Abstract
Although the auditory cortex is known to be essential for normal sound localization in the horizontal plane, its contribution to vertical localization has not so far been examined. In this study, we measured the acuity with which ferrets could discriminate between two speakers in the midsagittal plane before and after silencing activity bilaterally in the primary auditory cortex (A1). This was achieved either by subdural placement of Elvax implants containing the GABAA receptor agonist muscimol or by making aspiration lesions after determining the approximate location of A1 electro-physiologically. Psychometric functions and minimum audible angles were measured in the upper hemifield for 500-, 200-, and 40-ms noise bursts. Muscimol-Elvax inactivation of A1 produced a small but significant deficit in the animals’ ability to localize brief (40-ms) sounds, which was reversed after removal of the Elvax implants. A similar deficit in vertical localization was observed after bilateral aspiration lesions of A1, whereas performance at longer sound durations was unaffected. Another group of ferrets received larger lesions, encompassing both primary and nonprimary auditory cortical areas, and showed a greater deficit with performance being impaired for long- and short-duration (500- and 40-ms, respectively) stimuli. These data suggest that the integrity of the auditory cortex is required to successfully utilize spectral localization cues, which are thought to provide the basis for vertical localization, and that multiple cortical fields, including A1, contribute to this task.
Introduction
Sound localization in the horizontal plane is solved principally by exploiting binaural cues, differences in both the time of arrival and level of sounds at the two ears (Strutt 1907). By contrast, accurately localizing a sound in elevation is based on spectral cues arising from the direction-dependent filtering effects of the external ears (Hofman and Van Opstal 2003; Humanski and Butler 1988; Middlebrooks 1992; Morimoto 2001). Despite the extensive subcortical processing of these sound localization cues, it is well established that the auditory cortex plays a critical role in the ability of carnivores and primates to localize sounds in space. Thus removal or inactivation of the cortex impairs their ability to localize sounds in the horizontal plane (Beitel and Kaas 1993; Heffner and Heffner 1990; Jenkins and Masterton 1982; Jenkins and Merzenich 1984; Kavanagh and Kelly 1987; Malhotra and Lomber 2007; Malhotra et al. 2004; Smith et al. 2004). However, to date, no study has investigated the role of auditory cortex in localization in the vertical plane.
The initial processing of auditory localization cues takes place in largely separate, parallel pathways in the brain stem (reviewed by Yin 2002; Young and Davis 2002). Binaural convergence in the superior olivary complex gives rise to neuronal sensitivity to interaural time and intensity/level differences (ITDs and ILDs, respectively), whereas behavioral (May 2000; Sutherland et al. 1998) and electrophysiological (Young et al. 1992) evidence suggests that spectral cues are first extracted at the level of the dorsal cochlear nucleus (DCN). The principal output neurons of the DCN provide the dominant input to type O cells in the inferior colliculus (IC) (Davis et al. 2002), which are therefore hypothesized to encode spectral information in the midbrain (Ramachandran et al. 1999). However, more recent studies using virtual acoustic space stimuli, which allow acoustic cue values to be manipulated independently, indicate there is actually considerable integration of spatial information by IC neurons (Chase and Young 2005).
As with the behavioral data, most recording studies of the auditory cortex have focused on the representation of sound azimuth. In some cases, however, tuning in both azimuth and elevation has been reported, at least for stimuli close to unit threshold, in the primary auditory cortex (A1) (Brugge et al. 1994; Middlebrooks and Pettigrew 1981; Mrsic-Flogel et al. 2005), as well as in higher-level cortical fields (Xu et al. 1998). These physiological data therefore suggest that, as in the IC, cortical neurons are sensitive to multiple localization cues. Indeed, the spatial response fields of high-frequency neurons in ferret A1 are shaped by their sensitivity to ILDs and pinna-based spectral cues (Campbell et al. 2006; Mrsic-Flogel et al. 2005).
Together, these data predict that loss of activity in A1 should impair elevation judgments. In this study, we used both reversible, pharmacological inactivation, and permanent aspiration lesions to assess the contribution of A1 to the ability of ferrets to localize sounds in the midsagittal plane, where ILDs and ITDs are thought to contribute little, if at all, to localization. Because inactivation of other cortical fields has also been shown to impair azimuthal localization (Malhotra and Lomber 2007; Malhotra et al. 2004), we extended the study to examine the effects of removing a larger area of auditory cortex, encompassing more than A1, on localization in the midsagittal plane.
Methods
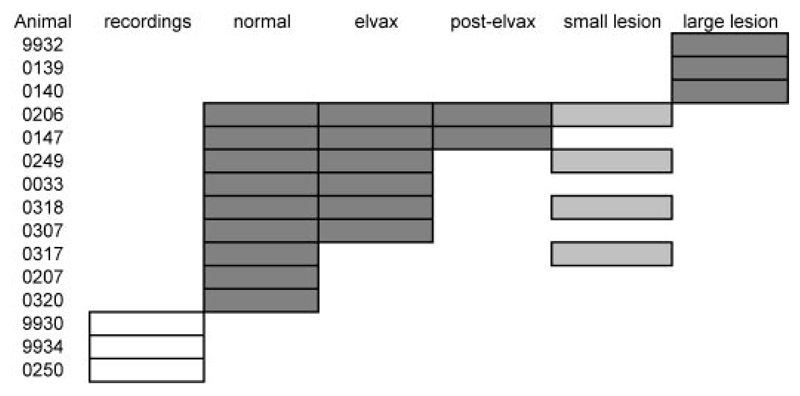
All experiments were carried out in accordance with the Animals (Scientific Procedures) Act 1986 and under license from the UK Home Office. A total of 15 pigmented ferrets (Mustela putorius) of both sexes (ten female, five male, 1 to 3 yr of age) were used in this study. Figure 1 lists the contribution of each individual to this study.
Fig. 1.
Summary of animals used in this study. Gray boxes indicate that an animal was tested for its ability to localize sound in the midsagittal plane in the condition indicated. For example, animal F0206 was trained and tested at all stimulus durations, providing control data (“normal”). These measurements were then repeated with 40-ms noise bursts after inactivating primary auditory cortex (A1) bilaterally with muscimol-Elvax (“elvax”), again after Elvax removal (“post-elvax”), and once more after making bilateral aspiration lesions of A1 (“small lesion”); “large lesion” refers to animals in which more extensive regions of auditory cortex were removed bilaterally. Dark gray boxes indicate that the animal was used for behavioral testing only, whereas light gray boxes (i.e., all A1 small lesions) indicate that electrophysiological recordings were made as well, before aspirating the cortex. Empty boxes indicate that the animal was used for electrophysiological recordings only.
Of 12 animals used for behavioral testing, nine contributed control data, six of which subsequently had A1 reversibly inactivated on both sides of the brain. Three of these animals then went on to receive bilateral aspiration lesions that were restricted to A1, although histo-logical analysis revealed that in one case (F0318; see Fig. 5) the lesion was effective unilaterally only. One ferret provided control data before having A1 removed bilaterally and three additional animals received larger lesions encompassing most of the ectosylvian gyrus, and therefore nonprimary as well as primary auditory fields, about 10 mo before testing on the vertical localization task. During this time, these three animals were used in another sound localization experiment. Three additional animals were used for physiological recordings to confirm the efficacy of newly made batches of muscimol-Elvax.
Fig. 5.
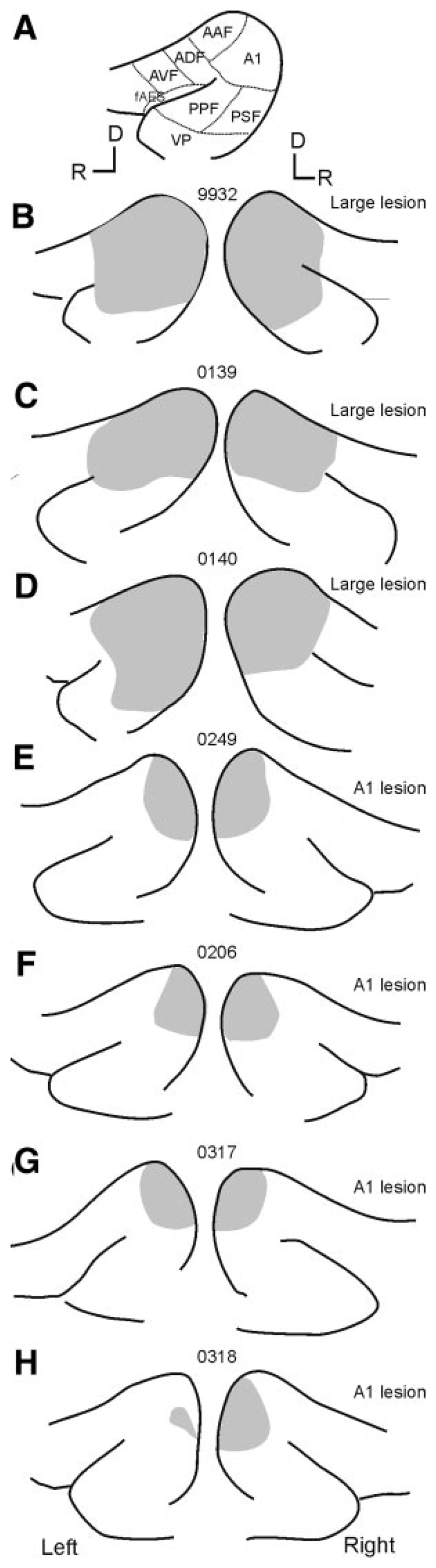
Schematics showing the size and location of the aspiration lesions made in each animal. In each case the suprasylvian sulcus and the pseudosyl-vian sulcus are shown with the lesion shaded in gray. A: schematic showing the location of auditory cortical fields (abbreviations as in Fig. 4). B–D: animals F9932, F0139, and F0140 received “large” lesions that included both the primary auditory fields and varying extents of the more ventral nonprimary fields. More restricted lesions were made in the other animals (E–H) after electrophysiological identification of the ventral low-frequency border of A1. D, dorsal; R, rostral.
