Abstract
Current therapies for common types of cancer such as renal cell cancer often are ineffective and unspecific, and novel pharmacological targets and approaches are in high demand. Here we show the unexpected possibility for rapid and selective killing of renal cancer cells through activation of calcium-permeable non-selective transient receptor potential canonical (TRPC) calcium channels by the sesquiterpene (-)-Englerin A that was found to be a highly-efficient, fast-acting, potent, selective and direct stimulator of TRPC4 and of TRPC5 channels. TRPC4/5 activation through a high-affinity extracellular (-)-Englerin A binding site may open up novel opportunities for drug discovery aimed at renal cancer.
Keywords: Natural products, renal cancer, ion channels, TRPC4, calcium ions
Renal cell carcinoma (RCC) is a frequently occurring, particularly challenging malignancy[1] as it is often diagnosed with poor prognosis at the metastatic stage. Only few non-curative treatments with serious side effects are available for RCC.[2] Therefore, the discovery of innovative targets for RCC treatment and small molecule modulators of their function is of major current interest. Highly potent natural products may enable the identification of novel targets for drug discovery.[3]
The sesquiterpene (-)-Englerin A[4,5] 1 is a very potent and selective inhibitor of renal cancer cell growth compared to cancer cell lines of different origin and, even more remarkably, compared to normal kidney cells. Sourbier et al. recently suggested that Englerin A directly activates PKCtheta, which results in phosphorylation of insulin receptor substrate 1 (IRS1) and the transcription factor HSF1 to simultaneously cause insulin resistance and glucose dependence.[4d] While investigating the bioactivity of Englerin A and derivatives with enhanced potency,[4b] to our surprise, we determined that the renal cancer cell line A498 that is most sensitive for (-)-Englerin A among the cell lines investigated by Ratnayake et al.[4a, 4c] does not express PKCtheta (Supporting Figure 1). Thus, at least in this cell line the natural product must target a different protein. In addition, in time-resolved investigations we found that Englerin A-induced cytotoxicity is manifested already within minutes (Supporting Figure 2, Supporting Movies 1 and 2). This finding excludes regulation of gene transcription which typically occurs on the time scale of hours as the primary mode of action and is also indicative of a different primary target. Here we report that (-)-Englerin A is a selective and potent activator of the transient receptor potential Ca2+ channel C4 (TRPC4) in RCC cells and that Englerin A induces cell death by elevated Ca2+ influx and Ca2+ cell overload.
Initial attempts to identify the cellular target of (-)-Englerin A by means of affinity-based chemical proteomics experiments remained fruitless, indicating that the cellular target of Engerin A may be a low abundant protein and/or a membrane protein.
Low abundance G-protein-coupled receptors (GPCRs) and ion channels usually respond to stimuli within milliseconds to seconds, and typically cannot be identified by established chemical proteomics methods. Investigation of several prototypic GPCRs and ion channels as potential (-)EA targets revealed only occasional weak inhibition (Supporting Table 1 and 2). However, a report linking transient receptor potential canonical channel 4 (TRPC4) to renal cell carcinoma[6] led us to pursue functional studies of this protein. This hypothesis was underscored by gene expression analysis of the NCI60 cell line panel that had revealed a highest degree of expression of TRPC4 in A498 cells among the NCI60 cell lines (http://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS4296:224219_s_at). TRPC4 is one of 6 members of the membrane-spanning TRPC family of human proteins, a subset of the TRP super-family [7] which assembles as homo- or hetero- tetramers to form Ca2+-permeable non-selective cationic channels [8]. Despite much effort towards understanding TRPC4 channels, the absence of highly potent, selective and efficacious modulators [8b] has been a major limitation on progress.
