Abstract
Sensory brain areas are usually characterized by their responses to external stimuli; however, neuroimaging studies have now shown that activation of auditory cortex occurs spontaneously and can be induced during silence by stimulus expectancy or mental imagery.
The ultimate goal of sensory neuroscience is to understand how the responses of neurons to different stimuli give rise to our perception of the world and enable us to control our actions within it. The usual approach to this is to measure stimulus-driven activity either directly using electrophysiological approaches or more indirectly by neuroimaging. Using these methods, we can identify those brain regions that are activated by, for example, different types of sound and attempt to relate this to the experience of hearing.
But presenting sound to the listener is not the only way of eliciting responses in the auditory areas of the cerebral cortex. For one thing, there is growing evidence that activity in the auditory cortex of humans and other species can be heavily influenced by inputs from other sensory modalities [1]. These findings are rapidly changing our views about the stimulus specificity of cortical areas that have long been viewed as belonging exclusively to one or other of the senses. Even more remarkably, several recent studies [2–4] have shown that the auditory cortex can be reliably activated in the absence of any external stimulus.
Listening for the Sound
It is well established that attention can modulate neuronal responses to sensory stimuli. Studies in the visual system, in particular, have shown that information processing in the cortex can be altered dramatically when attention is shifted to different aspects of a stimulus, or from one region of space or another [5]. Attention therefore has a profound influence on what aspects of the sensory world we actually perceive and respond to.
In a recent neuroimaging study of auditory attention, Voisin and colleagues [2] asked subjects to indicate, by pressing a button, when they heard a sound that was rapidly increased in intensity following a silent period of variable duration. They observed increased activity in the auditory cortex on the side opposite to where the sound was expected to occur while the subjects listened in silence, as well as enhanced responses to the stimulus itself. Frontal cortical areas believed to play a higher-level role in attentional control were also activated on the right side of the brain, irrespective of the side from which the sound was anticipated.
Activity in these cortical areas was correlated with the duration of the period in which the subjects were attentively listening in silence, arguing against a more general arousal effect associated with performing a behavioural task. Expectation of a stimulus has previously been found to modulate activity in visual cortical areas [6,7]. It therefore seems likely that priming by top-down attentional mechanisms before the occurrence of a stimulus is a common property of sensory cortex.
Imagining the Sound
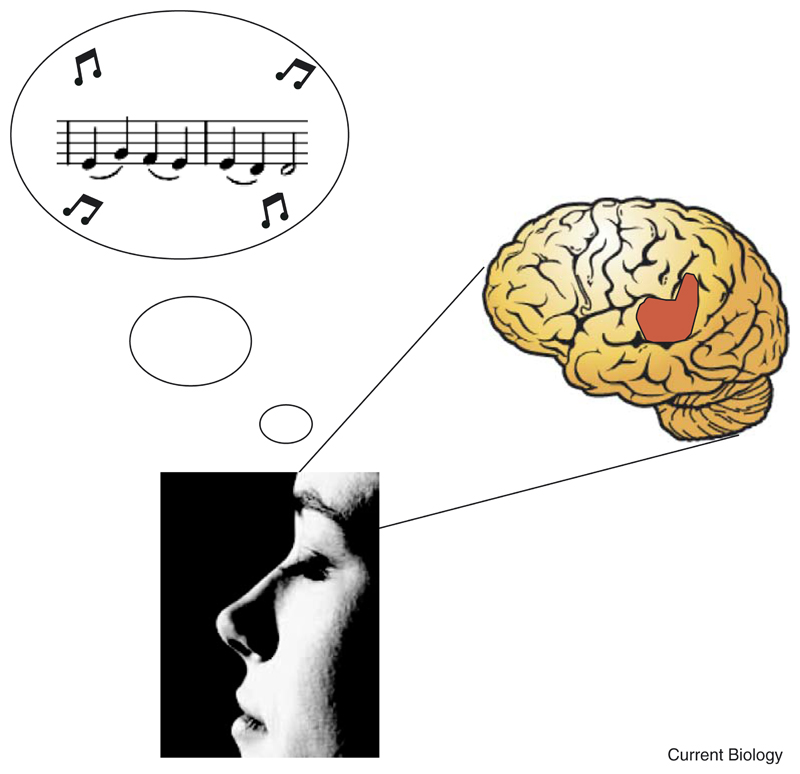
The attended sounds used in the study by Voisin and colleagues [2] were noise bursts which varied in their frequency content and which had no meaning to the subjects. It is therefore unlikely that the increase in activity in the cortex prior to the auditory stimulus was a result of mental imagery. Imagining a meaningful object or event is, however, another very effective way of activating the cortex in the absence of a real stimulus (Figure 1) and has been used for a number of years as a tool for studying the formation of short-term representations in the brain [8].
Figure 1.
Neuroimaging can be used to measure brain activation during mental imagery tasks, such as playing a tune in one’s head.
Not surprisingly, the cortical regions engaged by visual and auditory imagery tasks overlap closely with those that are active during perception of external stimuli, though certain differences have been reported to exist [9–12]. For instance, although higher-level sensory areas are reliably activated in both conditions, some studies have failed to find activity in the primary cortical areas during mental imagery. While this may reflect methodological difficulties in identifying the precise location of the signals in neuroimaging experiments or the complexity of the imagery task, it nonetheless raises the possibility that the primary cortical areas can — under certain conditions — be dissociated from conscious perception.
