Abstract
Background
During the COVID-19 lockdown, referrals via the 2-week-wait urgent pathway for suspected cancer in England, UK, are reported to have decreased by up to 84%. We aimed to examine the impact of different scenarios of lockdown-accumulated backlog in cancer referrals on cancer survival, and the impact on survival per referred patient due to delayed referral versus risk of death from nosocomial infection with severe acute respiratory syndrome coronavirus 2.
Methods
In this modelling study, we used age-stratified and stage-stratified 10-year cancer survival estimates for patients in England, UK, for 20 common tumour types diagnosed in 2008–17 at age 30 years and older from Public Health England. We also used data for cancer diagnoses made via the 2-week-wait referral pathway in 2013–16 from the Cancer Waiting Times system from NHS Digital. We applied per-day hazard ratios (HRs) for cancer progression that we generated from observational studies of delay to treatment. We quantified the annual numbers of cancers at stage I–III diagnosed via the 2-week-wait pathway using 2-week-wait age-specific and stage-specific breakdowns. From these numbers, we estimated the aggregate number of lives and life-years lost in England for per-patient delays of 1–6 months in presentation, diagnosis, or cancer treatment, or a combination of these. We assessed three scenarios of a 3-month period of lockdown during which 25%, 50%, and 75% of the normal monthly volumes of symptomatic patients delayed their presentation until after lockdown. Using referral-to-diagnosis conversion rates and COVID-19 case-fatality rates, we also estimated the survival increment per patient referred.
Findings
Across England in 2013–16, an average of 6281 patients with stage I–III cancer were diagnosed via the 2-week-wait pathway per month, of whom 1691 (27%) would be predicted to die within 10 years from their disease. Delays in presentation via the 2-week-wait pathway over a 3-month lockdown period (with an average presentational delay of 2 months per patient) would result in 181 additional lives and 3316 life-years lost as a result of a backlog of referrals of 25%, 361 additional lives and 6632 life-years lost for a 50% backlog of referrals, and 542 additional lives and 9948 life-years lost for a 75% backlog in referrals. Compared with all diagnostics for the backlog being done in month 1 after lockdown, additional capacity across months 1–3 would result in 90 additional lives and 1662 live-years lost due to diagnostic delays for the 25% backlog scenario, 183 additional lives and 3362 life-years lost under the 50% backlog scenario, and 276 additional lives and 5075 life-years lost under the 75% backlog scenario. However, a delay in additional diagnostic capacity with provision spread across months 3–8 after lockdown would result in 401 additional lives and 7332 life-years lost due to diagnostic delays under the 25% backlog scenario, 811 additional lives and 14 873 life-years lost under the 50% backlog scenario, and 1231 additional lives and 22 635 life-years lost under the 75% backlog scenario. A 2-month delay in 2-week-wait investigatory referrals results in an estimated loss of between 0·0 and 0·7 life-years per referred patient, depending on age and tumour type.
Interpretation
Prompt provision of additional capacity to address the backlog of diagnostics will minimise deaths as a result of diagnostic delays that could add to those predicted due to expected presentational delays. Prioritisation of patient groups for whom delay would result in most life-years lost warrants consideration as an option for mitigating the aggregate burden of mortality in patients with cancer.
Funding
None.
Introduction
After the announcement by the UK Government on March 23, 2020, of implementation of a nationwide lockdown to combat the COVID-19 pandemic, hospital referrals for non-COVID-19-related illnesses have decreased substantially.1 As the lockdown is lifted, a surge in presentations for non-COVID-19-related medical issues is anticipated.
Research in context.
Evidence before this study
We searched PubMed, with no language or date restrictions, on March 10, 2020, for observational studies of cancer pathway delays in English using the terms ([“cancer” OR “neoplasm”] AND [“delay” OR “interval” OR “wait”] AND [“diagnosis” OR “treatment]). Studies typically reported data extracted from institutional, regional, or national databases. Overall, studies are highly heterogeneous in design and findings, including the durations of delay studied, the duration of survival follow-up, the method by which impact is captured (percentages, odds ratios, hazard ratios), and how and when staging is done. Each study typically focused on a single tumour type and most did not stratify impact by stage of cancer. To our knowledge, no study had modelled the impact in lives and life-years lost of systematic delays in referral pathways in a standardised fashion across tumour types until we recently reported a health-care resource analysis focused on systemic delays at point of surgery
Added value of this study
Across multiple tumour types, we used a standardised approach using per-day fatality hazard ratios to quantify the effect of different durations of delay on survival, examining both the referred patient and the diagnosed patient, and examining delays for individual tumour types and subtypes and aggregated across major tumour types. This study focuses specifically on cancers diagnosed via the 2-week wait pathway because this pathway is most amenable to interventions. Although pertinent to ongoing forecasting of the impact of COVID-19-related delays, these models could apply to any systemic delays to cancer pathways.