In HEK 293 cells over-expressing human TRPC4 (Supporting Figure 3a) 3 nM (–)EA evoked sustained elevation of the intracellular Ca2+ concentration within 1 minute (Figure 1a). At the higher concentration of 100 nM it caused an even larger increase in Ca2+ that contrasted markedly with the small or undetectable responses to previously reported TRPC4 activators (Figure 1b and Supporting Figure 4a and b). The response to 100 nM (–)EA was abolished by removal of extracellular Ca2+ and unaffected by prior depletion of intracellular Ca2+ stores suggesting mediation by Ca2+ entry rather than Ca2+ release (Figure 1c and Supporting Figure 4). (+)Englerin A[4b] was ineffective at elevating Ca2+ in TRPC4-expressing cells even at the higher concentration of 1 µM (Supporting Figure 4c), showing stereoisomeric specificity. In support of TRPC4 channel activation by (–)EA, whole-cell voltage-clamp recordings revealed large (–)EA-evoked currents with a seat-like inflection in the current-voltage relationship (I-V) (Figure 1d), a finger-print of TRPC4 and closely-related channels.[9] The concentration of (–)EA required for 50 % activation (EC50) was 11.2 nM (Figure 1e). We tested if (–)EA affects other TRP channels, starting with TRPC4’s closest relative, TRPC5. There was striking activation of Ca2+ entry in HEK 293 cells over-expressing TRPC5 and the potency was again impressive (EC50 7.6 nM) (Figure 1f and Supporting Figure 3b). In contrast, cells over-expressing TRPC6, TRPM2 or TRPV4 (Supporting Figure 3c and Supporting Figure 5) lacked responses to (–)EA. The data suggest (–)EA as a potent, efficacious and selective activator of TRPC4 and TRPC5 channels. TRPC4/5 channels are promiscuously stimulated by agonists acting via GPCRs [8a,9a,10]. We therefore strongly blocked all G protein activity by exposing the intracellular face of excised membranes to a high concentration of the stable guanosine diphosphate analogue GDP-β-S and recorded TRPC4 channel currents. Application of 100 nM (–)EA to the extracellular surface of membranes rapidly and strongly activated hundreds of TRPC4 channels leading to macroscopic currents that were so large that they resembled whole-cell currents (Figure 1g). Currents returned to baseline quickly after wash-out of (–)EA and a second similar response was readily evoked when (–)EA application was repeated (Figure 1g). The evoked current was suppressed reversibly by 5 μM of the reported TRPC4 inhibitor ML204[11] (Figure 1g). 10 nM (–)EA was also effective at evoking currents in the excised membrane patch and the concentration-dependence was similar to that seen in Ca2+ measurement studies (Figure 1g). The data suggest that (–)EA does not act via G protein signaling.
Figure 1. (–)EA is a potent and highly efficacious activator of TRPC4/5 channels.
