Abstract
Despite extensive subcortical processing, the auditory cortex is believed to be essential for normal sound localization. However, we still have a poor understanding of how auditory spatial information is encoded in the cortex and of the relative contribution of different cortical areas to spatial hearing. We investigated the behavioral consequences of inactivating ferret primary auditory cortex (A1) on auditory localization by implanting a sustained release polymer containing the GABAA agonist muscimol bilaterally over A1. Silencing A1 led to a reversible deficit in the localization of brief noise bursts in both the horizontal and vertical planes. In other ferrets, large bilateral lesions of the auditory cortex, which extended beyond A1, produced more severe and persistent localization deficits.
To investigate the processing of spatial information by high-frequency A1 neurons, we measured their binaural-level functions and used individualized virtual acoustic space stimuli to record their spatial receptive fields (SRFs) in anesthetized ferrets. We observed the existence of a continuum of response properties, with most neurons preferring contralateral sound locations. In many cases, the SRFs could be explained by a simple linear interaction between the acoustical properties of the head and external ears and the binaural frequency tuning of the neurons. Azimuth response profiles recorded in awake ferrets were very similar and further analysis suggested that the slopes of these functions and location-dependent variations in spike timing are the main information-bearing parameters.
Studies of sensory plasticity can also provide valuable insights into the role of different brain areas and the way in which information is represented within them. For example, stimulus-specific training allows accurate auditory localization by adult ferrets to be relearned after manipulating binaural cues by occluding one ear. Reversible inactivation of A1 resulted in slower and less complete adaptation than in normal controls, whereas selective lesions of the descending corticocollicular pathway prevented any improvement in performance. These results reveal a role for auditory cortex in training-induced plasticity of auditory localization, which could be mediated by descending cortical pathways.
Keywords: Auditory localization, Ferret, Reversible inactivation, Corticofugal pathway, Virtual acoustic space, Binaural, Plasticity, Training
1. Introduction
An ability to localize sounds accurately and rapidly is of fundamental importance for the way in which humans and other species perceive and interact with their environment. This process plays a key role in directing attention toward objects or events of interest and enables many species to find potential mates or prey, or to avoid and escape from approaching predators. Moreover, spatial hearing helps to discriminate sounds in noisy environments and therefore aids source identification.
Determining the direction of a sound-source relies on the detection and interpretation of monaural and binaural spatial cues generated by the interaction between sound waves and the head and external ears (King et al., 2001). Localization in the horizontal plane is based primarily on binaural disparity cues, with interaural time differences (ITDs) dominating at low frequencies and interaural level differences (ILDs) at higher frequencies. With narrow-band sounds, the same ITD or ILD can be generated by a range of sound directions, referred to as a ‘cone of confusion’. As the frequency content of the sound is increased, however, these spatial ambiguities can be resolved by the peaks and notches imposed on the sound spectrum by the external ears. In addition to allowing listeners to distinguish between front and back, the spectral cues generated by each external ear provide the basis for localization in the vertical plane and, under certain conditions, for localizing sounds using one ear alone (Wightman and Kistler, 1993; Van Wanrooij and Van Opstal, 2004).
The relative contribution of each of these spatial cues to our ability to determine the direction of a sound-source therefore varies with the frequency content and level of the sound, as well as the region of space from which it originates. Consequently, maintenance of accurate sound localization over a range of stimulus parameters requires that information provided by the different cues be combined appropriately within the brain. This is further complicated by the fact that the cue values corresponding to different directions in space depend on the physical dimensions of the head and ears, which undergo substantial changes during development and vary from one individual to another (Clifton et al., 1988; Middlebrooks and Green, 1990; Middlebrooks, 1999; Schnupp et al., 2003). Together, these findings suggest that the ability to localize accurately has to be learned by experience of the cues available to individual listeners.
The extraction of monaural and binaural localization cues begins in largely separate, parallel pathways within the brainstem (Yin, 2002; Young and Davis, 2002). These pathways converge in the inferior colliculus (IC) in the midbrain, which has been the subject of many physiological studies of spatial hearing (McAlpine, 2005) and where sensitivity to more than one localization cue is typically seen (e.g. Chase and Young, 2006). ILDs and spectral cues are also integrated in another midbrain nucleus, the superior colliculus, to form a neural map of auditory space, which, together with topographic representations of other sensory modalities, is used to direct gaze shifts toward novel stimuli (King et al., 2001; King, 2005).
Given the extensive processing of spatial information that takes place subcortically, it is not immediately clear what additional contributions are made at the level of the cortex. Nevertheless, it is widely accepted that an intact auditory cortex is required for accurate sound localization. Here we review recent behavioral and physiological studies in which we have examined the role of the primary auditory cortex (A1) both in normal spatial hearing and in the recalibration of localization responses in response to changes in auditory spatial cues values.
