natl Acad Sci U S A. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–17. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Burgess NK, Stanley AM, Fleming KG. Determination of membrane protein molecular weights and association equilibrium constants using sedimentation equilibrium and sedimentation velocity. Methods Cell Biol. 2008;84:181–211. doi: 10.1016/S0091-679X(07)84007-6. [DOI] [PubMed] [Google Scholar]
- 10.Vukoti K, Kimura T, Macke L, Gawrisch K, Yeliseev A. Stabilization of functional recombinant cannabinoid receptor CB(2) in detergent micelles and lipid bilayers. PLoS One. 2012;7:e46290. doi: 10.1371/journal.pone.0046290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Micelles protect membrane complexes from solution to vacuum. Science. 2008;321:243–6. doi: 10.1126/science.1159292. [*This is a report of a membrane protein complex ejected from a detergent micelle into the gas phase of a mass spectrometer with cytoplasmic and membrane domains intact] [DOI] [PubMed] [Google Scholar]
- 12.Reading E, et al. The role of the detergent micelle in preserving the structure of membrane proteins in the gas phase. Angew Chem Int Ed Engl. 2015;54:4577–81. doi: 10.1002/anie.201411622. [DOI] [PubMed] [Google Scholar]
- 13.Liko I, et al. Dimer interface of bovine cytochrome c oxidase is influenced by local posttranslational modifications and lipid binding. Proc Natl Acad Sci U S A. 2016;113:8230–5. doi: 10.1073/pnas.1600354113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, et al. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science. 2011;334:380–385. doi: 10.1126/science.1210148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susac L, Eddy MT, Didenko T, Stevens RC, Wuthrich K. A2A adenosine receptor functional states characterized by (19)F-NMR. Proc Natl Acad Sci U S A. 2018;115:12733–12738. doi: 10.1073/pnas.1813649115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [*This study reports the structure of an unmodified wild-type GPCR, allowing insights into the propagation of signals in vision] [DOI] [PubMed] [Google Scholar]
- 17.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–73. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen SG, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–55. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chae PS, et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–8. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caffrey M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr F Struct Biol Commun. 2015;71:3–18. doi: 10.1107/S2053230X14026843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen HY, et al. PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature. 2018;559:423–427. doi: 10.1038/s41586-018-0325-6. [*This study reports the use of mass spectrometry to uncover the role of PIP2 in stabilizing downstream coupling of class A GPCRs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020 doi: 10.1038/s41586-020-1953-1. [*This paper reports the cryo-EM structure of an arrestin-bound receptor, also revealing a PIP2 molecule forming a bridge between the membrane side of the receptor and arrestin] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Liu D, Wu K, Lei J, Yan N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science. 2019;363:1303–1308. doi: 10.1126/science.aaw2493. [DOI] [PubMed] [Google Scholar]
- 25.Urner LH, et al. Modular detergents tailor the purification and structural analysis of membrane proteins including G-protein coupled receptors. Nat Commun. 2020;11:564. doi: 10.1038/s41467-020-14424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorwart MR, Wray R, Brautigam CA, Jiang Y, Blount PS. aureus MscL is a pentamer in vivo but of variable stoichiometries in vitro: implications for detergent-solubilized membrane proteins. PLoS Biol. 2010;8:e1000555. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reading E, et al. The Effect of Detergent, Temperature, and Lipid on the Oligomeric State of MscL Constructs: Insights from Mass Spectrometry. Chem Biol. 2015;22:593–603. doi: 10.1016/j.chembiol.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chipot C, et al. Perturbations of Native Membrane Protein Structure in Alkyl Phosphocholine Detergents: A Critical Assessment of NMR and Biophysical Studies. Chem Rev. 2018;118:3559–3607. doi: 10.1021/acs.chemrev.7b00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemieux MJ, Reithmeier RA, Wang DN. Importance of detergent and phospholipid in the crystallization of the human erythrocyte anion-exchanger membrane domain. J Struct Biol. 2002;137:322–32. doi: 10.1016/s1047-8477(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 30.Drachmann ND, et al. Comparing crystal structures of Ca(2+) -ATPase in the presence of different lipids. FEBS J. 2014;281:4249–62. doi: 10.1111/febs.12957. [DOI] [PubMed] [Google Scholar]
- 31.Laganowsky A, et al. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta K, et al. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature. 2017;541:421–424. doi: 10.1038/nature20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders MR, Findlay HE, Booth PJ. Lipid bilayer composition modulates the unfolding free energy of a knotted alpha-helical membrane protein. Proc Natl Acad Sci U S A. 2018;115:E1799–E1808. doi: 10.1073/pnas.1714668115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karabadzhak AG, et al. Bilayer Thickness and Curvature Influence Binding and Insertion of a pHLIP Peptide. Biophys J. 2018;114:2107–2115. doi: 10.1016/j.bpj.