(a-c, e-f) Measurements of the free intracellular calcium ion (Ca2+ i) concentration shown as the fura-2 fluorescence (F) ratio or change (Δ) in this ratio. Extracellular Ca2+ was 1.5 mM unless otherwise indicated. (a) Genetically-modified HEK 293 cells not induced (HEK (Tet-)) or induced using tetracycline (Tet+) to over-express TRPC4 (HEK-TRPC4). After 1 min of recording 3 nM (–)EA was applied to the extracellular medium as indicated by the vertical dashed line (N=5 each). Representative of n=4. (b-e) Genetically-modified HEK 293 cells induced to over-express TRPC4 (HEK-TRPC4). (b) Extracellular application of 100 µM carbachol (CCh) and then, in addition, 100 nM (–)EA (N=6 each). Representative of n=3. (c) Application of 100 nM (–)EA in the presence (1.5 Ca2+ e) or absence (0 Ca2+ e) of extracellular 1.5 mM Ca2+ (N=6 each). Representative of n=3. (d) Whole-cell voltage-clamp recording of membrane current (I) from a single-cell during ramp changes in membrane voltage (V) from –100 to +100 mV, shown during the application of extracellular vehicle (dimethyl sulfoxide and pluronic acid) and then 100 nM (–)EA. The arrow points to the seat-like inflection in the I-V. Typical of n=12 (4 with standard pipette solution, 8 with aspartate solution). (e) Concentration-response data for (–)EA (n/N=4/18-19). The fitted curve is a Hill equation indicating 50 % maximum effect (EC50) at 11.2 nM. (g) As for (e) except the cells were genetically-modified HEK 293 cells induced to over-express TRPM2 (HEK-TRPM2) (n/N=3/18). (g-i) Ionic currents across outside-out membrane patches from genetically-modified HEK 293 cells induced to over-express TRPC4 (HEK-TRPC4). (g) As indicated by the inset diagram 1 mM guanosine 5′-[β-thio]diphosphate (GDP-β-S) was in the patch pipette and (–)EA (EA) was bath-applied to the extracellular surface of the membrane (indicated by horizontal bars above the experimental traces). ML204 (ML) was also bath-applied. The vehicle (dimethyl sulfoxide and pluronic acid) was kept constant throughout the recording. Ramp changes in membrane voltage from –100 to +100 mV were applied every 10 s and the currents sampled at –100 and +100 mV are displayed. Typical of n=4. (h) As for (g) except, as indicated by the inset diagram, GDP-β-S (and ATP) were omitted from the patch pipette and the pipette contained 100 nM (–)EA. Typical of n=3. (i) From the experiment shown in (h), full current traces during two ramp changes in voltage, one before ((–)EA i) and the other after bath-application of (–)EA ((–)EA e+i). The arrow points to the seat-like inflection in the I-V.
We then recorded from excised membrane patches but in the absence of any nucleotides or other regulatory co-factors and with the intracellular face of the channels exposed to 100 nM (–)EA from the outset. Although there were constitutive currents (Figure 1h) they did not exhibit the I-V characteristics of TRPC4 channels (Figure 1i), suggesting TRPC4-independent background currents and that (–)EA did not activate TRPC4 channels via an intracellular site. In contrast 100 nM (–)EA subsequently applied to the extracellular surface of the same patch repeatedly activated currents that were ML204 sensitive (Figure 1h) and exhibited TRPC4’s seat-like inflection in the I-V (Figure 1i). The data suggest that (–)EA activates TRPC4 channels directly via a site exposed externally or accessible only via the external leaflet of the bilayer.