2. Inactivation of auditory cortex impairs sound localization
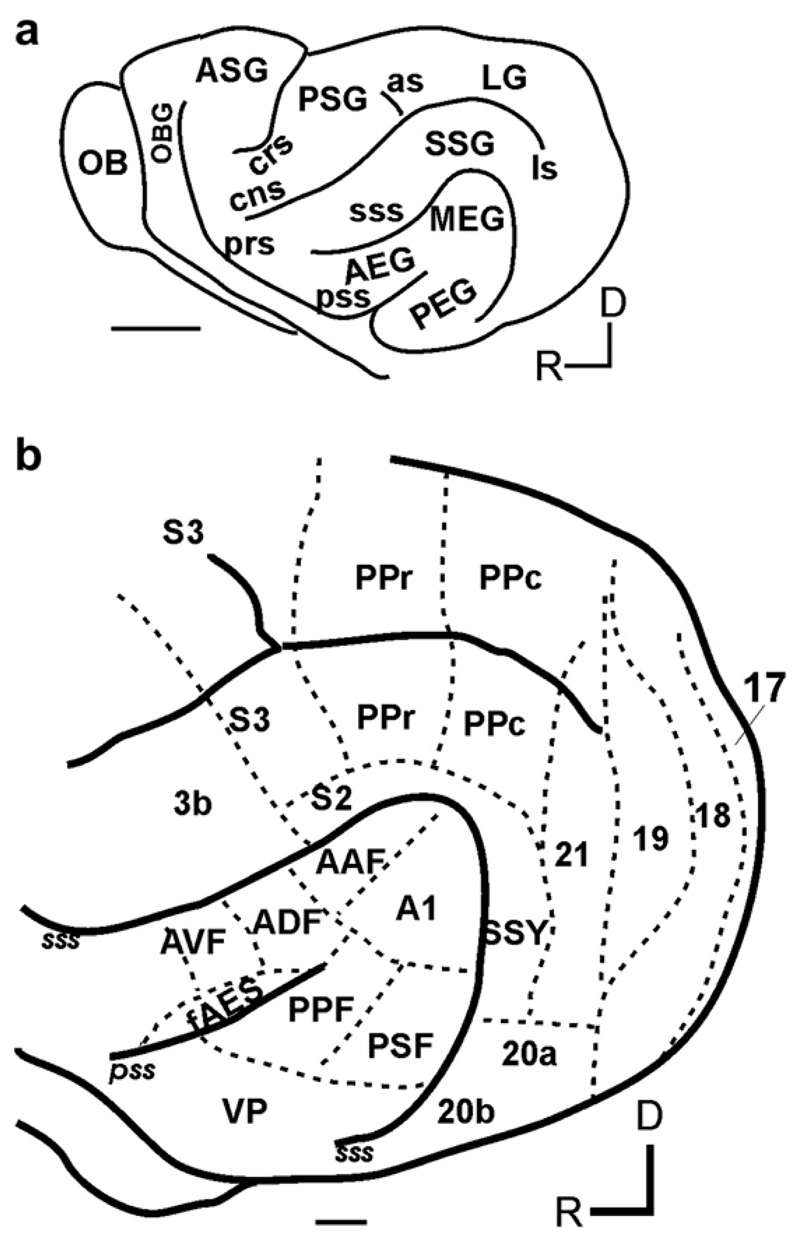
Although electrophysiological recordings from cortical neurons have been carried out in a number of species, studies of auditory localization have focused primarily on cats, ferrets and non-human primates (reviewed by King and Campbell, 2005). In each case, auditory cortex comprises one or more primary (or ‘core’ areas in primates) and several non-primary (belt and para-belt) areas. Fig. 1 illustrates our current view of the organization of cortical fields in the ferret, which, largely because of its particular suitability for behavioral and developmental studies, is being used increasingly for research on cortical mechanisms of auditory processing and plasticity.
Fig. 1.
(a) Lateral view of the ferret brain showing the major sulci and gyri. (b) Sensory cortical areas in the ferret. The locations of known auditory, visual, somatosensory and posterior parietal fields are indicated. Scale bar = 5 mm in a and 1mm in b, respectively. (Abbreviations: AEG, anterior ectosylvian gyrus; ASG, anterior sigmoid gyrus; as, ansinate sulcus; cns, coronal sulcus; crs, cruciate sulcus; LG, lateral gyrus; ls, lateral sulcus; MEG, middle ectosylvian gyrus; OB, olfactory bulb; OBG, orbital gyrus; PEG, posterior ectosylvian gyrus; prs, presylvian sulcus; PSG, posterior sigmoid gyrus; pss, pseudosylvian sulcus; SSG, suprasylvian gyrus, sss, suprasylvian sulcus; A1, primary auditory cortex; AAF, anterior auditory field; PPF, posterior pseudosylvian field; PSF, posterior suprasylvian field; ADF, anterior dorsal field; AVF, anterior ventral field; SSY, suprasylvian field; fAES, anterior ectosylvian sulcal field; PPc, caudal posterior parietal cortex; PPr, rostral posterior parietal cortex; 3b, primary somatosensory cortex; S2, secondary somatosensory cortex; S3, tertiary somatosensory cortex; D, dorsal; R, rostral.)
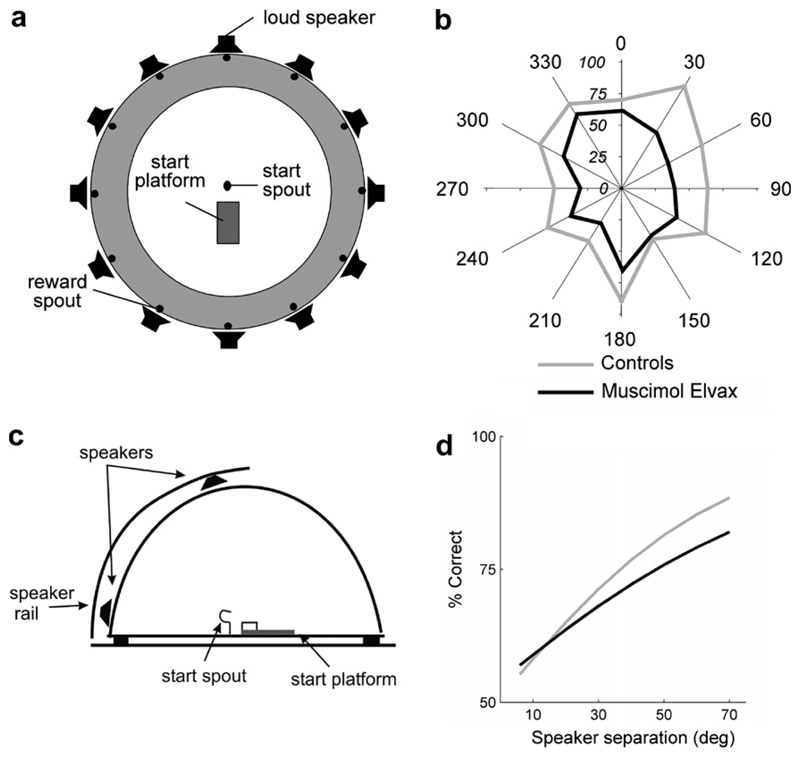
The principal evidence that auditory cortex is needed for normal spatial hearing comes from the finding that, in carnivores and primates, cortical lesions result in auditory localization deficits, as expressed by a greatly impaired ability to approach, discriminate or even orient toward sound-sources within the contralateral hemifield (e.g. Jenkins and Masterton, 1982; Jenkins and Merzenich, 1984; Kavanagh and Kelly, 1987; Heffner and Heffner, 1990; Beitel and Kaas, 1993). Although the magnitude and nature of the reported deficits varied with the size of the lesions, impaired performance has consistently been observed following damage to A1 alone. More recently, attention has focused on the behavioral consequences of producing a local and reversible inactivation of specific areas of auditory cortex. In keeping with the results from the lesion studies, both unilateral cryogenic inactivation (Malhotra et al., 2004) and pharmacological inactivation of A1 using muscimol-releasing Elvax implants (Smith et al., 2004) result in contralateral deficits, whereas bilateral inactivations lead to increased localization errors at all positions tested within the horizontal plane (Fig. 2a, b). In addition to these azimuthal deficits, we found, using muscimol-Elvax, that activity within A1 is required for discrimination by ferrets of sound-source locations within the vertical plane (Fig. 2c, d). While these studies highlight the importance of A1 in auditory localization, Malhotra et al. (2004) also observed localization deficits in the cat after cooling both the posterior auditory field (PAF) and the anterior ectosylvian sulcal (AES) cortex but not the anterior auditory field (AAF). This is consistent with a segregation of auditory function in higher-level cortical fields beyond A1 (Belin and Zatorre, 2000).