Testing chamber
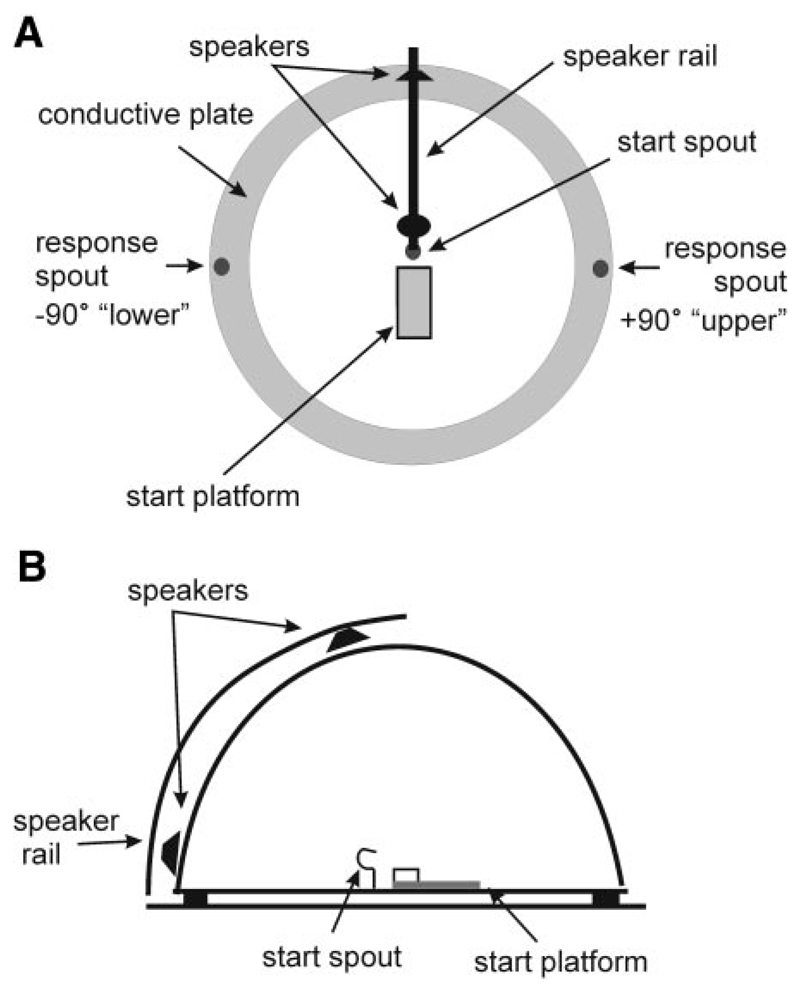
Figure 2 shows the testing chamber used in this task, consisting of a circular arena (radius 75 cm) enclosed by a hemispheric 25-mm2 wire mesh. The arena was housed in a double-walled sound-attenuated room, the walls and ceiling of which were lined with 50-mm-thick MelaTech sound-absorbing foam (The Noise Control Center, Leicestershire, UK). A raised start platform and spout were attached to the arena floor and two reward spouts were located around the perimeter at +90 and –90°. Stimuli were generated by TDT System II hardware (Tucker-Davis Technologies, Alachua, FL) and presented using two 50-W dome tweeters (Audax midrange YN43W, Audax UK, Amble-stone, UK) mounted on a curved rail positioned outside the mesh enclosure in front of the start platform and therefore lying on the midsagittal plane when the animal stood on the start platform. The speakers were matched for level at each angle from which stimuli were presented by placing a Bru¨el & Kjær type 4134 1/2-in. microphone at the start spout (where the ferret’s head would be when a trial was initiated), which was connected to a type 1618 measuring amplifier (Bru¨el & Kjær, Nærum, Denmark). The amplitude spectrum of the broadband stimulus (0- to 30-kHz bandwidth) was randomly varied on each trial by dividing it in 1/5th-octave intervals and varying the level of each interval with a Gaussian distribution over a ±5-dB range.
Fig. 2.
Floor plan (A) and side view (B) of the testing arena used to measure vertical localization. Animals were trained to discriminate between two broadband sound sources located on the midsagittal plane and differing only in their vertical locations. The testing chamber was circular in shape (radius 75 cm). To initiate a trial the ferret stood on the central platform and licked the start spout. This triggered the presentation of a noise burst from one of 2 speakers located on a curved rail outside the wire-mesh dome that enclosed the chamber. Response spouts were located at +90 and –90°.
Pyschophysical procedure
To initiate a trial, water-restricted ferrets were trained to stand on the start platform and to make contact, by licking, with the central spout. There was a variable delay (300–1,000 ms) before the stimulus was presented, during which time the animal was required to maintain contact with the spout. This ensured that the head was located at the center of the chamber and appropriately oriented when the stimulus was delivered from one of the two speakers. Animals were closely monitored by an overhead camera during training and testing to ensure that the head was maintained in the correct position until the stimulus was presented. One ferret initially had a tendency to rotate her head to one side when licking the spout, but we were able to train her not to do this by fitting a horizontal extension to the spout during the initial phase of training. This extension was removed during data collection. In about 12% of trials, a drop of water was presented at the start spout before stimulus presentation to motivate the animals to initiate a new trial.
We found it easier to train the ferrets on the vertical localization task if they had previously been trained and tested to perform an azimuth sound-localization task, in which broadband-noise bursts were presented from one of 12 speakers located around the periphery of the chamber, with correct responses rewarded by delivering water from a reward spout positioned at each speaker location (for details of the azimuth setup see Parsons et al. 1999). The animals were switched to the elevation task by using the reward spouts at ±90° only and by systematically moving the speakers at those locations toward the midsagittal plane, separating them in elevation until the speaker initially located at –90° was placed on the horizon directly in front of the animal and the speaker that had been located at +90° was also in the midsagittal plane and 70° above the horizon. Thus animals were rewarded for responding to their right, at +90°, when sounds came from the top speaker and were rewarded from the spout to their left (–90°) when sounds originated from the speaker located on the horizon. Animals quickly (<2 days) learned to make this association.
Correct responses were rewarded with three to four water drops (calibrated and matched in volume at each spout). Incorrect responses were not rewarded and were followed by correction trials, whereby the animal received up to two additional presentations of the same stimulus. If these were also mislocalized, one or more “easy trials” were presented, in which looped noise bursts were delivered from that location until the animal responded correctly. These trials were recorded but did not contribute to the score of the animal. Trials in which water was delivered at the central start spout were also excluded from the data analysis.
During testing, the ferrets were deprived of water in their home cages and kept on their normal diet of dry food pellets. They received their water by rewarding the correct trials in their twice-daily testing sessions. During a single testing session, the animals typically performed 100–150 trials and this was terminated when they appeared to be satiated and stopped initiating trials. Animals were tested for a maximum of 14 consecutive days, after which they had ≥4 days with unrestricted water access before beginning a new testing block. During testing, the animals were weighed daily to ensure that their weight stayed within 2SD of their mean baseline weight. They also received a small amount of wet food after their second daily testing session.
Animals were initially trained on the elevation task by presenting a looped noise stimulus at 75 dB SPL with the two speakers separated by 70°. Once they were achieving scores >85%, single noise bursts were presented, initially with a duration of 1,000 ms, which was further reduced to 500, then 200, and finally to 40 ms. Stimuli at the three shortest durations were presented at three different levels (70, 75, and 80 dB SPL), randomly interleaved, to further reduce the possibility of any nonspatial cues being available to the animals. Animals were kept at the maximum speaker separation for each stimulus duration until they attained equivalent scores (>85% correct). The speaker separation was reduced only when this level of performance was reached. After the cortex had been lesioned, this criterion score was not achieved in some cases and so the speaker separation was kept at 70° until 1,000 trials had been performed. Psychometric functions were then measured by systematically reducing the speaker separation in 10° steps provided that the scores achieved in two consecutive sessions (each containing a minimum of 75 trials) were statistically matched (P > 0.01, binomial distribution), or after four testing sessions had been completed at each angle. We kept reducing the speaker separation until the average performance fell below chance (57.5% for 150 trials, 56.5% for 200 trials using the confidence intervals from the binomial probability density function for 50% performance, at the 0.01 significance level) or until the smallest attainable separation of the speakers (6°) had been reached. If an animal performed above chance level at the smallest speaker separation, then the speakers were swapped to ensure that the animals really were performing a localization task rather than using nonspatial cues.
Psychometric functions were measured for the control animals using 500-, 200-, and 40-ms noise bursts, with at least two functions obtained at 40 ms. Two psychometric functions were measured in the presence of muscimol-Elvax and at least one more after its removal (post-Elvax condition). If a trained animal performed > 1,000 trials at a given duration with the maximum speaker separation and failed to achieve a score above chance, then the animal was adjudged not to be able to perform the task at that stimulus duration and the speaker separation was not reduced.
Data analysis
Data were analyzed using MATLAB (The MathWorks, Natick, MA). Each testing run typically consisted of >150 trials at each of eight speaker separations and was analyzed separately. Psychometric functions were fitted to the mean percentage correct scores at each speaker separation using binomial logistic regression. The goodness of fit of an optimized model was assessed using the deviance statistic (Berry et al. 2001). The deviance (D) has two important statistical properties: first, D is χ 2 distributed with a number of degrees of freedom that equals the number of stimulus parameter combinations tested (in our case eight) minus the number of free parameters (k) in the model. Thus if 1 – χ 2(D, 8 – k) < α, where α is the confidence interval (here α = 0.05), then we can reject the hypothesis that the model provides an adequate fit of the data. The model fits for our data typically produced deviances of around 5, meaning that these data and the model were statistically indistinguishable. Minimum audible angles (MAAs) were calculated from the fitted function as the speaker separation at which the animal obtained a score of 75% correct.