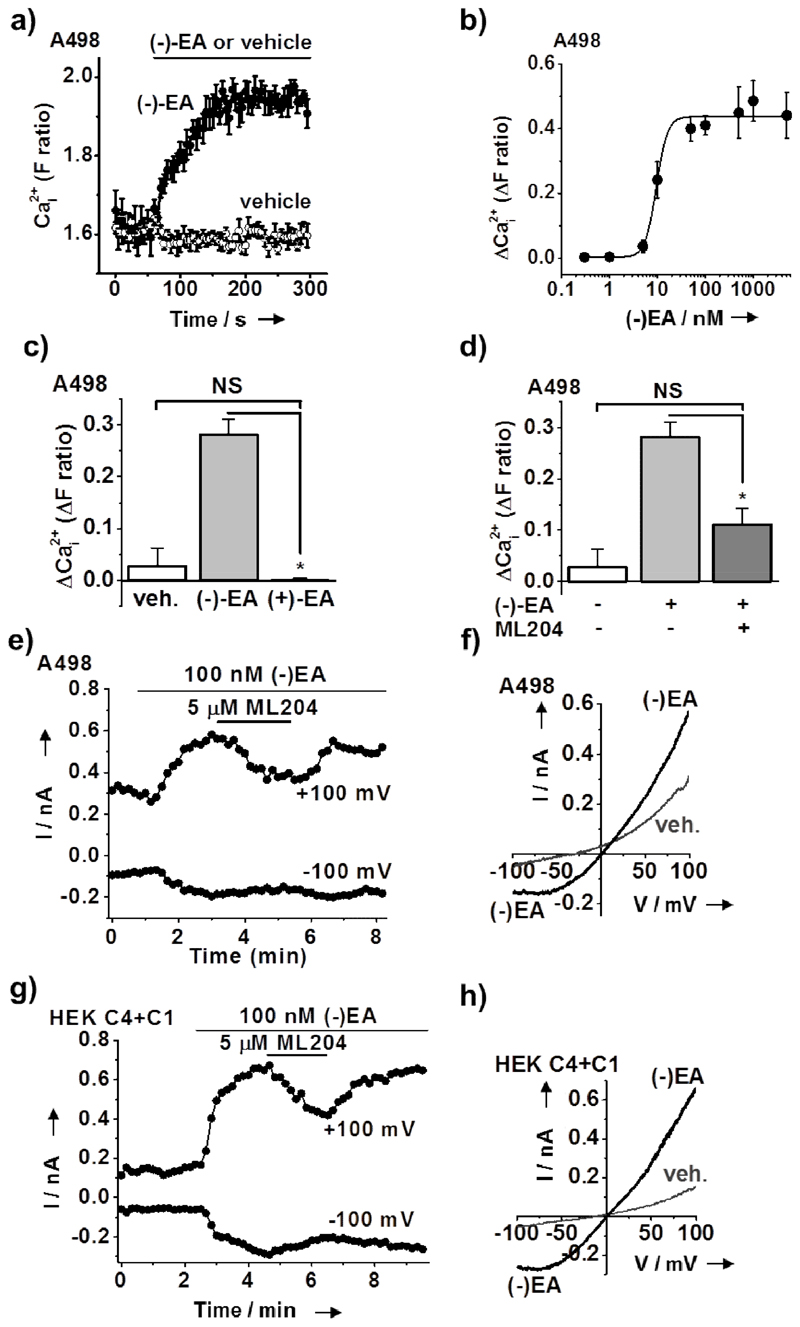
(–)EA evoked intracellular Ca2+ elevations in A498 cells as well with an EC50 of 10 nM (Figure 2a, b) whereas 1 μM (+)Englerin A was ineffective (Figure 2c). Moreover the Ca2+ elevations were ML204 sensitive, consistent with Ca2+ entry involving TRPC4 (Figure 2d) and supported by the finding that TRPC4 but not TRPC5 is detected in A498 cells (Supporting Figure 6). In whole-cell voltage-clamp recordings 100 nM (–)EA evoked ML204-sensitive current (Figure 2e) but the characteristic seat-like inflection of the TRPC4 I-V was lacking (Figure 2f). We hypothesized that the different shape was due to native co-expression of the broadly-expressed TRPC1 protein, which does not produce channels on its own but forms heteromers with TRPC4 and removes the seat-like inflection in the I-V [9c,12]. We therefore co-expressed TRPC4 with TRPC1 in HEK 293 cells to generate over-expressed heteromers, which almost perfectly reproduced the response of native A498 channels to (–)EA (Figure 2g and h). The data suggest that (-)EA also activates TRPC1/4 channels and that these heteromeric channels are the ones activated by (–)EA in A498 cells.
Figure 2. (–)EA activated endogenous TRPC4-containing channels of renal carcinoma cells are a mechanism for selective drug-induced cell death.
(a-d) Measurements of the free intracellular calcium ion (Ca2+ i) concentration in A498 cells shown as the fura-2 fluorescence (F) ratio or change (Δ) in this ratio. (a) Example effect of extracellular application of (–)EA or its vehicle control (n/N=1/5 each). (b) Concentration-response data (n/N=3/45) with a fitted Hill equation indicating an EC50 of 9.5 nM. (c) Mean responses after 4 min exposure to vehicle, 1 µM (–)EA, or 1 µM (+)EA (n/N=4/23 each). (d) Mean responses after 4 min exposure to vehicle, 1 µM (–)EA, or 1 µM (–)EA in the presence of 5 µM ML204 (n/N=4/23 each). (e) Whole-cell voltage-clamp recording of membrane current from a single A498 cell during ramp changes in membrane voltage from -100 to +100 mV applied every 10 s. Only current sampled at –100 and +100 mV is displayed. 100 nM (–)EA and 5 µM ML204 were bath-applied as indicated by the horizontal bars. Representative from n=11 (standard pipette solution) and n=5 (aspartate pipette solution). (f) From the experiment shown in (e) full current traces during two ramp changes in voltage, one during the initial application of vehicle (veh.) (dimethyl sulfoxide and pluronic acid) and the other after the application of (–)EA and before ML204. (g, h) As for (e, f) except genetically-modified HEK 293 cells induced to over-express TRPC4 and transiently express TRPC1 (HEK C4+C1). Representative from n=3 (standard pipette solution).