Implications of all the available evidence
Incorporating previous observational studies of delay and examining crudely estimated, non-naturalistic per-patient delays, our models predict that COVID-19-related delays in presentation, diagnosis, and subsequent treatment, will result in loss of life and life-years that varies widely according to patient age and tumour type. Data regarding the true duration and extent of service disruption and per-patient cancer pathway delay across the UK as a result of the COVID-19 lockdown are currently immature. Direct predictions regarding attributable cancer deaths will be possible once more accurate patient-level data become available
Any delay in cancer treatment has the real risk of patients' tumours progressing from being curable (with near-normal life expectancy) to becoming non-curable (with very reduced life expectancy). Specific pathways have been established in the UK for referral from primary care for urgent specialist evaluation and investigation of individuals with so-called red flag symptoms suggestive of a specific cancer type, termed the 2-week-wait pathway. Reductions of up to 84% have been reported in 2-week-wait referrals in March–May, 2020 (Lawler M, unpublished).2, 3, 4 Large backlogs of patients accrued as a consequence of the lockdown are predicted to first place pressure on diagnostic services in secondary care, and affect other areas of the pathway thereafter.5
We aimed to address two key questions relating to this potential surge in presentations of symptomatic patients. First, we explored the effect of a range of scenarios of provision of additional diagnostic capacity to address patient backlogs, assuming no prioritisation of patient groups. For each scenario, we assessed the degree of so-called diagnostic delay incurred on top of the so-called presentation delay accrued during lockdown. Second, accounting for the risk of death associated with nosocomial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, we examined the gain in survival and life-years through prompt investigation per patients referred via 2-week-wait investigatory referral by age group and tumour referral group (the high-level grouping of tumour types via which referrals are made [appendix 1 pp 4–5]). To do these analyses, we developed a model using data on 10-year cancer survival for 2008–17 (stratified by age and cancer stage) combined with a per-day hazard ratio (HR) for delay and applied it to age-specific and stage-specific case and referral volumes based on the 2-week-wait pathway.6
Methods
Data sources
In this modelling study, we obtained patient numbers, age-stratified and cancer stage-stratified 5-year (2013–17) and age-stratified 10-year (2008–17) cancer survival data for all diagnoses, and those cancers associated with surgical resection for non-haematological malignancies, from Public Health England's National Cancer Registration and Analysis Service (NCRAS), including by subtype of breast cancer (appendix 1 pp 1–3). We obtained data on route to diagnosis by age and cancer stage from National Health Services (NHS) England Clinical Commissioning Groups collections.7 We used data on proportion of referrals for suspected cancer that translated into cancer diagnoses (the diagnostic-conversion rate) from Cancer Waits-Faster Diagnosis Standard data for west London, UK, for 2019–20.8 We used data from across England for cancer cases diagnosed when the patient was aged 30 years or older. We concentrated our analysis on the 20 most common cancers referred via 2-week-wait pathways, for which we analysed NCRAS survival data from 2 314 822 cancer cases (2008–17) and 2-week-wait diagnoses for 385 156 cancer cases (2013–16) (appendix 1 pp 4–5). We calculated life expectancy on the basis of UK Office of National Statistics life tables for 2016–18.9 We estimated nosocomial infection rates and median duration of hospital stay for treatment of each cancer type on the basis of aggregated and anonymised information from three large UK surgical oncology centres (Gronthoud F, unpublished). For the calculation of the case-fatality rate associated with unselected COVID-19 infection, we used published data from China because UK COVID-19 case-fatality rate estimates were only available for patients who had been admitted to hospital.10, 11
Model development
We used net survival estimates in which crude survival has been adjusted for background age-specific death rates in the general population to reflect cancer-specific mortality. Since long-term survival rates for most cancers are only known 5–10 years after diagnosis, we used 10-year cancer stage-specific survival data in our calculations. Because 10-year stage-specific survival data are not directly available for recent cancer cohorts in England, we estimated these data using established methods, by taking the ratio of stage-specific to all-stage 5-year survival data and applying it to 10-year all-stage survival data.12 We used the midpoint per 10-year age group for life expectancy to estimate life-years gained, averaged per patient.9
To calculate COVID-19-related mortality in patients with cancer, we first estimated peri-surgical mortality from nosocomial infection as the product of operation-specific duration of surgical admission, age-specific case-fatality rates, and the rate of nosocomial infection per day (1%, 2%, 5%, or 10%). Then we estimated COVID-19-related mortality in the community ascribing the patient a year of active cancer management status; this estimate was the product of the likelihood of community-acquired COVID-19 during the year (1%, 10%, 20%, or 50%), age-specific case-fatality rates, and the increase in COVID-19 case-fatality rate as a consequence of cancer as a comorbidity (two times or five times).10, 11
For the calculation of the HR for per-day delay in management, we used published data on the impact of delay in cancer surgery on overall survival for stage I–III disease to estimate per-day HRs associated with delay to definitive treatment.13, 14, 15, 16, 17, 18, 19, 20, 21 Since sufficient observational data were only available to generate summary delay HRs for breast, colorectal, and bladder cancers, we assigned delay HRs to other tumours on the basis of similarity of 5-year survival, categorising tumour progressiveness as being low (with a 5-year survival for stage II disease being >90%), moderate (50–90%), or high (<50%). Because of the scarcity of published observational data on tumours of high progressiveness (eg, oesophageal and gastric cancers), we conservatively considered this group as having a similar delay HR as moderately progressive tumours (appendix 1 pp 1–3). Finally, we assumed that delay to treatment for stage IV cancer would not affect 10-year survival.
Because patients younger than 60 years with stage I–III cancers typically have treatment with curative intent, we generated the stage-specific ratio from this group of those having major resection to those having other definitive treatment (eg, endoscopic resection or curative radiotherapy). We applied this ratio to age-specific and cancer stage-specific strata for those aged 60 years and older having major resection to estimate the proportion of patients who have been diagnosed who are having other types of definitive treatment.
To estimate 10-year survival for individuals diagnosed with stage I–III cancer who have no delay in treatment, we used NCRAS 10-year survival data and adjusted for COVID-19-related peri-surgical and community mortality. To estimate 10-year survival associated with delay, we applied the delay HR relating to the specified number of days of delay, along with the COVID-19-related peri-surgical and community mortality, to the NCRAS 10-year survival (formulas are in appendix 1 [pp 1–3]). We conservatively assumed that no additional downstream delays would occur after the diagnostic delay.
We quantified the annual numbers of cancers diagnosed via the 2-week-wait pathway using the 2-week-wait age-specific and stage-specific breakdowns. From these, our outcome measures were estimated aggregate number of lives lost and life-years lost in England for per-patient delays of 1–6 months.
