Abstract
The hippocampus is the key site for learning and memory and for processing of spatial information in the brain. It is divided into three main subregions: the dentate gyrus (DG), the CA3 area, and the CA1 region, which are linearly interconnected to form a so-called trisynaptic circuit. Thus, the DG sits in a strategic position to gate the flow of information from the neocortex into the hippocampal network. The granule cells (GCs), the main cell type in the DG, receive ‘where’ and ‘what’ information from the medial and lateral entorhinal cortex, respectively. How they process this mixed information remains enigmatic. By characterizing the spatial information encoded by the excitatory postsynaptic potentials (EPSPs) in GCs, we demonstrated that the majority of GCs received spatially tuned synaptic input. However, only a minority of GCs successfully converted spatially tuned input to spatially tuned output. Furthermore, we found that mature GCs were highly heterogeneous in terms of their dendritic morphology and intrinsic excitability, which contributes to the sparse and heterogeneous firing of GCs. Finally, we discuss the possible origin of this neural heterogeneity and its potential role in enlarging the computational power of the DG, facilitating pattern separation in this network.
Keywords: Dentate gyrus, granule cells, intracellular in vivo recordings, place cells, EPSPs, spatial navigation
Background
It is widely accepted that the hippocampus plays a key role in learning and memory and represents a primary site for the processing of spatial information. This was most clearly demonstrated by the detailed analysis of the patient Henry Molaison. In attempt to treat his epilepsy, he went through a bilateral surgical removal of two-thirds of his hippocampus and some associated regions. Although the surgery was partially successful in controlling his epilepsy, he became unable to form new episodic memories. Episodic memory is the memory of daily events. One of the key factors to form episodic memory is to remember where the event took place. Back in 1970’s, O’Keefe and Dostrovsky found that hippocampal neurons responded specifically to the current location of the animal. They called these cells “place cells”1. Different place cells were found to generate spikes at different locations, collectively forming an internal map of the spatial environment. These findings suggested that the hippocampus represents the brain’s cognitive map 2.
The DG sits in a strategic position, gating the flow of information from the entorhinal cortex to the downstream CA3 region. It is thought to contribute to episodic memory by functioning as a pattern separator3–5. In the mouse, the DG contains approximately one million mature GCs, while ~700 newborn GCs are integrated daily into the dentate network throughout life. The number of GCs is five times larger than the number of entorhinal cells projecting to them. Furthermore, GCs are highly connected to inhibitory interneurons, forming a lateral inhibition microcircuit6. Both divergent excitatory connectivity and lateral inhibition permit decorrelation of highly overlapping cortical inputs into distinct neuronal representations in the DG, which leads to a separation of similar contexts (pattern separation)7–9.
As the “gateway” into the hippocampus, GCs receive their primary cortical input from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC) via the perforant path. The MEC innervates the middle third of the molecular layer and encodes robust spatial and movement-related signals, which are encoded by grid cells, border cells, and head direction cells10–12. The LEC innervates the outer third of the molecular layer and provides information about local landmarks and individual objects 13,14. In addition, mossy cells in the hilar region and pyramidal cells in the CA3 region that participate in encoding spatial information backproject to the GCs15–17. The spatial tuning properties of identified GCs have only recently been elucidated, using extracellular recordings and Ca2+ imaging with rigorous cellular identification16–20. Within the population of active GCs in the DG, only a minor subset of GCs fired in a specific location of the environment, thereby displaying a single ‘place field’16–20. Despite the well-characterized anatomical projections and the spatial tuning properties of GCs, how GCs integrate diverse synaptic inputs to form a unified place field remains to be determined.
Highly similar synaptic input
Intracellular recordings using whole-cell patch-clamp recording offer a unique possibility to simultaneously record incoming and outgoing signals of the GCs. Mice were head-fixed, and trained to run for a water reward on a linear treadmill enriched with three types of cues to allow them to determine their “location”. Whole-cell patch-clamp recording from single GCs was combined with local field potential recording in the DG molecular layer to record neuronal population activity. To differentiate between types of excitatory neurons, recorded cells were filled with biocytin during recording, allowing subsequent reconstruction of their morphological properties. Synaptic EPSP events were detected by a novel and efficient detection algorithm that we developed based on supervised machine learning and Wiener filtering21. Analysis of the EPSP spatial tuning vector as a function of position demonstrated that ~50% of GCs received spatially tuned synaptic input. Furthermore, fine-structure analysis of EPSP frequency against position by Fourier transformation revealed that GCs abundantly received conjunctive place-grid-like synaptic input22.