Since (–)EA does not cause death of all types of cancer cells, we explored the (-)EA-resistant colorectal adenocarcinoma cell line HT29.[4a] HT-29 cells were confirmed as resistant to (–)EA-induced cell death (Supporting Figure 7a) and then studied by whole-cell patch-clamp recording. No (–)EA-activated current or ML204 sensitivity was detected (Supporting Figure 7b). The data suggest that (–)EA-activated current occurs only in cell types with susceptibility to (–)EA-induced cell death.
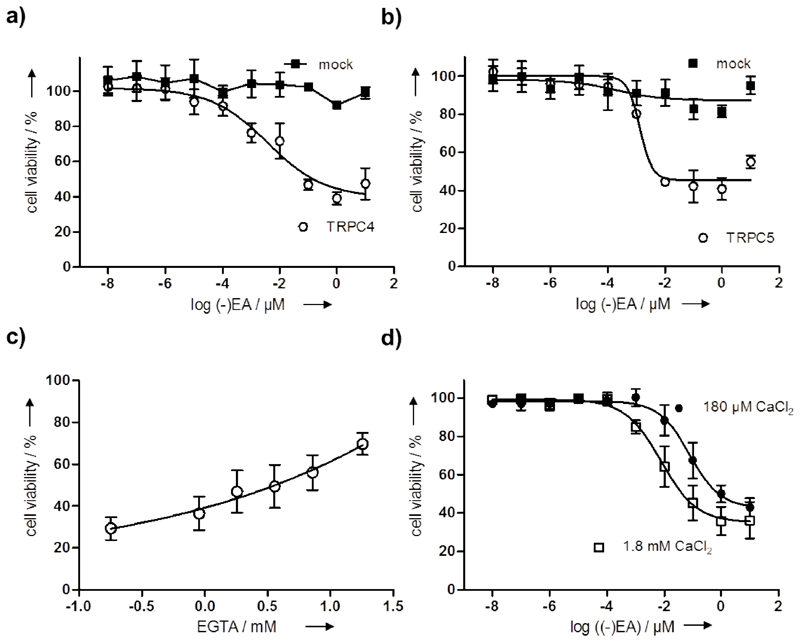
The relationship of (–)EA-activated TRPC4/5 channels to cell viability was investigated by attempting to confer (–)EA-induced death on HEK 293 cells, which are normally resistant.[4b] We compared HEK 293 cells over-expressing TRPC4, TRPC5 or TRPM2. (–)EA had no effect on HEK 293 cells over-expressing TRPM2 but potently suppressed HEK 293 cell viability when TRPC4 or TRPC5 was over-expressed (Figure 3a, b, Supporting Figure 8 and Supporting Movies 3-6). To determine whether Ca2+ influx is the cause for cell death, we pre-incubated A498 cells with increasing concentrations of the cell-impermeable Ca2+ chelator EGTA to reduce the amount of free Ca2+ in the culture medium prior to addition of 100 nM (-)EA. Interestingly, (-)EA-mediated reduction of cell viability was dose-dependently rescued by EGTA (Figure 3c). In addition, lowering the Ca2+ concentration in the culturing medium from 1.8 mM to 180 µM resulted in 10fold higher IC50 for inhibition of cell viability (Figure 3d) indicating that Ca2+ influx and thus Ca2+ cell overload are the cause for the devastating influence of (-)EA in this cell line. These results are in agreement with the finding that overexpression of constitutively-active mutants of TRPC4 or TRPC5 confers Ca2+-dependent death on HEK 293 cells.[14] The data suggest that activation of TRPC4/5 channels is a mechanism for (–)EA-induced cell death caused by Ca2+ overload.