Statistical analyses were performed using the “R” package, an open-source implementation of S-plus (www.r-project.org; Venables 2002). The data were fitted with a mixed-effects general linear model (GLM, using the glmmPQL function; Venables and Ripley 1999), which takes into account repeated observations on the same animal. In other words, “animal ID” is treated as a random effect. The model described the proportion of correct responses as a function of the fixed effects “speaker separation” and “condition” (i.e., control, muscimol-Elvax, post-Elvax, A1 lesion). In the form our model was fitted, the coefficient for “speaker separation” described the slope of the function and “condition” described the y-intercept with respect to the control group. We also calculated the separation–condition interactions, which test whether there is a significant difference in the slope between controls and each one of the experimental manipulations. A cortical manipulation, such as A1 inactivation, could therefore produce a function that differs significantly from the controls in either slope or intercept.
Implantation of Elvax slices
Inactivation of auditory cortex was achieved using the slow-release ethylene-vinyl acetate copolymer Elvax 40-W containing the γ-ami-nobutyric acid type A (GABAA) agonist muscimol at a concentration of 75 mM. Details of how the muscimol-Elvax was prepared are given in Smith et al. (2004). Muscimol-Elvax was cut into 200μm-thick sheets using a Vibratome and stored at 4°C until required. The sheets were then trimmed to the desired size to lie over A1. Because trimming the Elvax leaves cut edges that briefly increase drug release, the sheets were rinsed for 24 h in phosphate-buffered saline (37°C) immediately before surgery.
Six trained ferrets received bilateral implants of muscimol-Elvax (Fig. 1). They were anesthetized by intramuscular (im) injection of Saffan (0.3% Alphadolone acetate, 0.9% Alphaxalone; 2 ml/kg; Mallinckrodt Veterinary, Uxbridge, UK). The EEG and heart rate were monitored throughout and core temperature was maintained at 38°C. The animal was placed in a stereotaxic frame and, after deflecting the temporal muscles, craniotomies were made bilaterally over the dorsal part of the ectosylvian gyrus and surrounding cortical areas. A slit was then made in the dura caudal to the suprasylvian sulcus, immediately behind A1, which occupies the caudal two thirds of the middle ectosylvian gyrus (MEG). The Elvax sheet was carefully slipped beneath the dura and moved into position over A1. The Elvax was positioned to avoid the rostromedial part of the gyrus, where the anterior auditory field (AAF) is thought to be located (Bizley et al. 2005; Kowalski et al. 1995). No electrophysiological recordings were carried out in these animals, to ensure that the cortex remained undamaged. Instead, the Elvax placement was made on the basis of previous descriptions of auditory cortex obtained by optical imaging (Nelken et al. 2004; Versnel et al. 2002) and electrophysiological recording (Bizley et al. 2005; Phillips et al. 1988). After placing the Elvax, the bone flap was replaced, the temporal muscle and scalp were sutured, and the animal was dosed with perioperative analgesic (20 μg/kg imVetergesic; Alstoe Animal Health, Melton Mowbray, UK).
After further behavioral testing, which recommenced 4 days after implantation, surgery to remove the Elvax was performed in a similar way. The Elvax was carefully pulled from beneath the dura, having first noted its position and the extent of any dura regrowth. In all but one animal (F0318), we observed no obvious change in the position of the implant over the ectosylvian gyrus and no change in the appearance of the cortex. In the case of F0318 the left implant was fully encapsulated by dura and its position had shifted caudally such that it was no longer fully covering the estimated location of A1. This is the only case (out of >20 animals in this and other studies in this laboratory) in which we observed encapsulation and displacement of the Elvax.
Cortical lesions
Surgery to expose auditory cortex was performed as described earlier, but under Domitor-ketamine anesthesia to facilitate cortical recordings (n = 4, Fig. 1). Animals were first anesthetized by im injection of Saffan (as before) and after intubation of the trachea and catheterization of the radial vein, anesthesia was maintained by intravenous infusion of Ketaset (ketamine hydrochloride, 2.5 mg·kg–1,h–1; Fort Dodge Animal Health, Southampton, UK) and Domitor (medetomidine hydrochloride, 15 mg·kg–1·h–1; Pfizer, Sandwich, UK). A small number of electrophysiological recordings was performed in each animal (see following text for methods) to localize the ventral, low-frequency border of A1. Because isofre-quency contours are continuous across A1 and AAF, it is not possible to use a reversal in the tonotopic gradient to distinguish these primary fields (Nelken et al. 2004). The medial extent of A1 was therefore estimated on the basis of previous electrophysiological recording studies (Bizley et al. 2005; Kowalski et al. 1993), whereas its dorsal and caudal borders were defined in relation to the sulcal pattern. At the end of the recordings, a lesion was made by aspiration after creating a small incision through the pia with a fine surgical blade, taking care to avoid damaging the underlying white matter. The void space in the cortex was filled with absorbable gel foam and the skull replaced.
Three additional animals received much larger aspiration lesions of the auditory cortex that included both primary and nonprimary fields. Because the intention was to remove most of the auditory cortex, no recordings were carried out before making the lesions. The animals were recovered as detailed earlier.
Electrophysiological recordings
In addition to the electrophysiological recordings that were made from auditory cortex in the four animals from which A1 was removed, we recorded from visual cortex in three additional ferrets that did not participate in behavioral testing (Fig. 1). These recordings were carried out to confirm that new batches of muscimol-Elvax, to be used for implantion in behaving animals, were effective in silencing neural activity and, in one case, to show that drug-free Elvax had no effect on cortical activity. As in our previous study (Smith et al. 2004), we recorded from visual cortex because its neurons tend to produce stronger and more robust responses than those in auditory cortex, making it easier to determine the spatial extent of the cortical inactivation produced by muscimol-Elvax.
Animals used to assess cortical inactivation were anesthetized as described earlier for the lesioned animals. A craniotomy was made over visual cortex and the dura reflected. All electrophysiological recordings were carried out in a darkened anechoic chamber. A light-emitting diode was positioned in front of the contralateral eye and responses were recorded to stationary light flashes to determine the location of the primary visual cortex (V1). A sheet of either muscimol-Elvax or drug-free Elvax, in which one or more small holes (2-mm diameter) had previously been bored and replugged, was then placed onto V1 and left in position for a minimum of 2 h. After this time surface-normal penetrations were made into the cortex by unplugging the holes that had been made in the Elvax before implantation and also in the cortex adjacent to the implant.
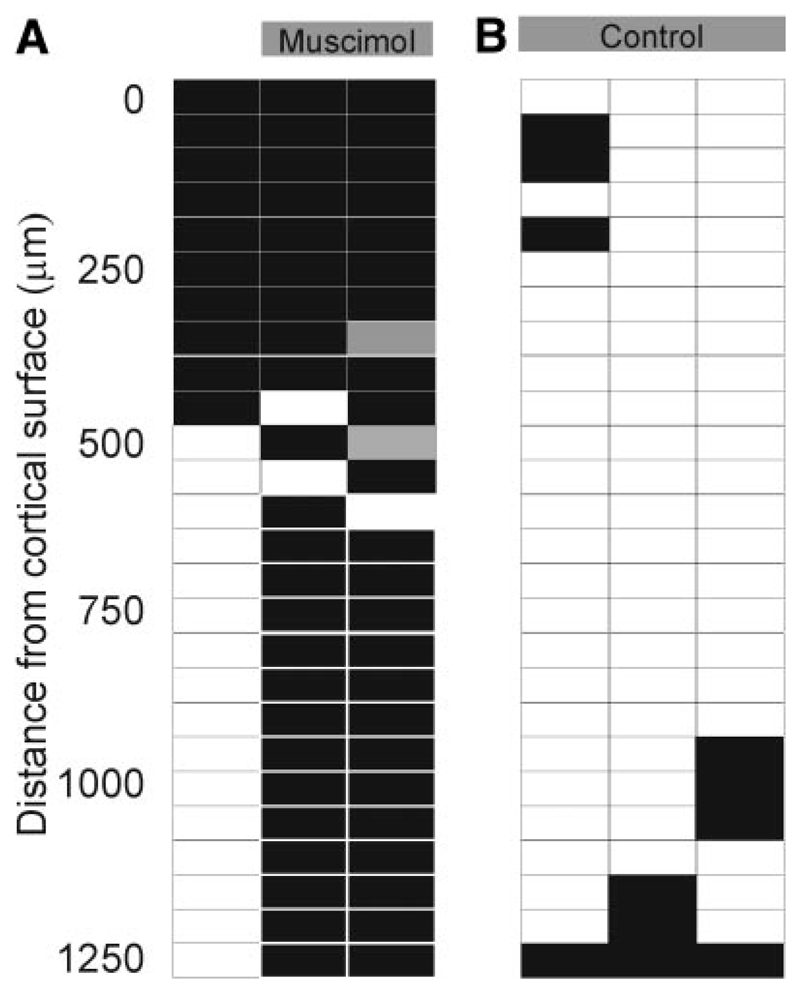
Figure 3 illustrates the results of one of these recording experiments. Very little cortical activity was encountered in two penetrations made through small holes in the muscimol-Elvax (Fig. 3A ), whereas visually evoked responses were recorded at most sites beneath the drug-free Elvax (Fig. 3B ) implanted in the other hemisphere of the same animal. Visually evoked activity was also recorded after the first 500 μm in a single electrode penetration made adjacent to the muscimol-Elvax implant (Fig. 3A ), suggesting that inactivation of cortical neurons was restricted, particularly in the deeper layers, to the vicinity of the implant. These results are consistent with those presented in the more detailed study by Smith et al. (2004) in which recordings were made from both V1 and A1.
Fig. 3.
Effects of muscimol-Elvax (A) and drug-free Elvax (B) implants on activity in ferret visual cortex. Recordings were made 2 h after implantation. Surface-normal electrode penetrations were made either directly beneath the Elvax (through a small hole that had been bored before implantation) or close to the lateral edge of the implant. Recordings were made every 50 μm and each sampling site is represented by a box: black boxes indicate that no activity was recorded, gray boxes indicate spontaneous activity, and white boxes mark positions where visual activity was recorded. Muscimol-Elvax largely eliminated activity throughout the depth of the cortex, whereas the drug-free control implants appeared to have no effect on the activity of the cortex.
