Abstract
Our sensory systems fill in information obscured by other, competing signals to maintain a stable representation of the world. A correlate of the continuity illusion, in which sounds are perceived to continue despite being interrupted by other sounds, has now been found in the auditory cortex.
Imagine that you are sitting in the audience of a play and someone coughs at a crucial moment. Irritating though this can be, the auditory system actually possesses a remarkable capacity to fill in the sound that is masked by the cough or by other background sounds through a process known as auditory induction. This is obviously a very useful property, as it potentially allows speech or other sounds of interest to be followed in the presence of extraneous sounds produced by other objects.
The impact of auditory induction on speech intelligibility was demonstrated by showing that replacing a phoneme from a recorded sentence by a loud cough or tone leads to the illusion that the missing speech sound is still present [1] (Figure 1). Moreover, if bursts of noise are inserted at different places in a recording, the missing segments are restored by the brain and the speech is heard as though it is continuous [2]. This is part of a more general property of our sensory systems to fill in missing information, which has been shown to occur for a variety of sounds [3–5] and also in other stimulus modalities [6]. Although the neurophysiological basis for the auditory continuity illusion is not well understood, recent evidence suggests that it might have its origin in the response properties of neurons in the auditory cortex [7].
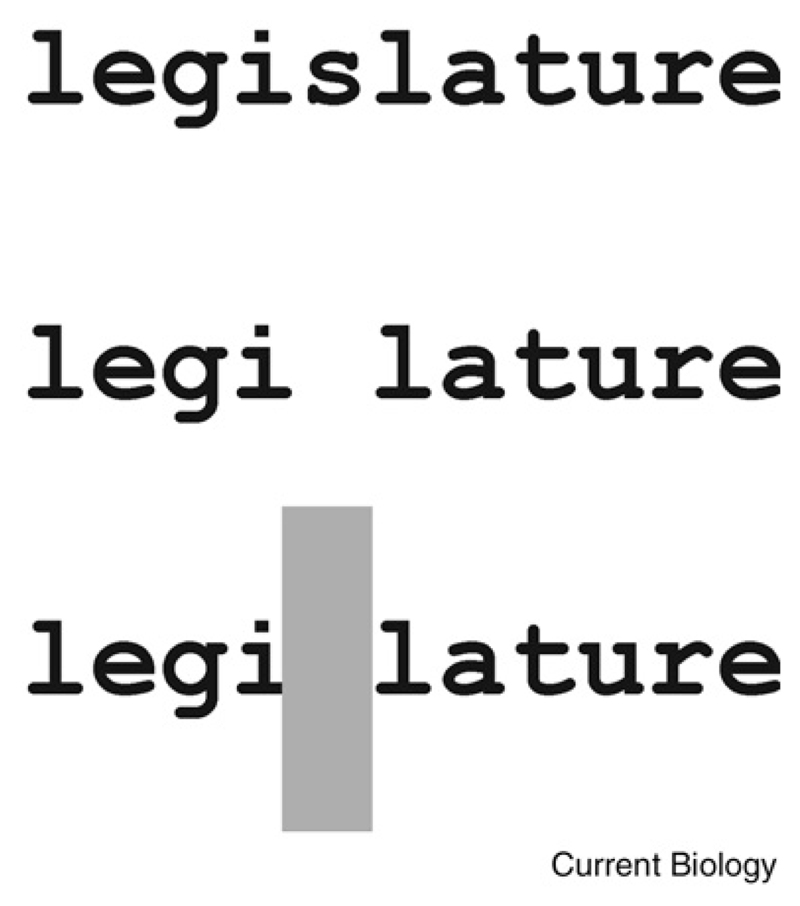
Figure 1.
Auditory induction — also known as phonemic restoration when speech is used — is an illusion in which a sound is perceived to be continuous when a gap in the sound is replaced by noise.
If the ‘s’ in ‘legislature’ is omitted, this is clearly heard as an interruption in the word. But if noise of the same duration is played in its place, the ‘s’ is heard as clearly as any of the phonemes that are present and the word becomes intelligible again.
Induction and Auditory Scene Analysis
One of the most challenging tasks performed by the auditory system is to group together the sound frequency components associated with a particular object, such as a speaker’s voice, and separate them from those belonging to other objects that are present at the same time. It is also necessary for sounds to be grouped sequentially, so that the same auditory object can be recognized when it occurs at different times. These principles are fundamental to our ability to make sense of our complex acoustic environment.