Figure 3. TRPC4/5 are involved in the (-)EA-mediated decrease of cell viability.
(a-b) HEK293 cells were transiently transfected with plasmids for ectopic expression of TRPC4 (a) or TRPC5 (b). 8 h later cell were replated and allowed to grow for 48 h. Cells were treated with different concentrations of (-)EA for 4 h prior to determination of cell viability using the WST-1 reagent. Data are mean values (N=4) ± s.d. and are normalized to cells treated with DMSO. Data were fitted using four-parameter Hill equation and are representative of three independent experiments. (c) Different concentrations of EGTA and 100 nM (-)EA were added to A498 cells prior to subsequent determination of cell viability. Data are shown as mean values ± s.d. (n=3, N=4). (d) A498 cells were incubated for 24 h in medium containing 180 µM CaCl2 or 1.8 mM CaCl2 prior to addition of different concentrations of (-)EA followed by determination of cell viability. Data are shown as mean values ± s.d. (n=3, N=4) and were fitted using four-parameter Hill equation indicating IC50 of 77.7 nM in presence of 180 µM CaCl2 and 7.5 nM in presence of 1.8 mM CaCl2.
TRPC channels have remained enigmatic despite much investigation.[8,10] Progress towards understanding the channels has been handicapped by the absence of selective highly efficacious activators and lack of potent or selective small-molecule inhibitors.[8b] (–)EA or analogues of it now open up entirely novel opportunities for studies aimed at better understanding the biology of TRPC4/5 channels.
Moreover, the results suggest an unanticipated opportunity for addressing the problem of renal cell carcinoma through small-molecule activation of TRPC4 or TRPC4-containing channels.
Supplementary Material
Contributor Information
Mathias Christmann, Email: mathias.christmann@fu-berlin.de.
David J Beech, Email: d.j.beech@leeds.ac.uk.
Herbert Waldmann, Email: herbert.waldmann@mpi-dortmund.mpg.de.
References
- [1].a) Haase VH. Exp Cell Res. 2012;318:1057–1067. doi: 10.1016/j.yexcr.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. Eur Urol. 2011;60:1317–1317. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- [2].a) Esper P. Semin Oncol Nusr. 2012;28:170–179. doi: 10.1016/j.soncn.2012.05.006. [DOI] [PubMed] [Google Scholar]; b) Vasudev NS, Selby PJ, Banks RE. BMC Med. 2012;10:112. doi: 10.1186/1741-7015-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Koehn FE, Carter GT. Nat Rev Drug Discovery. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]; b) Wilson RM, Danishefsky SJ. J Org Chem. 2006;71:8329–8351. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]; c) Kaiser M, Wetzel S, Kumar K, Waldmann H. Cell Mol Life Sci CMLS. 2008;65:1186–1201. doi: 10.1007/s00018-007-7492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bon RS, Waldmann H. Acc Chem Res. 2010;43:1103–1114. doi: 10.1021/ar100014h. [DOI] [PubMed] [Google Scholar]; e) Wetzel S, Bon RS, Kumar K, Waldmann H. Angew Chem. 2011;123:10900–11018. doi: 10.1002/anie.201007004. [DOI] [PubMed] [Google Scholar]; f) Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) van Hattum H, Waldmann H. J Am Chem Soc. 2014;136:11853–11859. doi: 10.1021/ja505861d. [DOI] [PubMed] [Google Scholar]; h) Tao L, Zhu F, Qin C, Zhang C, Xu F, Tan CY, Jiang YY, Chen YZ. Nat Biotechnol. 2014;32:979–980. doi: 10.1038/nbt.3034. [DOI] [PubMed] [Google Scholar]