For recordings made from auditory cortex (before aspiration of A1), broadband-noise bursts or pure tones were presented at a range of sound levels using closed-field headphones (Panasonic model RPHV 297; Panasonic, Bracknell, UK). The frequency tuning properties and peristimulus time histogram profiles were used, in accordance with our previous studies (Bizley et al. 2005), to delimit the borders of A1.
MRI scanning
Four of the ferrets with cortical lesions underwent magnetic resonance imaging (MRI) to visualize the site and extent of the lesions before recommencing behavioral testing. These animals were anesthetized by a single im dose of Ketaset and Domitor. Each was immobilized with a custom holder with a tooth bar to immobilize the head, placed in the scanner, during which time the ECG was measured and temperature maintained at 38°C. MRI was performed with a 7-T horizontal bore magnet interfaced to a Varian Inova spectrometer (Varian, Palo Alto, CA). A multislice data set of T2-weighted images was acquired using a fast spin-echo sequence with a repetition time (TR) of 3 s and an echo time (TE) of 58 ms. Eleven horizontal slices (1-mm slice thickness) spanning the whole brain were acquired with a 5 × 5-cm field of view and 128 × 125 matrix. Total acquisition time was 1 min 20 s.
Histological reconstruction of lesion sites
After completion of behavioral testing, all ferrets in which aspiration lesions had been made were transcardially perfused with 4% paraformaldehyde. The brains were photographed and then cryoprotected in 30% sucrose before cutting 50-μm coronal sections using a freezing microtome. Sections were mounted and stained with cresyl violet. The extent of the lesion was then reconstructed using a Leica DMR microscope (Leica Microsystems, Heerbrugg, Switzerland) and the thalamus was examined for evidence of degeneration within the medial geniculate body.
Results
Muscimol-Elvax inactivation
The spatiotemporal characteristics of the cortical inactivation produced by muscimol-Elvax were previously determined and documented (Smith et al. 2004). In that study, recordings made from both visual and auditory cortices demonstrated that Elvax implants of the same dimensions and containing the same concentration of muscimol as those used here silence neurons in all layers of the cortex for ≤6 wk after implantation and that cortical activity returns within a few hours of removing the Elvax. Each batch of muscimol-Elvax was tested for efficacy as described in methods.
Lesion sites
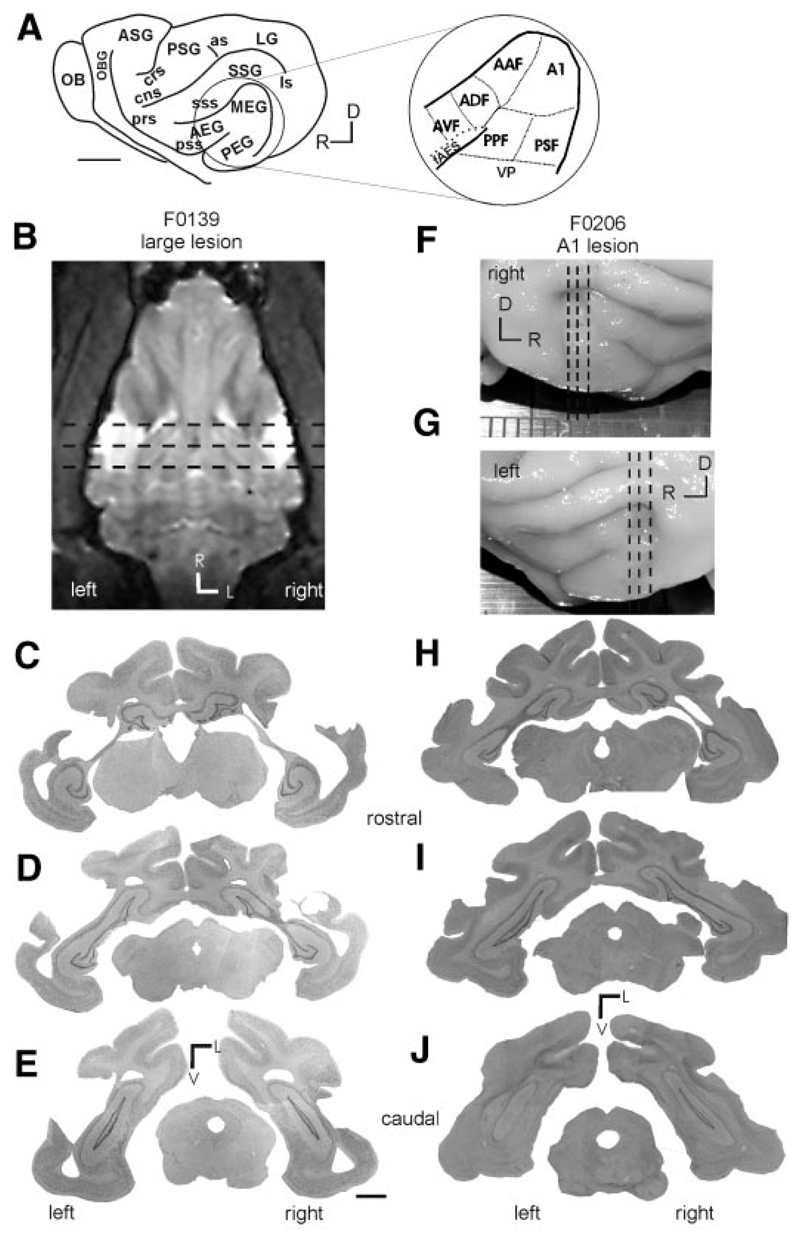
In all cases, the location of the aspiration lesions was reconstructed histologically. Figure 4 shows in detail the lesion sites for F0139 (large lesion; Fig. 4, B–E ) and F0206 (A1 lesion; Fig. 4, F–J ) and Fig. 5 summarizes for all seven animals the extent of cortical damage on surface views of the ectosylvian gyrus. Histological analysis confirmed that in three of the four cases the lesions that we attempted to restrict to A1 were of an appropriate size and that the white matter remained intact with no evidence for degeneration in the MGB (Fig. 4, H–J ). In one case (F0318, Fig. 5H ), the caudal part of the MEG was removed on the right side only, with the left-side lesion being restricted to more ventral A1 and, even there, the deeper cortical layers remained intact. In the three animals with “large” cortical lesions, the areas removed included A1 and AAF on the MEG, as well as more ventral fields. In each case, extensive retrograde degeneration was observed in the ventral division of the medial geniculate body. In the case of F9932 considerable proportions of the posterior ectosylvian gyrus (PEG) and the dorsal anterior ectosylvian gyrus (AEG) were removed bilaterally (Fig. 5B ; see Fig. 4A for the location of these regions). Animals F0139 (Figs. 4, B–E and 5C ) and F0140 (Fig. 5D ) had more restricted lesions with some of the nonprimary auditory fields remaining intact on both sides in F0139 and at least unilaterally in the case of F0140.
Fig. 4.
Location of the cortical lesions in 2 of the ferrets (0139 and 0206) used in this study. A: schematic of a ferret brain illustrating the location of the major gyri (OB, olfactory bulb; OBG, orbital gyrus; ASG, anterior sigmoid gyrus; PSG, posterior sigmoid gyrus; LG, lateral gyrus; SSG, suprasylvian gyrus; MEG, middle ectosyslvian gyrus; PEG, posterior ectosyslvian gyrus; AEG, anterior ectosyslvian gyrus) and sulci (prs, presylvian sulcus; prs, perirhinal sulcus; cng, coronal sulcus; as, anterior sigmoid; ls, lateral sulcus; sss, suprasylvian sulcus; pss, pseudosylvian sulcus). Inset: auditory cortex, located on the ectosyslvian gyrus, with functional subdivisions marked (A1, primary auditory cortex; AAF, anterior auditory field; PPF, posterior pseudo-sylvian field; PSF, posterior suprasylvian field; VP, ventral posterior field; ADF, anterior dorsal field; AVF, anterior ventral field; fAES, anterior ecto-syslvian sulcal field). B: horizontal MRI slice showing the location (in white) of the large bilateral temporal lobe lesions in animal F0139. C–E: coronal sections stained for Nissl substance taken at the approximate locations indicated by the dashed lines in B. F and G: photographs of the brain of animal F0206, in which A1 had been aspirated bilaterally. H–J: coronal sections stained for Nissl substance taken at the approximate locations marked by the dashed lines in F and G. Note that this lesion has maintained the integrity of the white matter.