Sparse but heterogeneous AP output
Early in vivo electrophysiological recordings suggested that GCs fire action potentials (APs) at high frequency in the center of a place field and during short-term memory tasks23,24. In contrast, more recent electrophysiological recordings, analysis of immediate early genes (IEG) expression, and Ca2+ imaging experiments indicated that GCs fire only sparsely in a given environment, while a major fraction is completely silent7,16–19,25. These confusing findings likely result from the relative strengths and limitations of the applied methods. Extracellular electrophysiological recordings provide a high temporal resolution but lack cellular identification. Thus, it is likely that the GC activity recorded by this method is biased by the activity of the most active neurons, such as immature GCs and mossy cells16,17,24. In contrast, IEG methods provide spatial resolution and cellular identification, but lack temporal resolution26. Neuronal activity, inferred from Ca2+, signals might be insufficiently sensitive to differentiate single spikes from bursts17,18,20. Using intracellular recording from identified GCs allowed us to obtain “ground truth” data and revealed that the activity of mature GCs was sparse but highly heterogeneous. The activity level ranged from complete silence to a mean firing rate as high as 3 Hz, with firing patterns varying from single APs to bursts and “superbursts”. Different firing patterns may have different impact on the downstream CA3 network. When the superbursts are applied as stimuli at single mossy fiber boutons in vitro, they are capable of inducing post-tetanic potentiation in postsynaptic CA3 pyramidal cells, and to facilitate AP initiation in postsynaptic cells. This may enhance pattern completion in the CA3 region27.
Gating mechanisms of input-output conversion
Surprisingly, while the majority of mature GCs receive significant spatially tuned synaptic input, only a small subset of highly active GCs (with firing rate above 1 Hz) could successfully convert this spatially tuned input into spatially tuned output. What are the mechanisms underlying the sparse but heterogeneous firing pattern of GCs? Differences in extrinsic synaptic input or in intrinsic properties of GCs may cause this variation. Our results demonstrate that neither somatic EPSP amplitudes and frequency nor the degree of spatial tuning showed any correlation to the firing rate of GCs. Thus, extrinsic factors did not explain the heterogeneous activity of the GCs. We next tested whether the intrinsic properties of GCs contributed to the heterogeneous activity. The input resistance of all recorded GCs was in the range of 200–400 MΩ, indicating that they were mature GCs. However, the intrinsic excitability characterized by relative threshold, maximal rate of rise, and peak amplitude of APs showed a highly significant correlation with the firing frequency. In addition, dendritic topological analysis revealed that highly active GCs had significantly more complex dendritic trees than sparsely active GCs, corroborating previous observations19. Together, these results indicate that mature GCs are highly heterogeneous, with intrinsic excitability and dendritic morphology contributing to their heterogeneous firing patterns.
Neuronal heterogeneity in the DG network
How does the heterogeneity of mature GCs originate? Several possible explanations have to be considered. First, it is possible that different activity levels correspond to different maturational stages of GCs. A unique and intriguing feature of the DG is its neurogenesis, which continuously adds new adult-born GCs into the existing dentate network throughout life28. Although newborn GCs show an initial period of hyperexcitability followed by a later period of reduced excitability29, there is increasing evidence that newborn cells undergo further maturation of both excitability and synaptic output for an extended period of time. Alternatively, the active cells may belong to recently formed or reactivated engrams, consistent with the finding that engram cells are more excitable than non-engram cells30. In this framework, it is possible that silent GCs become active in other behavioral contexts. Alternatively, silent GCs may represent a “reserve” for future encoding of information. Finally, it is conceivable that “silent” GCs fire at extremely low average frequency, contributing to extremely sparse encoding on information. As sparsification contributes to decorrelation, such a mechanism could add to pattern separation. Further work is required to distinguish between these possibilities.
Conclusion and Perspectives
Our results provide new insights onto how GCs contribute to higher-order computations at both single-cell and population level. At the single-cell level, GC activity is controlled by intrinsic neuronal properties, allowing GCs to convert a broadly tuned input into a more precisely tuned output. At the population level, our results suggest a parallel model of information processing in which spatial input information is distributed onto a small population of more active cells and a large population of less active neurons. Neuronal maturation over an extended time period or engram formation could underlie the heterogeneity to the DG network, and provide a continuous updating of the GC code, analogous to more barcodes being available to link to books in a library.