- [4].Bioactivity of (-)Englerin A and analogues [Google Scholar]; a) Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Org Lett. 2009;11:57–60. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Radtke L, Willot M, Sun HY, Ziegler S, Sauerland S, Strohmann C, Frohlich R, Habenberger P, Waldmann H, Christmann M. Angew Chem. 2011;123:4084. doi: 10.1002/anie.201007790. Angew. Chem. Int. Ed. 2011, 50, 3998-4002. [DOI] [PubMed] [Google Scholar]; c) Sulzmaier FJ, Li ZW, Nakashige ML, Fash DM, Chain WJ, Ramos JW. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048032. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Sourbier C, Scroggins BT, Ratnayake R, Prince TL, Lee S, Lee MJ, Nagy PL, Lee YH, Trepel JB, Beutler JA, et al. Cancer Cell. 2013;23:228–237. doi: 10.1016/j.ccr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Williams RT, Yu AL, Diccianni MB, Theodorakis EA, Batova A. J Exp Clin Cancer Res. 2013;32 doi: 10.1186/1756-9966-32-57. For further studies concerning the bioactivity of Englerin A see ref. 5g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Total syntheses of (-)Englerin A [Google Scholar]; a) Willot M, Radtke L, Konning D, Frohlich R, Gessner VH, Strohmann C, Christmann M. Angew Chem. 2009;121:9269–9272. doi: 10.1002/anie.200905032. Angew. Chem. Int. Ed. 2009, 48, 9105-9108. [DOI] [PubMed] [Google Scholar]; b) Molawi K, Delpont N, Echavarren AM. Angew Chem. 2010;122:3595–3597. doi: 10.1002/anie.201000890. Angew. Chem. Int. Ed. 2010, 49, 3517-3519. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Kang QA, Ng SY, Chen DYK. J Am Chem Soc. 2010;132:8219–8222. doi: 10.1021/ja102927n. [DOI] [PubMed] [Google Scholar]; d) Xu J, Caro-Diaz EJE, Theodorakis EA. Org Lett. 2010;12:3708–3711. doi: 10.1021/ol1015652. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Zhou QH, Chen XF, Ma DW. Angew Chem. 2010;122:3591–3594. Angew. Chem. Int. Ed. 2010, 49, 3513-3516. [Google Scholar]; f) Li ZW, Nakashige M, Chain WJ. J Am Chem Soc. 2011;133:6553–6556. doi: 10.1021/ja201921j. [DOI] [PubMed] [Google Scholar]; g) Pouwer RH, Richard JA, Tseng CC, Chen DYK. Chem Asian J. 2012;7:22–35. doi: 10.1002/asia.201100780. [DOI] [PubMed] [Google Scholar]; h) Zahel M, Kessberg A, Metz P. Angew Chem. 2013;125:5500–5502. doi: 10.1002/anie.201301247. Angew. Chem. Int. Ed. 2013, 52, 5390-5392. [DOI] [PubMed] [Google Scholar]
- [6].Veliceasa D, Ivanovic M, Hoepfner FT, Thumbikat P, Volpert OV, Smith ND. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- [7].a) Damann N, Voets T, Nilius B. Cur Biol CB. 2008;18:R880–889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]; b) Moran MM, McAlexander MA, Biro T, Szallasi A. Nat Rev Drug discovery. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- [8].a) Abramowitz J, Birnbaumer L. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bon RS, Beech DJ. Br J Pharmacol. 2013;170:459–474. doi: 10.1111/bph.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. The J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]; b) Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, et al. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, Kim SY, Jeon JH, Kim HJ, So I. Pflugers Arch. 2014;466:491–504. doi: 10.1007/s00424-013-1332-y. [DOI] [PubMed] [Google Scholar]
- [10].Beech DJ. Circ J. 2013;77:570–579. doi: 10.1253/circj.cj-13-0154. [DOI] [PubMed] [Google Scholar]
- [11].Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, et al. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- [13].Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ. Nature Biotechnol. 2005;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- [14].Beck A, Speicher T, Stoerger C, Sell T, Dettmer V, Jusoh SA, Abdulmughni A, Cavalie A, Philipp SE, Zhu MX et al. J Biol Chem. 2013;288:19471–19483. doi: 10.1074/jbc.M113.478305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.