Scenarios analysed using the model
We assessed the scenario of a 3-month period of lockdown during which a proportion of symptomatic patients delayed their presentation until after lockdown (ie, backlog patients), set at 25%, 50%, and 75% of normal monthly volumes. We assumed normal volumes of incident symptomatic patients presenting after lockdown. We considered different scenarios of extra capacity for catching up on this backlog applied across months 1–8 after lockdown. The backlog patients were assigned an average presentational delay of 2 months. Backlog and incident patients then accrued diagnostic delay in rounded whole months. We estimated the attributable lives and life-years lost, compared with the default position in which all backlog patients would complete investigatory referral within month 1 after lockdown. We modelled all backlog patients presenting in month 1 after lockdown or with variable presentation across months 1–3 (appendix 2).
We then did a per-patient risk–benefit analysis for 2-week-wait investigatory referral. A 2-week-wait investigatory referral was assigned as being 0·5 days of exposure to nosocomial infection. We combined per-day rates of nosocomial infection with the age-specific COVID-19 case-fatality rates to quantify the COVID-19-related fatality associated with investigatory referral. We combined this estimate with a so-called technical fatality risk for invasive investigations (eg, a one in 10 000 risk of death from perforation from colonoscopy)22 to produce a combined per-referral mortality.
Using the diagnostic-conversion rates, we estimated the survival benefit per patient from an investigatory referral for each age group and tumour group. We considered the potential to delay referral by 2, 4, and 6 months against varying rates of nosocomial infection per investigatory referral (5% being very high, 2·5% being high, 1% being moderate, and 0·5% being low). By age group and tumour type, we compared the benefit of prompt investigatory referral versus different periods of delay or no referral (absolute survival benefit). We estimated benefit in proportional survival and life-years gained from gain in cancer survival versus the combined fatality risk (COVID-19 and technical).
Statistical analysis
We combined individual log(HR)s, by stage and days of delay, using weighted linear regression to calculate the summary per-day delay-log(HR) (ie, delay HR) and SD of this estimate (ie, SE), expressing it as a percentage of the estimate. We did multivariate sensitivity analyses across ranges of parameter estimates, including 2 SD of delay HR and per day nosocomial infection rates of 1%, 2%, 5%, and 10%. Unless otherwise specified, we applied the default values for likelihood of community-acquired COVID-19, which was 20%, and per-day rate of nosocomial infection, which was 2%, which were selected to be conservatively high. For cancer-related increase in mortality due to community-acquired COVID-19, we used a default value of two times, which is at the low-to-intermediate end of the published estimates (reflecting a non-metastatic cancer population; appendix 1 p 1). Assumptions and parameter estimates are justified in detail in appendix 1 (pp 1–3). We did all analyses using STATA (version 15).
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
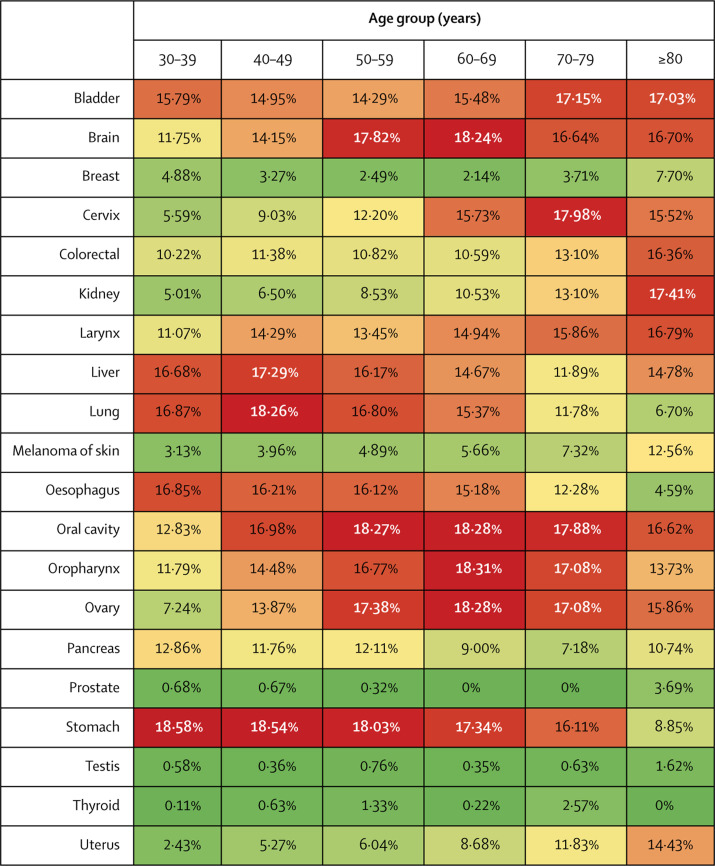
For several cancers, including those of the colorectum, oesophagus, lung, liver, bladder, pancreas, stomach, larynx, and oropharynx, a 3-month delay to diagnosis is predicted to result in a reduction in long-term (10-year) survival of more than 10% in most age groups (figure 1 ). Delays of 6 months are predicted to reduce 10-year mortality by more than 30% in many of these tumour types (appendix 1 p 6). Differences in the effects of a 3-month diagnostic delay were observed for different cancer stages across all tumour types and for different subtypes and stages of breast cancer (appendix 1 p 7).
Figure 1.
Reduction in 10-year net survival incurred from a 3-month delay for the 20 most common tumour types, by age group
Red indicates the highest decile of survival decrement, scaling down through orange and yellow to pale green, which indicates the lowest decile of survival decrement.