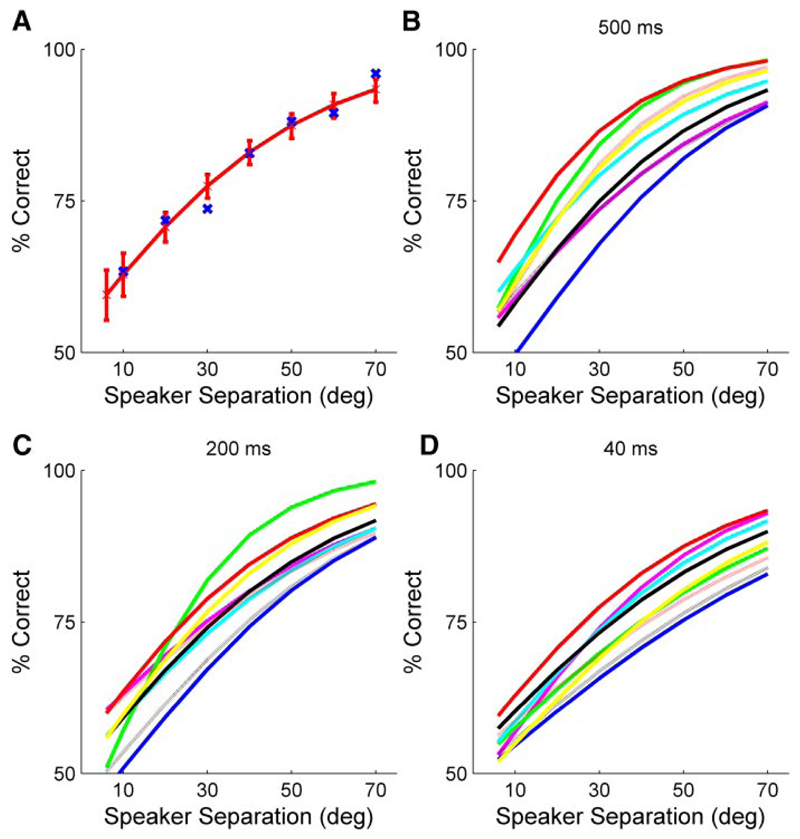
Behavioral results
All control (or prelesion) animals rapidly learned to associate the difference in vertical location of the speakers with the left and right response reward spouts. Data were collected at a range of stimulus durations (500, 200, and 40 ms). At least 150 trials were collected per speaker separation and a binomial logistic regression was used to fit a psychometric function to these data, as shown in Fig. 6A . Here the raw scores are plotted, with the fitted function and the 95% confidence bounds for this function. In subsequent figures only the fitted function is shown. Threshold (taken as 75% correct) values were estimated from these fitted functions. Figure 6, B–D shows control data collected at each of the three stimulus durations. As in the horizontal plane (Parsons et al. 1999; Smith et al. 2004), the ferrets performed best at the longest stimulus duration (500 ms). The mean (±SD) thresholds measured from the fitted psychometric functions for these nine animals were 27.0 ± 7.7° at 500 ms, 31.7 ± 6.3° at 200 ms, and 35.3 ± 9.6° at 40 ms. Although there was clearly some variability between animals, the performance of individual ferrets was found to be consistent across testing runs even when they were retested several weeks later.
Fig. 6.
Localization in the midsagittal plane. A: raw data (% correct scores for 40-ms noise bursts as a function of the vertical angular separation of the speakers) and the fitted psychometric function, from which the minimum audible angle (MAA) was calculated, for animal F0249. These data were obtained before any cortical manipulation. B–D: control data for all the animals. Each line represents the psychometric function from a different animal for stimulus durations of 500 ms (B), 200 ms (C), and 40 ms (D). Mean MAAs, corresponding to the 75% score, were 27.0 ± 7.7° at 500 ms, 31.7 ± 6.3° at 200 ms, and 35.3 ± 9.6° at 40 ms.
Deficits in performance after reversible inactivation or removal of A1
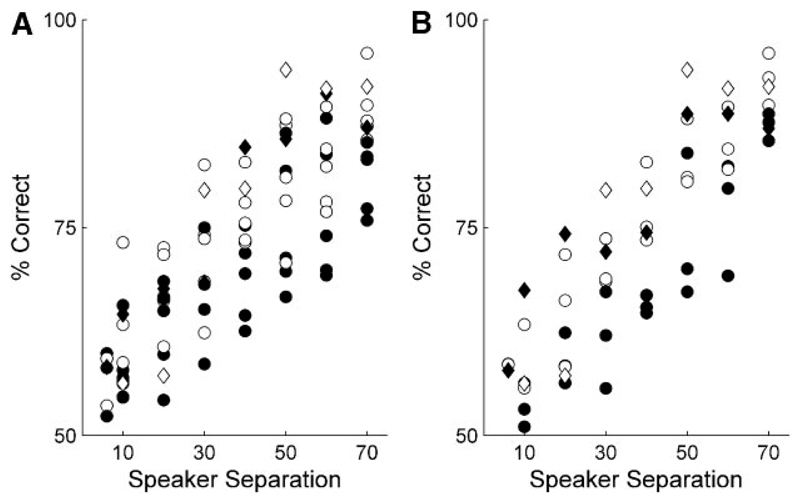
Figure 7 plots the scores achieved when 40-ms noise bursts were presented at each speaker separation for all animals before and after A1 inactivation (Fig. 7A ) or removal (Fig. 7B ). Although they were still able to perform this task, lower scores tended to be made at all speaker angles when the function of A1 was removed. The only exception to this was F0318 (indicated by the filled and open diamonds in Fig. 7), which was subsequently shown to have incomplete inactivation/lesion of the left A1 (see following text).
Fig. 7.
Effects of loss of activity in A1 on sound localization in the midsagittal plane. A: data from 6 animals showing the mean percentage correct score for each animal at the speaker separation indicated before implantation of muscimol-Elvax (open symbols) and with the implants in place (filled symbols). B: data from 4 animals showing the scores obtained before (open symbols) and after bilateral removal of A1 (filled symbols). Note that the ferrets tend to make lower scores after inactivation or aspiration of A1. One exception to this was F0318, whose data are indicated by the diamonds in A, in which the Elvax sheet became encapsulated by dura and displaced from the surface of the left A1. This is the same animal, again indicated by the diamonds in B, whose auditory cortex lesion was effective unilaterally only.
Elvax Inactivation
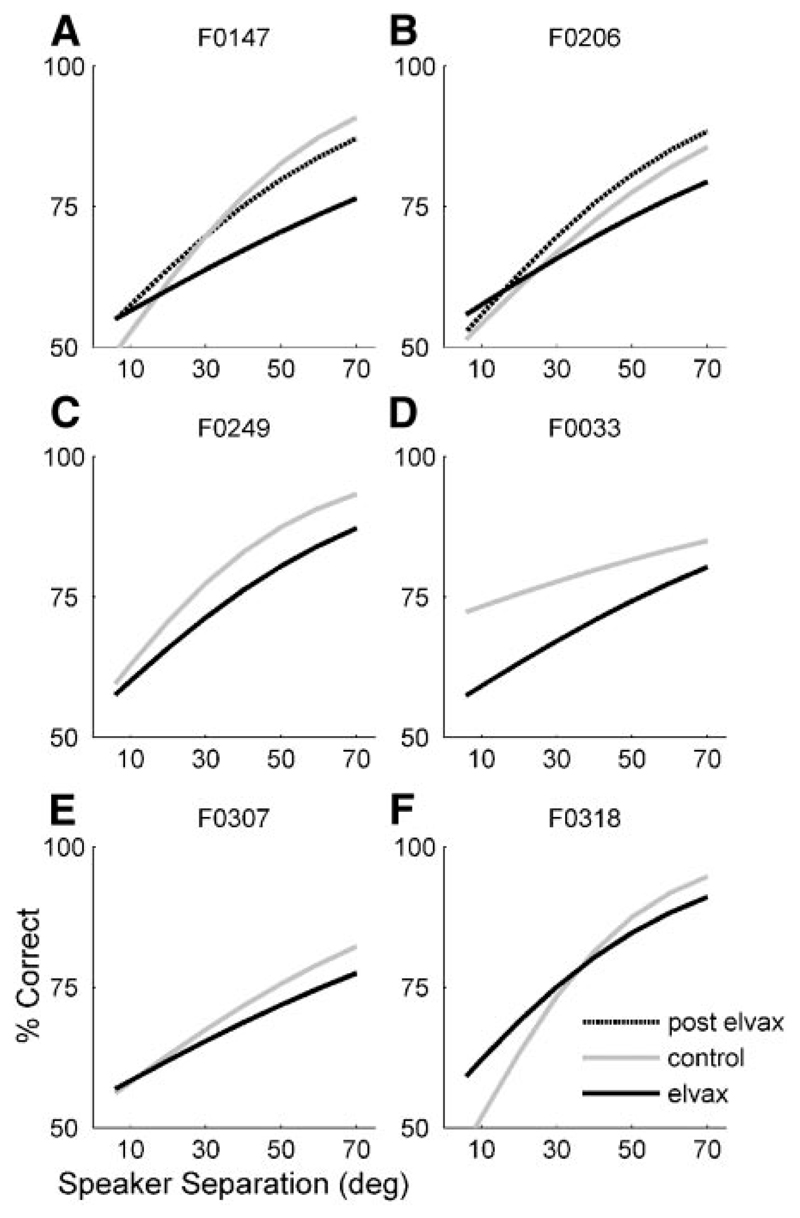
Due to the limited time course over which the cortex is completely silenced by muscimol-Elvax (~6 wk; Smith et al. 2004), psychometric functions were measured only at the shortest stimulus duration (40 ms). The pre-Elvax thresholds of the six ferrets in this group were 33.7 ± 7.7°, which rose to 50.1 ± 13.6° in the presence of the muscimol-Elvax implants. Figure 8 plots the psychometric functions from each of these animals before and during A1 inactivation. In five of six animals there was a small but clear drop in performance as a result of inactivating A1 (Fig. 8, A–E ). This effect was statistically significant when modeled with a mixed-effects GLM [pre-Elvax vs. Elvax: slope, t = 1.8, P = 0.07 (ns); intercept, t = –5.8, P ≤ 0.001], indicating that performance was degraded at all speaker separations. The remaining animal (F0318; Fig. 8F ) did not show a clear effect of the muscimol-Elvax, but, in this case, we subsequently found that the Elvax sheet that had been placed over the left cortex was heavily encapsulated with dura regrowth and had shifted caudally away from A1. The Elvax sheet on the right side, however, was correctly positioned and in direct contact with the pial surface of A1. Two animals were retested after the Elvax implants were removed and their scores were found to be indistinguishable from those measured before cortical inactivation [Fig. 8, A and B ; mixed-effects GLM condition: pre-Elvax vs. post-Elvax: slope, t = 0.38, P = 0.71 (ns); intercept, t = –0.71, P = 0.48 (ns)]. Thus removal of the Elvax reversed the deficit observed when the implants were in place, indicating that the change in localization acuity was related specifically to A1 inactivation rather than to a nonspecific effect of the surgery.
Fig. 8.