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landreh M, Costeira-Paulo J, Gault J, Marklund EG, Robinson CV. Effects of Detergent Micelles on Lipid Binding to Proteins in Electrospray Ionization Mass Spectrometry. Anal Chem. 2017;89:7425–7430. doi: 10.1021/acs.analchem.7b00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoonens M, Catoire LJ, Giusti F, Popot JL. NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci U S A. 2005;102:8893–8. doi: 10.1073/pnas.0503750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese AN, Watkinson TG, Henderson PJ, Radford SE, Ashcroft AE. Amphipols outperform dodecylmaltoside micelles in stabilizing membrane protein structure in the gas phase. Anal Chem. 2015;87:1118–26. doi: 10.1021/ac5037022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chien CH, et al. An Adaptable Phospholipid Membrane Mimetic System for Solution NMR Studies of Membrane Proteins. J Am Chem Soc. 2017;139:14829–14832. doi: 10.1021/jacs.7b06730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elter S, et al. The use of amphipols for NMR structural characterization of 7-TM proteins. J Membr Biol. 2014;247:957–64. doi: 10.1007/s00232-014-9669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diver MM, Cheng Y, Julius D. Structural insights into TRPM8 inhibition and desensitization. Science. 2019;365:1434–1440. doi: 10.1126/science.aax6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean MA, Gregory MC, Sligar SG. Nanodiscs: A Controlled Bilayer Surface for the Study of Membrane Proteins. Annu Rev Biophys. 2018 doi: 10.1146/annurev-biophys-070816-033620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Autzen HE, Julius D, Cheng Y. Membrane mimetic systems in CryoEM: keeping membrane proteins in their native environment. Curr Opin Struct Biol. 2019;58:259–268. doi: 10.1016/j.sbi.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokogawa M, Fukuda M, Osawa M. Nanodiscs for Structural Biology in a Membranous Environment. Chem Pharm Bull (Tokyo) 2019;67:321–326. doi: 10.1248/cpb.c18-00941. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–51. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu W, et al. Structure and activity of lipid bilayer within a membrane-protein transporter. Proc Natl Acad Sci U S A. 2018;115:12985–12990. doi: 10.1073/pnas.1812526115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Postis V, et al. The use of SMALPs as a novel membrane protein scaffold for structure study by negative stain electron microscopy. Biochim Biophys Acta. 2015;1848:496–501. doi: 10.1016/j.bbamem.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C, et al. Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature. 2018;557:123–126. doi: 10.1038/s41586-018-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brady NG, Li M, Ma Y, Gumbart JC, Bruce BD. Non-detergent isolation of a cyanobacterial photosystem I using styrene maleic acid alternating copolymers. RSC Advances. 2019;9:31781–31796. doi: 10.1039/c9ra04619d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall SCL, et al. An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale. 2018;10:10609–10619. doi: 10.1039/c8nr01322e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan J, Xia S, Liu X, Lieberman J, Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557:62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng W, de Souza Santos M, Li Y, Tomchick DR, Orth K. High-resolution cryo-EM structures of the E. coli hemolysin ClyA oligomers. PLoS One. 2019;14:e0213423. doi: 10.1371/journal.pone.0213423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–5. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang QX, Chester DW, Sigworth FJ. Spherical reconstruction: a method for structure determination of membrane proteins from cryo-EM images. J Struct Biol. 2001;133:119–31. doi: 10.1006/jsbi.2001.4376. [DOI] [PubMed] [Google Scholar]
- 54.Markones M, et al. Stairway to Asymmetry: Five steps to lipid-asymmetric proteoliposomes. Biophysical Journal. 2019 doi: 10.1016/j.bpj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson ML, et al. The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution. Elife. 2018;7 doi: 10.7554/eLife.34085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frauenfeld J, et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat Methods. 2016;13:345–51. doi: 10.1038/nmeth.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlson ML, et al. Profiling the Escherichia coli membrane protein interactome captured in Peptidisc libraries. Elife. 2019;8 doi: 10.7554/eLife.46615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeev-Ben-Mordehai T, Vasishtan D, Siebert CA, Whittle C, Grunewald K. Extracellular vesicles: a platform for the structure determination of membrane proteins by Cryo-EM. Structure. 2014;22:1687–92. doi: 10.1016/j.str.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeev-Ben-Mordehai T, Vasishtan D, Siebert CA, Grunewald K. The full-length cell-cell fusogen EFF-1 is monomeric and upright on the membrane. Nat Commun. 2014;5:3912. doi: 10.1038/ncomms4912. [*This study reports the cryo-EM structure of a cell–cell fusogen imaged while in a membrane environment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chorev DS, et al. Protein assemblies ejected directly from native membranes yield complexes for mass spectrometry. Science. 2018;362:829–834. doi: 10.1126/science.aau0976. [*This paper reports a mass spectrometry study of membrane protein complexes ejected directly from their membrane environments including bacterial and mitochnodrial membranes] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danev R, Yanagisawa H, Kikkawa M. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem Sci. 2019;44:837–848. doi: 10.1016/j.tibs.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Laverty D, et al. Cryo-EM structure of the human alpha1beta3gamma2 GABAA receptor in a lipid bilayer. Nature. 2019;565:516–520. doi: 10.1038/s41586-018-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu S, et al. Structure of a human synaptic GABAA receptor. Nature. 2018;559:67–72. doi: 10.1038/s41586-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phulera S, et al. Cryo-EM structure of the benzodiazepine-sensitive alpha1beta1gamma2S tri-heteromeric GABAA receptor in complex with GABA. Elife. 2018;7 doi: 10.7554/eLife.39383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose RJ, Damoc E, Denisov E, Makarov A, Heck AJ. High-sensitivity Orbitrap mass analysis of intact macromolecular assemblies. Nat Methods. 2012;9:1084–6. doi: 10.1038/nmeth.2208. [*This study demonstrates a high-resolution Orbitrap for native MS of soluble protein complexes] [DOI] [PubMed] [Google Scholar]