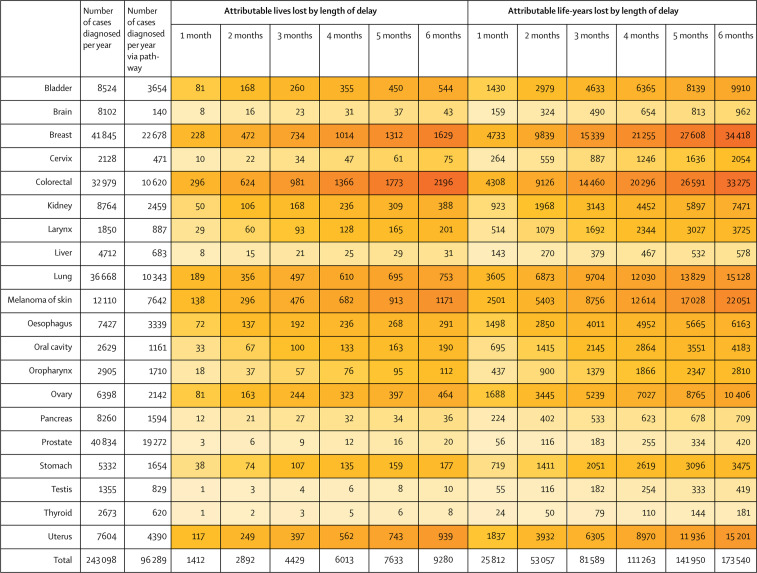
The aggregated impact of universal delays in the 2-week-wait pathway on lives lost and life-years lost varies widely by tumour type (figure 2 ). These predicted outcomes are driven by aspects of the model including age-specific incidence, the proportion of cancers diagnosed via the 2-week-wait pathway, the proportion of cancers diagnosed as stage I–III via the 2-week-wait pathway, and the aggressiveness of the tumour (appendix 1 pp 4–5). Attributable lives lost are highest for colorectal cancer and attributable life-years lost are highest for breast cancer. The aggregate effect from delays in prostate cancer pathways is predicted to be low, predominantly due to the high proportion of indolent cases inherent to the model. Similarly, pancreatic, gastric, and liver cancers only contribute moderately to the estimated total lives and life-years lost because fewer patients present via the 2-week-wait pathway and high proportions have stage IV disease at presentation (appendix 1 pp 4–5).
Figure 2.
Annual lives and life-years lost attributable to delay, aggregated for all patients diagnosed via the 2-week-wait pathway for the 20 most common tumour types
Based on 10-year net survival data for England, UK, 2008–17. Greatest decrements in lives and life-years lost are shown in darker shades of orange.
Across the 20 cancer types, on average an estimated 243 098 cancers are diagnosed annually. Of these, an estimated 96 289 are diagnosed via the 2-week-wait pathway, of which 75 369 (78·3%) are diagnosed at stage I–III (table ). 20 293 (26·9%) of 75 369 patients would be predicted to die due to cancer within 10 years of diagnosis, representing a loss of 304 129 life-years. A uniform per-patient delay of 1 month would be predicted to result in attributable additional lives lost of 1412 and life-years lost of 25 812 and a per-patient delay of 6 months would be attributed to an additional 9280 lives and 173 540 life-years lost over the subsequent 10 years for an annual cohort of cancer cases diagnosed via 2-week wait at stage I–III (figure 2).
Table.
Cancer diagnoses made through the 2-week-wait pathway for 2013–16
|
Proportion of annual cancer diagnoses that are 2-week-wait diagnoses |
Proportion by age group (years) |
Proportion by cancer stage |
Diagnostic conversion rate |
Estimated annual 2-week-wait referrals |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | ≥80 | Stage I | Stage II | Stage III | Stage IV | Stage I–III | Proportion of any cancer | Proportion of cancers in tumour referral group | |||
| Bladder | 3654/8524 (42·9%) | 0·3% | 2·1% | 7·4% | 23·4% | 36·2% | 30·6% | 51·9% | 29·1% | 6·7% | 12·3% | 87·7% | 16·9% | 98·2% | 21 624 |
| Brain | 140/8102 (1·7%) | 8·7% | 8·4% | 16·4% | 31·2% | 25·2% | 10·1% | N/A | N/A | N/A | N/A | N/A | 1·0% | 100·0% | 13 982 |
| Breast | 22 678/41 845 (54·2%) | 6·1% | 19·1% | 16·2% | 16·3% | 20·5% | 21·8% | 31·2% | 50·9% | 13·0% | 4·9% | 95·1% | 4·9% | 99·3% | 462 822 |
| Cervix | 471/2128 (22·1%) | 16·6% | 15·8% | 18·1% | 19·7% | 17·3% | 12·4% | 29·3% | 40·1% | 15·0% | 15·6% | 84·4% | 3·1% | 97·4% | 15 183 |
| Colorectal | 10 620/32 979 (32·2%) | 0·8% | 3·1% | 13·0% | 21·5% | 33·0% | 28·5% | 15·4% | 28·2% | 32·5% | 23·9% | 76·1% | 2·8% | 78·4% | 379 272 |
| Kidney | 2459/8764 (28·1%) | 2·3% | 8·1% | 17·7% | 27·8% | 27·5% | 16·7% | 45·3% | 11·4% | 21·8% | 21·6% | 78·4% | 16·9% | 98·2% | 14 551 |
| Larynx | 887/1850 (48·0%) | 0·5% | 5·2% | 19·4% | 33·3% | 28·2% | 13·4% | 36·6% | 19·3% | 17·8% | 26·3% | 73·7% | 2·9% | 74·0% | 30 599 |
| Liver | 683/4712 (14·5%) | 0·7% | 1·9% | 10·1% | 25·2% | 34·3% | 27·7% | 7·6% | 10·6% | 15·6% | 66·1% | 33·9% | 5·7% | 85·9% | 11 989 |
| Lung | 10 343/36 668 (28·2%) | 0·3% | 2·2% | 10·2% | 30·2% | 35·8% | 21·3% | 15·4% | 9·9% | 27·9% | 46·8% | 53·2% | 10·9% | 93·7% | 94 893 |
| Melanoma* | 7642/12 110 (63·1%) | 10·4% | 14·2% | 17·8% | 23·0% | 20·3% | 14·3% | 71·5% | 20·4% | 6·5% | 1·6% | 98·4% | 4·4% | 98·1% | 173 673 |
| Oesophagus | 3339/7427 (45·0%) | 0·4% | 3·0% | 12·9% | 29·0% | 31·0% | 23·8% | 7·4% | 16·1% | 41·2% | 35·3% | 64·7% | 5·7% | 85·9% | 58 571 |
| Oral cavity | 1161/2629 (44·1%) | 2·9% | 9·7% | 22·4% | 29·4% | 20·9% | 14·8% | 27·3% | 15·8% | 10·4% | 46·5% | 53·5% | 2·9% | 74·0% | 40 022 |
| Oropharynx | 1710/2905 (58·9%) | 1·4% | 12·1% | 34·7% | 33·8% | 14·2% | 3·8% | 2·8% | 6·1% | 13·4% | 77·6% | 22·4% | 2·9% | 74·0% | 58 960 |