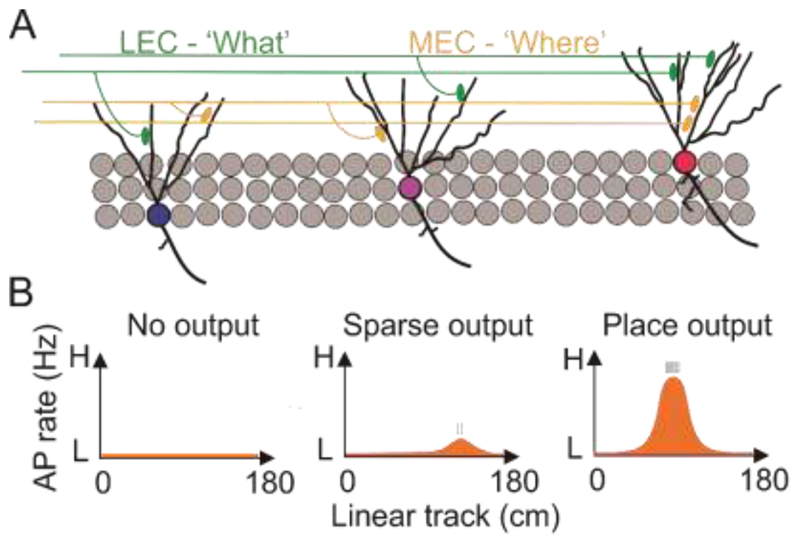
Fig. 1. Parellel model of information processing in the denate gyrus.
(A) Granule cells receive information of ‘what’ and ‘where’ from LEC and MEC, respectively. (B) Similar place tuned synaptic inputs cause distinct spatial output depending on intrinsic properties.
Acknowledgements
Dr. Jonas acknowledges the support of the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 692692) and the Fond zur Förderung der Wissenschaftlichen Forschung (Z 312-B27, Wittgenstein award). Dr. Zhang acknowledges an IST fellowship jointly funded by IST and the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement.
References
- 1.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-886(76)900558. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford University Press; 1978. [Google Scholar]
- 3.O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a tradeoff. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 4.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 5.Jonas P, Lisman J. Structure, function, and plasticity of hippocampal dentate gyrus microcircuits. Frontiers in Neural Circuits. 2014;9:107. doi: 10.3389/fncir.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinoza C, Guzman SJ, Zhang X, Jonas P. Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat Commun. 2018;9 doi: 10.1038/s41467-018-0689. 4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded Input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 11.Sargolini F. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 12.Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, et al. Egocentric coding of external items in the lateral entorhinal cortex. Science. 2018;362:945–949. doi: 10.1126/science.aau4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0369. 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharfman HE. Progress in Brain Research. Vol. 163. Elsevier; 2007. The CA3 “backprojection” to the dentate gyrus; pp. 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senzai Y, Buzsáki G. Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron. 2017;93:691–704.e5. doi: 10.1016/j.neuron.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GoodSmith D, et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron. 2017;93:677–690.e5. doi: 10.1016/j.neuron.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielson NB, et al. Distinct Contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90:101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamantaki M, Frey M, Berens P, Preston-Ferrer P, Burgalossi A. Sparse activity of identified dentate granule cells during spatial exploration. eLife. 2016;5:e20252. doi: 10.7554/eLife.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainmueller T, Bartos M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature. 2018;558:292–296. doi: 10.1038/s41586-018-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Schlögl A, Vandael D, Jonas P. MOD: A novel machine-learning optimal-filtering method for accurate and efficient detection of subthreshold synaptic events in vivo. 2020 doi: 10.1101/2020.07.04.186478. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Schlögl A, Jonas P. Selective routing of spatial information flow from input to output in hippocampal granule cells. Neuron. 2020;107 doi: 10.1016/j.neuron.2020.07.006. 12121225.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 24.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 25.Pilz G-A, et al. Functional imaging of dentate granule cells in the adult mouse hippocampus. J Neurosci. 2016;36:7407–7414. doi: 10.1523/JNEUROSCI.3065-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla MK, et al. Sparse, environmentally selective expression ofArc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 27.Vandael D, Borges-Merjane C, Zhang X, Jonas P. Short-term plasticity at hippocampal mossy fiber synapses is induced by natural activity patterns and associated with vesicle pool engram formation. Neuron. 2020;107:509–521.e7. doi: 10.1016/j.neuron.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn H, Dickinson-Anson H, Gage F. Neurogenes is in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 30.Pignatelli M, et al. Engram cell excitability state determines the efficacy of memory retrieval. Neuron. 2019;101:274–284.e5. doi: 10.1016/j.neuron.2018.11.029. [DOI] [PubMed] [Google Scholar]