Fig. 2.
The auditory cortex is needed for normal sound localization: (a) Setup used for measuring localization in the horizontal plane. Ferrets were trained to stand on the start platform and initiate a trial by licking the start spout. Each trial consisted of a broadband noise burst of variable duration and level presented randomly from 1 of 12 speakers positioned at 30° intervals in the horizontal plane. The animals were rewarded for approaching and licking the reward spout associated with the speaker that had been triggered. (b) The polar plot shows the mean percentage scores achieved when localizing 40-ms noise bursts by a group of four control ferrets and four animals in which A1 had been inactivated bilaterally by placing sheets of a slow-release polymer containing the GABAA agonist muscimol on the cortex. These animals achieved lower scores than the normal controls at all stimulus angles. From Smith et al. (2004). (c) Setup used for measuring localization in the vertical plane. The animals had to discriminate between stimuli presented from one of two speakers positioned in the midsagittal plane. Because it was not possible for the animals to approach the sound-source directly, they were rewarded for responding at a reward spout to their right (90°) when the sound was presented from the upper speaker, and at a spout to their left (270°) when sound was presented from the lower speaker. (d) Psychometric functions fitted to the data from the same ferrets before (control) and after inactivating A1 bilaterally with muscimol-Elvax. In five (out of six) animals contributing to these data, A1 inactivation produced a significant drop in performance. From Bizley et al. (submitted).
3. Spatial processing in the primary auditory cortex
The spatial selectivity of cortical neurons has been examined by recording spike activity in response to sounds delivered either in the free field from loudspeakers positioned in real space around the animal’s head (e.g. Middlebrooks and Pettigrew, 1981; Imig et al., 1990; Rajan et al., 1990; Stecker et al., 2005) or over earphones in virtual acoustic space (VAS) (Brugge et al., 1994, 1996; Mrsic-Flogel et al., 2001, 2003, 2005). These studies have shown that, in keeping with the behavioral deficits produced by unilateral lesions or inactivation, most A1 neurons respond best to stimuli on the contralateral side of the animal and that their spatial receptive fields tend to expand, often quite considerably, with increasing sound level. Although there is some indication that neurons tuned to particular regions of space occur in clusters, there is no evidence for a topographic representation of space equivalent to that found in the superior colliculus.
A number of studies have attempted to define the acoustical basis for the spatial receptive fields of cortical neurons. The binaural response properties of A1 neurons have been probed in a variety of species using dichotic presentation of spectrally-simple sounds (e.g. Imig and Adrian, 1977; Irvine et al., 1996; Rutkowski et al., 2000; Zhang et al., 2004; Campbell et al., 2006). In most of these studies, neurons were assigned to different binaural classes on the basis of their responses to independent stimulation of each ear and then subclassified according to the binaural interactions observed when both ears were stimulated. It has been reported that neurons with similar binaural properties orthogonally span the cortical layers to form a columnar organization and are arranged across the cortical surface into clusters or patches (e.g. Imig and Adrian, 1977; Rutkowski et al., 2000). This is broadly consistent with the clustered distribution of spatial receptive fields observed in some free-field studies. However, using a multivariate statistical analysis, we found that although binaural response functions certainly vary in shape among different neurons (Fig. 3a–f), principal components analysis of those shapes provided no evidence for the existence of discrete classes. Instead, binaural response functions form a roughly Gaussian point cloud in principal component space (Fig. 3g, h; for details see Campbell et al., 2006). Thus, across the population of A1 neurons, sensitivity to ILDs appears to be distributed continuously from a minority showing ipsilateral dominance to the majority that respond most strongly to values corresponding to the contralateral side of space.
Fig. 3.
Examples of neuronal sensitivity to interaural level differences (ILDs) in A1 of anesthetized ferrets: (a, b) Neurons sensitive to ILDs favoring the contralateral ear. (c, d) Neurons sensitive predominantly to average binaural level (ABL), but less so to ILD. (e, f) Neurons with non-monotonic responses in both ILD and ABL. The gray scale indicates the mean evoked spike rate (Hz) per stimulus presentation. Negative rates are sound level combinations for which the evoked spike rate was below spontaneous levels. To aid visualization, response surfaces were smoothed with a low-pass filter (2D Gaussian, σ = 0.66) and interpolated. These examples are not members of discrete classes since principal components analysis of the population of binaural response function shapes reveals a continuous distribution of response properties (g, h). From Campbell et al. (2006).