- 66.Gault J, et al. High-resolution mass spectrometry of small molecules bound to membrane proteins. Nat Methods. 2016;13:333–6. doi: 10.1038/nmeth.3771. [*This study covers the development of the Orbitrap platform for membrane proteins and demonstrates that the chain length of lipids could be distinguished while bound to the membrane protein] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fort KL, et al. Expanding the structural analysis capabilities on an Orbitrap-based mass spectrometer for large macromolecular complexes. Analyst. 2017;143:100–105. doi: 10.1039/c7an01629h. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, et al. Selective binding of a toxin and phosphatidylinositides to a mammalian potassium channel. Nat Commun. 2019;10:1352. doi: 10.1038/s41467-019-09333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liko I, Allison TM, Hopper JT, Robinson CV. Mass spectrometry guided structural biology. Curr Opin Struct Biol. 2016;40:136–144. doi: 10.1016/j.sbi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Abbas Y, Wu D, Robinson CV, Rubinstein J. Structure of the mammalian synaptic vesicle V-ATPase. Science. doi: 10.1126/science.aaz2924. in press. [*This study reports the cryo-EM structure of the V-type ATPase isolated from rat brain synaptic vesicles. MS was used to define the isoform and subunit stoichiometry and composition of the intact V1 complex] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hellwig N, et al. Native mass spectrometry goes more native: investigation of membrane protein complexes directly from SMALPs. Chem Commun (Camb) 2018 doi: 10.1039/c8cc06284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keener JE, et al. Chemical Additives Enable Native Mass Spectrometry Measurement of Membrane Protein Oligomeric State within Intact Nanodiscs. J Am Chem Soc. 2019;141:1054–1061. doi: 10.1021/jacs.8b11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sousa JS, Mills DJ, Vonck J, Kuhlbrandt W. Functional asymmetry and electron flow in the bovine respirasome. Elife. 2016;5 doi: 10.7554/eLife.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jussupow A, Di Luca A, Kaila VRI. How cardiolipin modulates the dynamics of respiratory complex I. Sci Adv. 2019;5:eaav1850. doi: 10.1126/sciadv.aav1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu F, et al. Structure and mechanism of the uracil transporter UraA. Nature. 2011;472:243–246. doi: 10.1038/nature09885. [DOI] [PubMed] [Google Scholar]
- 76.Yu X, et al. Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Research. 2017;27:1020–1033. doi: 10.1038/cr.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong X, et al. Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science. 2018;361 doi: 10.1126/science.aas8935. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, et al. Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched. Cell. 2018;175:1352–1364 e14. doi: 10.1016/j.cell.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian H, et al. Inhibition of tetrameric Patched1 by Sonic Hedgehog through an asymmetric paradigm. Nat Commun. 2019;10:2320. doi: 10.1038/s41467-019-10234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bakelar J, Buchanan SK, Noinaj N. The structure of the beta-barrel assembly machinery complex. Science. 2016;351:180–6. doi: 10.1126/science.aad3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han L, et al. Structure of the BAM complex and its implications for biogenesis of outer-membrane proteins. Nat Struct Mol Biol. 2016;23:192–6. doi: 10.1038/nsmb.3181. [DOI] [PubMed] [Google Scholar]
- 82.Iadanza MG, et al. Lateral opening in the intact beta-barrel assembly machinery captured by cryo-EM. Nat Commun. 2016;7:12865. doi: 10.1038/ncomms12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Celia H, et al. Structural insight into the role of the Ton complex in energy transduction. Nature. 2016;538:60–65. doi: 10.1038/nature19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maki-Yonekura S, et al. Hexameric and pentameric complexes of the ExbBD energizer in the Ton system. Elife. 2018;7 doi: 10.7554/eLife.35419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Celia H, et al. Cryo-EM structure of the bacterial Ton motor subcomplex ExbB-ExbD provides information on structure and stoichiometry. Commun Biol. 2019;2:358. doi: 10.1038/s42003-019-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Danev R, Baumeister W. Expanding the boundaries of cryo-EM with phase plates. Curr Opin Struct Biol. 2017;46:87–94. doi: 10.1016/j.sbi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz O, et al. Laser phase plate for transmission electron microscopy. Nat Methods. 2019;16:1016–1020. doi: 10.1038/s41592-019-0552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou A, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. Elife. 2015;4:e10180. doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raschle T, Hiller S, Etzkorn M, Wagner G. Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr Opin Struct Biol. 2010;20:471–9. doi: 10.1016/j.sbi.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]