| Ovary | 2142/6398 (33·5%) | 4·2% | 8·4% | 21·0% | 28·9% | 25·4% | 12·0% | 31·9% | 7·8% | 41·7% | 18·6% | 81·4% | 3·1% | 97·4% | 69 112 |
| Pancreas | 1594/8260 (19·3%) | 0·2% | 2·1% | 9·4% | 26·0% | 36·1% | 26·1% | 5·8% | 14·7% | 13·7% | 65·8% | 34·2% | 5·7% | 85·9% | 27 962 |
| Prostate | 19 272/40 834 (47·2%) | 0·0% | 0·9% | 9·6% | 32·9% | 38·2% | 18·2% | 27·9% | 21·6% | 26·0% | 24·5% | 75·5% | 16·9% | 98·2% | 114 037 |
| Stomach | 1654/5332 (31·0%) | 0·4% | 3·0% | 12·9% | 29·0% | 31·0% | 23·8% | 8·3% | 18·5% | 27·2% | 46·1% | 53·9% | 5·7% | 85·9% | 29 024 |
| Testis | 829/1355 (61·2%) | 61·7% | 22·4% | 10·9% | 3·4% | 1·2% | 0·4% | 86·6% | 7·8% | 3·1% | 2·5% | 97·5% | 9·0% | 75·0% | 9213 |
| Thyroid | 620/2673 (23·2%) | 28·3% | 19·0% | 18·1% | 15·1% | 12·2% | 7·3% | 44·4% | 10·0% | 19·0% | 26·7% | 73·3% | 2·9% | 74·0% | 21 388 |
| Uterus | 4390/7604 (57·7%) | 0·2% | 2·3% | 19·1% | 35·8% | 29·2% | 13·4% | 75·7% | 7·5% | 11·0% | 5·9% | 94·1% | 3·1% | 97·4% | 141 614 |
Data shown are proportion of all diagnoses made via 2-week-wait pathway, with a breakdown by cancers diagnosed via this pathway by age and cancer stage, diagnostic conversion rate, and average annual referrals. Diagnostic conversion rates reflect all diagnoses of invasive cancers (exceptions are that breast includes carcinoma in situ, skin excludes basal cell carcinomas, urology excludes pTa bladder tumours). N/A=not applicable.
Of the skin.
On the basis of preliminary estimates of 2-week-wait referral decreases, we estimated the predicted effects of 25%, 50%, and 75% reductions in presentations over a 3-month lockdown period (appendix 2).2, 3, 4 Based on data for 2013–16, for these 20 cancer types on average an estimated 149 000 2-week-wait referrals are made each month, resulting in 8024 diagnoses of cancer, of which 6281 are diagnosed at stage I–III (appendix 1 pp 4–5). 1691 (27%) of these 6281 patients will typically die from their cancer within 10 years.7 We estimated the national toll of presentational delay accrued over a 3-month lockdown period to be 181 attributable additional lives and 3316 life-years lost for a backlog rate of 25%, 361 additional lives and 6632 life-years lost for a backlog rate of 50%, and 542 additional lives and 9948 life-years lost assuming a backlog rate of 75%, with an average presentational delay of 2 months per patient. Assuming the patients all present in month 1 after lockdown, normal diagnostic capacity would need to work at least 175% under the 25% backlog scenario, 250% under the 50% backlog scenario, and 325% under the 75% backlog scenario to clear the backlog (appendix 2). However, it is unlikely that all extra diagnostic capacity required can be provided in a single month; therefore, we estimated the additional lives and life-years that might be lost due to subsequent diagnostic delays. Rapid provision of additional capacity over months 1–3 would result in 90 additional lives and 1662 live-years lost due to diagnostic delays for the 25% backlog scenario, 183 additional lives and 3362 life-years lost under the 50% backlog scenario, and 276 additional lives and 5075 life-years lost under the 75% backlog scenario (appendix 2). Conversely, delayed additional capacity provided across months 3–8 after lockdown would result in 401 additional lives and 7332 life-years lost due to diagnostic delays under the 25% backlog scenario, 811 additional lives and 14 873 life-years lost under the 50% backlog scenario, and 1231 additional lives and 22 635 life-years lost under the 75% backlog scenario (appendix 2).
We assessed the risk–benefit balance per individual for investigatory referral, considering different rates of nosocomial infection. First, we considered absolute survival benefit, comparing prompt referral, diagnosis, and management with no referral or subsequent medical intervention (appendix 1 p 9). There was a per-patient survival benefit from referral for nearly all tumour types and age groups at a nosocomial infection risk of 1% or less. If the risk of infection is high (≥2·5% per referral), for patients older than 70 years, the risk associated with investigatory referral might exceed the absolute survival benefit for tumour-referral groups with poorer outcomes, such as upper gastrointestinal (pancreas, oesophagus, liver, and stomach) and brain tumours (appendix 1 p 9).