Psychophysical studies in humans suggest that the same grouping rules apply to auditory induction. In order for the illusory percept of a continuous sound to be produced, the sounds occurring before and after the interrupting noise have to be grouped into a single auditory stream [8]. Similarly, judgments about whether a continuous sound is present or not can be influenced by whether the component frequencies of the interrupted sounds are grouped simultaneously into a single object [5]. Thus, identifying the neural substrate for auditory induction should provide valuable insights into the more general process of auditory scene analysis.
Neural Correlates of Auditory Induction
By recording event-related potentials in humans, Micheyl and colleagues [9] found that the auditory continuity illusion can be measured using the mismatch negativity signal, which is thought to be generated primarily within the auditory cortex when a sudden deviant sound is presented among a sequence of standard stimuli. Neural network modelling also points to the involvement of early cortical areas in auditory induction [10]. Interestingly, both studies suggest that the illusion can occur without having to direct attention toward the sounds that give rise to it, implying that top-down influences are not required.
Isolating the likely origin of the continuity illusion to the auditory cortex — or possibly even to subcortical processing levels — potentially paves the way for exploring the underlying mechanisms by recording from individual neurons in animals. But the feasibility of doing this obviously depends on demonstrating that perceptual filling-in occurs in species other than humans. This is difficult to do, but there is evidence that monkeys experience this illusory percept [11,12] and electophysiological recordings in anaesthetized cats [13] and awake monkeys [7] suggest that some neurons in the primary auditory cortex (A1) respond in a manner that is consistent with induction.
In the more recent of these recording studies, Petkov et al. [7] compared the responses of monkey A1 to tone pips interrupted by a loud noise to those evoked by the individual stimulus components or to the stimuli that should have been perceived had induction occurred. Some neurons responded to continuous tones in a sustained fashion (Figure 2A), whereas others gave phasic responses that signalled when the stimulus was turned on or off. The sustained neurons were deemed to fill in the missing tone if introducing a silent gap in the middle of the stimulus reduced the subsequent activity of the neuron (Figure 2B), which was reversed by filling the gap with noise (Figure 2C), even though no response was evoked by the noise alone. Onset neurons appeared to represent the missing tone by no longer registering the end of the gap when it was filled with noise, whereas offset neurons failed to detect the start of the gap, although whether these responses actually reflect induction depends critically on how the neurons respond to the interrupting noise by itself.
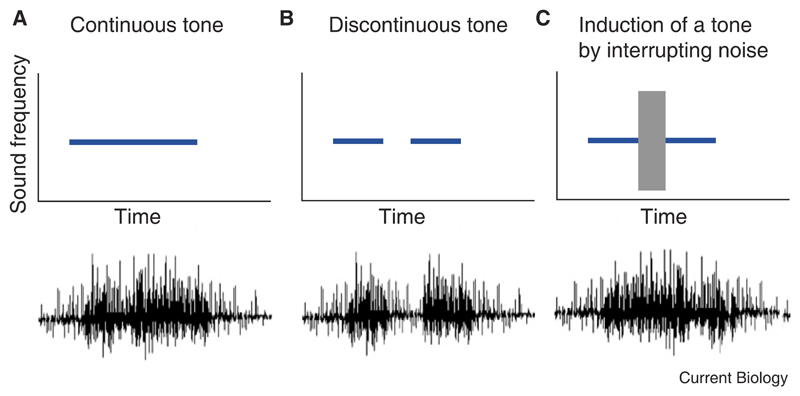
Figure 2.
A neurophysiological correlate of auditory induction has been described by Petkov et al. [7] in monkey primary auditory cortex.
Schematic illustrating one of the response types observed in this study. This neuron gives a sustained response to a continuous tone (A) and shows a reduction in activity when a gap is introduced in the middle of the tone (B). However, if the gap in the discontinuous tone is replaced by loud noise—which, so long as it contains energy at the induced frequency, would produce the percept of a continuous tone — the neuron again responds in a sustained fashion (C).