Effect of bilateral inactivation of A1 by muscimol-Elvax on psychometric functions in the midsagittal plane. Stimuli were 40-ms noise bursts. Data from individual animals are shown in each panel. Each line shows the fitted psychometric function obtained before implantation of muscimol-Elvax (gray line) and with the implants in place (black line) for a different ferret. Two animals were retested after the muscimol-Elvax implants had been removed (A, B, stippled line). Note that the data in F are from F0318, the animal in which the Elvax had become encapsulated by dura and displaced from the surface of the cortex.
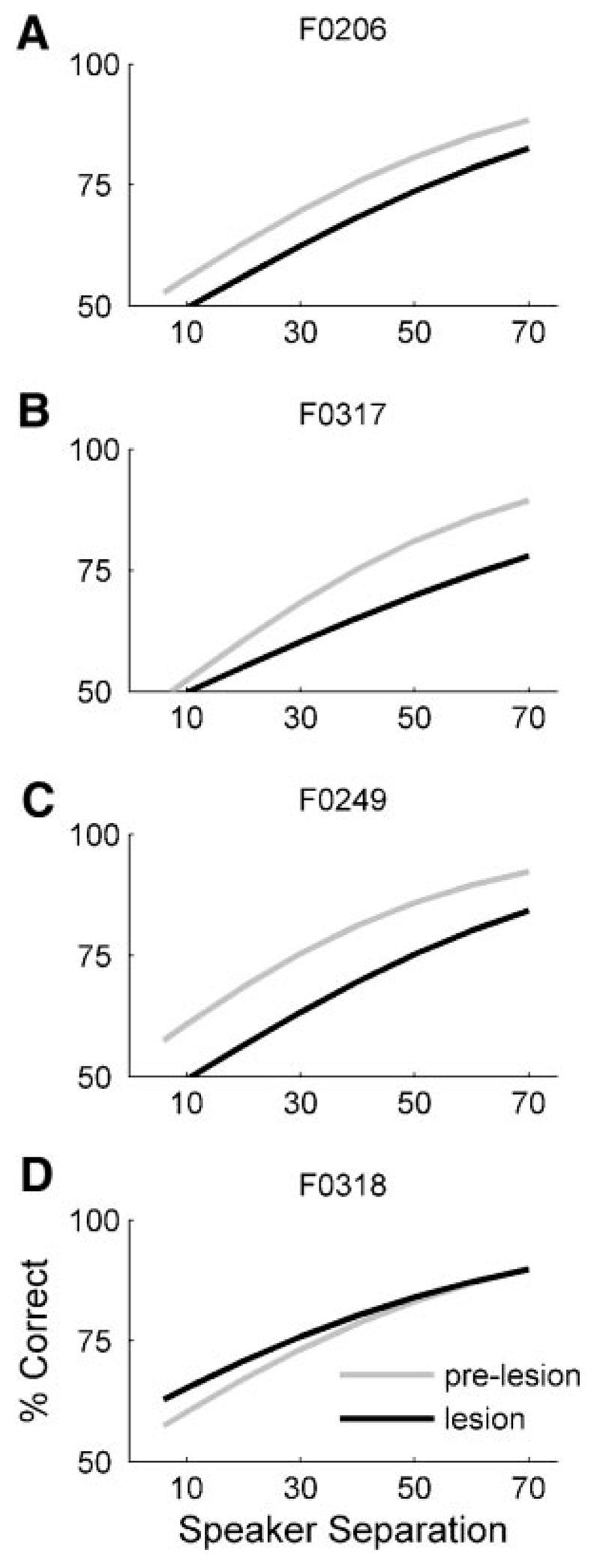
Lesions
Three animals had complete bilateral lesions of A1. In these cases there was a clear deficit in performance with 40-ms noise bursts that appeared similar in magnitude to that observed after cortical inactivation with muscimol-Elvax (Fig. 9, A–C ). MAAs increased from a mean of 32.3 ± 16.3° before the lesions were made to 43.4 ± 13.3° afterward. This result was statistically significant [mixed-effects GLM, prelesion vs. A1 lesion: slope, t = –2.3, P = 0.023; intercept, t = –1.86, P = 0.065 (ns)]. A fourth animal (F0318) was lesioned bilaterally, but subsequent histological analysis revealed that the appropriate region of cortex had been removed on the right side only, with a large part of the left A1 remaining intact (see Fig. 5H ). In keeping with the results obtained from the same animal with muscimol-Elvax (Fig. 8F ), which was found to be positioned appropriately on one side only, this ferret did not show a sound localization deficit in the midsagittal plane (Fig. 9D ). None of the animals tested with A1 lesions showed a deficit in vertical localization at longer stimulus durations.
Fig. 9.
Effect of lesioning A1 on localization in the midsagittal plane. Stimuli were 40-ms noise bursts. Data from individual animals are shown in each panel. Each line shows the fitted psychometric function obtained before (gray lines) and after (black lines) A1 was removed bilaterally. Note that the data in D are from F0318, the animal in which A1 turned out to be lesioned on one side only.
Large cortical lesions
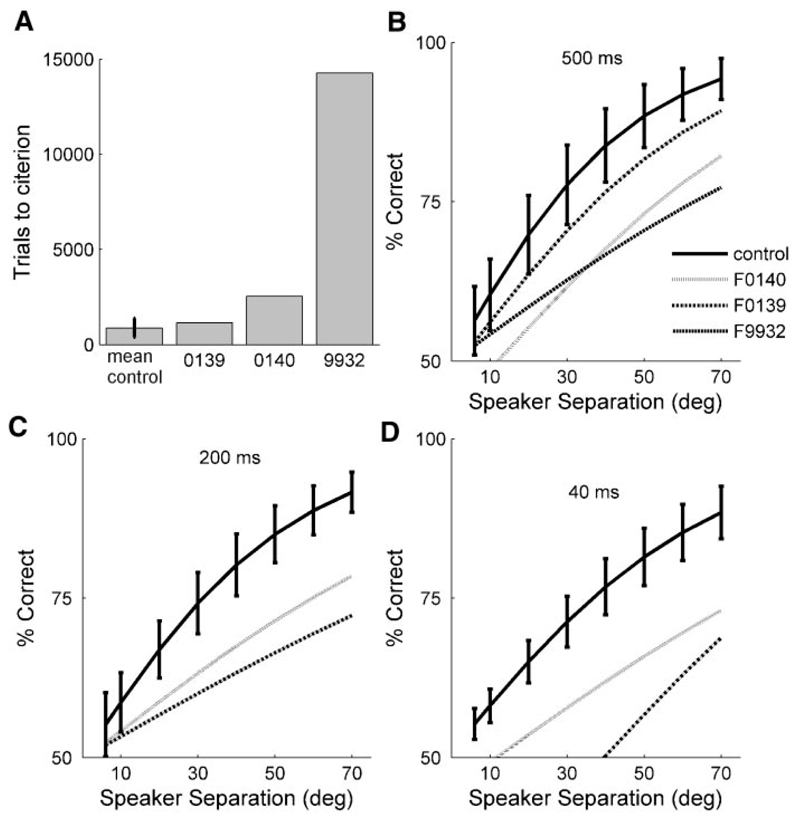
Although bilateral inactivation or removal of A1 resulted in a significant deficit in vertical localization, these animals could still do the task and achieved above chance scores at most speaker separations (Figs. 8 and 9). To investigate the possibility that other cortical fields might also be contributing to the ability of the animals to perform this task, we attempted to measure psychometric functions from three additional ferrets in which larger aspiration lesions had been made that encompassed A1, AAF, and much of the ventral, nonprimary auditory fields (see Figs. 4 and 5). Because the vertical localization abilities of these animals had not been tested before removal of the cortex, they could not provide their own control data, although they had, like all other animals, been trained previously to carry out an azimuth localization task in the same chamber and had achieved scores before surgery that fell within the normal range of values.
The capacity of these animals to learn the elevation task was judged by how many trials they took to reach an 85% correct criterion level when tested with 500-ms noise bursts at the maximum speaker separation (70°). Compared with the control animals, task acquisition was unimpaired in the case of F0139 (Fig. 10A ), which had the most restricted lesions of the three ferrets (Fig. 5C ). F0140, which had a relatively large lesion of the left cortex and a more restricted right cortical lesion (Fig. 5D ), took approximately twice as many trials to reach the criterion level of performance, whereas F9932, which had the largest cortical lesions (Fig. 5B ), took much longer (Fig. 10A ).
Fig. 10.
Effect of lesioning larger regions of auditory cortex on localization in the midsagittal plane. A: bar chart showing the mean (±SD) number of trials taken by the control animals and by each the animals with large bilateral lesions of auditory cortex to reach a criterion level of performance (>85% correct with 500-ms noise bursts at the maximum speaker separation). B–D: each line shows the fitted psychometric function for these animals at different stimulus durations. Mean (±SD) scores achieved by the control animals (n = 9) are shown by the solid lines and error bars. Other lines show the psychometric functions for the 3 ferrets with large cortical lesions, which all lie below the range of values obtained for the control group. Animal F9932 was unable to perform above the level expected by chance with either 200- or 40-ms noise bursts.
Although it is possible that the greater number of trials taken by cases F0140 and F9932 to reach the 85% correct criterion reflects, at least in part, the lack of training on the elevation task before auditory cortex ablation, the data from F0139 show that ferrets with large cortical lesions are capable of acquiring the task, and therefore of associating the stimulus source with the appropriate response spout, just as readily as control animals. Moreover, all three lesioned animals eventually reached the criterion level of performance.
However, the psychometric functions measured with 500-ms noise bursts for all three ferrets with large cortical lesions revealed that they performed consistently less well than the controls at all speaker separations (Fig. 10B ). At shorter stimulus durations, F9932 was unable to perform the task, scoring consistently at around the level expected by chance even at the maximum speaker separation. By contrast, F0140 and F0139 were able to perform the vertical localization task at both 200 and 40 ms, although their psychometric functions fell well below those measured for the control animals (Fig. 10, C and D ).