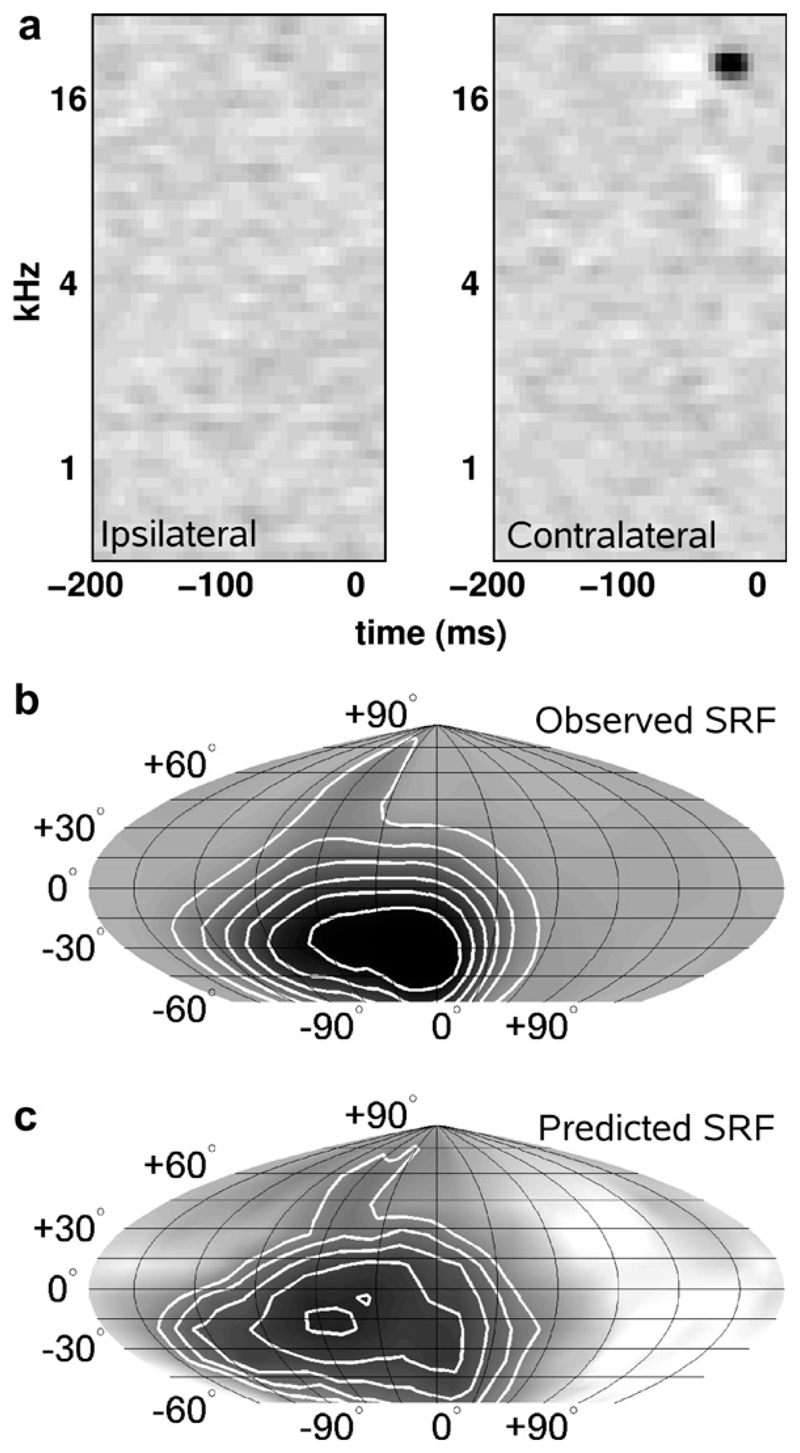
Binaural interactions alone are insufficient to account for the representation of sound-source location in A1. At near-threshold sound levels, high-frequency neurons in cat (Middlebrooks and Pettigrew, 1981; Rajan et al., 1990; Brugge et al., 1994) and ferret (Mrsic-Flogel et al., 2003, 2005) tend to be tuned in space to directions that correspond to the acoustical axis of the contralateral external ear. Moreover, using individualized VAS stimuli, we have shown that the location and shape of the spatial receptive fields can be well predicted by a simple filter model that integrates sound energy in the VAS stimuli according to the neurons’ frequency sensitivity to inputs from each ear (Schnupp et al., 2001; Mrsic-Flogel et al., 2005; Fig. 4). For many neurons, changes in spatial tuning with increasing sound level could be accounted for by this linear model for the cortical processing of spatial cues, although these changes were less well predicted for neurons that received excitatory inputs from both ears and which were likely sensitive to ITDs.
Fig. 4.
Predicting spatial responses from the frequency tuning of neurons in A1. Examples of frequency-time response fields (FTRFs) for each ear (a), together with the observed (b) and predicted (c) spatial receptive fields (SRFs) of a neuron recorded in A1 of an anesthetized ferret. The FTRFs were measured by reverse correlation to random chord stimuli presented to each ear. The observed SRFs were generated by presenting noise bursts from 224 virtual sound directions, covering 360° in azimuth and from –60° to +90° in elevation. The predicted SRFs were generated by convolving the FTRFs with the energy spectrum vectors of the VAS stimuli for each ear and each position in space (from Schnupp et al., 2001).