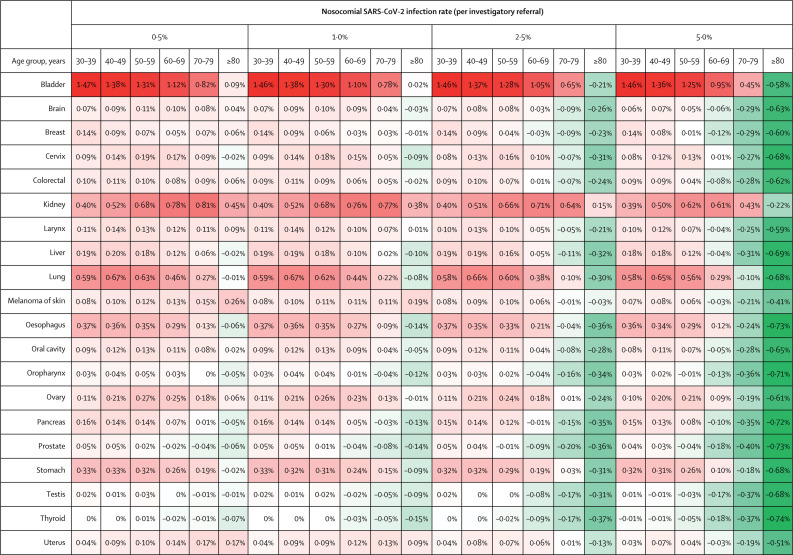
Second, we sought to address a common dilemma for primary care physicians—namely, to establish in which patients referral could be delayed by a few months, either to await reduction in nosocomial infection rates or to reduce pressure on diagnostic services. A 2-month delay in 2-week-wait investigatory referrals results in an average loss of between 0·0 and 0·7 life-years per referred patient, depending on age and tumour type (appendix 1 p 12). We compared risk of death from investigatory referral with delay-associated increase in risk of cancer death per patient referred (figure 3 ; appendix 1 pp 10–11). Factors associated with benefit of immediate referral over delay included younger patient age (<70 years), high tumour progressiveness, high diagnostic-conversion rates, and higher proportion of cases diagnosed with stage I–III disease (appendix 1 pp 1, 4, 10, 12). For those younger than 60 years, provided daily nosocomial infection rates are 2·5% or lower, even for short delays (2 months) the delay-related cancer-fatality rate largely exceeds investigation-related fatality. However, for patients older than 70 years, when the nosocomial infection rate is higher than 1%, investigation-related fatality for several tumour types of good prognosis (eg, prostate, testicular, and thyroid) or very poor prognosis (eg, pancreas, liver, oropharynx) is predicted to be greater than delay-related cancer fatality for as long as 6 months (appendix 1 pp 10–11). Bladder and kidney cancers exemplify tumour types for which prompt referral is most impactful, since these groups have a high diagnostic-conversion rate, the tumours are moderately progressive, but are predominantly stage I–III at diagnosis. In the event of stable, low nosocomial infection rate (≤0·5% per referral), we determined life-years lost for delayed referrals (appendix 1 p 12). For those referred with symptoms of bladder cancer, for a 2-month delay the average decrement per referred patient is 0·69 life-years for those aged 30–39 years and 0·10 life-years for those aged 70–79 years; for those referred with symptoms of brain tumour, the average decrement is 0·03 life-years for those aged 30–39 years and 0·01 for those aged 70–79 years.
Figure 3.
Per-patient net survival gain from urgent investigatory referral compared with 2-month delay for the 20 most common tumour types, with varying rates of nosocomial SARS-CoV-2 infection, by age group
Red indicates benefit of urgent investigatory referral and green indicates no benefit. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In multivariate sensitivity analyses, outcomes from the model were mostly sensitive to changes in the estimated per-day delay HR. Varying the delay HR by 2 SDs (eg, 16%) in the scenario of a 2% nosocomial infection rate, the total lives lost annually for the 2-week-wait population attributable to a 2-month delay ranged from 2412 to 3378, and attributable life-years lost ranged from 44 192 to 62 055 (appendix 1 p 13). Using a proportionately higher per-day delay HR for tumours of high progressiveness (delay HR = 0·0105 vs default delay HR = 0·0056) increased the impact of a 2-month delay to 3772 lives lost and 72 053 life-years lost. Varying the rate of nosocomial infection, the community infection rate, and the cancer mortality multiplier had a small effect on the impact of delay on survival.
Discussion
For most solid cancers, 10-year survival is generally considered to equate to cure, reflecting the proportion of stage I–III tumours for which their surgery (or radical radiotherapy) has enabled the restoration to normal or near-normal life expectancy. Our estimates suggest that, for many cancers, delays to treatment of 2–6 months will lead to a substantial proportion of patients with early-stage tumours progressing from having curable to incurable disease. However, this varies widely between tumour types, reflecting variation in the proportion of patients diagnosed through the 2-week-wait pathway, the proportion diagnosed with stage I–III tumours, the age profile of patients diagnosed with those cancers, and the diagnostic-conversion rate, which inevitably means that the overall impact of delays in referral via the 2-week-wait pathway is far from uniform between cancers.
During the lockdown in the UK in March–June, 2020, substantial temporal and geographical variation has been seen in rates of patient deferment in accessing urgent referral for cancer symptoms, with estimates as high as 84% (Lawler M, unpublished).2, 3 Substantial additional mortality from diagnostic delay on top of the presentational delay accrued during patient deferment is possible, especially if additional diagnostic capacity for catching up with the backlog is delayed. The additional capacity must include not only expanded technical provision for endoscopy, imaging, interventional radiology, and nuclear medicine, but also increased staffing for specialist assessment and pathology. Delivery will be further challenged by new requirements for personal protective equipment, physical distancing, and infection control. Innovative solutions will be required to deliver this extra capacity in a timely fashion, which might include procurement of private sector provision, expanded roles for health-care professionals such as endoscopy nurses, and pathway adaptation—eg, use of faecal immunochemical testing (FIT) for triage of colorectal cancer referrals.
Investment in expansion of capacity for NHS diagnostics and treatment is a priority if cancer services are to become more resilient to future extrinsic disruption, which could include additional waves of COVID-19. Additionally, more responsive informatic connections between primary care, diagnostic, and treatment services would enable improved agility in adaption of pathways and prioritisation of referrals. Furthermore, pre-emptive public education is required to discourage patients from deferring presentation of cancer symptoms along with modification of pathways to and through primary care.