Both bilateral reversible inactivation and removal of A1 alone resulted in a comparable increase in MAA compared with the control data obtained from the same animals. Because we have no prelesion elevation data for the animals with the larger cortical lesions, it is necessary to compare their MAAs with those measured in other, control animals. At a stimulus duration of 500 ms, A1 removal had no discernible effect on vertical localization, whereas the MAAs measured for the ferrets with large cortical lesions were, with a mean value of 50.7 ± 11.1°, almost double those obtained for the control group (27.0 ± 7.7°). Performance at 200 ms was again unaffected A1 removal. By contrast, MAAs could not be measured for two of the animals (F0140 and F9932) with large cortical lesions (i.e., they scored <75% correct at the maximum speaker separation). In the other case (F0139), the MAA was 60° compared with 31.7 ± 6.3° in the control animals. At 40 ms, where significant deficits in performance first appeared after A1 inactivation/removal, MAAs could not be measured in any of the ferrets with large cortical lesions.
Discussion
In this study, we found that bilateral reversible inactivation or removal of A1 impaired the ability of ferrets to discriminate between two sound sources located in the midsagittal plane. Animals with larger lesions that encompassed other auditory cortical fields exhibited larger deficits in vertical localization. These results are consistent with previous studies in ferrets (Kavanagh and Kelly 1987; Smith et al. 2004) and other species (e.g., Beitel and Kaas 1993; Heffner and Heffner 1990; Jenkins and Masterton 1982; Jenkins and Merzenich 1984; Malhotra and Lomber 2007; Malhotra et al. 2004) showing that an intact auditory cortex is required for normal sound localization in the horizontal plane. It therefore appears that the cortex plays a key role in the processing of both monaural and binaural spatial cues that underlie the ability of mammals to determine the direction of a sound source.
The only previous lesion studies of vertical localization in mammals showed that section of the dorsal and intermediate acoustic stria, the output pathways from the dorsal and pos-teroventral cochlear nuclei, impairs the ability of cats to make head-orienting responses toward sound sources that differ in elevation (May 2000; Sutherland et al. 1998). This behavioral deficit is consistent with physiological evidence indicating a role for the dorsal cochlear nucleus in the processing of spectral localization cues (Young and Davis 2002), which provide the principal basis by which the vertical direction of a sound source is determined. Although head-orienting responses can be used as a measure of how accurately ferrets localize sound in the horizontal plane (Smith et al. 2004), we found that, once trained to approach the lateral reward spouts, the animals used in this study tended not to look up toward elevated sound sources in the midsagittal plane. Moreover, in previous studies of localization in the horizontal plane, approach-to-target responses have shown more consistent deficits than orienting movements after cortical lesions. Indeed, we have previously reported that bilateral inactivation of ferret A1 does not affect the accuracy of orienting movements for sound sources in the horizontal plane, but does impair the ability of the animals to identify the location of the sound by walking toward it (Smith et al. 2004). Consequently, as in a previous study in which we investigated the role of the pinna-based spectral cues in sound localization (Parsons et al. 1999), we trained the ferrets to approach one of two reward spouts, located to either side of the head, according to the relative vertical location of the sound sources. Despite the complexity of this task, all the prelesion ferrets and two of the three animals with large cortical lesions rapidly learned to make this association. Moreover, the control animals were consistent in their performance, both with each other and in repeated runs carried out at the same stimulus duration.
We adopted the same reversible inactivation technique used in a previous study of horizontal localization (Smith et al. 2004) to examine the role of A1 in vertical localization. By incorporating [3H]muscimol, we demonstrated in that study that relatively constant levels of muscimol are released from the Elvax implants for >5 mo (Smith et al. 2004). Moreover, electrophysiological recordings carried out in both visual and auditory cortices showed that the inactivation was long-lasting and confined to the region around the implant, but did not affect thalamocortical afferents. Based on these measurements, which were confirmed by a more limited set of recordings in the present study, the full depth of cortex beneath the implants should have been silenced when the behavioral data were obtained with the muscimol-Elvax implants in place.
We found that the localization of brief sounds presented in the midsagittal plane was significantly impaired when muscimol-Elvax was applied to the region of the ectosylvian gyrus occupied by A1, although these deficits were fairly modest and were reversed after removal of the implants. This is consistent with the effects of A1 inactivation on horizontal localization, which caused the animals to make more and larger errors, although they still performed well above chance (Smith et al. 2004). The similarity in the magnitude of the deficits observed in the present study, where the animals had to respond to different locations from those where the sound was presented, to the deficits observed in the azimuth task, where animals approached the actual sound-source location, suggests that cortical inactivation resulted in a general impairment in spatial hearing rather than a reduced ability to make the association between stimulus and reward location.
The most persistent deficit observed in the horizontal plane after bilateral inactivation of A1 was an increased incidence of front-back errors. This led Smith et al. (2004) to propose that A1 might be particularly concerned with the processing of pinna-based spectral cues, which are important for resolving the front-back locations of sound sources (Heffner et al. 1996; Musicant and Butler 1984; Oldfield and Parker 1984; Parsons et al. 1999) as well as for vertical localization (Hofman and Van Opstal 2003; Wightman and Kistler 1997). Our discovery that localization accuracy in the midsagittal plane is reduced when A1 is silenced is consistent with this possibility and provides behavioral support for the finding that the spatial receptive fields of ferret A1 neurons are shaped by their sensitivity to spectral as well as binaural cues (Mrsic-Flogel et al. 2001, 2005).
A possible reason as to why the impairment in auditory localization in the presence of muscimol-Elvax was not complete could be that A1 was not fully inactivated. Our electro-physiological data (present study and Smith et al. 2004) indicate that it is extremely unlikely that the effects of the muscimol had worn off by the time the behavioral data had been collected. On the other hand, because we opted–to avoid damaging the cortex–not to map out the borders of A1 electrophysiologically before Elvax implantation, it is possible that the inactivation did not include the full extent of A1. However, any error in the placement of the Elvax will most likely have occurred at the low-frequency border, rather than the high-frequency end where spectral cues should be processed. With the exception of the one animal (F0318) that did not show any deficit in localization performance, we found that the Elvax implants did not move to any noticeable extent and remained apposed to the surface of the cortex. Nevertheless, we cannot rule out the possibility that some neurons within the dorsal and lateral suprasylvian sulci, which border and may or may not be part of A1, were still responsive to sound.
Because muscimol can silence cortical neurons without apparently affecting thalamocortical afferents or fibers of passage (Smith et al. 2004), it might be expected that the ensuing behavioral deficits would be less pronounced than those produced by lesions or cryogenic inactivation. However, when A1 was removed bilaterally after first mapping its ventral border electrophysiologically, we observed a similar deficit in the capacity of the ferrets to discriminate between two sound-source locations in the midsagittal plane to that found in the presence of muscimol-Elvax. This suggests that the modest impairment produced by pharmacological inactivation is unlikely to be due to sparing of neuronal activity within A1. Rather, it would appear that, at least for the relative localization task used in this study, the integrity of A1 is required for normal performance in the vertical plane, but that other brain areas can to a large extent support this function in the absence of A1. Because animals have to recover for a few days after surgery to aspirate the cortex or implant Elvax, it is also possible that some recovery of function involving other brain regions can occur and that a greater deficit would have been produced had A1 been “switched off” while the animal was actually performing the task, as shown for azimuthal localization after cryogenic inactivation of cat A1 (Malhotra and Lomber 2007; Malhotra et al. 2004).
It is interesting to note that we were unable to observe a vertical localization deficit in the one case (F0318) in which A1 was effectively inactivated (Fig. 8F ) or lesioned (Fig. 9D ) on one side only. Unilateral A1 lesions produce azimuth localization deficits that are restricted to the opposite hemifield, a finding consistent with the fact that A1 neurons encode predominantly contralateral space (Jenkins and Masterton 1982; Jenkins and Merzenich 1984; Kavanagh and Kelly 1987; Smith et al. 2004). Although it is not possible to draw firm conclusions on the basis of a single example, the data from this animal imply that auditory cortex on one side only might be sufficient to support vertical localization abilities in the midline.
In this study, we chose to focus on auditory localization in the upper hemifield only because ferrets are terrestrial mammals. However, spectral cues allow sounds to be localized by humans both above and below the horizon (e.g., Hofman and Van Opstal 2003). Moreover, spatial receptive fields in ferret A1 extend below the horizon and their centroid vectors span both upper and lower hemifields (Mrsic-Flogel et al. 2005). Consequently, we anticipate that loss of activity in auditory cortex would equally affect the ability of ferrets to localize sounds below the horizon.
Contribution of cortical areas beyond A1
In keeping with other studies (Heffner 1978; Heffner and Masterton 1975; Kavanagh and Kelly 1987), we found that larger localization deficits were produced when the aspiration lesions extended beyond A1. Indeed, the number of trials performed before these animals achieved a criterion level of performance at the maximum speaker separation seemed to scale with the size of the lesions. As with the effects of cortical lesions or inactivation on azimuth localization (Heffner 1978; Heffner and Heffner 1990; Smith et al. 2004), these localization deficits were most pronounced with single brief sounds, raising the possibility that the impaired localization performance actually reflects a deficit in temporal rather than spatial processing. Indeed, bilateral cortical lesions in ferrets have been shown to result in larger gap detection thresholds (Kelly et al. 1996). However, we found that vertical localization was also impaired, albeit to a smaller extent, at the longer stimulus durations of 200 and 500 ms, suggesting that a deficit in temporal resolution alone is insufficient to explain the present data. Moreover, at these stimulus durations, the animal could start to orient its head toward the speaker while the sound was still playing, potentially allowing additional dynamic spatial cues to be obtained (Smith et al. 2004). Although this might contribute to the better localization performance observed with increasing stimulus duration, both in control animals and those with cortical lesions, the additional information available from head movements cannot fully overcome the spatial deficits exhibited after auditory cortex removal.