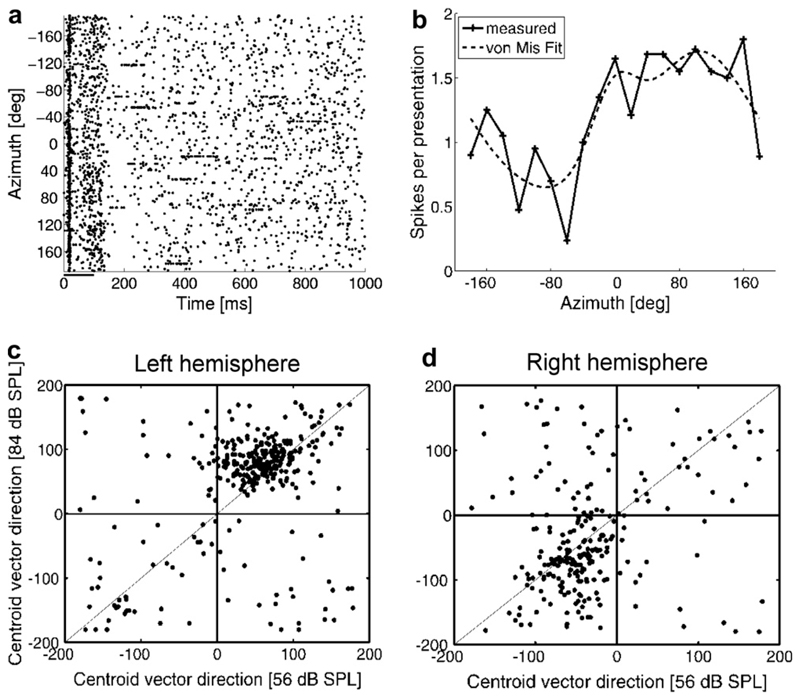
The great majority of electrophysiological recording studies of spatial coding in the auditory cortex have been carried out in anesthetized animals. An important issue for this as well as other aspects of auditory processing is the extent to which these responses differ in the unanesthetized cortex. Mickey and Middlebrooks (2003) found that the spatial receptive fields of neurons recorded in A1 of awake cats were typically more sharply tuned and less affected by variations in sound level than those recorded under anesthesia, although they were still very large, often spanning a hemifield in width. Our own recordings in awake, head-restrained ferrets have shown that the centroid direction vectors of most A1 neurons are found in the anterior, contralateral quadrant (Schulz et al., 2006; Fig. 5). As in the anesthetized animal, the preferred sound directions of these neurons tended to shift laterally (Fig. 5c, d) and their spatial tuning broadened with increasing sound level.
Fig. 5.
Spatial selectivity in A1 of awake ferrets: (a) Raster plot showing the response of one unit to free-field stimuli presented at the azimuthal angles given on the ordinate. Positive numbers indicate the right hemifield and negative numbers locations to the animal’s left. Each dot represents the time of occurrence of an action potential, as shown on the abscissa. Each row of dots indicates the spike pattern for a single presentation of a 100-ms noise burst, which began at 0 ms. (b) Azimuth response profile for this unit, which was smoothed by fitting with a von Mises (von Mis in key) spherical function. This unit was recorded in the left A1 and was broadly tuned to the contralateral hemifield. Centroid direction vectors indicate the overall directional preference of the response profiles and were calculated by modeling the response profile as a circle of unit radius, whose “mass density” in each direction was given by the observed response strength in the corresponding direction. These are shown in c and d for all units recorded from three diVerent animals and plotted according to the sound level used and the hemisphere in which they were recorded.
In contrast to the changes observed in A1 spatial receptive fields, the accuracy of auditory localization in humans remains relatively constant over a wide range of stimulus levels (Macpherson and Middlebrooks, 2000). However, although the spatial receptive fields expand with increasing level, the mutual information between stimulus location and the responses of adult ferret A1 neurons remains essentially unchanged (Mrsic-Flogel et al., 2003; Schulz et al., 2006). Moreover, it has been reported that both azimuth-rate functions (Stecker et al., 2005) and ILD-rate functions (Campbell et al., 2006) are steepest near the midline, which is consistent with this being the region of greater spatial acuity. Thus, some features of the spatial response properties of A1 neurons can account for auditory localization behavior.
In addition to the location-dependent variations in spike count that are used to define spatial receptive fields, it has been demonstrated in recordings made from A1 (Brugge et al., 1996; Mickey and Middlebrooks, 2003; Reale et al., 2003; Mrsic-Flogel et al., 2005) as well as other cortical fields (Furukawa and Middlebrooks, 2002; Stecker et al., 2003) that spatial information can be conveyed by the timing of spikes. Indeed, quantitative analysis of the spike discharge patterns recorded in A1 of anesthetized ferrets (Nelken et al., 2005) and the secondary auditory cortex of anesthetized cats (Furukawa and Middlebrooks, 2002) indicated that more information is carried by spike timing than by spike count. This highlights the importance of considering temporal measures of the response when attempting to assess how spatial and other signals are encoded within the auditory system. Although the limited evidence available so far suggests that the magnitude of the response may be a more important factor in the absence of anesthesia, onset latency still appears to carry much of the information about sound-source direction (Mickey and Middlebrooks, 2003).
4. Recalibrating sound localization and the auditory cortex
Efforts to understand the cortical mechanisms underlying perception and behavior need to take into account the capacity of the cortex to reorganize throughout life in response to experience. Indeed, cortical plasticity should be thought of as part of the normal operation of both sensory and motor systems. There is now extensive evidence that neuronal response properties in auditory cortex can be shaped by learning or by other changes in sensory inputs (reviewed by Ohl and Scheich, 2005). However, despite recent demonstrations that spatial discrimination can improve with practice (Wright and Fitzgerald, 2001) and that adults can adapt to altered localization cue values (Shinn-Cunningham et al., 1998; Van Wanrooij and Van Opstal, 2005; Kacelnik et al., 2006), we know very little about the physiological basis for this plasticity.