Diagnostic delays will affect patient groups differently. For younger patients (<70 years), all delays should be avoided. Our data show that survival decrement for even small delays (ie, 2 months) is substantial for most tumours. Conversely, for older groups (≥70 years), per-referral risk of death from nosocomial infection is much higher and might exceed the average decrement of a moderate delay, in particular for more indolent cancer types (eg, prostate cancer) or cancers with a poor overall prognosis (eg, upper gastrointestinal tract cancers). Even in the absence of concerns about nosocomial infection, if there are pressures on diagnostic capacity, prioritisation and deprioritisation of patients according to tumour referral group and age warrants consideration as a strategy to mitigate the population-level cost of diagnostic delays in terms of lives and life-years lost.
Whether or not deaths due to SARS-CoV-2 infection, both direct and indirect (eg, compromise in collateral health-care delivery) will be outweighed by the positive impacts on mortality (eg, reduced air pollution, fewer road-traffic accidents, and handwashing) as a result of the COVID-19 and lockdown period is a matter of debate. Although our analyses examine cancer-specific survival only, the estimations of life-years gained would be altered by any sizeable shifts in life expectancy.
Here we used data for England, but cancer survival is similar across most economically developed countries, so we believe our estimates of the impact of delay for each tumour type are broadly applicable. Overall, in areas where cancer incidence, population structure, and background rates of population mortality are broadly similar to those in England, our model would provide insights relevant to other health systems, although pathways to diagnosis for different cancers, eligibility criteria, and proportions of different cancers ascertained differ between countries. Issues of capacity and delays in diagnosis are of global interest as part of moving towards benchmarked metrics (eg, International Cancer Benchmarking Partnership).3, 23
Our analysis focused only on invasive disease in common adult tumour types. Additional analyses might extend across rarer cancers, tumours of childhood, and non-invasive lesions such as dysplastic colonic adenomas. We only considered the impact of delay on patients with stage I–III disease having treatment with curative intent. Additional analyses will be required to assess the impact of delays for those having non-curative treatments.
As with all modelling, the accuracy of our predictions is contingent on the validity of assumptions and parameter estimates. Although we identified suitable observational data for delays in treatment for stages I–III for three tumour types, uniform application of these delay HRs across tumour types and over time will invariably oversimplify the complex, dynamic, tumour type-specific, age-specific, and stage-specific nature of cancer progression. To enable systematic insights across tumour types, routine capture of delays in referral pathways should be incorporated into all national cancer data collections.
Our analyses at the level of referral are subject to the limitations of data collection for diagnostic-conversion rates, which were only available at the level of tumour-referral group, precluding analyses specific to age stratum or tumour type-specific symptomatology. Furthermore, our analysis does not capture the impact of delay on survival when a 2-week-wait referral resulted in diagnosis of a different cancer outside of the index tumour-referral group (appendix 1 pp 4–5).
The current model presents a what-if prediction, in which we have included what we believe to be plausible estimates of delay applied in a simplistic non-naturalistic manner. Delay patterns will likely be complex and vary between individuals, by tumour type, over time, and by geographical location. The severity of local COVID-19 patterns, method-specific diagnostic capacity, and organisation of cancer services will all have an effect, as will local variation in pathway innovations in both diagnostics (FIT triage, colonography) and treatment (a priori use of radiotherapy and hormonal treatments). Initiatives such as DATA-CAN, the UK Health Data Research Hub for Cancer, are assembling accurate real-world data quantifying in detail true delays and patient volumes and distributions; these data could be applied to our models to refine our predictions. Over the coming months, we will also be able to quantify whether the post-lockdown surge in presentations directly mirrors the deficit during lockdown in standard 2-week-wait presentations, or whether a proportion of these patients genuinely self-resolve (ie, do not need medical attention).24
The availability of models such as those we have used will also enable more agile prospective resource planning in the face of future instances of systematic disruption of cancer services, which could include future major waves of COVID-19, other pandemics, or economic contractions.
Although the linear elements vary for the different routes to diagnosis (urgent, routine, emergency, and screening), at each step convergence exists in the resources used for diagnostics and treatment. For diagnostics, cross-competition will exist between tumour-referral groups for routine radiology, interventional radiology, and endoscopy resources. For each tumour type, a hierarchy of investigation exists. For example, patients referred for suspected lung cancer typically receive a CT scan, but only a subset of patients have an endobronchial ultrasound or bronchoscopy; nevertheless, subsequent PET-CT for staging might be the narrowest of bottlenecks in the lung pathway. To optimise recovery, integrated time-course health system analyses across different routes to diagnosis will be required, accounting for all the linear steps up to and including surgical and adjuvant treatment and considering local variation in bottlenecks to capacity.6
The impact of COVID-19-related disruption on cancer care is likely to be an ongoing issue until a vaccine or effective treatment is identified. Our modelling suggests a clinically significant impact in lives and life-years lost if delays to the 2-week-wait pathway are extensive and prolonged. Unlike acute pathologies, such as stroke and myocardial infarction, the true excess mortality due to COVID-19-related disruption to cancer pathways will not be fully evident for 10 years or longer.
Acknowledgments
Acknowledgments
AS, CT, RH, and MEJ are supported by the Institute of Cancer Research. MEJ also received funding from Breast Cancer Now. BT and AG are supported by Cancer Research UK (C61296/A27223). CL and CT receive support from the Movember foundation. RH is supported by Cancer Research UK (C1298/A8362) and Bobby Moore Fund for Cancer. GL is supported by a Cancer Research UK Advanced Clinician Scientist Fellowship Award (C18081/A18180) and is Associate Director of the multi-institutional CanTest Collaborative funded by Cancer Research UK (C8640/A23385). DCM is supported by Cancer Research UK (C57955/A24390). AS is in receipt of an Academic Clinical Lectureship from National Institute for Health Research and Biomedical Research Centre post-doctoral support. EM receives post-doctoral support from Health Data Research UK and Cancer Focus Northern Ireland grants. ML is funded by Health Data Research UK and UK Research and Innovation Industrial Strategy Challenge Fund.