Rather than instructing them to imagine a particular sound, Kraemer and colleagues [3] imaged the brain while their subjects listened passively to excerpts of popular music in which several segments, lasting a variable amount of time, had been replaced by equivalent periods of silence. When the subjects were familiar with the soundtrack, they reported the experience of the music continuing during the silent gaps, whereas this was not the case when the songs were unknown to them. Activation of the secondary (or association) auditory cortex was correspondingly greater during the silent gaps present in familiar songs than during those embedded in unknown songs.
Although the imagery is harder to control in this type of study, it nonetheless provides evidence that the auditory cortex is recruited during a spontaneous imagining experience of the sort that occurs in everyday life. Interestingly, Kraemer and colleagues [3] also found that activation spread to the left primary auditory cortex during silent gaps in a familiar instrumental piece of music, whereas this did not happen if the music contained lyrics. This again suggests that the nature of the imagined event may determine the distribution of activity within the brain.
Spontaneous Activation
It is evident from these studies that sensory areas of the cortex can be activated in the absence of an external stimulus, either as a result of attention or by ‘hearing’ or ‘seeing’ an event inside the head. Another recent study [4] has shown that increases in activity in both primary and secondary auditory cortices can occur spontaneously and intermittently during silence. Although it is unclear whether any auditory experience was associated with these spontaneous activations, which originate in speech-sensitive regions in the left hemisphere, it is possible that abnormalities in these endogenous signals could provide a basis for auditory hallucinations.
The functional magnetic resonance imaging (fMRI) study by Hunter and colleagues [4] found that spontaneous activation of auditory cortex was accompanied by activity in the anterior cingulate cortex. Activation of structures in the frontal lobes has also been reported when subjects imagine a sound [13–15] and, as mentioned before, when listening in silence [2]. Thus, it would appear that frontal cortical areas can increase activity in specific regions of sensory cortex in different circumstances, such as in preparation for an expected sensory stimulus or during imagery.
Investigation of the way in which baseline activity in auditory or other sensory areas of the cortex is modulated by attention or when generating mental images provides a valuable way of probing their functional connectivity and contributions to conscious experience without having to use an external stimulus. The cortical regions activated under these conditions appear to be those responsible for processing real stimuli. Consequently, research in this field is likely to advance our understanding of where specific stimulus information is encoded and stored in the brain as well as how top-down processes initiate activity within low-level sensory areas.
References
- 1.Schroeder C, Foxe J. Multisensory contributions to low-level, ‘unisensory’ processing. Curr Opin Neurobiol. 2005;15:454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P. Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci. 2006;26:273–278. doi: 10.1523/JNEUROSCI.2967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer DJ, Macrae CN, Green AE, Kelley WM. Musical imagery: sound of silence activates auditory cortex. Nature. 2005;434:158. doi: 10.1038/434158a. [DOI] [PubMed] [Google Scholar]
- 4.Hunter MD, Eickhoff SB, Miller TW, Farrow TF, Wilkinson ID, Woodruff PW. Neural activity in speech-sensitive auditory cortex during silence. Proc Natl Acad Sci; USA. 2006. pp. 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treue S. Visual attention: the where, what, how and why of saliency. Curr Opin Neurobiol. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 6.Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- 7.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 8.Ganis G, Thompson WL, Mast F, Kosslyn SM. The brain’s mind’s images: the cognitive neuroscience of mental imagery. In: Gazzaniga MS, editor. In The Cognitive Neurosciences III. Cambridge, MA: MIT Press; 2004. pp. 931–941. [Google Scholar]
- 9.Kosslyn SM, Thompson WL. When is early visual cortex activated during visual mental imagery? Psychol Bull. 2003;129:723–746. doi: 10.1037/0033-2909.129.5.723. [DOI] [PubMed] [Google Scholar]
- 10.Zatorre RJ, Halpern AR. Mental concerts: musical imagery and auditory cortex. Neuron. 2005;47:9–12. doi: 10.1016/j.neuron.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Bunzeck N, Wuestenberg T, Lutz K, Heinze HJ, Jancke L. Scanning silence: mental imagery of complex sounds. Neuroimage. 2005;26:1119–1127. doi: 10.1016/j.neuroimage.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Amedi A, Malach R, Pascual-Leone A. Negative BOLD differentiates visual imagery and perception. Neuron. 2005;48:859–872. doi: 10.1016/j.neuron.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 13.McGuire PK, Silbersweig DA, Murray RM, David AS, Frackowiak RS, Frith CD. Functional anatomy of inner speech and auditory verbal imagery. Psychol Med. 1996;26:29–38. doi: 10.1017/s0033291700033699. [DOI] [PubMed] [Google Scholar]
- 14.Halpern AR, Zatorre RJ. When that tune runs through your head: a PET investigation of auditory imagery for familiar melodies. Cereb Cortex. 1999;9:697–704. doi: 10.1093/cercor/9.7.697. [DOI] [PubMed] [Google Scholar]
- 15.Hoshiyama M, Gunji A, Kakigi R. Hearing the sound of silence: a magnetoencephalographic study. Neuroreport. 2001;12:1097–1102. doi: 10.1097/00001756-200105080-00010. [DOI] [PubMed] [Google Scholar]