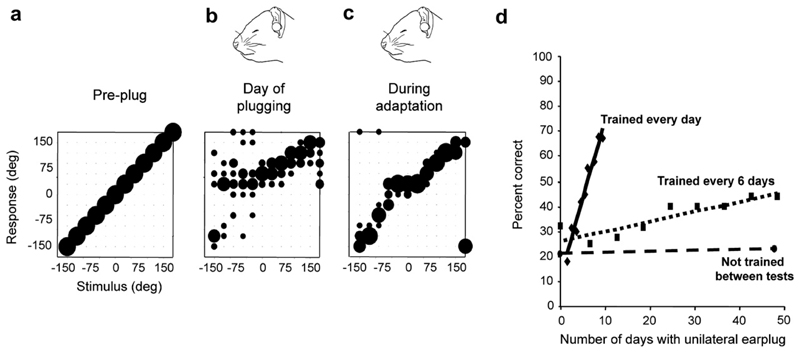
We examined the effects of altering binaural cues in adult ferrets by reversible occlusion of one ear (Kacelnik et al., 2006; Fig. 6). As expected, this procedure degraded the ability of the animals to localize sounds in the horizontal plane (Fig. 6a, b). However, their performance then improved, indicating that they were able to adapt to the presence of the earplug (Fig. 6c). This adaptation occurred only if the animals received auditory localization training, with the extent and rate of adaptation being determined by the frequency of training (Fig. 6d). By contrast, monaural occlusion for 42 days without training did not result in adaptation, despite daily exposure to normal animal-house sounds, as the animals localized just as poorly at the end of this period as when the earplug was Wrst introduced (Fig. 6d).
Fig. 6.
Plasticity of spatial hearing in adult ferrets: (a–c): Stimulus-response plots showing the distribution of responses (ordinate) made by a ferret as a function of stimulus location in the horizontal plane (abscissa). The size of the dots indicates, for a given speaker angle, the proportion of responses made to different locations. Correct responses are those that fall on the diagonal line, whereas all other responses represent errors of different magnitude. Prior to occlusion of the left ear, the animal achieved 100% correct scores at all stimulus directions (a), but performed poorly, particularly on the side of the earplug, when the left ear was occluded (b). Further testing with the earplug still in place, however, led to a recovery in localization accuracy (c). (d) Mean change in performance (averaged across all speaker locations) over time in three groups of ferrets with unilateral earplugs. No change was found in trained ferrets (n = 3) that received an earplug for 6 weeks, but were tested only at the start and end of this period (circles and dashed regression line). Two other groups of animals received an equivalent amount of training while the left ear was occluded. Although the earplug was in place for less time, a much faster rate of improvement was observed in the animals that received daily training (n = 3; diamonds and solid regression line) compared to those that were tested every 6 days (n = 6; squares and dotted regression line). From Kacelnik et al. (2006).
Although we have previously shown that adaptation to a unilateral earplug over the course of postnatal development is mirrored by adjustments to the auditory space map in the superior colliculus (King et al., 2000), we reasoned that the auditory cortex is likely to be involved in rapid, training-induced plasticity in adulthood. We conWrmed this by showing that bilateral inactivation of A1 significantly slowed the rate at which the animals relearned to localize sound (Fig. 7). It has yet to be determined how neuronal response properties, whether in A1 or elsewhere, change during this form of adaptive plasticity. However, the very small and transient after-effect observed following earplug removal suggests that adaptation involves learning to ignore the altered cues. Instead, there appears to be a reweighting toward other auditory cues, in particular the spectral information provided by the contralateral pinna and concha, that are less affected by the earplug (Kacelnik et al., 2006).
Fig. 7.
Plasticity of auditory localization depends on the auditory cortex. Change in performance (averaged across all speaker locations) over time in three groups of ferrets that received daily training with unilateral earplugs. Compared to the rapid and near complete recovery in localization accuracy observed in control animals (n = 3; black symbols and regression line), a significantly slower improvement was observed in animals in which A1 had been reversibly inactivated using muscimol-Elvax implants (n = 4; gray symbols and regression line). Moreover, no improvement in performance was observed in ferrets in which targeted apoptotic degeneration of corticocollicular neurons had been induced using a photoactivation technique (n = 3; open symbols and light gray regression line).
In addition to plasticity in the cortical representation of sound-source location, it is also necessary to consider the possibility that experience-driven changes in behavior could be mediated by descending corticofugal projections. Neurons in the deeper layers of the auditory cortex project back to the medial geniculate body, inferior colliculus and brainstem, and are therefore well placed to influence different aspects of auditory processing (Winer, 2006). In particular, it has been proposed that descending corticocollicular pathways might be involved in learning-induced plasticity (Suga and Ma, 2003). We have recently characterized the anatomical organization of the auditory corticocollicular projections in the ferret by placing tracer injections into different regions of the cortex and the inferior colliculus (Bajo et al., 2006a; Fig. 8). We then attempted to eliminate A1 layer V neurons that project to the IC using a targeted neuronal degeneration technique that has been shown to induce apoptotic cell death in specific populations of neurons (Madison and Macklis, 1993). Preliminary results indicate that ablation of corticocollicular neurons impairs adaptation (Fig. 7; Bajo et al., 2006b), suggesting that signals transmitted by descending cortical pathways are likely to mediate training-induced plasticity of auditory localization.
Fig. 8.
Organization of the descending auditory corticocollicular projection in the ferret: (a) Retrogradely-labelled neurons were found in layer V across the entire extent of the ectosylvian gyrus following fluororuby (dextran tetramethylrhodamine) injections in the IC. Neurons were located in the shaded regions of auditory cortex shown in the insets. Scale bar = 1mm. (b) Projections originating in the primary cortical fields on the MEG target the lateral nucleus of the IC on the same side and the dorsal cortex and dorsal part of the central nucleus on both sides. Neurons in the secondary auditory cortical fields on the PEG have the same targets as those located in primary areas, but exclude the central nucleus. Neurons located in the AEG primarily innervate the tegmental midbrain. (c) Examples of anterograde labelling observed in different regions of the ipsilateral IC after an injection of fluororuby in the MEG. The terminal fields have a distinctive orientation in every IC subdivision. Scale bars = 100 μm. (Abbreviations: A1, primary auditory field; AEG, anterior ectosylvian gyrus; AAF, anterior auditory field; ADF, anterior dorsal field; AEG, anterior ectosylvian gyrus; AES, anterior ectosylvian area; AVF, anterior ventral field; CN, central nucleus of the IC; contra, contralateral to the injection site; Cn, cuneiform nucleus; D, dorsal; DC, dorsal cortex of the IC; ipsi, ipsilateral to the injection site; LL, lateral lemniscus; LN, lateral nucleus of the IC; M, medial; MEG, middle ectosylvian gyrus; PAG, periaqueductal gray; PEG,, posterior ectosylvian gyrus; Pn, pontine nuclei; PPF, posterior pseudosylvian field; PSF, posterior suprasylvian feld; VP, ventro-posterior area.) From Bajo et al. (2006a).