Contributors
AS, CT, and RH designed the model. JB generated and did appropriate check to assert completeness and accuracy of data in the NCRAS datasets. SS generated and did appropriate checks to assert completeness and accuracy of data from the Clinical Commissioning Group-Cancer Waiting Times datasets. MEJ provided cancer progression models. BT provided mitigation models. AS and CT wrote the code for the model. MEJ, JB, ML, EM, CT, RH, AS, GL, ER, DCM, and MW provided epidemiological expertise in the parameterisation of the model and relevant literature. FG provided microbiology expertise in the estimation of nosocomial infection rates. SAB, SJ, DLN, JL, EK, CS, and NN provided details of clinical pathways. BT, AS, AG, and CL assembled the figures. CT drafted the manuscript, with substantial contribution from AS, RH, GL, ML, and EM. All authors contributed to the final manuscript.
Declaration of interests
ML reports personal fees and grants from Pfizer and personal fees from Roche outside of the submitted work. CS reports grants from Pfizer and Boehringer Ingelheim; grants and personal fees from Bristol Myers Squibb, AstraZeneca, Ono Pharmaceutical, and Roche-Ventana; personal fees from Novartis, MSD, Illumina, Celgene, GlaxoSmithKline, Genentech, Medicxi, and Sarah Canon Research Institute; personal fees and stock options from GRAIL; stock options from EPIC Biosciences and Apogen Biotech; and personal fees and being a co-founder of Achilles Therapeutics during the conduct of the study. CS has patents issued for an immune checkpoint intervention in cancer (PCT/EP2016/071471), for a method for treating cancer based on identification of clonal neo-antigens (PCT/EP2016/059401), for methods for lung cancer detection (PCT/US2017/028013), for a method of detecting tumour recurrence (PCT/GB2017/053289), for a method for treating cancer (PCT/EP2016/059401), for a method of treating cancer by targeting insertion/deletion mutations (PCT/GB2018/051893), for a method of identifying insertion/deletion mutation targets (PCT/GB2018/051892), for a method for determining whether an HLA allele is lost in a tumour (PCT/GB2018/052004), for a method for identifying responders to cancer treatment (PCT/GB2018/051912), and for a method of predicting survival rates for cancer patients (PCT/GB2020/050221). JL reports grants and personal fees from Achilles Therapeutics, Bristol-Myers Squibb, MSD, Nektar, Novartis, Pfizer, Roche, and Immunocore; personal fees from AstraZeneca, Boston Biomedical, Eisai, EUSA Pharma, GlaxoSmithKline, Ipsen, Imugene, Incyte, iOnctura, Kymab, Merck Sorono, Pierre Fabre, Secarna, Vitaccess, and Covance; and grants from Aveo and Pharmacyclics outside of the submitted work. All other authors declare no competing interests.
Supplementary Materials
References
- 1.Philpotts E. GPs made 30% fewer referrals to secondary care during March. Pulse. May 14, 2020. http://www.pulsetoday.co.uk/clinical/gps-made-30-fewer-referrals-to-secondary-care-during-march/20040818.article
- 2.Daily updates: Thursday 14 May. Providers NHS. 2020. https://nhsproviders.org/topics/covid-19/coronavirus-member-support/national-guidance/government-updates/daily-updates
- 3.Lai AG, Pasea L, Banerjee A. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. 2020 doi: 10.1101/2020.05.27.20083287. published online June 1. (preprint) [DOI] [Google Scholar]
- 4.Bower E. Urgent referrals rejected for one in three GPs during COVID-19 outbreak. GPonline. May 5, 2020. https://www.gponline.com/urgent-referrals-rejected-one-three-gps-during-covid-19-outbreak/article/1682282
- 5.Bostock N. Patients waiting more than a month for urgent cancer checks amid COVID-19 crisis. GPonline. May 20, 2020. https://www.gponline.com/patients-waiting-month-urgent-cancer-checks-amid-covid-19-crisis/article/1681673
- 6.Sud A, Jones M, Broggio J. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.05.009. published online May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHS England; Aug 16, 2018. CCG Cancer Assessment 2017/18.https://www.england.nhs.uk/publication/ccg-cancer-assessment-2017-18/ [Google Scholar]
- 8.The Cancer Waiting Times Data Collection (CWT) NHS Digital. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/cancerwaitingtimescwt
- 9.National life tables: UK. Office for National Statistics. Sept 25, 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables
- 10.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2:113–122. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson TM, Dickman PW, Eloranta S, Lambert PC. Estimating and modelling cure in population-based cancer studies within the framework of flexible parametric survival models. BMC Med Res Methodol. 2011;11:96. doi: 10.1186/1471-2288-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Kung PT, Wang YH, Kuo WY, Kao SL, Tsai WC. Effect of length of time from diagnosis to treatment on colorectal cancer survival: a population-based study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 15.Mano R, Vertosick EA, Hakimi AA. The effect of delaying nephrectomy on oncologic outcomes in patients with renal tumors greater than 4cm. Urol Oncol. 2016;34:239. doi: 10.1016/j.urolonc.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516–523. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 17.Chu AT, Holt SK, Wright JL. Delays in radical cystectomy for muscle-invasive bladder cancer. Cancer. 2019;125:2011–2017. doi: 10.1002/cncr.32048. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–115. doi: 10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 19.May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231–235. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 20.Bleicher RJ, Ruth K, Sigurdson ER. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–236. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 23.International Cancer Benchmarking Partnership (ICBP) Cancer Research UK. 2020. https://www.cancerresearchuk.org/health-professional/data-and-statistics/international-cancer-benchmarking-partnership-icbp
- 24.Dinmohamed AG, Visser O, Verhoeven RHA. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.