It is also unlikely that elevated hearing thresholds result in the localization deficits we describe here. Although we did not measure detection thresholds in the present study, Kavanagh and Kelly (1988) reported that, in most animals tested, bilateral ablation of ferret A1 has no effect on auditory sensitivity except at very high frequencies and that the hearing losses that were observed recovered to a very large degree. These animals were, however, impaired in their ability to localize sound in the horizontal plane. We used different sound levels in the present study (70, 75, and 80 dB SPL) and saw no effect of level on performance in the vertical localization task.
Seven different auditory cortical fields have so far been identified in the ferret (Bajo et al. 2007; Bizley et al. 2005; Nelken et al. 2004). As in the cat (Huang and Winer 2000), afferents from the ventral division of the medial geniculate body in the ferret innervate not only A1 but also both AAF and the fields on the PEG (Bizley 2005), providing an alternative route for information in the lemniscal pathway to activate auditory cortex. It seems likely that these projections underlie the reduced ability of ferrets to localize sound after inactivation or removal of A1. Consequently, the loss of areas outside A1 in F9932, coupled with the extensive degeneration of neurons within the ventral MGB, probably explains the inability of this animal to discriminate sound sources located in the midsagittal plane.
Lomber and colleagues (Malhotra and Lomber 2007; Malhotra et al. 2004) found that cats were impaired in their ability to localize sounds in the horizontal plane after cryogenic inactivation of A1, the posterior auditory field (PAF), or the anterior ectosylvian sulcal (fAES) field. On the other hand, cooling of other auditory cortical fields had no effect on sound localization. This supports the notion that parallel processing streams may exist for the processing of spatial and object information within the auditory cortex (Bendor and Wang 2006; Rauschecker and Tian 2000) and suggests that activity in A1, PAF, and fAES is required for normal auditory localization in the horizontal plane. Examination of the effects of inactiva-tion of each of these areas on vertical localization should provide further useful insights into their relative contributions to spatial hearing.
Acknowledgments
We thank S. Spires, O. Kacelnik, V. Bajo, D. Kumpik, and R. Campbell for assistance with the ferret testing; P. Cordery for making the Elvax; N. Sibson for the MRI; and R. Campbell for statistical advice.
Grants
This work was supported by Wellcome Trust through Senior and Principal Research Fellowships to A. J. King and a studentship to J. K. Bizley.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact
References
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–369. doi: 10.1152/jn.1993.70.1.351. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. Cortical representations of pitch in monkeys and humans. Curr Opin Neurobiol. 2006;16:391–399. doi: 10.1016/j.conb.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry G, Matthews JNS, Armitage P. Statistical Methods in Medical Research. Oxford, UK: Blackwell Science; 2001. [Google Scholar]
- Bizley JK. Organization and Function of Ferret Auditory Cortex (PhD thesis) Oxford, UK: Univ. of Oxford; 2005. [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Hind JE, Chan JCK, Musicant AD, Poon PW. Simulation of free-field sound sources and its application to studies of cortical mechanisms of sound localization in the cat. Hear Res. 1994;73:67–84. doi: 10.1016/0378-5955(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Campbell RAA, Schnupp JWH, Shial A, King AJ. Binaural-level functions in ferret auditory cortex: evidence for a continuous distribution of response properties. J Neurophysiol. 2006;95:3742–3755. doi: 10.1152/jn.01155.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. Limited segregation of different types of sound localization information among classes of units in the inferior colliculus. J Neurosci. 2005;25:7575–7585. doi: 10.1523/JNEUROSCI.0915-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Ramachandran R, May BJ. Auditory processing of spectral cues for sound localization in the inferior colliculus. J Assoc Res Otolaryngol. 2003;1:148–163. doi: 10.1007/s10162-002-2002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner H. Effect of auditory cortex ablation on localization and discrimination of brief sounds. J Neurophysiol. 1978;41:963–976. doi: 10.1152/jn.1978.41.4.963. [DOI] [PubMed] [Google Scholar]
- Heffner H, Masterton B. Contribution of auditory cortex to sound localization in the monkey (Macaca mulatta) J Neurophysiol. 1975;38:1340–1358. doi: 10.1152/jn.1975.38.6.1340. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Sound localization in chinchillas, III:effect of pinna removal. Hear Res. 1996;99:13–21. doi: 10.1016/s0378-5955(96)00074-3. [DOI] [PubMed] [Google Scholar]
- Hofman PM, Van Opstal J. Binaural weighting of pinna cues in human sound localization. Exp Brain Res. 2003;148:458–470. doi: 10.1007/s00221-002-1320-5. [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat:laminar and areal patterns of input. J Comp Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Humanski RA, Butler RA. The contribution of the near and far ear toward localization of sound in the sagittal plane. J Acoust Soc Am. 1988;83:2300–2310. doi: 10.1121/1.396361. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Masterton RB. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization by the ferret (Mustela putorius) J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Hearing in the ferret (Mustela putorius): effects of primary auditory cortical lesions on thresholds for pure tone detection. J Neurophysiol. 1988;60:879–888. doi: 10.1152/jn.1988.60.3.879. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap detection thresholds in the ferret (Mustela putorius) Behav Neurosci. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- Kowalski N, Versnel H, Shamma SA. Comparison of responses in the anterior and primary auditory fields of the ferret cortex. J Neurophysiol. 1995;73:1513–1523. doi: 10.1152/jn.1995.73.4.1513. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Lomber SG. Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and nonprimary auditory cortical areas in the cat. J Neurophysiol. 2007;97:26–43. doi: 10.1152/jn.00720.2006. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res. 2000;148:74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Narrow-band sound localization related to external ear acoustics. J Acoust Soc Am. 1992;92:2607–2624. doi: 10.1121/1.404400. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Pettigrew JD. Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location. J Neurosci. 1981;1:107–120. doi: 10.1523/JNEUROSCI.01-01-00107.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M. The contribution of two ears to the perception of vertical angle in sagittal planes. J Acoust Soc Am. 2001;109:1596–1603. doi: 10.1121/1.1352084. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Jenison RL, Schnupp JWH. Listening through different ears alters spatial response fields in ferret primary auditory cortex. J Neurophysiol. 2001;86:1043–1046. doi: 10.1152/jn.2001.86.2.1043. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, King AJ, Schnupp JWH. Encoding of virtual acoustic space stimuli by neurons in ferret primary auditory cortex. J Neurophysiol. 2005;93:3489–3503. doi: 10.1152/jn.00748.2004. [DOI] [PubMed] [Google Scholar]
- Musicant AD, Butler RA. The influence of pinnae-based spectral cues on sound localization. J Acoust Soc Am. 1984;75:1195–1200. doi: 10.1121/1.390770. [DOI] [PubMed] [Google Scholar]
- Nelken I, Bizley JK, Nodal FR, Ahmed B, Schnupp JWH, King AJ. Large-scale organization of ferret auditory cortex revealed using continuous acquisition of intrinsic optical signals. J Neurophysiol. 2004;92:2574–2588. doi: 10.1152/jn.00276.2004. [DOI] [PubMed] [Google Scholar]
- Oldfield SR, Parker SP. Acuity of sound localisation: a topography of auditory space. II. Pinna cues absent. Perception. 1984;13:601–617. doi: 10.1068/p130601. [DOI] [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JWH, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J Neurophysiol. 1999;82:2294–2309. doi: 10.1152/jn.1999.82.5.2294. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Judge PW, Kelly JB. Primary auditory cortex in the ferret (Mustela putorius): neural response properties and topographic organization. Brain Res. 1988;443:281–294. doi: 10.1016/0006-8993(88)91622-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Davis KA, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. I. Classification based on frequency response maps. J Neurophysiol. 1999;82:152–163. doi: 10.1152/jn.1999.82.1.152. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what”and “where” in auditory cortex. Proc Natl Acad Sci; USA. 2000. pp. 11800–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Parsons CH, Lanyon RG, Bizley JK, Akerman CJ, Baker GE, Dempster AC, Thompson ID, King AJ. An investigation of the role of auditory cortex in sound localization using muscimol-releasing Elvax. Eur J Neurosci. 2004;19:3059–3072. doi: 10.1111/j.0953-816X.2004.03379.x. [DOI] [PubMed] [Google Scholar]
- Strutt JW. On our perception of sound direction. Philos Mag. 1907;13:214–232. [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK. Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behav Brain Res. 1998;97:1–12. doi: 10.1016/s0166-4328(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TCT. The coding of spatial location by single units in the lateral superior olive of the cat. II. The determinants of spatial receptive fields in azimuth. J Neurosci. 2002;22:1468–1479. doi: 10.1523/JNEUROSCI.22-04-01468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. New York: Springer-Verlag; 1999. [Google Scholar]
- Versnel H, Mossop JE, Mrsic-Flogel TD, Ahmed B, Moore DR. Optical imaging of intrinsic signals in ferret auditory cortex: responses to narrowband sound stimuli. J Neurophysiol. 2002;88:1545–1558. doi: 10.1152/jn.2002.88.3.1545. [DOI] [PubMed] [Google Scholar]
- Xu L, Furukawa S, Middlebrooks JC. Sensitivity to sound-source elevation in nontonotopic auditory cortex. J Neurophysiol. 1998;80:882–894. doi: 10.1152/jn.1998.80.2.882. [DOI] [PubMed] [Google Scholar]
- Yin TCT. Neural mechanisms of encoding binaural localization cues in the auditory brainstem. In: Oertel D, Fay RR, Popper AN, editors. Integrative Functions in the Mammalian Auditory Pathway. New York: Springer-Verlag; 2002. pp. 99–159. [Google Scholar]
- Young ED, Davis KA. In: Integrative Functions in the Mammalian Auditory Pathway. Oertel D, Fay RR, Popper AN, editors. New York: Springer-Verlag; 2002. pp. 160–206. [Google Scholar]
- Young ED, Spirou GA, Rice JJ, Voigt HF. Neural organization and responses to complex stimuli in the dorsal cochlear nucleus. Philos Trans R Soc Lond B Biol Sci. 1992;336:407–413. doi: 10.1098/rstb.1992.0076. [DOI] [PubMed] [Google Scholar]