5. Concluding remarks
Although it has been known for many years that the auditory cortex is required for normal sound localization, our understanding of how sound-source direction is represented there is still incomplete. Several studies have shown that spatial information can be carried by the timing as well as the number of spikes produced by cortical neurons. However, evidence for a direct link between these putative neural codes and auditory spatial perception is lacking and will require recordings to be made from cortical neurons whilst animals perform localization tasks. While A1 is clearly necessary for normal sound localization, both recording and reversible inactivation studies suggest that further processing of spatial information takes place in higher cortical areas, such as PAF in the cat (Stecker et al., 2003) or the caudomedial field in monkeys (Recanzone et al., 2000). Consequently, the localization deficits induced by lesions or inactivation of A1 could at least in part be due to an interruption in the flow of signals to those more specialized cortical areas.
In view of the extensive processing of spatial information that takes place at subcortical levels, an important issue that remains to be investigated is what extra the cortex does. Given the growing evidence that neurons in auditory cortex are sensitive to the context in which sounds are presented, it is possible that individual sound-sources are effectively localized using brainstem circuitry, whereas the cortex is more involved in the localization of sounds in the presence of other, competing sources. The task of representing behaviorally-relevant features of auditory objects is also likely to be facilitated by the plasticity of cortical responses that has been observed over multiple time scales (Nelken, 2004). In the context of auditory localization, our finding that lesions of A1 and, more specifically, of corticocollicular neurons in layer V of the cortex interfere with the capacity of the brain to accommodate altered spatial cues highlights the importance of the cortex and its descending projections in the dynamic coding of sensory information.
Acknowledgements
The authors’ research work was funded by the Wellcome Trust.
References
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2006a;2006 doi: 10.1093/cercor/bhj164. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. Role of auditory cortex and descending corticocollicular projections in adaptation to altered binaural cues by adult ferrets. Assoc Res Otolaryngol Abstr. 2006b;29:56. [Google Scholar]
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–369. doi: 10.1152/jn.1993.70.1.351. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ. ‘What’, ‘where’ and ‘how’ in auditory cortex. Nature Neurosci. 2000;3:965–966. doi: 10.1038/79890. [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Parsons CH, King AJ. The role of auditory cortex in sound localization in the mid-sagittal plane. doi: 10.1152/jn.00444.2007. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Hind JE, Chan JCK, Musicant AD, Poon PW. Simulation of free-field sound sources and its application to studies of cortical mechanisms of sound localization in the cat. Hear Res. 1994;73:67–84. doi: 10.1016/0378-5955(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Hind JE. The structure of spatial receptive fields of neurons in primary auditory cortex of the cat. J Neurosci. 1996;16:4420–4437. doi: 10.1523/JNEUROSCI.16-14-04420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RAA, Schnupp JWH, Shial A, King AJ. Binaural-level functions in ferret auditory cortex: evidence for a continuous distribution of response properties. J Neurophysiol. 2006;95:3742–3755. doi: 10.1152/jn.01155.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase S, Young ED. Spike-timing codes enhance the representation of multiple simultaneous sound-localization cues in the inferior colliculus. J Neurosci. 2006;26:3889–3898. doi: 10.1523/JNEUROSCI.4986-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton RK, Gwiazda J, Bauer JA, Clarkson MG. Growth in head size during infancy: implications for sound localization. Dev Psychol. 1988;24:477–483. [Google Scholar]
- Furukawa S, Middlebrooks JC. Cortical representation of auditory space: information-bearing features of spike patterns. J Neurophysiol. 2002;87:1749–1762. doi: 10.1152/jn.00491.2001. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Adrian HO. Binaural columns in the primary field (A1) of cat auditory cortex. Brain Res. 1977;138:241–257. doi: 10.1016/0006-8993(77)90743-0. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Irons WA, Samson FR. Single-unit selectivity to azimuthal direction and sound pressure level of noise bursts in cat high-frequency primary auditory cortex. J Neurophysiol. 1990;63:1448–1466. doi: 10.1152/jn.1990.63.6.1448. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Rajan R, Aitkin LM. Sensitivity to interaural intensity differences of neurons in primary auditory cortex of the cat. I. Types of sensitivity and effects of variations in sound pressure level. J Neurophysiol. 1996;75:75–96. doi: 10.1152/jn.1996.75.1.75. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Masterton RB. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:e71. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contributions of auditory cortex to sound localization in the ferret (Mustela putorius) J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol. 2005;14:R335–R338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- King AJ, Campbell RAA. Cortical processing of sound-source location. Acta Acustica/Acustica. 2005;91:399–408. [Google Scholar]
- King AJ, Parsons CH, Moore DR. Plasticity in the neural coding of auditory space in the mammalian brain. Proc Natl Acad Sci USA. 2000;97:11821–11828. doi: 10.1073/pnas.97.22.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Schnupp JWH, Doubell TP. The shape of ears to come: dynamic coding of auditory space. Trends Cog Sci. 2001;5:261–270. doi: 10.1016/s1364-6613(00)01660-0. [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Localization of brief sounds: effects of level and background noise. J Acoust Soc Am. 2000;108:1834–1849. doi: 10.1121/1.1310196. [DOI] [PubMed] [Google Scholar]