Not all of the A1 neurons recorded by Petkov et al. [7] behaved in this fashion, but they argue that, at a population level, the activity of these neurons is compatible with the continuity illusion. They also simulated auditory nerve fibre responses, which — unlike many of the neurons recorded in A1 — were found to be dominated by the interrupting noise and did not show a correlate of induction. This is important because psychoacoustic measurements in humans are often interpreted in terms of the sensitivity of peripheral auditory neurons. These results therefore suggest that the neural processing that enables perceptual filling-in to occur emerges within the central auditory pathway and — in keeping with the earlier electrophysiological and modelling studies — is manifest at the level of the auditory cortex.
The data described by Petkov et al. [7] do not show that A1 is the neural substrate of the auditory continuity illusion, merely that the response properties of some of its neurons are compatible with this important phenomenon. Establishing a more direct link between auditory induction and the activity of neurons in A1 or other brain regions would necessitate the more challenging experiment of recording in awake, behaving animals whilst they experience illusory continuity. Nevertheless, neurophysiological investigations of this and other auditory perceptual illusions are likely to play an increasingly important role in our attempts to explore the brain processes that enable our complex acoustic world to be interpreted.
References
- 1.Warren RM. Perceptual restoration of missing speech sounds. Science. 1970;167:392–393. doi: 10.1126/science.167.3917.392. [DOI] [PubMed] [Google Scholar]
- 2.Bashford JA, Riener KR, Warren RM. Increasing the intelligibility of speech through multiple phonemic restorations. Percept. Psychophys. 1992;51:211–217. doi: 10.3758/bf03212247. [DOI] [PubMed] [Google Scholar]
- 3.Thurlow WR. An auditory figure-ground effect. Am J Psychol. 1957;70:653–654. [PubMed] [Google Scholar]
- 4.Lyzenga J, Carlyon RP, Moore BCJ. Dynamic aspects of the continuity illusiuon: perception of level and of depth, rate and phase of modulation. Hear Res. 2005;210:30–41. doi: 10.1016/j.heares.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Darwin CJ. Simultaneous grouping and auditory continuity. Percept Psychophys. 2005;67:1384–1390. doi: 10.3758/bf03193643. [DOI] [PubMed] [Google Scholar]
- 6.Paradiso MA, Blau S, Huang X, MacEvoy SP, Rossi AF, Shalev G. Lightness, filing-in, nd the fundamental role of context in visual perception. Prog Brain Res. 2006;155:109–123. doi: 10.1016/S0079-6123(06)55007-1. [DOI] [PubMed] [Google Scholar]
- 7.Petkov CI, O’Connor KN, Sutter ML. Encoding of illusory continuity in primary auditory cortex. Neuron. 2007;54:153–165. doi: 10.1016/j.neuron.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bregman AS, Colantonio C, Ahad PA. Is a common grouping mechanism involved in the phenomena of illusory continuity and stream segregation? Percept. Psychophys. 1999;61:195–205. doi: 10.3758/bf03206882. [DOI] [PubMed] [Google Scholar]
- 9.Micheyl C, Carlyon RP, Shtyrov Y, Hauk O, Dodson T, Pullvermuüller F. The neurophysiological basis of the auditory continuity illusion: a mismatch negativity study. J Cog Neurosci. 2003;15:747–758. doi: 10.1162/089892903322307456. [DOI] [PubMed] [Google Scholar]
- 10.Husain FT, Lozito TP, Ulloa A, Horwitz B. Investigating the neural basis of the auditory continuity illusion. J Cog Neurosci. 2005;17:1275–1292. doi: 10.1162/0898929055002472. [DOI] [PubMed] [Google Scholar]
- 11.Miller CT, Dibble E, Hauser MD. Amodal completion of acoustic signals by a nonhuman primate. Nature Neurosci. 2001;4:783–784. doi: 10.1038/90481. [DOI] [PubMed] [Google Scholar]
- 12.Petkov CI, O’Connor KN, Sutter ML. Illusory sound perception in macaque monkeys. J Neurosci. 2003;23:9155–9161. doi: 10.1523/JNEUROSCI.23-27-09155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugita Y. Neuronal correlates of auditory induction in the cat cortex. Neuroreport. 1987;8:1155–1159. doi: 10.1097/00001756-199703240-00019. [DOI] [PubMed] [Google Scholar]