- Madison RD, Macklis JD. Noninvasively induced degeneration of neocortical pyramidal neurons in vivo: selective targeting by laser activation of retrogradely transported photolytic chromophore. Exp Neurol. 1993;121:153–159. doi: 10.1006/exnr.1993.1082. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- McAlpine D. Creating a sense of auditory space. J Physiol. 2005;566:21–28. doi: 10.1113/jphysiol.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey BJ, Middlebrooks JC. Representation of auditory space by cortical neurons in awake cats. J Neurosci. 2003;23:8649–8663. doi: 10.1523/JNEUROSCI.23-25-08649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC. Individual differences in external-ear transfer functions reduced by scaling in frequency. J Acoust Soc Am. 1999;106:1480–1492. doi: 10.1121/1.427176. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Directional dependence of interaural envelope delays. J Acoust Soc Am. 1990;87:2149–2162. doi: 10.1121/1.399183. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Pettigrew JD. Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location. J Neurosci. 1981;1:107–120. doi: 10.1523/JNEUROSCI.01-01-00107.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Jenison RL, Schnupp JWH. Listening through different ears alters spatial response fields in ferret primary auditory cortex. J Neurophysiol. 2001;86:1043–1046. doi: 10.1152/jn.2001.86.2.1043. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Schnupp JWH, King AJ. Acoustic factors govern developmental sharpening of spatial tuning in the auditory cortex. Nature Neurosci. 2003;6:981–988. doi: 10.1038/nn1108. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Schnupp JWH. Encoding of virtual acoustic space stimuli by neurons in ferret primary auditory cortex. J Neurophysiol. 2005;93:3489–3503. doi: 10.1152/jn.00748.2004. [DOI] [PubMed] [Google Scholar]
- Nelken I. Processing of complex stimuli and natural scenes in the auditory cortex. Curr Opin Neurobiol. 2004;14:474–480. doi: 10.1016/j.conb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nelken I, Chechik G, Mrsic-Flogel TD, King AJ, Schnupp JWH. Encoding stimulus information by spike numbers and mean response time in primary auditory cortex. J Comput Neurosci. 2005;19:199–221. doi: 10.1007/s10827-005-1739-3. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Leaning-induced plasticity in animal and human auditory cortex. Curr Opin Neurobiol. 2005;15:470–477. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rajan R, Aitkin LM, Irvine DRF, McKay J. Azimuthal sensitivity of neurons in primary auditory cortex of cats. I. Types of sensitivity and the effects of variations in stimulus parameters. J Neurophysiol. 1990;64:872–887. doi: 10.1152/jn.1990.64.3.872. [DOI] [PubMed] [Google Scholar]
- Reale RA, Jenison RL, Brugge JF. Directional sensitivity of neurons in the primary auditory (AI) cortex: effects of sound-source intensity level. J Neurophysiol. 2003;89:1024–1038. doi: 10.1152/jn.00563.2002. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML, Su TK. Correlation between the activity of single auditory cortical neurons and sound-localization behavior in the macaque monkey. J Neurophysiol. 2000;83:2723–2739. doi: 10.1152/jn.2000.83.5.2723. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Wallace MN, Shackleton TM, Palmer AR. Organisation of binaural interactions in the primary and dorsocaudal fields of the guinea pig auditory cortex. Hear Res. 2000;145:177–189. doi: 10.1016/s0378-5955(00)00087-3. [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, Mrsic-Flogel TD, King AJ. Linear processing of spatial cues in primary auditory cortex. Nature. 2001;414:200–204. doi: 10.1038/35102568. [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, Booth J, King AJ. Modeling individual differences in ferret external ear transfer functions. J Acoust Soc Am. 2003;113:2021–2030. doi: 10.1121/1.1547460. [DOI] [PubMed] [Google Scholar]
- Schulz AL, Nodal FR, King AJ. Population code of sound source azimuth position in awake ferrets. FENS Abstr. 2006;3 A038.21. [Google Scholar]
- Shinn-Cunningham BG, Durlach NI, Held RM. Adapting to supernormal auditory localization cues. J Acoust Soc Am. 1998;103:3656–3666. doi: 10.1121/1.423088. [DOI] [PubMed] [Google Scholar]
- Smith AL, Parsons CH, Lanyon RG, Bizley JK, Akerman CJ, Baker GE, Dempster AC, Thompson ID, King AJ. An investigation of the role of auditory cortex in sound localization using muscimol-releasing Elvax. Eur J Neurosci. 2004;19:3059–3072. doi: 10.1111/j.0953-816X.2004.03379.x. [DOI] [PubMed] [Google Scholar]
- Stecker GC, Mickey BJ, Macpherson EA, Middlebrooks JC. Spatial sensitivity in field PAF of cat auditory cortex. J Neurophysiol. 2003;89:2889–2903. doi: 10.1152/jn.00980.2002. [DOI] [PubMed] [Google Scholar]
- Stecker GC, Harrington IA, Middlebrooks JC. Location coding by opponent neural populations in the auditory cortex. PLoS Biol. 2005;3:e78. doi: 10.1371/journal.pbio.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24:4163–4171. doi: 10.1523/JNEUROSCI.0048-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Releaning sound localization with a new ear. J Neurosci. 2005;25:5413–5424. doi: 10.1523/JNEUROSCI.0850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. Sound localization. In: Yost WA, Popper AN, Fay RR, editors. Human Psychophysics. Springer-Verlag; New York: 1993. pp. 155–192. [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2006;212:1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Wright BA, Fitzgerald MB. Different patterns of human discrimination learning for two interaural cues to sound-source location. Proc Natl Acad Sci USA. 2001;98:12307–12312. doi: 10.1073/pnas.211220498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TCT. Neural mechanisms of encoding binaural localization cues in the auditory brainstem. In: Fay RR, Popper AN, editors. Integrative Functions in the Mammalian Auditory Pathway. Springer-Verlag; New York: 2002. pp. 99–159. [Google Scholar]
- Young ED, Davis KA. Circuitry and function of the dorsal cochlear nucleus. In: Fay RR, Popper AN, editors. Integrative Functions in the Mammalian Auditory pathway. Springer-Verlag; New York: 2002. pp. 160–206. [Google Scholar]
- Zhang J, Nakamoto KT, Kitzes LM. Binaural interaction revisited in the cat primary auditory cortex. J Neurophysiol. 2004;91:101–117. doi: 10.1152/jn.00166.2003. [DOI] [PubMed] [Google Scholar]